Abstract
Purslane is a widespread succulent herb that exhibits various pharmacological effects. The purpose of this study was to evaluate the protective effect of Portulaca oleracea L. (purslane) on streptozotocin-induced diabetes in mice. Oral glucose-tolerance tests were carried out to assess blood glucose levels and body weight and food intake were recorded. The biochemical parameters anti-aspartate aminotransferase, alanine aminotransferase, insulin, triglycerides, total cholesterol, IL-6, IL-1β, and TNFα were also measured. The pathological condition of liver tissues were examined by hematoxylin–eosin staining. Rho, ROCK1, ROCK2, NFκBp65, p-NFκBp65, IκBα, and p-IκBα expression in liver tissue were analyzed by Western blot. Purslane increased body weight and decreased food intake. Purslane also significantly reduced concentrations of glucose, anti-aspartate aminotransferase, alanine aminotransferase, triglycerides, total cholesterol, IL-6, IL-1β, and TNFα in serum. Serum insulin was elevated with purslane treatment. In addition, pathologic liver changes in diabetic mice were also alleviated by purslane. Obtained data revealed that purslane restored the levels of Rho–NFκB signaling-related proteins in comparison with those of diabetic mice. Above all, it can be assumed that purslane might play a positive role in regulating streptozotocin-induced liver injury through suppressing the Rho–NFκB pathway.
Keywords: Portulaca oleracea L., diabetes, liver injury, Rho-NFκB
Introduction
Purslane, a member of the Portulacaceae family, is an annual succulent herb which is distributed as turfgrass weed or field crop in diverse geographical environments throughout the world. Purslane is one of the most useful medicinal plants and has been termed a “global panacea” by the World Health Organization.1 Research has confirmed the higher nutritional quality of purslane in most cultivated vegetables, because of its higher levels of α-linolenic acid, ascorbic acid, and β-carotene.2–4 As a traditional Chinese medicine, purslane is now widely used for its various pharmacological effects, including wound-healing, analgesic, antiaging, antioxidative,5 and anti-inflammatory activities.6,7 Evidence has emerged indicating the hepatoprotective effect of purslane on CCl4-induced liver damage and acetaminophen-induced liver injury through anti-inflammatory and antioxidative properties.8,9 We thought it was worth studying whether purslane could improve streptozotocin (STZ)-induced liver injury in diabetes.
Diabetes mellitus is a serious and complex metabolic disease and called the “silent killer”, due to the large number of patients and many chronic complications.10 It is caused by decreased tissue response to insulin and/or impaired insulin deficiency and is characterized by elevated blood glucose.11 There are 387 million people living with diabetes nowadays, and the number is expected to reach 592 million by 2035, according to the International Diabetes Federation.12,13 Diabetes patients fail to utilize glucose fully, such that it accumulates in the blood, leading to high blood glucose.14 Consequently, the liver is involved in the synthesis and metabolism of glucose. Secondary to absolute or relative lack of insulin, disturbances in carbohydrate, protein, and fat metabolism occur in diabetes mellitus, which might be reflected by high levels of triglycerides (TGs) and total cholesterol (TC).15
ROCK is a well-known downstream effector of the small GTPases and is closely involved in the productions of inflammatory molecules.16 Dysregulation of the Rho–ROCK signaling pathway is responsible for the invasion and metastasis in hepatocellular carcinoma.17 Moreover, Rho–ROCK signaling is believed to be implicated in liver-graft injury.18 As a critical downstream molecule, nuclear factor kappa B (NFκB) governs the transcriptions of inflammatory cytokines and is involved in the pathogenesis of STZ-induced hepatic damage.19 The present study aimed to evaluate the effect of purslane on STZ-induced liver injury in mice through the Rho–NFκB signaling pathway.
Materials and methods
Main reagents and kits
Purslane was purchased from a market in Yucheng, Henan, China. Metformin (Met) tablets were acquired from Simcare Drug Store (Nanjing, China). STZ was provided by Sigma-Aldrich (St Louis, MO, USA). Insulin enzyme-linked immuno-sorbent assay (ELISA) kits were supplied by KeyGen Biotech Co Ltd (Nanjing, China). Glucose, TGs, TC, anti-aspartate aminotransferase (AST), and alanine aminotransferase (ALT) commercial kits were purchased from Jiancheng Bioengineering Institute (Nanjing, China). ELISA kits for determination of interleukin (IL)-6, IL-1β, and tumor necrosis factor alpha (TNFα) were produced by R&D Systems (Minneapolis, MN, USA). Primary antibodies against Rho, ROCK1, ROCK2, NFκBp65, p-NFκBp65, IκBα, and p-IκBα were produced by Cell Signaling Technology (Beverly, MA, USA). All other chemicals and reagents used for this study were of analytical grade and purchased from approved organizations.
Preparation of purslane extract
Generally, purslane was washed thoroughly with water, dried in the shade. Powdered purslane (12 kg) was ground and extracted in ethanol (80 L) at room temperature for 7 days, and the filtered extract from ethanol was obtained under reduced pressure. The solvent was evaporated under vacuum to get rid of ethanol. Then, a crude extract purslane was achieved. Before experimental application, purslane extract was dissolved in dimethyl sulfoxide (DMSO), and the final concentration of DMSO was less than 0.05%.
Animals
A total of 50 male mice (8 weeks old) weighing 20–22 g, obtained from the Comparative Medicine Centre of Yangzhou University, were maintained in a temperature- and humidity-controlled animal facility under a 12-hour light–dark cycle. Mice were provided with water and food pellets ad libitum. All mice were required to acclimatize to the laboratory environment for 7 days before the experiment. All the experimental procedures were conducted according to the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals. All animal-handling procedures were performed according to the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health and followed the guidelines of the Animal Welfare Act. All animal experiments were approved by the Experimental Animal Ethical Committee of Second Military Medical University.
Experimental protocol
Body weight and the weight of food intake of all the animals were recorded as the “initial body weight” and “food intake before”. Food and water intake of all groups of animals were monitored during the experimental protocol. A fixed amount of chow was given to each mouse and replenished the next day. Mice were divided into five groups randomly as follows (n=8 per group): control group, STZ group, STZ + Met (10 mg/kg, orally) group, STZ + purslane (100 mg/kg, orally), and STZ + purslane (200 mg/kg, orally) group. A single dose of 135 mg/kg of STZ was injected intraperitoneally in citrate buffer at pH 4.2–4.5 prepared at the time of use. Met and purslane were administered for 28 consecutive days. Purslane (100 and 200 mg/kg) and Met (10 mg/kg) were intragastrically administered by gavage. Prior to killing the mice, their body weight was recorded as “final body weight” and the weight of food intake recorded as “food intake after”.
Sample preparation
After treatment with Met or purslane for 28 days, mice were killed on day 30. Blood samples were collected from the orbit and centrifuged at 4,500 rpm for 15 minutes. Liver tissues were harvested subsequently and set aside at −80°C for histological observation and protein quantification.
Oral glucose-tolerance test
Fasting glucose was detected 15 minutes before the oral glucose-tolerance test. Mice were orally administered glucose (1.5 g/kg body weight) on day 28. Blood glucose level was monitored via blood obtained from the tail vein using glucose commercial kits at 0 (prior to glucose administration), 30, 60, 90, and 120 minutes.
Insulin assay
The serum insulin level of each blood sample was measured by ELISA using a commercial kit according to the manufacturer’s instructions.
Biochemical analysis
AST, ALT, serum TGs, and TC were detected by commercial kits on the basis of the manufacturer’s instructions.
Cytokine measurement
Serum levels of IL-6, TNFα, and IL-1β were measured by ELISA according to the manufacturer’s instructions. The samples were added to 96-well ELISA plates and incubated with a biotin-conjugated anticytokine antibody. The color reaction was accomplished with the substrate solution and then was terminated by the stopping solution. The optical density of each well was recorded at 450 nm using microplate spectrophotometry. All measurements were performed in triplicate.
Histological analysis of liver
Liver tissues were fixed in 10% (v/v) neutral buffered formalin for 24 hours, embedded in paraffin wax, cut into 4 μm thickness, deparaffinized in xylene, and processed with graded ethanol series. Sections were stained with hematoxylin and eosin and observed by light microscopy (Nikon, Tokyo, Japan) at 200× magnification.
Western blot
Briefly, liver tissues (100 mg) were minced and homogenized in 1 mL ice-cold radioimmunoprecipitation-assay buffer, followed by centrifugation at 12,000 rpm for 5 minutes at 4°C. The supernatant was collected to a new clean centrifuge tube and bicinchoninic acid protein-assay kit (Beyotime, Haimen, China) was used for quantification. Equal amounts of 30 μg protein were subjected to 10% sodium dodecyl sulfate polyacrylamide-gel electrophoresis and transferred onto polyvinylidene difluoride membranes. The target blots were incubated in blocking solution containing 5% dried skim milk. Subsequently, the membrane was exposed to the appropriate concentration of specific antibody overnight at 4°C. Incubation with horseradish peroxidase-conjugated secondary antibody was carried out at room temperature for 1 hour after washing with Tris-buffered saline–polysorbate 20 three times. Antibody-reactive sheets were visualized by a KeyGen advanced enhanced-chemiluminescence system kit and a gel-imaging system (Tanon Science and Technology, Shanghai, China).
Statistical analysis
All data are presented as mean ± standard deviations. One-way analysis of variance followed by Tukey’s multiple-comparison test was conducted to assess differences between groups. Values were considered statistically significantly different at P<0.05. Calculations were made using GraphPad Prism 5.0.
Results
Effect of purslane on changes in body weight and food intake
Changes in body weight and food intake of control and experimental mice are presented in Table 1. Compared with the control group, body weight was significantly (P<0.001) decreased in the STZ-stimulated group. On the contrary, treatment with Met (10 mg/kg) and purslane (100 and 200 mg/kg) remarkably reduced final body weight compared with the STZ group (P<0.01). Moreover, the food intake of the STZ group was dramatically increased compared with the control group (P<0.001), whereas the administration of Met (10 mg/kg) and Purslane (100 and 200 mg/kg) notably reduced levels of food intake (P<0.01).
Table 1.
Effect of purslane on body weight and food intake in normal and experimental mice
| Groups | Body weight (g)
|
Food intake
|
||
|---|---|---|---|---|
| Initial | Final | Before | After | |
| Control | 19.17±1.12 | 32.83±1.57 | 3.52±1.24 | 3.94±1.5 |
| STZ | 19.36±1.35 | 16.59±2.03### | 3.63±1.07 | 7.13±2.31### |
| Met (10 mg/kg) | 19.21±1.07 | 25.45±1.4** | 3.27±1.42 | 5.16±1.88** |
| Purslane (100 mg/kg) | 19.29±1.2 | 22.08±1.72** | 3.44±1.3 | 5.94±1.72** |
| Purslane (200 mg/kg) | 19.15±1.33 | 24.35±1.27** | 3.49±1.16 | 5.3±1.65** |
Notes:
P<0.001 compared with control;
P<0.01 compared with model. Data shown as mean ± standard deviation.
Abbreviations: STZ, streptozotocin; Met, metformin.
Effect of purslane on blood glucose and serum insulin levels
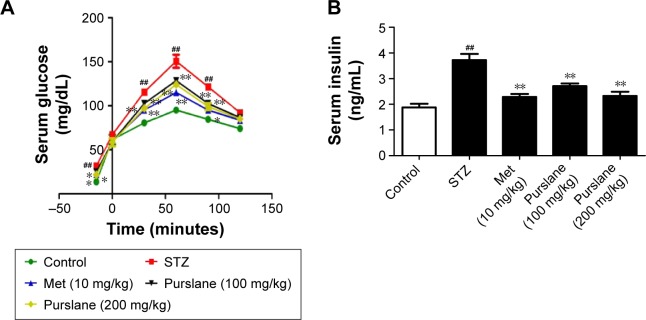
As shown in Figure 1, STZ stimulation increased the level of blood glucose (P<0.01) and decreased the level of serum insulin (P<0.01). However, purslane (100 and 200 mg/kg) treatment significantly restored normal levels and effectively maintained blood glucose balance (P<0.01). Serum glucose levels presented an apparent peak at 60 minutes. There was little difference at 90 minutes in serum glucose between Met (10 mg/kg) and purslane (100 and 200 mg/kg), with purslane (200 mg/kg) resulting more decrease than purslane (100 mg/kg). Meanwhile, the effect of Met treatment on serum insulin level was slightly more pronounced than purslane (100 and 200 mg/kg) treatment.
Figure 1.
Effects of purslane on blood glucose and serum insulin levels.
Notes: (A) OGTT; (B) serum insulin levels. Mice were intraperitoneally injected with 135 mg/kg of STZ. Met (10 mg/kg) and purslane (100 and 200 mg/kg) were intragastrically administered for 28 consecutive days. Values expressed as mean ± standard deviation. ##P<0.01 compared with control; *P<0.05, **P<0.01 compared with model.
Abbreviations: OGTT, oral glucose-tolerance test; STZ, streptozotocin; Met, metformin.
Effect of purslane on TG and TC levels
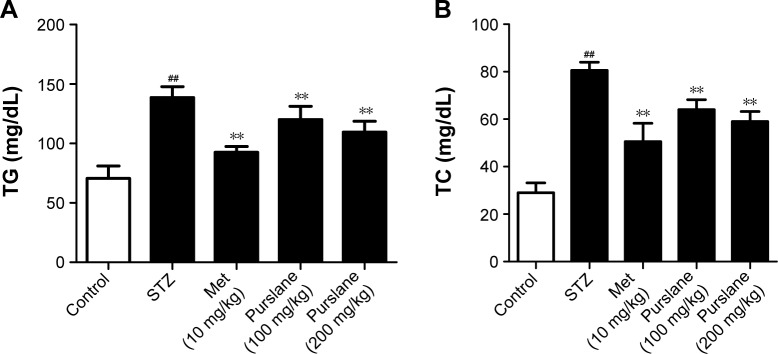
Serum TG (P<0.01) and TC (P<0.01) levels in the STZ-treated group were significantly elevated compared with those in the control group, while purslane (100 and 200 mg/kg) administration markedly reduced their abnormally high expression in diabetic mice (P<0.01), which were slightly less efficient than Met administration (P<0.01) (Figure 2).
Figure 2.
Effects of purslane on (A) TG and (B) TC levels.
Notes: Mice were intraperitoneally injected with 135 mg/kg of STZ. Met (10 mg/kg) and purslane (100 and 200 mg/kg) were intragastrically administered for 28 consecutive days. Values expressed as mean ± standard deviation. ##P<0.01 compared with control; **P<0.01 compared with model.
Abbreviations: TG, triglyceride; TC, total cholesterol; STZ, streptozotocin; Met, metformin.
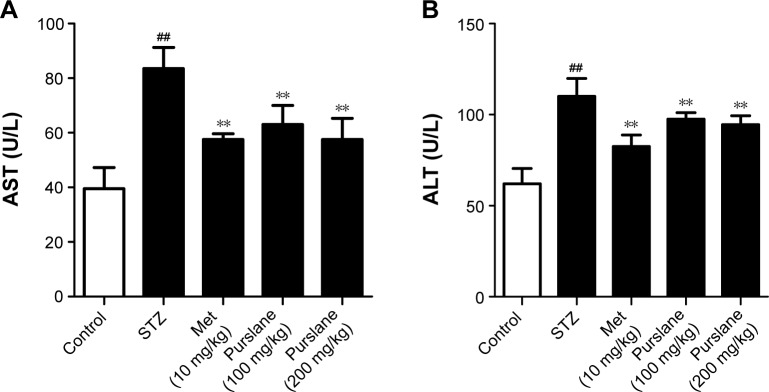
Effect of purslane on AST and ALT
AST and ALT concentrations in diabetic mice were relatively higher than those in the control group (P<0.01). As expected, purslane treatment dramatically controlled elevated levels of AST (P<0.01) and ALT (P<0.01) in comparison with those in the STZ-challenged group (Figure 3).
Figure 3.
Effects of purslane on (A) AST and (B) ALT concentrations.
Notes: Mice were intraperitoneally injected with 135 mg/kg of STZ. Met (10 mg/kg) and purslane (100 and 200 mg/kg) were intragastrically administered for 28 consecutive days. Values expressed as mean ± standard deviation. ##P<0.01 compared with control; **P<0.01 compared with model.
Abbreviations: STZ, streptozotocin; Met, metformin; ALT, alanine aminotransferase; AST, anti-aspartate aminotransferase.
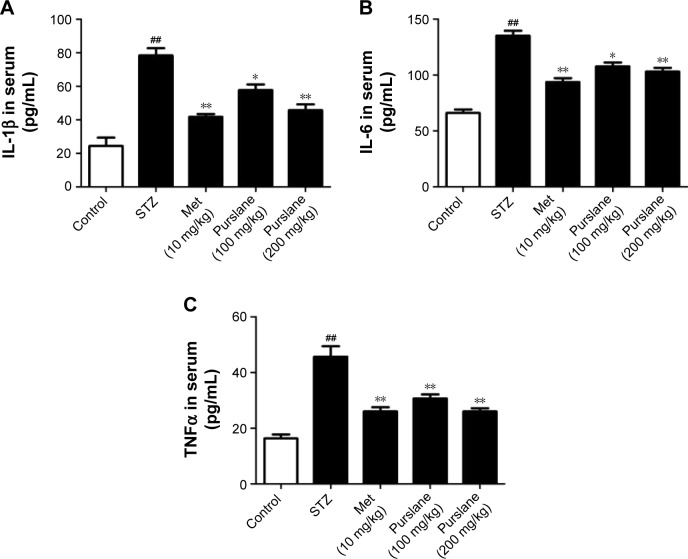
Effect of purslane on inflammatory cytokines in serum
Cytokines participate in the initiation and amplification of the inflammatory cascade of diabetes. As shown in Figure 4, IL-1β, IL-6, and TNFα levels in serum were significantly increased in the STZ group compared with the control group. Purslane (100 and 200 mg/kg) and Met (10 mg/kg) treatment remarkably inhibited the generation of IL-1β, IL-6, and TNFα in serum. In this study, purslane decreased the synthesis and release of inflammatory cytokines in STZ-induced liver injury.
Figure 4.
Effects of purslane on content of (A) IL-1β, (B) IL-6, and (C) TNFα in serum.
Notes: Mice were intraperitoneally injected with 135 mg/kg of STZ. Met (10 mg/kg) and purslane (100 and 200 mg/kg) were intragastrically administered for 28 consecutive days. Values expressed as mean ± standard deviation. ##P<0.01 compared with control; *P<0.05, **P<0.01 compared with model.
Abbreviations: IL, interleukin; TNFα, tumor necrosis factor alpha; STZ, streptozotocin; Met, metformin.
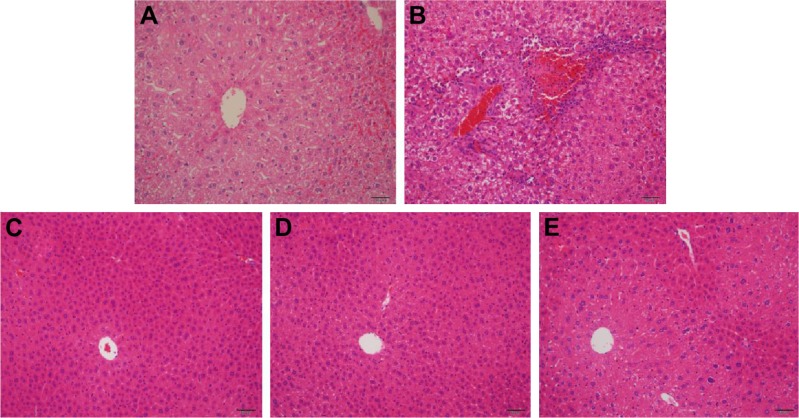
Effect of purslane on liver pathological changes
As exhibited in Figure 5, scarce obvious histological alteration was viewed in liver specimens of the control group. Notably, diabetic mice presented pathological changes as follows: fatty changes in centrilobular liver portions and inflammatory response in the liver. Nevertheless, mice treated with purslane showed great improvement in hepatic damage symptoms. Our results suggest that treatment with purslane could attenuate histopathology in STZ-induced liver injury.
Figure 5.
Effect of purslane on liver pathological changes.
Notes: Mice were intraperitoneally injected with 135 mg/kg of STZ. Met (10 mg/kg) and purslane (100 and 200 mg/kg) were intragastrically administered for 28 consecutive days. (A) Liver section from control group; (B) liver section from STZ group; (C) liver section from STZ + Met (10 mg/kg) group; (D) liver section from STZ + purslane (100 mg/kg) group; (E) liver section from STZ + purslane (200 mg/kg) group. Blue represents inflammatory cell infiltration. Scale bars =50 μM.
Abbreviations: STZ, streptozotocin; Met, metformin.
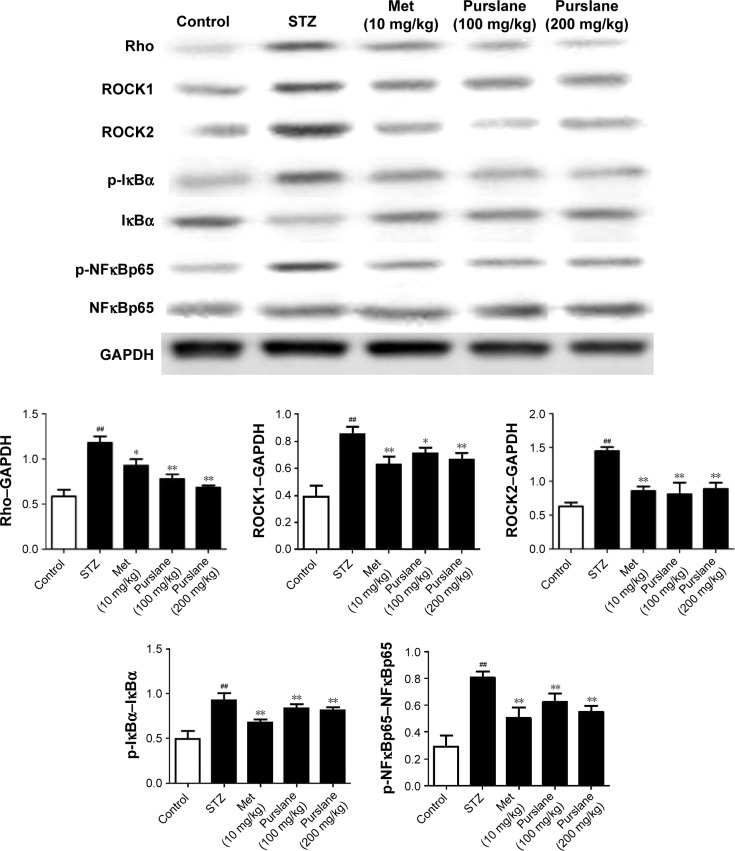
Effect of purslane on Rho–NFκB pathway-related proteins
To investigate further the possible antidiabetic mechanism, expressions of Rho–NFκB pathway-related proteins were also detected in liver tissues. As presented in Figure 6, Rho, ROCK1, ROCK2, p-NFκBp65, and p-IκBα expression were upregulated in diabetic mice (P<0.01). On the contrary, administration of purslane and Met evidently downregulated Rho, ROCK1, and ROCK2 expression and inhibited IκBα, NFκBp65 phosphorylation (P<0.01 or P<0.1).
Figure 6.
Effect of purslane on Rho–NFκB pathway-related proteins.
Notes: Mice were intraperitoneally injected with 135 mg/kg of STZ. Met (10 mg/kg) and purslane (100 and 200 mg/kg) were intragastrically administered for 28 consecutive days. Values expressed as mean ± standard deviation. ##P<0.01 compared with control; *P<0.05, **P<0.01 compared with model.
Abbreviations: STZ, streptozotocin; Met, metformin; NFκB, nuclear factor kappa B; GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
Discussion
Diabetes has long been a threat to human health and degrades the quality of life. An ideal antidiabetic drug should improve glucose metabolism and insulin resistance in diabetic patients without causing any adverse effect. Nevertheless, long-term therapy and prevention with conventional available antidiabetic drugs brings negative side effects.20 The World Health Organization Expert Committee on Diabetes has announced the great potential of natural plants and functional foods as alternative treatments for diabetes on the basis of over 400 reported traditional medicines.21 Therefore, the development of effective and safe natural resources against diabetes seems to be essential. Our findings demonstrated that purslane dramatically recovered the levels of blood glucose and insulin, as well as other biochemical indicators linked to diabetes symptoms in serum.
The metabolic characteristics of diabetes include imbalanced glucose metabolism and insulin resistance, frequently accompanied by dyslipidemia.22 The imbalance between glucose metabolism and insulin resistance in the current study could have resulted from damage stimulated by STZ, which works either directly or indirectly by enhancing blood glucose levels. Therefore, the goals of managing diabetes mellitus are to balance the diet, optimize control of blood glucose level, and normalize disturbances in lipid metabolism.23 Consequently, upregulated levels of glucose and downregulated levels of insulin were observed in diabetic mice in the present study, as suggested by Met treatment, which is usually applied as a positive-control medicine.24 As expected, purslane significantly recovered blood glucose balance and elevated insulin concentration, which reflected the hypoglycemic effects of purslane in diabetic mice.
Hypercholesterolemia and hypertriglyceridemia in STZ-induced diabetic animals are well documented. Glucose in diabetic mice fails to convert to carbohydrates, due to the lack of insulin. However, massive accumulation of glucose metabolizes to fatty acids in the liver.25 The overproduction of serum fatty acids by STZ-induced diabetics facilitates the conversion of excessive fatty acids into phospholipids and cholesterol in the liver.26 Accordingly, blood TC and TG levels are also strongly influenced. Since normal levels of blood glucose and insulin levels in blood were closely associated with blood TC and TG, the obtained data further verified the hepatoprotective effects of purslane in diabetic mice.
To illustrate further the molecular mechanisms related to the liver-recovery abilities of purslane in STZ-stimulated mice, Western blot analysis was used to determine the protein expressions of Rho-mediated inflammatory signaling. ROCKα/ROCK2 and ROCKβ/ROCK1 are two isoforms of ROCK, which have been identified as Rho effectors and play important roles in the pathogenesis of liver diseases.27 Excessive ROCK activities promote pro-inflammatory pathways, including enhanced expression of adhesion molecules and increased production of inflammatory cytokines.28 As expected, our results showed that purslane reduced inflammatory cytokines in serum and also inhibited expression of Rho, ROCK1, ROCK2, p-NFκBp65, and p-IκBα in liver tissue.
Given the relation between efficacy and dose, Met was demonstrated to have a more inhibitory effect against diabetes compared with purslane. However, our findings also suggest the potential role of purslane in improvement of diabetes. Purslane improved insulin levels and relieved chronic inflammation through the Rho–NFκB pathway, thus decreasing the concentration of blood glucose. The present study revealed that purslane might be an effective alternative treatment for liver injury in diabetes, possibly via alleviating expression of Rho–NFκB pathway-related proteins. Further research on purslane should be undertaken.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (No. 81102774, 81573653, 81402680).
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Ercisli S, Coruh I, Gormez A, Sengul M. Antioxidant and antibacterial activities of Portulaca oleracea L. grown wild in Turkey. Ital J Food Sci. 2008;20:533–542. [Google Scholar]
- 2.Bruix J, Reig M, Sherman M. Evidence-based diagnosis, staging, and treatment of patients with hepatocellular carcinoma. Gastroenterology. 2016;150:835–853. doi: 10.1053/j.gastro.2015.12.041. [DOI] [PubMed] [Google Scholar]
- 3.Xin H, Hou Y, Xu Y, et al. Portulacerebroside A: new cerebroside from Portulaca oleracea L. Chin J Nat Med. 2008;6(6):401–403. [Google Scholar]
- 4.Xin H, Xu Y, Hou Y, et al. Two novel triterpenoids from Portulaca oleracea L. Helvetica Chimica Acta. 2008;91:2075–2080. [Google Scholar]
- 5.Kim JY, Oh HM, Kwak SC, et al. Purslane suppresses osteoclast differentiation and bone resorbing activity via inhibition of Akt/GSK3β-c-Fos-NFATc1 signaling in vitro and prevents lipopolysaccharide-induced bone loss in vivo. Biol Pharm Bull. 2015;38:66–74. doi: 10.1248/bpb.b14-00567. [DOI] [PubMed] [Google Scholar]
- 6.Barakat LA, Mahmoud RH. The antiatherogenic, renal protective and immunomodulatory effects of purslane, pumpkin and flax seeds on hypercholesterolemic rats. N Am J Med Sci. 2011;3:411–417. doi: 10.4297/najms.2011.3351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Panth N, Paudel KR, Karki R. Phytochemical profile and biological activity of Juglans regia. J Integr Med. 2016;14(5):359–373. doi: 10.1016/S2095-4964(16)60274-1. [DOI] [PubMed] [Google Scholar]
- 8.Eidi A, Mortazavi P, Moghadam JZ, Mardani PM. Hepatoprotective effects of Portulaca oleracea extract against CCl4-induced damage in rats. Pharm Biol. 2014;53:1–10. doi: 10.3109/13880209.2014.957783. [DOI] [PubMed] [Google Scholar]
- 9.Liu XF, Zheng CG, Shi HG, et al. Ethanol extract from Portulaca oleracea L. attenuated acetaminophen-induced mice liver injury. Am J Transl Res. 2015;7:309–318. [PMC free article] [PubMed] [Google Scholar]
- 10.Campbell IW. Type 2 diabetes mellitus: ‘the silent killer’. Pract Diabetes Int. 2001;18:187–191. [Google Scholar]
- 11.American Diabetes Association Diagnosis and classification of diabetes mellitus. Diabetes Care. 2010;33(Suppl 1):S62–S69. doi: 10.2337/dc10-S062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aguiree F, Brown A, Cho NH, et al. IDF Diabetes Atlas. 6th ed. Brussels: International Diabetes Federation; 2013. [Google Scholar]
- 13.Wu MC. [Traditional Chinese medicine in prevention and treatment of liver cancer: function, status and existed problems] J Chin Integr Med. 2003;1(3):163–164. doi: 10.3736/jcim20030302. Chinese. [DOI] [PubMed] [Google Scholar]
- 14.Chehade JM, Gladysz M, Mooradian AD. Dyslipidemia in type 2 diabetes: prevalence, pathophysiology, and management. Drugs. 2013;73:327–339. doi: 10.1007/s40265-013-0023-5. [DOI] [PubMed] [Google Scholar]
- 15.Chen T, Gao J, Xiang P, et al. Protective effect of platycodin D on liver injury in alloxan-induced diabetic mice via regulation of Treg/Th17 balance. Int Immunopharmacol. 2015;26:338–348. doi: 10.1016/j.intimp.2015.04.001. [DOI] [PubMed] [Google Scholar]
- 16.Chen T, Guo Q, Wang H, et al. Effects of esculetin on lipopolysaccharide (LPS)-induced acute lung injury via regulation of RhoA/Rho kinase/NF-κB pathways in vivo and in vitro. Free Radic Res. 2015;49:1459–1468. doi: 10.3109/10715762.2015.1087643. [DOI] [PubMed] [Google Scholar]
- 17.Wong CC, Wong CM, Tung EK, Man K, Ng IO. Rho-kinase 2 is frequently overexpressed in hepatocellular carcinoma and involved in tumor invasion. Hepatology. 2009;49:1583–1594. doi: 10.1002/hep.22836. [DOI] [PubMed] [Google Scholar]
- 18.Zhu L, Chen T, Chang X, et al. Salidroside ameliorates arthritis-induced brain cognition deficits by regulating Rho/ROCK/NF-κB pathway. Neuropharmacology. 2016;103:134–142. doi: 10.1016/j.neuropharm.2015.12.007. [DOI] [PubMed] [Google Scholar]
- 19.Jain SK, Rains JL, Croad JL. Effect of chromium niacinate and chromium picolinate supplementation on lipid peroxidation, TNF-α, IL-6, CRP, glycated hemoglobin, triglycerides, and cholesterol levels in blood of streptozotocin-treated diabetic rats. Free Radic Biol Med. 2007;43:1124–1131. doi: 10.1016/j.freeradbiomed.2007.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guo X, Yoshitomi H, Gao M, et al. Guava leaf extracts promote glucose metabolism in SHRSP.Z-Leprfa/Izm rats by improving insulin resistance in skeletal muscle. BMC Complement Altern Med. 2013;13:52. doi: 10.1186/1472-6882-13-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bailey CJ, Day C. Traditional plant medicines as treatments for diabetes. Diabetes Care. 1989;12:553–564. doi: 10.2337/diacare.12.8.553. [DOI] [PubMed] [Google Scholar]
- 22.Li A, Liu YG, Wang LY, Zhang SY, Xu LT, Wang SM. Ergosterol alleviates kidney injury in streptozotocin-induced diabetic mice. Evid Based Complement Alternat Med. 2015;2015:691594. doi: 10.1155/2015/691594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Saravanan G, Ponmurugan P. Ameliorative potential of S-allylcysteine: effect on lipid profile and changes in tissue fatty acid composition in experimental diabetes. Exp Toxicol Pathol. 2012;64:639–644. doi: 10.1016/j.etp.2010.12.007. [DOI] [PubMed] [Google Scholar]
- 24.Yang Y, Zhang DM, Liu JH, et al. Wuling san protects kidney dysfunction by inhibiting renal TLR4/MyD88 signaling and NLRP3 inflammasome activation in high fructose-induced hyperuricemic mice. J Ethnopharmacol. 2015;169:49–59. doi: 10.1016/j.jep.2015.04.011. [DOI] [PubMed] [Google Scholar]
- 25.Hu QH, Zhang X, Wang Y, Kong LD. [Mangiferin promotes uric acid excretion and kidney function improvement and modulates related renal transporters in hyperuricemic mice] Yao Xue Xue Bao. 2010;45:1239–1246. Chinese. [PubMed] [Google Scholar]
- 26.Ma CH, Kang LL, Ren HM, Zhang DM, Kong LD. Simiao pill ameliorates renal glomerular injury via increasing Sirt1 expression and suppressing NF-κB/NLRP3 inflammasome activation in high fructose-fed rats. J Ethnopharmacol. 2015;172:108–117. doi: 10.1016/j.jep.2015.06.015. [DOI] [PubMed] [Google Scholar]
- 27.Lee DH, Shi J, Jeoung NH, et al. Targeted disruption of ROCK1 causes insulin resistance in vivo. J Biol Chem. 2009;284:11776–11780. doi: 10.1074/jbc.C900014200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen T, Wang R, Jiang W, et al. Protective effect of astragaloside IV against paraquat-induced lung injury in mice by suppressing Rho signaling. Inflammation. 2016;39:483–492. doi: 10.1007/s10753-015-0272-4. [DOI] [PubMed] [Google Scholar]