Abstract
Introduction: Advanced cancer patients participating in phase 1 clinical trials experience considerable symptom burden. Palliative care (PC) may benefit these individuals by providing supportive care during clinical research participation. This study investigates integration of a PC intervention among phase 1 trial participants with advanced cancer.
Methods and Materials: This study is a multisite randomized clinical trial testing a concurrent PC intervention among phase 1 trial participants. Baseline demographic and clinical characteristics and descriptive baseline assessment findings were examined for all participants to date. Self-report assessments included quality of life (QOL) using the Functional Assessment of Cancer Therapy-General, spirituality using the Functional Assessment of Chronic Illness Therapy-Spirituality, and overall distress using the Distress Thermometer. Clinical trial retention and healthcare utilization were assessed through chart audit at study completion.
Results: The study has enrolled 178 participants to date. The average age is 60.3 years, the majority was Caucasian (57.9%), and participants had an average of 1.7 comorbidities. Overall QOL was 77.6 (±15.1). Responses were most favorable for social/family well-being (22.6 ± 4.6), lowest for emotional well-being (14.9 ± 5.1), and average overall distress was 3.6 (±2.7). Healthcare utilization at study completion (n = 134) identified low rates of supportive care referrals, with approximately half of participants referred to social work (50.8%), and fewer referred for pain (43%), resource centers (44%), and physical therapy (18%).
Conclusion: Phase 1 clinical trial participants experience unmet QOL needs at baseline and levels of distress that merit clinical intervention. Although this study is in progress, initial findings support the potential benefits of PC among this population.
Keywords: : clinical trials, oncology, palliative care, palliative care intervention
Introduction
For patients confronted with an advanced cancer diagnosis, there comes a time when treatment for incurable cancer exhausts standard therapy options. Clinical trial participation may be offered to patients with advanced disease, presenting a significant decision for the patient and provider weighing the potential risks of participation against the potential for clinical benefit.1,2 Although patients that ultimately choose to participate in Phase 1 trials are among the healthiest of those with advanced cancer, these patients still experience a considerable symptom burden.3–5
When faced with the complexities of symptom management and advance care planning, including goals of care discussions in the context of an incurable cancer diagnosis, palliative care (PC) offers the opportunity for better communication, symptom control, and increased knowledge about treatment options and goals for the duration of their illness.6
PC is patient and family-centered care that anticipates, prevents, and treats suffering to maintain the greatest quality of life (QOL) for patients by addressing their physical, intellectual, emotional, social, and spiritual needs.6–8 This requires care coordinated and provided by an interdisciplinary team of providers through a collaborative process during the entire course of the illness.6
The American Society of Clinical Oncology (ASCO), in a 2016 update of the provisional clinical opinion on the integration of PC into treatment for patients diagnosed with cancer, recommended that all advanced cancer patients receive interdisciplinary PC team consultation early in their disease course and concurrent with active treatments.9 Although there is increasing support for the integration of PC as a standard in routine oncology care, PC referral rates remain at 25% and lower among many physicians.10 Provider perception likely impacts this, as many feel PC may not be appropriate for patients participating in potentially curative treatment, or that the suggestion of PC may attenuate the hope of patients receiving treatment.4
In a 2015 policy statement, the ASCO recognized that phase 1 trials play a critical role in the continued development of innovative cancer therapies.11 Phase 1 trials rely on patient participation, and these trials often offer a low risk of serious harm and some potential prospect of clinical benefit to patients that have exhausted other therapy options.12,13 However, rates of participation in clinical trials are very low, with only 3%–5% of patients participating in clinical trials.12
For patients that do participate in phase 1 clinical trials, research has found that they often experience a similar or greater symptom burden when compared with those not enrolled in these trials.3–5 These can include higher levels of symptoms such as fatigue and sleep disturbance.5,14 Conversely, patients participating in phase 1 clinical trials report relatively low levels of symptoms such as vomiting.5 The symptoms induced by Phase I trials have changed as the majority of trials at both centers are now immunotherapy and/or targeted treatments and not traditional chemotherapy.
PC offered with active treatment provides the opportunity to manage the complexities of symptom burden and end-of-life discussions while also allowing patients to engage in clinical research.2,13 The overall purpose of the study is to use a randomized clinical trial to test the efficacy of concurrent PC for patients receiving disease-directed treatments on phase 1 trials. This article reports on a NCI-funded study in progress to describe characteristics and PC needs of participants enrolled to date in this ongoing study.
Methods
Study design
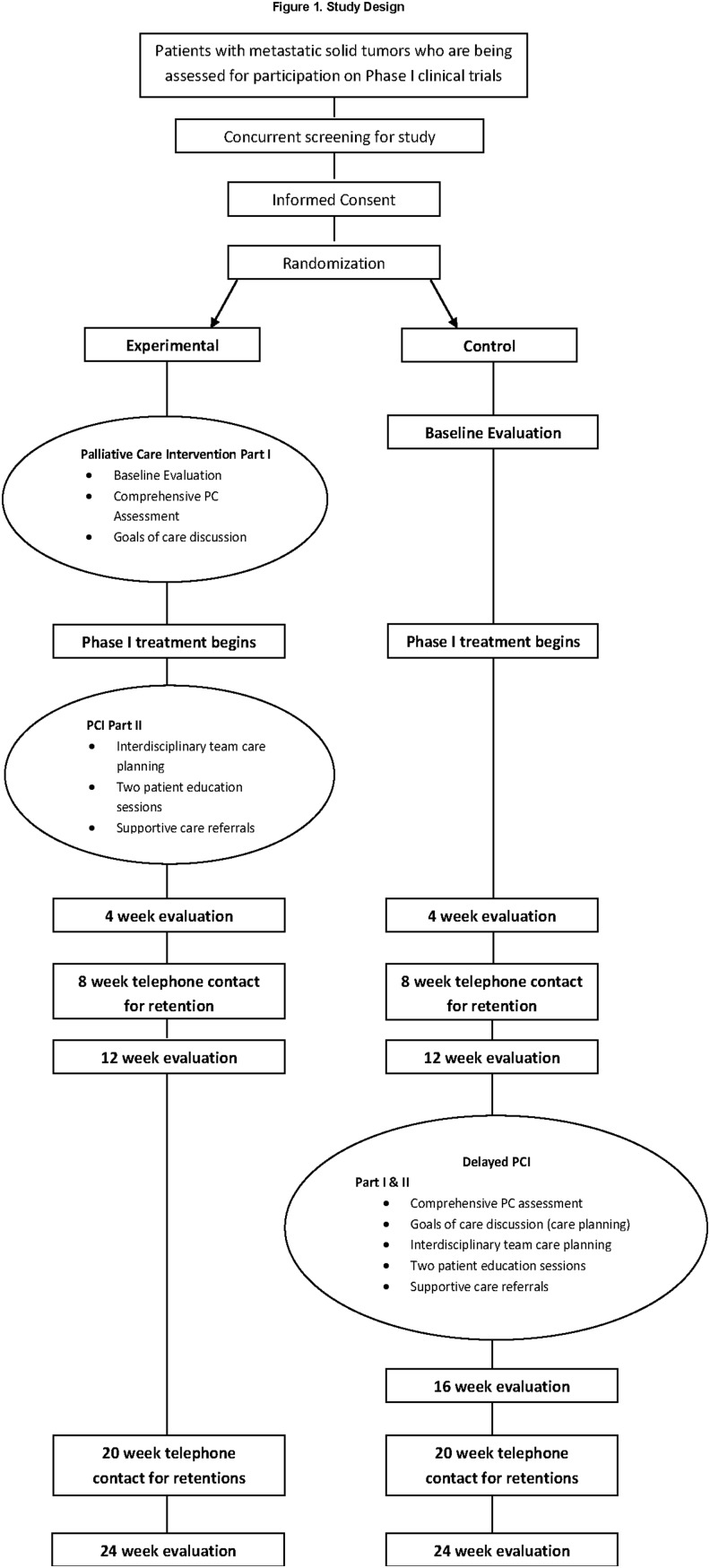
This study is a multisite, randomized clinical trial testing a palliative care intervention (PCI) administered concurrently to patients that are participants in phase 1 clinical trials. The study sites are the City of Hope Medical Center and the Sidney Kimmel Comprehensive Cancer Center of the Johns Hopkins Medical Institutions. Patients being assessed for participation in ongoing phase 1 clinical trials are concurrently screened for eligibility to participate in the current PCI study. Interested and eligible participants are then randomized to either the experimental (early PC) or control (delayed PC) groups. The study began in 2014 and is now in year 3 of active accrual. This article presents the baseline characteristics of participants enrolled in the study through October 2016. This study has been approved by both the City of Hope and Johns Hopkins Institutional Review Boards.
The final sample size is N = 480. This was based on a power analysis using the key dependent variables of QOL, Psychological Distress, Symptom Severity, and Symptom Distress from the primary arms of the study with power estimates of .90–99. The investigators decided to conduct this preliminary analysis, although not originally planned, as we felt that descriptive data from the first 2 years (n = 178) would be informative to the PC community in recognizing this population as a group with potential need for PC. We did not conduct any outcome analysis, thus IRB approval of this preliminary analysis was not required.
Participants
This study includes patients ages 21 and older diagnosed with solid tumor cancers who are eligible for participation in phase 1 clinical trials of investigational cancer therapies. Potential participants for this study must have signed informed consent for participation in a phase 1 clinical trial, and must be able to read and understand English. Patients were excluded from participating in the study if they were diagnosed with hematologic cancers, as this is a population distinct from the solid tumor populations.
Intervention
This interdisciplinary intervention utilizes the National Consensus Project (NCP) Clinical Practice Guidelines for Quality Palliative Care as a foundation for the conceptual framework and content.6 The intervention is initiated before phase 1 treatment beginning with a comprehensive PC assessment using the instruments described below.
The intervention also includes an interdisciplinary care planning meeting, including the investigators and the patient's oncologist if available. The oncologist can also participate by phone and if not available, the team prepares a summary of the patient discussion e-mailed to the physician. At the meeting, the research nurse who collected the baseline assessment presents the information, summarized into QOL categories of physical, psychological, social, and spiritual well-being. The assessment also documents pertinent information about the patient's disease history, Phase I trial, Advanced Directive if completed, and family support.
Before initiating the study, physicians in both sites were trained by one of the study PIs in Goals of Care conversations using established protocols emphasizing what information the patient has been told, what is the patient's understanding, and the physician's best estimate of the patient's survival. Each patient in the intervention group is discussed to identify support needs and to identify referrals needed, including PC. Fifteen minutes is allocated for each case discussion. In the following 2–3 weeks the study nurse also arranges two teaching sessions with the patient, and family caregiver if available, and these are conducted in person or by phone. The teaching sessions include written materials and are also organized according to the four QOL domains.
This is an innovative design that brings together PC clinicians and medical oncologists to provide optimum care to support patients through clinical trials and is adapted from an intervention used previously with lung cancer patients.15 The design is presented as Figure 1.
FIG. 1.
Study Design.
Measures
Demographic and clinical characteristics
This article presents data on patients (n = 178) accrued thus far. Demographic information was collected through self-report at baseline and included age, ethnicity, education level, religious affiliation, marital status, living situation, employment, annual income, past treatment, comorbidities, social support, and functional status. Chart audits are performed for each patient at the end of study participation to collect data on clinical trial retention and healthcare utilization, including supportive care referrals, scheduled and unscheduled ambulatory encounters, hospital admissions, and hospice referrals.
Patient-reported outcomes
The Functional Assessment of Cancer Therapy-General (FACT-G) was used to assess QOL. The FACT-G consists of four QOL domains: physical well-being, social/family well-being, emotional well-being, and functional well-being.16 The scale consists of 27 items asking how true each is for the respondent during the past 7 days, and each item is rated on a 5-point Likert-type scale, with 0 being not at all and 4 being very much.16 The tool yields an overall QOL score ranging from 0 to 108, with higher scores indicating better QOL, as well as scores for each of the four domains. The internal consistency and reliability measure revealed a Cronbach's coefficient alpha of 0.89 for the total FACT-G.16
The Functional Assessment of Chronic Illness Therapy-Spirituality (FACIT-Sp-12) was used to assess the spiritual well-being of participants.17 The instrument consists of three subscales, assessing the role of faith in illness, a sense of meaning and peace. The overall scale consists of 12 questions, with higher scores indicating better QOL/spiritual well-being. The items are rated on a Likert-type scale from 0 (not at all) to 4 (very much) and data from a validation study demonstrate good internal consistency and reliability, and a significant relationship to the QOL of cancer patients.17
The distress thermometer was used to evaluate psychological distress among participants. This low subject burden tool is used for evaluating patient distress over the past week. Respondents rate their distress on a scale of 1 to 10, with a rating over 3 indicating the need for intervention.18,19
Data collection
Upon enrollment, all study participants completed assessments at baseline. Follow-up time points for this study include assessments at 4, 8, 12, 18, and 24 weeks for both the intervention and control groups. In addition, there was another follow-up assessment at the 16-week time point for the control group after receiving the delayed PCI.
Statistical analysis
For this analysis of characteristics of the participants enrolled in the study to date, frequency data was calculated for demographic and clinical characteristics as well as the patient-reported outcome measures from baseline assessments of all participants. All data were examined for participants as a single group, with results reported in aggregate for the control and experimental groups combined in the form of the sample size and proportion or the mean and standard deviations, as appropriate. The SAS® statistical software package was used for the purposes of this analysis. We did not do interim analysis of between-group comparisons since the sample size would not be sufficient given our power calculations.
Results
Demographic characteristics
To date, 178 patients have enrolled and participated in the study. Of these, 90 participants (50.6%) have been randomized to the intervention group and 88 participants (49.4%) have been randomized to the control group. Demographic data are summarized in Table 1.
Table 1.
Participating Patient Demographics
| Demographic variables | All patients (n = 178) |
|---|---|
| Treatment arm, N (%) | |
| Experimental | 90 (50.6) |
| Control | 88 (49.4) |
| Age (y), mean (range) | 60.3 (26.0–83.0) |
| Age (y), N (%) | |
| < 50 | 35 (19.7) |
| 50–54 | 22 (12.4) |
| 55–59 | 22 (12.4) |
| 60–64 | 24 (13.5) |
| 65–69 | 43 (24.2) |
| 70–74 | 13 (7.3) |
| 75–79 | 13 (7.3) |
| 80+ | 6 (3.4) |
| Gender, N (%) | |
| Female | 101 (56.7) |
| Male | 77 (43.3) |
| Race/Ethnicity, N (%) | |
| African American | 13 (7.3) |
| Asian | 20 (11.2) |
| Caucasian | 103 (57.9) |
| Hispanic Latino | 21 (11.8) |
| Middle Eastern | 2 (1.1) |
| Native Hawaiian/Pacific Islander | 3 (1.7) |
| More than one race | 16 (9.0) |
| Education, N (%) | |
| Did not complete high school | 3 (1.7) |
| High school | 46 (25.8) |
| College | 84 (47.2) |
| Graduate/professional school | 40 (22.5) |
| Did not specify | 5 (2.8) |
| Religion, N (%) | |
| Protestant | 69 (38.8) |
| Catholic | 50 (28.1) |
| Jewish | 5 (2.8) |
| Other/not stated | 25 (14.2) |
| None | 29 (16.3) |
| Other household members (living with), N (%) | |
| Alone | 20 (11.2) |
| Children w/or w/out other relatives | 13 (7.3) |
| Parents, friends, and/or relatives | 21 (11.8) |
| Spouse | 75 (42.1) |
| Spouse, and children or other relatives | 49 (27.5) |
| Employment status, N (%) | |
| Employed full time | 35 (19.7) |
| Employed part time | 27 (15.2) |
| Homemaker | 10 (5.6) |
| Retired | 71 (39.9) |
| Unemployed | 33 (18.5) |
| Other/unspecified | 2 (1.2) |
| Family income, N (%) | |
| $10,000 or less | 4 (2.2) |
| $10,001 to $20,000 | 7 (3.9) |
| $20,001 to $30,000 | 10 (5.6) |
| $30,001 to $40,000 | 16 (9.0) |
| $40,001 to $50,000 | 39 (21.9) |
| Greater than $50,000 | 102 (57.3) |
| Type of cancer, N (%) | |
| Bladder | 4 (2.2) |
| Breast | 10 (5.6) |
| Cervical | 6 (3.4) |
| Colon | 30 (16.9) |
| Lung | 37 (20.8) |
| Ovarian | 15 (8.4) |
| Pancreatic | 17 (9.6) |
| Prostate | 6 (3.4) |
| Rectal | 11 (6.2) |
| Other | 42 (23.6) |
| Number of comorbidities, mean (range) | 1.7 (0.0–8.0) |
QOL and symptoms at baseline
Aggregate results using the FACT-G (Table 2) identified that the mean overall QOL score for participants was 77.6 (±15.1). With higher scores indicating best outcomes, participant scores were lowest for the emotional well-being subscale, with an average score of 14.9 (±5.1). Worry that their condition will get worse (1.3 ± 1.4) and worry about dying (2.3 ± 1.5) were the lowest scores in this domain, along with feeling sad (2.7 ± 1.1), feeling nervous (2.7 ± 1.0), and losing hope with fighting their illness (2.8 ± 1.2).
Table 2.
Functional Assessment of Cancer Therapy-General Questions at Baseline*
| Item | FACT-G questions | Mean (SD) |
|---|---|---|
| 1 | Worry condition will get worse | 1.3 (1.4) |
| 2 | Satisfied with sex life | 1.8 (1.6) |
| 3 | Lack energy | 2.1 (1.2) |
| 4 | Content with quality of life | 2.2 (1.2) |
| 5 | Worry about dying | 2.3 (1.5) |
| 6 | Able to work | 2.5 (1.2) |
| 7 | Have pain | 2.6 (1.1) |
| 8 | Feel nervous | 2.7 (1.0) |
| 9 | Feel sad | 2.7 (1.1) |
| 10 | Work is fulfilling | 2.7 (1.1) |
| 11 | Enjoying things for fun | 2.7 (1.3) |
| 12 | Able to enjoy life | 2.8 (1.1) |
| 13 | Losing hope with fighting illness | 2.8 (1.2) |
| 14 | Sleeping well | 2.8 (1.2) |
| 15 | Trouble meeting family needs | 2.9 (1.2) |
| 16 | Accepted illness | 3.1 (1.0) |
| 17 | Coping with illness | 3.1 (1.1) |
| 18 | Forced in bed | 3.2 (1.1) |
| 19 | Bothered by side effects | 3.4 (0.9) |
| 20 | Close to friends | 3.4 (0.9) |
| 21 | Feel ill physically | 3.4 (0.9) |
| 22 | Support from friends | 3.4 (0.9) |
| 23 | Family accepted illness | 3.4 (1.1) |
| 24 | Have nausea | 3.6 (0.8) |
| 25 | Satisfied with communication about illness | 3.6 (0.8) |
| 26 | Emotional support from family | 3.6 (0.9) |
| 27 | Feel close to partner | 3.6 (1.0) |
| Physical well-being subscale (score range 0–28) (Items Phys 3, 7, 15, 18, 19, 21, 24) | 21.2 (5.1) | |
| Social well-being subscale (score range 0–28) (Items Soc 2, 20, 22, 23, 25, 26, 27) | 22.6 (4.6) | |
| Emotional well-being subscale (score range 0–24) (Items Emot 1, 5, 8, 9, 13, 17) | 14.9 (5.1) | |
| Functional well-being subscale (score range 0–28) (Items Func 4, 6, 10, 11, 12, 14, 16) | 19.0 (5.7) | |
| Overall FACT-G Index (score range 0–108) | 77.6 (15.1) | |
| Mean Overall FACT-G Index | 2.9 (0.6) |
Scores range from 0–4, with higher scores indicating better outcomes.
Bolded values delineate individual questions from subscale summary scores.
FACT-G, functional assessment of cancer therapy-general.
Within the domain of functional well-being, scores were lowest for contentment with QOL (2.2 ± 1.2) and ability to work (2.5 ± 1.2). Within the same domain, participants reported better outcomes for sleeping well (2.8 ± 1.2) and having accepted their illness (3.1 ± 1.0).
Participants reported higher QOL for the physical well-being domain (21.2 ± 5.1). Lack of energy was the lowest for the physical well-being domain (2.1 ± 1.2).. Participants did not report high levels of feeling physically ill (3.4 ± 0.9) or being bothered by side effects (3.4 ± 0.9).
The most positive QOL outcomes were reported for the social/family well-being scale, with an average of 22.6 (±4.6). Satisfaction with sex life was the lowest score low satisfaction in this domain (1.8 ± 1.6), but respondents felt close to their partner (3.6 ± 1.0), were satisfied with communication about their illness (3.6 ± 0.8), and felt emotional support from their family (3.6 ± 0.9).
Spiritual well-being at baseline
Spiritual well-being, assessed using the FACIT-Sp-12 measure, is outlined in Table 3. Participants reported better outcomes for the meaning and peace subscale than the faith subscale, with lower scores in the faith domain for illness strengthening their faith (2.0 ± 1.7), comfort in their faith (2.5 ± 1.6), and strength in faith (2.6 ± 1.6). Participants did not report feeling their life lacks meaning or purpose (3.6 ± 0.8) as a concern and reported better QOL outcomes for feeling their lives were productive (3.6 ± 0.7) and having a reason for living (3.7 ± 0.6). Tables 2 and 3 also identify the items associated with each subscale of the FACT and FACIT.
Table 3.
Functional Assessment of Chronic Illness Therapy-Spirituality-12 Questions at Baseline*
| Item | FACIT-SP-12 questions | Mean (SD) |
|---|---|---|
| 11 | Illness strengthened faith–spiritual beliefs | 2.0 (1.7) |
| 9 | Comfort in faith or spiritual beliefs | 2.5 (1.6) |
| 10 | Strength in faith or spiritual beliefs | 2.6 (1.6) |
| 1 | Feel peaceful | 2.8 (1.0) |
| 7 | Sense of harmony with self | 2.8 (1.1) |
| 12 | Things will be okay | 2.9 (1.3) |
| 4 | Have trouble with peace of mind | 3.1 (0.9) |
| 6 | Able to reach deep into myself for comfort | 3.2 (1.0) |
| 5 | Feel sense of purpose in life | 3.4 (1.0) |
| 8 | Life lacks meaning and purpose | 3.6 (0.8) |
| 3 | Life is productive | 3.6 (0.7) |
| 2 | Have reason for living | 3.7 (0.6) |
| Peace subscale (items 1,4,6,7) | 13.3 (2.4) | |
| Meaning subscale (items 2,3,5,8) | 12.9 (3.1) | |
| Faith subscale (items 9–12) | 10.0 (5.4) |
Scores range from 0–4, with higher scores indicating better outcomes.
FACIT-SP, functional assessment of chronic illness therapy-spirituality.
Psychological distress and healthcare resource utilization
Overall distress as evaluated using the Distress Thermometer averaged 3.6 (±2.7) among participants at baseline, indicating overall distress levels that met the cutoff for requiring clinical intervention. Preliminary analysis for those participants that have completed the study (n = 134) and for whom chart abstraction has been completed also identified pertinent trends regarding healthcare utilization. Regardless of study arm assignment, upon completion of the study, only 58% of participants have an advance care directive and only 72% have a designated proxy decision maker. Only 40% of participants have a Do Not Resuscitate order, with 42% being full code status, and 18% having an unknown status. Considering rates of supportive care referrals, approximately half of participants had been referred to social work (50.8%) and nutrition services (50%), with lower rates of referral for pain (43%), resource centers (44%), chaplain services (24%), and physical therapy (18%).
Discussion
Examination of the demographic characteristics of participants enrolled to date in this study found that a considerable portion are working either full time or part time, are older than might have been expected, and are managing comorbid conditions in addition to their cancer diagnoses. In terms of their QOL, participants are experiencing better QOL outcomes for physical well-being, whereas QOL scores are lowest within the domains of functional and emotional well-being. Final analysis of healthcare utilization will be reported at completion of the trial, but the preliminary results uncovered a dearth of supportive care referrals, even for patients that were part of this study, during which the intervention calls for supportive care referrals. This demonstrates conflicting messages during the end-of-life stage even for participants enrolled in a PCI trial.
Previous research related to symptom burden and QOL among phase 1 trial participants has been limited. Studies have reported that although phase 1 participants have been found to have better performance status than nonparticipants, they experience a similar symptom burden.20 In terms of physical functioning, participants in phase 1 trials have previously reported better control over physical symptoms such as vomiting, supported by evidence from the current analysis.5 However, fatigue has been reported to affect higher numbers of patients on phase 1 trials, consistent with reports of concerns about lack of energy in this study.4,21 Pain has also been reported as an uncontrolled symptom of patients on phase 1 clinical trials, but this was not as great a concern for the current participants.4,21
Satisfaction with sex life was another low scoring item for participants in the current study. Previous research has identified that among advanced cancer patients on phase 1 trials, for 57% of females and 68% of males, sex life was a subject of interest, and the majority of both male and female participants wanted to preserve a good-quality sex life, regardless of metastatic cancer diagnosis.22 Regarding the emotional and functional well-being of phase 1 trial participants, an exploratory study identified that participants had lower emotional well-being after participation, and the domain of emotional well-being was also a concern for participants in this study.23 Another study identified poorer functioning in those with more advanced cancer, specifically physical, social, role, and emotional functioning.21 Furthermore, the current study found distress to be at a level that would require clinical intervention, findings which are congruent with another study of lung cancer patients with similar levels of distress at baseline (3.8/10).24
The spiritual well-being of participants in this study found that the peace and meaning outcomes were fairly positive, and this is consistent with previous research. Another study of phase 1 trial participants found that there was a renewed hope for participants on phase 1 clinical trials, and this supported a feeling of meaning and purpose in their lives.25 This is consistent with the greater meaning and purpose that participants felt at baseline in the current study.
The healthcare utilization and advance care planning findings in this study are also consistent with findings from the existing literature. Research has identified unmet PC needs at all phases of the cancer trajectory,21 and a previous study of patients referred to PC by phase 1 versus nonphase 1 oncologists found no significant difference in timing of PC referrals.20 Furthermore, patients on phase 1 trials value the close medical and psychological attention that comes with trial participation,25 and findings from previous research support the importance of a simultaneous care model.20 Advance care planning is complex in the context of cancer care, and has relational, emotional, and social components among advanced cancer patients facing this life-limiting illness.26 The preliminary findings from this study identified trends of lower rates of advance care planning than might be expected given the supportive care intervention, and this warrants further investigation.
It is important to acknowledge the potential limitations of these findings. While a randomized clinical trial, the current report is limited in that it provides an aggregate interim analysis of QOL and spirituality domains at baseline, which should be considered when interpreting the findings. Although this may limit inferences that can be drawn from the findings, this study provides insight into aspects of the QOL concerns and healthcare utilization trends among these participants that may be of clinical interest. The investigators acknowledge a limitation in that patients participating in clinical trials are different from nonclinical trial participants in factors such as age, function, and goals as cited above.
The investigators anticipated that this could be challenging as little has been previously done to integrate PC in the Phase I clinical trial population. The study has been successfully implemented and is at 90% of the accrual goal projected for this point in the study. Attrition was planned at 30% for the 3-month follow-up, which is the key outcome point and is currently at 32%. No study design changes have been required. The study has required close collaboration with clinical trial nurses and oncologists, but has been well supported in both settings.
Phase 1 clinical trial participants experience unmet QOL needs and can benefit from simultaneous PC during participation in these clinical trials. The current report provides preliminary evidence of these areas of unmet need, as well as evidence of a lack of supportive care and healthcare utilization trends, even after participation in an integrated PC. These findings are relevant and important to clinical practice, and identifying and addressing the unmet needs of phase 1 trial participants may help to improve QOL and reduce the symptom burden within this patient population.
Acknowledgment
This research is supported by the National Cancer Institute grant 1R01CA177562-01A1.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.American Cancer Society: Clinical Trials: What You Need to Know www.cancer.org/treatment/treatments-and-side-effects/clinical-trials/what-you-need-to-know.html (last accessed June5, 2017)
- 2.Sun V, Cooke L, Chung V, et al. : Feasibility of a palliative care intervention for cancer patients in phase I clinical trials. J Palliat Med 2014;17:1365–1368 [DOI] [PubMed] [Google Scholar]
- 3.Finlay E, Lu HL, Henderson HR, et al. : Do phase 1 patients have greater needs for palliative care compared with other cancer patients? Cancer 2009;115:446–453 [DOI] [PubMed] [Google Scholar]
- 4.Hui D, Park M, Liu D, et al. : Attitudes and beliefs toward supportive and palliative care referral among hematologic and solid tumor oncology specialists. Oncologist 2015;20:1326–1332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Parsons HA, Baracos VE, Dhillon N, et al. : Body composition, symptoms, and survival in advanced cancer patients referred to a phase I service. PLoS One 2012;7:e29330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dahlin C. (ed): Clinical Practice Guidelines for Quality Palliative Care. Pittsburgh PA: National Consensus Project for Palliative Care, 2013 [Google Scholar]
- 7.Medicare and Medicaid Programs: Hospice Conditions of Participation. Washington, DC: Centers for Medicare & Medicaid Services, 2008 [PubMed] [Google Scholar]
- 8.National Quality Forum: A national framework and preferred practices for palliative and hospice care quality: A consensus report www.qualityforum.org/publications/2006/12/A_National_Framework_and_Preferred_Practices_for_Palliative_and_Hospice_Care_Quality.aspx (last accessed June5, 2017)
- 9.Ferrell BR, Temel JS, Temin S, et al. : Integration of palliative care into standard oncology care: American society of clinical oncology clinical practice guideline update. J Clin Oncol 2017;35:96–112 [DOI] [PubMed] [Google Scholar]
- 10.Smith CB, Nelson JE, Berman AR, et al. : Lung cancer physicians' referral practices for palliative care consultation. Ann Oncol 2012;23:382–387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weber JS, Levit LA, Adamson PC, et al. : American society of clinical oncology policy statement update: The critical role of phase I trials in cancer research and treatment. J Clin Oncol 2015;33:278–284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bell JA, Balneaves LG: Cancer patient decision making related to clinical trial participation: An integrative review with implications for patients' relational autonomy. Support Care Cancer 2015;23:1169–1196 [DOI] [PubMed] [Google Scholar]
- 13.Cassel JB, Del Fabbro E, Arkenau T, et al. : Phase I cancer trials and palliative care: Antagonism, irrelevance, or synergy? J Pain Symptom Manage 2016;52:437–445 [DOI] [PubMed] [Google Scholar]
- 14.George GC, Iwuanyanwu EC, Anderson KO, et al. : Sleep quality and its association with fatigue, symptom burden, and mood in patients with advanced cancer in a clinic for early-phase oncology clinical trials. Cancer 2016;122:3401–3409 [DOI] [PubMed] [Google Scholar]
- 15.Ferrell B, Sun V, Hurria A, et al. : Interdisciplinary palliative care for patients with lung cancer. J Pain Symptom Manage 2015;50:758–767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cella DF, Tulsky DS, Gray G, et al. : The functional assessment of cancer therapy scale: Development and validation of the general measure. J Clin Oncol 1993;11:570–579 [DOI] [PubMed] [Google Scholar]
- 17.Peterman AH, Fitchett G, Brady MJ, et al. : Measuring spiritual well-being in people with cancer: The functional assessment of chronic illness therapy—spiritual well-being scale (FACIT-Sp). Ann Behav Med 2002;24:49–58 [DOI] [PubMed] [Google Scholar]
- 18.Jacobsen PB, Donovan KA, Trask PC, et al. : Screening for psychologic distress in ambulatory cancer patients. Cancer 2005;103:1494–1502 [DOI] [PubMed] [Google Scholar]
- 19.Roth AJ, Kornblith AB, Batel-Copel L, et al. : Rapid screening for psychologic distress in men with prostate carcinoma: A pilot study. Cancer 1998;82:1904–1908 [DOI] [PubMed] [Google Scholar]
- 20.Hui D, Parsons H, Nguyen L, et al. : Timing of palliative care referral and symptom burden in phase 1 cancer patients: A retrospective cohort study. Cancer 2010;116:4402–4409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Beernaert K, Pardon K, Van den Block L, et al. : Palliative care needs at different phases in the illness trajectory: A survey study in patients with cancer. Eur J Cancer Care 2016;25:534–543 [DOI] [PubMed] [Google Scholar]
- 22.Rouanne M, Massard C, Hollebecque A, et al. : Evaluation of sexuality, health-related quality-of-life and depression in advanced cancer patients: A prospective study in a phase I clinical trial unit of predominantly targeted anticancer drugs. Eur J Cancer 2013;49:431–438 [DOI] [PubMed] [Google Scholar]
- 23.Atherton PJ, Szydlo DW, Erlichman C, et al. : What can phase I clinical trials tell us about quality of life? A pilot study (MC0115). Clin Res Trials 2015;1:11–14 [Google Scholar]
- 24.Kim JY, Sun V, Raz DJ, et al. : The impact of lung cancer surgery on quality of life trajectories in patients and family caregivers. Lung Cancer 2016;101:35–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Godskesen T, Ngyren P, Nordin K, et al. : Phase 1 clinical trials in end-stage cancer: Patient understanding of trial premises and motives for participation. Support Care Cancer 2013;21:3137–3142 [DOI] [PubMed] [Google Scholar]
- 26.Johnson S, Butow P, Kerridge I, et al. : Advance care planning for cancer patients: A systematic review of perceptions and experiences of patients, families, and healthcare providers. Psychooncology 2016;25:362–386 [DOI] [PubMed] [Google Scholar]