Abstract
The CB2 cannabinoid agonist LY2828360 lacked both toxicity and efficacy in a clinical trial for osteoarthritis. Whether LY2828360 suppresses neuropathic pain has not been reported, and its signaling profile is unknown. In vitro, LY2828360 was a slowly acting but efficacious G protein–biased CB2 agonist, inhibiting cAMP accumulation and activating extracellular signal-regulated kinase 1/2 signaling while failing to recruit arrestin, activate inositol phosphate signaling, or internalize CB2 receptors. In wild-type (WT) mice, LY2828360 (3 mg/kg per day i.p. × 12 days) suppressed chemotherapy-induced neuropathic pain produced by paclitaxel without producing tolerance. Antiallodynic efficacy of LY2828360 was absent in CB2 knockout (KO) mice. Morphine (10 mg/kg per day i.p. × 12 days) tolerance developed in CB2KO mice but not in WT mice with a history of LY2828360 treatment (3 mg/kg per day i.p. × 12 days). LY2828360-induced antiallodynic efficacy was preserved in WT mice previously rendered tolerant to morphine (10 mg/kg per day i.p. × 12 days), but it was absent in morphine-tolerant CB2KO mice. Coadministration of LY2828360 (0.1 mg/kg per day i.p. × 12 days) with morphine (10 mg/kg per day × 12 days) blocked morphine tolerance in WT but not in CB2KO mice. WT mice that received LY2828360 coadministered with morphine exhibited a trend (P = 0.055) toward fewer naloxone-precipitated jumps compared with CB2KO mice. In conclusion, LY2828360 is a slowly signaling, G protein–biased CB2 agonist that attenuates chemotherapy-induced neuropathic pain without producing tolerance and may prolong effective opioid analgesia while reducing opioid dependence. LY2828360 may be useful as a first-line treatment in chemotherapy-induced neuropathic pain and may be highly efficacious in neuropathic pain states that are refractive to opioid analgesics.
Introduction
Morphine suppresses many types of pain, but tolerance, physical dependence, and unwanted side effects limit its clinical use (Trang et al., 2007). Identification of therapeutic strategies for blocking opioid tolerance and dependence has therefore evolved as an area of intense research interest (Habibi-Asl et al., 2014; Mansouri, et al., 2015; Hassanipour et al., 2016; Hosseinzadeh et al., 2016). Adjunctive pharmacotherapies that combine mechanistically distinct analgesics represent one such approach. Opioid and cannabinoid CB1 G protein–coupled receptors are often coexpressed in the central nervous system (CNS) (Pickel et al., 2004) and can functionally interact by receptor heterodimerization or signaling cross-talk (Bushlin et al., 2010). Although activation of both receptors produces analgesia, undesirable pharmacologic effects limit their use (Manzanares et al., 1999; Massi et al., 2001). An alternative approach aims at harnessing the therapeutic potential of cannabinoid CB2 receptors to suppress pathologic pain without producing CB1-mediated cannabimimetic effects (for review, see Guindon and Hohmann, 2008; Dhopeshwarkar and Mackie, 2014). CB2 receptors are primarily expressed on immune cells but may be induced in the CNS in response to injury (for review, see Mechoulam and Parker, 2013). Activation of cannabinoid CB2 receptors produces antinociceptive efficacy in many preclinical pain models without the unwanted side effects associated with CNS CB1 receptor activation. CB2 receptors have also been implicated in facilitating morphine antinociception in normal and inflammatory conditions (Lim et al., 2005; Merighi et al., 2012; Desroches et al., 2014); however, whether CB2 agonists suppress morphine tolerance or dependence in neuropathic pain models is unknown.
LY2828360 (Fig. 1) is a potent CB2 receptor agonist with similar affinity for human and rat CB2 receptors (Hollinshead et al., 2013). In a human CB2 functional assay, approximately 87% maximal stimulation of CB2 was observed at 20 nM concentrations, whereas only 15% maximal stimulation of CB1 was observed at 100 µM concentrations (Hollinshead et al., 2013). LY2828360 showed good CNS penetration and potent oral activity in a preclinical model of joint pain induced by intra-articular monoiodoacetic acid (Hollinshead et al., 2013). In the monoiodoacetic acid model, LY2828360 (0.3 mg/kg p.o.) produced a dose-related reversal of pain using incapacitance testing, demonstrating equivalent efficacy to the nonsteroidal anti-inflammatory drug diclofenac (Hollinshead et al., 2013). No specific risks or discomforts associated with LY2828360 were observed in patients with osteoarthritic pain who have taken LY2828360 up to a dose of 80 mg for 4 weeks (Pereira et al., 2013) (www.clinicaltrials.gov identifier: NCT01319929). Unfortunately, LY2828360 and placebo treatments did not differ in achieving the primary endpoint in patients with osteoarthritic knee pain in this phase 2 clinical trial. Evaluations of LY2828360 antinociceptive efficacy have not appeared in the published literature despite that LY2828360-associated improvements were noted in exploratory pain models (clinicaltrials.gov identifier: NCT01319929) (Pereira et al., 2013).
Fig. 1.
Chemical structure of CB2 receptor agonist LY2828360, drawn by ChemBioDraw Ultra (version 14.0).
The signaling profile of LY2828360 is unknown. We therefore performed a thorough characterization of the signaling of LY2828360 with stably expressed mouse and human CB2 receptors by using a range of cell-based in vitro signaling assays: arrestin recruitment, CB2 receptor internalization, inhibition of forskolin-stimulated cAMP (cyclase) accumulation, extracellular signal-regulated kinase (ERK1/2) phosphorylation, and myo-inositol phosphate 1 (IP1) accumulation. Moreover, to our knowledge, LY2828360 has never been evaluated in an animal model of neuropathic pain. Our previous studies showed that the CB2 agonist AM1710 suppressed neuropathic pain induced by the chemotherapeutic agent paclitaxel through a CB2-specific mechanism without producing tolerance or physical dependence (Deng et al., 2015). We therefore used the same paclitaxel model of peripheral neuropathy to evaluate whether LY2828360 would suppress chemotherapy-induced neuropathic pain in a CB2-dependent manner using both CB2KO and WT mice. We investigated whether repeated administration of LY2828360 would produce tolerance to the antinociceptive effects of the CB2 agonist in paclitaxel-treated mice. Comparisons were made with the opioid analgesic morphine administered under identical conditions. In addition, we evaluated whether LY2828360 would produce antiallodynic efficacy in mice that were rendered tolerant to morphine and, conversely, whether development of morphine tolerance would be attenuated in mice with a history of chronic LY2828360 treatment. We also evaluated whether coadministration of a low dose of LY2828360 with a maximally efficacious dose of morphine would attenuate morphine tolerance. In all studies, pharmacologic specificity was established using WT and CB2KO mice. Finally, to assess physical dependence, we challenged mice with either vehicle or the opioid antagonist naloxone to evaluate whether LY2828360 would impact naloxone-precipitated opioid withdrawal in mice previously rendered tolerant to morphine.
Materials and Methods
Subjects.
Adult male CB2KO mice [B6.129P2-CNR2 (tm1Dgen/J), bred at Indiana University] and WT mice (bred at Indiana University or purchased from Jackson Laboratory, Bar Harbor, ME) on a C57BL/6J background, weighing 25–33 g, were used in this study. Animals were single-housed several days before initiating pharmacologic manipulations. All mice were maintained in a temperature-controlled facility (73 ± 2°F, 45% humidity, 12-hour light/dark cycle, lights on at 7 AM); food and water were provided ad libitum. All experimental procedures were approved by the Bloomington Institutional Animal Care and Use Committee of Indiana University and followed the guidelines of the International Association for the Study of Pain (Zimmermann, 1983).
Drugs and Chemicals.
Paclitaxel (Tecoland Corporation, Irvine, CA) was dissolved in a cremophor-based vehicle made of Cremophor EL (Sigma-Aldrich, St. Louis, MO), ethanol (Sigma-Aldrich), and 0.9% saline (Aqualite System; Hospira, Inc., Lake Forest, IL) at a ratio of 1:1:18 as previously published (Deng et al., 2015). LY2828360 (8-(2-chlorophenyl)-2-methyl-6-(4-methylpiperazin-1-yl)-9-(tetrahydro-2H-pyran-4-yl)-9H-purine) was obtained from Eli Lilly and company (Indianapolis, IN) and synthesized by Eli Lilly (Indianapolis, IN) as previously described (Hollinshead et al., 2013). Morphine (Sigma-Aldrich), or LY2828360, was dissolved in a vehicle containing a 2:1:1:18 ratio of dimethylsulfoxide (DMSO) (Sigma-Aldrich), ALKAMULS EL-620 (Rhodia, Cranbury, NJ), ethanol, and saline. Naloxone (Sigma-Aldrich) was dissolved in saline as indicated. Drugs were administered via intraperitoneal injection to mice in a volume of 10 ml/kg. CP55940 [(2)-cis-3-[2-hydroxy-4-(1,1-dimethylheptyl)phenyl]-trans-4-(3-hydroxypropylcyclohexanol] was obtained from the National Institute of Drug Abuse Drug Supply Service (Bethesda, MD). Pertussis toxin (PTX; cat. no. BML-G100-0050) was purchased from Enzo Lifesciences (Farmingdale, NY).
Cell Culture.
Human embryonic kidney (HEK) 293 cells stably expressing mouse CB2 receptors (HEK mCB2) or human CB2 receptors (HEK hCB2) were generated, expanded, and maintained in Dulbecco’s modified Eagle’s medium with 10% fetal bovine serum and penicillin/streptomycin (GIBCO, Carlsbad, CA) at 37°C in 5% CO2. For ease of immunodetection, an amino-terminal hemagglutinin epitope tag was introduced into the CB1 and CB2 receptors.
Arrestin Recruitment.
To determine arrestin recruitment, assays were performed using an enzyme complementation approach (Dhopeshwarkar and Mackie, 2016). PathHunter Chinese hamster ovary (CHO) K1 CNR2 (cat. no. 93-0472C2) cells were purchased from DiscoveRx (Fremont, CA). This cell line is engineered wherein an N-terminal deletion mutant of β-galactosidase (β gal) enzyme acceptor is fused with arrestin while a complementary smaller fragment (C-terminal) is fused with C-terminal domain of the mouse CB2 cannabinoid receptor. Upon receptor activation, recruitment of arrestin leads to the formation of an active β galactosidase enzyme, which then acts on substrate to emit light that can be detected as luminescence. These cell lines were thawed, grown, and maintained in Pathunter AssayComplete media (cat. no. 92-0018GF2).
Quantification of cAMP Levels.
cAMP assays were optimized using PerkinElmer’s LANCE ultra-cAMP kit (cat. no. TRF0262; PerkinElmer, Boston, MA) per the manufacturer’s instructions. All assays were performed at room temperature using 384-optiplates (cat. no. 6007299; PerkinElmer). Briefly, cells were resuspended in 1× stimulation buffer (1× Hanks’ balanced salt solution, 5 mM HEPES, 0.5 mM IBMX, 0.1% bovine serum albumin (BSA), pH 7.4, made fresh on the day of experiment). Cells (HEK CB2) were incubated for 1 hour at 37°C, 5% CO2 and humidified air and then transferred to a 384-optiplate (500 cells/μl, 10 µl), followed by stimulation with drugs/compounds and forskolin (2 µM final concentration) made in 1× stimulation buffer, as appropriate, for 5 minutes. For time-course experiments, cells were treated with CP55940 or LY282360 (in the presence of 2 µM forskolin final concentration) for defined times. For experiments with PTX, cells were treated overnight with 300 ng/ml PTX at 37°C in 5% CO2. Cells were then lysed by addition of 10 μl Eu-cAMP tracer working solution (4×, made fresh in 1× lysis buffer supplied with the kit, under subdued light conditions) and 10 μl Ulight anti-cAMP working solution (4×, made fresh in 1× lysis buffer) and further incubated for 1 hour at room temperature. Plates were then read with the TR FRET mode on an Enspire plate reader (PerkinElmer).
Detection of Phosphorylated ERK1/2.
HEK-mCB2 or hCB2 were seeded on poly-d-lysine coated 96-well plates (75,000 cells/well) and grown overnight at 37°C, in 5% CO2 humidified air. The following day, media was replaced by serum free DMEM, and plates were further incubated for 5 hours at 37°C in 5% CO2 humidified air. For experiments involving PTX, cells were treated overnight with PTX (300 ng/ml) and the next day serum-starved for 5 hours. After serum starvation, the cells were challenged with drugs/compounds for the indicated time. After drug incubation, plates were emptied and quickly fixed with ice-cold 4% paraformaldehyde for 20 minutes, followed by ice-cold methanol with the plate maintained at −20°C for 15 minutes. Plates were then washed with Tris-buffered saline (TBS)/0.1% Triton X-100 for 25 minutes (5 × 5-minute washes). The wash solution was then replaced by Odyssey blocking buffer and incubated further for 90 minutes with gentle shaking at room temperature. Blocking solution was then removed and replaced with blocking solution containing anti-phospho-ERK1/2 antibody (1:150; Cell Signaling Technology, Danvers, MA) and was shaken overnight at 4°C. The next day, plates were washed with TBS containing 0.05% Tween-20 for 25 minutes (5 × 5-minute washes). Secondary antibody, donkey anti-rabbit conjugated with IR800 dye (Rockland, Limerick, PA), prepared in blocking solution, was added, and plates were gently shaken for 1 hour at room temperature. The plates were then again washed five times with TBS/0.05% Tween-20 solution. The plates were patted dry and scanned using LI-COR Odyssey scanner (LI-COR, Inc., Lincoln, NE) phosphorylated ERK1/2 (pERK1/2) activation (expressed in percentages) was calculated by dividing the average integrated intensities of the drug-treated wells by the average integrated intensities of vehicle-treated wells. All assays were performed in triplicate unless otherwise noted.
On-Cell Western for Receptor Internalization.
HEK CB2 cells were grown to 95% confluence in DMEM + 10% fetal bovine serum + 0.5% Pen/Strep. Cells were washed once with HEPES-buffered saline/BSA (BSA @ 0.08 mg/ml) with 200 μl/well. Drugs were applied at the indicated concentrations to cells, after which they were incubated for 90 minutes at 37°C. Cells were then fixed with 4% paraformaldehyde for 20 minutes and washed four times (300 μl per well) with TBS. Blocking buffer (Odyssey blocking buffer; LI-COR, Inc., Lincoln, NE) was applied at 100 μl per well for 1 hour at room temperature. Anti- hemagglutinin antibody (mouse monoclonal, 1:200; Covance, Princeton, NJ) diluted in Odyssey blocking buffer was then applied for 1 hour at room temperature. After this, the plate was washed five times (300 μl/well) with TBS. Secondary antibody diluted (anti-mouse 680 antibody 1:800, LI-COR, Inc.,) in blocking buffer was then applied for 1 hour at room temperature, after which the plate was washed five times (300 μl/well) with TBS. The plate was imaged using an Odyssey scanner (700 channel, 5.5 intensity, LI-COR, Inc.,).
IP1 Accumulation Assay.
Accumulation of IP1, a downstream metabolite of IP3, was measured by using IP-One HTRFkit (cat. no. 62, IPAPEB; Cisbio, Bedford, MA). Functional coupling of CB2 receptor to Gq G protein leads to phospholipase Cβ (PLC) activation and initiation of the IP hydrolysis cascade. Accumulated IP3 is quickly dephosphorylated to IP2 and then to IP1. This assay takes advantage of the fact that accumulated IP1 is protected from further dephosphorylation by the addition of lithium chloride, and IP1 levels can be easily quantified using an homogeneous time-resolved fluorescence (HTRF) assay. HEK mCB2 cells were detached from ∼50% confluent plates using versene. Cells (10 µl, 5000 cells) were resuspended in 1× stimulation buffer (containing lithium chloride, supplied with the kit) and were incubated for 1 hour at 37°C, 5% CO2, and humidified air and then transferred to a 384-optiplate, followed by stimulation with drugs/compounds made in DMSO/ethanol as appropriate, for defined time points. Cells were then lysed with 5 µl of IP1-d2 dye (made fresh in lysis buffer, supplied with the kit), followed by the addition of 5 µl Ab-Cryptate dye (made fresh in lysis buffer). Plates were incubated further for 60 minutes at room temperature and then read in HTRF mode on an Enspire plate reader. All cell-based assay experiments were performed in triplicate unless otherwise stated.
General In Vivo Experimental Protocol.
In all studies, the experimenter was blinded to the treatment condition, and mice were randomly assigned to experimental conditions. Paclitaxel (4 mg/kg i.p.) was administered four times on alternate days (cumulative dose, 16 mg/kg i.p.) to induce neuropathic pain as described previously by our group (Deng et al., 2015). Control mice received an equal volume of cremophor-vehicle. Development of paclitaxel-induced allodynia was assessed on day 0, 4, 7, 11, and 14.
Effects of pharmacologic manipulations were assessed at 30 minutes after drug administration during the maintenance phase of paclitaxel-induced neuropathy (i.e., beginning day 18–20 after initial paclitaxel injection).
In experiment 1, we assessed the dose response and time course of acute administration of LY2828360 on mechanical and cold allodynia in WT (C57BL/6J) mice treated with paclitaxel or its cremophor-based vehicle.
In experiments 2 and 3, pharmacologic manipulations were performed once daily for 12 consecutive days in each of the two phases of chronic treatment. Four days separated phase 1 and phase 2 chronic dosing in all studies comprising two phases of chronic dosing. Experiments 2 and 3 were performed concurrently using overlapping cohorts that were tested with a single vehicle (phase 1), vehicle (phase 2) group.
In experiment 2, we examined the antiallodynic efficacy of chronic systemic administration of LY2828360 (3 mg/kg per day i.p. × 12 days) or vehicle administered during phase 1 using paclitaxel-treated WT and CB2KO mice. We then assessed the antiallodynic efficacy of chronic systemic administration of vehicle or morphine (10 mg/kg per day i.p. × 12 days) administered during phase 2 in the same animals. Responsiveness to mechanical and cold stimulation was evaluated on treatment days 1, 4, 8, and 12 during phase 1 and on treatment days 16, 19, 23, and 27 during phase 2 (i.e., phase 2 started on day 16).
In experiment 3, we assessed the antiallodynic efficacy of chronic administration of LY2828360 (3 mg/kg per day i.p. × 12 days in phase 2) or vehicle in paclitaxel-treated WT and CB2KO mice that previously developed tolerance to morphine. To induce morphine tolerance, mice received repeated once daily injections of morphine (10 mg/kg per day i.p. × 12 days) in phase 1 treatment; vehicle or LY2828360 (3 mg/kg per day i.p. × 12 days) was administered chronically in phase 2.
In experiment 4, we evaluated the impact of coadministration of morphine (10 mg/kg i.p. × 12 days) with a submaximal dose of LY 2828360 (0.1 mg/kg per day i.p. × 12 days) in WT and CB2 KO mice.
In experiment 5, we evaluated whether chronic administration of LY2828360 would attenuate morphine-dependent withdrawal symptoms that were precipitated using the opioid receptor antagonist naloxone. After the last injection of morphine (on day 28 for two-phase treatments, on day 13 for coadministration treatment), we challenged WT or CB2KO mice from experiments 2, 3, and 4 with vehicle, followed 30 minutes later by naloxone (5 mg/kg i.p.) to precipitate opioid receptor-mediated withdrawal. Mice were video-recorded for subsequent scoring of withdrawal-like behaviors for a 30-minute interval after challenge with vehicle or naloxone.
Assessment of Mechanical Allodynia.
Paw withdrawal thresholds (grams) to mechanical stimulation were measured in duplicate for each paw using an electronic von Frey anesthesiometer supplied with a 90-g probe (model Alemo 2390–5; IITC, Woodland Hills, CA) as described previously (Deng et al., 2012). Mice were placed on an elevated metal mesh table and allowed to habituate under individual, inverted plastic cages to the testing platform for at least 20 minutes until exploratory behavior had ceased. After the habituation period, a force was applied to the midplantar region of the hind paw with a semiflexible tip connected to the anesthesiometer. Mechanical stimulation was terminated when the animal withdrew its paw, and the value of the applied force was recorded in grams. Mechanical paw withdrawal thresholds were obtained in duplicate for each paw and are reported as the mean of duplicate determinations from each animal, averaged across animals, for each group.
Assessment of Cold Allodynia.
Response time (seconds) spent attending to (i.e., elevating, licking, biting, or shaking) the paw stimulated with acetone (Sigma-Aldrich) was measured in triplicate for each paw to assess cold allodynia as previously published (Deng et al., 2012, 2015). An acetone bubble (approximately 5 to 6 µl) formed at the end of a blunt 1-ml syringe hub was gently applied to the plantar surface of the hind paw. Care was taken not to apply mechanical stimulation to the hind paw with the syringe itself. The total time the animal spent attending to the acetone-stimulated paw (i.e., elevation, shaking, or licking) was recorded over 1 minute after acetone application. Acetone was applied three times to each paw with a 3-minute interval between applications. Values for each animal were calculated as the mean of six determinations of acetone responsiveness derived from each mouse.
Evaluation of Opioid Receptor-Mediated Withdrawal Symptoms.
WT (C57BL/6J) mice and CB2KO mice that received either vehicle or morphine (10 mg/kg per day, i.p.) or a combination of morphine with LY2828360 (10 mg/kg per day i.p. morphine coadministered with 0.1 mg/kg per day i.p. LY2828360) for 12 days were challenged with vehicle followed by naloxone (5 mg/kg i.p.) to induce opioid withdrawal beginning 30 minutes after the last injection of the test drugs. Mice were video-taped, and the number of jumps was scored in 5-minute intervals for a total observation period of 30 minutes after challenge with either saline or naloxone (5 mg/kg i.p.).
Statistical Analyses.
Paw withdrawal thresholds (mechanical) and duration of acetone-evoked behavior (cold) were calculated for each paw and averaged. Analysis of variance for repeated measures was used to determine the time course of paclitaxel-induced mechanical and cold allodynia. One-way analysis of variance was used to identify the source of significant interactions at each time point and compare postinjection responses with baseline levels, followed by Bonferroni’s post hoc tests (for comparisons between groups). Appropriate comparisons were also made using Bonferroni’s post hoc tests or planned comparison t tests (unpaired or paired, as appropriate). All statistical analyses were performed using IBM-SPSS Statistics version 24.0 (SPSS Inc., an IBM company, Chicago, IL). P < 0.05 was considered statistically significant. Sample size calculations and power analyses were performed using Statmate 2.0 for windows (Graphpad Prism Software, San Diego CA, www.graphpad.com).
Results
LY2828360 Displays a Delayed, G Protein–Biased Signaling Profile at CB2 Receptors.
A range of cell-based in vitro signaling assays were used to dissect the signaling of LY2828360 at CB2 receptors.
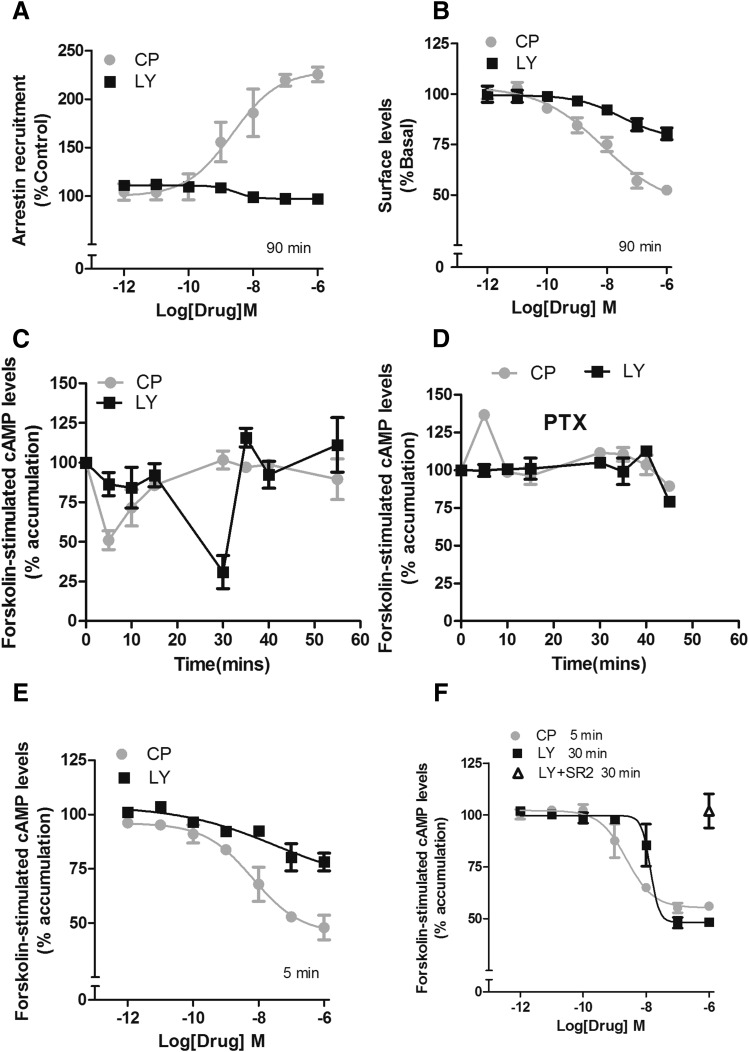
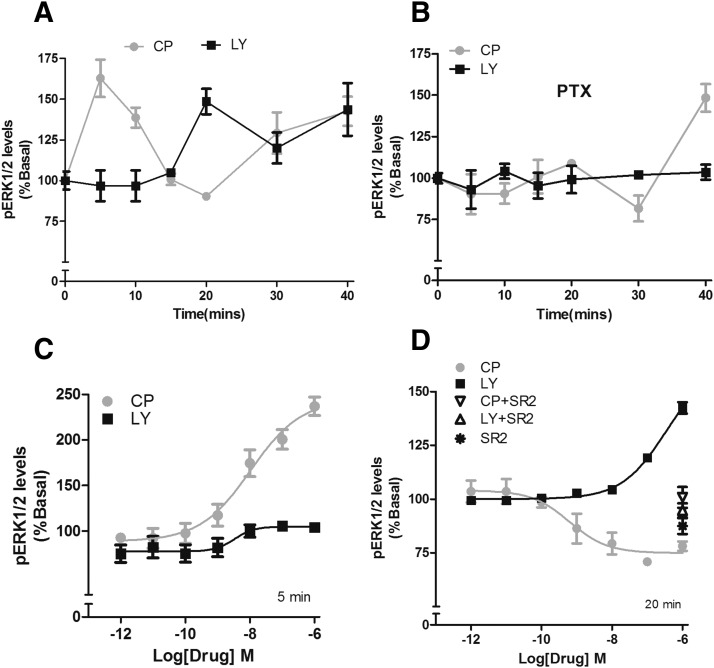
In an arrestin recruitment assay evaluating mouse CB2 receptors, CP55940 recruited arrestin in a concentration-dependent manner, whereas LY2828360 failed to do so after a 90-minute drug incubation (Fig. 2A). Recruitment of arrestin is necessary for many forms of receptor sequestration and internalization (Luttrell and Lefkowitz, 2002). In congruency, LY2828360 failed to internalize the receptor (Fig. 2B). Strikingly, CP55940 (1 µM) induced a rapid (∼5 minutes) and efficacious inhibition of forskolin-stimulated adenylyl cyclase, and LY2828360 (1 µM) induced an efficacious inhibition only after 30 minutes (Fig. 2C). CB2 receptor inhibition of adenylyl cyclase is mediated by inhibitory Gi/o G proteins (Dhopeshwarkar and Mackie, 2014). Thus, to confirm whether delayed inhibition by LY2828360 was mediated by Gi/o proteins, cells were pretreated with PTX, 300 ng/ml, overnight). After PTX treatment, LY2828360 no longer inhibited cAMP accumulation at 30 minutes (Fig. 2D), confirming involvement of inhibitory G proteins. Next, full-concentration response experiments were performed two times when maximal inhibition of forksolin-stimulated cAMP accumulation was observed. At 5 minutes, CP55940 potently and efficaciously inhibited cAMP accumulation, whereas LY2828360 had no effect (Fig. 2E; Table 1). Conversely, at 30 minutes, LY2828360 was potent, efficacious, and CB2 receptor mediated (Fig. 2F). CP55940 (1 µM) was efficacious in stimulating ERK1/2 phosphorylation (pERK1/2) at 5, 10, 30, and 40 minutes, whereas LY2828360 (1 µM) increased pERK1/2 only at later times (20, 30, and 40 minutes). ERK1/2 activation by LY2828360 was completely abolished by pretreatment of cells with PTX (300 ng/ml; overnight) (Fig. 3, A and B), demonstrating G protein dependence. In contrast, only the early phase of CP55940 stimulation of pERK1/2 was PTX sensitive, consistent with the delayed phase of pERK1/2 activation by CP55940 being arrestin-mediated. A full concentration response experiment revealed that LY2828360 failed to increase pERK1/2 at 5 minutes but was potent and efficacious at 20 minutes and required CB2 receptors as it was blocked by SR144528 (Fig. 3, C and D; Table 1). To determine whether the slow, biased signaling of LY2828360 was specific for mouse CB2 receptors, we next evaluated LY2828360 signaling via hCB2 receptors. As with mCB2, LY2828360 failed to internalize hCB2 receptors (Supplemental Fig. S1A) and exhibited time- dependent delayed inhibition of cAMP accumulation (Supplemental Fig. S1, B, D, and E) and ERK1/2 phosphorylation (Supplemental Fig. S1, F, G, and I). As with mouse CB2, these effects were abolished by PTX (Supplemental Fig. S1, C and H) and blocked by SR144528 (Supplemental Fig. S1I), confirming the involvement of Gi/o proteins and CB2 receptors respectively. Finally, LY2828360 did not affect IP1 accumulation via mouse or human CB2 receptors (Supplemental Fig. S2, A and B). Potencies and efficacies of CP55940 and LY2828360 in the signaling assays described at mouse and human CB2 receptors are summarized in Tables 1 and 2, respectively (Tables 1 and 2).
Fig. 2.
LY2828360 displays a delayed signaling profile at mouse CB2 receptors. (A) In CHO cells stably expressing mCB2 receptors, CP55940 recruited arrestin in a concentration-dependent manner, whereas LY2828360 failed to do so after 90-minute drug incubation. (B) In HEK cells stably transfected with mCB2, CP55940 concentration dependently internalized the mCB2; LY2828360 was less potent and efficacious. (C) In a forskolin-stimulated cAMP time course assay, CP55940 (1 µM) was efficacious and rapid in inhibiting forskolin-stimulated cAMP accumulation at 5 minutes, whereas LY2828360 (1 µM) was efficacious only after 30 minutes. (D) After PTX treatment, CP55940 (1 µM) modestly increased cAMP accumulation at 5 minutes, whereas LY2828360 (1 µM) failed to affect cyclase levels at all time points examined/tested. (E) CP55940 was potent and efficacious in inhibiting forskolin-stimulated cAMP accumulation at 5 minutes, whereas LY2828360 failed to affect cAMP levels at this time point. (F) After 30-minute incubation, however, LY2828360 concentration dependently inhibited forskolin-stimulated cAMP accumulation, and this inhibition was completely blocked by 1 µM SR144528(SR2). Forskolin-stimulated cAMP assays were performed in duplicate. All other assays were performed in triplicate. All data were plotted and analyzed using GraphPad Prism 4.
TABLE 1 .
Potencies and efficacies of CP55940 and LY2828360 in arrestin, internalization, cyclase, and pERK1/2 assays at mouse CB2 receptors
Duration of drug incubation is expressed in minutes. All assays were performed in triplicates except cAMP accumulation assays, which were performed in duplicate. EC50, 95% confidence intervals (CI), and the maximal effect (Emax) (mean ± S.E.M.) were obtained by plotting and analyzing the data using GraphPad Prism 4.
| CP55940 |
LY2828360 |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Drug Incubation | EC50 | 95% CI | Emax | ± S.E.M. | EC50 | 95% CI | Emax (%) | ±S.E.M. | |
| Arrestin | min 90 | nM 2.3 | 0.4–12.2 | % 125 | ±1.6 | nM ND | ND | % 97.9 | ±1.5 |
| internalization | 90 | 7.4 | 1.1–19.3 | 49.1 | ±1.2 | 30.7 | 1.4–626.5 | 19.1 | ±2.4 |
| Cyclase | 05 | 6.6 | 1.7–12.2 | 52.8 | ±3.6 | ND | ND | 18.9 | ±5.8 |
| 30 | — | — | — | — | 13.6 | 10.4–45.3 | 53.4 | ±1.9 | |
| pERK1/2 | 05 | 10.5 | 2.2–17.9 | 136.2 | ±4.1 | ND | ND | 4.1 | ±2.5 |
| 20 | 1.5 | 0.1–3.7 | 20.3 | ±3.4 | 339 | 128.8–345.8 | 43.6 | ±2.3 | |
ND, Not determined or cannot be determined.
Fig. 3.
LY282360 displays a delayed CB2 receptor– and G protein–dependent signaling profile in activating pERK1/2. (A) In HEK cells stably expressing mouse CB2 receptors, CP55940 (1 µM) increased phosphorylated ERK1/2 at 5-, 10-, 30-, and 40-minute time points, whereas LY2828360 (1 µM) had no effect at 5- and 10-minute time points but increased ERK1/2 phosphorylation at 20, 30, and 40 minutes. (B) PTX treatment abolished the 20-minute phosphorylation of ERK1/2 by LY2828360 (1 µM) and abolished the CP55940-mediated phosphorylation of ERK1/2 at the 5-minute time point, but it was retained at the 40-minute time point after PTX treatment. (C) CP55940 concentration dependently increased ERK1/2 phosphorylation at 5 minutes, whereas LY2828360 failed to affect pERK1/2 levels at this time point. (D) Conversely, after 20 minutes of treatment, CP55940 decreased ERK1/2 phosphorylation, whereas LY2828360 increased ERK1/2 phosphorylation, in a concentration- dependent manner. Both effects were blocked by the CB2 receptor antagonist SR144528 (1 µM) (SR2). All pERK1/2 assays were performed in triplicate. All the experimental data were plotted and analyzed using GraphPad Prism 4.
TABLE 2 .
Potencies and efficacies of CP55940 and LY2828360 in internalization, cyclase, and pERK1/2 assays at human CB2 receptors
Duration of drug incubation is expressed in minutes. cAMP accumulation assays were performed in duplicate. All other assays were performed in triplicate. EC50, 95% confidence intervals (CI), and the maximal effect (Emax, mean ± S.E.M.) were obtained by plotting and analyzing the data using GraphPad Prism 4.
| CP55940 |
LY2828360 |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Drug Incubation | EC50 | 95% CI | Emax | ± S.E.M. | EC50 | 95% CI | Emax | ±S.E.M. | |
| Internalization | min 90 | nM 3 | 0.3–15.6 | % 33.9 | ±4.6 | nM ND | ND | % 10.2 | ±7.1 |
| Cyclase | 05 | 12.3 | 2.9–18.3 | 59.6 | ±8.3 | ND | ND | ND | ND |
| 35 | — | — | — | — | 16.7 | 4.6–59.6 | 42.8 | ±2.7 | |
| pERK1/2 | 05 | 3.77 | 0.4–12.7 | 95.7 | ±9.1 | ND | ND | 22.1 | ±5.8 |
| 30 | 23.3 | 10.1–53.9 | 49.4 | ±1.6 | 33.5 | 9.1–107.1 | 32.3 | ±1.9 | |
ND, Not determined or cannot be determined.
Effects of Acute Administration of LY2828360 in Paclitaxel-Treated WT Mice.
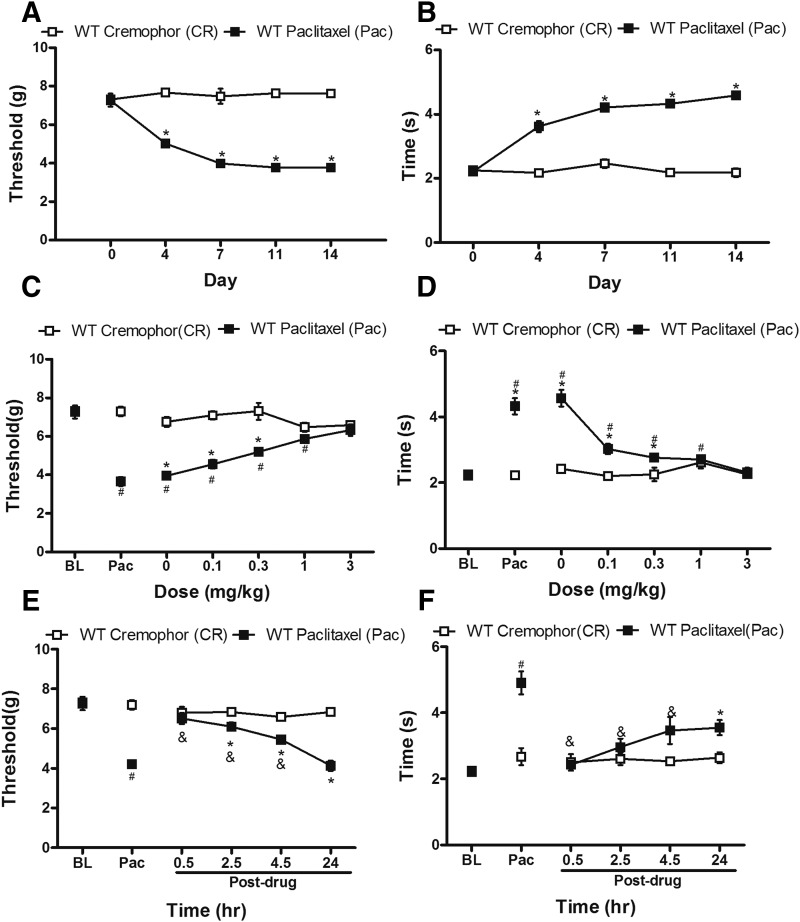
Paclitaxel decreased paw-withdrawal thresholds (F1, 10 = 249.98, P = 0.0001) and increased acetone-evoked behaviors (F1, 10 = 342.95, P = 0.0001), consistent with our previous studies showing development of mechanical and cold allodynia after paclitaxel treatment in mice (Deng et al., 2015). Thus, mechanical (Fig. 4A) and cold (Fig. 4B) allodynia developed by day 4 (P = 0.0001) after initial paclitaxel dosing and was maintained with high stability in paclitaxel-treated WT mice relative to cremophor-vehicle treatment from day 7 onward (P = 0.0001).
Fig. 4.
Paclitaxel produced hypersensitivities to mechanical (A) and cold (B) stimulation. Nonchemotherapy control mice received cremophor-based vehicle in lieu of paclitaxel. Dose response of LY2828360, administered systemically (i.p.), on the maintenance of (C) mechanical and (D) cold allodynia in paclitaxel-treated WT (C57BL/6J) mice. The time course of LY2828360, administered systemically (3 mg/kg i.p.), on the maintenance of (E) mechanical and (F) cold allodynia in paclitaxel-treated WT mice. Data are expressed as mean ± S.E.M. (n = 6/group). *P < 0.05 vs. control, one-way analysis of variance at each time point, followed by Bonferroni’s post hoc test. #P < 0.05 vs. baseline before paclitaxel, repeated measures analysis of variance. &P < 0.05 vs. baseline after paclitaxel, repeated measures analysis of variance. BL, pre-paclitaxel baseline; Pac, baseline after paclitaxel.
In WT mice, acute systemic administration of LY2828360 suppressed paclitaxel-induced mechanical (F1, 10 = 125.902, P = 0.0001; Fig. 4C) and cold (F1, 10 = 29.167, P = 0.0001; Fig. 4D) allodynia in a dose-dependent manner. The high dose of LY2828360 (3 mg/kg i.p.) fully reversed paclitaxel-induced allodynia and normalized responses to pre-paclitaxel baseline levels (P = 0.167 mechanical; P = 0.53 cold) (Fig. 4, C and D); however, neuropathic pain was prominent in paclitaxel-treated mice receiving doses of LY2828360 lower than 0.3 mg/kg i.p. compared with control mice that received the cremophor-vehicle in lieu of paclitaxel (P = 0.001 mechanical; P = 0.044 cold).
To study the duration of antinociceptive action of LY2828360, the maximally efficacious dose (3 mg/kg i.p.) was administered to paclitaxel-treated mice and responsiveness to mechanical and cold stimulation was evaluated at 0.5, 2.5, 4.5, and 24 hours postinjection. LY2828360 produced time-dependent suppressions of paclitaxel-evoked mechanical (F1, 10 = 38.604 P = 0.0001; Fig. 4E) and cold (F1, 10 = 4.993, P < 0.05 cold; Fig. 4F) hypersensitivities and suppression of allodynia was maintained for at least 4.5 hours postinjection (P = 0.001 mechanical, P = 0.022 cold) relative to drug preinjection levels (i.e., Pac). At 24 hours postinjection, paclitaxel-induced mechanical allodynia had returned (P = 1 mechanical; P = 0.125 cold) to drug preinjection levels of hypersensitivity (Fig. 4, E and F). Residual suppression of cold allodynia was absent by 72 hours after LY2828360 treatment (data not shown).
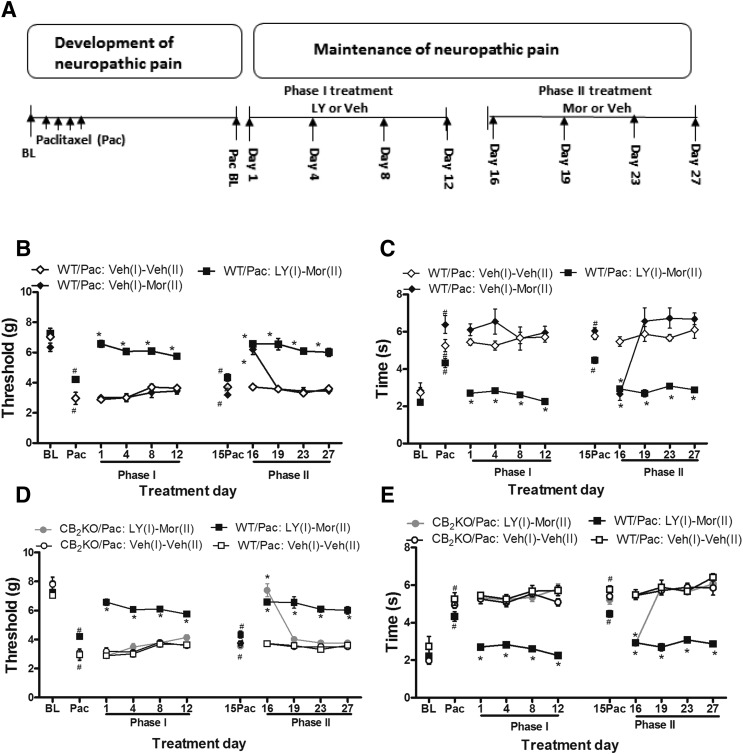
Previously Chronic Administration of LY2828360 Blocked the Development of Tolerance to the Antiallodynic Effects of Morphine in WT but Not in CB2KO Mice.
To study the effects of LY2828360 treatment on the development of tolerance to morphine, pharmacologic manipulations were used in two phases of treatment during the maintenance of neuropathic pain (Fig. 5A). In WT mice, phase 1 treatment with LY2828360 (3 mg/kg per day i.p. × 12 days) suppressed paclitaxel-induced mechanical (F2, 15 = 183.929, P = 0.0001; Fig. 5B) and cold (F2, 15 = 64.218, P = 0.0001; Fig. 5C) hypersensitivities relative to phase 1 vehicle treatments. LY2828360 markedly suppressed paclitaxel-induced mechanical and cold allodynia throughout the observation interval (P = 0.0001 mechanical; P = 0.016 cold; Fig. 5, B and C). Mechanical and cold hypersensitivities were largely normalized by LY2828360 (3 mg/kg i.p. × 12 days) with responses returning to baseline (i.e., pre-paclitaxel) levels (P = 0.138 mechanical; P = 0.182 cold). The antiallodynic efficacy of LY2828360 was stable throughout phase 1 treatment (P = 0.310 mechanical, P = 0.314 cold) without the development of tolerance (Fig. 5, B and C).
Fig. 5.
History of chronic LY2828360 treatment blocked the development of morphine tolerance in WT but not in CB2KO mice. (A) The testing scheme used to evaluate the two phases of treatment during the maintenance of neuropathic pain. History of chronic LY2828360 (3 mg/kg per day i.p. × 12 days in phase 1) treatment suppressed paclitaxel-induced (B) mechanical (C) cold allodynia in WT mice. History of chronic LY2828360 (3 mg/kg per day i.p. × 12 days in phase 1) blocked the development of tolerance to the antiallodynic effects of morphine (10 mg/kg per day × 12 days in phase 2) in WT but not in CB2KO mice for both mechanical (D) and cold (E) modalities. Data are expressed as mean ± S.E.M. (n = 6/group). *P < 0.05 versus Veh (1)-Veh (2), one-way analysis of variance at each time point, followed by Bonferroni’s post hoc test. #P < 0.05 vs. baseline before paclitaxel, repeated measures analysis of variance.
On day 15, 3 days after the completion of phase 1 treatment, paclitaxel-induced mechanical and cold allodynia had returned to levels comparable to those observed before the initiation of phase 1 treatment (i.e., Pac; P = 0.379 mechanical, P = 0.62 cold; Fig. 5, B and C). Mechanical and cold allodynia were maintained in these mice relative to pre-paclitaxel levels (i.e., baseline; P < 0.005 mechanical, P < 0.006 cold). In paclitaxel-treated WT mice, chronic morphine treatment during phase 2 of mice previously receiving vehicle during phase 1 [WT/Pac: Veh (vehicle) (1)-Mor (morphine) (2)] only suppressed paclitaxel-induced mechanical and cold allodynia on day 16 (P = 0.0001 mechanical, P = 0.0001 cold) and then failed to suppress paclitaxel-induced mechanical (P = 1) and cold (P = 1) allodynia on subsequent test days (i.e., days 19, 23, and 27) relative to vehicle-treated mice [WT/Pac: Veh (1)-Veh (2); Fig. 5, B and C]. Thus, morphine tolerance rapidly developed to the antiallodynic effects of phase 2 morphine in paclitaxel-treated mice receiving vehicle in phase 1.
By contrast, in WT mice receiving LY2828360 during phase 1, phase 2 morphine [WT/Pac: LY (1)-Mor (2); 10 mg/kg i.p. × 12 days] sustainably suppressed paclitaxel-induced mechanical (F2, 15 = 91.428, P = 0.0001) (Fig. 5B) and cold (F2, 15 = 40.979, P = 0.0001; Fig. 5C) hypersensitivities relative to mice pretreated with vehicle in phase 1 [WT/Pac: Veh (1)-Mor (2); P = 0.0001] (Fig. 5, B and C). This suppression was present and stable throughout phase 2 for both mechanical (P < 0.05) and cold (P < 0.009) modalities compared with drug preinjection levels in phase 2 (i.e., day 15). Morphine-induced antiallodynic efficacy was stably maintained throughout the observation interval after LY2828360 pretreatment for each stimulus modality (P = 0.222 mechanical, P = 0.535 cold). Thus, a previous history of chronic treatment with LY2828360 prevented the development of morphine tolerance in paclitaxel-treated WT mice for both stimulus modalities.
In paclitaxel-treated CB2KO mice, phase 1 LY2828360 (3 mg/kg per day i.p. × 12 days) treatment failed to suppress mechanical (P > 0.05) or cold (P > 0.05) allodynia relative to vehicle treatment on any day (Fig. 5, D and E). In these same CB2KO mice, subsequent phase 2 morphine treatment [CB2KO/Pac: LY (1) - Mor (2)] suppressed only mechanical (P = 0.0001) and cold (P = 0.0001) allodynia on the initial day of morphine dosing (i.e., day 16) relative to vehicle treatment [CB2KO/Pac: Veh (1)-Veh (2)]. Paclitaxel-induced allodynia was fully reinstated at subsequent time points (i.e., on days 19, 23, and 27; P = 1 mechanical, P = 0.269 cold). The antiallodynic efficacy of initial morphine administration (i.e., on day 16) was similar in WT mice and CB2KO mice (P = 0.203 mechanical; P = 1 cold). Phase 2 morphine administration continued to suppress paclitaxel-induced allodynia (P = 0.0001 mechanical; P = 0.0001 cold) in WT mice previously receiving LY2828360 [WT/Pac: LY (1)-Mor (2)] but not in the CB2KO mice at subsequent time points (i.e., days 19, 23, and 27), suggesting that pretreatment with LY2828360 did not block the development of morphine tolerance in CB2KO mice.
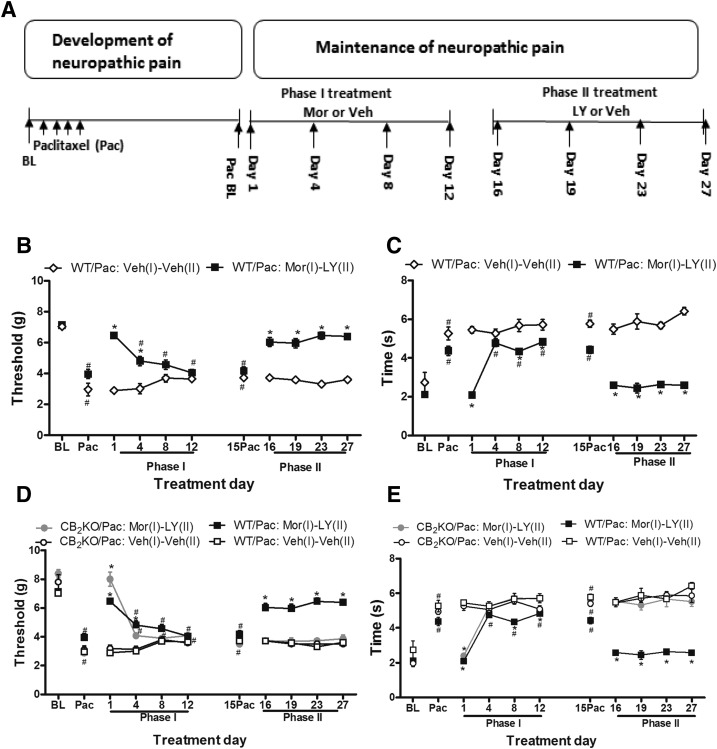
Chronic LY2828360 Treatment Suppresses Paclitaxel-Induced Mechanical and Cold Allodynia in WT Mice but Not in CB2KO Mice Previously Rendered Tolerant to Morphine.
To evaluate whether LY2828360 has antiallodynic efficacy in morphine-tolerant mice, we first dosed paclitaxel-treated WT and CB2KO mice chronically with morphine during phase 1 (10 mg/kg per day i.p. × 12 days) and continued with chronic LY2828360 administration (3 mg/kg per day i.p. × 12 days) (Fig. 6A) in phase 2. In phase 1, morphine administration suppressed paclitaxel-induced mechanical (F1, 10 = 83.817 P = 0.0001) and cold (F1, 10 = 99.443, P = 0.0001) allodynia relative to vehicle treatment. On day 1, morphine fully reversed paclitaxel-induced allodynia and normalized responses to pre-paclitaxel levels (i.e., baseline; P = 0.062 mechanical; P = 1.0 cold) but not on subsequent test days (i.e., day 4, 8, 12; Fig. 6, B and C). Antiallodynic efficacy of morphine was decreased on subsequent test days relative to pre-paclitaxel levels of responsiveness (P = 0.005 mechanical; P = 0.0001 cold). Thus, tolerance developed to the antiallodynic effects of morphine (i.e., on day 4, 8 and 12) (Fig. 6, B and C).
Fig. 6.
Chronic LY2828360 treatment showed sustained antiallodynic efficacy in morphine-tolerant WT mice but not in CB2KO mice. (A) Testing scheme used to evaluate the two phases of treatment during the maintenance of neuropathic pain. Chronic LY2828360 (3 mg/kg per day i.p. × 12 days in phase 2) treatment suppressed paclitaxel-induced mechanical (B and D) and cold (C and E) allodynia in WT mice but not in CB2KO mice previously rendered tolerant to morphine (10 mg/kg per day i.p. × 12 days in phase 1). Data are expressed as mean ± S.E.M. (n = 6/group). Veh (1)-Veh (2) group is replotted from Fig. 5. *P < 0.05 vs. Veh (1)-Veh (2), one-way analysis of variance at each time point, followed by Bonferroni’s post hoc test. #P < 0.05 vs. baseline before paclitaxel, repeated measures analysis of variance.
To evaluate whether LY2828360 produces antiallodynic effects in mice previously rendered tolerant to morphine, LY2828360 (3 mg/kg per day i.p. × 12 days) was administered during phase 2 to paclitaxel-treated mice that previously receiving morphine during phase 1. Phase 2 LY2828360 (3 mg/kg per day i.p. × 12 days) treatment fully reversed paclitaxel-induced allodynia and normalized responsiveness to pre-paclitaxel baseline levels in WT mice that previously developed morphine tolerance in phase 1 (P = 0.112 mechanical; P = 0.103 cold; Fig. 6, B and C). Thus, prior morphine tolerance does not attenuate LY2828360-induced antiallodynic efficacy in phase 2 in WT mice. Antiallodynic efficacy of LY2828360 was also stable throughout the chronic dosing period (P = 1.0 mechanical; P = 1.0 cold), suggesting that tolerance did not develop to phase 2 LY2828360 treatment in WT mice (Fig. 6, B and C).
To further evaluate the mechanism of action underlying the antiallodynic efficacy of LY2828360, we compared the efficacy of phase 2 LY2828360 treatment in CB2KO and WT mice that were rendered tolerant to morphine during phase 1. Acute morphine increased paw withdrawal thresholds and reduced cold response times in paclitaxel-treated CB2KO mice relative to the vehicle treatment on day 1 of phase 1 dosing (P = 0.0001 mechanical; P = 0.0001 cold) (Fig. 6, D and E). The antiallodynic effects of phase 1 morphine were attenuated on day 4 (P = 0.058 mechanical; P = 0.992 cold) and morphine antiallodynic efficacy was completely absent on day 8 and day 12 of chronic dosing (P = 1.0 mechanical; P = 1.0 cold; Fig. 6, D and E). Chronic administration of LY2828360 in phase 2 (3 mg/kg per day, i.p. × 12 days) did not alter responsiveness to mechanical or cold stimulation in paclitaxel-treated CB2KO mice relative to the vehicle treatment at any time point (P = 0.252 mechanical; P = 0.299 cold) (Fig. 6, D and E). Thus, chronic administration of LY2828360 produced antiallodynic efficacy in paclitaxel-treated WT mice but not CB2KO with the same histories of morphine treatment (P = 0.0001 mechanical, P = 0.0001 cold).
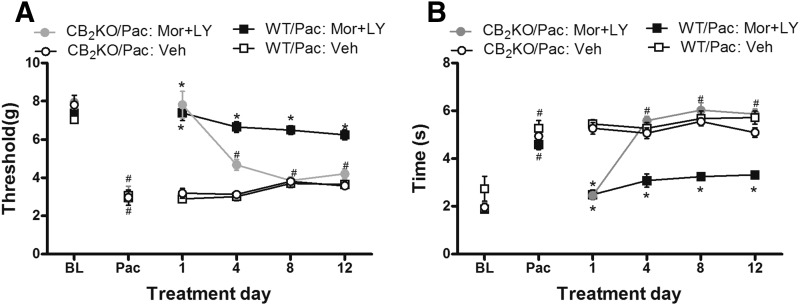
Chronic Coadministration of Low-Dose LY2828360 with Morphine Blocked Morphine Tolerance in WT but Not in CB2 KO Mice.
In WT mice, coadministration of a submaximal dose of LY2828360 (0.1 mg/kg per day i.p. × 12 days) with morphine (10 mg/kg per day × 12 days) suppressed paclitaxel-induced mechanical (F3, 20 = 111.039 P = 0.0001) (Fig. 7A) and cold (F3, 20 = 56.823 P = 0.0001; Fig. 7B) hypersensitivities relative to vehicle treatment (P = 0.0001). Coadministration of the CB2 agonist with morphine fully reversed paclitaxel-induced mechanical allodynia and normalized responses to pre-paclitaxel baseline levels throughout the observation period (P = 0.078). Coadministration of the CB2 agonist with morphine also normalized cold responsiveness on days 1 and 4 (P = 0.156) of chronic dosing to pre-paclitaxel baseline levels. By contrast, in CB2KO mice, sustained antiallodynic efficacy was absent in paclitaxel-treated mice receiving LY2828360 coadministered with morphine; the combination treatment reversed only paclitaxel-induced mechanical (P = 0.0001) and cold (P = 0.0001) allodynia relative to vehicle on day 1 (Fig. 7, A and B). Antiallodynic efficacy of morphine coadministered with LY2828360 was greater in WT mice relative to CB2KO mice on subsequent days of chronic dosing (i.e., days 4, 8, and 12; P = 0.0001 mechanical; P = 0.0001 cold) (Fig. 7, A and B). In paclitaxel-treated WT mice, the combination of morphine with LY2828360 produced a stable, sustained antiallodynic efficacy throughout the dosing period (P = 0.344 mechanical; P = 0.995 cold), demonstrating that morphine tolerance failed to develop in the coadministration condition (Fig. 7, A and B).
Fig. 7.
Chronic coadministration of low-dose LY2828360 (0.1 mg/kg per day i.p. × 12 days) with morphine (10 mg/kg per day i.p. × 12 days) blocked development of morphine tolerance in WT but not in CB2KO mice tested for both (A) mechanical and (B) cold allodynia. Data are expressed as mean ± S.E.M. (n = 6/group). *P < 0.05 vs. WT-Veh, one-way analysis of variance at each time point, followed by Bonferroni’s post hoc test. #P < 0.05 vs. baseline before paclitaxel, repeated measures analysis of variance.
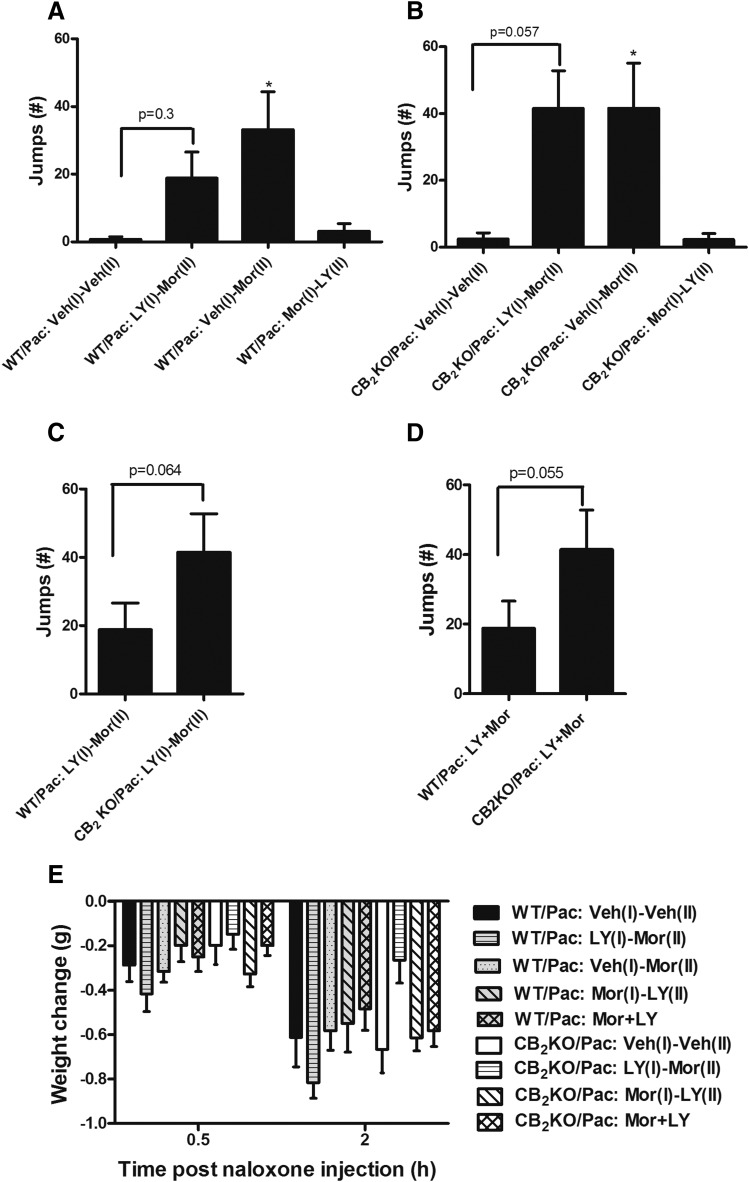
Naloxone-Precipitated Withdrawal is Attenuated in Morphine Tolerant WT but Not CB2KO Mice with a History of LY2828360 Treatment.
In paclitaxel-treated WT mice, naloxone challenge produced characteristic jumping behavior that differed between groups (F3, 22 = 5.657, P = 0.005) (Fig. 8A). Post hoc comparisons revealed that paclitaxel-treated WT mice that received morphine during phase 2 but vehicle during phase 1 [i.e., WT/Pac: Veh (1)-Mor (2) group] exhibited a greater number of jumps relative to paclitaxel-treated WT mice that received vehicle during both phases [WT/Pac: Veh (1)-Veh (2); P = 0.007]. The number of naloxone-precipitated jumps did not differ between groups that received phase 1 LY2828360 followed by phase 2 morphine treatment [WT/Pac: LY (1)-Mor (2)] and those that received phase 1 vehicle followed by phase 2 vehicle treatment [WT/Pac: Veh (1)-Veh (2); P = 0.3]. Also, the number of jumps did not differ between phase 2 morphine-treated mice that received either LY2828360 or vehicle during phase 1 [WT/Pac: Veh (1)-Mor (2) vs. WT/Pac: LY (1)-Mor (2), P = 0.831]. Naloxone challenge did not precipitate withdrawal in paclitaxel-treated WT mice receiving morphine in phase 1 [WT/Pac: Mor (1)-LY (2) vs. WT/Pac: Veh (1)-Veh (2) P = 1] (Fig. 8A).
Fig. 8.
Impact of LY2828360 treatment on naloxone-precipitated opioid withdrawal in CB2 KO and WT mice. Naloxone (5 mg/kg i.p.) precipitates jumping in WT (A) and CB2KO (B) mice receiving morphine (10 mg/kg per day i.p. × 12 days) during phase 2 of chronic dosing. (C) A trend (P = 0.064) toward lower numbers of naloxone-precipitated jumps was observed in WT compared with CB2KO mice with similar histories of LY2828360 (3 mg/kg per day × 10 days during phase 1), followed by morphine (10 mg/kg per day i.p. × 12 days during phase 2) treatment. (D) Naloxone-precipitated (5 mg/kg i.p.) jumping trended lower in WT mice (P = 0.055) receiving coadministration of LY2828360 (0.1 mg/kg per day i.p. × 12 days) with morphine (10 mg/kg per day i.p. × 12 days) compared with CB2KO mice with the same histories of drug treatment. Naloxone did not precipitate jumping behavior in the absence of morphine. (E) Changes in body weight were greater at 2 hours compared with 0.5 hour after naloxone challenge. Data are expressed as mean ± S.E.M. (n = 6–8/group) *P < 0.05 vs. Veh (I)-Veh (II), one-way analysis of variance followed by Bonferroni’s post hoc test or one tailed t test as appreciate.
Similarly, naloxone challenge altered the number of jumps in paclitaxel-treated CB2KO mice (F3, 21 = 5.696 P = 0.005; Fig. 8B). In paclitaxel-treated CB2KO mice, naloxone injection precipitated jumping in mice receiving phase 1 vehicle followed by phase 2 morphine treatment versus mice receiving vehicle during both phases of chronic dosing [CB2KO/Pac: Veh (1)-Veh (2) vs. CB2KO/Pac: Veh (1)-Mor (2), P = 0.044]. The number of jumps trended higher in paclitaxel-treated CB2KO mice receiving LY2828360 in phase 1 and morphine in phase 2 relative to CB2KO mice that received vehicle during both phases [CB2KO/Pac: LY (1)-Mor (2) vs. CB2KO/Pac: veh (1)-Veh (2) group; P = 0.057]. In paclitaxel-treated CB2KO mice, the number of jumps did not differ between phase 2 morphine-treated mice that received either LY2828360 or vehicle during phase 1 [CB2KO/Pac: LY (1)-Mor (2) vs. CB2KO/Pac: Veh (1)-Mor (2), P = 1]. A trend toward fewer naloxone-precipitated jumps was observed in WT relative to CB2KO mice (P = 0.064; Fig. 8C) that received the same histories of phase 1 LY2828360 followed by phase 2 morphine treatment. Similarly, coadministration of LY2828360 with morphine also trended to produce a lower number of naloxone-precipitated jumps in WT compared with CB2KO mice (P = 0.055; Fig. 8D). The observed power of the marginally significant unpaired t test comparing impact of LY2828360 on morphine-dependent WT and CB2KO mice was 40%. A sample size of 20/group would be required to detect a statistically significant impact of LY2828360 on WT and CB2KO animals based on the observed S.D., sample size and magnitude difference observed between means.
Body weight change from baseline (i.e., postvehicle) differed as a function of time after naloxone challenge (F1, 48 = 144.18, P = 0.0001) but did not differ between groups, and the interaction between time and group was not significant. A trend toward group differences in post-naloxone body weight was observed at 2 hours (F8, 48 = 2.033, P = 0.062) but not at 0.5 hour (F8, 48 = 1.460, P = 0.197) postinjection (Fig. 8E).
Discussion
Here we show that the CB2 agonist LY2828360 is a slowly acting but efficacious G protein–biased CB2 agonist that inhibits cAMP accumulation and activates ERK1/2 signaling in vitro. In vivo, chronic systemic administration of the CB2 agonist LY2828360 suppressed chemotherapy-induced neuropathic pain without producing tolerance. The observed antiallodynic efficacy was absent in CB2KO mice, demonstrating mediation by CB2 receptors. Sustained efficacy of LY2828360 was observed in mice with a history of morphine tolerance. Moreover, both chronic LY2828360 dosing completed before morphine dosing and coadministration of LY2828360 with morphine strongly attenuated development of tolerance of morphine. LY2828360 also trended to decrease naloxone precipitated withdrawal signs in WT but not in CB2KO mice.
LY2828360 also displays an intriguing, yet interesting, signaling profile at mouse and human CB2 receptors. Our results suggest that LY2828360 is a slowly acting CB2-receptor agonist strongly biased toward Gi/oG protein signaling with little effect on arrestin or Gq signaling, which contrasts strongly with the balanced agonist CP55940, which rapidly inhibited cAMP accumulation and increased pERK1/2. This ability of a ligand to selectively activate a subset of signaling pathways is termed biased agonism or functional selectivity (Kenakin, 2011) and has emerged as an important pharmacologic concept. For example, a “biased” agonist may activate a pathway that is therapeutically more relevant and shun pathways that lead to untoward effects. More recently, “kinetic bias” has emerged as another important pharmacologic concept that emphasizes the time scale of the activation of a particular pathway (Klein Herenbrink et al., 2016). It remains to be determined whether the marked kinetic and G-protein bias of LY2828360 explains either its remarkable opioid sparing property or its failure in clinical trials for osteoarthritis pain (Pereira et al., 2013).
Tolerance limits therapeutic utility of an analgesic (Rosenblum et al., 2008). In the present study, the antiallodynic efficacy of LY2828360 was fully maintained in neuropathic mice that received once daily administration of the maximally effective dose of LY2828360 over 12 consecutive days. Antiallodynic efficacy of LY2828360 (3 mg/kg i.p.) lasted more than 4.5 hours after acute administration. Responsiveness to mechanical and cold stimulation returned to baseline after 1 and 3 days, respectively. Our data are consistent with our previous studies showing that CB2 agonist AM1710 suppresses paclitaxel-induced neuropathic pain without producing tolerance or physical dependence after either 8 days of once daily (i.p.) dosing (Deng et al., 2015) or chronic infusion over 4 weeks (Rahn et al., 2014).
A striking novel observation of our study was that prior chronic treatment with LY2828360 for 12 days prevented subsequent development of tolerance to the antiallodynic effect of morphine. By contrast, tolerance to morphine developed in CB2KO mice identically treated with chronic LY2828360 in phase 1 followed by chronic morphine treatment in phase 2. Moreover, in paclitaxel-treated WT mice, coadministration of morphine with a low dose of LY2828360 was fully efficacious in alleviating neuropathic pain and blocking the development of morphine tolerance. These observations suggest that analgesic efficacy and, potentially, the therapeutic ratio of morphine could be improved by adjunctive treatment that combines an opioid with a CB2 agonist to treat neuropathic pain while simultaneously limiting the development of tolerance, dependence, and potentially other adverse side effects of the opioid analgesic. Our results are in line with a recent study suggesting that coadministration of a low dose of the CB2 receptor agonist AM1241 combined with morphine reduced the morphine tolerance in Walker 256 tumor-bearing rats (Zhang et al., 2016), although mediation by CB2 receptors was not assessed. AM1241 produced a modest enhancement of opioid-mediated antinociception in the hotplate test and in a test of mechanical sensitivity in tumor-bearing rats (Zhang et al., 2016); however, tolerance developed to the antiallodynic effects of the combination treatment assessed with mechanical but not thermal (hot plate) stimulation, suggesting that therapeutic benefit of the adjunctive treatment may be ligand- and/or modality-dependent. Coadministration of CB2 agonist JWH133 also exhibited opioid-sparing effects in the formalin model of inflammatory pain (Yuill et al., 2017). The mechanism underlying these therapeutically advantageous properties remains incompletely understood. In tumor-bearing mice, AM1241 upregulated µ-opioid receptor expression in the spinal cord and dorsal root ganglia (DRG) (Zhang et al., 2016). Another study suggested CB2 agonist upregulated µ-opioid receptor expression levels, whereas the CB2 antagonist inhibited µ-opioid receptor expression level in Jurkat T cells (Börner et al., 2006) and in mouse brainstem (Páldy et al., 2008). Mitogen-activated protein kinase (MAPK) activation and glial proinflammatory mediator release have also been linked to morphine tolerance (Raghavendra et al., 2002; Mika et al., 2007). CB2 agonists could alleviate morphine tolerance by an interaction between microglial opioid and CB2 receptors and/or by reduction of glial and MAPK activation (Badalà et al., 2008; Tumati et al., 2012). CB2 activation is correlated with increasing anti-inflammatory gene expression in the dorsal horn and reductions in mechanical and thermal hypersensitivities. Coadministration of morphine with the CB2 agonist JWH015 synergistically inhibited preclinical inflammatory, postoperative, and neuropathic pain in a dose- and time-dependent manner (Grenald et al., 2017). The observed synergism may involve activation of CB2 receptors on immune cells and subsequent inhibition of the inflammatory process coupled with morphine’s well characterized ability to inhibit nociceptive signaling (Grenald et al., 2017). In keratinocytes in peripheral paw tissue, AM1241 stimulated the release of the endogenous opioid β-endorphin, which acted at local neuronal MORs to inhibit nociception through a naloxone-dependent mechanism (Ibrahim et al., 2005); however, naloxone sensitivity is not a class effect of CB2 agonists and cannot account for AM1241 antinociception (Rahn et al., 2008) but may depend upon levels of endogenous analgesic tone.
Some effects of cannabinoid receptor agonists and antagonists on morphine antinociceptive tolerance remain controversial. Coadministration of the CB2 receptor agonist JWH-015 with morphine increased morphine analgesia and morphine antinociceptive tolerance (Altun et al., 2015). By contrast, the CB2 receptor antagonist JTE907 decreased morphine analgesia and attenuated morphine antinociceptive tolerance in rats using tail-flick and hot-plate tests of antinociception (Altun et al., 2015). Differences in experimental paradigms, biased signaling of the CB2 agonist used, or the presence or absence of a pathologic pain state could account for these disparities.
An emerging challenge for pain management is how to treat pain in the morphine-tolerant individual. Dose escalation is typically used in early unimodal treatment (de Leon-Casasola et al., 1993), which may enhance potential for abuse (Rosenblum et al., 2008). The combination of two or more analgesic agents with different mechanisms was proposed as an analgesic strategy (Raffa et al., 2010). Our study has important implications for the clinical management of neuropathic pain because chronic LY2828360 treatment showed sustained antiallodynic efficacy in neuropathic mice previously rendered tolerant to morphine. This observation is unlikely to be due to pharmacokinetic factors because morphine dosing ceased for 4 days in our study before introduction of phase 2 LY2828360 chronic treatment.
Physical dependence is another major side effect of opioid treatment, which can lead to a withdrawal syndrome when the user stops taking the drug; however, most studies of opioid dependence have used naïve animals rather than animals subjected to a neuropathic pain state (Lynch et al., 2010). The opioid receptor antagonist naloxone precipitates a spectrum of autonomic and somatic withdrawal signs in morphine-dependent animals (Morgan and Christie, 2011). In the present study, in paclitaxel-treated WT mice, chronic phase 1 pretreatment with LY2828360 produced a trend toward reducing naloxone-precipitated withdrawal jumps without reducing pain relief in the same animals where LY2828360 blocked development of morphine tolerance. This trend was absent in CB2KO mice receiving identical treatments. In fact, our studies raise the possibility that CB2 receptor signaling may attenuate opioid antagonist-precipitated withdrawal because CB2KO mice trended to show higher levels of naloxone-precipitated jumping compared with WT mice when pretreated with CB2 agonist. Moreover, coadministration of low-dose LY2828360 with morphine mimicked these effects and trended to decrease naloxone-precipitated withdrawal jumping in paclitaxel-treated WT mice compared with CB2KO mice (P = 0.055). Thus, LY2828360 may be efficacious in decreasing morphine withdrawal symptoms. Variability in withdrawal jumps and inadequate statistical power could account for the failure to observe more robust statistical differences in jumps between groups; the primary endpoints evaluated here were mechanical and cold responsiveness, not naloxone-induced jumping. Observations from both these studies are, nonetheless, broadly consistent with the hypothesis that CB2 receptor activation may attenuate signs of opioid withdrawal. Stimulation of microglial CB2 receptors by the CB2 agonist suppressed microglial activation (Ehrhart et al., 2005), which has been linked to morphine withdrawal behaviors. Thus, depletion of spinal lumbar microglia decreased withdrawal behaviors and attenuated the severity of withdrawal without affecting morphine antinociception (Burma NE, et al., 2017). The mechanism underlying these observations remains to be explored.
In summary, our observations suggest that CB2 agonists may be useful as a first-line treatment of suppressing chemotherapy-induced neuropathic pain. Our results suggest that CB2 agonists may be useful for suppressing neuropathic pain with sustained efficacy in opioid-recalcitrant pain states without the development of tolerance or dependence.
Acknowledgments
The authors thank Ben Cornett for assistance with mouse husbandry and genotyping.
Abbreviations
- AM1710
3-(1, 1-dimethyl-heptyl)-1-hydroxy-9-methoxy-benzo(c) chromen-6-one
- BSA
bovine serum albumin
- CB1 or CB2
cannabinoid receptor 1 or 2
- CHO
Chinese hamster ovary
- CNS
central nervous system
- CP55940
(2)-cis-3-[2-hydroxy-4-(1,1-dimethylheptyl)phenyl]-trans-4-(3-hydroxypropyl)cyclohexanol
- DMSO
dimethylsufoxide
- ERK
extracellular signal-regulated kinases
- Gi
inhibitory G proteins
- HEK
human embryonic kidney
- IP1
myo-inositol phosphate 1
- KO
knockout
- LY2828360
(8-(2-chlorophenyl)-2-methyl-6-(4-methylpiperazin-1-yl)-9-(tetrahydro-2H-pyran-4-yl)-9H-purine)
- pERK 1/2
phosphorylated ERK1/2
- PTX
pertussis toxin
- TBS
Tris-buffered saline
- WT
wild type
Authorship Contributions
Participated in research design: Lin, Dhopeshwarkar, Mackie, Hohmann.
Conducted experiments: Lin, Dhopeshwarkar, Huibregtse.
Performed data analysis: Lin, Dhopeshwarkar.
Wrote or contributed to the writing of the manuscript: Lin, Dhopeshwarkar, Mackie, Hohmann.
Footnotes
This research was supported by the National Institutes of Health National Institute on Drug Abuse [Grants DA041229, DA009158, and DA021696] and National Cancer Institute [Grant CA200417].
 This article has supplemental material available at molpharm.aspetjournals.org.
This article has supplemental material available at molpharm.aspetjournals.org.
References
- Altun A, Yildirim K, Ozdemir E, Bagcivan I, Gursoy S, Durmus N. (2015) Attenuation of morphine antinociceptive tolerance by cannabinoid CB1 and CB2 receptor antagonists. J Physiol Sci 65:407–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badalà F, Nouri-mahdavi K, Raoof DA. (2008) NIH public access. Computer (Long Beach Calif) 144:724–732. [Google Scholar]
- Börner C, Höllt V, Kraus J. (2006) Cannabinoid receptor type 2 agonists induce transcription of the mu-opioid receptor gene in Jurkat T cells. Mol Pharmacol 69:1486–1491. [DOI] [PubMed] [Google Scholar]
- Burma NE, Bonin RP, Leduc-pessah H, Baimel C, Cairncross ZF, Mousseau M, Shankara JV, Stemkowski PL, Baimoukhametova D, Bains JS, et al. (2017) Blocking microglial pannexin-1 channels alleviates morphine withdrawal in rodents. Nature Publishing Group 23: 355–360. [DOI] [PubMed] [Google Scholar]
- Bushlin I, Rozenfeld R, Devi LA. (2010) Cannabinoid – opioid interactions during neuropathic pain and analgesia. Curr Opin Pharmacol 10: 80–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Leon-Casasola OA, Myers DP, Donaparthi S, Bacon DR, Peppriell J, Rempel J, Lema MJ. (1993) A comparison of postoperative epidural analgesia between patients with chronic cancer taking high doses of oral opioids versus opioid-naive patients. Anesth Analg 76:302–307.https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=8424506&dopt=Abstract [PubMed] [Google Scholar]
- Deng L, Guindon J, Cornett BL, Makriyannis A, Mackie K, Hohmann AG. (2015) Chronic cannabinoid receptor 2 activation reverses paclitaxel neuropathy without tolerance or cannabinoid receptor 1-dependent withdrawal. Biol Psychiatry 77:475–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng L, Guindon J, Vemuri VK, Thakur GA, White FA, Makriyannis A, Hohmann AG. (2012) The maintenance of cisplatin- and paclitaxel-induced mechanical and cold allodynia is suppressed by cannabinoid CB2 receptor activation and independent of CXCR4 signaling in models of chemotherapy-induced peripheral neuropathy. Mol Pain 8:71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desroches J, Bouchard JF, Gendron L, Beaulieu P. (2014) Involvement of cannabinoid receptors in peripheral and spinal morphine analgesia. Neuroscience 261:23–42. [DOI] [PubMed] [Google Scholar]
- Dhopeshwarkar A, Mackie K. (2014) CB2 cannabinoid receptors as a therapeutic target—what does the future hold? Mol Pharmacol 86: 430–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhopeshwarkar A, Mackie K. (2016) Functional selectivity of CB2 cannabinoid receptor ligands at a canonical and noncanonical pathway. J Pharmacol Exp Ther 358:342–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrhart J, Obregon D, Mori T, Hou H, Sun N, Bai Y, Klein T, Fernandez F, Tan J, Shytle RD. (2005) Stimulation of cannabinoid receptor 2 (CB2) suppresses microglial activation. J Neuroinflammation 2:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grenald SA, Young MA, Wang Y, Ossipov MH, Ibrahim MM, Largent-Milnes TM, Vanderah TW. (2017) Synergistic attenuation of chronic pain using mu opioid and cannabinoid receptor 2 agonists. Neuropharmacology 116:59–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guindon J, Hohmann AG. (2008) Cannabinoid CB2 receptors: a therapeutic target for the treatment of inflammatory and neuropathic pain. Br J Pharmacol 153:319–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habibi-Asl B, Vaez H, Najafi M, Bidaghi A, Ghanbarzadeh S. (2014) Attenuation of morphine-induced dependence and tolerance by ceftriaxone and amitriptyline in mice. Acta Anaesthesiol Taiwan 52:163–168. [DOI] [PubMed] [Google Scholar]
- Hassanipour M, Amini-Khoei H, Shafaroodi H, Shirzadian A, Rahimi N, Imran-Khan M, Rezayat SM, Dehpour A. (2016) Atorvastatin attenuates the antinociceptive tolerance of morphine via nitric oxide dependent pathway in male mice. Brain Res Bull 125:173–180. [DOI] [PubMed] [Google Scholar]
- Hollinshead SP, Tidwell MW, Palmer J, Guidetti R, Sanderson A, Johnson MP, Chambers MG, Oskins J, Stratford R, Astles PC. (2013) Selective cannabinoid receptor type 2 (CB2) agonists: optimization of a series of purines leading to the identification of a clinical candidate for the treatment of osteoarthritic pain. J Med Chem 56:5722–5733. [DOI] [PubMed] [Google Scholar]
- Hosseinzadeh H, Parvardeh S, Masoudi A, Moghimi M, Mahboobifard F. (2016) Attenuation of morphine tolerance and dependence by thymoquinone in mice. Avicenna J Phytomed 6:55–66. [PMC free article] [PubMed] [Google Scholar]
- Ibrahim MM, Porreca F, Lai J, Albrecht PJ, Rice FL, Khodorova A, Davar G, Makriyannis A, Vanderah TW, Malan TP, et al. (2005) CB2 cannabinoid receptor activation produces antinociception by stimulating peripheral release of endogenous opioids. Proc Natl Acad Sci U S A 102: 3093–3098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenakin T. (2011) Functional selectivity and biased receptor signaling. J Pharmacol Exp Ther 336:296–302. [DOI] [PubMed] [Google Scholar]
- Klein Herenbrink C, Sykes DA, Donthamsetti P, Canals M, Coudrat T, Shonberg J, Scammells PJ, Capuano B, Sexton PM, Charlton SJ, et al. (2016) The role of kinetic context in apparent biased agonism at GPCRs. Nat Commun 7:10842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim G, Wang S, Mao J. (2005) Central glucocorticoid receptors modulate the expression of spinal cannabinoid receptors induced by chronic morphine exposure. Brain Res 1059:20–27. [DOI] [PubMed] [Google Scholar]
- Luttrell LM, Lefkowitz RJ. (2002) The role of β - arrestins in the termination and transduction of G-protein-coupled receptor signals. J Cell Sci 115: 455–465. [DOI] [PubMed] [Google Scholar]
- Lynch WJ, Nicholson KL, Dance ME, Morgan RW, Foley PL. (2010) Animal models of substance abuse and addiction : implications for science, animal welfare, and society. Comp Med 60:177–188. [PMC free article] [PubMed] [Google Scholar]
- Mansouri MT, Khodayar MJ, Tabatabaee A, Ghorbanzadeh B, Naghizadeh B. (2015) Modulation of morphine antinociceptive tolerance and physical dependence by co-administration of simvastatin. Pharmacol Biochem Behav 137:38–43. [DOI] [PubMed] [Google Scholar]
- Manzanares J, Corchero J, Romero J, Fernández-Ruiz JJ, Ramos JA, Fuentes JA. (1999) Pharmacological and biochemical interactions between opioids and cannabinoids. Trends Pharmacol Sci 20:287–294. [DOI] [PubMed] [Google Scholar]
- Massi P, Vaccani A, Romorini S, Parolaro D. (2001) Comparative characterization in the rat of the interaction between cannabinoids and opiates for their immunosuppressive and analgesic effects. J Neuroimmunol 117:116–124. [DOI] [PubMed] [Google Scholar]
- Mechoulam R, Parker LA. (2013) The endocannabinoid system and the brain. Annu Rev Psychol 64:21–47. [DOI] [PubMed] [Google Scholar]
- Merighi S, Gessi S, Varani K, Fazzi D, Mirandola P, Borea PA. (2012) Cannabinoid CB(2) receptor attenuates morphine-induced inflammatory responses in activated microglial cells. Br J Pharmacol 166:2371–2385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mika J, Osikowicz M, Makuch W, Przewlocka B. (2007) Minocycline and pentoxifylline attenuate allodynia and hyperalgesia and potentiate the effects of morphine in rat and mouse models of neuropathic pain. Eur J Pharmacol 560:142–149. [DOI] [PubMed] [Google Scholar]
- Morgan MM, Christie MJ. (2011) Analysis of opioid efficacy, tolerance, addiction and dependence from cell culture to human. Br J Pharmacol 164:1322–1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Páldy E, Bereczki E, Sántha M, Wenger T, Borsodi A, Zimmer A, Benyhe S. (2008) CB(2) cannabinoid receptor antagonist SR144528 decreases mu-opioid receptor expression and activation in mouse brainstem: role of CB(2) receptor in pain. Neurochem Int 53:309–316. [DOI] [PubMed] [Google Scholar]
- Pereira A, Chappell A, Dethy J, Hoeck H, Arendt-Nielsen L, Verfaille S, Boulanger B, Jullion A, Johnson M, McNearney T. (2013). A proof-of concept (poc study including experimental pain models (epms) to assess the effects of a CB2 agonist (LY2828360) in the treatment of patients with osteoarthritic (OA) knee pain. Clin Pharmacol Ther 93: S56–S57. [Google Scholar]
- Pickel VM, Chan J, Kash TL, Rodríguez JJ, MacKie K. (2004) Compartment-specific localization of cannabinoid 1 (CB1) and mu-opioid receptors in rat nucleus accumbens. Neuroscience 127:101–112. [DOI] [PubMed] [Google Scholar]
- Raffa RB, Pergolizzi JN, Tallarida Jr. RJ. (2010) Analgesic Combinations. J Pain 11:701–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghavendra V, Rutkowski MD, DeLeo JA. (2002) The role of spinal neuroimmune activation in morphine tolerance/hyperalgesia in neuropathic and sham-operated rats. J Neurosci 22:9980–9989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahn EJ, Deng L, Thakur GA, Vemuri K, Zvonok AM, Lai YY, Makriyannis A, Hohmann AG. (2014) Prophylactic cannabinoid administration blocks the development of paclitaxel-induced neuropathic nociception during analgesic treatment and following cessation of drug delivery. Mol Pain 10:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahn EJ, Zvonok AM, Thakur GA, Khanolkar AD, Makriyannis A, Hohmann AG. (2008) Selective activation of cannabinoid CB2 receptors suppresses neuropathic nociception induced by treatment with the chemotherapeutic agent paclitaxel in rats. J Pharmacol Exp Ther 327:584–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenblum A, Marsch LA, Joseph H, Portenoy RK. (2008) Opioids and the treatment of chronic pain: controversies, current status, and future directions. Exp Clin Psychopharmacol 16:405–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trang T, Sutak M, Jhamandas K. (2007) Involvement of cannabinoid (CB1)-receptors in the development and maintenance of opioid tolerance. Neuroscience 146:1275–1288. [DOI] [PubMed] [Google Scholar]
- Tumati S, Largent-Milnes TM, Keresztes A, Ren J, Roeske WR, Vanderah TW, Varga EV. (2012) Repeated morphine treatment-mediated hyperalgesia, allodynia and spinal glial activation are blocked by co-administration of a selective cannabinoid receptor type-2 agonist. J Neuroimmunol 244:23–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuill MB, Hale DE, Guindon J, Morgan DJ. (2017) Anti-nociceptive interactions between opioids and a cannabinoid receptor 2 agonist in inflammatory pain. Mol Pain 13:1744806917728227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M, Wang K, Ma M, Tian S, Wei N, Wang G. (2016) Low-dose cannabinoid type 2 receptor agonist attenuates tolerance to repeated morphine administration via regulating μ-opioid receptor expression in walker 256 tumor-bearing rats. Anesth Analg 122:1031–1037. [DOI] [PubMed] [Google Scholar]
- Zimmermann M. (1983) Ethical guidelines for investigations of experimental pain in conscious animals. Pain 16:109–110. [DOI] [PubMed] [Google Scholar]