Abstract
Objective(s):
The neurodegeneration and loss of memory function are common consequences of aging. Medicinal plants have potent protective effects against chronic neurodegenerative diseases. The aim of this study was to investigate the beneficial effects and molecular mechanisms of crocin on brain function in D-galactose (D-gal)-induced aging model in rats.
Materials and Methods:
Male Wistar rats weighing 220 ± 20 g were randomly divided into six groups: control, D-gal (400 mg/kg, SC), D-gal (400 mg/kg) plus crocin (7.5, 15, 30 mg/kg, IP) and crocin alone at dose of 30 mg/kg for 8 weeks. The neuroprotective effects of crocin were evaluated by Morris water maze, determination of malondialdehyde (MDA) levels and Western blot analysis.
Results:
Crocin significantly inhibited the neurotoxic effects of D-gal through improvement of spatial learning and memory functions as well as the reduction of MDA levels. It was also found that administration of crocin up-regulated pAkt/Akt and pErk/Erk ratio which were decreased by chronic D-gal treatment. In addition, the elevated level of carboxymethyl lysine (CML), as an advance glycation product (AGE), NF-κB p65, TNFα and IL1β significantly decreased in crocin treated rats compared to D-gal group.
Conclusion:
These findings suggest that crocin is able to enhance memory function in D-gal aging model through anti-glycative and anti-oxidative properties which finally can suppress brain inflammatory mediators (IL-1, TNF and NF-κB) formations and increase PI3K/Akt and Erk/MAPK pathways activity. Therefore, crocin can be considered as healthcare product to prevent age-related brain diseases such as Alzheimer.
Keywords: Advance glycation product, Brain aging, Crocin, D-galactose, Inflammation
Introduction
Recently because of prolonged life span, aging process is the main purpose of literatures to find its related mechanisms. Aging is a gradual and progressive process that decreases the functions of several organs. Alzheimer disease (AD) with loss of memory and learning ability is the most common neurodegenerative disorder in elderly (1). The main approved mechanism of brain aging is oxidative stress and vulnerability to reactive oxygen species (ROS) accumulation (2). Also, many studies have shown the critical roles of advanced glycation end products (AGEs) in induction of AD process. AGEs participate in the AD progression by elevation of ROS production and inflammatory responses (3). These heterogeneous compounds are formed under excessive glucose or specific diets throughout several reactions that Millard reaction is the most common pathway. The Millard and same reactions induce dicarbonyl compounds, which finally produce stable irreversible products called AGEs. Pentosidine and N-(carboxymethyl) lysine)CML(as predominant
AGEs are created in the complex processes of glycation and oxidation (4). AGEs interaction with the receptor of AGEs (RAGE), a multi ligand receptor, starts AGEs-related molecular damages. AGE-RAGE interaction leads to ROS elevation and alteration the activity of mitogen-activated protein kinase (MAPK) (5), Janus kinase (JNK) (6) and Rho- Guanine triphos-phatases (Rho-GTPases) (7) which triggers nuclear factor kappa B (NF-κB) expression (8). NF-kappa B has an important role in developing inflammatory responses and tissue damages through increasing pro-inflammatory cytokines, induction of oxidative stress and apoptosis (9).
The activation of the AGE/RAGE pathway in brain tissue and neuronal cells has a critical role in enhancing oxidative stress (10). In D-galactose (D-gal) treated rats the brain level of glycative products and ROS significantly increased which elevated the expression of inflammatory markers such as cyclooxygenase-2 (COX-2), interleukin-1beta (IL-1β), interleukin-6, tumor necrosis factor-alpha (TNF-α) and prostaglandin E2 (PGE2) (11). D-gal injection in mice induced disturbance in learning and memory performance, increased malondialdehyde (MDA) level, nitric oxide production, inducible nitric oxide synthase activity, and decreased glutathione (GSH) content, superoxide dismutase (SOD) and glutathione peroxidase (GPx) activities in the brain tissue due to phosphatidylinositol 3-kinase (PI3K) and protein kinase B (Akt) genes down regulation (1). Additionally, it has been found that D-gal induced learning and memory impairment through reduction of phospho Erk1/2 (pErk 1/2) expression in hippocampus (12). It has been proved that activation of extracellular-signal-regulated kinase 1 and 2/ mitogen-activated protein kinases (Erk/MAPK) and Akt/PI3K signaling pathways protected neurons against age-related and drug-induced damages (13).
Crocin is a yellow carotenoid which is usually extracted from Crocus sativus L. stigmas (saffron) and Gardenia jasminoides fruits (14). Saffron was used for a long time as food colorant and multi-functional drug in the folk medicine for antispasmodic, expectorant, stomachic, aphrodisiac and emmenagogue properties (15). The main constituents of saffron are picrocrocin (bitter principles), safranal (volatile agents), crocetin and its glycoside crocin (dye materials) (16). Pharmacological effects of crocin have been shown in the several novel studies, including anticancer (17, 18), anti-depression (19, 20), anti-anxiety(21), anti-inflammatory (22), memory improvement (23-25), anti-Parkinson (26), anti-diabetic, anti-glycative (27) and hepatoprotective (28-29) effects. The protective effects of crocin have been proved in the central nervous system (CNS) with the abundant animal and human studies (30). In our previous study, crocin attenuated acrolein-induced tau hyper phosphorylation and oxidative stress in rat cerebral cortex via modulating MAPKs signaling pathways (31). According to different memory impairment models, the impacts of crocin on memory enhance-ment might be related to the antioxidant, anti-hyperglycemic, anti-hypoinsulinemic properties of this compound (32-34). In addition, beta amyloid induced-memory deficit was inhibited via anti-apoptotic and anti-oxidative effects of crocin (35). Therefore, in the current study, we evaluated the protective effects of crocin in D-gal-induced aging model in rats. The molecular mechanisms of aging through formation of glycation products, induction of oxidative stress and alteration of MAPK/Akt/NF-κB signaling pathway were considered.
Materials and Methods
Materials
D-gal (99% purity), 2-Thiobarbituric acid (TBA) and 5, 5’-dithiobis-(2- nitrobenzoic acid) (DTNB) were purchased from Sigma. Rabbit polyclonal CML antibody and nuclear extraction kit were purchased from Abcam. Mouse monoclonal beta actin and phospho-p44/42 MAPK (pErk1/2), rabbit monoclonal NF-κB p65, Akt, phospho Akt, p44/42 MAPK (Erk1/2) and antirabbit IgG labeled with horseradish peroxidase were purchased from Cell Signaling. Polyvinylidene fluoride (PVDF) membrane was provided from Bio-Rad.
Crocin extraction
Crocin crystals were extracted and purified from saffron stigmas (purchased from Novin Saffron, Ghaen, Khorasan province, Northeast of Iran), in accordance to the crystallization method as previously described (36).
Animal and experimental design
Male Wistar rats, 220±20 g were housed in colony rooms with 12/12 hr light/dark cycle at 21±2 °C and had free access to food and water. All animal experiments were done according to Mashhad University of Medical Sciences, Ethical Committee Acts. In order to induce brain aging and memory impairment, animal were exposed to D-gal (400 mg/kg/day) subcutaneous (SC) for 56 days. For our study, rats were randomly divided into 6 groups (n=5 in each group) and treatments were done as follows:
1) Control, Normal saline
3) D-gal 400 mg/kg/day + crocin 7.5 mg/kg/day intraperitoneal (IP)
4) D-gal 400 mg/kg/day + crocin 15 mg/ kg/day IP
5) D-gal 400 mg/kg/day + crocin 30 mg/kg/day IP
6) Crocin 30 mg/kg/day IP
At the end of 56 days treatment period, after behavioral examination, rats were euthanized and hippocampi were dissected. Brain samples were snap-frozen in liquid nitrogen and stored at −80 °C until use.
Morris water maze
The acquisition and retention of memory were evaluated using Morris water maze. The water maze apparatus (136 cm in diameter and 60 cm in height) was filled to a depth of 25 cm with 22 ±1 °C water. The pool was divided into four equal quadrants, northeast (NE), northwest (NW), southeast (SE), southwest (SW), with an invisible platform (13 cm in diameter, 2 cm below the water surface) in the center of the NW quadrant. Spatial learning was tested from day 51 to 55 with 4 trials a day. The animals were forced to swim to find the hidden platform starting from the 4 different quadrants during the test. The rats were artificially guided to the hidden platform if they could not find it in 60 sec. They were allowed to spend 20 sec on the platform. Swimming activity was monitored using a video camera mounted overhead. The latency time and traveled distance to escape onto the hidden platform were recorded by video tracking software. Memory retention was evaluated by a probe trial on day 56. In the probe trial, the platform was removed from the water maze apparatus and the animal was allowed to swim freely in the pool for 60 sec. The swim speed and the time spent in the NW quadrant, where the platform was previously located, were recorded (39).
Measurement of lipid peroxidation
MDA level as a marker of lipid peroxidation was measured in hippocampus of rat. MDA reacts with thiobarbituric acid (TBA), as a thiobarbituric acid reactive substance (TBARS), to induce a pink colored complex. The maximum absorbance of complex is at 532 nm. Briefly, 3 ml phosphoric acid (1%), 1 ml TBA (0.6%) and 0.5 ml of brain tissue homogenate 10% in KCl were mixed and heated for 45 min in a boiling water bath. After cooling, 4 ml of n-butanol was added to the mixture and vortex-mixed for 1 min followed by centrifugation at 3000 g for 10 min. The organic layers were transferred to a fresh tube and absorbance was recorded by spectrophotometer (Jenway 6105 uv/vis, UK) at 532 nm (40). MDA levels were measured through standard curve, using malondialdehyde tetra -butylammonium and expressed as nmol/g tissue.
Western blot
The total proteins were extracted from rat’s hippocampus using lysis buffer containing 50 mM Tris-HCl (pH: 7.4), 2 mM EDTA, 2 mM EGTA, 10 mM NaF, 1 mM sodium orthovanadate (Na3VO4), 10 mM β glycerophosphate, 0.2% W/V sodium deoxycholate, 1 mM phenylmethylsulfonyl fluoride (PMSF), and complete protease inhibitor cocktail. Nuclear proteins were extracted with a nuclear extraction kit accor-ding to the instructions. Protein concentrations were analyzed using Bio-Rad protein assay. Samples were separated by 10% sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis and 15% SDS which was used for CML protein. Then samples transferred to polyvinylidene fluoride membranes by electrophoretic transfer. After that, blots were blocked in 5% non-fat milk (in 5% BSA for pErk and pAkt) in tris buffered saline with Tween 20 (TBST) for 2 hr (1 hr block for CML, pErk and pAkt) at room temperature. Membranes were incubated with the primary antibodies at 1000-fold dilutions anti-TNFα (6671 Abcam), anti-IL1β antibody (9722 Abcam), anti-NF-κB p65 (#4764 Cell Signaling), anti-Erk (1/2) antibody (#9102 Cell Signaling), anti-Akt antibody (#9272 Cell Signaling), anti-pAkt antibody (#9271 Cell Signaling), anti-β-actin (#3700 Cell Signaling) and anti-lamin B1 (#12586 Cell Signaling) for 2 hr at room temperature. The incubation properties for anti-CML (ab27684) were at 4 °C over- night. After repeated wash, immunoblots were in -cubated with Horseradish-peroxidase conjugated anti-rabbit antibody (#7074 Cell Signaling) and anti-mouse antibody (#7076 Cell Signaling) at 1:3000 dilutions 90 min at room temperature. Finally, protein bands were detected by enhance in chemiluminescnces (ECL) reagent and Alliance 4.7 Geldoc (UK). Quantified densitometric analysis performed using UVtec software (UK) and results were normalized to β-actin and lamin B1(41).
Statistical analysis
The results were expressed as mean ± SD. Statistical analyses were performed with ANOVA followed by Tukey–Kramer test to compare the differences between the means. Differences were considered statistically significant when P<0.05.
Results
Crocin treatment reduced memory impairment induced by D-gal in rat
Morris water maze (MVM) test was performed to evaluate the spatial learning and memory ability of rats. As shown in Figure 1A, the escape latency declined progressively during the five training days. The D-gal-treated rats spent longer period in finding the platform than the control group especially in the fourth and fifth days (P<0.05 and P<0.001, respectively). Treatment of rats with different doses of crocin (7.5, 15 and 30 mg/kg) significantly reduced the escape latency compared with D-gal group on the fifth day (P<0.001). In the sixth day on probe trial, the time spent in the target quadrant (hidden platform location) was shown in Figure 1B. D-gal treated rats spent less time (P<0.05) in the target quadrant as compared to the control group. In the crocin treated rats (15 and 30 mg/kg), the probe trial test showed a significant increase in the time spent in the target quadrant compared with D-gal group (P<0.001 and P<0.05, respectively). These results revealed that D-gal treatment had significant cognitive impairments and crocin has potent memory enhancement. Figures 1C and 1D show the mean swimming speeds of different groups of treated rats during the training days and probe day, respectively. There were no significant differences in swimming speeds between all groups (control, D-gal, D-gal+crocin, crocin). Also, swim paths in probe trial day (Figure 1E), show retention of memory that represented with more time spending and crossing over the target quadrant. D-gal group spent less time in target quadrant in comparison to the control or crocin (15 and 30 mg/kg) treated groups.
Figure 1.
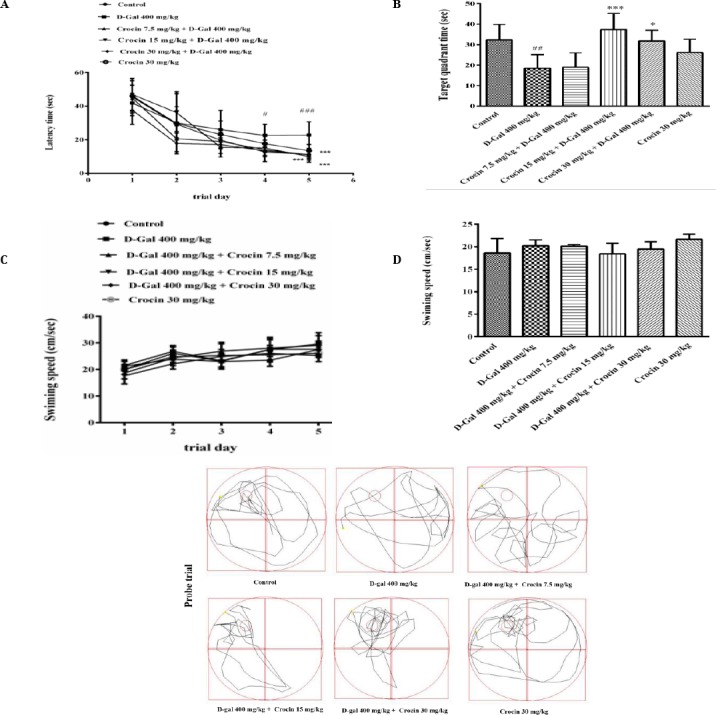
Spatial learning and memory of Wistar rats were evaluated by the Morris water maze. (A) D-gal treatment caused taking more time to learn the hidden platform position compared with the control group (# P<0.05 and ### P<0.001). After administration of crocin, the rats showed learning improvement during 5 days of training significantly in the fifth day compare to D-gal group (*** P<0.001). (B) Treatment with crocin also compensated the defects of time spending in target quadrant caused by D-gal (## P<0.01 vs control, *** P<0.001 and *P<0.05 vs D-gal group). (C) and (D) there were no differences in swim speed between control group and any of the treated groups (P>0.05), calculated in the training days and probe day, respectively. (E) Swim traces in probe test. Data are expressed as means±SD and were analyzed through one-way ANOVA coupled with Tukey-Kramer multiple comparisons test (n=5)
Crocin treatment reduced the level of MDA which elevated by D-gal
Alterations in the oxidative stress of rat hippocam-pus were investigated by measuring MDA formation. D-gal treatment increased significantly the level of MDA in comparison to control group (P<0.001). Crocin (7.5 and 30 mg/kg) decreased MDA formation significantly when injected with D-gal (P<0.01 and P<0.05, respectively) (Figure 2).
Figure 2.
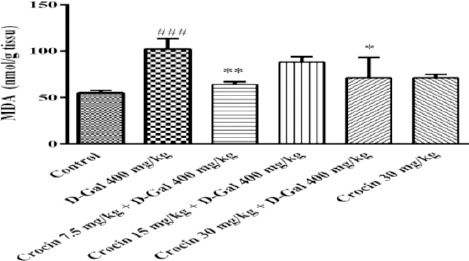
Changes in the malondialdehyde (MDA) levels of rat’s hippocampus. All values are expressed as mean±SD, n=5. ###P<0.01 vs control group, **P<0.01 and *P<0.05 vs D-gal treated rats
Figure 3.
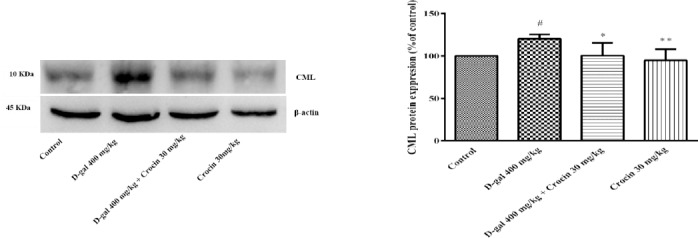
Expressions of carboxymethyl lysine (CML) protein in the hippocampus of rats. (A) Western blots of CML protein. (B) The quantitative protein levels of CML. Data are expressed as the mean ± SD of four separate experiments. #P<.05 vs. control, *P<0.05, **P<0.01 vs. D-gal treated rats
Anti-glyction activity of crocin on CML formation induced through D-gal treatment
The level of CML, as a most abundant AGE was determined by Western blot analysis in rat hippocampus. The results showed that D-gal treatment significantly increased CML formation when compared to control group (P<0.05). The CML expression significantly decreased in the crocin treated rats as compare to D-gal group (P<0.05).
Crocin improved Akt and Erk activities suppression induced by D-gal
PI3K/AKT and MAPK/Erk pathways promote cell survival and proliferation. Western blot analysis showed D-gal treatment decreased pErk/Erk ratio in comparison to control (P<0.05). Crocin treatment significantly increased MAPK activity through the elevation of pErk/Erk ratio (P<0.001) (Figure 4). Also, the pAkt/Ak ratio in the hippocampus of D-gal treated rats was less than control (P<0.01), while administration of crocin markedly elevated phosphorylation of Akt protein (P< 0.0001 vs D-gal group) (Figure 5).
Figure 4.
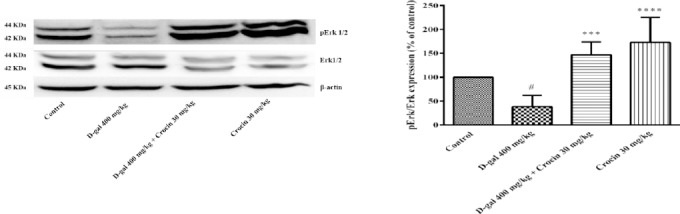
The activity of Erk 1/2 is presented in the four separate experiments. (A) Western blots of total Erk 1/2 and pErk 1/2 proteins. (B) The ratio of pErk/Erk was determined from density analysis of the bands. Data are expressed as the mean±SD. #P<0.05 vs. control, ***P<0.001 and ****P<0.0001 vs. D-gal treated rats
Figure 5.
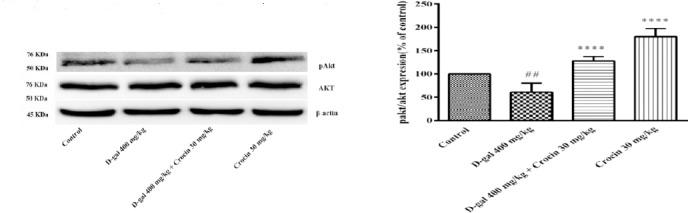
The activity of Akt is presented in the four separate experiments. (A) Western blots of total Akt and pAkt proteins. (B) The ratio of pAkt/Akt was determined from density analysis of the bands. Data are expressed as the mean±SD. ##P<0.01 vs. control, ****P<0.0001 and ****P<0.0001 vs. D-gal treated rats
Anti-inflammatory activity of crocin on IL-1β, TNFα and nuclear NF-κB p65 formation induced through D-gal treatment
NFkB-p65 a subunit of NF-kappa-B transcription complex plays a critical role in the inflammatory responses and cell survival. Pro-inflammatory cytokines, TNFα and IL-1β are both activators and transcriptional targets of NF-κB. As shown in Figure 6, a high dose treatment of rats with D-gal (400 mg/kg) for 56 days significantly increased nuclear NF-κB p65 expression in comparison to control group (P<0.05). Administration of crocin (30 mg/kg) decreased NF-κB p65 protein level in rat hippocampus (P<0.01 vs D-gal group). Additionally, IL-1β and TNFα expression in the hippocampus of D-gal treated rats markedly elevated (P<0.01 and P<0.05 vs control, respectively) (Figure 7 and 8). Crocin treatment significantly decreased IL-1β and TNFα formations in comparison to D-gal-treated animals (P<0.01 and P<0.05, respectively).
Figure 6.
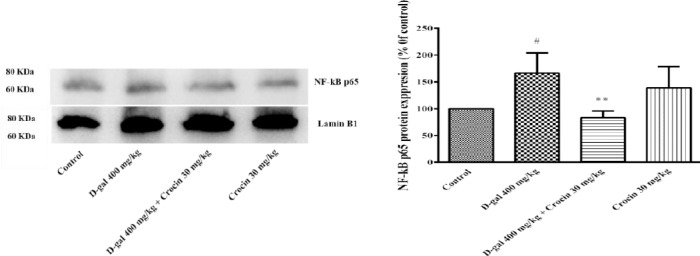
Expressions of nuclear NF-κB protein in the hippocampus of rats. (A) Western blots of NF-κB protein. (B) The quantitative protein levels of NF-κB. Data are expressed as the mean ± SD of four separate experiments. #P<0.05 vs. control and **P<0.01 vs. D-gal treated rats
Figure 7.
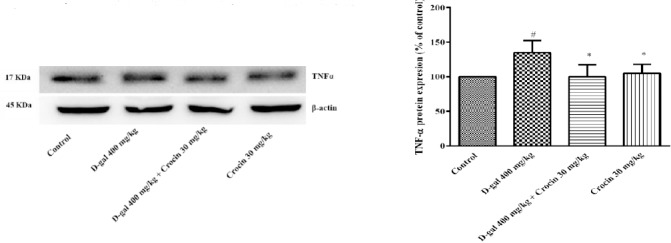
Expressions of TNFα protein in the hippocampus of rats. (A) Western blots of TNFα protein. (B) The quantitative protein levels of TNFα. Data are expressed as the mean ± SD of four separate experiments. #P<0.05 vs. control and *P<0.05 vs. D-gal treated rats
Figure 8.
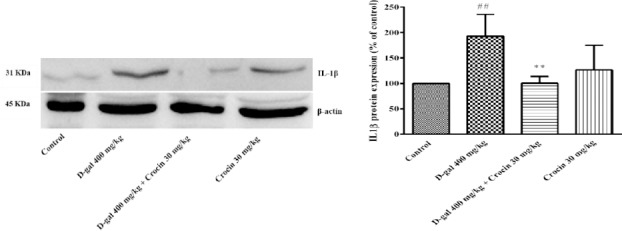
Expressions of IL-1β protein in the hippocampus of rats. (A) Western blots of IL-1β protein. (B) The quantitative protein levels of IL-1β. Data are expressed as the mean±SD of four separate experiments. ##P<0.01 vs. control and **P<0.01 vs. D-gal treated rats
Discussion
Aging is a slow and gradual biological process, associated with multiple physiological and pathological changes including formation of AGEs and free radicals damage (42-43). Due to the multifactorial nature of aging, various theories have been proposed that the glycation and glycoxidation hypothesis of aging are the main factors in aging process (44). Brain aging induced by high dose of D-gal treatment is a well-known model for studying age-related oxidative damages and memory disorders such as Alzheimer. D-gal induces aging through the formation of ROS and AGEs (45, 46). This study investigated the protective effects of crocin against aging model induced by D-gal on cognitive function of Wistar rats and its molecular mechanisms. Our data proved that crocin as the main constitute of saffron, has potent improving effects on neurotoxicity of D-gal.
A long time spending to find the hidden platform during the five retrieval trial days in the MVM test indicates the impairment of learning memory. Reten- tion memory impairment presented as less time spen- ding in the target quadrant (hidden platform location) in the last day (39). The data presented above agree with other studies which showed the disability of senescent rats (56 days injection of 400 mg/kg D-gal) in the acquisition of information and retention of acquired memory (46). In several research projects have shown the protective effects of crocin on memory impairment induced by different models (24, 47). According to our data, chronic administration of different doses of crocin reduced memory decay induced by D-gal. The analysis of speeds shows that chronic D-gal and crocin treatment did not affect motor or physical activity.
Oxidative stress is a major hypothesis in the induction and progression of neural degeneration (48). Generally age-related oxidative stress is due to the production of free radicals or oxidative agents, reduction of antioxidant agent and impairment the repair of oxidative damages (49). Many reports have shown chronic administration of D-gal leads to significant increase of free radicals and neuronal damages in the animal brain (50, 51). MDA level is an important marker of lipid peroxidation under oxidative conditions (52). In the current study, senescent rats induced by D-gal showed a significant elevation in the level of MDA in the hippocampus. Since crocin has obvious antioxidant properties (53), MDA levels induced by D-gal significantly decreased in the crocin-treated rats. This is due to the facts that saffron and crocin are able to reduce ROS and corticosterone response. These two mechanisms decrease the need for antioxidant enzymes (47).
It has been reported that chronic administration of high dose of D-gal increases oxidative stress directly or via activation of AGE/RAGE pathway (10). The existence of higher AGEs levels associates with neuronal aging and cognitive decline in the senescence adults (54). According to investigations, it has concluded that AGEs formation occurs under both excess glucose existing and oxidative conditions. Whereas, AGE-RAGE interaction increases intracellular oxidative stress via activation of nicotinamide adenine dinucleotide phosphate-oxidase (NADPH oxidase), which produces superoxide (55). In the current study, CML expression was higher in hippocampus of D-gal treated rats than untreated group. Crocin (30 mg/kg/day) could prevent AGE formation induced by D-gal administration. Many research projects reported that the antiglyctive activity of saffron and its active constituent is due to reduction in glucose levels via elevation of insulin sensitivity. Therefore, the suppression of AGEs formation leads to decrease in lipid peroxidation and oxidative stress (56).
Mitogen-activated protein kinases (MAPKs) play distinct roles in proliferation, differentiation, and neuronal viability. Erk 1/2 activity, a member of the mitogen-activated MAPK family, decreased in the hippocampus, frontal cortex and striatum of aging rats (57). One of the underlying mechanisms of oxidative stress in the impairment of brain function is through the suppression of Erk phosphorylation (58). In addition, the activity of the Phosphatidyl -inositol 3-kinase (PI3K)/Akt pathway has been implicated in hippocampal synaptic plasticity and cell survival (59). In the several research studies, it has been demonstrated that amyloid beta-induced apoptosis in Alzheimer’s disease through the inactive- tion of Akt and Erk1/2 (60, 61). According to few previous studies, chronic treatment with high dose of D-gal significantly diminished brain pAkt and pErk 1/2 expression because of excessive oxidative stress formation (1, 12). The present study showed that crocin carotenoid not only elevated pAkt and pErk 1/2 levels, but also significantly decreased nuclear NF-κB expression compare to D-gal treated rats.
The accumulation of AGEs molecules such as CML and pentosidine and oxidative stress in the brain aging induced by D-gal activates NF-κB formation. It is well known that the transcriptional activity of NF-κB starts the pathological cascade, which finally increases ROS formation and stimulates pro-inflammatory cytokines such as TNF-alpha and interleukin IL-1β (11, 55). Generally, inflammatory processes in the brain increase the development of Alzheimer’s disease (62). In addition, pro-inflamma- tory mediators like IL-1, lipopolysaccharides (LPS), and TNF activate NF-κB formation and cause its translocation from the cytoplasm to the nucleus cells to start transcriptional activity (63). All of inflammatory mediators (IL-1, TNF and NF-κB) which activated through chronic treatment with D-gal significantly decreased in hippocampus of crocin treated rats.
Conclusion
In summary, our results demonstrated that crocin attenuated D-gal-induced memory impairment and synaptic dysfunction, through decreasing ROS and AGEs formation. The inhibitory effects of crocin on oxidative stress may activate MAPK and Akt signaling pathways. On the other hand, crocin finally modulate NF-KB, IL-1, TNF and other signaling molecules during inflammation and neurodegenera -tion in the hippocampus of D-gal treated rats. The suggested mechanism about the neuroprotective effects of crocin against D-gal-induced memory dysfunction, neuroinflammation, and neurodegenera tion are shown in Figure 9.
Figure 9.
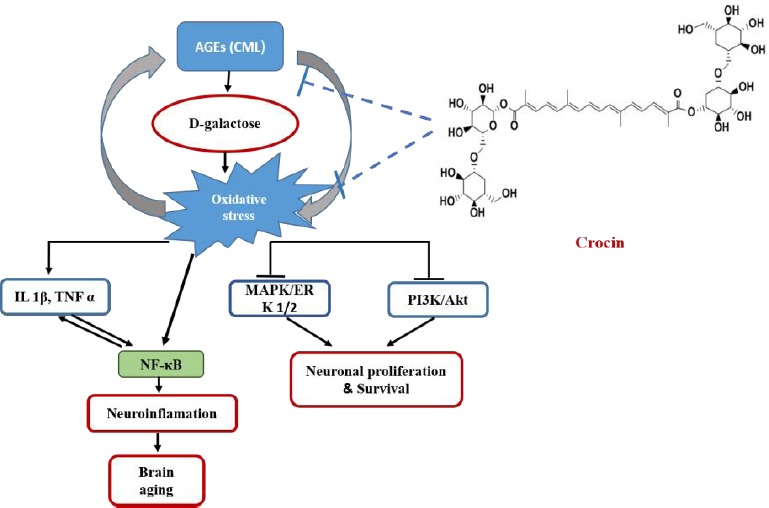
Proposed Schematic mechanism of crocin against D-gal neurotoxicity. This diagram shows possible mechanisms of crocin on synaptic plasticity, which prevented memory impairment, through MAPK and Akt pathways activation in D-gal induced aging rat model. In addition, crocin decreases ROS induction, AGEs formation and neuroinflammation
Acknowledgment
The authors are thankful to the Vice Chancellor of Research, Mashhad University of Medical Sciences for financial supports. The results described in this paper are part of a PhD thesis.
References
- 1.Zhou Y, Dong Y, Xu Q, He Y, Tian S, Zhu S, et al. Mussel oligopeptides ameliorate cognition deficit and attenuate brain senescence in D-galactose-induced aging mice. Food Chem Toxicol. 2013;59:412–420. doi: 10.1016/j.fct.2013.06.009. [DOI] [PubMed] [Google Scholar]
- 2.Park D-S, Lee S-H, Choi Y-J, Bae D-K, Yang Y-H, Yang G-E, et al. Improving effect of silk peptides on the cognitive function of rats with aging brain facilitated by D-galactose. Biomol Ther. 2011;19:224–230. [Google Scholar]
- 3.Kuhla A, Ludwig SC, Kuhla B, Münch G, Vollmar B. Advanced glycation end products are mitogenic signals and trigger cell cycle reentry of neurons in Alzheimer's disease brain. Neurobiol Aging. 2015;36:753–761. doi: 10.1016/j.neurobiolaging.2014.09.025. [DOI] [PubMed] [Google Scholar]
- 4.Nowotny K, Jung T, Hohn A, Weber D, Grune T. Advanced glycation end products and oxidative stress in type 2 diabetes mellitus. Biomolecules. 2015;5:194–222. doi: 10.3390/biom5010194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li JH, Wang W, Huang XR, Oldfield M, Schmidt AM, Cooper ME, et al. Advanced glycation end products induce tubular epithelial-myofibroblast transition through the RAGE-ERK1/2 MAP kinase signaling pathway. Am J Pathol. 2004;164:1389–1397. doi: 10.1016/S0002-9440(10)63225-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tanikawa T, Okada Y, Tanikawa R, Tanaka Y. Advanced glycation end products induce calcification of vascular smooth muscle cells through RAGE/p38 MAPK. J Vasc Res. 2009;46:572–580. doi: 10.1159/000226225. [DOI] [PubMed] [Google Scholar]
- 7.Hirose A, Tanikawa T, Mori H, Okada Y, Tanaka Y. Advanced glycation end products increase endothelial permeability through the RAGE/Rho signaling pathway. FEBS Lett. 2010;584:61–66. doi: 10.1016/j.febslet.2009.11.082. [DOI] [PubMed] [Google Scholar]
- 8.Lin L, Park S, Lakatta EG. RAGE signaling in inflammation and arterial aging. Front Biosci. 2009;14:1403–1413. doi: 10.2741/3315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Libermann TA, Baltimore D. Activation of interleukin-6 gene expression through the NF-kappa B transcription factor. Mol Cell Biol. 1990;10:2327–2334. doi: 10.1128/mcb.10.5.2327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang K-L, Lou D-D, Guan Z-Z. Activation of the AGE/RAGE system in the brains of rats and in SH-SY5Y cells exposed to high level of fluoride might connect to oxidative stress. Neurotoxicol Teratol. 2015;48:49–55. doi: 10.1016/j.ntt.2015.01.007. [DOI] [PubMed] [Google Scholar]
- 11.Tsai SJ, Yin MC. Anti-glycative and anti-inflammatory effects of protocatechuic acid in brain of mice treated by D-galactose. Food Chem Toxicol. 2012;50:3198–3205. doi: 10.1016/j.fct.2012.05.056. [DOI] [PubMed] [Google Scholar]
- 12.Wu H, Zhao J, Chen M, Wang H, Yao Q, Fan J, et al. The anti-aging effect of erythropoietin via the ERK/Nrf2-ARE pathway in aging rats. J Mol Neurosci. 2017;61:449–458. doi: 10.1007/s12031-017-0885-1. [DOI] [PubMed] [Google Scholar]
- 13.Wang X, Wang Z, Yao Y, Li J, Zhang X, Li C, et al. Essential role of ERK activation in neurite outgrowth induced by alpha-lipoic acid. Biochim Biophys Acta. 2011;1813:827–838. doi: 10.1016/j.bbamcr.2011.01.027. [DOI] [PubMed] [Google Scholar]
- 14.Bandegi AR, Rashidy-Pour A, Vafaei AA, Ghadrdoost B. Protective effects of Crocus Sativus L. extract and crocin against chronic-stress induced oxidative damage of brain, liver and kidneys in rats. Adv Pharm Bull. 2014;4:493–499. doi: 10.5681/apb.2014.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cao P-C, Xiao W-X, Yan Y-B, Zhao X, Liu S, Feng J, et al. Preventive effect of crocin on osteoporosis in an ovariectomized rat model. Evid Based Complement Alternat Med. 2014;2014:825181. doi: 10.1155/2014/825181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moshiri M, Vahabzadeh M, Hosseinzadeh H. Clinical applications of saffron (Crocus sativus) and its constituents:a review. Drug Res. 2015;65:287–295. doi: 10.1055/s-0034-1375681. [DOI] [PubMed] [Google Scholar]
- 17.Soeda S, Ochiai T, Shimeno H, Saito H, Abe K, Tanaka H, et al. Pharmacological activities of crocin in saffron. J Nat Med. 2007;61:102–111. [Google Scholar]
- 18.Abdullaev FI. Cancer chemopreventive and tumoricidal properties of saffron (Crocus sativus L.) Exp Biol Med. 2002;227:20–25. doi: 10.1177/153537020222700104. [DOI] [PubMed] [Google Scholar]
- 19.Vahdati Hassani F, Naseri V, Razavi BM, Mehri S, Abnous K, Hosseinzadeh H. Antidepressant effects of crocin and its effects on transcript and protein levels of CREB, BDNF, and VGF in rat hippocampus. Daru. 2014;22:16–25. doi: 10.1186/2008-2231-22-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang Y, Han T, Zhu Y, Zheng C-J, Ming Q-L, Rahman K, et al. Antidepressant properties of bioactive fractions from the extract of Crocus sativus L. J Nat Med. 2010;64:24–30. doi: 10.1007/s11418-009-0360-6. [DOI] [PubMed] [Google Scholar]
- 21.Hosseinzadeh H, Noraei NB. Anxiolytic and hypnotic effect of Crocus sativus aqueous extract and its constituents, crocin and safranal, in mice. Phytother Res. 2009;23:768–774. doi: 10.1002/ptr.2597. [DOI] [PubMed] [Google Scholar]
- 22.Ding Q, Zhong H, Qi Y, Cheng Y, Li W, Yan S, et al. Anti-arthritic effects of crocin in interleukin-1beta-treated articular chondrocytes and cartilage in a rabbit osteoarthritic model. Inflamm Res. 2013;62:17–25. doi: 10.1007/s00011-012-0546-3. [DOI] [PubMed] [Google Scholar]
- 23.Essa MM, Vijayan RK, Castellano-Gonzalez G, Memon MA, Braidy N, Guillemin GJ. Neuroprotective effect of natural products against Alzheimer's disease. Neurochem Res. 2012;37:1829–1842. doi: 10.1007/s11064-012-0799-9. [DOI] [PubMed] [Google Scholar]
- 24.Hosseinzadeh H, Ziaei T. Effects of Crocus sativus L. stigma extract and its constituents, crocin and safranal, on intact memory and scopolamine-induced learning deficits in rats performing the Morris water maze task. J Med Plants. 2006;3:40–50. [Google Scholar]
- 25.Pitsikas N, Zisopoulou S, Tarantilis PA, Kanakis CD, Polissiou MG, Sakellaridis N. Effects of the active constituents of Crocus sativus L. crocins on recognition and spatial rats'memory. Behav Brain Res. 2007;183:141–146. doi: 10.1016/j.bbr.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 26.Rao SV, Muralidhara Yenisetti SC, Rajini PS. Evidence of neuroprotective effects of saffron and crocin in a Drosophila model of parkinsonism. Neurotoxicology. 2016;52:230–242. doi: 10.1016/j.neuro.2015.12.010. [DOI] [PubMed] [Google Scholar]
- 27.Hazman O, Aksoy L, Buyukben A. Effects of crocin on experimental obesity and type-2 diabetes. Turk J Med Sci. 2016;46:1593–1602. doi: 10.3906/sag-1506-108. [DOI] [PubMed] [Google Scholar]
- 28.Yousefsani BS, Pourahmad J, Hosseinzadeh H. The Mechanism of protective effect of crocin against liver mitochondrial toxicity caused by Arsenic III. Toxicol Mech Methods. 2017 Aug;31:1–10. doi: 10.1080/15376516.2017.1368054. doi:10.1080/15376516.2017.1368054. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 29.Vahdati Hassani F, Mehri S, Abnous K, Birner-Gruenberger R, Hosseinzadeh H. Protective effect of crocin on BPA-induced liver toxicity in rats through inhibition of oxidative stress and downregulation of MAPK and MAPKAP signaling pathway and miRNA-122 expression. Food Chem Toxicol. 2017;107:395–405. doi: 10.1016/j.fct.2017.07.007. [DOI] [PubMed] [Google Scholar]
- 30.Alavizadeh SH, Hosseinzadeh H. Bioactivity assessment and toxicity of crocin:a comprehensive review. Food Chem Toxicol. 2014;64:65–80. doi: 10.1016/j.fct.2013.11.016. [DOI] [PubMed] [Google Scholar]
- 31.Rashedinia M, Lari P, Abnous K, Hosseinzadeh H. Protective effect of crocin on acrolein-induced tau phosphorylation in the rat brain. Acta Neurobiol Exp. 2015;75:208–219. [PubMed] [Google Scholar]
- 32.Hosseinzadeh H, Sadeghnia HR, Ghaeni FA, Motamedshariaty VS, Mohajeri SA. Effects of saffron (Crocus sativus L.) and its active constituent, crocin, on recognition and spatial memory after chronic cerebral hypoperfusion in rats. Phytother Res. 2012;26:381–386. doi: 10.1002/ptr.3566. [DOI] [PubMed] [Google Scholar]
- 33.Tamaddonfard E, Farshid AA, Asri-Rezaee S, Javadi S, Khosravi V, Rahman B, et al. Crocin improved learning and memory impairments in streptozotocin-induced diabetic rats. Iran J Basic Med Sci. 2013;16:91–100. [PMC free article] [PubMed] [Google Scholar]
- 34.Ahmadi M, Rajaei Z, Hadjzadeh MA, Nemati H, Hosseini M. Crocin improves spatial learning and memory deficits in the Morris water maze via attenuating cortical oxidative damage in diabetic rats. Neurosci Lett. 2017;642:1–6. doi: 10.1016/j.neulet.2017.01.049. [DOI] [PubMed] [Google Scholar]
- 35.Asadi F, Jamshidi AH, Khodagholi F, Yans A, Azimi L, Faizi M, et al. Reversal effects of crocin on amyloid beta-induced memory deficit:Modification of autophagy or apoptosis markers. Pharmacol Biochem Behav. 2015;139:47–58. doi: 10.1016/j.pbb.2015.10.011. [DOI] [PubMed] [Google Scholar]
- 36.Hadizadeh F, Mohajeri SA, Seifi M. Extraction and purification of crocin from saffron stigmas employing a simple and efficient crystallization method. Pak J Biol Sci. 2010;13:691–698. doi: 10.3923/pjbs.2010.691.698. [DOI] [PubMed] [Google Scholar]
- 37.Haider S, Liaquat L, Shahzad S, Sadir S, Madiha S, Batool Z, et al. A high dose of short term exogenous D-galactose administration in young male rats produces symptoms simulating the natural aging process. Life Sci. 2015;124:110–119. doi: 10.1016/j.lfs.2015.01.016. [DOI] [PubMed] [Google Scholar]
- 38.Wang T, Di G, Yang L, Dun Y, Sun Z, Wan J, et al. Saponins from Panax japonicus attenuate D-galactose-induced cognitive impairment through its anti-oxidative and anti-apoptotic effects in rats. J Pharm Pharmacol. 2015;67:1284–1296. doi: 10.1111/jphp.12413. [DOI] [PubMed] [Google Scholar]
- 39.Vorhees CV, Williams MT. Morris water maze:procedures for assessing spatial and related forms of learning and memory. Nat Protoc. 2006;1:848–858. doi: 10.1038/nprot.2006.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mehri S, Abnous K, Khooei A, Mousavi SH, Shariaty VM, Hosseinzadeh H. Crocin reduced acrylamide-induced neurotoxicity in Wistar rat through inhibition of oxidative stress. Iran J Basic Med Sci. 2015;18:902–908. [PMC free article] [PubMed] [Google Scholar]
- 41.Ghasemi T, Abnous K, Vahdati F, Mehri S, Razavi BM, Hosseinzadeh H. Antidepressant effect of Crocus sativus aqueous extract and its effect on CREB, BDNF, and VGF transcript and protein levels in rat hippocampus. Drug Res. 2015;65:337–343. doi: 10.1055/s-0034-1371876. [DOI] [PubMed] [Google Scholar]
- 42.Dammann P, Sell DR, Begall S, Strauch C, Monnier VM. Advanced glycation end-products as markers of aging and longevity in the long-lived Ansell's mole-rat (Fukomys anselli) J Gerontol A Biol Sci Med Sci. 2011;67:573–583. doi: 10.1093/gerona/glr208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rolewska P, Al-Robaiy S, Santos AN, Simm A, Silber R-E, Bartling B. Age-related expression, enzymatic solubility and modification with advanced glycation end-products of fibrillar collagens in mouse lung. Exp Gerontol. 2013;48:29–37. doi: 10.1016/j.exger.2012.04.012. [DOI] [PubMed] [Google Scholar]
- 44.Masoro EJ. Use of rodents as models for the study of “normal aging”:conceptual and practical issues. Neurobiol Aging. 1991;12:639–643. doi: 10.1016/0197-4580(91)90114-y. [DOI] [PubMed] [Google Scholar]
- 45.Kumar A, Prakash A, Dogra S. Centella asiatica attenuates D-Galactose-induced cognitive impairment, oxidative and mitochondrial dysfunction in mice. Int J Alzheimers Dis. 2011;2011:347569. doi: 10.4061/2011/347569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Haider S, Liaquat L, Shahzad S, Sadir S, Madiha S, Batool Z, et al. A high dose of short term exogenous D-galactose administration in young male rats produces symptoms simulating the natural aging process. Life Sci. 2015;124:110–119. doi: 10.1016/j.lfs.2015.01.016. [DOI] [PubMed] [Google Scholar]
- 47.Ghadrdoost B, Vafaei AA, Rashidy-Pour A, Hajisoltani R, Bandegi AR, Motamedi F, et al. Protective effects of saffron extract and its active constituent crocin against oxidative stress and spatial learning and memory deficits induced by chronic stress in rats. Eur J Pharmacol. 2011;667:222–229. doi: 10.1016/j.ejphar.2011.05.012. [DOI] [PubMed] [Google Scholar]
- 48.Ansari MA, Scheff SW. Oxidative stress in the progression of Alzheimer disease in the frontal cortex. J Neuropathol Exp Neurol. 2010;69:155–167. doi: 10.1097/NEN.0b013e3181cb5af4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gilca M, Stoian I, Atanasiu V, Virgolici B. The oxidative hypothesis of senescence. J Postgrad Med. 2007;53:207–213. doi: 10.4103/0022-3859.33869. [DOI] [PubMed] [Google Scholar]
- 50.Banji D, Banji OJ, Dasaroju S, Kranthi KC. Curcumin and piperine abrogate lipid and protein oxidation induced by D-galactose in rat brain. Brain Res. 2013;1515:1–11. doi: 10.1016/j.brainres.2013.03.023. [DOI] [PubMed] [Google Scholar]
- 51.Mao Z, Zheng Y-l, Zhang Y-q, Han B-p, Zhu X-w, Chang Q, et al. The anti-apoptosis effects of daidzein in the brain of D-galactose treated mice. Molecules. 2007;12:1455–1470. doi: 10.3390/12071455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.GawełS Wardas M, Niedworok E, Wardas P. Malondialdehyde (MDA) as a lipid peroxidation marker. Wiad lek. 2003;57:453–455. [PubMed] [Google Scholar]
- 53.Hosseinzadeh H, Shamsaie F, Mehri S. Antioxidant activity of aqueous and ethanolic extracts of Crocus sativus L. stigma and its bioactive constituents, crocin and safranal. Pharmacogn Mag. 2009;5:419–424. [Google Scholar]
- 54.Liu YY, Nagpure BV, Wong PT, Bian JS. Hydrogen sulfide protects SH-SY5Y neuronal cells against d-galactose induced cell injury by suppression of advanced glycation end products formation and oxidative stress. Neurochem Int. 2013;62:603–609. doi: 10.1016/j.neuint.2012.12.010. [DOI] [PubMed] [Google Scholar]
- 55.Luevano-Contreras C, Chapman-Novakofski K. Dietary advanced glycation end products and aging. Nutrients. 2010;2:1247–1265. doi: 10.3390/nu2121247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rajaei Z, Hadjzadeh M-A-R, Nemati H, Hosseini M, Ahmadi M, Shafiee S. Antihyperglycemic and antioxidant activity of crocin in streptozotocin-induced diabetic rats. J Med Food. 2013;16:206–210. doi: 10.1089/jmf.2012.2407. [DOI] [PubMed] [Google Scholar]
- 57.Mo L, Ren Q, Duchemin A-M, Neff NH, Hadjiconstantinou M. GM1 and ERK signaling in the aged brain. Brain Res. 2005;1054:125–134. doi: 10.1016/j.brainres.2005.06.068. [DOI] [PubMed] [Google Scholar]
- 58.Yufune S, Satoh Y, Akai R, Yoshinaga Y, Kobayashi Y, Endo S, et al. Suppression of ERK phosphorylation through oxidative stress is involved in the mechanism underlying sevoflurane-induced toxicity in the developing brain. Sci Rep. 2016;6:21859. doi: 10.1038/srep21859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.LesnéS Gabriel C, Nelson DA, White E, MacKenzie ET, Vivien D, et al. Akt-dependent expression of NAIP-1 protects neurons against amyloid-βtoxicity. J Biol Chem. 2005;280:24941–24947. doi: 10.1074/jbc.M413495200. [DOI] [PubMed] [Google Scholar]
- 60.Liu J, He J, Huang L, Dou L, Wu S, Yuan Q. Neuroprotective effects of ginsenoside Rb1 on hippocampal neuronal injury and neurite outgrowth. Neural Regen Res. 2014;9:943–950. doi: 10.4103/1673-5374.133137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhao R, Zhang Z, Song Y, Wang D, Qi J, Wen S. RETRACTED:Implication of phosphatidylinositol-3 kinase/Akt/glycogen synthase kinase-3βpathway in ginsenoside Rb1's attenuation of beta-amyloid-induced neurotoxicity and tau phosphorylation. J Ethnopharmacol. 2011;133:1109–1116. doi: 10.1016/j.jep.2010.11.054. [DOI] [PubMed] [Google Scholar]
- 62.Granic I, Dolga AM, Nijholt IM, van Dijk G, Eisel UL. Inflammation and NF-κB in Alzheimer's disease and diabetes. J Alzheimer's Dis. 2009;16:809–821. doi: 10.3233/JAD-2009-0976. [DOI] [PubMed] [Google Scholar]
- 63.Shih R-H, Wang C-Y, Yang C-M. NF-kappaB signaling pathways in neurological inflammation:a mini review. Front Mol Neurosci. 2015;8:77. doi: 10.3389/fnmol.2015.00077. [DOI] [PMC free article] [PubMed] [Google Scholar]


