Abstract
Objective
To elucidate the factors associated with high left ventricular mass index (LVMI) and to test the hypothesis that high LVMI is associated with worse outcome in severe aortic stenosis (AS).
Methods
We analysed 3282 patients with LVMI data in a retrospective multicentre registry enrolling consecutive patients with severe AS in Japan. The management strategy, conservative or initial aortic valve replacement (AVR), was decided by the attending physician. High LVMI was defined as LVMI >115 g/m2 for males and >95 g/m2 for females. We compared the risk between normal and high LVMI in the primary outcome measures compromising aortic valve-related death and heart failure hospitalisation.
Results
Age was mean 77 (SD 9.6) years and peak aortic jet velocity (Vmax) was 4.1 (0.9) m/s. The factors associated with high LVMI (n=2374) included female, body mass index ≥22, absence of dyslipidemia, left ventricular ejection fraction <50%, Vmax ≥4 m/s, regurgitant valvular disease, hypertension, anaemia and end-stage renal disease. In the conservative management cohort (normal LVMI: n=691, high LVMI: n=1480), the excess adjusted 5-year risk of high LVMI was significant (HR: 1.53, 95% CI 1.26 to 1.85, p<0.001). In the initial AVR cohort (normal LVMI: n=217, high LVMI: n=894), the risk did not differ significantly between the two groups (HR: 0.96, 95% CI 0.60 to 1.55, p=0.88). There was a significant interaction between the initial treatment strategy and the risk of high LVMI (p=0.016).
Conclusions
The deleterious impact of high LVMI on outcome was observed in patients managed conservatively, but not observed in patients managed with initial AVR.
Trial registration number
Keywords: aortic stenosis
Introduction
Left ventricular hypertrophy (LVH) is a common finding in patients with cardiovascular disease (CVD) and having CVD risk factors.1 LVH is a physiological adaptation that is an attempt to normalise increased wall stress and to maintain cardiac output in hypertensive patients. However, this cascade of compensatory responses alters the myocardium, causing changes in ventricular mass as well as in myocardial cellular structure that lead to development of fibrosis.2 The presence of high left ventricular (LV) mass or a high left ventricular mass index (LVMI) is reportedly an independent predictor of increased cardiovascular morbidity and mortality both in the general populations and in hypertensive populations.3 Adverse consequences of LVH, such as myocardial ischaemia, diastolic dysfunction and impairment of systolic function, are related to these adverse cardiovascular outcomes. In patients with aortic stenosis (AS), the development of LVH is also considered as an adaptive response that maintains LV wall stress close to normal against the increased afterload due to the stenosis of the aortic valve. As AS gradually progresses, this afterload increases gradually. However, the maladaptive rather than beneficial effects of LVH are similarly reported in patients with AS.4–8 Surgical aortic valve replacement (AVR) or transcatheter aortic valve implantation (TAVI) in patients with severe AS results in regression of LV mass and a corresponding decrease in cardiovascular event risk.9 10 However, there are no previous large studies focusing on the prognostic impact of the LVMI in patients with severe AS stratified by the initial treatment strategies managed either conservatively or surgically with AVR. Therefore, it is hypothesised that a high LVMI is related to increased cardiovascular mortality in severe AS, and its prognostic impact of high LVMI differs between the treatment strategies. We sought to investigate the factors associated with a high LVMI in order to understand the pathophysiology of maladaptive responses to AS and the prognostic impact of high LVMI according to the initial treatment strategies in a large Japanese observational database of consecutive patients with severe AS.
Methods
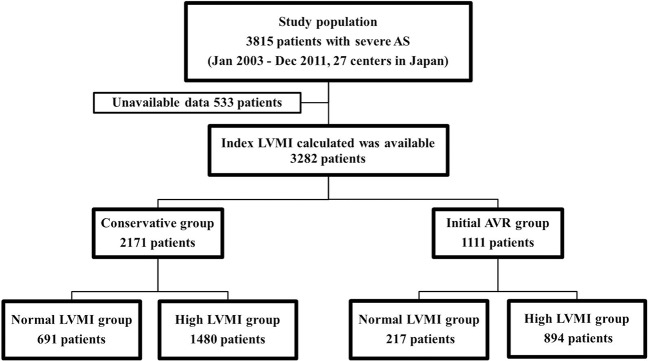
The CURRENT AS registry (Contemporary outcomes after sURgery and medical tREatmeNT in patients with severe Aortic Stenosis registry) is a retrospective multicentre registry that enrolled consecutive patients with severe AS from 27 centres (the on-site surgical facilities in 20 centres) in Japan between January 2003 and December 2011 (list of investigators in supplementary materials). We searched the hospital database for transthoracic echocardiography and enrolled consecutive patients who had met the definition of severe AS (peak aortic jet velocity (Vmax) >4.0 m/s, mean aortic pressure gradient (PG) >40 mm Hg or aortic valve area (AVA) <1.0 cm2) for the first time during the study period.11 We excluded patients with a history of percutaneous balloon valvuloplasty or surgical aortic valve repair/replacement/plasty. The study design and patient enrolment in the registry were previously described in detail.12 Among the 3815 patients enrolled in the registry, the final study population consisted of 3282 patients whose LVMI had been calculated at the time of the initial echocardiography after excluding 533 patients due to the missing data for calculation of their LVMI (online supplementary tables 1 and 2). In the present analysis, we investigated the factors associated with high LVMI in the entire study population and compared the long-term clinical outcomes between the high LVMI and normal LVMI groups. The latter analyses were stratified according to the initial treatment strategies: a conservative management cohort of 2171 patients and an initial AVR cohort of 1111 patients (figure 1). The decision of the initial treatment strategy was based on the physicians’ discretion. Patients initially not planned to be treated with AVR/TAVI were assigned in the conservative cohort, and patients planned to be treated with AVR/TAVI were assigned in the initial AVR cohort. The protocol was approved by the institutional review board of each participating centre. Given the retrospective nature of the study, written informed consent was waived, and all of the patients agreed to participate in the study when contacted for follow-up. The patient record/information was anonymised prior to analysis.
Figure 1.
Study patient flow. Normal LVMI was defined as LVMI ≤115 g/m2 for male patients and ≤95 g/m2 for female patients. AVR, aortic valve replacement; AS, aortic stenosis; LVMI, left ventricular mass index.
LV mass was calculated with the formula recommended by the American Society of Echocardiography (ASE) and was indexed to the body surface area as follows: LV mass=0.8×1.04 [(LVDd + LVPWTd + IVSTd)3− (LVDd)3]+0.6, where LVDd was the LV diastolic diameter, IVSTd was the diastolic interventricular septal wall thickness and LVPWTd was the diastolic LV posterior wall thickness.13 In line with the ASE recommendations, high LVMI was defined as LVMI >115 g/m2 for male patients and >95 g/m2 for female patients. As an additional sensitivity analysis, we categorised patients into four groups: no LVH, mild LVH, moderate LVH and severe LVH according to the ASE guidelines.13 The values for these groups were provided in supplementary methods. The data from the two-dimensional transthoracic echocardiography were analysed at baseline. The LV ejection fraction (LVEF) was measured using the Teichholz method or the modified Simpson’s rule method.
The primary outcome measure for the present analysis was a composite of the aortic valve-related death and hospitalisation for heart failure during the 5-year follow-up period. The secondary outcome measures included all-cause mortality as well as the individual components of the composite primary outcome measure. Aortic valve-related death included aortic procedure-related death, sudden death, death caused by heart failure potentially related to the aortic valve and death due to aortic valve endocarditis. Heart failure hospitalisation was defined as hospitalisation for worsening heart failure requiring intravenous drug therapy. The cause of death was classified according to the Valve Academic Research Consortium definitions and was adjudicated by a clinical event committee.14
Statistical analysis
The categorical variables were presented as numbers and percentages and were compared using a χ2 test or Fisher’s exact test. The continuous variables were expressed as mean (SD) or median (IQR). Based on their distributions, the continuous variables were compared using the Student’s t-test or the Wilcoxon rank sum test between two groups, and the one-way analysis of variance or the Kruskal-Wallis test between four groups. To analyse the factors associated with high LVMI, we used a multivariable logistic regression model involving the following potential independent clinically relevant variables: age, sex, body mass index (BMI), echocardiographic parameters, presence of other valve diseases, aetiology of AS, medical history and comorbidities, as shown in table 1. The adjusted ORs and 95% CIs were calculated.
Table 1.
Baseline clinical and echocardiographic characteristics in the conservative management and initial AVR cohorts
| Variable | All patients | Conservative management cohort | Initial AVR cohort | ||||
| Normal LVMI (n=691) | High LVMI (n=1480) | p Value | Normal LVMI (n=217) | High LVMI (n=894) | p Value | ||
| Clinical characteristics | |||||||
| Age, years*† | 77.0 (9.6) | 77.8 (9.6) | 79.5 (9.2) | <0.001 | 71.6 (9.4) | 73.5 (8.8) | 0.0051 |
| Age ≥80 years | 1378 (42) | 321 (46) | 787 (53) | 0.0035 | 40 (18) | 230 (26) | 0.025 |
| Male* | 1275 (39) | 345 (50) | 457 (31) | <0.001 | 121 (56) | 352 (39) | <0.001 |
| BMI <22*‡ | 1822 (56) | 398 (58) | 878 (59) | 0.45 | 109 (50) | 437 (49) | 0.72 |
| BSA, m2 | 1.46 (0.18) | 1.47 (0.18) | 1.42 (0.18) | <0.001 | 1.54 (0.18) | 1.49 (0.18) | <0.001 |
| Hypertension* | 2326 (71) | 492 (71) | 1089 (74) | 0.25 | 133 (61) | 612 (68) | 0.044 |
| Current smoking* | 180 (5) | 31 (4) | 72 (5) | 0.70 | 15 (7) | 62 (7) | 0.99 |
| Diabetes mellitus | 806 (25) | 187 (27) | 365 (25) | 0.23 | 58 (27) | 196 (22) | 0.13 |
| On insulin therapy* | 177 (5) | 41 (6) | 82 (6) | 0.71 | 12 (6) | 42 (5) | 0.61 |
| Coronary artery disease* | 1036 (32) | 231 (33) | 441 (30) | 0.088 | 78 (36) | 286 (32) | 0.27 |
| Prior symptomatic stroke* | 428 (13) | 119 (17) | 209 (14) | 0.060 | 22 (10) | 78 (9) | 0.51 |
| Atrial fibrillation or flutter*† | 698 (21) | 157 (23) | 351 (24) | 0.61 | 43 (20) | 147 (16) | 0.24 |
| Serum creatinine, mg/dL* | 0.9 (0.56) | 0.89 (0.7–1.22) |
0.91 (0.7–1.49) | <0.001 | 0.79 (0.65–0.97) | 0.83 (0.69–1.16) | 0.022 |
| ESRD*§ | 486 (15) | 77 (11) | 270 (18) | <0.001 | 16 (7) | 123 (14) | 0.011 |
| Anaemia*¶ | 1784 (54) | 333 (48) | 873 (59) | <0.001 | 93 (43) | 485 (54) | 0.0026 |
| Liver cirrhosis (Child-Pugh B or C)* | 34 (1) | 6 (1) | 22 (1) | 0.23 | 0 (0) | 6 (0.7) | 0.60 |
| Malignancy currently under treatment*† | 133 (4) | 53 (8) | 57 (4) | <0.001 | 2 (1) | 21 (2) | 0.29 |
| Chronic lung disease (moderate or severe)* | 97 (3) | 21 (3) | 57 (4) | 0.34 | 2 (1) | 17 (2) | 0.56 |
| STS score (PROM), % | 3.8 (4.5) | 3.8 (2.3–6.2) |
4.8 (2.8–8.4) |
<0.001 | 2.2 (1.5–3.7) |
2.9 (1.8–5.0) |
<0.001 |
| Symptoms at index echocardiography | 1707 (52) | 181 (26) | 690 (47) | <0.001 | 147 (68) | 689 (77) | 0.0039 |
| Chest pain | 439 (13) | 46 (7) | 120 (8) | 0.23 | 60 (28) | 213 (24) | 0.25 |
| Syncope | 173 (5) | 18 (3) | 51 (3) | 0.29 | 26 (12) | 78 (9) | 0.15 |
| Chronic exertional dyspnoea | 1353 (41) | 147 (21) | 601 (41) | <0.001 | 84 (39) | 521(58) | <0.001 |
| Admission for heart failure at index echocardiography*† | 621 (19) | 66 (10) | 318 (21) | <0.001 | 21 (10) | 216 (24) | <0.001 |
| Echocardiographic variables | |||||||
| Vmax, m/s | 4.1 (0.9) | 3.6 (0.7) | 4.0 (0.9) | <0.001 | 4.4 (0.7) | 4.8 (0.8) | <0.001 |
| Vmax >4 m/s*† | 1863 (57) | 204 (30) | 740 (50) | <0.001 | 165 (76) | 754 (84) | 0.0037 |
| Peak aortic PG, mm Hg | 72 (32) | 53 (20) | 66 (29) | <0.001 | 80 (25) | 94 (33) | <0.001 |
| Mean aortic PG, mm Hg | 41 (20) | 30 (12) | 38 (18) | <0.001 | 47 (16) | 56 (20) | <0.001 |
| AVA (equation of continuity), cm2 | 0.72 (0.18) | 0.80 (0.14) | 0.74 (0.19) | <0.001 | 0.69 (0.16) | 0.64 (0.18) | <0.001 |
| Low gradient AS** | 1215 (37) | 426 (62) | 645(44) | <0.001 | 41 (19) | 103 (12) | 0.0029 |
| Low gradient AS with preserved LVEF†† | 966 (29) | 377 (55) | 491 (33) | <0.001 | 37 (17) | 61 (7) | <0.001 |
| AVA index, cm2/m2 | 0.50 (0.13) | 0.55 (0.11) | 0.52 (0.13) | <0.001 | 0.46 (0.11) | 0.43 (0.12) | 0.011 |
| LV end-diastolic diameter, mm | 46.1 (7.0) | 42.3 (5.5) | 47.1 (6.7) | <0.001 | 42.5 (5.1) | 48.4 (7.2) | <0.001 |
| LV end-systolic diameter, mm | 30.3 (7.9) | 27.2 (5.8) | 31.2 (7.9) | <0.001 | 26.1 (5.5) | 32.3 (8.7) | <0.001 |
| LVEF, % | 63 (13) | 65 (11) | 62 (14) | <0.001 | 69 (9) | 62 (14) | <0.001 |
| LVEF <68%*† | 1928 (59) | 393 (57) | 919 (62) | 0.021 | 92 (42) | 524 (59) | <0.001 |
| LVMI, g/m2 | 123 (101–151) | 89 (79–99) | 133 (117–157) | <0.001 | 91 (82–100) | 145 (124–206) | <0.001 |
| Any combined valvular disease (moderate or severe)*† | 1310 (40) | 199 (29) | 662 (45) | <0.001 | 60 (28) | 389 (44) | <0.001 |
| TR pressure gradient ≥40 mm Hg* | 516 (16) | 79 (11) | 269 (18) | <0.001 | 16 (7) | 152 (17) | <0.001 |
p Values were calculated from a χ2 test or Fisher’s exact test for categorical variables, and Student’s t-test or Wilcoxon rank sum test for continuous variables.
Values are number (%), mean (SD) or median (IQR).
*Potential risk-adjusting variables selected for Cox proportional hazard models.
†Potential risk-adjusting variables selected for parsimonious Cox proportional hazard models.
‡BMI was calculated as weight in kilograms divided by height in metres squared.
§ESRD was defined as creatinine level >2 mg/dL and/or haemodialysis.
¶Anaemia was defined by the WHO criteria (haemoglobin <12.0 g/dL in women and <13.0 g/dL in men).
**Low gradient AS=AVA<1 cm2, mean PG ≤40 mm Hg and Vmax ≤4 m/s.
††Low gradient AS with preserved LVEF=AVA <1 cm2, mean PG ≤40 mm Hg, Vmax ≤4 m/s and LVEF ≥50%.
AS, aortic stenosis; AVA, aortic valve area; AVR, aortic valve replacement; BMI, body mass index; BSA, body surface area; ESRD, end-stage renal disease; LV, left ventricular; LVEF, left ventricular ejection fraction; LVMI, left ventricular mass index; PG, pressure gradient; PROM, predicted risk of mortality; STS, Society of Thoracic Surgeons; TR, tricuspid regurgitation; Vmax, peak aortic jet velocity.
Moreover, we compared the 5-year clinical outcomes between the high LVMI and normal LVMI groups in the conservative management and initial AVR cohorts according to the intention-to-treat principle, regardless of the actual performance of AVR. Subgroup analyses for the primary outcome measure were also performed based on Vmax, LVEF and acute heart failure presentation at baseline. Acute heart failure presentation was defined as hospitalisation for heart failure. The cumulative incidences of clinical events were estimated using the Kaplan-Meier method, and the intergroup differences were assessed with a log-rank test. Multivariable Cox proportional hazards models were used to estimate the risk of high LVMI relative to normal LVMI for the primary and secondary outcomes. The results were expressed as HRs and its 95% CIs. We selected the 22 clinically relevant risk-adjusting variables presented in table 1 for the primary outcome measure and all-cause mortality in the main analysis, with the centres incorporated as the stratification variable. This was consistent with our previous report,12 except for the addition of acute heart failure presentation as a risk-adjusting variable. Given the small number of patients with an event, we used the seven clinically relevant risk-adjusting variables presented in table 1 for aortic valve death, heart failure hospitalisation and for the additional analysis of four LVH classifications. Proportional hazard assumptions for the normal LVMI and high LVMI groups were assessed on the plots of log (time) versus log [−log (survival)] stratified by the variable, and verified to be acceptable, as well as other risk-adjusting variables previously verified.12 We also evaluated the interaction between the initial treatment strategies and the effect of high LVMI relative to normal LVMI for the clinical outcomes. In the subgroup analysis, we used 21 risk-adjusting variables in the conservative management cohort, and six risk-adjusting variables in the initial AVR cohort, without adjustment for multiple tests. We also evaluated the interactions between the subgroup factors and the effect of high LVMI relative to normal LVMI for the clinical outcomes.
All the statistical analyses were conducted by physicians (EM, TT and TK) and a statistician (TM) using JMP V.10.0.2 or SAS V.9.4. All the reported p values were two tailed, and p values <0.05 were considered statistically significant.
Results
Factors associated with high LVMI
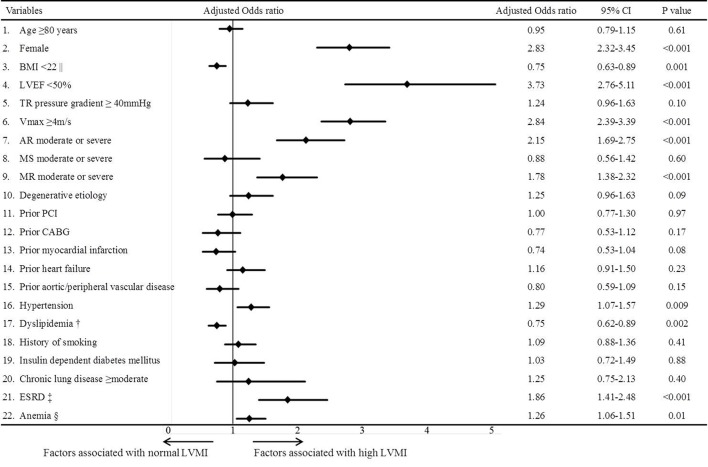
A total of 2374 patients (1480 patients in the conservative management cohort and 894 patients in the initial AVR cohort) had high LVMI, and 908 patients (691 patients in the conservative management cohort and 217 patients in the initial AVR cohort) had normal LVMI (figure 1). The baseline characteristics of the entire study population are presented in online supplementary table 3. According to the multivariable logistic regression analysis, the female sex, LVEF <50%, Vmax ≥4 m/s, aortic regurgitation, mitral regurgitation, hypertension, anaemia and end-stage renal disease (serum creatinine >2 mg/dL or haemodialysis) were independent factors associated with high LVMI, while dyslipidemia and a BMI <22 were independent negative factors associated with high LVMI (figure 2).
Figure 2.
Multivariable logistic regression analysis for the factors associated with high LVMI. LVMI=left ventricular mass index. || BMI was calculated as weight in kilograms divided by height in metres squared. † Dyslipidemia was defined as total cholesterol levels ≥240 mg/dL, high-density lipoprotein cholesterol levels <40 g/dL or the use of statin. ‡ ESRD was defined as creatinine level >2 mg/dL and/or haemodialysis. § Anaemia was defined by the WHO criteria (haemoglobin <12.0 g/dL in women and <13.0 g/dL in men). AR, aortic regurgitation; BMI, body mass index; CABG, coronary artery bypass grafting; ESRD, end-stage renal disease; LVEF, left ventricular ejection fraction; LVMI, left ventricular mass index; MR, mitral regurgitation; MS, mitral stenosis; PCI, percutaneous coronary intervention; TR, tricuspid regurgitation; Vmax, peak aortic jet velocity.
heartjnl-2016-311022supp001.pdf (29.7KB, pdf)
Baseline clinical and echocardiographic characteristics: normal versus high LVMI groups
The differences in the baseline clinical and echocardiographic characteristics of the high and normal LVMI groups were generally consistent in both the conservative management and initial AVR cohorts (table 1 and online supplementary table 4). The patients in the high LVMI group were older than those in the normal LVMI group, were more often female and were more likely to have a smaller body surface area, hypertension, end-stage renal disease, anaemia, higher surgical risk scores, symptoms, greater severity of AS, larger LV dimensions, lower LVEF, greater LV wall thickness, combined valvular disease and pulmonary hypertension (table 1 and online supplementary table 4).
Clinical outcomes: normal versus high LVMI groups
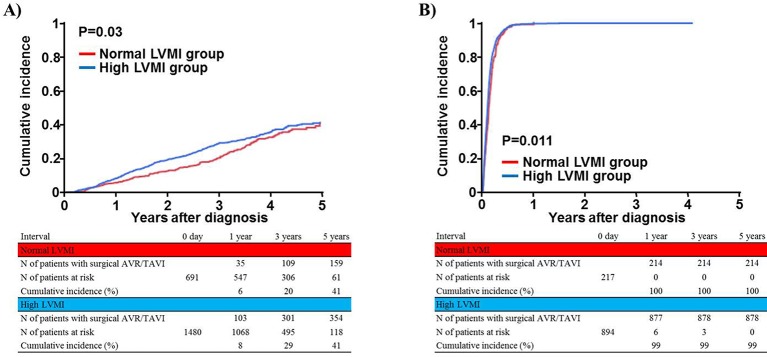
The median follow-up duration after the index echocardiography was 1204 (IQR: 824) days, with a 94% follow-up rate at 2 years. The cumulative 5-year incidence of surgical AVR or TAVI was significantly higher in the high LVMI group than in the normal LVMI group in both the conservative management and initial AVR cohorts (figure 3A and B).
Figure 3.
Cumulative incidence of surgical AVR or TAVI during follow-up: normal versus high LVMI groups. (A) Conservative management cohort and (B) initial AVR cohort. AVR, aortic valve replacement; LVMI, left ventricular mass index; TAVI, transcatheter aortic valve implantation.
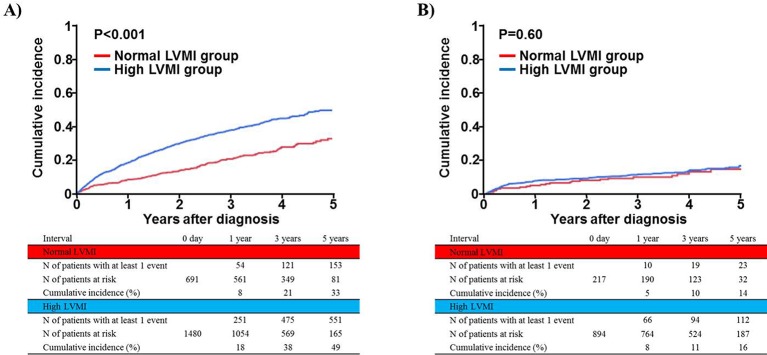
In the conservative management cohort, the cumulative 5-year incidences of the primary and secondary outcome measures were significantly higher in the high LVMI group than in the normal LVMI group (table 2 and figure 4A). After adjusting for confounders, the excess risk of high LVMI relative to normal LVMI for the primary outcome measure was remained significant in the conservative management cohort. The adjusted risk for all-cause death did not differ significantly between high and normal LMI groups (table 2).
Table 2.
Clinical outcomes of patients in the conservative management and initial AVR cohorts
| Normal LVMI N of patients with event/N of patients at risk (cumulative 5-year incidence (%)) |
High LVMI N of patients with event/N of patients at risk (cumulative 5-year incidence (%)) |
Unadjusted | Adjusted | |||||
| HR (95% CI) |
p Value | HR (95% CI) |
p Value | p Value for interaction | ||||
| A composite of aortic valve-related death or hospitalisation due to heart failure | Conservative management cohort | 164/691 (33) | 582/1480 (49) | 1.93 (1.63 to 2.31) |
<0.001 | 1.53 (1.26 to 1.85) |
<0.001 | 0.016 |
| Initial AVR cohort | 26/217 (14) | 123/894 (16) | 1.12 (0.75 to 1.75) |
0.59 | 0.96 (0.60 to 1.55) |
0.88 | ||
| All-cause death | Conservative management cohort | 257/691 (42) | 684/1480 (52) | 1.36 (1.18 to 1.57) |
<0.001 | 1.11 (0.94 to 1.30) |
0.23 | 0.39 |
| Initial AVR cohort | 34/217 (19) | 176/894 (23) | 1.19 (0.84 to 1.75) |
0.33 | 0.92 (0.61 to 1.39) |
0.69 | ||
| Aortic valve-related death | Conservative management cohort | 86/691 (17) | 335/1480 (31) | 1.98 (1.57 to 2.53) |
<0.001 | 1.52 (1.18 to 1.96) |
0.001 | 0.42 |
| Initial AVR cohort | 8/217 (5) | 51/894 (6) | 1.53 (0.77 to 3.48) |
0.24 | 1.42 (0.65 to 3.08) |
0.38 | ||
| Heart failure hospitalisation | Conservative management cohort | 130/691 (28) | 471/1480 (43) | 1.98 (1.64 to 2.42) |
<0.001 | 1.64 (1.33 to 2.02) |
<0.001 | 0.0034 |
| Initial AVR cohort | 18/217 (10) | 76/894 (12) | 0.99 (0.61 to 1.71) |
0.98 | 0.89 (0.50 to 1.57) |
0.68 | ||
Other abbreviations are same as in table 1.
AVR, aortic valve replacement; LVMI, left ventricular mass index.
Figure 4.
Cumulative incidence of the primary outcome measure (a composite of aortic valve-related death or hospitalisation due to heart failure): normal versus high LVMI groups. (A) Conservative management cohort and (B) initial AVR cohort. AVR, aortic valve replacement; LVMI, left ventricular mass index.
In the initial AVR cohort, the cumulative 5-year incidence of the primary outcome did not differ significantly between the high and normal LVMI groups (table 2 and figure 4B). After adjusting for confounders, the risks for the primary and secondary outcomes did not differ significantly between the high and normal LVMI groups in the initial AVR cohort (table 2). There was a significant interaction between the initial treatment strategies and the effect of high LVMI relative to normal LVMI for the primary outcome measure (table 2).
When the severity of LVH was classified into four groups (online supplementary table 5), the increasing LVMI was associated with incrementally higher risk for primary outcome measures (online supplementary figure 1 and online supplementary table 6). There was a significant interaction between initial treatment strategies and the effect of LVH severity on the primary outcome measure (interaction p=0.034 and online supplementary table 6).
Subgroup analysis in the conservative management and initial AVR cohorts: normal versus high LVMI groups
In the subgroup analyses stratified by Vmax and LVEF, there were no significant interactions between the subgroup factors and the effect of high LVMI for the primary outcome measure in both the conservative management and initial AVR cohorts (online supplementary table 7). There was a significant interaction between acute heart failure presentation and the effect of high LVMI for the primary outcome measure in the conservative management cohort (p<0.001, online supplementary table 7).
Discussion
The main finding of this study are the following: (1) female sex, BMI ≥22, low LVEF, severity of AS, valvular regurgitation, hypertension, anaemia, absence of dyslipidemia and renal dysfunction were independently associated with high LVMI in patients with severe AS. (2) The deleterious impact of LVMI on the outcomes found in patients with conservative treatment had in contrast no effect on the outcomes in patients who were managed with AVR or TAVI.
The underlying mechanisms of development of LVH are multifactorial in hypertensive patients, although the traditional theory was hypertrophy for adaptation.15
Several studies have shown that the degree of LVH is poorly related the severity of flow obstruction in AS.5 The previously reported factors influencing the LV response in addition to the pressure overload include age,16 sex,17 and obesity18 in patients with AS and diabetes,19 and kidney disease20 in patients who underwent surgical AVR or TAVI. Other factors associated with high LVMI remain unknown in patients with severe AS. Among the factors associated with increased LVMI in the present study, hypertension, concomitant valvular regurgitation, greater peak aortic valve gradient, anaemia and kidney dysfunction are the chronic factors that increase the afterload and cardiac workload through hemodynamics. Female sex is one factor associated with the increased LVMI. Previous studies have reported that there was no significant difference in the rate of progression of AS between male and female patients.21 Therefore, the noted difference in LVMI is more likely to be influenced by the differences in the ventricular response. Women are usually less prone to myocardial hypertrophy, mainly because of the protective effects of oestrogen on cardiomyocytes and fibroblasts.22 However, female sex is an increased risk of myocardial hypertrophy caused by hypertensive stress after menopause.23 This study showed that LVEF <50% was associated with high LVMI. A recent study has suggested that increased LV mass is a predictor of LV dysfunction, although a causal relationship between low LVEF and high LVMI was not determined in the present study.5 The present study demonstrated that dyslipidemia was a negative factor associated with high LVMI. Dyslipidemia was defined based on total cholesterol levels ≥240 mg/dL, high-density lipoprotein cholesterol levels <40 g/dL or the use of statin in CURRENT AS registry. Statins have been demonstrated to decrease LVMI in hypertensive patients.24 It is proposed that its mechanism is related to the cholesterol-independent beneficial effects induced by anti-inflammatory mechanisms.25 In this study, 74% of the patients (903/1226) received statin therapy (online supplementary table 4).
High LVMI adversely affected the outcomes of patients with severe AS, when managed conservatively. This finding is consistent with that of previous studies. High LVMI is independently associated with increased cardiovascular mortality in 1656 asymptomatic patients with mild to moderate AS4 and in 201 asymptomatic patients with severe AS6 under conservative management. We analysed 3282 consecutive patients with severe AS stratified by the initial treatment strategies, demonstrating that high LVMI as compared with normal LVMI was associated with higher risk for long-term AS-related clinical outcomes in patients managed conservatively, but not in patients managed with the initial AVR strategy.
Risk prediction in severe AS before the development of congestive heart failure remains a challenge. Echocardiographic markers for developing symptoms or death are aortic valve calcification, a rapid increase in the PG, very high aortic valve velocities,26 LVEF <50%10 and other echocardiographic parameters8 as well as LV hypertrophy.4 6 7 In the present study, high LVMI, regardless of the severity of Vmax, was associated with an excess risk for the long-term AS-related outcomes and would be an additional risk predictor beyond Vmax in patients with severe AS managed conservatively.
In contrast, high LVMI did not have any adverse impact on long-term AS-related outcomes in patients managed surgically. There is no previous study about the relationship between preoperative LVMI and postoperative outcomes. However, surgical AVR and TAVI were reported to reduce postoperative LVMI during follow-up.9 10 This effect is called reverse remodelling of LVH after AVR. Early reverse remodelling is thought to be due to the regression of myocyte hypertrophy after the decrease of afterload,4 and its late regression is a consequence of remodelling of the interstitial fibrosis, which may develop over years.27 In addition, the sooner the regression of LVMI was observed, the better outcome was observed.9 One of the reasons for having no effect of high LVMI on the prognosis after AVR might be related with a line of evidence suggesting that the higher preoperative LVMI is a correlate of maximum LV mass regression after AVR.10 The no effect of high LVMI was in contrast to the adverse impact of LV dysfunction on postoperative prognosis, although it remains unclear what matters most among the factors such as low LVEF,10 low gradient or/and low flow28 in patients with severe AS managed with surgical AVR or TAVI.
Limitations
This study has several limitations. First, this retrospective study was performed without randomisation of patient selection. Therefore, the decision on the initial treatment strategy was based on physician discretion. Second, the echocardiographic measurement was not performed in a core laboratory but in each participating centre. Therefore, we could not deny the possibility for variations in the echocardiographic measurement of LVMI or LVEF. Third, information about the cardiac output and stress echocardiography was not collected in this study. In clinical practice, a variable proportion of patients with severe AS on the basis of AVA <1.0 cm2 alone by echocardiography have less severe Vmax and/or mean PG (low gradient severe AS).29 A large proportion of patients with preserved EF were included in the normal LVMI group, and they might have small ventricular cavities, increased afterload and subtle systolic dysfunction with cardiac fibrosis.30 The information about cardiac output would help for the understanding of the pathophysiology. Forth, we did not analyse the changes in LVMI and LVEF during follow-up, and therefore, we could not assess the relation of LV remodelling and reverse LV remodelling with the long-term outcomes. Fifth, there remain unmeasured confounders affecting the long-term prognosis, although we conducted extensive statistical adjustment for the measured confounders. Sthix, several subgroup analyses have a risk for multiple comparison as well as small sample size with low statistical power. Finally, missing data included the aged patients with fewer comorbidities and higher EuroSCOREs (European System for Cardiac Operative Risk Evalution).
Conclusions
In patients with severe AS managed conservatively, high LVMI as compared with normal LVMI was associated with higher long-term risk for AS-related clinical events, while high LVMI relative to normal LVMI did not have effect in patients managed with initial AVR.
Key messages.
What is already known on this subject?
Several factors, including age, sex, obesity, diabetes and kidney disease influence the left ventricular (LV) hypertrophy in patients with aortic stenosis (AS). A high left ventricular mass index (LVMI) is associated with increased mortality in asymptomatic patients with mild to moderate AS who were managed conservatively.
What might this study add?
Female sex, low ejection fraction, severity of AS, valvular regurgitation, hypertension, anaemia, absence of dyslipidemia and renal dysfunction were independently associated with high LVMI in patients with severe AS. The deleterious impact of high LVMI on the outcomes found in patients with conservative treatment had in contrast no effect on the outcomes in patients who were managed surgically.
How might this impact on clinical practice?
Considering the different effects of high LVMI on outcomes between treatment strategies, ventricular response is important for the risk stratification and the timing of surgical or transcatheter intervention in patients with severe AS.
heartjnl-2016-311022supp002.pdf (528.8KB, pdf)
Footnotes
Contributors: TakaK had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: EM-M, TakaK, TM, TT and TakeK. Acquisition, analysis or interpretation of data: all authors. Drafting of the manuscript: EM-M, TakaK, TM and TK. Critical revision of the manuscript for important intellectual content: all authors. Statistical analysis: EM-M, TakaK, TM and TT. Administrative, technical or material support: TK. Study supervision: TM and TK.
Competing interests: None declared.
Ethics approval: The institutional review board of Kyoto University and each participating centre.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1. Koren MJ, Devereux RB, Casale PN, et al. Relation of left ventricular mass and geometry to morbidity and mortality in uncomplicated essential hypertension. Ann Intern Med 1991;114:345–52. 10.7326/0003-4819-114-5-345 [DOI] [PubMed] [Google Scholar]
- 2. Lorell BH, Carabello BA. Left ventricular hypertrophy: pathogenesis, detection, and prognosis. Circulation 2000;102:470–9. 10.1161/01.CIR.102.4.470 [DOI] [PubMed] [Google Scholar]
- 3. Verdecchia P, Porcellati C, Reboldi G, et al. Left ventricular hypertrophy as an independent predictor of acute cerebrovascular events in essential hypertension. Circulation 2001;104:2039–44. 10.1161/hc4201.097944 [DOI] [PubMed] [Google Scholar]
- 4. Gerdts E, Rossebø AB, Pedersen TR, et al. Relation of left ventricular mass to Prognosis in initially asymptomatic mild to moderate aortic valve Stenosis. Circ Cardiovasc Imaging 2015;8:e003644 10.1161/CIRCIMAGING.115.003644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kupari M, Turto H, Lommi J. Left ventricular hypertrophy in aortic valve Stenosis: preventive or promotive of systolic dysfunction and heart failure? Eur Heart J 2005;26:1790–6. 10.1093/eurheartj/ehi290 [DOI] [PubMed] [Google Scholar]
- 6. Cioffi G, Faggiano P, Vizzardi E, et al. Prognostic effect of inappropriately high left ventricular mass in asymptomatic severe aortic Stenosis. Heart 2011;97:301–7. 10.1136/hrt.2010.192997 [DOI] [PubMed] [Google Scholar]
- 7. Pellikka PA, Sarano ME, Nishimura RA, et al. Outcome of 622 adults with asymptomatic, hemodynamically significant aortic Stenosis during prolonged follow-up. Circulation 2005;111:3290–5. 10.1161/CIRCULATIONAHA.104.495903 [DOI] [PubMed] [Google Scholar]
- 8. Hachicha Z, Dumesnil JG, Pibarot P. Usefulness of the valvuloarterial impedance to predict adverse outcome in asymptomatic aortic Stenosis. J Am Coll Cardiol 2009;54:1003–11. 10.1016/j.jacc.2009.04.079 [DOI] [PubMed] [Google Scholar]
- 9. Lindman BR, Stewart WJ, Pibarot P, et al. Early regression of severe left ventricular hypertrophy after transcatheter aortic valve replacement is associated with decreased hospitalizations. JACC Cardiovasc Interv 2014;7:662–73. 10.1016/j.jcin.2014.02.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Une D, Mesana L, Chan V, et al. Clinical impact of changes in left ventricular function after aortic valve replacement: analysis from 3112 patients. Circulation 2015;132:741–7. 10.1161/CIRCULATIONAHA.115.015371 [DOI] [PubMed] [Google Scholar]
- 11. Nishimura RA, Otto CM, Bonow RO, et al. 2014 AHA/ACC Guideline for the management of patients with Valvular Heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice guidelines. Circulation 2014;129:e521–e643. 10.1161/CIR.0000000000000031 [DOI] [PubMed] [Google Scholar]
- 12. Taniguchi T, Morimoto T, Shiomi H, et al. Initial Surgical Versus Conservative strategies in patients with asymptomatic severe aortic Stenosis. J Am Coll Cardiol 2015;66:2827–38. 10.1016/j.jacc.2015.10.001 [DOI] [PubMed] [Google Scholar]
- 13. Lang RM, Badano LP, Mor-Avi V, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr 2015;28:1–39. e14. 10.1016/j.echo.2014.10.003 [DOI] [PubMed] [Google Scholar]
- 14. Kappetein AP, Head SJ, Généreux P, et al. Updated standardized endpoint definitions for transcatheter aortic valve implantation: the Valve Academic Research Consortium-2 consensus document. J Am Coll Cardiol 2012;60:1438–54. 10.1016/j.jacc.2012.09.001 [DOI] [PubMed] [Google Scholar]
- 15. Peer M, Boaz M, Zipora M, et al. Determinants of left ventricular hypertrophy in hypertensive patients: identification of high-risk patients by metabolic, vascular, and inflammatory risk factors. Int J Angiol 2013;22:223–8. 10.1055/s-0033-1348880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Salcedo EE, Korzick DH, Currie PJ, et al. Determinants of left ventricular hypertrophy in patients with aortic Stenosis. Cleve Clin J Med 1989;56:590–6. 10.3949/ccjm.56.6.590 [DOI] [PubMed] [Google Scholar]
- 17. Lee JM, Park SJ, Lee SP, et al. Gender difference in ventricular response to aortic Stenosis: insight from cardiovascular magnetic resonance. PLoS One 2015;10:e0121684 10.1371/journal.pone.0121684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lund BP, Gohlke-Bärwolf C, Cramariuc D, et al. Effect of obesity on left ventricular mass and systolic function in patients with asymptomatic aortic Stenosis (a Simvastatin Ezetimibe in aortic Stenosis [SEAS] substudy). Am J Cardiol 2010;105:1456–60. 10.1016/j.amjcard.2009.12.069 [DOI] [PubMed] [Google Scholar]
- 19. Nakamura T, Toda K, Kuratani T, et al. Diabetes Mellitus impairs left ventricular mass regression after Surgical or Transcatheter aortic valve replacement for severe aortic Stenosis. Heart Lung Circ 2016;25:68–74. 10.1016/j.hlc.2015.05.019 [DOI] [PubMed] [Google Scholar]
- 20. Benedetto U, Melina G, Angeloni E, et al. Moderate chronic kidney disease and left ventricular hypertrophy after aortic valve replacement for aortic valve Stenosis. J Thorac Cardiovasc Surg 2010;139:881–6. 10.1016/j.jtcvs.2009.05.041 [DOI] [PubMed] [Google Scholar]
- 21. Otto CM, Burwash IG, Legget ME, et al. Prospective study of asymptomatic valvular aortic Stenosis. clinical, echocardiographic, and exercise predictors of outcome. Circulation 1997;95:2262–70. 10.1161/01.CIR.95.9.2262 [DOI] [PubMed] [Google Scholar]
- 22. Fazal L, Azibani F, Vodovar N, et al. Effects of biological sex on the pathophysiology of the heart. Br J Pharmacol 2014;171:555–66. 10.1111/bph.12279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Luczak ED, Leinwand LA. Sex-based cardiac physiology. Annu Rev Physiol 2009;71:1–18. 10.1146/annurev.physiol.010908.163156 [DOI] [PubMed] [Google Scholar]
- 24. Warita S, Kawasaki M, Tanaka R, et al. Effects of pitavastatin on cardiac structure and function and on prevention of atrial fibrillation in elderly hypertensive patients: a prospective study of 2-years' follow-up. Circ J 2012;76:2755–62. 10.1253/circj.CJ-12-0722 [DOI] [PubMed] [Google Scholar]
- 25. Takemoto M, Node K, Nakagami H, et al. Statins as antioxidant therapy for preventing cardiac myocyte hypertrophy. J Clin Invest 2001;108:1429–37. 10.1172/JCI13350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Rosenhek R, Zilberszac R, Schemper M, et al. Natural history of very severe aortic Stenosis. Circulation 2010;121:151–6. 10.1161/CIRCULATIONAHA.109.894170 [DOI] [PubMed] [Google Scholar]
- 27. Bjørnstad JL, Neverdal NO, Vengen OA, et al. Alterations in circulating activin A, GDF-15, TGF-beta3 and MMP-2, -3, and -9 during one year of left ventricular reverse remodelling in patients operated for severe aortic Stenosis. Eur J Heart Fail 2008;10:1201–7. 10.1016/j.ejheart.2008.09.010 [DOI] [PubMed] [Google Scholar]
- 28. Herrmann HC, Pibarot P, Hueter I, et al. Predictors of mortality and outcomes of therapy in low-flow severe aortic Stenosis: a Placement of aortic transcatheter valves (PARTNER) trial analysis. Circulation 2013;127:2316–26. 10.1161/CIRCULATIONAHA.112.001290 [DOI] [PubMed] [Google Scholar]
- 29. Clavel MA, Magne J, Pibarot P. Low-gradient aortic stenosis. Eur Heart J 2016;37:2645–57. 10.1093/eurheartj/ehw096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hachicha Z, Dumesnil JG, Bogaty P, et al. Paradoxical low-flow, low-gradient severe aortic Stenosis despite preserved ejection fraction is associated with higher afterload and reduced survival. Circulation 2007;115:2856–64. 10.1161/CIRCULATIONAHA.106.668681 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
heartjnl-2016-311022supp001.pdf (29.7KB, pdf)
heartjnl-2016-311022supp002.pdf (528.8KB, pdf)