Abstract
Objective(s):
Oxidative stress has a pivotal role in the pathogenesis of diabetic retinopathy (DR). Juglans regia L. (JRL) leaf extract has hypoglycemic and antioxidative properties. This study aimed to determine the ameliorative effects of JRL against diabetic retinopathy.
Materials and Methods:
The DR rat model was generated by injection of streptozotocin (STZ). A subset of the diabetic rats received JRL or metformin after the onset of hyperglycemia. Histopathology and immunohistochemistry of apoptotic and inflammatory factors were assessed along with biochemical assessments of lipid peroxidation and antioxidant status.
Results:
Lipid peroxidation level and catalase activity significantly improved after JRL consumption (P<0. 001). Degeneration of the retina attenuated after JRL consumption. Attenuation of the caspase-3, COX-2, PARP, and S100B expression could be detected significantly (P<0. 001) in the JRL-treated rats. While, blood glucose level decreased after JRL consumption (P<0. 001).
Conclusion:
JRL leaf extract exert protective effects against diabetic retinopathy.
Keywords: Antioxidant, Apoptosis, Diabetic retinopathy, Hyperglycemia, Inflammation, Juglans regia L. leaf
Introduction
Diabetic retinopathy is one of the most common long term complications of diabetes mellitus which is leading to blindness in working-age adults (1). In this regard, several mechanisms account for the development of diabetic retinopathy such as microvascular damages, inflammation, metabolic disorders, haemodynamic changes, and oxidative stress (2-4). Among these factors, oxidative stress has a pivotal role in the development of diabetic retinopathy. Increased oxidative stress after hyperglycemia is caused mainly due to autooxidative glycosylation, advanced glycation end products (AGEs) formation, and increasing polyol pathway activity (5). Therefore, it has been postulated that the use of antioxidant supplements may offer some protection against this complication through free radical scavenging actions. Within the previous decades, a rapidly growing number of natural phenolic compounds, secondary metabolites of plants, with antioxidant effects have been described. The seeds, green husks, and leaves of the Persian or common walnut (Juglans regia L.), the best-known member of the Juglans genus (6, 7), are a rich source of these molecules.
They have been traditionally used in Iranian folk medicine for treatment of several diseases such as infections, inflammations, and diabetes and its complications. Flavonoids, phenolic acids, and naphtoquinones are considered as major phenolic compounds in J. regia leaves (8-10). There is accumulating evidence that attributed the beneficial effects of J. regia leaf extract to a variety of biological activities, including anti-oxidative (10, 11), anti-inflammatory (12), anti-carcinogenic (13), anti-microbial (14), and anti-fungal properties (15). Recently, there are a few experimental studies on the hypoglycemic effect of J. regia leaf extract in diabetes mellitus. These studies documented that administration of J. regia leaf extract significantly reduced fast blood sugar (FBS) and glycated hemoglobin (HbA1c) compared to control groups (16-19). Moreover, results of two clinical trial studies have shown that FBS and HbA1c significantly decreased after consumption of 100 mg J. regia leaf extract for 3 months (20) and 200 mg J. regia leaf extract for 2 months (21) compared to placebo groups. An in vitro study also reported that walnut leaf extract inhibits protein tyrosine phosphatase 1B (PTP1B) and enhances glucose-uptake (22).
Due to the potent antioxidant and hypoglycemic properties of J. regia leaf extract, we investigated for the first time the protective effects of the extract against diabetic retinopathy as a common serious complication of diabetes.
Materials and Methods
Extract preparation and GC-MS
Fresh leaves of J. regia were collected during July-August 2014 from cultivated trees in Khorramabad (Lorestan Province, Western Iran) and authenticated by Natural Resources Research Center of Lorestan Province. Briefly, the leaves were dried and pulverized and then stored in dark at room temperature. Methanol was added to the pulverized leaves for 72 hr and then filtered through filter paper. The obtained extracts were concentrated at 40 °C. Gas chromatography-mass spectrometry (GC-MS) was carried out using a Hewlett-Packard 6859 with a quadrupole detector, on a HP-5 column, operating at 70 eV ionization energy, using the same temperature program and carrier gas as above. Retention indices were measured by retention times of n-alkanes that were injected after the extract (23).
Animals
Male adult Sprague-Dawley rats were used (250-275 g) (Laboratory Animal Research Center, Sari, Iran). They were kept in the laboratory under constant conditions of temperature (23±2 ºC) and light/dark cycle (12 hr/12 hr) for at least one week before and through the experimental work. All procedures were done according to the guidelines of the university’s animal care codes (IR.MAZUMS.REC.95.S171) to minimize the animal’s suffering and were fed a standard rat chow and drinking water ad libitum throughout the study period.
Induction of diabetes and experimental design
Diabetes was induced by a 55-mg/kg single dose of streptozotocin (Santa Cruz Biotechnology) diluted in 0.1 M citrate buffer with pH-4.5. Blood samples were collected from tail vein 48 hr after streptozotocin (STZ) administration and estimated plasma glucose levels using a commercial glucometer and test strips (Accu-Chek® Active test meter). Rats with plasma glucose level more than 250 mg/dl were considered as diabetics and were further considered for study. J. regia leaf extract (200 mg/kg/day) and metformin (350 mg/kg/day) was administered by oral gavages. The doses and treatment schedules were based on previous studies (16, 18, 24, 25) and pilot experiments in our laboratory.
Animal groups
The animals were randomly allocated to five groups, each containing 7 rats: (Ι) Control group, which received citrate buffer intraperitoneally and isotonic saline orally for the duration of the study; (II) Control+ J. regia leaf extract (JRL) group, which received JRL orally (200 mg/kg/day) for a period of two months; (III) Diabetic group, which received single injection of STZ (55 mg/kg) intraperitoneally and were also given isotonic saline orally for the duration of the study; (IV) Diabetic+JRL group, which received JRL (200 mg/kg/day) starting one week after STZ injection for a period of two months; (V) Diabetic+Metformin group (positive control group), which received Metformin (350 mg/kg/day) starting one week after STZ injection for a period of two months. Blood glucose levels were measured weekly until the end of the experiment.
Biochemistry
The retina samples (the left side retina) were thoroughly cleaned of blood and then were immediately frozen and stored in a -80 °C freezer for assays of tissue malondialdehyde (MDA) levels (26) and catalase (CAT) activities (27). The absorbance of the supernatant was measured by spectrophotometery. MDA levels and CAT activities were expressed as micromoles per milligram of protein and as nanomoles trolox equivalent per milligram of protein, respectively.
Histopathology
The retina samples (the right side retina) were obtained and then were immediately fixed in 10% buffered formaldehyde and embedded in paraffin. Five-micrometer serial sections were prepared from the paraffin-embedded blocks using microtome. For histopathological assessment, some tissue sections were deparaffinized with xylene, stained with hematoxylin and eosin (H&E), and studied by light microscopy. Morphometric computer-assisted image analysis of the retina was done using Image_J software (MacBiophotonics ImageJ 1.41a) using methods previously described (28). All the histological assessments were performed in a blinded fashion.
Immunohistochemistry
For immunohistochemistry, some sections were incubated with anti-caspase 3 (1:100, Abcam), anti-COX 2 (1:100, Abcam), anti-S100B (1:1000, Abcam) and anti-PARP (1:100, Abcam) overnight at 4 °C. Then followed by incubation with secondary antibody conjugated with horseradish peroxidase (Abcam) and detected by DAB for 10 min. Afterwards, they were dehydrated and mounted. Quantitative analysis was assessed by densitometry using Image_J software (five immunohistochemical photographs from each sample). Data are expressed as a percentage of total tissue surface area.
Statistical analysis
Statistical analysis was carried out in SPSS (Version 15, Chicago, IL, USA). Results were presented as mean values (±SD). The K-S test was used in order to evaluate the normality of the data. Also, the Tukey’s multiple comparison tests and the analysis of the variance were used to compare each two groups and data among the groups, respectively. A value of P<0.05 was considered significant.
Results
GC-MS analysis
The principal components identified in J. regia leaf extract were 2-ß-pinene (17.09%), α-pinene (13.29%), trans-caryophyllene (10.58%), and germacrene D (8.90%). Other minor identified constituents were dl-limonene (3.85%), terpine-4-ol (3.70%), ß-slinene (3.25%), and methyl salicylate (3.07%).
Blood glucose levels
Fasting blood sugar (FBS) levels for all groups are shown in Table 1. Administration of STZ in the diabetic group produced a significant elevation (P<0.001) in FBS level and the hyperglycemia was maintained throughout experimental period compared to the control and control+ JRL groups. At the end of the experiment, the FBS levels in the diabetic+JRL group were significantly lower than that in the diabetic group (P<0.001), While the differences between diabetic+JRL and diabetic+ metformin group were not significant (P>0.05).
Table 1.
Effect of the J. regia (JRL) leaf extract on fasting blood sugar (FBS) level
| Experimental Groups | FBS (mg/dl) 0d | FBS (mg/dl) 7d | FBS (mg/dl) 30d | FBS (mg/dl) 60d |
|---|---|---|---|---|
| Control | 86.00±8.57 | 98.60±9.12 | 94.60±16.43 | 97.20±15.07 |
| Control+JRL | 92.00±11.11 | 90.40±9.96 | 94.80±12.09 | 93.80±11.65 |
| Diabetic | 86.40±8.08 | 408.00±99.88* | 442.60±62.03* | 430.00±70.81* |
| Diabetic+JRL | 85.20±8.70 | 373.80±45.35* | 271.40±67.01*,** | 227.80±65.59*,** |
| Diabetic+Metformin | 89.20±6.70 | 359.40±49.36* | 268.40±47.09*,** | 237.70±21.56*,** |
Data are represented in Mean±SD.
P<0.001 versus Control and Control+JRL groups;
P<0.001 versus Diabetic group. Time (d) after STZ induction
Biochemical analysis
MDA levels for all groups at the end of the experiment are shown in Table 2. Administration of STZ in the diabetic group produced a significant elevation (P<0.001) in lipid peroxidation level compared to Control and Control+JRL groups. The MDA levels in the Diabetic+JRL group were significantly lower than that those in the Diabetic group (P<0.001), While the differences between diabetic+JRL and diabetic+ Metformin group were significant (P<0.01).
Table 2.
Effect of the J. regia (JRL) leaf extract on biochemical markers of rat retina affected by STZ-induced diabetic retinopathy
| Experimental Groups | MDA µmol/mg-protein | CAT unit/mg-protein |
|---|---|---|
| Control | 99.31±0.44 | 8.66±0.2 |
| Control+JRL | 94.02±0.02 | 7.41±0.25 |
| Diabetic | 147.30±0.84* | 2.36±0.50## |
| Diabetic+JRL | 94.29±4.90** | 8.11±2.11*** |
| Diabetic+Metformin | 113.60±3.79**,# | 8.87±0.53*** |
Data are represented in Mean±SD.
P<0.001 versus Control and Control+JRL groups;
P<0.001 versus Diabetic group;
P<0.01 versus Diabetic+JRL group;
P<0.01 versus Control and Control+JRL groups;
P<0.01 versus Diabetic group
Catalase (CAT) activity levels for all groups at the end of the experiment are shown in Table 2. Administration of STZ in the diabetic group produced a significant (P<0.01) decrease in CAT activity compared to the control and control+JRL groups. The CAT activities in the diabetic+JRL group were significantly (P<0.01) higher than that in the diabetic group, While the differences between diabetic+JRL and diabetic+metformin group were not significant (P>0.05).
Histopathologic changes
Histological examination of the retina in the diabetic animals (Figure 1A) revealed histopathological changes including, ganglion cell layer shrinkage, new vessel formation in the junction between ganglion cell layer and inner plexiform layer. Furthermore, the retinas of diabetic rats somewhat disorganized and thinner than those of normal rats. Treatment with J. regia leaf extract (Figure 1B) and Metformin ameliorated the dramatic histological alternations but did not reach the normal structural pattern. No detectable injury was shown in control and control+JRL groups. Computer-aided morphometric analyses of the retina in the experimental groups are shown in Table 3.
Figure 1.
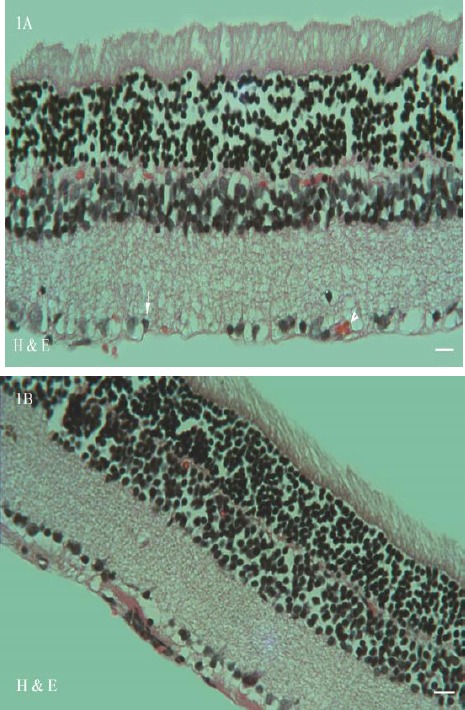
Photomicrographs of the retina in diabetic group (1A) revealed histopathological changes such as ganglion cell layer shrinkage (arrow) and new vessel formation (arrowhead). Treatment with JRL and metformin ameliorated the dramatic histological alternations (1B) (stained with hematoxylin and eosin; original magnification: ×400, bar: 100µm)
Table 3.
Effect of the J. regia (JRL) leaf extract on morphometric analysis of the diabetic retina
| Experimental Groups | Retinal thickness (µm) | Number of cells in GCL/100µm |
|---|---|---|
| Control | 790±64.97 | 6.2±1.20 |
| Control+JRL | 806±51.68 | 6.15±1.40 |
| Diabetic | 584.1±29.18* | 3.13±1.30* |
| Diabetic+JRL | 736.5±56.63** | 5.66±1.23** |
| Diabetic+Metformin | 686.5±46.53**,# | 4.13±2.45**,# |
Data are represented in mean±SD.
P<0.001 versus control and control+JRL groups;
P<0.001 versus diabetic group;
P<0.001 versus diabetic+JRL group
Immunohistochemical assessment
Figures 2-5 show the immunohistochemical staining of caspase-3, COX-2, PARP, and S100B, respectively. Administration of STZ in the diabetic group increased the expression of caspase-3 (Figure 2A), COX-2 (Figure 3A), PARP (Figure 4A), and S100B (Figure 5A), while J. regia leaf extract treatment in the diabetic+JRL (Figure B) and metformin in the diabetic+ metformin groups reduced the degree of positive staining for caspase-3 (Figure 2B), COX-2 (Figure 3B), PARP (Figure 4B), and S100B (Figure 5B) compared to the diabetic group. The histograms of the quantitative analysis of caspase-3, COX-2, PARP, and S100B positive staining in the experimental groups are shown in Figures 2C, 3C, 4C, and 5C respectively.
Figure 2.
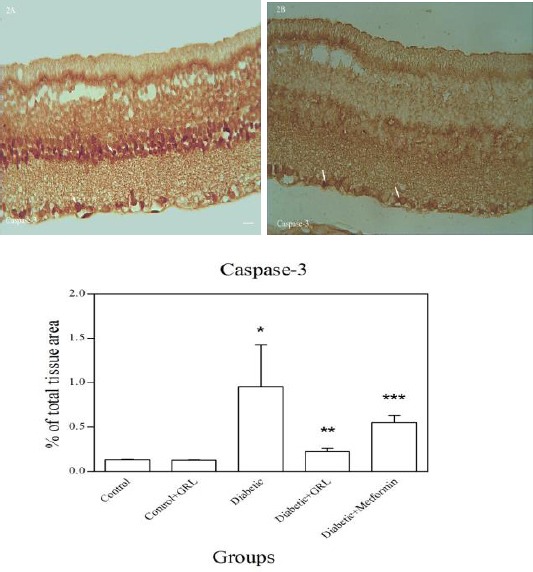
Light photomicrographs show immunohistochemical expression of caspase-3 in diabetic (2A) and diabetic+JRL (2B) group. The positive staining of caspase-3 is presented by a brown color of cytoplasm (arrows) (original magnification: ×400, bar: 100 µm). Densitometry analysis of immunohistochemical photomicrographs for caspase-3 was assessed. Data are expressed as a percentage of total tissue area (2C). *P<0.001 versus control and control+JRL groups; **P<0.001 versus diabetic group; ***P<0.05 versus diabetic group
Figure 5.
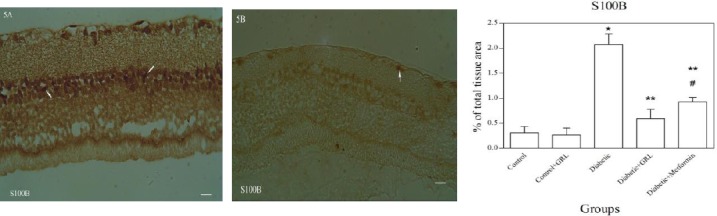
Light photomicrographs show immunohistochemical expression of S100B in diabetic (5A) and diabetic+JRL (5B) groups. The positive staining of S100B is presented by a brown color of cytoplasm (arrows) (original magnification: ×400, bar: 100µm). Densitometry analysis of immunohistochemical photomicrographs for S100B was assessed. Data are expressed as a percentage of total tissue area (5C). *P<0.001 versus control and control+JRL groups; **P<0.001 versus diabetic group; #P<0.05 versus diabetic + JRL group
Figure 3.
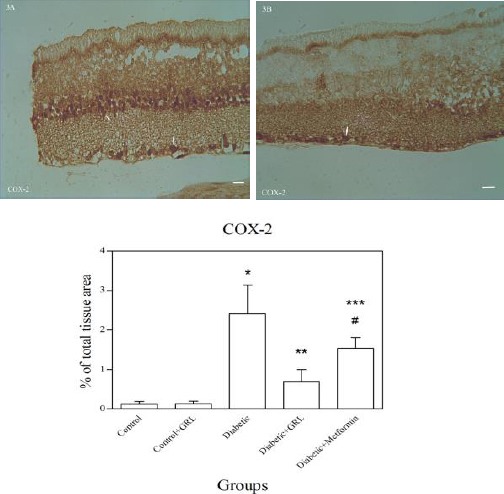
Light Photomicrographs show immunohistochemical expression of COX-2 in Diabetic (3A) and Diabetic + JRL (3B) groups. The positive staining of COX-2 is presented by a brown color of cytoplasm (arrows) (original magnification: ×400, bar: 100µm). Densitometry analysis of immunohistochemical photomicrographs for COX-2 was assessed. Data are expressed as a percentage of total tissue area (3C). *P<0.001 versus Control and Control+JRL groups; **P<0.001 versus Diabetic group; ***P<0.05 versus Diabetic group; #P<0.05 versus Diabetic+JRL group
Figure 4.
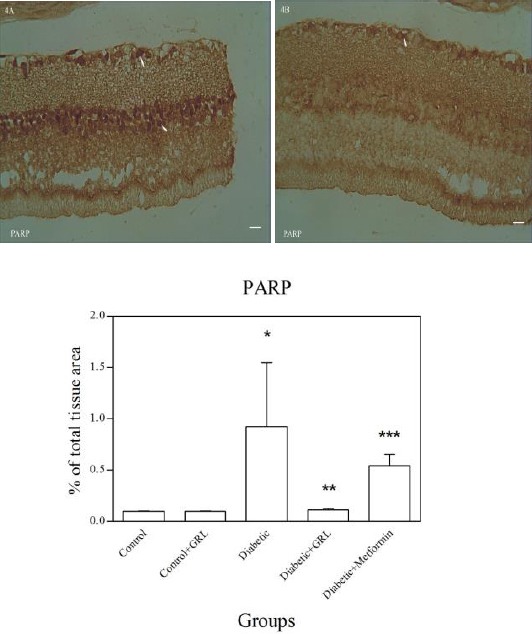
Light photomicrographs show immunohistochemical expression of PARP in diabetic (4A) and diabetic+JRL (4B) groups. The positive staining of PARP is presented by a brown color of cytoplasm (arrows) (original magnification: ×400, bar: 100 µm). Densitometry analysis of immunohistochemical photomicrographs for PARP was assessed. Data are expressed as a percentage of total tissue area (4C). *P<0.01 versus control and control+JRL groups; **P<0.001 versus diabetic group; ***P<0.05 versus diabetic group
Discussion
The main findings of the current study showed that administration of J. regia leaf extract attenuates criteria of retinopathy in STZ-induced diabetic rats, in addition to hypoglycemic effects. The hypoglycemic effects of J. regia leaf extract in this study, have been previously proven by the experimental (18, 19) and human clinical trial studies (20, 21). In this regard, the activity was attributed to the antioxidant capacity of the polyphenols present in walnut leaves (29), its effects on glucose-uptake due to inhibition of protein tyrosine phosphatase 1B (22), and its effects on beta cells regeneration and its anti-inflammatory properties (30). One of the most common complications of diabetes mellitus is retinopathy. Free radical induced oxidative stress has been implicated to play an important role in the pathogenesis of diabetic retinopathy (31). Meanwhile, the retina is vulnerable to oxidative stress due to polyunsaturated fatty acids high content, high oxygen uptake, and high glucose oxidation (32). In this regard, we observed a significant increase in lipid peroxidation and a significant reduction of catalase activity in the retina of diabetic rats. Treatment with J. regia leaf extract ameliorated lipid peroxidation and improved antioxidant status in the retina of diabetic rats. Free radicals reactive oxygen species and nitrogen species have been implicated as a potential contributor to the pathogenesis of diabetic complications (32-34) which seems appropriate for therapeutic interventions such as the use of free radical scavengers. J. regia leaves contain a large amount of phenolic compounds, well-known free radicals scavengers. Phenolic acids, naphthoquinones, and flavonoids are the main phenolic compounds in fresh J. regia leaves (10, 29, 35, 36). In this regard, a study of antioxidant activity of J. regia leaf extract by the reducing power assay and the scavenging effect on DPPH radicals revealed that walnut leaves cultivars have high antioxidant properties (9). In vitro study indicated that flavonoids from Juglans regia leaves could reduce the reactive oxygen species level in RAW264.7 cells (10). Carvalho et al. documented that J. regia leaf extract significantly protected AAPH-induced oxidative hemolysis of human erythrocytes in a time- and concentration-dependent manner (13). In another study, the antioxidant potential of ethanolic extract of Juglans regia leaves was measured and the highest ability to chelate Fe2+, high reducing power, high antiradical activity, and relatively low prevention of lipid oxidation documented (37). Results of an in vivo study demonstrated that administration of walnut leaf extract increased the antioxidant enzymes superoxide dismutase and catalase against CCI4-induced oxidative damage in rat liver (38).
Several lines of evidence obtained in experimental and clinical studies demonstrated that inflammatory-like processes play a critical role in the development of the early and late stages of the retinopathy. In this regard, it was well documented that the enhanced inflammatory response in the early stages of diabetic retinopathy was mediated by NF-kB axis activation; so that selective inhibition of the NF-kB reduces retinal degeneration and expression of proinflammatory proteins such as COX-2, TNFɑ, PARP, iNOS, and IL-1ß (39-41). On the other hand, microglial activation which is observed in diabetic retinopathy causing the release of inflammatory factors (42). Our immunohistocehmical assessments showed that increased COX-2 and PARP expression in diabetic rats significantly attenuated after treatment with J. regia leaf extract. Meanwhile, S100B expression, as a marker of glial cell activation, which is upregulated in diabetic retina attenuated after treatment with J. regia leaf extract. Hosseinzadeh et al. documented that the aqueous and ethanolic extracts of J. regia leaves have anti-inflammatory effect against xylene-induced ear swelling in mice, which is mediated by a membrane-stabilizing effect that reduces capillary permeability and/or release of inflammatory mediators (30). In the other study, it was shown that J. regia leaf extract exhibited anti-inflammatory activity against carrageenan-induced hind paw edema model in mice. However, the mechanism underlying this phenomenon is not clear (12).
Apoptosis is a key mechanism of degenerative diseases, which is triggered by some factors such as hyperglycemia toxicity. In vivo studies revealed that hyperglycemia affected the cell survival and induced apoptotic changes within the inner layers of the retina including inner plexiform layer and ganglion cell layer secondarily to the caspase-dependent pathway (43, 44). These findings were confirmed after administration of a specific inhibitor of caspase-3 which was significantly reduced the intensity of apoptosis in the diabetic retina (44). Our immunohistochemical results showed that administration of STZ considerably increased the expression of caspase-3, which plays a critical role in apoptosis. Also, our results showed that these up- and down-regulations significantly attenuated after J. regia leaf extract consumption. Javidanpour et al. documented the proliferative effects of J. regia leaf extract on pancreatic ß-cells in STZ-induced diabetic rats (16). Results of another study demonstrated that walnut leaf extract has a hepatoprotective effect against carbon tetrachloride-induced cell death (38). On the contrary, some studies demonstrated that walnut leaf extract showed a higher antiproliferative efficiency than green husk and seed extracts against various cancer cell line such as human renal, oral, breast and colon cancer cell lines (13, 45), which is more likely related to its phenolic constituents. Also, it was found that walnut extracts suppressed proliferation and induced apoptosis of cancer cells in a dose- and time-dependent manner by modulating expression of apoptosis-related genes namely Bax, caspase-3, Bcl2, and tp53 (46, 47).
Conclusion
Results of this study suggest that JRL leaf extract exert protective effects against STZ-induced diabetic retinopathy in rats which might be associated with its anti-oxidative, anti-inflammatory, and anti-apoptotic properties, in addition to hypoglycemic effects.
Acknowledgment
This work was supported financially by Molecular and Cell Biology Research Center, Faculty of Medicine, Mazandaran University of Medical Sciences (grant number, 2266), Sari. Iran.
Conflict of Interest
The authors declare no conflict of interest.
References
- 1.Klein BE. Overview of epidemiologic studies of diabetic retinopathy. Ophthalmic Epidemiol. 2007;14:179–183. doi: 10.1080/09286580701396720. [DOI] [PubMed] [Google Scholar]
- 2.Joussen AM, Poulaki V, Le ML, Koizumi K, Esser C, Janicki H, et al. A central role for inflammation in the pathogenesis of diabetic retinopathy. FASEB J. 2004;18:1450–1452. doi: 10.1096/fj.03-1476fje. [DOI] [PubMed] [Google Scholar]
- 3.Guzman DC, Olguín HJ, García EH, Peraza AV, de la Cruz DZ, Soto MP. Mechanisms involved in the development of diabetic retinopathy induced by oxidative stress. Redox Rep. 2016:1–7. doi: 10.1080/13510002.2016.1205303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tarr JM, Kaul K, Chopra M, Kohner EM, Chibber R. Pathophysiology of diabetic retinopathy. ISRN Ophthalmol. 2013;2013:343560. doi: 10.1155/2013/343560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brownlee M. The pathobiology of diabetic complications:a unifying mechanism. Diabetes. 2005;54:1615–1625. doi: 10.2337/diabetes.54.6.1615. [DOI] [PubMed] [Google Scholar]
- 6.Lim TK. Edible medicinal and non-medicinal plants. Vol. 3. Dordrecht Heidelberg London New York: Springer; 2012. pp. 8661–8667. [Google Scholar]
- 7.Sorber DG, Kimball MH. Use of ethylene in harvesting the Persian walnut (Juglans Regia) in California. Issue 996 of Technical bulletin, U.S. Department of Agriculture. 1950 [Google Scholar]
- 8.Pereira JA, Oliveira I, Sousa A, Ferreira IC, Bento A, Estevinho L. Bioactive properties and chemical composition of six walnut (J regia) cultivars. Food Chem Toxicol. 2008;46:2103–2111. doi: 10.1016/j.fct.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 9.Pereira JA, Oliveira I, Sousa A, Valentão P, Andrade PB, Ferreira IC, et al. Walnut (J regia) leaves:phenolic compounds, antibacterial activity and antioxidant potential of different cultivars. Food Chem Toxicol. 2007;45:2287–2295. doi: 10.1016/j.fct.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 10.Zhao MH, Jiang ZT, Liu T, Li R. Flavonoids in Juglans regia L. leaves and evaluation of in vitro antioxidant activity via intracellular and chemical methods. Scientific World Journal. 2014;2014:303878. doi: 10.1155/2014/303878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Almeida IF, Fernandes E, Lima JL, Costa PC, Bahia MF. Walnut (Juglans regia) leaf extracts are strong scavengers of pro-oxidant reactive species. Food Chem. 2008;106:1014–1020. [Google Scholar]
- 12.Erdemoglu N, Küpeli E, Yeşilada E. Anti-inflammatory and antinociceptive activity assessment of plants used as remedy in Turkish folk medicine. J Ethnopharmacol. 2003;89:123–129. doi: 10.1016/s0378-8741(03)00282-4. [DOI] [PubMed] [Google Scholar]
- 13.Carvalho M, Ferreira PJ, Mendes VS, Silva R, Pereira JA, Jerónimo C, et al. Human cancer cell antiproliferative and antioxidant activities of Juglans regia L. Food Chem Toxicol. 2010;48:441–447. doi: 10.1016/j.fct.2009.10.043. [DOI] [PubMed] [Google Scholar]
- 14.Rather MA, Dar BA, Dar MY, Wani BA, Shah WA, Bhat BA, et al. Chemical composition, antioxidant and antibacterial activities of the leaf essential oil of Juglans regia L. and its constituents. Phytomedicine. 2012;19:1185–1190. doi: 10.1016/j.phymed.2012.07.018. [DOI] [PubMed] [Google Scholar]
- 15.Noumi E, Snoussi M, Hajlaoui H, Valentin E, Bakhrouf A. Antifungal properties of Salvadora persica and Juglans regia L. extracts against oral Candida strains. Eur J Clin Microbiol Infect Dis. 2010;29:81–88. doi: 10.1007/s10096-009-0824-3. [DOI] [PubMed] [Google Scholar]
- 16.Javidanpour S, Fatemi Tabtabaei SR, Siahpoosh A, Morovati H, Shahriari A. Comparison of the effects of fresh leaf and peel extracts of walnut (Juglans regia L.) on blood glucose and β-cells of streptozotocin-induced diabetic rats. Vet Res Forum. 2012;3:251–255. [PMC free article] [PubMed] [Google Scholar]
- 17.Jelodar G, Mohsen M, Shahram S. Effect of walnut leaf, coriander and pomegranate on blood glucose and histopathology of pancreas of alloxan induced diabetic rats. Afr J Tradit Complement Altern Med. 2007;4:299–305. doi: 10.4314/ajtcam.v4i3.31223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Asgary S, Parkhideh S, Solhpour A, Madani H, Mahzouni P, Rahimi P. Effect of ethanolic extract of Juglans regia L. on blood sugar in diabetes-induced rats. J Med Food. 2008;11:533–538. doi: 10.1089/jmf.2007.0611. [DOI] [PubMed] [Google Scholar]
- 19.Mohammadi J, Delaviz H, Malekzadeh JM, Roozbehi A. The effect of hydro alcoholic extract of Juglans regia leaves in streptozotocin-nicotinamide induced diabetic rats. Pak J Pharm Sci. 2012;25:407–411. [PubMed] [Google Scholar]
- 20.Hosseini S, Huseini HF, Larijani B, Mohammad K, Najmizadeh A, Nourijelyani K, et al. The hypoglycemic effect of Juglans regia leaves aqueous extract in diabetic patients:A first human trial. Daru. 2014;22:19. doi: 10.1186/2008-2231-22-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hosseini S, Jamshidi L, Mehrzadi S, Mohammad K, Najmizadeh AR, Alimoradi H, et al. Effects of Juglans regia L. leaf extract on hyperglycemia and lipid profiles in type two diabetic patients:a randomized double-blind, placebo-controlled clinical trial. J Ethnopharmacol. 2014;152:451–456. doi: 10.1016/j.jep.2014.01.012. [DOI] [PubMed] [Google Scholar]
- 22.Pitschmann A, Zehl M, Atanasov AG, Dirsch VM, Heiss E, Glasl S. Walnut leaf extract inhibits PTP1B and enhances glucose-uptake in vitro. J Ethnopharmacol. 2014;152:599–602. doi: 10.1016/j.jep.2014.02.017. [DOI] [PubMed] [Google Scholar]
- 23.Vandendool H, Kratz PD. A generalization of the retention index system including linear temperature programmed gas-liquid partition chromatography. J Chromatogr. 1963;11:463–471. doi: 10.1016/s0021-9673(01)80947-x. [DOI] [PubMed] [Google Scholar]
- 24.Gong CY, Lu B, Hu QW, Ji LL. Streptozotocin induced diabetic retinopathy in rat and the expression of vascular endothelial growth factor and its receptor. Int J Ophthalmol. 2013;6:573–577. doi: 10.3980/j.issn.2222-3959.2013.05.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tzeng TF, Liou SS, Tzeng YC, Liu IM. Zerumbone, a Phytochemical of Subtropical Ginger, Protects against Hyperglycemia-Induced Retinal Damage in Experimental Diabetic Rats. Nutrients. 2016;8:pii:E449. doi: 10.3390/nu8080449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mihara S, Uchiyama M. Determination of malonaldehyde precursor in tissues by thiobarbituric acid test. Anal Biochem. 1978;86:271–278. doi: 10.1016/0003-2697(78)90342-1. [DOI] [PubMed] [Google Scholar]
- 27.Aebi H. Catalase in vitro. Methods Enzymol. 1984;105:121–126. doi: 10.1016/s0076-6879(84)05016-3. [DOI] [PubMed] [Google Scholar]
- 28.Jiang Y, Walker RJ, Kern TS, Steinle JJ. Application of isoproterenol inhibits diabetic-like changes in the rat retina. Exp Eye Res. 2010;9:171–179. doi: 10.1016/j.exer.2010.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Amaral JS, Seabra RM, Andrade PB, Valentao P, Pereira JA, Ferreres F. Phenolic profile in the quality control of walnut (Juglans regia L.) leaves. Food Chem. 2004;88:373–379. [Google Scholar]
- 30.Hosseinzadeh H, Zarei H, Taghiabadi E. Antinociceptive, anti-inflammatory and acute toxicity effects of juglans regia L. Leaves in mice. Iran Red Crescent Med J. 2011;13:27–33. [PMC free article] [PubMed] [Google Scholar]
- 31.Kowluru RA, Chan PS. Oxidative stress and diabetic retinopathy. Exp Diabetes Res. 2007;2007:43603. doi: 10.1155/2007/43603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Anderson RE, Rapp LM, Wiegand RD. Lipid peroxidation and retinal degeneration. Curr Eye Res. 1984;3:223–227. doi: 10.3109/02713688408997203. [DOI] [PubMed] [Google Scholar]
- 33.Wu Y, Tang L, Chen B. Oxidative stress:implications for the development of diabetic retinopathy and antioxidant therapeutic perspectives. Oxid Med Cell Longev. 2014;2014:752387. doi: 10.1155/2014/752387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pacher P, Obrosova IG, Mabley JG, Szabó C. Role of nitrosative stress and peroxynitrite in the pathogenesis of diabetic complications. Emerging new therapeutical strategies. Curr Med Chem. 2005;12:267–275. doi: 10.2174/0929867053363207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Solar A, Colarič M, Usenik V, Stampar F. Seasonal variations of selected flavonoids, phenolic acids and quinones in annual shoots of common walnut (Juglans regia L.) Plant Science. 2006;170:453–461. [Google Scholar]
- 36.Cosmulescu S, Trandafir I, Nour V. Seasonal variation of the main individual phenolics and juglone in walnut (Juglans regia) leaves. Pharm Biol. 2014;52:575–580. doi: 10.3109/13880209.2013.853813. [DOI] [PubMed] [Google Scholar]
- 37.Gawlik-Dziki U, Durak A, Pecio Ł, Kowalska I. Nutraceutical potential of tinctures from fruits, green husks, and leaves of Juglans regia L. ScientificWorldJournal. 2014;2014:501392. doi: 10.1155/2014/501392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Eidi A, Moghadam JZ, Mortazavi P, Rezazadeh S, Olamafar S. Hepatoprotective effects of Juglans regia extract against CCl4-induced oxidative damage in rats. Pharm Biol. 2013;51:558–565. doi: 10.3109/13880209.2012.749920. [DOI] [PubMed] [Google Scholar]
- 39.Kowluru RA, Koppolu P, Chakrabarti S, Chen S. Diabetes-induced activation of nuclear transcriptional factor in the retina, and its inhibition by antioxidants. Free Radic Res. 2003;37:1169–1180. doi: 10.1080/10715760310001604189. [DOI] [PubMed] [Google Scholar]
- 40.Nagai N, Izumi-Nagai K, Oike Y, Koto T, Satofuka S, Ozawa Y, et al. Suppression of diabetes-induced retinal inflammation by blocking the angiotensin II type 1 receptor or its downstream nuclear factor-kappaB pathway. Invest Ophthalmol Vis Sci. 2007;48:4342–4350. doi: 10.1167/iovs.06-1473. [DOI] [PubMed] [Google Scholar]
- 41.Zheng L, Howell SJ, Hatala DA, Huang K, Kern TS. Salicylate-based anti-inflammatory drugs inhibit the early lesion of diabetic retinopathy. Diabetes. 2007;56:337–345. doi: 10.2337/db06-0789. [DOI] [PubMed] [Google Scholar]
- 42.Krady JK, Basu A, Allen CM, Xu Y, LaNoue KF, Gardner TW, et al. Minocycline reduces proinflammatory cytokine expression, microglial activation, and caspase-3 activation in a rodent model of diabetic retinopathy. Diabetes. 2005;54:1559–1565. doi: 10.2337/diabetes.54.5.1559. [DOI] [PubMed] [Google Scholar]
- 43.Borrie SC, Duggan J, Cordeiro MF. Retinal cell apoptosis. Expert Rev Ophthalmol. 2009;4:27–45. [Google Scholar]
- 44.Li YH, Zhuo YH, Lü L, Chen LY, Huang XH, Zhang JL, et al. Caspase-dependent retinal ganglion cell apoptosis in the rat model of acute diabetes. Chin Med J (Engl) 2008;121:2566–2571. [PubMed] [Google Scholar]
- 45.Salimi M, Majd A, Sepahdar Z, Azadmanesh K, Irian S, Ardestaniyan MH, et al. Cytotoxicity effects of various Juglans regia (walnut) leaf extracts in human cancer cell lines. Pharm Biol. 2012;50:1416–1422. doi: 10.3109/13880209.2012.682118. [DOI] [PubMed] [Google Scholar]
- 46.Alshatwi AA, Hasan TN, Shafi G, Syed NA, Al-Assaf AH, Alamri MS, et al. Validation of the Antiproliferative Effects of Organic Extracts from the Green Husk of Juglans regia L. on PC-3 Human Prostate Cancer Cells by Assessment of Apoptosis-Related Genes. Evid Based Complement Alternat Med. 2012;2012:103026. doi: 10.1155/2012/103026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hasan TN, B LG, Shafi G, Al-Hazzani AA, Alshatwi AA. Anti-proliferative effects of organic extracts from root bark of Juglans Regia L. (RBJR) on MDA-MB-231 human breast cancer cells:role of Bcl-2/Bax, caspases and Tp53. Asian Pac J Cancer Prev. 2011;12:525–530. [PubMed] [Google Scholar]


