Abstract
Background
Human neuroimaging studies indicate that the loss of brain volume associated with substance abuse may be recovered during abstinence. Subcortical and prefrontal cortical regions involved in reward and decision-making are among the regions most consistently implicated in damage and recovery from substance abuse, but the relative capacities of these different brain regions to recover volume during abstinence remains unclear, and it is unknown whether recovery capacities depend on the substance that was abused.
Methods
Voxel-based morphometry in a prison inmate sample (n=107) of long-term abstinent former regular users (FRUs) and former light users (FLUs) of alcohol, cocaine, and/or cannabis. Cross-sectional indicators of volume recovery were operationalized as 1) positive correlation between abstinence duration and volume in FRUs and 2) absence of lower volume in FRUs compared to FLUs.
Results
In FRUs of alcohol, abstinence duration positively correlated with volume in subcortical regions (particularly the putamen and amygdala) but not prefrontal regions; lower prefrontal but not subcortical volume was observed in FRUs compared to FLUs. In FRUs of cocaine, abstinence duration positively correlated with volume in both subcortical regions (particularly the nucleus accumbens) and prefrontal regions; lower volume was not observed in either subcortical or prefrontal regions in FRUs. In FRUs of cannabis, abstinence duration positively correlated with subcortical but not prefrontal volume; lower prefrontal but not subcortical volume was observed in FRUs.
Conclusions
Subcortical structures displayed indicators of volume recovery across FRUs of all three substances, whereas prefrontal regions displayed indicators of volume recovery only in FRUs of cocaine.
Keywords: Brain volume, MRI, substance abuse, addiction, prefrontal cortex, striatum
INTRODUCTION
Neurogenesis and synaptic plasticity confer a capacity for repair and reorganization after certain kinds of neurological damage. A number of studies have investigated the brain's ability to recover from damage resulting from substance abuse. Studies have repeatedly linked substance abuse to decreased gray matter volume in abusers of alcohol(1, 2), cocaine(3, 4), cannabis(5, 6) and multiple other substances(7) in regions involved in the brain's reward and decision-making circuitry. These include subcortical structures such as the striatum(2, 3, 8), amygdala(1, 2, 8) and hippocampus(7, 8) (but see also(9, 10)), as well as prefrontal cortical structures(2, 4, 5, 11). At the same time, there is increasing evidence that abstinence from substance abuse can facilitate the recovery of volume lost during abuse. For instance, there are reported associations between abstinence from abuse and increased volume in regions of the prefrontal cortex(12, 13) (but see(2, 11, 14)), striatum(2), insula(2), and parietal lobe(13).
Yet, due to the variation in experimental designs and subject profiles between studies and the almost exclusive focus on individuals abstinent from alcohol, a number of important questions regarding abstinence-facilitated recovery still remain unanswered. First, it is unclear whether the capacity for volume recovery is uniform throughout all brain regions affected by substance abuse, or if recovery capacities, and perhaps recovery time courses, vary by region. Indeed, a synthesis of the extant literature suggests that the latter alternative may be the case. Though some studies that have examined relatively short periods of abstinence(13, 15) have found an association between abstinence and increased volume in the prefrontal cortex, several studies comparing brain volumes between abstinent substance abusers and healthy subjects find that parts of the prefrontal cortex still display lower volume in abstinent abusers after a period of abstinence(2, 11, 14). In contrast, findings related to subcortical structures suggest that these regions may have the capacity to recover volume to pre-abuse levels over a long period of abstinence(16). This pattern of findings suggests regional differences in volume recovery capacities during abstinence.
Furthermore, given that different substances have different modes of neurotoxicity(17, 18), it is not known whether recovery capacities might vary depending on the substance that was formerly abused. For instance, whereas cocaine is thought to cause cell death by eliciting uncontrolled autophagy via the nitric oxide-glyceraldehyde-3-phosphate dehydrogenase signaling pathway(17), reports suggest that alcohol causes cell death by increasing levels of proinflammatory cytokines and oxidative enzymes(18). Given the predominant focus on abstinent alcohol abusers, there is presently insufficient data in the literature to address whether recovery depends on the substance abused.
The current study uses voxel-based morphometry in a prison inmate sample (n=107) of long-term abstinent former regular users (FRUs) and former light users (FLUs) of alcohol, cocaine, and/or cannabis in order to examine whether recovery capacities might be region-specific and substance-specific. Since in a cross-sectional design volume recovery cannot be measured directly, we examined two potential indicators of volume recovery: 1) a positive correlation between volume and abstinence duration in FRUs, and 2) the absence of lower volume in FRUs compared to FLUs.
METHODS and MATERIALS
Participants
Participants (n=124) from a medium-security Wisconsin correctional facility were selected based on the following inclusion criteria: age less than 45 years; IQ greater than 70; no history of psychosis or bipolar disorder; no history of significant head injury or post-concussion symptoms; no current use of psychotropic medications; and completed interview assessments for substance use and psychopathy (see below). Informed consent was obtained both orally and in writing. Out of these 124 participants, 15 subjects who reported never having used any of the three substances of interest (alcohol, cocaine, or cannabis) were excluded because they had no history of substance use and thus no period of abstinence. Of the remaining 109 subjects, two were excluded due to nonsensical self-report data (i.e., negative abstinence durations), leaving a final sample of 107. Supersets of this sample have been used in previous reports from our group on psychopathy(19, 20).
Substance Use and Abstinence Assessment
Substance use and abstinence data were obtained using the Addiction Severity Index (ASI)(21), which measures subjects' histories with a range of substances of abuse. Subjects in this sample were labeled as former regular users (FRUs) of a substance if they met the ASI criterion for “regular use”, which constitutes use of a substance at least three times a week (usually to the point of intoxication or to the point where it compromises other normal activities), or use during two-day binges. Subjects who reported past use of a substance, but did not meet the criteria for “regular use”, were labeled as former light users (FLUs) of that substance. Subject characteristics, including substance abuse and abstinence data for the three primary substances of abuse examined here, are summarized in Table 1. While the ASI provides data on the history of use of a range of different substances (i.e., alcohol, cocaine, cannabis, heroin, nicotine, methamphetamine, amphetamine, hallucinogens, inhalants, methadone, and other opiates), we limited our investigation to substances that allowed both FRU and FLU groups of at least 15 subjects each: alcohol (62 FRUs, 45 FLUs), cocaine (25 FRUs, 20 FLUs) and cannabis (80 FRUs, 17 FLUs) Among FRUs of alcohol, 30.6% were also FRUs of cocaine and 83.9% were also FRUs of cannabis; among FRUs of cocaine, 76.0% were also FRUs of alcohol and 96.0% were also FRUs of cannabis; among FRUs of cannabis, 62.5% were also FRUs of alcohol and 28.8% were also FRUs of cocaine (see Table S1). A total of n=18 subjects were FRUs of all three substances.
Table 1.
Participant characteristics
| All (n=107) | Alcohol (n=107) | Cocaine (n=45) | Cannabis (n=97) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| FRUs (n=62) | FLUs (n=45) | p | FRUs (n=25) | FLUs (n=20) | p | FRUs (n=80) | FLUs (n=17) | p | ||
| Age | 31.4 (7.4) | 31.3 (8.1) | 31.5 (6.4) | 0.93 | 33.9 (8.0) | 29.8 (9.5) | 0.12 | 30.2 (6.8) | 35.4 (8.3) | <.01 |
| IQ | 85.0 (34.7) | 85.8 (35.1) | 84.0 (35.0) | 0.80 | 90.1 (35.6) | 91.3 (32.3) | 0.91 | 81.9 (37.8) | 90.6 (26.3) | 0.37 |
| Factor 2 score | 13.4 (4.0) | 13.7 (3.8) | 12.9 (4.3) | 0.32 | 14.6 (3.2) | 13.4 (4.0) | 0.27 | 14.2 (3.5) | 10.9 (3.9) | <.01 |
| Abstinence Duration (months) | N/A | 72.7 (62.4) | 85.4(77.7) | 0.35 | 98.4 (77.5) | 95.0 (101.2) | 0.90 | 66.9 (60.0) | 187.8 (108.3) | <.01 |
| Duration of Abuse (months) | N/A | 77.2 (62.6) | N/A | N/A | 50.1 (68.5) | N/A | N/A | 108.7 (71.3) | N/A | N/A |
| Age of First Use | N/A | 12.4 (3.0) | 14.4 (3.4) | <.01 | 17.4 (3.0) | 20.0 (5.3) | 0.0 5 | 12.8 (2.7) | 15.4 (1.5) | <.01 |
| Total Intracranial Volume (mL) | 1510.9 (129.1) | 1514.1 (124.6) | 1506.6 (136.4) | 0.78 | 1508.2 (108.2) | 1514.5 (133.0) | 0.86 | 1520.8 (129.9) | 1462.0 (135.0) | 0.10 |
| Race, % (n) | ||||||||||
| Caucasian | 54(58) | 60(37) | 47(21) | 0.10a | 80(20) | 75(15) | 0.84a | 49(39) | 59(10) | 0.68a |
| African-American | 43(46) | 40(25) | 47(21) | 16(4) | 25(5) | 48(38) | 41(7) | |||
| Hispanic | 2(2) | 0(0) | 4(2) | 0(0) | 0(0) | 3(2) | 0(0) | |||
| Native American | 1(1) | 0(0) | 2(1) | 4(1) | 0(0) | 1(1) | 0(0) | |||
All data are Mean (SD) except where noted.
pa: Fisher's Exact Test
Abstinence durations for both FRUs and FLUs of each substance were calculated by subtracting each subject’s self-reported last date of use of the substance from the date of the subject’s MRI scan. Distinct abstinence durations were calculated for alcohol (mean=6.1 years in FRUs; range= 0.8 to 20.1 years in FRUs), cocaine (mean=8.2 years in FRUs; range=1.2 to 26.2 years in FRUs), and cannabis (mean=5.5 years in FRUs; range=0.3 to 21.8 years in FRUs). Mean abstinence duration was not significantly different between FRUs of alcohol and cocaine (p=0.19) or FRUs of alcohol and cannabis (p=0.58), but was significantly different between FRUs of cocaine and cannabis (p=0.04).
MRI Acquisition and Preprocessing
MRI data were acquired with the Mind Research Network’s Siemens 1.5T Avanto Mobile MRI System equipped with a 12-channel head coil. All participants underwent scanning on correctional facility grounds. A high-resolution T1-weighted structural image was acquired for each subject using a four-echo magnetization-prepared rapid gradient-echo sequence (TR=2530 ms; TE=1.64, 3.5, 5.36 and 7.22 ms; flip angle=7°; FOV=256x256 mm2; matrix=128x128; slice thickness=1.33 mm; no gap; voxel size=1x1x1.33 mm3;128 interleaved sagittal slices). All four echoes were averaged into a single high-resolution image(22). Preprocessing and analyses of structural MRI data were conducted in Statistical Parametric Mapping software (SPM12; http://www.fil.ion.ucl.ac.uk/spm). Preprocessing consisted of manual realignment of T1 images; segmentation into gray matter, white matter, and cerebrospinal fluid; normalization to Montreal Neurological Institute (MNI)-152 space; modulation after normalization to preserve volume; and smoothing with an 8mm full-width at half-maximum (FWHM) Gaussian kernel(23).
Analytic Strategy
In order to disentangle the effects of different substances, we partitioned the sample into three overlapping sets and examined each separately. The first set consisted of FRUs and FLUs of alcohol, the second of FRUs and FLUs of cocaine, and the third of FRUs and FLUs of cannabis. Within each set of subjects, two main analyses were performed to examine indicators of volume recovery. First, the relation between gray matter volume and duration of abstinence from the substance of interest was assessed; second, gray matter volume was compared between FRUs and FLUs of the substance.
We also conducted group-by-abstinence duration interaction analyses for each set of subjects, allowing us to assess whether there was a statistically significant difference in the way that volume was associated with abstinence duration between the FRU and FLU groups for each substance.
We first conducted these analyses using a voxel-wise, region-of-interest (ROI)-based approach. We used the following ROIs that previous studies have shown display lower volume in association with substance abuse: nucleus accumbens(2), putamen(24), caudate(3), globus pallidus(25), amygdala(1), hippocampus(7), medial orbitofrontal cortex (mOFC)(14), dorsolateral prefrontal cortex (DLPFC)(15), and anterior cingulate cortex (ACC)(4). ROIs were generated from Individual Brain Atlases using Statistical Parametric Mapping (IBASPM) (http://www.thomaskoenig.ch/Lester/ibaspm.htm) in the Wake Forest University (WFU) Pick Atlas Toolbox. Effects were assessed using peak-height, corrected for multiple comparisons using a family-wise error (FWE) rate of pFWE<0.05. Significance was also assessed relative to a Bonferroni corrected level of pFWE ≤0.006 to account for the nine ROIs examined.
To investigate potential relationships outside of these a priori ROIs, we then repeated these analyses using an exploratory voxel-wise whole-brain approach, evaluating significance with cluster thresholding. We used a less conservative uncorrected threshold for these analyses (p<0.01 uncorrected) which we corrected for multiple comparisons by conducting a Monte Carlo simulation using AlphaSim(26) to determine a 758 voxel extent threshold for p<0.05 corrected.
Several potentially confounding sources of variance existed in this sample, requiring the use of models with covariates accounting for differences in substance use history (i.e., duration of abuse; age of first use), differences in psychopathy severity, and most importantly, the fact that many subjects were FRUs of multiple substances (see Covariates). To ensure that findings were not highly sensitive to the model construction we repeated the main ROI and whole-brain analyses with “slimmed” models that only included age, race, and intracranial volume as covariates. Additionally, to further reduce the complexity of the statistical analyses, we examined the association between abstinence duration and gray matter volume independent of the substance abused. For this analysis, we pooled all subjects who met the criterion for regular use of alcohol, cocaine, or cannabis into one group (n=93) and used a simple model in which gray matter volume was regressed on abstinence duration controlling for age, race, and intracranial volume. Abstinence duration values from subjects who were regular users of only one substance were taken from the abstinence duration from that substance; for subjects who were regular users of more than one substance, the lowest (most recent) abstinence duration among the substances was used.
Covariates
As volumetric analyses require a control for individual variation in overall brain size, we included total intracranial volume (ICV; gray matter (GM) + white matter (WM) + cerebrospinal fluid (CSF)) as a covariate in all models. Neither GM, WM, CSF, nor ICV correlated with the duration of abstinence from any of the three substances, and neither GM, WM, CSF, nor ICV was significantly different between FRUs and FLUs of any of the three substances. Age was included as a covariate in all models as well because GM has been shown to decrease with age during adulthood(27). We found the expected negative correlation between age and GM in each of the three subgroups of participants (Alcohol: r=−.50, p<0.001; Cocaine: r=−.56, p=0.027; Cannabis: r=−.41, p<0.001), as well as the expected positive correlations between age and CSF (Alcohol: r=.26, p<0.041; Cocaine: r=.54, p=0.006; Cannabis: r=.34, p=0.002). Furthermore, age was strongly positively correlated with abstinence duration (Alcohol: r=.59, p<0.001; Cocaine: r=.36, p=0.073; Cannabis: r=.50, p<0.001).
In a previous report on a superset of this sample(19) gray matter volume of the striatum and prefrontal cortex was shown to increase with the Factor 2 (impulsive-antisocial) dimension of psychopathy as measured by the PCL-R(28) (Supplementary Methods). Since the striatum and prefrontal cortex are primary regions of interest in this study, Factor 2 score was included as a covariate in all full models. Race has also been shown to significantly relate to gray matter volume in this sample(19), so race, coded as Caucasian or non-Caucasian, was also included as a covariate in all models. Furthermore, substance use has been shown in a sample of rats to have differential effects and degrees of severity depending on the age of the user(29). Thus, for all full models, the age of first use of the substance of interest was included as a covariate.
In addition, covariates were included in the full models to rule out the influence of other substances. These covariates were coded as binary variables marking each subject as either a FRU or non-FRU (defined as either a FLU or non-user) of each other substance. In addition to the three substances of interest here, the ASI includes data on subjects' use of at least eight other substances. Thus, in order to simplify the model we used a statistical diagnostic to select covariates marking FRU or non-FRU only for substances that were most likely to influence group differences (for between-group analyses) or individual differences (for within-group analyses). The statistical diagnostics used to select these other substance covariates are discussed in the Supplementary Methods.
Given the large number of covariates in the full models, we ensured that problematic levels of multicollinearity were not present among the variables. We present the variance inflation factor (VIF) and tolerance for each variable in a representative within-FRU analysis for each substance that regresses putamen volume on abstinence duration of the substance of interest (Table S2). VIF was between 1 and 2 for most variables and was between 2 and 4 for the age and abstinence duration variables (which, as reported earlier, were highly correlated within each substance set), indicating somewhat elevated but not problematic levels of multicollinearity between these variables(30). Furthermore, the beta coefficients reveal that these two variables pick up substantively different variance in the models, as the beta coefficients for age were negative in all models (the expected correlation between age and gray matter volume), whereas the beta coefficients for abstinence duration were positive (Table S2). In other words, there was a negative association between age and brain volume, but a positive association between abstinence duration and brain volume.
RESULTS
Alcohol
Within-group ROI analyses using the full model revealed that within FRUs, but not within FLUs, the duration of abstinence positively correlated with volume in the right and left nucleus accumbens, right putamen, right globus pallidus, and right amygdala. The relationships in the right putamen and right amygdala were also observed in the slimmed model, which included only age, race, and intracranial volume as covariates. Group-by-abstinence duration interaction analyses did not reveal any significant relationships. See Table 2.
Table 2.
ROI Analyses: Positive associations between volume and abstinence duration
| Full Model (FRUs) | Slimmed Model (FRUs) | Interaction | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Region | Peak Coordinates | t value | pFWE | Peak Coordinates | t-value | pFWE | Peak Coordinates | t-value | pFWE | |
| Alcohol | R Nucleus Accumbens | (14, 6, −8) | 3.14 | 0.013 | ||||||
| L Nucleus Accumbens | (−16, 10, −15) | 2.82 | 0.023 | |||||||
| R Putamen | (20, 15, 0) | 3.94 | 0.010 | (32, −18, −9) | 3.43 | 0.034 | ||||
| R Globus Pallidus | (16, 10, −3) | 3.34 | 0.020 | |||||||
| R Amygdala | (27, 0, −18) | 3.06 | 0.025 | (27, −2, −15) | 3.08 | 0.023 | ||||
| (24, −9, −21) | 2.75 | 0.048 | ||||||||
| R Hippocamp | (33, −20, −9) | 3.62 | 0.026 | |||||||
| (27, −16, −21) | 3.43 | 0.043 | ||||||||
| (32, −16, − 12) | 3.39 | 0.047 | ||||||||
| Cocaine | L Nucleus Accumbens | (−12, 6, −15) | 3.63 | 0.011 | ||||||
| R Nucleus Accumbens | (6, 10, −10) | 3.78 | 0.011 | (8, 12, −10) | 3.05 | 0.028 | (18, 6, −12) | 3.59 | 0.006* | |
| (16, 6, −15) | 3.68 | 0.01 | (8, 12, −12) | 3.12 | 0.017 | |||||
| R Putamen | 3 | (28, 0, −6) | 3.82 | 0.019 | ||||||
| (21, 6, −12) | 3.75 | 0.022 | ||||||||
| R Globus Pallidus | (27, 0, −6) | 3.81 | 0.008 | |||||||
| R Hippocampus | (21, −6, −22) | 4.88 | 0.015 | |||||||
| R Amygdala | (22, 0, −18) | 4.95 | 0.003* | (27, −2, −14) | 3.30 | 0.018 | ||||
| R Medial OFC | (3, 10, −10) | 5.59 | 0.003* | (6, 8, −14) | 3.30 | 0.028 | ||||
| (3, 18, −14) | 4.03 | 0.039 | ||||||||
| L DLPFC | (−30, 58, 6) | 6.62 | 0.010 | |||||||
| (−16, 68, 0) | 5.49 | 0.048 | ||||||||
| Cannabis | R Globus Pallidus | (22, 6, 2) | 2.98 | 0.038 | ||||||
| (24, −2, 6) | 2.98 | 0.039 | ||||||||
| R Putamen | (24, 9, 0) | 3.38 | 0.032 | |||||||
Significant after Bonferroni correction for multiple comparisons (p ≤ 0.006)
Between-group ROI analyses using the full model revealed lower volume in the left DLPFC in FRUs compared to FLUs. This relationship was not observed in the slimmed model. See Table 3.
Table 3.
ROI Analyses: Regions of less volume in Former Regular Users compared to Former Light Users
| Full Model | ||||
|---|---|---|---|---|
| Region | Peak Coordinates | t-value | pFWE | |
| Alcohol | L DLPFC | (−51, 20, 24) | 4.47 | 0.011 |
| Cannabis | L Medial OFC | (−4, 20, −16) | 4.54 | 0.001* |
| L Medial OFC | (−6, 58, −12) | 3.37 | 0.028 | |
| L Medial OFC | (−2, 30, −22) | 3.23 | 0.040 | |
| R Medial OFC | (2, 18, −14) | 3.94 | 0.006* | |
| R Medial OFC | (4, 48, −14) | 3.86 | 0.008 | |
| R ACC | (2, 20, −10) | 3.79 | 0.018 | |
| L ACC | (−2, 20, −10) | 3.75 | 0.020 | |
Significant after Bonferroni correction for multiple comparisons (p ≤ 0.006)
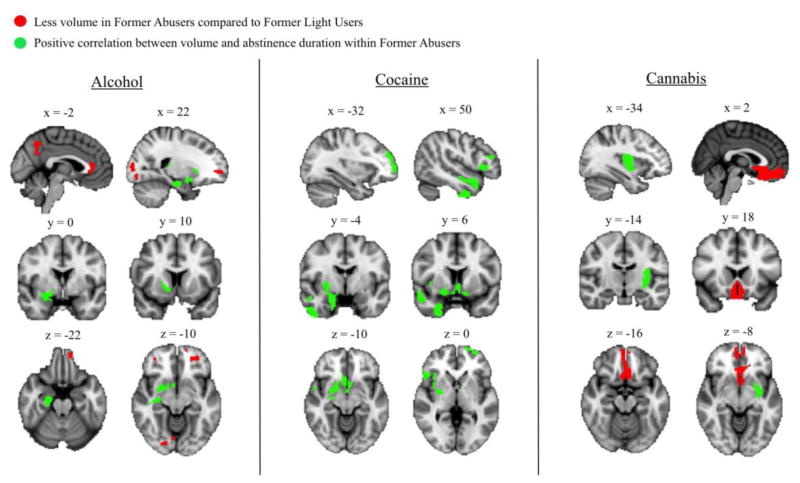
Exploratory whole-brain analyses corroborated the ROI findings (Figures 1–2, Table 4) and also found regions of lower volume in the mOFC, ACC, posterior cingulate, precuneus, and occipital lobe in FRUs compared to FLUs (Figure 1, Table 5).
Figure 1.
Results of whole-brain analyses separated by substance set. Red clusters indicate areas where volume is significantly (k>758; p<0.05 corrected) less in former regular users (FRUs) compared to former light users (FLUs) of the substance. Green clusters indicate areas where volume significantly (k>758; p<0.05 corrected) positively correlates with abstinence duration within FRUs of the substance.
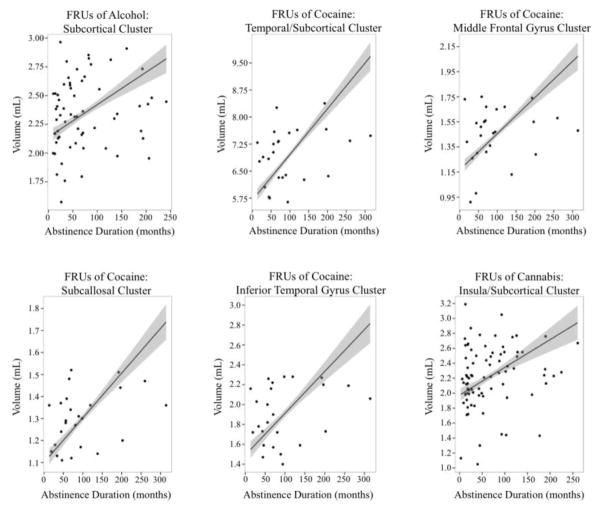
Figure 2.
Plots of the relationship between abstinence duration and volume within the significant clusters identified in the full model whole-brain analyses for FRUs of each substance.
Table 4.
Whole-Brain Analyses: Positive associations between volume and abstinence duration
| Region at Peak Coordinates | Other Regions in Cluster | Peak Coordinates | Cluster Size | |
|---|---|---|---|---|
| Alcohol | R Putamen | R Amygdala R Hippocampus R Globus Pallidus R Accumbens R Parahippocampal Gyrus R Thalamus |
(20, 15, 0) | 1081 |
| Cocaine | R Temporal Pole | R Putamen L Accumbens R Amygdala R Hippocampus R Parahippocampal Gyrus R Middle Temporal Gyrus R Superior Temporal Gyrus |
(38, 12, −38) | 4527 |
| L Anterior Middle Frontal Gyrus |
(−30, 58, 6) | 892 | ||
| R Subcallosal Cortex |
L Subcallosal Cortex | (3, 12, −10) | 862 | |
| R Anterior Inferior Temporal Gyrus |
(52, −16, −39) | 1203 | ||
| Cannabis | L Insular Cortex | L Putamen | (−34, −16, 14) | 1520 |
Table 5.
Whole-Brain Analyses: Regions of less volume in Former Regular Users compared to Former Light Users
| Region at Peak Coordinates | Other Regions in Cluster | Peak Coordinates | Cluster Size | Percent less volume in Cluster | |
|---|---|---|---|---|---|
| Alcohol | L Medial Orbitofrontal Cortex |
L Anterior Cingulate Gyrus | (−10, 52, −26) | 1858 | 18.5% |
| R Superior Lateral Occipital Cortex |
R Angular Gyrus R Middle Temporal Gyrus |
(45, −70, 27) | 973 | 13% | |
| R Middle Frontal Gyrus |
(36, 36, 26) | 1175 | 18.2% | ||
| R Precuneus | R Posterior Cingulate Gyrus L Precuneus L Posterior Cingulate Gyrus |
(8, −54, 22) | 1761 | 8.7% | |
| R Lingual Gyrus |
R Occipital Fusiform Gyrus R Lateral Occipital Cortex |
(15, −87, −3) | 1173 | 12.5% | |
| Cannabis | L Subcallosal Cortex |
R Anterior Cingulate Gyrus L Medial Orbitofrontal Cortex R Medial Orbitofrontal Cortex R Subcallosal Cortex |
(−4, 20, −16) | 3160 | 12.6% |
Cocaine
Within-group ROI analyses using the full model revealed that within the FRU group, but not within the FLU group, the duration of abstinence positively correlated with volume in the right and left nucleus accumbens, right hippocampus, right amygdala, right mOFC, left DLPFC, and right and left ACC. The relationship in the right nucleus accumbens was also observed in the slimmed model. Group-by-abstinence duration interaction analyses revealed significant relationships in right nucleus accumbens, right putamen, right globus pallidus, right amygdala and right mOFC, indicating that the relationship between abstinence duration and volume in these regions was stronger in FRUs than in FLUs. See Table 2.
Between-group ROI analyses using both the full model and slimmed model did not reveal any regions of lower volume in FRUs compared to FLUs.
Exploratory whole-brain analyses corroborated the ROI findings and also found positive relationships between abstinence duration and volume in the temporal lobe in FRUs but not FLUs (Figures 1–2, Table 4); furthermore, group-by-abstinence duration interaction analysis revealed a significant cluster that included areas of the right striatum, amygdala, hippocampus, parahippocampal gyrus, insula, and subcallosal cortex (Figure 3, Table S3).
Figure 3.
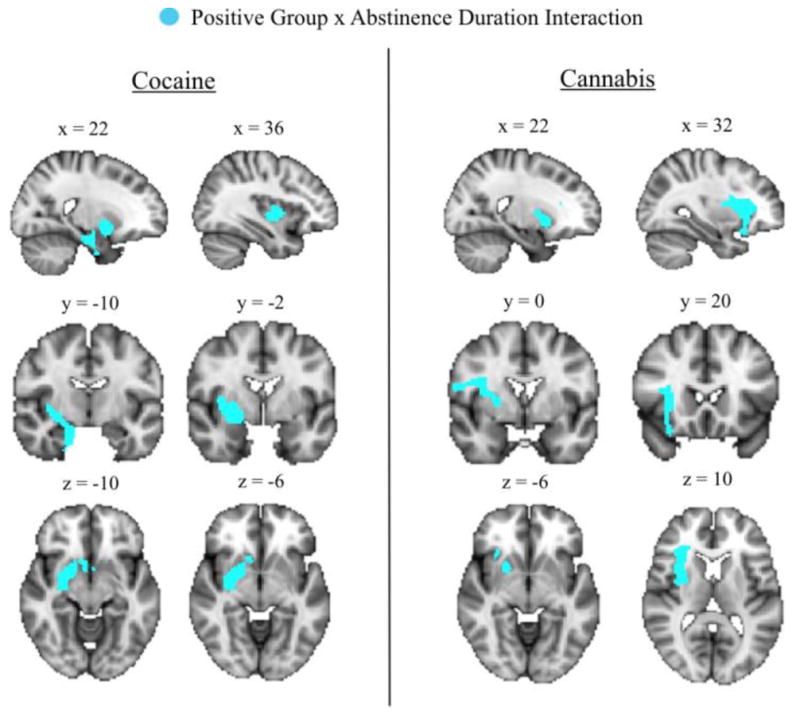
Results of whole-brain group-by-abstinence duration interaction analyses separated by substance set. Light blue cluster indicate areas where the positive correlation between volume and abstinence duration was significantly (k>758; p<0.05 corrected) stronger in FRUs compared to FLUs. No significant interaction clusters were identified in the alcohol group.
Cannabis
Within-group ROI analyses using both the full model and slimmed model did not reveal any significant relationships between abstinence duration and volume in FRUs or FLUs. Group-by-abstinence duration interaction analyses revealed significant relationships in right putamen and right globus pallidus, indicating that the relationship between abstinence duration and volume in these regions was stronger in FRUs than in FLUs. See Table 2.
Between-group ROI analyses using the full model revealed lower volume in the right and left mOFC and right and left ACC (Table 3). These relationships were not observed in the slimmed model.
Exploratory whole-brain analyses corroborated the ROI findings and also found a positive correlation between duration of abstinence and volume in the putamen and insula in FRUs but not FLUs (Figures 1–2, Tables 4–5); furthermore, group-by-abstinence duration interaction analysis revealed a significant cluster that included areas of the right interior insula, striatum, inferior frontal gyrus, orbitofrontal cortex, and precentral gyrus (Figure 3, Table S3).
Results from whole-brain analyses using the slimmed model are presented in the Supplementary Results and Table S4.
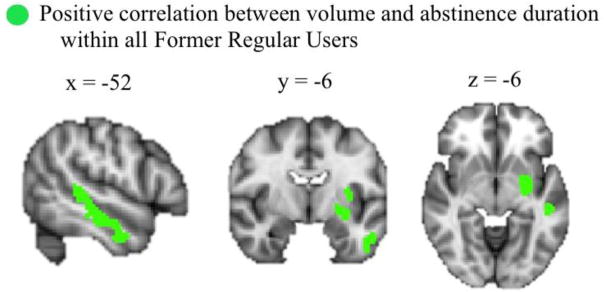
All FRUs
Within-group ROI analyses revealed a positive correlation between duration of abstinence and volume in the left globus pallidus with peak coordinates (−27, −12, −4); t=2.93, pFWE=0.042. Exploratory whole-brain analyses corroborated the ROI findings and also found a positive correlation between duration of abstinence and volume in the putamen, insula, and temporal lobe (Figure 4, Table S5).
Figure 4.
Results of whole-brain analysis for all former regular users. Green clusters indicate areas where volume significantly (k>758; p<0.05 corrected) positively correlates with abstinence duration.
Follow-up Analyses
In recognition of the large sample overlap – particularly between the alcohol FRU and cannabis FRU groups – we conducted follow-up analyses using “clean”, non-overlapping subsamples of alcohol FRUs who were not FRUs of either cocaine or cannabis (n=9) and cannabis FRUs who were not FRUs of either cocaine or alcohol (n=25). A “clean” cocaine subsample was not available (n=0). The results from the “clean” alcohol FRU group were generally consistent with the findings from the larger alcohol group, and the results from the “clean” cannabis FRU group were generally consistent with the findings from the larger cannabis group (see Supplementary Results; Tables S6–S7; Figure S1). These findings support the conclusion that the main models were largely successful in isolating substance-specific effects.
DISCUSSION
In an incarcerated sample of long-term abstinent former regular substance users, we found that positive correlations between volume and abstinence duration in FRUs and absence of lower volume in FRUs compared to FLUs – potential indicators of volume recovery – were observed in subcortical structures in relation to all three substances, but were only observed in prefrontal regions in relation to cocaine.
First, these findings suggest that subcortical structures may have a more robust capacity to recover volume during long-term abstinence than prefrontal regions. This finding is consistent with at least one study that found an absence of lower subcortical volume in long-term abstinent alcoholics compared to nonalcoholics(16) – in line with the interpretation that subcortical structures may recover volume to pre-abuse levels after long-term abstinence – and another study which found that volume in subcortical but not prefrontal regions positively correlated with duration of abstinence in former alcoholics(2).
However, the present findings also suggest that despite the lack of indicators of prefrontal volume recovery in FRUs of alcohol and cannabis, prefrontal volume recovery may occur in FRUs of cocaine. While evidence for a degree of prefrontal volume recovery after short-term abstinence has previously been observed in both alcohol(13, 15) and cocaine(31), our results suggest that continuing long-term prefrontal volume recovery may only be sustained after cocaine abuse. Furthermore, though some studies find lower prefrontal volume in FRUs of cocaine after short-term abstinence compared to non-abusers(11), this does not preclude the possibility that volume may return to pre-abuse levels after longer-term abstinence. Nonetheless, it is important to note the smaller group sizes for the cocaine FRUs and FLUs compared to the other substance groups, and that the absence of group differences here may be partially attributable to a lack of power.
The interpretation of the results from this study requires consideration of several additional limitations. The cross-sectional nature of our data precludes the conclusion that any relationships observed here between abstinence duration and volume are causal. Relatedly, the absence of data chronicling how the brains of these subjects changed during active substance abuse prevents us from definitively concluding that these relationships reflect the recovery of volume lost during substance abuse. However, two observations support these interpretations. First, most of the areas identified here in which volume positively correlated with abstinence duration are regions that previous studies have identified as those associated with volume loss during active substance abuse. Second, we observed significant correlations between volume and abstinence duration only within the FRU groups, and never within the FLU groups. Taken together, the fact that the volume-abstinence duration relationships were found in regions known to experience volume loss during substance abuse – and were present only within former regular substance users – suggests that the phenomenon being reflected in the data may be an abstinence-facilitated recovery of volume lost during abuse.
Another important consideration is that the abstinence durations of the subjects in this study are relatively long (5–8 years, on average). While this allows us to make inferences about long-term, sustained volume recovery, we are unable to detect possible instances of recovery with shorter time courses. It may be that some regions, such as the prefrontal cortex, recover volume primarily during the first few weeks or months of abstinence(32), but then do not experience appreciable recovery thereafter. Relatedly, it is important to note that while mean abstinence duration was greater than 5.5 years for all three substances, mean abstinence duration from cocaine (8.2 years) was significantly longer than that from cannabis (5.5 years). This difference is unlikely to have introduced variability across substances in whether certain regions were correlated with abstinence duration, but may have introduced some variability across substances in whether certain regions had lower volume in FRUs compared to FLUs.
A further issue – one that complicates the interpretation of the between-group findings – is that previous studies provide evidence for preexisting differences between the brains of individuals who go on to abuse substances and those who do not. For instance, Cheetham and colleagues (2014)(33) found that alcohol-related problems at age 16 are predicted by smaller volume in the paralimbic anterior cingulate cortex at age 12. Thus, it is possible that our observation of lower prefrontal volume in FRUs of alcohol and cannabis compared to FLUs may not entirely reflect a lack of robust recovery in this region, but partly or wholly reflect a preexisting difference in which drug abusers have smaller prefrontal volumes at baseline than non-abusers. Indeed, it is even possible that prefrontal volume in these FRU groups have recovered volume to baseline, but that recovery occurred and ended during the early periods of abstinence.
Another limitation of this study is that a significant number of subjects were FRUs of multiple substances, making it difficult to definitively disentangle the effects of abuse and abstinence for individual substances, and requiring the use of models with numerous covariates. Furthermore, because subjects who were regular users of multiple substances were placed in multiple FRU groups, these groups were not statistically independent. In particular, there was substantial overlap between the alcohol and cannabis groups. While statistical methods were applied to isolate the effects of individual substances to the greatest extent possible, and follow-up analyses using “clean” subsamples helped to confirm that isolation of substance-specific effects had been largely successful, follow-up studies could aim to recruit independent samples of abusers of single substances.
Despite these limitations, this study is the first to compare the relationship between abstinence duration and gray matter volume across FRUs of multiple substances, and has provided evidence that these relationships are both region-specific and substance-specific. An important implication of these findings is that abuse of certain substances – particularly alcohol and cannabis – may result in longer lasting and perhaps more permanent volume loss than others, especially in the prefrontal cortex. As such, substance abuse treatment programs for these substances may wish to emphasize rehabilitation strategies – both behavioral and pharmaceutical – that target the prefrontal cortex and the cognitive functions that it subserves.
Supplementary Material
Acknowledgments
We thank the many individuals at the Wisconsin Department of Corrections who made this research possible, and are especially indebted to Warden Judy Smith, Warden Randy Hepp, and Dr. Kevin Kallas. This work was supported by grants from the National Institutes of Health [grant numbers MH070539, DA026505, MH087525, MH090169].
Footnotes
FINANCIAL DISCLOSURES
The authors report no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Fein G, Landman B, Tran H, McGillivray S, Finn P, Barakos J, et al. Brain atrophy in long-term abstinent alcoholics who demonstrate impairment on a simulated gambling task. Neuroimage. 2006;32:1465–1471. doi: 10.1016/j.neuroimage.2006.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Makris N, Oscar-Berman M, Jaffin SK, Hodge SM, Kennedy DN, Caviness VS, et al. Decreased volume of the brain reward system in alcoholism. Biol Psychiatry. 2008;64:192–202. doi: 10.1016/j.biopsych.2008.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barros-Loscertales A, Garavan H, Bustamante JC, Ventura-Campos N, Llopis JJ, Belloch V, et al. Reduced striatal volume in cocaine-dependent patients. Neuroimage. 2011;56:1021–1026. doi: 10.1016/j.neuroimage.2011.02.035. [DOI] [PubMed] [Google Scholar]
- 4.Franklin TR, Acton PD, Maldjian JA, Gray JD, Croft JR, Dackis CA, et al. Decreased gray matter concentration in the insular, orbitofrontal, cingulate, and temporal cortices of cocaine patients. Biol Psychiat. 2002;51:134–142. doi: 10.1016/s0006-3223(01)01269-0. [DOI] [PubMed] [Google Scholar]
- 5.Battistella G, Fornari E, Annoni JM, Chtioui H, Dao K, Fabritius M, et al. Long-Term Effects of Cannabis on Brain Structure. Neuropsychopharmacology. 2014;39:2041–2048. doi: 10.1038/npp.2014.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lorenzetti V, Lubman DI, Whittle S, Solowij N, Yucel M. Structural MRI findings in long-term cannabis users: what do we know? Subst Use Misuse. 2010;45:1787–1808. doi: 10.3109/10826084.2010.482443. [DOI] [PubMed] [Google Scholar]
- 7.Thompson PM, Hayashi KM, Simon SL, Geaga JA, Hong MS, Sui YH, et al. Structural abnormalities in the brains of human subjects who use methamphetamine. Journal of Neuroscience. 2004;24:6028–6036. doi: 10.1523/JNEUROSCI.0713-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wrase J, Makris N, Braus DF, Mann K, Smolka MN, Kennedy DN, et al. Amygdala volume associated with alcohol abuse relapse and craving. Am J Psychiatry. 2008;165:1179–1184. doi: 10.1176/appi.ajp.2008.07121877. [DOI] [PubMed] [Google Scholar]
- 9.Das D, Cherbuin N, Anstey KJ, Sachdev PS, Easteal S. Lifetime cigarette smoking is associated with striatal volume measures. Addict Biol. 2012;17:817–825. doi: 10.1111/j.1369-1600.2010.00301.x. [DOI] [PubMed] [Google Scholar]
- 10.Chang L, Alicata D, Ernst T, Volkow N. Structural and metabolic brain changes in the striatum associated with methamphetamine abuse. Addiction. 2007;102(Suppl 1):16–32. doi: 10.1111/j.1360-0443.2006.01782.x. [DOI] [PubMed] [Google Scholar]
- 11.Fein G, Di Sclafani V, Meyerhoff DJ. Prefrontal cortical volume reduction associated with frontal cortex function deficit in 6-week abstinent crack-cocaine dependent men. Drug Alcohol Depend. 2002;68:87–93. doi: 10.1016/s0376-8716(02)00110-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.O'Neill J, Cardenas VA, Meyerhoff DJ. Effects of abstinence on the brain: quantitative magnetic resonance imaging and magnetic resonance spectroscopic imaging in chronic alcohol abuse. Alcohol Clin Exp Res. 2001;25:1673–1682. [PubMed] [Google Scholar]
- 13.Cardenas VA, Studholme C, Gazdzinski S, Durazzo TC, Meyerhoff DJ. Deformation-based morphometry of brain changes in alcohol dependence and abstinence. Neuroimage. 2007;34:879–887. doi: 10.1016/j.neuroimage.2006.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tanabe J, Tregellas JR, Dalwani M, Thompson L, Owens E, Crowley T, et al. Medial orbitofrontal cortex gray matter is reduced in abstinent substance-dependent individuals. Biol Psychiatry. 2009;65:160–164. doi: 10.1016/j.biopsych.2008.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pfefferbaum A, Sullivan EV, Mathalon DH, Shear PK, Rosenbloom MJ, Lim KO. Longitudinal changes in magnetic resonance imaging brain volumes in abstinent and relapsed alcoholics. Alcohol Clin Exp Res. 1995;19:1177–1191. doi: 10.1111/j.1530-0277.1995.tb01598.x. [DOI] [PubMed] [Google Scholar]
- 16.Sameti M, Smith S, Patenaude B, Fein G. Subcortical Volumes in Long-Term Abstinent Alcoholics: Associations With Psychiatric Comorbidity. Alcoholism-Clinical and Experimental Research. 2011;35:1067–1080. doi: 10.1111/j.1530-0277.2011.01440.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guha P, Harraz MM, Snyder SH. Cocaine elicits autophagic cytotoxicity via a nitric oxide-GAPDH signaling cascade. P Natl Acad Sci USA. 2016;113:1417–1422. doi: 10.1073/pnas.1524860113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Crews FT, Nixon K. Mechanisms of neurodegeneration and regeneration in alcoholism. Alcohol Alcohol. 2009;44:115–127. doi: 10.1093/alcalc/agn079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Korponay C, Pujara M, Deming P, Philippi C, Decety J, Kosson D, et al. Impulsive-antisocial dimension of psychopathy linked to enlargement and abnormal functional connectivity of the striatum. Biol Psychiatry: CNNI. doi: 10.1016/j.bpsc.2016.07.004. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Philippi CL, Pujara MS, Motzkin JC, Newman J, Kiehl KA, Koenigs M. Altered Resting-State Functional Connectivity in Cortical Networks in Psychopathy. J Neurosci. 2015;35:6068–6078. doi: 10.1523/JNEUROSCI.5010-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McLellan AT, Luborsky L, Woody GE, O'Brien CP. An improved diagnostic evaluation instrument for substance abuse patients. The Addiction Severity Index. J Nerv Ment Dis. 1980;168:26–33. doi: 10.1097/00005053-198001000-00006. [DOI] [PubMed] [Google Scholar]
- 22.Ly M, Motzkin JC, Philippi CL, Kirk GR, Newman JP, Kiehl KA, et al. Cortical thinning in psychopathy. Am J Psychiatry. 2012;169:743–749. doi: 10.1176/appi.ajp.2012.11111627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ashburner J, Friston KJ. Voxel-based morphometry--the methods. Neuroimage. 2000;11:805–821. doi: 10.1006/nimg.2000.0582. [DOI] [PubMed] [Google Scholar]
- 24.Yip SW, DeVito EE, Kober H, Worhunsky PD, Carroll KM, Potenza MN. Pretreatment measures of brain structure and reward-processing brain function in cannabis dependence: an exploratory study of relationships with abstinence during behavioral treatment. Drug Alcohol Depend. 2014;140:33–41. doi: 10.1016/j.drugalcdep.2014.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chang L, Cloak C, Patterson K, Grob C, Miller EN, Ernst T. Enlarged striatum in abstinent methamphetamine abusers: a possible compensatory response. Biol Psychiatry. 2005;57:967–974. doi: 10.1016/j.biopsych.2005.01.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ward D. Simultaneous inference for fMRI data 2000 [Google Scholar]
- 27.Walhovd KB, Fjell AM, Reinvang I, Lundervold A, Dale AM, Eilertsen DE, et al. Effects of age on volumes of cortex, white matter and subcortical structures. Neurobiol Aging. 2005;26:1261–1270. doi: 10.1016/j.neurobiolaging.2005.05.020. discussion 1275–1268. [DOI] [PubMed] [Google Scholar]
- 28.Hare RD. The Hare psychopathy checklist-revised. 2. Toronto: Multi-Health Systems; 2003. [Google Scholar]
- 29.Crews FT, Braun CJ, Hoplight B, Switzer RC, 3rd, Knapp DJ. Binge ethanol consumption causes differential brain damage in young adolescent rats compared with adult rats. Alcohol Clin Exp Res. 2000;24:1712–1723. [PubMed] [Google Scholar]
- 30.Hair JFJ, Anderson RE, Tatham RL, Black WC. Multivariate Data Analysis. 3. New York: Macmillan; 1995. [Google Scholar]
- 31.Parvaz MA, Moeller SJ, d'Oleire Uquillas F, Pflumm A, Maloney T, Alia-Klein N, et al. Prefrontal gray matter volume recovery in treatment-seeking cocaine-addicted individuals: a longitudinal study. Addict Biol. 2016 doi: 10.1111/adb.12403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Durazzo TC, Mon A, Gazdzinski S, Yeh P-H, Meyerhoff DJ. Serial longitudinal magnetic resonance imaging data indicate non-linear regional gray matter volume recovery in abstinent alcohol-dependent individuals. Addict Biol. 2015;20:956–967. doi: 10.1111/adb.12180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cheetham A, Allen NB, Whittle S, Simmons J, Yucel M, Lubman DI. Volumetric differences in the anterior cingulate cortex prospectively predict alcohol-related problems in adolescence. Psychopharmacology (Berl) 2014;231:1731–1742. doi: 10.1007/s00213-014-3483-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.