Abstract
Objective(s):
The purpose of this study was to investigate the change in plasma anandamide (AEA) levels throughout the normal menstrual cycle, and to analyze the relationship among AEA, sex steroids and gonadotrophins.
Materials and Methods:
The patients were fertile women with normal menstrual cycle, proposed to get in vitro fertilization (IVF) treatment due to oviduct obstruction or male infertility. Patients were divided into two groups, cross-sectional (n=79) and longitudinal (n=10). The plasma AEA levels were examined by the ultra-performance liquid chromatography tandem mass spectrometry (UPLC-MS/MS) system. The serum levels of follicle stimulating hormone (FSH), luteinizing hormone (LH), estradiol (E2), and progesterone (P) were measured by chemiluminescence.
Results:
The AEA levels in the late follicular phase were slightly higher than those in the early follicular phase. Subsequently, the AEA levels peaked at the time of ovulation in both two cohorts. Finally, the lowest AEA levels were measured in the luteal phase. Moreover, there were highly significant positive correlations between the plasma AEA concentration and the serum levels of FSH, LH and E2, whereas the AEA level was not correlated with P during the normal menstrual cycle.
Conclusion:
Our observations reveal a dynamic change in the plasma AEA level, which is closely associated with the levels of gonadotrophin and sex steroid hormones, suggesting that the hormones may be involved in the regulation of AEA levels during the menstrual cycle. Our studies help to design new strategies to improve implantation and treatments for reproductive diseases.
Keywords: Anandamide, Endocannabinoid, Gonadotrophin, Menstrual cycle, Sex steroid hormones
Introduction
Infertility has become a global health problem affecting human’s mental health, happiness and population change, but effective treatments are yet to be developed. Many studies revealed that the endocannabinoid system (ECS) influences multiple reproductive events, such as gametogenesis, fertilization, oviductal transport, embryo implantation and final outcome of pregnancy (1, 2). Therefore, to study the physiology of the ECS will contribute to the discovery of biomarkers predicting the reproductive potential of male and female gametes, and to provide novel strategies for infertility treatment.
The ECS is widely distributed in the hypothalamus-pituitary-ovarian axis, which is composed of the endocannabinoids, cannabinoid receptors and a variety of related enzymes (3). It is increasingly realized that the endocannabinoids have important effects on female reproductive functions (4). N-arachidonoyl-ethanolamine (anandamide, AEA) was the first identified endocanna -binoids, originally isolated from the brain tissue in 1992 (5), and 2-arachidonoyl-glycerol (2-AG) was discovered in 1995 (6, 7). AEA is a member of fatty acid amides, whereas 2-AG belongs to the monoacylglycerol class. Several years later, three additional ligands of cannabinoid receptors were proposed, including 2-arachidonyl-glyceryl ether (2-AGE), O-arachidonoyl-ethanolamine (virodhamine) and N-arachidonoyl-dopamine (NADA) (8-10). AEA is one of the most extensively studied endocannabinoids. The precursor of AEA is N-arachidonoylphosphatidylethanolamine (NAPE), which is cleaved into AEA and phosphatidic acid (PA) by NAPE-hydrolyzing phosopholipase D (NAPE-PLD) (11, 12). The synthesized AEA is transported into the extracellular space, where it activates cannabinoid receptors, such as endogenous cannabinoid receptor type 1 (CB1) and CB2, through the autocrine or paracrine pathway (13, 14). The CB1 and CB2 are widely distributed in endometrial tissues (15). The activity of AEA is terminated when it is transported back to the intracellular space, where it is degraded into amino acids and ethanolamine by fatty acid amide hydrolases-1 (FAAH-1) (16), FAAH-2 (17) or the lysosomal N-acylethanolamine-hydrolyzing acid amidase (18). FAAH is the material of hydrolytic enzyme endogenous cannabinoids, adjusting the concentration of endocannabinoids (16).
A number of studies indicate that AEA has a pivotal role in human reproduction. In particular, local concentration of AEA in the fallopian tube affected sperm viability, as low concentration (0.25 nM) of AEA analogue R-formamide facilitated human sperm activity, whereas the sperm motility was weakened when concentration was increased to 2.5 nM (19). Moreover, AEA could activate the CB1 receptor, and promote the adsorption of sperm by the fallopian tube, resulting in sperm migration to fertilization (20). After fertilization, the fertilized egg in the fallopian tube forms mulberry embryo via mitosis, which then develops into the blastocyst with inner cell mass and nourish ectoderm. The suitable receptivity between the blastocyst and uterine is important for a successful implantation. AEA ensures the synchronous development of the pre-implantation embryo and the endometrium, thereby facilitating embryo implantation during the implantation window (21, 22).
Hormones also play important roles in the regulation of AEA concentration, for successful fertilization, implantation and maintenance of early pregnancy (23). It was reported that the progesterone (P) enhanced the FAAH activity in lymphocytes through the transcription factor Ikaros, resulting in decreased AEA (24). However, P had little effect on expressions of endocannabinoid membrane transporter (EMT), NAPE-PLD and CB1 in lymphocytes. In addition, E2 could activate the NAPE-PLD, but suppress FAAH activity, leading to the release of AEA by endothelial cells, which regulated the cardiovascular and immune systems (25, 26). Although P and E2 are thought to be involved in the regulation of AEA, the evidence for this speculation is not yet reported. Furthermore, the interplay between ECS and sex steroid hormones or gonadotrophins throughout the menstrual cycle, as well as the exact mechanisms controlling plasma AEA levels, has not been well understood.
In this study, we analyzed variations in plasma AEA levels throughout the menstrual cycle. We found that the plasma AEA reached the highest level at ovulatory phase, whereas decreased to the lowest level at luteal phase. Further analysis demonstrated that the plasma AEA levels might be closely related to key ovarian sex steroid hormones and gonadotrophins involved in the regulation of the menstrual cycle. Overall, our research into AEA signaling may provide better understanding of the pathogenesis of infertility, and improve new strategies and treatments for infertility.
Materials and Methods
Patients and grouping strategy
The patients were women of childbearing age, who proposed to perform in vitro fertilization (IVF) treatment at Reproductive Center of the Second Hospital of Hebei Medical University between May and October 2015. The patients involved in the clinical trial were 20-38 years old, with the body mass index (BMI) of 19-25 kg/m2, nonsmokers, menstruating in regular cycles (defined as occurring every 26-32 days), not taking any medication or hormonal contraception for at least three months before recruitment into the studies, with normal endocrine secretion, excluding the possibilities of polycystic ovary syndrome, endometriosis and uterine fibroids. Hysterosa -lpingography suggested that patients might have oviduct obstruction.
The patients were divided into cross-sectional and longitudinal groups. In the cross-sectional group, the levels of AEA in patients’ plasma were examined during various phases of the menstrual cycle. In longitudinal group, patients acted as their own controls, and their plasma AEA, and serum levels of E2, P, follicle stimulating hormone (FSH) and luteinizing hormone (LH) were measured to investigate whether variations in AEA levels observed cross-sectionally were comparable to that observed longitudinally, and to investigate the relationship among AEA, sex steroid hormones and gonadotrophin during the menstrual cycle. In both study groups, the menstrual cycle was divided into four phases (early follicular, late follicular, ovulatory and luteal phases) based on the normal physiologic changes in the levels of sex steroid hormones and gonadotrophins, that occur throughout the ovulatory menstrual cycle. This study was approved by the Ethics Committee of The Second Hospital of Hebei Medical University.
Specimen collection
The patients were recruited for specimen collection at the defined periods of their menstrual cycle, based on their self-reported last menstrual period and auxiliary detection by type-B ultrasonic. A total of 79 unselected patients were included in cross-sectional group. Among them, 21 women were in the period of early follicular phase (day 2-6), whose follicular diameters were less than 6 mm; 16 women were in the late follicular phase (day 8-12, follicular diameter≤15 mm); 21 women were in the ovulatory phase (day 13-16, follicular diameter>16 mm), and 21 women were in the luteal phase (day 16-30). A total of 10 patients were recruited to longitudinal part of the study. A single sample of blood (5 ml) was taken from each patient in cross-sectional study to measure the plasma AEA level during four phases of menstrual cycle, while 10 ml of blood was collected from each patient in the longitudinal study for both plasma and serum. All samples were collected between 10:00 and 12:00 am to avoid any diurnal variation.
Preparation of plasma and serum
The blood for plasma was collected into anticoagulant tubes, followed by centrifugation (3,000 × rmp) at 4 °C for 10 min to separate plasma from cells, and was stored at -80 °C for AEA measurement. Blood for serum was collected into serum gel tubes, and was left to clot for 15 min before being centrifuged at 1,200×g for 30 min, and separated serum was stored at -80 °C for hormone measurements.
Measurement of plasma anandamide
Plasma AEA levels were measured by the ultra-performance liquid chromatography tandem mass spectrometry (UPLC-MS/MS) system (Waters, United States). Briefly, 1 ml of separated plasma was transferred to a tube, and 10 µl of deuterium-labeled AEA (AEA-d8, 1 µg/ml, Cayman Chemicals, United States) was added to estimate the efficiency of the lipid extraction. Protein was extracted by adding 1 ml of methylbenzene, followed by vortex for 30 sec and centrifugation at 4,000 × rpm for 10 min at 4 °C. The supernatant was transferred to a fresh small bottle and the methylbenzene was evaporated under a stream of nitrogen gas until the solid analyst appeared in the bottle. Finally, 5 ml of methanol:water (3:1 vol:vol) mixture was added, followed by vortex for 1 min. Reconstituted samples were injected into the UPLC-MS/MS system to be analyzed. The injection volumes for samples and standards were 25 μl.
The UPLC-MS/MS system is composed of an Acquity UPLC system connected in-line with a Quattro Primer tandem mass spectrometer (Waters Corporation, US). The chromatographic column was an ACQUITY UPLC BEH C18 column (100 mm×2.1mm, particle size: 1.7 μm) maintained at 60 °C. Mobile phases were A (2 mM ammonium acetate containing water) and B (2 mM ammonium acetate containing methyl alcohol). The flow velocity was 0.5 ml/min. The liquid chromatography gradient conditions were as follows: 75% B for 0.5 minute; linear to 79% B over the next 4.5 min, changing to 90% B over the next 0.5 min; then the column was equilibrated again at 75% B for 1 min. Samples were quantified using tandem electrospray mass spectrometry in positive ion mode (ES+). Source parameters including capillary voltage of 0.8 kV, source temperature of 150 °C, desolvation temperature of 600 °C, and desolvation gas flow of 1,100 l/hr.
Measurement of serum FSH, LH, E2 and P
The serum samples were measured by an automated ADVIA Centaur Assay System (Bayer Diagnostics, United Kingdom) in the clinical laboratory of the Second Hospital of Hebei Medical University.
Statistical analyses
All statistical analyses were calculated using SPSS 21.0. Data were normally distributed and were therefore presented as mean +/-SEM. Statistical significance was determined by Student’s t test. Pearson correlation was used to evaluate the relationship between different variables. P<0.05 was considered to be statistically significant.
Results
General characteristics of Subjects
The patients recruited to the study were fertile women with normal menstrual cycle, proposed to get IVF treatment due to oviduct obstruction or male infertility. A total of 79 patients were included in the cross-sectional group, and 10 patients were included in the longitudinal group. There were no statistically significant differences in the age and BMI of the patients in the two study groups. The average age of women in the cross-sectional group was 29.43±0.43 years (Table 1), while the average age of longitudinal group was 29.57±0.57 years (Table 1). The BMIs were 21.82±0.20 kg/m2 for cross-sectional group and 21.81±0.20 kg/m2 for longitudinal group, respectively (Table 1).
Table 1.
The basic characteristics of patients
| n | Age(y) | BMI (kg/m2) | |
|---|---|---|---|
| Cross-sectional arm | 79 | 29.43±0.43 | 21.82±0.20 |
| Longitudinal arm | 10 | 29.57±0.57 | 21.81±0.20 |
| t | 0.454 | -0.185 | |
| P | 1.21 | 0.84 |
Plasma AEA levels during the menstrual cycle
A number of studies proposed a strong link between AEA and human pregnancy, however little is known about the biochemistry, physiology and consequence of AEA in human reproduction. To observe the fluctuation in AEA levels throughout the normal menstrual cycle, blood samples of the patients in the cross-sectional and longitudinal groups were collected, and plasma AEA levels were measured by a UPLC-MS/MS system. First, we analyzed whether changes in plasma AEA levels observed cross-sectionally were comparable to the levels observed longitudinally. The results showed that plasma AEA levels of the cross-sectional group were comparable to that of the longitudinal group (Table 2). The four phases of menstrual cycle include the early follicular (day 2-6), late follicular (days 8-12), ovulatory (day 13-16) and luteal (day 16-30) phases, which were divided according to the normal physiologic changes in the levels of sex steroid hormones and gonadotrophins that occur throughout the ovulatory menstrual cycle.
Table 2.
Plasma AEA levels in volunteers in the two studies
| Early Follicular (ng/ml) | Late Follicular (ng/ml) | Ovulation (ng/ml) | Luteal (ng/ml) | |
|---|---|---|---|---|
| Cross-sectional arm (n=79) | 9.71±0.86 | 10.61±1.05 | 12.24±0.73 | 7.46±0.71 |
| Longitudinal arm (n=10) | 8.76±0.91 | 11.61±1.28 | 13.85±1.18 | 8.50±1.08 |
| t | -0.686 | 0.597 | 1.226 | 0.826 |
| P | 0.498 | 0.556 | 0.231 | 0.416 |
* Data are presented as mean±SEM. P values were calculated using unpaired Student’s t-test with Welch’s correction for unequal variances
In the cross-sectional arm of the study, the plasma AEA levels in the early follicular phase, late follicular phase and ovulatory phase were 9.71±0.86 ng/ml, 10.61±1.05 ng/ml and 12.24±0.73 ng/ml, respectively, which exhibited a gradually increasing trend. However, plasma AEA level in the luteal phases was reduced to 7.46±0.71 ng/ml (Figure 1 and Table 3). There was a statistically significant (P<0.05) rise in the plasma AEA level at the time of ovulation, compared with early follicular and luteal phases, but the difference between the ovulatory and late follicular phases was not statistically significant. The lowest plasma AEA level was observed in the luteal phase, which was significantly lower (P<0.05) than the other three phases (Figure 1 and Table 3).
Figure 1.
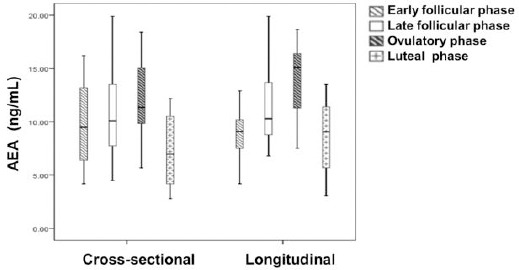
The plasma AEA levels throughout the menstrual cycle were measured. The plasma AEA levels of the patients in the cross-sectional (n=74) and longitudinal groups (n=10) were measured by a UPLC-MS/MS system. The four phases of menstrual cycle include the early follicular (day 2-6), late follicular (days 8-12), ovulatory (day 13-16) and luteal (day 16-30) phases. The results were presented as mean +/-SEM. The significance of the variation was summarized in Table 2 and 3
Table 3.
Plasma AEA levels in volunteers of four phases
| Cross-sectional arm (ng/ml, n=79) | Longitudinal arm (ng/ml, n=10) | |
|---|---|---|
| Early Follicular | 9.71±0.86 | 8.76±0.91 |
| Late Follicular | 10.61±1.05 | 11.61±1.28 |
| Ovulation | 12.24±0.73 | 13.85±1.18 |
| Luteal | 7.46±0.71 | 8.50±1.08 |
| F | 6.098 | 5.133 |
| P | 0.001** | 0.005** |
* Statistical significance is set at P<0.05
Similar trend was also observed in the longitudinal arm of the study. The plasma AEA level was increased from 8.76±0.91 ng/ml in the early follicular phase to 11.61±1.28 ng/ml in the late follicular phase, with the peak plasma AEA level (13.85±1.18 ng/ml) observed at the time of ovulation, followed by a statistically significant decrease to 8.50±1.08 ng/ml in the luteal phase (Figure 1 and Table 3). A statistically significant difference (P<0.05) was also observed between the highest plasma AEA level measured at the time of ovulation and at the early follicular and luteal phases. The lowest plasma AEA level in the late luteal phase was significantly lower (P<0.05) than that in the ovulatory phase.
Taken together, these data presented a fluctuating plasma AEA level during normal menstrual cycle. In both arms of the study, the plasma AEA level peaked at the ovulatory phase, while decreased to the minimum at the luteal phase.
The correlation between plasma AEA concentration and levels of follicle stimulating hormone, luteinizing hormone and estradiol
AEA is released from cell membrane phospholipid precursors, catalyzed by a calcium-dependent N-acyltransa -cylase, in response to hormones and neurotransmitters (11). The dedicated regulation of hormones is required for successful fertilization, implantation and maintenance of early pregnancy. To investigate the correlation between plasma AEA and hormones important in human reproduction will contribute to our understanding of AEA involvement in human fertility. FSH is synthesized and secreted by gonadotropic cells to stimulate the growth and recruitment of immature ovarian follicles in the ovary. It functions together with LH in the reproductive system, the latter of which supports theca cells in the ovaries that provide androgens and hormonal precursors for E2 production. E2 activates estradiol receptors, which regulate the expressions of many genes related to human pregnancy. In addition, P is a C-21 steroid hormone, which stimulates the release of leukemia inhibitory factor to promote implantation and pregnancy continuation. We examined serum concentrations of LH, FSH, E2 and P of patients in the longitudinal group by chemiluminescence, and then analyzed the relationship between plasma AEA and different hormone levels (Figure 2 and Table 4). There were highly statistically significant positive correlations between plasma AEA levels and serum levels of LH (P<0.001), FSH (P<0.001) and E2 (P=0.003), but not with serum P level (P=0.067).
Figure 2.
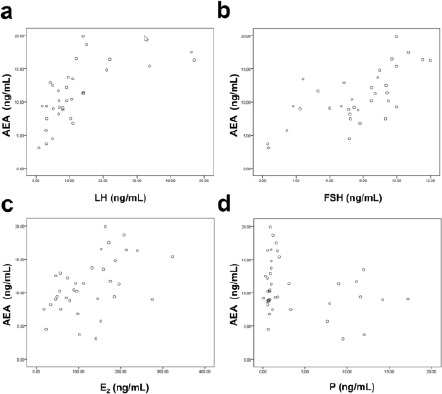
Pearson correlations between the plasma AEA level and several hormone levels. The levels of hormones were determined by chemiluminescence. Then the correlations between the plasma AEA levels and the levels of serum LH (a), FSH (b), E2 (c), and P (d) were constructed for the women in longitudinal groups (n=10). The R2 values and the significance of the correlations were summarized in Table 4. *, P< 0.05, **, P< 0.01, ***, P< 0.001
Table 4.
Correlations between plasma AEA levels with FSH, LH, E2 and P
| r | P | |
|---|---|---|
| FSH | 0.680 | <0.001*** |
| LH | 0.639 | <0.001*** |
| E2 | 0.493 | 0.003** |
| P | -0.296 | 0.067 |
* Statistical significance is set at P<0.05
Discussion
The menstrual cycle is tightly regulated by a panel of gonadotrophin and sex steroid hormones. During the follicular phase, the ovarian follicles mature through the stimulation by FSH, and become ready to release an egg (27, 28). Subsequently, E2 promotes the production of a large amount of LH, leading to the fully developed follicle to release its secondary oocyte, which is referred to as the ovulatory phase (29). The FSH and LH induce the remaining parts of the follicle to transform into the corpus luteum, which then continues to secrete P to maintain the new pregnancy (30). Although several factors have been identified to affect the ovum development and the endometrium receptivity for implantation, the mechanisms that regulate this process are not completely understood. Recent studies suggested that AEA might have a crucial role in embryo implantation and early fetal development (1). AEA is synthesized within the female reproductive tracts, and the AEA and CB1 receptor expressions in the uterine are higher than in the brain tissue (31). Moreover, the AEA levels in the implantation sites of mouse uterus were lower than the inter-implantation sites, because the downregulation of AEA levels was associated with uterine receptivity, whereas its upregulation was correlated with uterine refractoriness to embryo implantation (32). The site-specific levels of AEA in the mouse uterus might regulate implantation spatially by promoting the trophoblast differentiation at the site of blastocyst implantation (33). In addition, AEA has been shown to regulate the development of the mouse preimplantation embryo via CB1 receptors expressed on the embryonic cell surface (34). During the normal menstrual cycle, plasma levels of AEA vary widely at different phases, as well as between individual pregnancies. Some reports have shown that AEA level in the early follicular phase was higher than the late luteal phase of the menstrual cycle (35). However, these data were acquired from European women, whose physique and genetic backgrounds were greatly different from Asian women. Reliable reference values based on the local population are important for correct clinical diagnosis.
Here we reported that the plasma AEA level varied throughout the menstrual cycle. In both arms of the study, AEA levels were slightly upregulated in late follicular phase compared with the early follicular phase, followed by peaking at the time of ovulation, suggesting that AEA might have a role in folliculogenesis or ovulation. In line with our results, others also showed a direct correlation between the follicular fluid AEA level and follicle size, supporting the notion that AEA might be involved in folliculogenesis (36). However, there have been few published studies on the effects of AEA on folliculogenesis or ovulation, and further studies are required to investigate the effect of AEA on these processes. During the luteal phase of the menstrual cycle, the plasma AEA levels declined significantly in both arms of the study. This period of the menstrual cycle is referred to as the “implantation window”, where the endometrium undergoes structural and functional changes to be receptive to the invasion by the embryo. Proper cross-talk is established between the blastocyst and a receptive endometrium during this critical period, and any defect in this cross-talk may cause embryo abortion. Among the women who had undergone IVF or intracytoplasmic sperm injection (ICSI), there was a significant decrease in plasma AEA levels from the day of oocyte retrieval to that of embryo transfer, with a mean 43.2% decrease in pregnant group, whereas the mean difference in the decline of plasma AEA levels between the two periods was only 20.2% in the non-pregnant group, suggesting that a higher plasma AEA level at ovulation and a significantly lower level during implantation are required for successful pregnancy (22). Taken together, our results showed reliable reference plasma AEA levels throughout the menstrual cycle in Asian women, which might be used as a biomarker for the appropriate timing of embryo transfer.
The enzymes and hormones involved in the synthesis and degradation of endocannabinoids have critical roles in normalizing AEA levels for the successful implantation and early pregnancy maintenance. FAAH is the principle enzyme involved in the degradation of AEA. FAAH-deficient mice or animals administrated with FAAH inhibitors were severely impaired in their ability to hydrolyze AEA. It was reported that P could upregulate FAAH activity and consequently decrease AEA levels (24). However, we observed no correlation between plasma AEA level and serum P level, which suggested that P might not be the main regulator of AEA levels in normal cycling women. E2 was also involved in the maintenance of AEA levels. The studies have shown that E2 modulates CB1 receptor expression, and stimulates the release of AEA from endothelial cells by inhibiting FAAH (25). Moreover, E2 inhibited FAAH activity in murine uterine, supporting the possibility that E2 and AEA levels are closely linked (23). Our finding of the positive correlation between the plasma AEA and the serum E2 levels is consistently with previous studies, suggesting E2 is an important regulator of AEA during normal menstrual cycle. In addition to E2, FSH and LH may also be involved in the regulation of AEA based on our data. Previous study showed that treating mouse primary Sertoli cells with FSH enhanced the activity of the AEA hydrolase, whereas it did not affect the enzymes synthesizing AEA, nor CB1 and CB2 expressions (37). The regulation of FAAH by FSH has a critical impact on Sertoli cell proliferation, spermatogenesis and male reproduction (37). However, study in the regulatory role of FSH in female reproduction system was limited, and needs to be further explored. In addition, it is reported that downregulated LH could reduce E2 release from ovaries (38), suggesting a potential regulatory effect on AEA. Our data suggested that LSH, LH and E2 might regulate AEA concentration during the menstrual cycle in Asian women, but further studies are needed to explore in details how this regulation may occur. The cross-talk among AEA, E2, FSH and LH is interesting to be investigated as well.
Conclusion
In summary, our study established a fluctuating AEA level throughout the menstrual cycle. We found that AEA levels were closely associated with FSH, LH and E2. The novel information obtained in our study could facilitate our understanding of key molecular mechanisms that regulate the process of folliculogenesis, ovulation, early embryo development and oviductal transport, and contribute to the development of hormone-based therapeutic strategies for the treatment of human infertility.
Acknowledgment
None.
Conflict of interests
The authors declare that there is no conflict of interests.
References
- 1.Battista N, Bari M, and Maccarrone M. Endocannabinoids and Reproductive Events in Health and Disease. Handb Exp Pharmacol. 2015;231:341–365. doi: 10.1007/978-3-319-20825-1_12. [DOI] [PubMed] [Google Scholar]
- 2.Meccariello R, Battista N, Bradshaw HB, and Wang H. Updates in reproduction coming from the endocannabinoid system. Int J Endocrinol. 2014;2014:412354. doi: 10.1155/2014/412354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fride E. Endocannabinoids in the central nervous system--an overview. Prostaglandins Leukot Essent Fatty Acids. 2002;66:221–233. doi: 10.1054/plef.2001.0360. [DOI] [PubMed] [Google Scholar]
- 4.Fride E, and Shohami E. The endocannabinoid system:function in survival of the embryo, the newborn and the neuron. Neuroreport. 2002;13:1833–1841. doi: 10.1097/00001756-200210280-00001. [DOI] [PubMed] [Google Scholar]
- 5.Devane WA, Hanus L, Breuer A, Pertwee RG, Stevenson LA, Griffin G, et al. Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science. 1992;258:1946–1949. doi: 10.1126/science.1470919. [DOI] [PubMed] [Google Scholar]
- 6.Sugiura T, Kondo S, Sukagawa A, Nakane S, Shinoda A, Itoh K, et al. 2-Arachidonoylglycerol:a possible endogenous cannabinoid receptor ligand in brain. Biochem Biophys Res Commun. 1995;215:89–97. doi: 10.1006/bbrc.1995.2437. [DOI] [PubMed] [Google Scholar]
- 7.Mechoulam R, Ben-Shabat S, Hanus L, Ligumsky M, Kaminski NE, Schatz AR, et al. Identification of an endogenous 2-monoglyceride, present in canine gut, that binds to cannabinoid receptors. Biochem Pharmacol. 1995;50:83–90. doi: 10.1016/0006-2952(95)00109-d. [DOI] [PubMed] [Google Scholar]
- 8.De Petrocellis L, Cascio MG, Di Marzo V. The endocannabinoid system:a general view and latest additions. Br J Pharmacol. 2004;141:765–774. doi: 10.1038/sj.bjp.0705666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Porter AC, Sauer JM, Knierman MD, Becker GW, Berna MJ, Bao J, et al. Characterization of a novel endocannabinoid, virodhamine, with antagonist activity at the CB1 receptor. J Pharmacol Exp Ther. 2002;301:1020–1024. doi: 10.1124/jpet.301.3.1020. [DOI] [PubMed] [Google Scholar]
- 10.Bisogno T, Melck D, Bobrov M, Gretskaya NM, Bezuglov VV, De Petrocellis L, et al. N-acyl-dopamines:novel synthetic CB(1) cannabinoid-receptor ligands and inhibitors of anandamide inactivation with cannabimimetic activity in vitro and in vivo. Biochem J. 2000;351(Pt 3):817–824. [PMC free article] [PubMed] [Google Scholar]
- 11.Sugiura T, Kobayashi Y, Oka S, and Waku K. Biosynthesis and degradation of anandamide and 2-arachidonoylglycerol and their possible physiological significance. Prostaglandins Leukot Essent Fatty Acids. 2002;66:173–192. doi: 10.1054/plef.2001.0356. [DOI] [PubMed] [Google Scholar]
- 12.Okamoto Y, Morishita J, Tsuboi K, Tonai T, and Ueda N. Molecular characterization of a phospholipase D generating anandamide and its congeners. J Biol Chem. 2004;279:5298–5305. doi: 10.1074/jbc.M306642200. [DOI] [PubMed] [Google Scholar]
- 13.Howlett AC, Barth F, Bonner TI, Cabral G, Casellas P, Devane WA, et al. International Union of Pharmacology. XXVII. Classification of cannabinoid receptors. Pharmacol Rev. 2002;54:161–202. doi: 10.1124/pr.54.2.161. [DOI] [PubMed] [Google Scholar]
- 14.Pertwee RG, Ross RA. Cannabinoid receptors and their ligands. Prostaglandins Leukot Essent Fatty Acids. 2002;66:101–121. doi: 10.1054/plef.2001.0341. [DOI] [PubMed] [Google Scholar]
- 15.Pertwee RG, Ross RA, Craib SJ, Thomas A. (-)-Cannabidiol antagonizes cannabinoid receptor agonists and noradrenaline in the mouse vas deferens. Eur J Pharmacol. 2002;456:99–106. doi: 10.1016/s0014-2999(02)02624-9. [DOI] [PubMed] [Google Scholar]
- 16.McKinney MK, Cravatt BF. Structure and function of fatty acid amide hydrolase. Annu Rev Biochem. 2005;74:411–432. doi: 10.1146/annurev.biochem.74.082803.133450. [DOI] [PubMed] [Google Scholar]
- 17.Wei BQ, Mikkelsen TS, McKinney MK, Lander ES, Cravatt BF. A second fatty acid amide hydrolase with variable distribution among placental mammals. J Biol Chem. 2006;281:36569–36578. doi: 10.1074/jbc.M606646200. [DOI] [PubMed] [Google Scholar]
- 18.Tsuboi K, Sun YX, Okamoto Y, Araki N, Tonai T, Ueda N. Molecular characterization of N-acylethanolamine-hydrolyzing acid amidase, a novel member of the choloylglycine hydrolase family with structural and functional similarity to acid ceramidase. J Biol Chem. 2005;280:11082–11092. doi: 10.1074/jbc.M413473200. [DOI] [PubMed] [Google Scholar]
- 19.Schuel H, Burkman LJ, Lippes J, Crickard K, Mahony MC, Giuffrida A, et al. Evidence that anandamide-signaling regulates human sperm functions required for fertilization. Mol Reprod Dev. 2002;63:376–387. doi: 10.1002/mrd.90021. [DOI] [PubMed] [Google Scholar]
- 20.Gervasi MG, Rapanelli M, Ribeiro ML, Farina M, Billi S, Franchi AM, et al. The endocannabinoid system in bull sperm and bovine oviductal epithelium:role of anandamide in sperm-oviduct interaction. Reproduction. 2009;137:403–414. doi: 10.1530/REP-08-0204. [DOI] [PubMed] [Google Scholar]
- 21.El-Talatini MR, Taylor AH, Elson JC, Brown L, Davidson AC, Konje JC. Localisation and function of the endocannabinoid system in the human ovary. PLoS One. 2009;4:e4579. doi: 10.1371/journal.pone.0004579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.El-Talatini MR, Taylor AH, Konje JC. Fluctuation in anandamide levels from ovulation to early pregnancy in in-vitro fertilization-embryo transfer women, its hormonal regulation. Hum Reprod. 2009;24:1989–1998. doi: 10.1093/humrep/dep065. [DOI] [PubMed] [Google Scholar]
- 23.Karasu T, Marczylo TH, Maccarrone M, Konje JC. The role of sex steroid hormones, cytokines and the endocannabinoid system in female fertility. Hum Reprod Update. 2011;17:347–361. doi: 10.1093/humupd/dmq058. [DOI] [PubMed] [Google Scholar]
- 24.Maccarrone M, Valensise H, Bari M, Lazzarin N, Romanini C, Finazzi-Agro A. Progesterone up-regulates anandamide hydrolase in human lymphocytes:role of cytokines and implications for fertility. J Immunol. 2001;166:7183–7189. doi: 10.4049/jimmunol.166.12.7183. [DOI] [PubMed] [Google Scholar]
- 25.Maccarrone M, Bari M, Battista N, Finazzi-Agro A. Estrogen stimulates arachidonoylethanolamide release from human endothelial cells and platelet activation. Blood. 2002;100:4040–4048. doi: 10.1182/blood-2002-05-1444. [DOI] [PubMed] [Google Scholar]
- 26.Maccarrone M, Falciglia K, Di Rienzo M, Finazzi-Agro A. Endocannabinoids, hormone-cytokine networks and human fertility. Prostaglandins Leukot Essent Fatty Acids. 2002;66:309–317. doi: 10.1054/plef.2001.0354. [DOI] [PubMed] [Google Scholar]
- 27.Grisendi V, Spada E, Argento C, Plebani M, Milani S, Seracchioli R, et al. Age-specific reference values for serum FSH and estradiol levels throughout the reproductive period. Gynecol Endocrinol. 2014;30:451–455. doi: 10.3109/09513590.2014.893572. [DOI] [PubMed] [Google Scholar]
- 28.Ecochard R, Guillerm A, Leiva R, Bouchard T, Direito A, Boehringer H. Characterization of follicle stimulating hormone profiles in normal ovulating women. Fertil Steril. 2014;102:237–243.e235. doi: 10.1016/j.fertnstert.2014.03.034. [DOI] [PubMed] [Google Scholar]
- 29.Dimitraki M, Messini CI, Dafopoulos K, Gioka T, Koutlaki N, Garas A, et al. Attenuating activity of the ovary on LH response to GnRH during the follicular phase of the cycle. Clin Endocrinol (Oxf) 2014;80:439–443. doi: 10.1111/cen.12306. [DOI] [PubMed] [Google Scholar]
- 30.Honnma H, Asada Y, Baba T, Endo T. Continuous high-dose estrogen controls serum FSH and LH levels:new treatment strategy for extremely low ovarian reserve patients, two case reports. Gynecol Endocrinol. 2014;30:341–344. doi: 10.3109/09513590.2013.871524. [DOI] [PubMed] [Google Scholar]
- 31.Yang ZM, Paria BC, Dey SK. Activation of brain-type cannabinoid receptors interferes with preimplantation mouse embryo development. Biol Reprod. 1996;55:756–761. doi: 10.1095/biolreprod55.4.756. [DOI] [PubMed] [Google Scholar]
- 32.Schmid PC, Paria BC, Krebsbach RJ, Schmid HH, Dey SK. Changes in anandamide levels in mouse uterus are associated with uterine receptivity for embryo implantation. Proc Natl Acad Sci U S A. 1997;94:4188–4192. doi: 10.1073/pnas.94.8.4188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang J, Paria BC, Dey SK, Armant DR. Stage-specific excitation of cannabinoid receptor exhibits differential effects on mouse embryonic development. Biol Reprod. 1999;60:839–844. doi: 10.1095/biolreprod60.4.839. [DOI] [PubMed] [Google Scholar]
- 34.Paria BC, Das SK, Dey SK. The preimplantation mouse embryo is a target for cannabinoid ligand-receptor signaling. Proc Natl Acad Sci U S A. 1995;92:9460–9464. doi: 10.1073/pnas.92.21.9460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Habayeb OM, Taylor AH, Evans MD, Cooke MS, Taylor DJ, Bell SC, et al. Plasma levels of the endocannabinoid anandamide in women--a potential role in pregnancy maintenance and labor? J Clin Endocrinol Metab. 2004;89:5482–5487. doi: 10.1210/jc.2004-0681. [DOI] [PubMed] [Google Scholar]
- 36.El-Talatini MR, Taylor AH, Konje JC. The relationship between plasma levels of the endocannabinoid, anandamide, sex steroids, and gonadotrophins during the menstrual cycle. Fertil Steril. 2010;93:1989–1996. doi: 10.1016/j.fertnstert.2008.12.033. [DOI] [PubMed] [Google Scholar]
- 37.Schlosburg JE, Kinsey SG, Lichtman AH. Targeting fatty acid amide hydrolase (FAAH) to treat pain and inflammation. AAPS J. 2009;11:39–44. doi: 10.1208/s12248-008-9075-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gorzalka BB, Dang SS. Minireview:Endocannabinoids and gonadal hormones:bidirectional interactions in physiology and behavior. Endocrinology. 2012;153:1016–1024. doi: 10.1210/en.2011-1643. [DOI] [PubMed] [Google Scholar]


