Summary
Aims
The present study aimed to investigate microbial patterns associated with disease progression and coinfection by different Herpesviruses in generalized aggressive periodontitis (GAP).
Methods
Microbiological samples were obtained from active (AS) and non-active (n-AS) sites in 165 subjects affected by GAP and were analyzed for 40 bacterial species by the Checkerboard DNA-DNA Hybridization technique and for Herpes simplex 1 (HSV-1), Human Cytomegalovirus (CMV), and Epstein Bar virus (EBV) by PCR.
Common Factor Analysis and Multiple Regression Analysis were applied to disclose specific microbial patterns associated with the three viruses.
Results
Herpesviruses were detected in 37.6% of subjects. Detection of each of the searched viruses was associated with specific patterns of subgingival biofilm in AS. Logistic regression analyses evidenced several virus/bacteria associations: i) EBV with Aggregatibacter actinomycetemcomitans; ii) CMV with A. actinomycetemcomitans, Veillonella parvula, Parvimonas micra and Fusobacterium nucleatum subsp. polymorphum; iii) HSV-1 with Porphyromonas gingivalis, Tannerella forsythia, Fusobacterium periodonticum and Staphylococcus aureus.
Conclusions
Microbiological data suggest that Herpesviruses are probably not mere spectators of disease progression and that specific patterns of subgingival plaque are correlated with the presence of different Herpesviruses.
Keywords: generalized aggressive periodontitis, herpesviruses, microbiota, disease progression
Introduction
Periodontitis is a group of infectious disorders characterized by inflammation affecting periodontal tissues and causing an inconstant, episodic progression in breakdown of the teeth-supporting tissues. In some patients progression of periodontitis is characterized by shortened periods of remission, leading to a clinical presentation called aggressive periodontitis, that can be either generalized or localized (1–3). The reasons leading some patients to undergo generalized aggressive periodontitis (GAP) are poorly understood, and no definite useful molecular or microbiological markers or predictors of disease progression have been identified yet (4, 5). Recent reports suggest moreover that atypical pathogens are frequently associated with progression of periodontal lesions and resistance to conventional treatments in aggressive periodontitis and have disclosed interesting associations (6, 7). These atypical pathogens, including Staphylococcus aureus, Pseudomonas aeruginosa and different members of the family Enterobacteriaceae, are known to possess virulence factors that could account for the accelerated tissue destruction characterizing GAP.
Recent studies by our group have shown higher Staphylococcus aureus oral carriage rates in periodontitis affected patients as compared to healthy controls (8). Moreover, we showed that isolation of S. aureus from subgingival sites of GAP positively correlates with disease progression, with higher levels of inflammatory mediators and with faster periodontal breakdown (9).
Subgingival isolates of S. aureus from active sites of GAP were shown to possess a set of genes encoding for pathogenicity and virulence factors (9) which are already known to be involved in the pathogenesis of a variety of severe staphylococcal infections (10).
Among factors potentially able to trigger and amplify tissue destruction in GAP, co-infection by different Herpesviruses has been given wide consideration in the last decades.
Studies started about three decades ago have shown that HSV-1, EBV and CMV are detected with high prevalence and at high copy counts in progressive periodontal diseases (11–13).
Association and immunologic studies have provided a solid base of evidence supporting a periodontopathic role of Herpesviruses, although at present the specific molecular and cellular mechanisms which enable these viruses to exacerbate periodontitis are still to be clarified.
In a recent review of the literature Slots in summarizing results from studies performed by different groups in different countries evidenced that mean percentages of sites of aggressive periodontitis reported to be positive for detection of Herpesviruses are 49% for CMV, 45% for EBV and 63% for HSV (11). A detailed analysis of available data evidenced the existence of significant differences among published studies and a possible bias coming from the fact that in most studies sampling was performed regardless of disease activity.
In a recent report Stein et al. failed to find any association between aggressive periodontitis and the presence of Herpesviruses in a sample of patients in Germany (14).
Sunde et al. found that 40% and 12% of periodontitis sites were positive, respectively, for EBV and CMV, although viral loads were close to detection limits (15). Consequently they considered a role for these viruses in the pathogenesis of periodontal lesions unlikely and, possibly, only the consequence of contamination by blood or saliva or of an accumulation of lymphoid cells harbouring viruses in the inflamed tissue.
On the other hand many other Authors found a positive correlation between detection of Herpesviruses and severity and progression of periodontal lesions of both chronic and aggressive periodontitis (11, 16–19). Histomolecular analyses demonstrated that gingival epithelial cells of periodontitis affected sites are frequently infected by one or more Herpesviruses and that viral loads positively correlate with disease severity (20, 21).
Overall, experimental data collected by different research groups in different geographical areas suggest that human Herpesviruses could play a relevant role in both chronic and aggressive periodontitis (12–16), although some Authors hypothesize that the prevalence of HSV, CMV, and EBV in periodontal lesions could vary also on a geographical and ethnic base (22).
It is well known that many viruses are able to act as strong promoters of bacterial pathogenicity and virulence, thus facilitating the onset and progression of acute, aggressive infections in many districts of the human body (23–25). It is consequently conceivable that, as a consequence of specific molecular mechanisms of cooperation, co-infection by different Herpesviruses can influence the qualitative and quantitative composition of subgingival plaque and play a role in the progression of lesions in GAP.
The present study was consequently designed as an observational study aimed to characterize the subgingival microbiota of active sites of GAP and to correlate microbial patterns with the presence/absence of three species of Herpesviruses.
Materials and methods
Studied population and samples
Patients with a clinical diagnosis of GAP (1) and with at least one active site were selected for the present observational study as described previously (9). Briefly, microbiological samples were obtained from subjects attending 12 private dental practices for periodontal problems.
All patients were recruited from structures located in highly urbanized areas (Genua, Brescia, Piacenza, Savona, Milan) and consisted prevalently of subjects with high school or university degrees. Overall, 214 patients were selected during first visits, basing on an initial diagnosis of generalized aggressive periodontitis. The following criteria were used for inclusion in the study: age ranging ≥ 18 – ≤ 39 years; presence of ≥ 20 teeth in the mouth; presence of at least 6 teeth with Periodontal Probing Depth (PPD) ≥ 5 mm and with bleeding on probing (including at least one incisor and one first molar). The following criteria were used for exclusion from the study: presence of systemic diseases or conditions known to affect antibacterial defences, and/or soft tissues and epithelial barrier integrity; subjects who had assumed antibiotics, and/or topic antimicrobials, and/or who underwent periodontal treatment in the previous 6 months; pregnant or breast feeding subjects.
Periodontal status was assessed in terms of Visible Plaque Index (VPI), Gingival Bleeding Index (GBI), and Periodontal Probing Depth (PPD) at the beginning of the study (T0), and after 45 days (T45). Periodontal sites showing a significant increase in both PPD and GBI between T0 and T45 were considered as active sites (AS) and were distinguished from non-active sites (n-AS).
VPI and GBI were evaluated separately at the four main aspects of each tooth, while PPD was evaluated at six sites for each tooth, using a Michigan periodontal probe with Williams markings.
Enrolment in the study was confirmed upon detection of at least one AS between T0 and T45.
Each patient was examined at T0 and T45 by the same examiner.
All clinical evaluations were performed separately by two experienced clinicians (P.G. and M.P.), calibrated to provide consistent diagnoses as follows: twenty subjects were selected among those participating in the study. Each examiner recorded values of PPD at the 6 aspects of 6 selected teeth (2 molars, 2 premolars and 2 incisors) for each subject; measures were repeated after 48 hours at the same sites. Values recorded by the two examiners at the same time were used to calculate inter-examiner reproducibility. Values recorded by each examiner at different times were used to calculate intra-examiner reproducibility. Inter- and intra-examiner reproducibility was measured through Cohen’s weighted kappa which resulted 0.70 (intra-examiner 1), 0.88 (intra-examiner 2) and 0.75 (inter-examiner), suggesting substantial to almost perfect agreement (26).
One hundred and sixty-five of 214 patients (77.1%) (mean age 32.3±3.8 years, ages ranging 18 to 39) were selected for microbiological sampling. Of these, 81 were males (mean age 32.4±3.6 years, ages ranging 21 to 39) and 84 were females (mean age 32.3±4.1 years, ages ranging 18 to 39).
The sampled patients participated voluntarily, were explained the nature of the study and invited to sign an informed consent in conformity with the Helsinki Declaration on Ethical Principles for Medical Research Involving Human Subjects. During the 45 days of observation all patients were invited to maintain their standard oral hygiene procedures.
The study design was approved by the ethical committee of the University of Brescia.
Microbiological sampling
Following clinical evaluation at T45, samples of subgingival biofilm were obtained from one AS and one n-AS in each subject. Selection of AS and n-AS in each patient was made paying attention to select two sites resembling each other as much as possible with regard to position and PPD at the time of sampling.
Samples of subgingival biofilm were collected following careful removal of supragingival biofilm by a sterile Columbia Universal curette: a sterile nr. 40 endodontic paper point was inserted into the pocket and maintained in place for 60 seconds. Samples were then transferred into 1.5 ml screw cap vials containing 0.5 ml of sterile DNAse free molecular grade water, vortexed for 2 minutes, stored at −80° C and processed within 48 hours from sampling.
Purification of bacterial DNA
Total bacterial DNA was extracted and purified from each sample as described previously (27) using the Nucleospin Genomic DNA purification Kit (Macherey-Nagel GmbH Düren, Germany) according to instructions of the manufacturer. Purified DNA was quantified by the Qubit™ quantitation system (Invitrogen, Milan, Italy) and stored at −80° C until used.
Quantitation of specific bacterial DNA in samples
Forty selected bacterial species (listed in Tab. 1) were quantitatively detected in total DNA samples by a modified checkerboard DNA-DNA hybridization technique (CKB) (28, 29).
Table 1.
Bacterial strains used to prepare specific species probes for the modified checkerboard DNA-DNA Hybridization analysis.
| Bacterial Taxa | Strain |
|---|---|
| Actinomyces gerencseriae | CCUGa 32936T |
| Actinomyces israelii | CCUG 18307T |
| Actinomyces naeslundii | CCUG 18310T |
| Actinomyces oris | DSMb 23056 |
| Actinomyces odontolyticus | DSM 19120 |
| Veillonella parvula | DSM 2008 |
| Streptococcus gordonii | DSM 6777 |
| Streptococcus intermedius | DSM 20573 |
| Streptococcus mitis | DSM 12643 |
| Streptococcus oralis | DSM 20623 |
| Streptococcus sanguinis | DSM 20567 |
| Aggregatibacter actinomycetemcomitans | DSM 8324 |
| Capnocytophaga gingivalis | DSM 3290 |
| Capnocytophaga ochracea | DSM 7171 |
| Capnocytophaga sputigena | DSM 7273 |
| Eikenella corrodens | DSM 8340 |
| Campylobacter gracilis | DSM 19528 |
| Campylobacter rectus | DSM 3260 |
| Campylobacter showae | DSM 19458 |
| Eubacterium nodatum | DSM 3993 |
| Fusobacterium nucleatum ss. nucleatum | DSM 15643 |
| Fusobacterium nucleatum ss. polymorphum | DSM 20482 |
| Fusobacterium nucleatum ss. vincentii | DSM 19507 |
| Fusobacterium periodonticum | ATCCc 33693 |
| Parvimonas micra | ATCC 33270 |
| Prevotella intermedia | ATCC 25611 |
| Prevotella nigrescens | ATCC 33563 |
| Streptococcus constellatus | DSM 20575 |
| Tannerella forsythia | ATCC 43037 |
| Porphyromonas gingivalis | ATCC 33277 |
| Treponema denticola | DSM 14222 |
| Eubacterium saburreum | ATCC 33271 |
| Gemella morbillorum | DSM 20572 |
| Leptotrichia buccalis | DSM 1135 |
| Neisseria mucosa | DSM 17611 |
| Prevotella melaninogenica | ATCC25854 |
| Streptococcus anginosus | DSM 20563 |
| Selenomonas noxia | DSM 19578 |
| Treponema socranskii | ATCC 35536 |
| Staphylococcus aureus | ATCC 12600 |
Species specific probes were prepared by the Biotin DecaLabel™ DNA Labeling Kit (Fermentas, Thermo Fisher Scientific), and detected after high stringency hybridization using the Biotin Chromogenic detection kit (Fermentas). Quantitative determinations were performed following detection by a densitometric scanner (BioRad GS800 calibrated densitometer) using the Quantity One® analysis software (BioRad) and expressed as percent of total DNA counted in the sample. Samples giving no signal were all reported as scoring 0.1% of total bacterial DNA in the sample, to account for limits of sensitivity of the test.
Detection of Herpesviruses
A second set of samples was obtained from each selected AS and n-AS, as described above, to detect the presence of Herpes simplex type 1 (HSV-1), Human Cytomegalovirus (CMV) and Epstein Bar virus (EBV), using previously described specific PCR methods (30, 31).
Patients were split into four groups according to positivity for detection of HSV-1 (HSV+), EBV (EBV+), CMV (CMV+), and negativity for detection of any of the searched viruses (HHV−).
Statistics
The Student’s t-test for paired samples was performed to detect AS, i.e. those sites showing significant differences in clinical variables (PPD and GBI) between T0 and T45.
Significance of differences in the percentages of species-specific DNAs between AS and n-AS and, within AS, among the four groups (i.e. HSV+, EBV+, CMV+ and HHV−) were evaluated through the oneway analysis of variance (ANOVA) for dependent samples. The Tukey’s HSD test was performed as post hoc test to detect differences between pairs of groups.
In order to investigate the relative frequencies of subgingival microbiota potentially associated with EBV, CMV and HSV-1 in AS, three logistic regression analyses were designed using EBV, CMV and HSV-1, dichotomized into detected/undetected, as response variables and the relative frequencies as explanatory variables, adjusted for patient’s age and gender, VPI and GBI as confounders.
Preliminarily the existence of inter-correlated bacterial species was investigated by performing the correlation matrix with the Pearson’s correlation coefficients “r” among species (threshold value was set at r≥0.5). Common Factor Analysis (CFA) was then used to reduce the number of bacterial variables (32–34).
CFA generated a small set of non-inter-correlated factors (i.e. groups of inter-correlated bacterial species).
The association between each generated factor and EBV, CMV and HSV-1 was initially explored with logistic regression analysis. Factors resulting more strongly associated with EBV, CMV, and HSV-1 were first tested for multi-collinearity and then chosen for the multiple logistic regression analysis (35).
The goodness of fit of the models was assessed through the likelihood ratio c2 test (p<0.05) and pseudo-R2. The analysis was validated by splitting the sample into two subsamples of equal size, and assessing the coefficients using one subsample and the goodness of fit using the other subsample.
Statistical analysis was made by a blinded statistician (S.P.) who was not aware of the bacterial and viral taxa at the moment of analysis. The statistical software StatView 5.0.1 (SAS® Institute Inc., NC, USA) was used. The level of significance was set at 95%.
Results
By adopting the selection criteria described in the materials and methods section, 165 AS and 165 n-AS were selected for microbiological sampling. Both the AS group and the n-AS group comprised 45 upper molars, 41 lower molars, 19 upper premolars, 16 lower premolars, 31 upper incisors and 13 lower incisors. Overall, according to clinical values recorded at T0, AS and n-AS were comparable in terms of PPD (mean values being 5.5 mm and 5.6 mm respectively, P = 0.24), VPI (mean values being 1.6 and 1.5 respectively, P = 0.35), and GBI (mean values being 1.7 and 1.6 respectively, P = 0.39). In accordance with criteria adopted for selection, AS alone showed a significant increase in both PPD and GBI between T0 and T45, while values of VPI did not change significantly.
Detection of Herpesviruses in subgingival plaque
Sixty-two out of 165 subjects (37.6%) resulted positive for the detection of one of the 3 searched viruses. Prevalence was 14.5, 13.9 and 9.1% for HSV-1, EBV, and CMV respectively. None of the studied sites resulted positive for detection of more than one of the searched viruses. No difference was observed in positivity for the three searched viruses between AS and n-AS in the single patients. No statistically significant differences were observed with regard to age and gender among subjects divided in HSV+, EBV+,CMV+, and HHV−.
At T0, AS of CMV+ subjects showed significantly higher mean VPI values as compared to those of HHV− subjects (Tab. 2). The AS of both HSV+ and CMV+ subjects showed significantly greater increase in PPD between T0 and T45 as compared to those of HHV− subjects (Tab. 2).
Table 2.
Distribution of the sample according to positivity for detection of type 1 Herpes Simplex Virus (HSV+), Epstein Bar Virus (EBV+) and Human Cytomegalovirus (CMV+) or no Human Herpesvirus (HHV−). Mean values of Visible Plaque Index (VPI), Gingival Bleeding Index (GBI) and periodontal probing depth (PPD) detected in AS at T0 and their mean variations between T0 and T45 (ΔVPI, ΔGBI, ΔPPD).
| Patient group according to virus detection | Number out of 165 | VPI mean score (± SD) | GBI mean score (± SD) | PPD mean mm (± SD) | ΔVPI mean score T45-T0 | ΔGBI mean score T45-T0 | ΔPPD mean mm T45-T0 |
|---|---|---|---|---|---|---|---|
| HSV+ | 24 | 1.5 (±0.5) | 1.5 (±0.5) | 5.6 (±0.7) | 0.1 (±0.3) | 0.5 (±0.5) | 1.5* (±0.5) |
| EBV+ | 23 | 1.4 (±0.50) | 1.5 (±0.5) | 5.5 (±0.6) | 0.1 (±0.3) | 0.5 (±0.5) | 1.2 (±0.4) |
| CMV+ | 15 | 1.7* (±0.5) | 1.6 (±0.5) | 5.5 (±0.5) | 0.1 (±0.5) | 0.5 (±0.5) | 1.3* (±0.5) |
| HHV− | 103 | 1.5 (±0.50) | 1.7 (±0.5) | 5.6 (±0.6) | 0.0 (±0.3) | 0.4 (±0.5) | 1.1 (±0.3) |
Characterization of subgingival biofilm
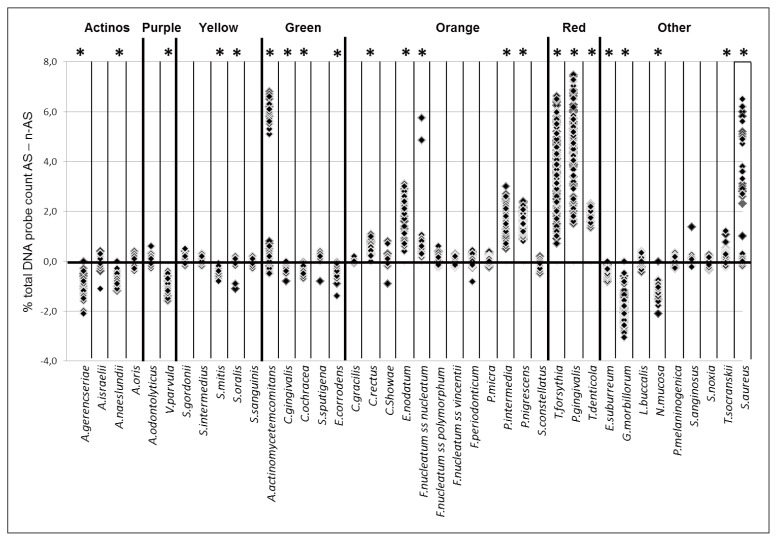
The quantitative detection of 40 bacterial species in subgingival biofilm samples by the CKB technique, as expected, showed the existence of several significant differences between AS and n-AS (Fig. 1). AS were overall characterized by the presence of significantly higher amounts of Aggregatibacter actinomycetemcomitans, Campylobacter rectus, Eubacterium nodatum, Fusobacterium nucleatum subsp. nucleatum, Prevotella intermedia, Prevotella nigrescens, Tannerella forsythia, Porphyromonas gingivalis, Treponema denticola, Treponema socranskii, and S. aureus as compared to n-AS (Fig. 1). As opposite, Actinomyces gerencseriae, Actinomyces naeslundii, Veillonella parvula, Streptococcus mitis, Capnocythophaga gingivalis, Capnocythophaga ochracea, Eikenella corrodens, Eubacterium saburreum, Gemella morbillorum, and Neisseria mucosa, were represented in significantly lower amounts in AS as compared to n-AS (Fig. 1).
Figure 1.
Plot of differences of percentages of total DNA probe count of the 40 test species in subgingival biofilm samples between 165 AS and 165 n-AS of generalized aggressive periodontitis. Significant differences between AS and n-AS for each species are indicated with an asterisk. Species were ordered and grouped according to the complexes described by Socransky et al., 1998 (28).
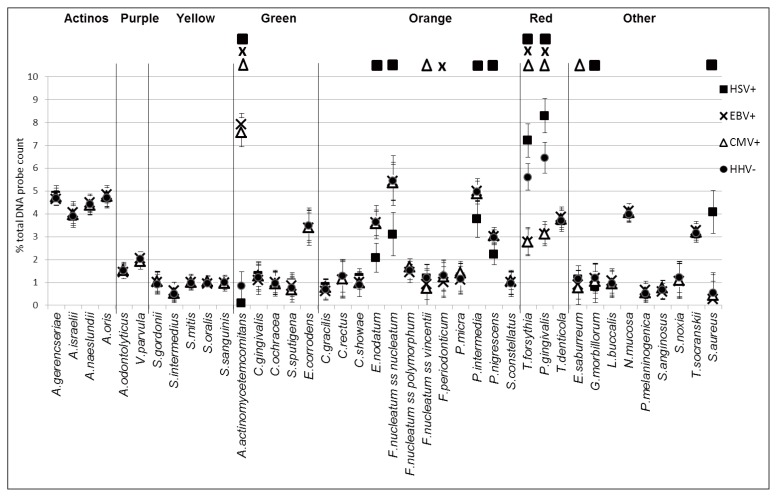
Significant differences were also detected in the quantitative composition of subgingival biofilm from AS of HSV+, EBV+, CMV+ subjects as compared to HHV− subjects (Fig. 2).
Figure 2.
Mean percentage (±standard deviation) of the total DNA probe count of the 40 test species in subgingival biofilm samples from 165 AS of generalized aggressive periodontitis distinguished according to positivity for detection of HSV-1 (HSV+), EBV (EBV+), CMV (CMV+) or no Herpesvirus (HHV−) specific sequences. Significance of differences for each species between AS of HSV+, or EBV+, or CMV+, and HHV− sites is indicated by replicating the symbol corresponding to each category at the top of the plot. Species were ordered and grouped according to the complexes described by Socransky et al., 1998 (28).
In fact, sites of HSV+ subjects were characterized by significantly higher amounts of T. forsythia, P. gingivalis, and S. aureus and by significantly lower amounts of A. actinomycetemcomitans, C. showae, E. nodatum, F. nucleatum subsp. nucleatum, P. intermedia, P. nigrescens and G. morbillorum as compared to HHV− subjects (Fig. 2).
Sites of EBV+ subjects were characterized by significantly higher amounts of A. actinomycetemcomitans and by significantly lower amounts of F. nucleatum subsp. vincentii, F. periodonticum, T. forsythia, P. gingivalis, and E. saburreum as compared to HHV-subjects (Fig. 2).
Sites of CMV+ subjects were characterized by significantly higher amounts of A. actinomycetemcomitans and by significantly lower amounts of F. nucleatum subsp. vincentii, T. forsythia, P. gingivalis, and E. saburreum as compared to HHV− subjects (Fig. 2).
Analysis of the association of subgingival microbiota with Herpesviruses.
Analysis of values of skewness and kurtosis showed that percentages of total DNA probe counts of the studied bacterial species followed a reasonably normal distribution (data not shown). Analysis of the Pearson’s correlation coefficients between relative frequencies of bacterial species strongly justified the use of CFA (data not shown). CFA generated 19 factors; of these, 15, showing eigenvalues >1, were selected as meaningful and subjected to factor rotation. After oblique and orthogonal rotation, the 15 selected factors accounted for variance proportions ranging between 4.1 and 13.2% (Tab. 3). All bacterial taxa were included in only one factor, excluding P. gingivalis and T. forsythia which were directly associated with factor 8 and inversely associated with factor 1 (Tab. 3).
Table 3.
Meaningful factors generated by Common Factor Analysis and bacterial species resulting strongly directly or inversely associated with each dimension (i.e. factor loading assessed with Pearson’s correlation coefficients between bacterial species and dimension ≥0.33).
| Factor | Accounted Variance (%) | Bacterial species associated with dimension | |
|---|---|---|---|
| Directly | Inversely | ||
| 1 | 13.2 | E.nodatum, P.intermedia, P.nigrescens | S.aureus, T.forsythia, P.gingivalis |
| 2 | 10.4 | A.israelii, T.denticola, T.socranskii | |
| 3 | 7.2 | C.rectus, S.noxia | |
| 4 | 7.0 | A.oris, N.mucosa | |
| 5 | 7.1 | E.suburreum, F.nucleatum subsp vincentii | |
| 6 | 6.4 | C.gracilis, S.anginosus | |
| 7 | 6.5 | A.odontolyticus, C.gingivalis | |
| 8 | 8.7 | P.gingivalis, T.forsythia, F.periodonticum | A.actinomycetemcomitans |
| 9 | 6.9 | S.intermedius, P.melaninogenica, S.constellatus | |
| 10 | 4.7 | G.morbillorum | L.buccalis |
| 11 | 4.3 | P.micra, F.nucleatum subsp. polymorphum | V.parvula |
| 12 | 4.8 | E.corrodens, S.sputigena | S.sanguinis |
| 13 | 4.1 | A.gerencseriae, A.naeslundii | S.gordonii |
| 14 | 4.1 | C.showae, C.ochracea | |
| 15 | 4.6 | S.mitis, S.oralis | |
According to the simple logistic regression analyses, three factors (factors 2, 4, and 8) were marginally (i.e., 0.20<p<0.05) or significantly (i.e., p≤0.05) associated with EBV, three (factors 5, 8, and 11) with CMV, and three (factors 1, 8, and 10) with HSV (Tab. 4).
Table 4.
Association (OR with 95% confidence interval -95CI) between each single factor and EBV, CMV and HSV-1, adjusted for confounders and assessed through logistic regression analysis. Factors with p<0.20 were included in the final logistic regression models.
| Factor | Association with OR (95CI) | ||
|---|---|---|---|
|
| |||
| EBV | CMV | HSV-1 | |
| 1 | Inverse 0.1 (0.1–0.3)** | ||
| 2 | Direct 1.4 (0.9–2.2)* | ||
| 4 | Direct 1.4 (0.9–2.2)* | ||
| 5 | Inverse 0.6 (0.4–1.1)* | ||
| 8 | Inverse 0.1 (0.03–0.2)** | Inverse 0.1 (0.05–0.3)** | Direct 4.1 (1.7–10.1)** |
| 10 | Inverse 0.7 (0.4–1.0)* | ||
| 11 | Direct 2.2 (1.2–4.1)** | ||
0.20<p<0.05: marginally associated; included in the final model.
p≤0.05: significantly associated; included in the final model.
Multiple logistic regression analysis showed that: 1) factor 8 was the only one being significantly and inversely associated with EBV; 2) factor 11 was significantly and directly associated with CMV, while factors 5 and 8 were significantly and inversely associated with CMV; 3) factor 8 was significantly and directly associated with HSV-1, while factors 1 and 10 were significantly and inversely associated with HSV-1 (Tab. 5). The three regression models were not disturbed by multi-collinearity, (VIFs = 0.10). The likelihood c2 tests resulted highly significant (P<0.0001), while the Pseudo-R2 values were all 0.6 for the three models. These tests suggest that the variables included in the regression models were highly predictive.
Table 5.
Multivariate association (OR with 95% confidence interval -95CI) between the meaningful factors and Epstein Bar Virus (EBV), Human Cytomegalovirus (CMV), and type 1 Herpes Simplex Virus (HSV-1), adjusted for confounders and assessed through logistic regression analysis.
| Factor | OR (95CI) | ||
|---|---|---|---|
|
| |||
| EBVa | CMVb | HSV-1c | |
| 1 | 0.1 (0.04–0.3) − p<0.0001 | ||
| 2 | 1.8 (0.8–4.0) − p=0.14d | ||
| 4 | 2.0 (0.9–4.5) − p=0.08d | ||
| 5 | 0.4 (0.2–0.9) − p=0.02d | ||
| 8 | 0.05 (0.01–0.2) − p<0.0001 | 0.07 (0.02–0.3) − p<0.0001 | 12.7 (2.1–75.5) − p=0.005 |
| 10 | 0.5 (0.2–1.0) − p=0.05d | ||
| 11 | 4.6 (1.6–13.6) − p=0.005 | ||
Whole model goodness of fit:
likelihood-ratio χ27df=84.16; p<0.0001. Pseudo-R2=0.63
likelihood-ratio χ27df=59.99; p<0.0001. Pseudo-R2=0.60
likelihood-ratio χ27df=83.22; p<0.0001. Pseudo-R2=0.61
validation analysis did not confirm the statistically significant association
Following validation analysis, factors 2 and 4 were no longer associated with EBV, factor 5 was no longer associated with CMV, and factor 10 was no longer associated with HSV-1. The likelihood c2 tests remained highly significant (P<0.0001).
Discussion
Powerful molecular methods of investigation introduced during the last few decades enabled a substantial improvement of our knowledge on different aspects of the oral and periodontal microbiota and on its interactions with host defenses.
Nevertheless, the etiopathogenesis of different clinical forms of periodontitis, the specific mechanisms accounting for its episodic progression and those being able to trigger the activation of sites are still debated (36, 37).
Most Authors agree to recognize in a limited number of microbial species, grouped in a few complexes, the principal and most frequent etiologic agents of common clinical forms of periodontal disease28. Nevertheless, it has been suggested that non typical bacterial species and some Herpesviruses might also cooperate to accelerate disease progression and its susceptibility to periodontal treatments (6, 7, 9, 11, 16, 17, 19, 22, 38).
The role of Herpesviruses in the etiopathogenesis of periodontal diseases is still the object of discussion and different Herpesviruses have been detected with variable prevalence in a number of different studies, starting to be published about three decades ago. Large part of these studies evidenced that HSV-1, EBV and CMV are detected with high prevalence in periodontal lesions, that they directly infect gingival epithelial cells and that viral loads positively correlate with disease severity (11–13, 16–21).
Although some recently published studies failed to find any association between aggressive periodontitis and the presence of Herpesviruses (14), or detected the three viruses at very low levels, and suggested that their detection could possibly be the consequence of contamination by blood or saliva or of lymphoid cells (15), available data, considered altogether suggest that human Herpesviruses probably play a relevant role in both chronic and aggressive periodontitis (12–16), and that differences can in large part be explained on the basis of geographical and ethnic variability (22).
In a critical review of the literature that was published recently, Slots evidenced that the possibility to correlate the presence of Herpesviruses with periodontal disease progression is biased by the fact that in most studies sampling was performed regardless of disease activity.
Recently we demonstrated that subjects affected by periodontal diseases show higher prevalence of S. aureus in their oral microbiota and that they commonly host highly toxigenic strains of such an opportunistic pathogen (9).
Moreover, we showed that the isolation of S. aureus from subgingival sites of GAP correlates with disease progression.
Having cumulatively considered the above observations, data on composition of the subgingival microbiota of GAP, and data on detection of Herpesviruses from subgingival sites of GAP, we designed the present study to characterize the subgingival microbiota of AS of GAP and to correlate microbial patterns found in these sites with the presence/absence of Herpesviruses.
To this purpose, one AS and one n-AS were selected in a population of subjects affected by GAP. Attention was dedicated at selecting two sites that differed almost only with regard to activity of the lesion; basing on a protocol of repeated clinical examination extending over a period of 3 months the time interval of 45 days was found as the most appropriate to detect actively progressing sites in the studied population (9). Two samples of subgingival biofilm were obtained from each selected site.
One sample for each studied site was processed to detect the presence of HSV-1, EBV, or CMV by standard PCR methods. The analyzed sites were consequently distinguished in four groups according to positivity for detection of HSV-1, EBV, CMV or negativity for Herpesviruses (HHV−).
Adoption of standard PCR methods and the fact that only two samples were analyzed from each subject could account for the relatively low number of sites that resulted positive for Herpesviruses as compared to data reported in other studies (11, 15, 16, 19, 22). The fact that we found full correspondence with regard to positivity/negativity for detection of viruses between experimental (AS) and control sites (n-AS) is not surprising, nor in contrast with previous studies in which experimental, diseased sites were compared to control healthy sites demonstrating the existence of significant differences in viral loads between the two groups (11, 15–17, 19–22).
Analysis of clinical data of patients, aggregated according to positivity/negativity for detection of each of the three searched viruses, evidenced that the four groups were comparable with regard to age and gender. At T0 they were also comparable with regard to VPI, GBI and PPD (Tab. 2). Exception to this were CMV+ ASs showing significantly higher VPI values at T0 as compared to those of HHV− subjects (P=0.04).
HSV-1+ and CMV+ ASs showed significantly greater increase of PPD between T0 and T45 as compared to HHV− ASs (P<0.01). These data suggest that at least CMV and HSV-1 are not mere spectators of the progression of GAP lesions.
The second sample from each studied site was used to analyze composition of the subgingival biofilm by means of a modified CKB technique (28, 29); main modifications regarded inclusion in the panel of an S. aureus specific probe, and column purification of metagenomic DNA, instead of simple alkaline lysis, in order to obtain more efficient DNA extraction from Gram positives and to reduce interference due to microbial proteins in crude lysates.
The comparative, semiquantitative analysis of the metagenome from subgingival biofilm samples obtained from AS and n-AS (Fig. 1) substantially confirmed previous observations by other Authors and reinforced evidence of the role played by certain known periodontopathogens and atypical pathogens (namely S. aureus in our study) in the progression of lesions of GAP (4–7, 9).
Data presented here confirm previous reports and suggest that the presence of Herpesviruses affects composition of the subgingival biofilm in GAP and prevalence of certain periodontopathogens (22).
Moreover, they show that virus specific microbial patterns characterize both AS and n-AS and that, during active progression of lesions, the prevalence of certain bacterial species in the subgingival biofilm changes in a virus specific manner.
To our knowledge this is the first investigation aiming to characterize microbial patterns associated with active progression of lesions of GAP and to evaluate at the same time if the presence/absence of three species of Herpesviruses is associated with specific microbial patterns.
In order to disclose specific microbial patterns associated with the presence/absence of each of the three Herpesviruses and with active progression of lesions required an articulated analysis of data, in which the relative frequencies of the searched bacterial species were evaluated considering the potential influence of general variables (age, gender, hygienic skills, reactivity of the host, local anatomy) and specific variables (co-variation of groups of inter-related bacterial species) (34).
Consequently data obtained by CKB from subgingival biofilm samples of AS, divided according to positivity/negativity for detection of HSV-1, EBV, or CMV were preliminary analyzed by CFA to disclose the existence of bacterial taxa whose relative frequencies varied among samples in an inter-correlated manner. CFA enabled to reduce the number of microbial variables and to prevent the negative impact of inter-correlation on results of logistic regression analysis (33–35).
At the end of CFA a small set of non-inter-correlated factors was generated, each factor being characterized by a group of inter-correlated bacterial species.
The association between each single factor and EBV, CMV or HSV-1, adjusted for individual characteristics and clinical indices, was explored with logistic regression analysis.
Those factors resulting more strongly associated with EBV, CMV, or HSV-1 were chosen for multiple logistic regression analysis.
Such an analytical process of microbiological and clinical data was able to return reliable information on bacterial species that are significantly associated with the three viruses and how.
This information can be summarized as follows: I) coinfection by EBV is directly associated with a higher prevalence of A. actinomycetemcomitans and inversely associated with that of P. gingivalis, T. forsythia and F. periodonticum; II) coinfection by CMV is directly associated with a higher prevalence of A. actinomycetemcomitans, V. parvula, P. micra and F. nucleatum subsp. polymorphum and inversely associated with that of P. gingivalis, T. forsythia and F. periodonticum; III) coinfection by HSV-1 is directly associated with a higher prevalence of P. gingivalis, T. forsythia, F. periodonticum and S. aureus and inversely associated with that of A. actinomycetemcomitans, E. nodatum, P. intermedia and P. nigrescens.
The observation that changes in the subgingival biofilm are virus specific suggests that they cannot be explained simply by the immunosuppressive effect of Herpesviruses and are probably consequence of specific mechanisms of cooperation between each of the 3 viruses and some bacterial species.
The existence of cooperative interactions involving different viruses and bacterial species, including S. aureus, is well known and can depend on different cellular and molecular mechanisms (23–25). Cooperative interactions have also been demonstrated between CMV and A. actinomycetemcomitans and are possibly involved in the pathogenesis of periodontal disease (39).
The existence of these associations certainly results from complex cooperative interactions and inter-relations among these species, that have been already described previously and possibly from the impact of virus infection on the subgingival environment, on the concentration of factors of inflammation, on local immunity, on the expression of proteins at the surface of epithelial cells, and on the expression of adhesins and toxins by microorganisms (28, 40–43).
Recent researches showed that polymicrobial synergy can occur during infection and that these interactions can affect health and disease (44).
The high prevalence of S. aureus in HSV-1+ sites and its correlation to lesion activity deserves consideration. A consistent mass of experimental and clinical data shows that polymicrobial infections involving S. aureus exhibit enhanced disease severity and morbidity (44).
S. aureus possesses and uses strategies to survive in many different ecological niches and to counterattack the competing bacteria. Such cooperative and competitive interactions enable this flexible opportunist to evolve rapidly towards persistent phenotypes characterized by multidrug resistance and increased virulence (45).
With regard to the possible role of S. aureus in the progression of lesions of GAP, we have previously shown that subgingival isolates from AS of GAP are characterized by a richer armamentarium of virulence and pathogenicity factors as compared to supragingival isolates (9).
Altogether data presented here and data of the recent literature suggest that although the presence of Herpesviruses is not necessary for the progression of periodontal lesions of GAP, it can facilitate it, possibly by promoting pathogenicity and virulence of periodontopathogenic and other bacteria in a virus and bacterial species dependent manner. Furthermore they suggest existence of a complex cooperative interaction promoted by HSV-1 infection, involving S. aureus and the periodontopathogens P. gingivalis, T. forsythia, and F. periodonticum, that could promote an accelerate progression of lesions of GAP.
Further studies will be necessary to demonstrate and characterize this cooperation at the cellular and molecular level.
Acknowledgements
This work was supported by a grant from MIUR (Project PRIN 2007LXNYS7 003) to CP. The Authors declare that they have no conflict of interests with regard to this work.
References
- 1.Armitage G. Development of a classification system for periodontal diseases and conditions. Ann Periodontol. 1999;4:1–6. doi: 10.1902/annals.1999.4.1.1. [DOI] [PubMed] [Google Scholar]
- 2.Loesche WJ, Grossman NS. Periodontal disease as a specific, albeit chronic, infection: diagnosis and treatment. Clin Microbiol Rev. 2001;14:727–752. doi: 10.1128/CMR.14.4.727-752.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Neely AL, Holford TR, Loe H, Anerud A, Boysen H. The natural history of periodontal disease in man. Risk factors for progression of attachment loss in individuals receiving no oral health care. J Periodontol. 2001;72:1006–1015. doi: 10.1902/jop.2001.72.8.1006. [DOI] [PubMed] [Google Scholar]
- 4.Mullally BH, Dace B, Shelburne CE, Wolff LF, Coulter WA. Prevalence of periodontal pathogens in localized and generalized forms of early-onset periodontitis. J Period Res. 2000;35:232–241. doi: 10.1034/j.1600-0765.2000.035004232.x. [DOI] [PubMed] [Google Scholar]
- 5.Kamma JJ, Nakou M, Gmür R, Baehni PC. Microbiological profile of early onset/aggressive periodontitis subjects. Oral Microbiol Immunol. 2004;19:314–321. doi: 10.1111/j.1399-302x.2004.00161.x. [DOI] [PubMed] [Google Scholar]
- 6.Da Silva-Boghossian CM, do Souto RM, Luiz RR, Colombo AP. Association of red complex, A. actinomycetemcomitans and non oral bacteria with periodontal diseases. Arch Oral Biol. 2011;56:899–906. doi: 10.1016/j.archoralbio.2011.02.009. [DOI] [PubMed] [Google Scholar]
- 7.Heller D, Da Silva-Boghossian CM, do Souto RM, Colombo AP. Subgingival microbial profiles of generalized aggressive and chronic periodontal diseases. Arch Oral Biol. 2012;57:973–980. doi: 10.1016/j.archoralbio.2012.02.003. [DOI] [PubMed] [Google Scholar]
- 8.Passariello C, Puttini M, Iebba V, Pera P, Gigola P. Influence of oral conditions on colonization by highly toxigenic Staphylococcus aureus strains. Oral Dis. 2012;18:402–409. doi: 10.1111/j.1601-0825.2011.01889.x. [DOI] [PubMed] [Google Scholar]
- 9.Passariello C, Lucchese A, Virga A, Pera F, Gigola P. Isolation of Staphylococcus aureus and progression of periodontal lesions in aggressive periodontitis. Eur J Inflamm. 2012;10:501–513. [Google Scholar]
- 10.Cheng AG, Kim HK, Burts ML, Krausz T, Schneewind O, Missiakas DM. Genetic requirements for Staphylococcus aureus abscess formation and persistence in host tissues. FASEB J. 2009;23:3393–3404. doi: 10.1096/fj.09-135467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Slots J. Periodontal herpesviruses: prevalence, pathogenicity, systemic risk. Periodontol. 2015;69:28–45. doi: 10.1111/prd.12085. [DOI] [PubMed] [Google Scholar]
- 12.Passariello C, Palamara AT, Garaci E, Pasquantonio G. Herpesviruses and periodontal disease: a cautionary tale. International Journal of Immunopathology and Pharmachology. 2009;22(2):263–268. doi: 10.1177/039463200902200202. [DOI] [PubMed] [Google Scholar]
- 13.Ambili R, Preeja C, Archana V, Nisha KJ, Seba A, Reejamol MK. Viruses: are they really culprits for periodontal disease? A critical review. J Investig Clin Dent. 2013;4:1–9. doi: 10.1111/jicd.12029. [DOI] [PubMed] [Google Scholar]
- 14.Stein JM, Said Yekta S, Kleines M, Ok D, Kasaj A, Reichert S, Schulz S, Scheithauer S. Failure to detect an association between aggressive periodontitis and the prevalence of herpesviruses. J Clin Periodontol. 2013;40:1–7. doi: 10.1111/jcpe.12021. [DOI] [PubMed] [Google Scholar]
- 15.Sunde PT, Olsen I, Enersen M, Beiske K, Grinde B. Human Cytomegalovirus and Epstein-Barr virus in apical and marginal periodontitis: a role in pathology? J Med Virol. 2008;80:1007–1011. doi: 10.1002/jmv.21180. [DOI] [PubMed] [Google Scholar]
- 16.Ting M, Contreras A, Slots J. Herpesvirus in localized juvenile periodontitis. J Period Res. 2000;35:17–25. doi: 10.1034/j.1600-0765.2000.035001017.x. [DOI] [PubMed] [Google Scholar]
- 17.Yapar M, Saygun I, Ozdemir A, Kubar A, Sahin S. Prevalence of human herpesviruses in patients with aggressive periodontitis. J Periodontol. 2003;74:1634–1640. doi: 10.1902/jop.2003.74.11.1634. [DOI] [PubMed] [Google Scholar]
- 18.Wu Y-M, Yan J, Ojcius DM, Chen L-L, Gu Z-Y, Pan J-P. Correlation between infections with different genotypes of human Cytomegalovirus and Epstein-Barr virus in subgingival samples and periodontal status of patients. J Clin Microbiol. 2007;45:3665–3670. doi: 10.1128/JCM.00374-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Das S, Krithiga GS, Gopalakrishnan S. Detection of human herpes viruses in patients with chronic and aggressive periodontitis and relationship between viruses and clinical parameters. J Oral Maxillofac Pathol. 2012;16:203–209. doi: 10.4103/0973-029X.98502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vincent-Bugnas S, Vitale S, Mouline CC, Khaali W, Charbit Y, Mahler P, Precheur I, Hofman P, Maryanski JL, Doglio A. EBV Infection is common in gingival epithelial cells of the periodontium and worsens during chronic periodontitis. PLoS One. 2013;8:e80336. doi: 10.1371/journal.pone.0080336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Saygun I, Kubar A, Sahin S, Sener K, Slots J. Quantitative analysis of association between herpesviruses and bacterial pathogens in periodontitis. J Periodontal Res. 2008;43:352–359. doi: 10.1111/j.1600-0765.2007.01043.x. [DOI] [PubMed] [Google Scholar]
- 22.Imbronito AV, Okuda OS, Maria de Freitas N, Moreira Lotufo RF, Nunes FD. Detection of herpesviruses and periodontal pathogens in subgingival plaque of patients with chronic periodontitis, generalized aggressive periodontitis, or gingivitis. J Periodontol. 2008;79:2313–2321. doi: 10.1902/jop.2008.070388. [DOI] [PubMed] [Google Scholar]
- 23.Avadhanula V, Rodriguez CA, Devincenzo JP, Wang Y, Webby RJ, Ulett GC. Respiratory viruses augment the adhesion of bacterial pathogens to respiratory epithelium in a viral species and cell type-dependent manner. J Virol. 2006;80:1629–1636. doi: 10.1128/JVI.80.4.1629-1636.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Passariello C, Schippa S, Conti C, Russo P, Poggiali F, Garaci E, Palamara AT. Rhinoviruses promote internalization of Staphylococcus aureus into non fully permissive cultured pneumocytes. Microbes Infect. 2006;8:758–766. doi: 10.1016/j.micinf.2005.09.013. [DOI] [PubMed] [Google Scholar]
- 25.Passariello C, Nencioni L, Sgarbanti R, Ranieri D, Torrisi MR, Ripa S, Garaci E, Palamara AT. Viral hemagglutinin is involved in promoting the internalisation of Staphylococcus aureus into human pneumocytes during influenza A H1N1 virus infection. Int J Med Microbiol. 2010;301:97–104. doi: 10.1016/j.ijmm.2010.06.008. [DOI] [PubMed] [Google Scholar]
- 26.Landis JR, Koch GG. The Measurement of observer agreement for categorical data. Biometrics. 1977;33(1):159–174. [PubMed] [Google Scholar]
- 27.Passariello C, Puttini M, Virga A, Gigola P. Microbiological and host factors are involved in promoting the periodontal failure of metaloceramic crowns. Clin Oral Invest. 2012;16:987–995. doi: 10.1007/s00784-011-0585-0. [DOI] [PubMed] [Google Scholar]
- 28.Socransky SS, Haffajee AD, Cugini MA, Smith C, Kent RL., Jr Microbial complexes in subgingival plaque. J Clin Periodontol. 1998;25:134–144. doi: 10.1111/j.1600-051x.1998.tb02419.x. [DOI] [PubMed] [Google Scholar]
- 29.Teles RP, Gursky LC, Faveri M, Rosa EA, Teles FR, Feres M, Haffajee A. Relationships between subgingival microbiota and GCF biomarkers in generalized aggressive periodontitis. J Clin Periodontol. 2010;37:313–323. doi: 10.1111/j.1600-051X.2010.01534.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nogueira ML, Siqueira RC, Freitas N, Amorim JB, Bonjardim CA, Ferreira PC, Oréfice F, Kroon EG. Detection of herpesvirus DNA by the polymerase chain reaction (PCR) in vitreous samples from patients with necrotising retinitis. J Clin Pathol. 2001;54:103–106. doi: 10.1136/jcp.54.2.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Baldanti F, Grossi P, Furione M, Simoncini L, Sarasini A, Comoli P, Maccario R, Fiocchi R, Gerna G. High levels of Epstein-Barr Virus DNA in blood of solid-organ transplant recipients and their value in predicting posttransplant lymphoproliferative disorders. J Clin Microbiol. 2000;38:613–619. doi: 10.1128/jcm.38.2.613-619.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bagley SC, White H, Golomb BA. Logistic regression in the medical literature: standards for use and reporting, with particular attention to one medical domain. J Clin Epidemiol. 2001;54:979–985. doi: 10.1016/s0895-4356(01)00372-9. [DOI] [PubMed] [Google Scholar]
- 33.Petti S, Scully C. Determinants of oral cancer at the national level: just a question of smoking and alcohol drinking prevalence? Odontology. 2010;98:144–152. doi: 10.1007/s10266-010-0133-4. [DOI] [PubMed] [Google Scholar]
- 34.Teles R, Sakellari D, Teles F, Konstantinidis A, Kent R, Socransky S, Haffajee A. Relationships among gingival crevicular fluid biomarkers, clinical parameters of periodontal disease, and the subgingival microbiota. J Periodontol. 2010;81:89–98. doi: 10.1902/jop.2009.090397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Petti S, Scully C. Association between different alcoholic beverages and leukoplakia among non- to moderate-drinking adults: a matched case-control study. Eur J Cancer. 2006;42:521–527. doi: 10.1016/j.ejca.2005.07.038. [DOI] [PubMed] [Google Scholar]
- 36.Filoche S, Wong L, Sissons CH. Oral biofilms: emerging concepts in microbial ecology. J Dent Res. 2010;89:8–18. doi: 10.1177/0022034509351812. [DOI] [PubMed] [Google Scholar]
- 37.Jenkinson HF. Beyond the oral microbiome. Environ Microbiol. 2011;13:3077–3087. doi: 10.1111/j.1462-2920.2011.02573.x. [DOI] [PubMed] [Google Scholar]
- 38.Bertoldi C, Pellacani C, Lalla M, Consolo U, Pinti M, Cortellini P, Cossarizza A. Herpes Simplex I virus impairs regenerative outcomes of periodontal regenerative therapy in intrabony defects: a pilot study. J Clin Periodontol. 2012;39:385–392. doi: 10.1111/j.1600-051X.2012.01850.x. [DOI] [PubMed] [Google Scholar]
- 39.Teughels W, Sliepen I, Quirynen M, Haake SK, Van Eldere J, Fives-Taylor P, Van Ranst M. Human Cytomegalovirus enhances A. actinomycetemcomitans adherence to cells. J Dent Res. 2007;86:175–180. doi: 10.1177/154405910708600213. [DOI] [PubMed] [Google Scholar]
- 40.Contreras A, Botero JE, Slots J. Biology and pathogenesis of cytomegalovirus in periodontal disease. Periodontol 2000. 2014;64:40–56. doi: 10.1111/j.1600-0757.2012.00448.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hung SL, Chiang HH, Wu CY, Hsu MJ, Chen YT. Effects of herpes simplex type 1 infection on immune functions of human neutrophils. J Periodontal Res. 2012;47:635–644. doi: 10.1111/j.1600-0765.2012.01476.x. [DOI] [PubMed] [Google Scholar]
- 42.Kajita K, Honda T, Amanuma R, Domon H, Okui T, Ito H, Yoshie H, Tabeta K, Nakajima T, Yamazaki K. Quantitative messenger RNA expression of Toll-like receptors and interferon-alpha 1 in gingivitis and periodontitis. Oral Microbiol Immunol. 2007;22:398–402. doi: 10.1111/j.1399-302X.2007.00377.x. [DOI] [PubMed] [Google Scholar]
- 43.Kanangat S, Postlethwaite A, Cholera S, Williams L, Schaberg D. Modulation of virulence gene expression in Staphylococcus aureus by interleukin 1-β: Novel implications in bacterial pathogenesis. Microbes Infect. 2007;9:408–415. doi: 10.1016/j.micinf.2006.12.018. [DOI] [PubMed] [Google Scholar]
- 44.Murray JL, Connell JL, Stacy A, Turner KH, Whiteley M. Mechanisms of synergy in polymicrobial infections. J Microbiol. 2014;52(3):188–199. doi: 10.1007/s12275-014-4067-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nair N, Biswas R, Götz F, Biswas L. Impact of Staphylococcus aureus on pathogenesis in polymicrobial infections. Infect Immun. 2014;82(6):2162–2189. doi: 10.1128/IAI.00059-14. [DOI] [PMC free article] [PubMed] [Google Scholar]