Abstract
Introduction: Pediatric Acute-onset Neuropsychiatric Syndrome (PANS) is characterized by the sudden onset of severe obsessive-compulsive symptoms and/or eating restriction along with at least two coinciding neuropsychiatric symptoms. When associated with group A Streptococcus, the syndrome is labeled Pediatric Autoimmune Neuropsychiatric Disorder Associated with Streptococcal infections (PANDAS). An abnormal immune response to infection and subsequent neuroinflammation is postulated to play an etiologic role. We evaluated the impact of nonsteroidal anti-inflammatory drug (NSAID) treatment on flare duration in PANS/PANDAS.
Methods: Patient inclusion criteria: Patients were included if they had at least one neuropsychiatric deterioration (“flare”) that met strict PANS/PANDAS research criteria and for which flare duration could be assessed. Flare inclusion criteria: Any flare that started before October 15, 2016 was included and followed until the flare resolved or until the end of our data collection (November 1, 2016). Flare exclusion criteria: Flares were excluded if they were incompletely resolved, treated with aggressive immunomodulation, or treated with NSAIDs late (>30 days of flare onset). Ninety-five patients met study inclusion criteria and collectively experienced 390 flares that met flare criteria. Data were analyzed using multilevel linear models, adjusting for demographics, disease, and treatment covariates.
Results: NSAID use was associated with a significantly shorter flare duration. Flares not treated with NSAIDs had a mean duration of approximately 12.2 weeks (95% CI: 9.3–15.1). Flares that occurred while the child was on NSAID maintenance therapy were approximately 4 weeks shorter than flares not managed with NSAIDs (95% CI: 1.85–6.24; p < 0.0001). Flares treated with NSAIDs within 30 days of flare onset were approximately 2.6 weeks shorter than flares not managed with NSAIDs (95% CI: 0.43–4.68; p = 0.02). Flares treated prophylactically and those treated early with NSAIDs did not differ in duration (p = 0.26). Among the flares that received NSAID treatment within the first 30 days, earlier intervention was modestly associated with shorter flare durations (i.e., for each day that NSAID treatment was delayed, flare duration increased by 0.18 weeks; 95% CI: 0.03–0.33; p = 0.02), though it was not statistically significant after controlling for covariates (p = 0.06).
Conclusion: NSAIDs given prophylactically or within 30 days of flare onset may shorten neuropsychiatric symptom duration in patients with new-onset and relapsing/remitting PANS and PANDAS. A randomized placebo-control clinical trial of NSAIDs in PANS is warranted to formally assess treatment efficacy.
Keywords: : PANS, PANDAS, NSAIDs
Introduction
Pediatric Acute-onset Neuropsychiatric Syndrome (PANS) is characterized by the abrupt and dramatic onset of obsessive-compulsive (OC) symptoms and/or severely restrictive food intake with at least two coinciding, equally debilitating symptoms (anxiety, mood dysregulation, irritability/aggression/oppositionality, behavioral regression, cognitive deterioration, sensory amplification, motor abnormalities, urinary frequency or enuresis, and sleep disturbances) (Swedo et al. 2012; Chang et al. 2015). When symptom onset is associated with group A Streptococcus (GAS), the disorder is termed Pediatric Autoimmune Neuropsychiatric Disorder Associated with Streptococcal infections (PANDAS) (Swedo et al. 1998). The etiology of PANS is currently unknown, but given the phenotypic overlap with Sydenham's chorea (Williams and Swedo 2015), as well as basic and clinical research (Hoffman et al. 2004; Yaddanapudi et al. 2010; Brimberg et al. 2012; Cox et al. 2013; Lotan et al. 2014; Kumar et al. 2015; Macrì et al. 2015; Cutforth et al. 2016; Dileepan et al. 2016), a neuro-inflammatory process is postulated.
The Stanford PANS Program, created to research the etiology of and treatment for this disorder, conducts a community-based interdisciplinary clinic that is designed to evaluate and treat youth with suspected PANS or PANDAS. Our program accepts patients who live locally (83% live within 90 miles). For our local cohort, we require frequent follow-up visits (every 1–2 weeks during flare and every 4–12 weeks during remissions).
In our clinic, patients' PANS symptoms often relapse and remit. Relapses (acute deteriorations in neuropsychiatric symptoms, referred to as “flares”) can be severely debilitating, often lasting weeks to months. As a flare resolves, neuropsychiatric symptoms improve; however, patients often report persistent fatigue, pain, and arthritis, similar to what is noted in other inflammatory conditions, including systemic lupus erythematosus (Benseler and Silverman 2007; Petri et al. 2016).
Treatment of PANS flares includes eliminating associated factors contributing to inflammation (e.g., infection), cognitive behavioral therapy (CBT), psychiatric medications, and addressing inflammation itself. Consensus guidelines for immunomodulatory interventions have been developed, recommending therapies depending on disease severity and course (Frankovich et al. 2017). The use of nonsteroidal anti-inflammatory drugs (NSAIDs) is included in these recommendations which are based on clinical experience, as there are limited reports on the efficacy of NSAIDs in PANS (Spartz et al. 2017).
When our PANS clinic opened in September 2012, clinicians initially prescribed NSAIDs for arthritis and pain, as this is the standard of care for juvenile arthritis. Interestingly, in addition to improvement in joint pain, temporal associations between NSAID introduction and psychiatric symptom improvement were noted (Spartz et al. 2017). These observations are congruent with burgeoning anecdotal experiences shared between parents of affected youth in online PANS/PANDAS forums.
To further investigate the link between NSAID use and PANS flare characteristics, we designed this retrospective case review with the following two aims (developed a priori): (1) to assess the impact of NSAID treatment on flare duration in patients with new-onset PANS and relapsing/remitting illness (primary analysis); (2) to evaluate the impact of timing of NSAID introduction on flare duration.
Methods
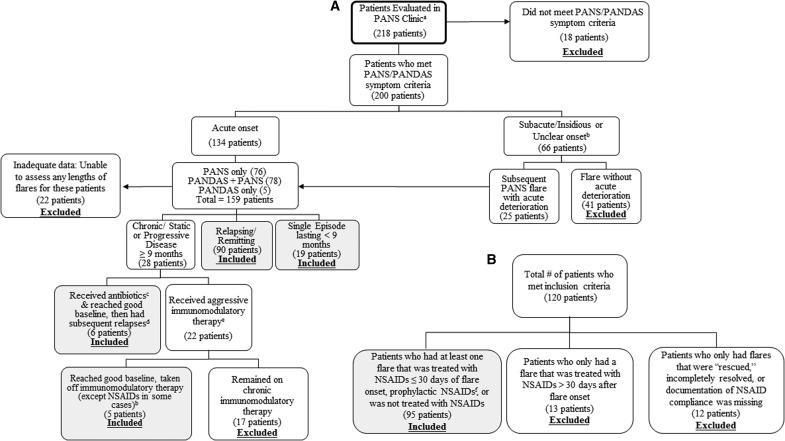
We reviewed clinical electronic medical records (EMR) of 218 consecutive patients of the Stanford PANS Clinic between September 1, 2012 and October 15, 2016 or by our PANS psychiatrist (MT) before starting the formal multidisciplinary clinic (Fig. 1A). Patients were prescreened before registering with our clinic to increase the probability that patients seen would meet criteria for PANS or PANDAS. The Stanford Panel on Human Subjects Institutional Review board approved this study. All definitions, inclusion criteria, exclusion criteria, statistical methods, and covariates listed later were developed a priori.
FIG. 1.
(A) Patients evaluated at the Stanford PANS clinic. (B) Patients who met study inclusion criteria. aPatients were prescreened before clinic entry, which is why a large fraction of patients ultimately met criteria. bThese patients did not meet full PANS/PANDAS criteria since the onset was not acute. cAntibiotics given for infection (i.e., group A Streptococcus, sinusitis, otitis media, Mycoplasma pneumoniae, etc.). dIn this group, only subsequent relapses were included in the flare analyses. eHigh-dose IVIG, intravenous methylprednisolone pulse(s) (30 mg/kg), plasma exchange, mycophenylate mofetil, rituximab, etc. fPatient was on NSAIDs before a subsequent flare for two reasons: (1) For treatment of arthritis and/or pain or (2) Attempts to remove NSAID resulted in psychiatric symptom recrudescence; thus, the patient was continued on NSAIDs chronically. PANS, Pediatric Acute-onset Neuropsychiatric Syndrome; PANDAS, Pediatric Autoimmune Disorder Associated with Streptococcal infections; NSAIDs, nonsteroidal anti-inflammatory drugs; IVIG, intravenous immunoglobulin.
Definitions of clinical course
Relapsing/remitting course is defined as an abrupt onset of symptom relapses (“flares”) followed by an approximate return to 90%–95% of preflare baseline functioning without the need for aggressive immunomodulatory therapy (e.g., intravenous immunoglobulin [IVIG], mycophenolate mofetil, plasma exchange, rituximab, etc.). Chronic-static course is defined as a course with persistent unchanging PANS symptoms lasting at least 9 months. Progressive disease is a chronic course in which PANS symptoms worsen in intensity over time.
Patient inclusion criteria
Patients were included in the analysis if they had at least one acute neuropsychiatric deterioration (“flare”) that strictly met both research criteria for PANS or PANDAS (Swedo et al. 1998, 2012; Chang et al. 2015) and flare inclusion criteria; and for which there was adequate documentation to assess flare duration (Fig. 1A). Patients with a single flare or relapsing/remitting course were included in the analyses (Fig. 1A). As noted in Figure 1A, eleven patients with chronic-static or progressive disease were included in the study because they eventually returned to their pre-PANS baseline without requiring chronic immunomodulatory therapy. These patients experienced subsequent deteriorations, which were included in the flare analyses.
Patient exclusion criteria
Patients were excluded if they required aggressive disease-modifying therapies such as rituximab, cyclophosphamide, mycophenolate mofetil, and/or chronic immunomodulatory therapy to sustain baseline functioning (i.e., monthly IVIG and/or monthly intravenous [IV] methylprednisolone pulses). Patients who remained chronic-static or progressive were excluded from the study (Fig. 1).
Study entry date
Study entry begins at the time of the first clearly documented neuropsychiatric deterioration (“flare”) that meets strict PANS/PANDAS research criteria and flare inclusion criteria. This “first flare” may have occurred before entry/registration in our PANS clinic. Study entry is therefore not necessarily coterminous with entry/registration in the PANS clinic.
Definition of flare duration
A flare is defined as an acute neuropsychiatric deterioration that meets strict PANS or PANDAS research criteria. Flares and flare durations were identified by using our standard psychiatric and neurological clinical assessments (documented in the EMR), our PANS questionnaires, which are based on tracking forms used in the recent PANDAS clinical trial (Williams et al. 2016), the PANS Global Impairment Scale, and documented communications (emails and phone calls) between parents and providers. The PANS Global Impairment Scale was developed by Dr. James Leckman and colleagues and the National Institute of Mental Health (personal communication). A flare was considered resolved when the patient was functioning at or near their preflare baseline based on clinician documentation, patient questionnaires, and parent recall.
Flare inclusion criteria
Any flare that started before October 15, 2016 was included and followed until the flare resolved or until the end of our data collection (November 1, 2016). Included flares could have occurred either during treatment in our PANS clinic or before clinic entry if there was adequate historical documentation to determine flare duration (weeks). Historical documentation reviewed includes medical records, therapy records, and patient questionnaires.
Flare exclusion criteria
We created exclusion criteria for individual patients and for individual flares. For example, a patient could have been eligible for study inclusion, while one or more of the patient's flares were excluded.
“Rescued” flares
“Rescued” flares are those that were severe enough to be treated with IVIG, high-dose IV methylprednisolone (30 mg/kg, maximum: 1 g/dose), or plasma exchange, in addition to NSAIDs, oral corticosteroids, and/or antibiotics. “Rescued” flares were excluded from the analyses because they required escalating immunomodulatory treatment to achieve resolution and were therefore clinically distinct from other flares (n = 61).
Incompletely resolved flares
Flares that were unresolved before a subsequent deterioration were excluded (n = 14).
Late NSAID-treated flares
When the initiation of NSAID treatment for a flare was more than 30 days after the flare onset, the flare was excluded (n = 44). These flares were not included in the analysis for two reasons: First, the amount of time until NSAID initiation is linearly related to flare duration. Therefore, flares with late NSAID use would likely last longer than other flares for reasons that are inconsequential to the analysis. Second, we hypothesized that flares treated in the late phase of disease may not be as responsive to NSAIDs, a phenomenon seen in other inflammatory diseases such as juvenile arthritis (Wallace 2006).
Other excluded flares
Flares that occurred fewer than 50 days after a single treatment with IVIG, a pulse of high-dose IV methylprednisolone (30 mg/kg), or plasma exchange were excluded. This time-frame was chosen for two reasons. First, we expected that these medications may exert effects for 50 days based on the following logic: (1) the half-life of immunoglobulin G levels in healthy patients is 3–4 weeks, but longer for other disorders (Silvergleid and Ballow 2016) and (2) methylprednisolone pulse effects often last 2–6 weeks in other autoimmune conditions, which is why many rheumatologic protocols use monthly methylprednisolone pulse regimes (Barile-Fabris et al. 2005; Then Bergh et al. 2006; Huber et al. 2012; Li et al. 2012). Second, many patients in our clinic experience a recrudescence of symptoms approximately 3–4 weeks after IVIG and/or IV methylprednisolone. It was unclear whether recrudescence of symptoms during this 50 day period was due to medication waning or a new onset flare, and we therefore excluded these flares.
Lastly, eight flares were excluded because the timing of NSAID initiation was not recorded, and three flares were excluded because patient compliance with NSAID prescription was not documented.
Definition of time since onset of PANS illness
Time since onset of PANS illness is defined as the number of weeks since the onset of the patient's initial neuropsychiatric deterioration that met PANS or PANDAS symptom criteria. For 79 out of 95 (83%) patients included in the study, the initial neuropsychiatric deterioration met strict PANS or PANDAS onset criteria (acute onset ≤72 hours). For the remaining 16 patients, the acuity of their initial flare onset was unclear. These 16 patients were still included in the study because they experienced a future flare that clearly met strict PANS or PANDAS research criteria.
Definition of NSAID treatment
NSAID treatment is defined as the use of NSAIDs that was started after the flare onset date and was prescribed for at least 7 days during a PANS flare. Prophylactic NSAID treatment is defined as the use of NSAIDs before the flare onset date, with subsequent use throughout the duration of the flare. Early NSAID treatment describes the introduction of NSAIDs within 30 days of the flare onset.
NSAID treatment protocol
PANS clinic patients are evaluated and treated for underlying infections on presentation to the clinic, in accordance with PANS consensus diagnostic guidelines (Chang et al. 2015) and PANS infection treatment guidelines (Cooperstock et al. 2017). Before January 2015, our clinicians did not routinely prescribe NSAIDs for treatment of PANS flares, but rather offered them to treat arthritis and/or pain. Starting January 2015, clinicians routinely recommended NSAIDs to treat new-onset PANS or PANS flares. Clinicians also offered NSAIDs for treatment of arthritis, pain, or residual neuropsychiatric symptoms. Naproxen was dosed at 10 mg/kg every 12 hours (maximum: 500 mg/dose). Ibuprofen was dosed at 10 mg/kg every 6–8 hours (maximum: 600 mg/dose). Sulindac was dosed at 2–4 mg/kg every 12 hours (maximum: 6 mg/kg/day, adult maximum dose = 400 mg/day divided twice a day). Celecoxib was dosed at 50–100 mg twice a day. These are the same dosage regimens used by pediatric rheumatologists to treat other inflammatory disorders. Many of our patients with PANS have fluid restriction (as determined by clinical history, high specific gravity on urine analyses, and/or high BUN/Cr ratio). With these patients, we reduced the dose or withheld NSAIDs. We monitored for headaches, allergic reactions, bruising, gastrointestinal side effects, transaminitis, renal function, and hematuria. Liver enzymes, renal function tests, and urinalysis were obtained every 3–6 months for patients on chronic NSAIDs.
Statistical analyses
For analyses that use data with repeated observations on the same patient (Table 3), we employed a multilevel linear model that accounts for within-subject correlation. For analyses with no repeated measures (i.e., one flare per patient; Table 4), we used a standard general linear model. We report the results of an initial model in which the target variable(s) of interest are evaluated without covariates, as are the results with the full complement of putative covariates. Both the multilevel linear and standard general linear models were adjusted for the following covariates: sex, age at flare onset, weeks since onset of PANS illness, antibiotic treatment during flare, prophylactic antibiotics on board before flare, previous flare treated with immunomodulation (IVIG, IV methylprednisolone, and/or plasma exchange), oral corticosteroids, number of psychiatric medications, and use of CBT during flare. Results of the models are presented as the regression parameter estimate and 95% confidence interval (CI); this is interpreted as the change in the dependent variable associated with a one-unit increase in the independent variable. Given that maximum likelihood estimation cannot be relied on to accommodate missing X-side data, multiple imputation routines in Mplus software (Muthén and Muthén 2012) were used to generate pooled results across 20 datasets, accounting for the multilevel structure of the data and the distribution of the variables. Given the exploratory nature of the analyses, p-values were not adjusted for multiple comparisons.
Table 3.
Effect of Prophylactic or Early NSAIDs, Compared with No NSAIDs, on Flare Duration (in Weeks) (Number of Patients = 95, Number of Flares = 390)
| Unadjusted model | Model adjusted for demographic/disease variables | Final model, adjusted for demographic/disease and treatment variables | ||||
|---|---|---|---|---|---|---|
| B | 95% CI | B | 95% CI | B | 95% CI | |
| NSAID Treatment (Reference = None) | ||||||
| Early (≤ 30 days) | −3.78** | (−6.10 to −1.46) | −3.27** | (−5.56 to −0.98) | −2.56* | (−4.68 to −0.43) |
| Prophylactic | −5.52** | (−7.62 to −3.42) | −4.28** | (−6.59 to −1.96) | −4.05** | (−6.24 to −1.85) |
| Age (years) at PANS flare | 0.08 | (−0.23–0.38) | −0.30 | (−0.60–0.01) | ||
| Sex (male/female) | −0.84 | (−2.84–1.16) | −0.95 | (−2.74–0.85) | ||
| Weeks since onset of PANS illness | −0.01 | (−0.02–0.0) | −0.01 | (−0.01–0.0) | ||
| Previous flare treated with aggressive immunomodulatory therapya | −3.00* | (−5.74 to −0.26) | −3.44** | (−5.95 to −0.92) | ||
| Patient on prophylactic antibiotics at onset of flare | −1.79 | (−3.73–0.15) | −0.93 | (−2.93–1.06) | ||
| Antibiotics used to treat infection during flareb | 1.87* | (0.34–3.40) | ||||
| Oral corticosteroids given during flare | −1.14 | (−2.98–0.70) | ||||
| Number of psychiatric medications during flare | 1.50** | (0.79–2.21) | ||||
| Cognitive behavioral therapy during flare | 4.13** | (2.04–6.22) | ||||
High-dose IVIG, intravenous methylprednisolone pulse(s) (30 mg/kg), or plasma exchange.
Common infections treated during flare include group A Streptococcus, sinusitis, otitis media, Mycoplasma pneumoniae, etc.
p < 0.01, *p < 0.05
Results are from a multilevel model; the unstandardized B value is interpreted as the expected change in flare duration, with a one-unit change in the independent variable. For NSAID use, this is interpreted as the difference between the level of NSAID use (Early or Prophylactic) and the None category, which is the reference. For other categorical variables, the second category is the reference. For example, in the final model, early NSAID use is associated with a duration that is 2.56 weeks shorter than when no NSAID was used, whereas prophylactic NSAID use is associated with a duration that is 4.05 weeks shorter. Prophylactic and Early treatment did not differ from one another in flare duration: Final model, B(SE) = 1.49 (1.32), p = 0.26.
B, beta; CI, confidence interval.
Table 4.
Effect of Time to NSAID on Flare Duration (in Weeks) for Early NSAID-Treated Flares (Number of Patients = 36, Number of Flares = 36)
| Unadjusted model | Adjusted for demographic/disease and treatment variables | Adjusted only for covariates with p < 0.20 | ||||
|---|---|---|---|---|---|---|
| B | 95% CI | B | 95% CI | B | 95% CI | |
| Time to NSAID (days)a | 0.18* | 0.03–0.33 | 0.15 | −0.01–0.32 | 0.17* | 0.03–0.31 |
| Age (years) at PANS flare | −0.06 | −0.59–0.48 | ||||
| Sex (male/female) | −0.94 | −3.83–1.95 | ||||
| Weeks since onset of PANS illness | 0 | −0.02–0.01 | ||||
| Previous flare treated with aggressive immunomodulatory therapyb | −1.07 | −5.99–3.85 | ||||
| Patient on prophylactic antibiotics at onset of flare | 0.18 | −2.97–3.33 | ||||
| Antibiotics used to treat infection during flarec | 2.52 | −0.21–5.25 | 2.64* | 0.28–5.00 | ||
| Oral corticosteroids given during flare | 0.27 | −2.70–3.23 | ||||
| Number of psychiatric medications during flare | 1.40 | −1.23–4.03 | ||||
| Cognitive behavioral therapy during flare | 0.66 | −3.01–4.33 | ||||
Number of days from flare onset to NSAID initiation.
High-dose IVIG, intravenous methylprednisolone pulse(s) (30 mg/kg), or plasma exchange.
Common infections treated during flare include group A Streptococcus, sinusitis, otitis media, Mycoplasma pneumoniae, etc.
p < 0.05
Results are from a standard general linear model with no repeated observations; the unstandardized B value is interpreted as the expected change in flare duration, with a one-unit change in the independent variable. For example, in the final model, for every day that NSAID initiation is delayed, the flare duration increases by 0.17 weeks. For categorical variables, the second category is the reference.
Results
Of the 120 eligible patients (Fig. 1B), 95 patients met all inclusion criteria and were included in the current analyses (Table 1). Thirteen patients were excluded because they received only late NSAID treatment, and 12 patients were excluded because the flares were “rescued,” the flares were incompletely resolved, or documentation of NSAID use was missing or unclear (Fig. 1B).
Table 1.
Participant Characteristics (Number of Patients = 95)
| M ± SD | Range | |
|---|---|---|
| Age at onset of PANS illness | 7.90 ± 3.44 | 2.21–17.7 |
| Flares per patient in dataset | ||
| Flares that received no NSAID treatment (Np = 82, Nf = 271) | 3.30 ± 2.77 | 1–13 |
| Flares that received early NSAID treatment (Np = 36, Nf = 43) | 1.19 ± 0.58 | 1–4 |
| Flares that received prophylactic NSAIDs (Np = 39, Nf = 76) | 1.95 ± 1.10 | 1–5 |
| n (%) | ||
|---|---|---|
| Male | 58/95 (61%) | |
| Obsessive-compulsive disorder | 85/95 (89%) | |
| Eating restrictiona | 49/94 (52%) | |
| Anxiety | 92/95 (97%) | |
| Mood dysregulationb | 93/95 (98%) | |
| Irritability/oppositionality/aggression | 89/95 (94%) | |
| Behavioral regressiona | 71/94 (76%) | |
| Academic deteriorationa | 64/91 (70%) | |
| Sensory or motor symptoms | 91/95 (96%) | |
| Somatic symptoms | 92/95 (97%) |
Some missing data: eating restriction and behavioral regression, n = 1. Academic deterioration, n = 4.
Mood dysregulation, including depression and/or emotional lability.
PANS, Pediatric Acute-onset Neuropsychiatric Syndrome; NSAIDs, nonsteroidal anti-inflammatory drugs; NP, number of patients; NF, number of flares.
Of the 517 eligible flares, 390 met flare inclusion criteria and were included in the current analyses (Table 2). The majority of flares were not treated with NSAIDs (n = 271), followed by prophylactic NSAID treatment (n = 76) and early NSAID treatment (n = 43). Accounting for multiple observations per individual, there were some significant differences (p < 0.05) among flare groups in covariates (Table 2).
Table 2.
Key Flare Characteristics (Covariates) Used in Analysis (Number of Patients = 95, Number of Flares = 390)
| Prophylactic NSAIDs | Early NSAID treatment | Not treated with NSAIDs | Comparison (p < 0.05)a | |
|---|---|---|---|---|
| Number of flares | 76 | 43 | 271 | N/A |
| Number of patients | 39 | 36 | 82 | N/A |
| Est. SE | Est. SE | Est. SE | ||
| Age (years) at PANS flare | 10.6 (0.46) | 11.2 (0.42) | 9.6 (0.37) | P:N, E:N |
| Male sex | 58% | 61% | 61% | None |
| Weeks since onset of PANS illness | 131.6 (1.17) | 160.0 (1.13) | 85.7 (1.13) | P:N, E:N |
| Previous flare treated with aggressive immunomodulatory therapyb | 13% (4%) | 15% (4%) | 10% (3%) | None |
| Patient on prophylactic antibiotics at onset of flare | 32% (7%) | 62% (7%) | 20% (4%) | P:E, P:N |
| Antibiotics used to treat infection during flarec | 58% (7%) | 37% (6%) | 57% (4%) | P:E, P:N |
| Oral corticosteroids given during flare | 35% (7%) | 36% (6%) | 18% (3%) | P:N, E:N |
| Number of psychiatric medications during flare | 0.9 (1.22) | 1.1 (1.19) | 0.8 (1.19) | P:N |
| Cognitive behavioral therapy during flare | 22% (6%) | 23% (6%) | 26% (4%) | None |
Significant differences between groups are demarcated by [group 1]:[group2], for example, P:N indicates that the Prophylactic and Not Treated groups differed significantly.
High-dose IVIG, intravenous methylprednisolone pulse(s) (30 mg/kg), or plasma exchange.
Common infections treated during flare include group A Streptococcus, sinusitis, otitis media, Mycoplasma pneumoniae, etc.
Contents of table are estimated values (with standard errors in parentheses) for the repeated-measures models, accounting for multiple observations per subject.
SE, standard error; P, prophylactic NSAIDs; E, treated with NSAIDs early (≤30 days of flare onset); N, not treated with NSAIDs; IVIG, intravenous immunoglobulin.
Both prophylactic and early NSAID treatment were associated with a significantly shorter flare duration than no NSAID use. Flares not treated with NSAIDs lasted approximately 12.2 weeks (95% CI: 9.3–15.1 weeks). Prophylactically treated flares were about 4 weeks shorter than flares not treated with NSAIDs (95% CI: 1.85–6.24, p < 0.0001), and early treated flares were about 2.5 weeks shorter (95% CI: 0.43–4.68, p = 0.018) (Table 3). Flare duration did not significantly differ between prophylactically and early treated NSAID flares (p = 0.26).
Given that early NSAID treatment is associated with shorter PANS flares, we hypothesized that a relationship may exist between the timing of NSAID introduction and flare duration. Among the early treated flares (within 30 days of flare onset, nf = 43), the majority of patients had only one flare (np = 36). Thus, the first flare for each patient was selected, resulting in a sample of 36 flares from 36 individuals. Within these early treated flares, the timing of NSAID was significantly associated with flare duration, such that each day that NSAID initiation was delayed was associated with an increase in flare duration of 0.18 weeks (95% CI: 0.03–0.33, p = 0.02) (Table 4). When all of the covariates were added to the model, this relationship became nonsignificant (p = 0.06). However the magnitude of the relationship remained unchanged, suggesting inadequate power due to the relatively small sample. When only covariates with at least marginal significance (p < 0.20) were retained, the effect of NSAID timing was again of a similar magnitude, but it was statistically significant (p = 0.016).
Of the 57 patients included in the analysis who had at least one flare treated with NSAIDs that met flare inclusion criteria, 11 (19%) patients had transient side effects. No patients developed clinically significant side effects. Side effects included abdominal pain (n = 5), skin rash (n = 1), bruising (n = 1), proteinuria (n = 3), and clinically insignificant transaminitis (n = 1). Abdominal pain self-resolved after stopping or reducing NSAID doses in four of the five patients; of these four, two patients tolerated NSAIDs when given to treat a subsequent PANS flare. The patient whose abdominal pain did not resolve after NSAID removal was believed to have abdominal pain amplification; when reintroducing the NSAID, the abdominal pain did not worsen. Skin rash and bruising self-resolved after stopping or reducing NSAID doses, and both of these patients tolerated NSAIDs when given to treat a subsequent PANS flare. Of the three patients with proteinuria, one had elevated urine protein/creatinine ratio (0.3) on a morning void, which resolved after removal of NSAIDs. In the other two patients, it is unclear whether the mild proteinuria (+1) observed on afternoon urinalyses was due to NSAIDs or orthostatic proteinuria. In one of these patients, the proteinuria resolved despite continued NSAID use. The other patient was lost to follow-up. One patient developed mildly elevated liver enzymes while on NSAIDs (AST = 53, normal range for age <50; ALT = 67, normal range for age <60); this patient had a concurrent viral illness that was believed to contribute to the transaminitis. The transaminitis self-resolved despite continued NSAID use.
Discussion
This is the first study to assess the impact of NSAIDs on flare duration in patients with PANS and/or PANDAS. Approximately one-third of the flares included in this study were treated with NSAIDs early or prophylactically. PANS flares treated with NSAIDs early or prophylactically appear to resolve more quickly than flares not treated with NSAIDs. Furthermore, earlier NSAID introduction was associated with faster improvement. This effect was not significant after entering covariates, which is likely a result of inadequate power; regardless of this, the effect was small, perhaps due to the restricted range of time to initiation (up to 30 days). Together, these data suggest that NSAID treatment may be superior to nontreatment and earlier initiation may be beneficial. Our findings have clear clinical importance since patients often present to specialty clinics several days to weeks after symptom onset, and they may still benefit from NSAID treatment.
Flare duration did not differ between flares treated with prophylactic NSAIDs and those treated with NSAIDs within 30 days of flare onset. It is unclear from this analysis whether this is a consequence of statistical power, or whether these results suggest that both treatment approaches may be similarly effective. In addition, it is likely that patients who received prophylactic NSAIDs were in a different disease state than those who received the NSAID after the flare began (i.e., those receiving prophylactic NSAID may have had arthritis or had a history of deteriorating after NSAIDs were stopped).
No patients developed clinically significant side effects; however, 19% reported transient side effects from NSAIDs. Many of the patients who did not tolerate NSAIDs tolerated a subsequent NSAID trial given for a future PANS flare. Therefore, an initial intolerance for NSAIDs should not dissuade NSAID use to treat a future PANS flare.
Wide variability in flare duration was observed in this study, which may be explained by the heterogeneity of the patient and flare groups with respect to disease severity, disease trajectory at the time of NSAID introduction, and flare trigger (i.e., bacterial, viral, etc.). Some patients in our clinic experience a dramatic and rapid response to NSAIDs, whereas others exhibit no response to NSAIDs (Spartz et al. 2017). Variability in response is also observed within individual patients; an individual may exhibit little-to-no response to an initial NSAID trial, but may respond to NSAIDs in a future milder flare (Spartz et al. 2017). These clinical observations, coupled with our current findings that prophylactic NSAIDs are associated with shorter flares, suggest that failure to have an initial response should not dissuade NSAID use for future flares. However, formal clinical trials are required to identify the predictors of response and to evaluate the risk-to-benefit ratio systematically.
Inflammation may play a role in triggering and/or exacerbating PANS/PANDAS symptoms in individuals. Animal and human research suggests that the adaptive immune response (autoantibodies and Th17 cells), neuroinflammation, and microglial activation all may contribute to the pathogenesis of poststreptococcal neuropsychiatric diseases (Kirvan et al. 2003, 2006a, 2006b; 2007; Hoffman et al. 2004; Yaddanapudi et al. 2010; Brimberg et al. 2012; Cox et al. 2013; Lotan et al. 2014; Kumar et al. 2015; Macrì et al. 2015; Williams and Swedo 2015; Xu et al. 2015; Carapetis et al. 2016; Cutforth et al. 2016; Dileepan et al. 2016). In addition, a positron emission tomography (PET) imaging study found greater microglial activation in patients with PANDAS compared with controls (Kumar et al. 2015). Another study found an association between the tumor necrosis factor (TNF)-α gene −308G/A polymorphism and patients with PANDAS (Luleyap et al. 2013). A recent animal model designed to evaluate the relationship between nasopharyngeal infection and neuroinflammation showed that multiple intranasal infections with live GAS generate GAS-specific Th17 cells that migrate along olfactory sensory axons into the brain. Migration of Th17 cells into the brain was associated with neurovascular damage, including increased blood-brain barrier (BBB) permeability and neuroinflammation (Dileepan et al. 2016). NSAIDs inhibit the cyclooxygenase (COX) enzyme, which influences multiple inflammatory mechanisms. Notably, human, postmortem, and animal studies show that NSAIDs can affect all the inflammatory pathways mentioned earlier, including reducing the Th17 response (Chizzolini et al. 2008; Napolitani et al. 2009), reducing TNF-α (Iñiguez et al. 1999, 2010), reducing microglial activation (Mackenzie and Munoz 1998; Klegeris et al. 2004), and reducing BBB permeability (Candelario-Jalil et al. 2007; Brooks et al. 2008). Of importance, we do not know whether shortened PANS flare durations associated with NSAIDs are direct effects (due to the anti-inflammatory properties of NSAIDs on neuroinflammation) or indirect effects (due to reduction in BBB permeability, thus reducing autoantibody access into the central nervous system).
Interestingly, NSAIDs have been observed to be beneficial in other psychiatric conditions. Two clinical trials in adult patients with OC disorder showed celecoxib to be helpful in reducing OC symptoms (Sayyah et al. 2011; Shalbafan et al. 2015). In addition, clinical trials have suggested some benefit of NSAIDs in depression, bipolar disorder, and schizophrenia (Müller et al. 2004; Abbasi et al. 2012; Arabzadeh et al. 2015).
Within our PANS cohort, we previously reported a high rate of arthritis and inflammatory back pain in our PANS cohort (Frankovich et al. 2015; Brown et al. 2016), for which NSAIDs are the cornerstone treatment. For these conditions, NSAIDs are the most effective when they are used early in the disease course and in milder disease. NSAIDs are also used for a number of inflammatory diseases, including juvenile arthritis, systemic lupus erythematosus, Behçet's disease, etc., but they are often used in the context of milder disease and in patients with concurrent arthritis and/or myalgia (Sirnsek et al. 1991; Lander et al. 2002; Wallace 2006). In juvenile arthritis, NSAIDs are often extended beyond the resolution of arthritis by 3–6 months to avoid the recurrence of arthritis that is associated with early discontinuation (Wallace 2006). We have found that in some patients with PANS, arthritis, pain, and psychiatric symptoms relapse after NSAIDs are removed, and they resolve when NSAIDs are reintroduced (Spartz et al. 2017).
As in other inflammatory diseases, more aggressive induction regimens and maintenance immunomodulatory therapy are often necessary to achieve durable remissions in PANS patients, especially in patients with moderate-to-severe disease, chronic-static or progressive courses, or frequent relapses. This study excluded patients who had a chronic-static or progressive disease (i.e., those who required aggressive immunomodulatory regimens). Thus, our results are not generalizable to these patient subgroups. However, NSAIDs may be an important adjunct therapy in these groups as they are often used in combination with other anti-inflammatory medications in more advanced juvenile arthritis.
Limitations
The design of this study was a retrospective chart review, which has several attendant limitations that we attempted to ameliorate with clear reporting of our decisions to include and exclude patients and flares. One major difficulty with this type of design is variability in the quality of documentation; many of the flares treated in the early days of our clinic did not have clear records of start and end dates. In addition, we failed to capture some data when patients did not follow up with our clinic regularly, or when they had flares between clinic visits that lacked proper documentation. However, we expect that the most clinically significant flares resulted in clinic visits, as the majority of our patients (83%) live within 90 miles of the clinic. Although many of these flares/patients were excluded (per flare criteria), flares meeting criteria but that had missing data were retained. The statistical methodology we selected is robust to the influence of these missing data.
Other limitations include the current absence of reliable and validated psychiatric assessment instruments that are specific to PANS. No single measure adequately reports impairment in all of the psychiatric domains affected in PANS and PANDAS. Although derived from validated instruments, the PANS Global Impairment Score has not been validated. Therefore, our primary analysis focused on flare duration rather than symptom level, an approach used in Sydenham's chorea, another poststreptococcal neuropsychiatric disease (Barash et al. 2005; Paz et al. 2006; Walker et al. 2007). Parent questionnaires, clinic visit data, email, and phone communications (recorded in the EMR and personal communication) were used to help inform the outcome variables. Another limitation is that the research assistant (KB) who conducted the retrospective chart review was not blinded to treatment status.
This study under-reports the true NSAID side effect rate since only patients who tolerated NSAIDs for a minimum of 7 days were included in this study, thus eliminating patients who may have had side effects in the first week of NSAID therapy. The true NSAID side effect rate may be as high as 33% (Spartz et al. 2017), but the vast majority of these side effects are clinically insignificant, transient, and self-resolved.
Conclusions
Our results suggest that NSAIDs given prophylactically or early in the disease flare are associated with shorter flare durations in patients with new-onset and relapsing/remitting PANS and PANDAS. Earlier treatment with NSAIDs for a PANS flare may lead to a faster remission. These findings are consistent with the impact of NSAIDs in other inflammatory diseases, including juvenile arthritis. A randomized placebo-control clinical trial of NSAIDs in PANS is warranted to formally assess NSAID treatment efficacy.
Clinical Significance
The results of this retrospective, observational study warrant a randomized, placebo-control trial to definitively evaluate the impact of NSAIDs treatment and prophylaxis on PANS symptoms and subsequent flares, and to systematically evaluate predictors of response. The high rate of relapse observed in patients on prophylactic NSAIDs calls for identification of other treatments or adjunct treatments that may result in a more durable remission. As in other inflammatory disorders, clinicians may consider adjunctive therapies such as corticosteroid bursts (Brown et al. 2017), daily low-dose corticosteroids (as with other inflammatory diseases such as lupus and asthma), hydroxychloroquine, and other corticosteroid-sparing agents in combination with NSAIDs. This study demonstrates that for PANS and PANDAS initial flares and relapses, NSAIDs are associated with shorter neuropsychiatric symptom duration when given early in disease flare or prophylactically. In the absence of clinical trial data, NSAIDs are recommended to treat mild-to-moderate PANS flares, with periodic trials off and side-effect monitoring to evaluate for efficacy in alleviating symptoms (Frankovich et al. 2017).
Acknowledgments
The authors would like to acknowledge Dr. Susan Swedo, MD, The National Institute of Mental Health, the PANDAS Physicians Network, Dr. Kiki Chang, MD, SPARK Translational Research Program, and all the staff and faculty at Lucille Packard Children's Hospital and Stanford PANS Clinic who make caring for these children possible. This work was supported (in part) by the Intramural Research Program of the National Institute of Mental Health and the PANDAS Physician Network.
Disclosures
No competing financial interests exist.
References
- Abbasi SH, Hosseini F, Modabbernia A, Ashrafi M, Akhondzadeh S: Effect of celecoxib add-on treatment on symptoms and serum IL-6 concentrations in patients with major depressive disorder: Randomized double-blind placebo-controlled study. J Affect Disord 141:308–314, 2012 [DOI] [PubMed] [Google Scholar]
- Arabzadeh S, Ameli N, Zeinoddini A, Rezaei F, Farokhnia M, Mohammadinejad P, Ghaleiha A, Akhondzadeh S: Celecoxib adjunctive therapy for acute bipolar mania: A randomized, double-blind, placebo-controlled trial. Bipolar Disord 17:606–614, 2015 [DOI] [PubMed] [Google Scholar]
- Barash J, Margalith D, Matitiau A: Corticosteroid treatment in patients with Sydenham's chorea. Pediatr Neurol 32:205–207, 2005 [DOI] [PubMed] [Google Scholar]
- Barile-Fabris L, Ariza-Andraca R, Olguín-Ortega L, Jara LJ, Fraga-Mouret A, Miranda-Limón JM, Fuentes de la Mata J, Clark P, Vargas F, Alcocer-Varela J: Controlled clinical trial of IV cyclophosphamide versus IV methylprednisolone in severe neurological manifestations in systemic lupus erythematosus. Ann Rheum Dis 64:620–625, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benseler SM, Silverman ED: Systemic Lupus Erythematosus. Rheum Dis Clin North Am 33:471–498, 2007 [DOI] [PubMed] [Google Scholar]
- Brimberg L, Benhar I, Mascaro-Blanco A, Alvarez K, Lotan D, Winter C, Klein J, Moses AE, Somnier FE, Leckman JF, Swedo SE, Cunningham MW, Joel D: Behavioral, pharmacological, and immunological abnormalities after streptococcal exposure: A novel rat model of Sydenham chorea and related neuropsychiatric disorders. Neuropsychopharmacology 37:2076–2087, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks T a, Nametz N, Charles R, Davis TP: Diclofenac attenuates the regional effect of lambda-carrageenan on blood-brain barrier function and cytoarchitecture. J Pharmacol Exp Ther 325:665–673, 2008 [DOI] [PubMed] [Google Scholar]
- Brown K, Farmer C, Farhadian B, Hernandez J, Thienemann M, Frankovich J: Pediatric Acute-onset Neuropsychiatric Syndrome (PANS)- response to oral corticosteroid bursts: An observational study of patients in an academic community-based PANS clinic. J Child Adolesc Psychopharmacol 2017. [Epub ahead of print]. DOI: 10.1089/cap.2016.0139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown KD, Mahony T, Thienemann M, Chang K, Frankovich J: High rate of Inflammatory Back Pain in Pediatric OCD-related psychiatric syndromes. Chicago: Int OCD Found 23rd Annu OCD Conf, July 28, 2016. (abstract/poster #38) [Google Scholar]
- Candelario-Jalil E, Taheri S, Yang Y, Sood R, Grossetete M, Estrada EY, Fiebich BL, Rosenberg GA: Cyclooxygenase inhibition limits blood-brain barrier disruption following intracerebral injection of tumor necrosis factor-alpha in the rat. J Pharmacol Exp Ther 323:488–498, 2007 [DOI] [PubMed] [Google Scholar]
- Carapetis JR, Beaton A, Cunningham MW, Guilherme L, Karthikeyan G, Mayosi BM, Sable C, Steer A, Wilson N, Wyber R, Zühlke L: Acute rheumatic fever and rheumatic heart disease. Nat Rev Dis Prim 2:15084, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang K, Frankovich J, Cooperstock M, Cunningham MW, Latimer ME, Murphy TK, Pasternack M, Thienemann M, Williams K, Walter J, Swedo SE: Clinical evaluation of youth with pediatric acute-onset neuropsychiatric syndrome (PANS): Recommendations from the 2013 PANS Consensus Conference. J Child Adolesc Psychopharmacol 25:3–13, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chizzolini C, Chicheportiche R, Alvarez M, De Rham C, Roux-Lombard P, Ferrari-Lacraz S, Dayer JM: Prostaglandin E2 synergistically with interleukin-23 favors human Th17 expansion. Blood 112:3696–3703, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooperstock M, Murphy TK, Pasternack M, Swedo SE: Clinical Management of Pediatric Acute-onset Neuropsychiatric Syndrome (PANS): Part III- Treatment and Prevention of Infections. J Child Adolesc Psychopharmacol, 2017. [Epub ahead of print]. DOI: 10.1089/cap.2016.0151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox CJ, Sharma M, Leckman JF, Zuccolo J, Zuccolo A, Kovoor A, Swedo SE, Cunningham MW: Brain Human Monoclonal Autoantibody from Sydenham Chorea Targets Dopaminergic Neurons in Transgenic Mice and Signals Dopamine D2 Receptor: Implications in Human Disease. J Immunol 191:5524–5541, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutforth T, Demille MMC, Agalliu I, Agalliu D: CNS autoimmune disease after Streptococcus pyogenes infections: Animal models, cellular mechanisms and genetic factors. Future Neurol 11:63–76, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dileepan T, Smith ED, Knowland D, Hsu M, Platt M, Bittner-Eddy P, Cohen B, Southern P, Latimer E, Harley E, Agalliu D, Cleary PP: Group A Streptococcus intranasal infection promotes CNS infiltration by streptococcal-specific Th17 cells. J Clin Invest 126:303–317, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankovich J, Swedo SE, Murphy T, Dale RC, Agalliu D, Williams K, Hornig M, Chugani H, Sanger T, Muscal E, Pasternack M, Cooperstock M, Gans H, Zhang Y, Cunningham M, Bernstein G, Bromberg R, Willet T, Brown K, Farhadian B, Chang K, Geller D, Hernandez J, Sherr J, Shaw R, Latimer E, Leckman J, Thienemann M: Clinical Management of Pediatric Acute-onset Neuropsychiatric Syndrome Part II - Use of Immunomodulatory Therapies. J Child Adolesc Psychopharmacol 2017. [Epub ahead of print]. DOI: 10.1089/cap.2016.0148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankovich J, Thienemann M, Pearlstein J, Crable A, Brown K, Chang K: Multidisciplinary clinic dedicated to treating youth with pediatric acute-onset neuropsychiatric syndrome: Presenting characteristics of the first 47 consecutive patients. J Child Adolesc Psychopharmacol 25:38–47, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman KL, Hornig M, Yaddanapudi K, Jabado O, Lipkin WI: A Murine Model for Neuropsychiatric Disorders Associated with Group A Beta-Hemolytic Streptococcal Infection. J Neurosci 24:1780–1791, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber AM, Robinson AB, Reed AM, Abramson L, Bout-Tabaku S, Carrasco R, Curran M, Feldman BM, Gewanter H, Griffin T, Haines K, Hoeltzel MF, Isgro J, Kahn P, Lang B, Lawler P, Shaham B, Schmeling H, Scuccimarri R, Shishov M, Stringer E, Wohrley J, Ilowite NT, Wallace C: Consensus treatments for moderate juvenile dermatomyositis: Beyond the first two months. Results of the second Childhood Arthritis and Rheumatology Research Alliance consensus conference. Arthritis Care Res 64:546–553, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iñiguez MA, Punzón C, Cacheiro-Llaguno C, Díaz-Muñoz MD, Duque J, Cuberes R, Alvarez I, Andrés EM, Buxens J, Buschmann H, Vela JM, Fresno M: Cyclooxygenase-independent inhibitory effects on T cell activation of novel 4,5-dihydro-3 trifluoromethyl pyrazole cyclooxygenase-2 inhibitors. Int Immunopharmacol 10:1295–1304, 2010 [DOI] [PubMed] [Google Scholar]
- Iñiguez MA, Punzon C, Fresno M: Induction of cyclooxygenase-2 on activated T lymphocytes: Regulation of T cell activation by cyclooxygenase-2 inhibitors. J Immunol 163:111–119, 1999 [PubMed] [Google Scholar]
- Kirvan CA, Cox CJ, Swedo SE, Cunningham MW: Tubulin Is a Neuronal Target of Autoantibodies in Sydenham's Chorea. J Immunol 178:7412–7421, 2007 [DOI] [PubMed] [Google Scholar]
- Kirvan CA, Swedo SE, Heuser JS, Cunningham MW: Mimicry and autoantibody-mediated neuronal cell signaling in Sydenham chorea. Nat Med 9:914–920, 2003 [DOI] [PubMed] [Google Scholar]
- Kirvan CA, Swedo SE, Kurahara D, Cunningham MW: Streptococcal mimicry and antibody-mediated cell signaling in the pathogenesis of Sydenham's Chorea. Autoimmunity 39:21–29, 2006a [DOI] [PubMed] [Google Scholar]
- Kirvan CA, Swedo SE, Snider LA, Cunningham MW: Antibody-mediated neuronal cell signaling in behavior and movement disorders. J Neuroimmunol 179:173–179, 2006b [DOI] [PubMed] [Google Scholar]
- Klegeris A, Maguire J, McGeer PL: S- but not R-enantiomers of flurbiprofen and ibuprofen reduce human microglial and THP-1 cell neurotoxicity. J Neuroimmunol 152:73–77, 2004 [DOI] [PubMed] [Google Scholar]
- Kumar A, Williams MT, Chugani HT: Evaluation of Basal Ganglia and Thalamic Inflammation in Children With Pediatric Autoimmune Neuropsychiatric Disorders Associated With Streptococcal Infection and Tourette Syndrome: A Positron Emission Tomographic (PET) Study Using C-11-[R]-PK11195. J Child Neurol 30:749–756, 2015 [DOI] [PubMed] [Google Scholar]
- Lander SA, Wallace DJ, Weisman MH: Celecoxib for systemic lupus erythematosus: Case series and literature review of the use of NSAIDs in SLE. Lupus 11:340–347, 2002 [DOI] [PubMed] [Google Scholar]
- Li SC, Torok KS, Pope E, Dedeoglu F, Hong S, Jacobe HT, Rabinovich CE, Laxer RM, Higgins GC, Ferguson PJ, Lasky A, Baszis K, Becker M, Campillo S, Cartwright V, Cidon M, Inman CJ, Jerath R, O'Neil KM, Vora S, Zeft A, Wallace CA, Ilowite NT, Fuhlbrigge RC: Development of consensus treatment plans for juvenile localized scleroderma: A roadmap toward comparative effectiveness studies in juvenile localized scleroderma. Arthritis Care Res 64:1175–1185, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotan D, Benhar I, Alvarez K, Mascaro-Blanco A, Brimberg L, Frenkel D, Cunningham MW, Joel D: Behavioral and neural effects of intra-striatal infusion of anti-streptococcal antibodies in rats. Brain Behav Immun 38:249–262, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luleyap HU, Onatoglu D, Yilmaz MB, Alptekin D, Tahiroglu AY, Cetiner S, Pazarbasi A, Unal I, Avci A, Comertpay G: Association between pediatric autoimmune neuropsychiatric disorders associated with streptococcal infections disease and tumor necrosis factor-α gene-308 g/a, −850 c/t polymorphisms in 4–12-year-old children in Adana/Turkey. Indian J Hum Genet 19:196–201, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackenzie IRA, Munoz DG: Nonsteroidal anti-inflammatory drug use and Alzheimer-type pathology in aging. Neurology 50:986–990, 1998 [DOI] [PubMed] [Google Scholar]
- Macrì S, Ceci C, Onori MP, Invernizzi RW, Bartolini E, Altabella L, Canese R, Imperi M, Orefici G, Creti R, Margarit I, Magliozzi R, Laviola G: Mice repeatedly exposed to Group-A β-Haemolytic Streptococcus show perseverative behaviors, impaired sensorimotor gating, and immune activation in rostral diencephalon. Sci Rep 5:13257, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller N, Ulmschneider M, Scheppach C, Schwarz MJ, Ackenheil M, Möller HJ, Gruber R, Riedel M: COX-2 inhibition as a treatment approach in schizophrenia: Immunological considerations and clinical effects of celecoxib add-on therapy. Eur Arch Psychiatry Clin Neurosci 254:14–22, 2004 [DOI] [PubMed] [Google Scholar]
- Muthén L, Muthén B: Mplus user's guide (7th ed.). Los Angeles: CA, Muthén & Muthén, 2012 [Google Scholar]
- Napolitani G, Acosta-Rodriguez EV, Lanzavecchia A, Sallusto F: Prostaglandin E2 enhances Th17 responses via modulation of IL-17 and IFN-Y production by memory CD4+ T cells. Eur J Immunol 39:1301–1312, 2009 [DOI] [PubMed] [Google Scholar]
- Paz JA, Silva CAA, Marques-Dias MJ: Randomized Double-Blind Study With Prednisone in Sydenham's Chorea. Pediatr Neurol 34:264–269, 2006 [DOI] [PubMed] [Google Scholar]
- Petri MA, Martin RS, Scheinberg MA, Furie RA: Assessments of fatigue and disease activity in patients with systemic lupus erythematosus enrolled in the Phase 2 clinical trial with blisibimod. Lupus 26:27–37, 2017 [DOI] [PubMed] [Google Scholar]
- Sayyah M, Boostani H, Pakseresht S, Malayeri A: A preliminary randomized double-blind clinical trial on the efficacy of celecoxib as an adjunct in the treatment of obsessive-compulsive disorder. Psychiatry Res 189:403–406, 2011 [DOI] [PubMed] [Google Scholar]
- Shalbafan M, Mohammadinejad P, Shariat SV, Alavi K, Zeinoddini A, Saleh M, Askari N, Akhondzadeh S: Celecoxib as an adjuvant to Fuivoxamine in moderate to severe obsessive-compulsive disorder: A double-blind, placebo-controlled, randomized trial. Pharmacopsychiatry 48:136–140, 2015 [DOI] [PubMed] [Google Scholar]
- Silvergleid AJ, Ballow M: Overview of intravenous immune globulin (IVIG) therapy. In: UpToDate. Edited by Post TW, Schrier SL, Stiehm ER, Tirnauer JS, and Feldweg AM. Waltham MA, UpToDate, 2016
- Sirnsek H, Dundar S, Telatar H: Treatment of Behqet Disease with lndomethacin. Int J Dermatol 30:54–57, 1991 [DOI] [PubMed] [Google Scholar]
- Spartz E, Freeman GM, Brown KD, Farhadian B, Thienemann M, Frankovich J: Course of neuropsychiatric symptoms after introduction or removal of non-steroidal anti-inflammatory drugs: A pediatric observational study. J Child Adolesc Psychopharmacol 2017. [Epub ahead of print]. DOI: 10.1089/cap.2016.0179 [DOI] [PubMed] [Google Scholar]
- Swedo S, Leckman J, Rose N: From research subgroup to clinical syndrome: Modifying the PANDAS criteria to describe PANS (pediatric acute-onset neuropsychiatric syndrome). Pediatr Ther 2:1–8, 2012 [Google Scholar]
- Swedo SE, Leonard HL, Garvey M, Mittleman B, Allen AJ, Perlmutter SJ, Lougee L, Dow S, Zamkoff J, Dubbert BK: Pediatric autoimmune neuropsychiatric disorders associated with streptococcal infections (PANDAS): Clinical description of the first 50 cases. Am J Psychiatry 155:264–271, 1998 [DOI] [PubMed] [Google Scholar]
- Then Bergh F, Kümpfel T, Schumann E, Held U, Schwan M, Blazevic M, Wismüller A, Holsboer F, Yassouridis A, Uhr M, Weber F, Daumer M, Trenkwalder C, Auer DP: Monthly intravenous methylprednisolone in relapsing-remitting multiple sclerosis - reduction of enhancing lesions, T2 lesion volume and plasma prolactin concentrations. BMC Neurol 6:19, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker AR, Tani LY, Thompson JA, Firth SD, Veasy LG, Bale JF: Rheumatic Chorea: Relationship to Systemic Manifestations and Response to Corticosteroids. J Pediatr 151:679–683, 2007 [DOI] [PubMed] [Google Scholar]
- Wallace CA: Current management of juvenile idiopathic arthritis. Best Pract Res Clin Rheumatol 20:279–300, 2006 [DOI] [PubMed] [Google Scholar]
- Williams KA, Swedo SE: Post-infectious autoimmune disorders: Sydenham's chorea, PANDAS and beyond. Brain Res 1617:144–154, 2015 [DOI] [PubMed] [Google Scholar]
- Williams KA, Swedo SE, Farmer CA, Grantz H, Grant PJ, D'Souza P, Hommer R, Katsovich L, King RA, Leckman JF: Randomized, Controlled Trial of Intravenous Immunoglobulin for Pediatric Autoimmune Neuropsychiatric Disorders Associated With Streptococcal Infections. J Am Acad Child Adolesc Psychiatry 55:860–867.e2, 2016 [DOI] [PubMed] [Google Scholar]
- Xu M, Kobets A, Du J-C, Lennington J, Li L, Banasr M, Duman RS, Vaccarino FM, DiLeone RJ, Pittenger C: Targeted ablation of cholinergic interneurons in the dorsolateral striatum produces behavioral manifestations of Tourette syndrome. Proc Natl Acad Sci U S A 112:893–898, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaddanapudi K, Hornig M, Serge R, De Miranda J, Baghban a, Villar G, Lipkin WI: Passive transfer of streptococcus-induced antibodies reproduces behavioral disturbances in a mouse model of pediatric autoimmune neuropsychiatric disorders associated with streptococcal infection. Mol Psychiatry 15:712–726, 2010 [DOI] [PubMed] [Google Scholar]