Abstract
Introduction: Biosamples and associated clinical data accelerate translational and clinical research discoveries. A lack of high quality biosamples both stalls projects and limits research advances. In this study, we targeted a wide audience of University of California (UC) biobanking stakeholders who were either involved with the collection or the utilization of biosamples to assess the scope of their biobanking activities and their interest in virtual biobanking or cooperating in the formation of the UC-wide biorepository.
Materials and Methods: Each institutional review board from the five UC medical campuses' provided a dataset of potential researchers involved with biobanking. Once identified, a brief six item web-based questionnaire was administered electronically to these researchers.
Results: Most survey participants (80%) responded “yes” (n = 348) that they were actively collecting biosamples for research, and 68% of participants indicated they would either definitely (30%, n = 131) or maybe (38%, n = 166) request biosample materials now or within the next year. An equal proportion of participants responded yes (42% or n = 184) and maybe (42% or n = 182) when asked if they would voluntarily contribute specimens to a UC-wide virtual biobank.
Discussion: The results presented above show high levels of support among UC biobanking stakeholders for both requesting material from and contributing material to a UC-wide virtual biobank. In addition, a considerable number of individual researchers on our five UC medical campuses are conducting biospecimen research (i.e., well over n = 435 respondents).
Keywords: : biobank, biosamples, virtual, stakeholder, perceptions
Introduction
Biosamples and associated clinical data accelerate translational and clinical research discoveries. A lack of high quality biosamples both stalls projects and limits research advances. With the continuing evolution in medicine toward personalized healthcare and clinical and translational science research, these resources are fundamental to effective population-based studies in diverse disciplines such as neoplastic, vascular, metabolic, and neurodegenerative diseases, as well as pharmacogenomics.1
Starting in the 1990's, networks of biobanks began to emerge and proliferate. These provide significantly more power for driving research than dispersed individual biobanks, as a robust sample size is often needed to give statistical rigor to a study.2 The countries with the most large-scale biobanks historically have been the United Kingdom (n = 15), United States (n = 14), Sweden (n = 12), France (n = 9), the Netherlands (n = 8), and Italy (n = 8), although Korea has recently created a national biobank incorporating 17 regional banks.3 Sixty percent of sponsors for biobanks are governmental or national institutes, and close to 17% are sponsored by nonprofit organizations, universities, or hospitals. In Kang and colleagues' review article on the increase in biobanks since the 1980s, one study they reviewed found that most biobanks (60%) recruited less than 100,000 subjects, while thirty percent of the biobanks recruited 100,000 to 1,000,000 subjects.4
More recently virtual biorepositories have emerged as a viable option for more broad-based sharing of research resources. A virtual biobank is an electronic database of biological specimens and other related information that exists virtually, independent of where the actual specimens are stored.5 These characteristics allow virtual biobanks to bring together a widely dispersed collection of biospecimens and associated genetic and other background data into one virtual location for ease of access by researchers. Virtual biobanks can quickly and efficiently help investigators locate specific biospecimens that would otherwise require contact with multiple individual biobanks.5 Such efficiency is particularly useful in the initial stages of research, to determine the feasibility of proposed experiments.
Although instruments have been developed to assess attitudes toward biobanking in the general population, surveys of researchers engaged in biobanking have been more limited. Furthermore, very little is known about researcher concerns and suggestions with respect to developing a biobank that would meet their research interests and needs.6
We conducted a two-phase survey of University of California (UC) biobanking stakeholders who collected biospecimens through five University of California Clinical and Translational Science Award-institutions (CTSAs), including UC Davis, UC Irvine, UC Los Angeles, UC San Diego, and UC San Francisco, which comprise a western regional CTSA network, UC Biomedical Research Acceleration, Integration and Development (UC BRAID). The survey was designed to gather researchers' opinions on creating a UC-wide virtual biobank. In particular, we were interested in eliciting participants' concerns and themes associated with virtual biobanks, operations, and resources for research activities.
Materials and Methods
We initiated the project by conducting individual semistructured interviews with biobanking leaders at the UC CTSAs with the purpose of determining the most appropriate study design, sampling frame, and stakeholder groups to include in the survey. The 30-minute interviews were audio recorded, and notes were transcribed following each interview session. From these interviews, it became clear that the most effective approach would be to conduct data collection by survey in two phases.
In phase one, we targeted a wide audience of researchers who had collected biosamples to assess the scope of their biobanking activities, their interest in virtual biobanking, and their willingness to participate in a more comprehensive survey.
In phase two of the survey, we followed up with a subset of these researchers to gather more in-depth information about their biobanking operations, whether a virtual biobank would be a useful resource for their research activities, whether they would contribute samples, and their concerns and suggestions regarding a virtual biobank network. This article presents the phase one results, from data collected December 2014 through May 2015. Phase two results will be presented in a future article that is focused on more in-depth researcher opinions on: collecting, storing, distributing, sourcing, maintaining, and sharing biosamples in a virtual biobank.
To develop the sampling framework, we contacted the institutional review boards (IRBs) offices at each of the five UC CTSA medical campuses to obtain a list of researchers who included human biospecimen collections in their IRB protocols in the past five years. Each of the campuses' IRB offices generated a database of researchers created using a key word search to identify biobankers, specifically. Keywords used to search the IRB protocol titles included the following: “tissue,” “biosample,” “bank,” and “specimen.” Biobanking leads at each campus reviewed the sampling frame to determine if key investigators were missing from the list and when necessary to provide additional investigators to be included in the phase one research sampling frame.
Although demographic and occupation information was deliberately not collected to minimize respondent burden for this short survey, it is possible to characterize the sample as follows: the majority of Principal Investigators who submit applications to the IRBs hold academic positions at one of the UC medical campuses and very few had nonacademic appointments; the dataset requested from the IRBs contained primarily biomedical researchers.
A brief six item web-based questionnaire was administered by email to the IRB-identified researchers who were involved in biobanking activities to obtain baseline information about their human sample collection and biobanking activities (see Fig. 1 Survey Questions). Respondents were given contextual information at the top of the survey stating, “We are exploring the possibility of developing a voluntary virtual biobank where researchers from across the UC campuses could choose to share and use each other's biospecimens. The biospecimens you choose to share would remain in your biobank. This network would source materials (and collection protocols and storage methods) virtually from the five UC-Medical Center Biorepositories and participating Principal Investigators.”
FIG. 1.

Survey to collect opinions on creating a virtual biobank.
Questions included five multiple-choice questions and one open-ended qualitative question that queried about general opinions on a virtual biobank. Each researcher who was eligible for inclusion in the sample was sent up to five email reminders inviting them to participate, and multiple emails were sent only to those who had not yet responded.
Results
Analytic sample
Table 1 presents the sampling frame and response rates by campus. After removing four incomplete records, the final analytic sample size for data analysis was n = 435. The response rates for each campus varied from 25% to 43%.
Table 1.
Biobanking Researcher Sample and Response by Campus
| Campus | Sampling frame denominator | Sampling frame numerator | Response rate (%) | Completed surveys |
|---|---|---|---|---|
| UCSF | 255 | 98 | 38 | ∼97 |
| UCI | 240 | 92 | 38 | ∼91 |
| UCD | 577 | 191 | 33 | ∼190 |
| UCSD | 53 | 19 | 25 | ∼18 |
| UCLA | 92 | 40 | 43 | ∼39 |
| Total/average | 1263 | 440 | 35 | 435 |
UCD, UC Davis; UCI, UC Irvine; UCLA, UC Los Angeles; UCSD, UC San Diego; UCSF, UC San Francisco.
Quantitative results
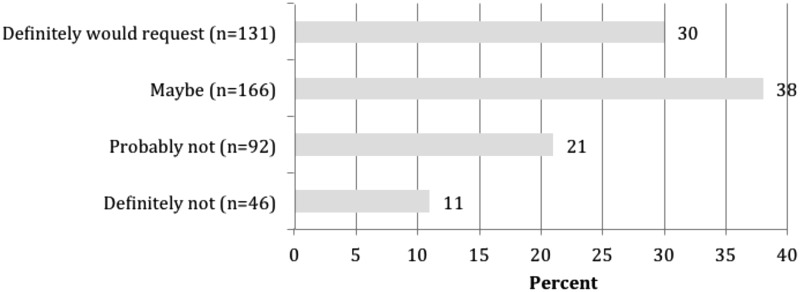
Response categories for the quantitative questions were developed using a combination of literature review, leadership interviews described above, and the collective expertise of the UC BRAID Biobanking Workgroup that led this project. Most participants (80%) responded “yes” (n = 348) that they were actively collecting biosamples for research and 20% (n = 88) were not collecting or had completed their collection (Fig. 2). When asked if they would be interested in requesting biosamples from a UC-wide biosample (tissue and fluid) network, 68% of participants indicated they would either definitely (30%, n = 131) or maybe (38%, n = 166) request material now or within the next year, 21% (n = 92) indicated they would probably not make a request, and 11% (n = 46) said definitely not (Fig. 2).
FIG. 2.
Percentage of participants who would request human tissues or fluids to their specification if a UC-wide virtual biobank existed. UC, University of California.
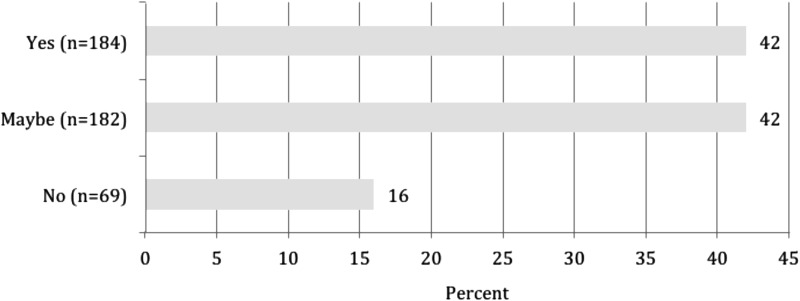
Participants were also asked if they would be interested in voluntarily sharing all or a portion of their biorepository samples and data with researchers across UC as part of the “virtual biobank.” An equal proportion of participants responded yes (42% or n = 184) and maybe (42% or n = 182), while only 16% said they would not be interested (Fig. 3). Respondents were allowed to select multiple reasons for answering “maybe.” These included: (1) only if the recipients agree to be collaborators (59% of “maybe” or n = 108); (2) only if my governance committee allows for this (59% or n = 107); (3) only if charging for all costs of organizing samples is provided (54% or n = 99); (4) only if registration is a requirement for access to the UC-wide biorepository (39% or n = 71), and (5) “other” (not further specified) (18% of “maybe's” or n = 33).
FIG. 3.
Percentage of participants who would or would not contribute biorepository samples if a UC-wide virtual biobank existed.
Participants who said they were not interested in voluntarily sharing biosamples (16% of total respondents) were also asked to select a reason, and multiple reasons could be selected. These included: (1) individuals did not have biosamples to share (47% or n = 33); (2) their current IRB consent does not allow this type of sharing (29% or n = 20); (3) patient consent or authorization does not allow for this (26% or n = 18); (4) “other” (not further specified) reason (17% or n = 12); (5) they were not interested (13% or n = 9); and (6) the sponsor did not allow for it (7% or n = 5).
We concluded the quantitative part of our survey by asking if participants would be interested in learning more about the development of a voluntary UC-wide virtual biobank. This was included to enable us to keep interested individuals informed of developments in this area. A total of 69% of individuals (n = 299) were interested in learning more and 31% (n = 136) were not. Two-thirds (n = 89) of those who were not interested in learning more also felt they were unlikely to request materials. One-third of those not interested in learning more (n = 44) were not interested in sharing specimens with the biobank.
Both those who responded that they would request materials from a virtual biobank, as well as those who responded that they would share their specimens in a virtual biobank, were significantly more likely to indicate that they were interested in learning more about the development of a voluntary UC-wide virtual biobank (p < 0.001). Finally, participants were asked if they were willing to participate in a more comprehensive follow-up survey, and 52% of researchers (n = 227) said they were, while 48% were not (n = 208).
Qualitative results
Through an open-ended survey question investigators were queried, “Regardless of whether you are in favor of a virtual UC-wide biobank or not, we are very interested in your opinions on this. Please provide us with additional information on your opinions about creating a virtual UC-wide biobank.” Among the 435 total respondents, 227 (52%) responded using a free text field. Two researchers coded the qualitative data into four broad response categories based on commonly recurring types of responses. In descending order of frequency, the categories created were as follows: Quality/Benefit (n = 119), Concern (tied with n = 119), Conditional (n = 55), and Suggestion (n = 54). In addition, the qualitative researchers included a separate Miscellaneous category (n = 84) for responses which did not fit neatly into any of the four main categories—responses that were either too vague to categorize, responses asking for more information, comments redirecting focus toward other aspects of research, and/or contextual comments regarding the investigators' biobanking background.
The responses within the “Quality/Benefit” category focused on potential benefits brought about by the creation of a virtual biobank, or more general positive attributes of a UC-based virtual biobank. The “Concern” category included responses regarding issues, such as limitations to the creation of a virtual biobank network, potential for exploitation of researchers and patients, as well as exorbitant costs and resource waste. All of the responses determined to be “Conditional” were predicated on investigators' potential use and support of the biobank being contingent upon implementation and/or regulatory guidelines, such as standard protocols or intellectual property safeguards. Finally, the “Suggestion” category included recommendations for the organization and maintenance of a virtual biobank or in some cases responses that suggested resources should be focused elsewhere.
In addition to characterizing the type of response (quality/benefit, concern, conditional, and suggestion), we coded six substantive themes, as follows: Sample/Data; Financial/Resources; Regulatory/Structure; Investigator/Researcher; Collaboration; and Research/Science.
The Sample/Data theme contained the highest frequency of coded responses in the qualitative analysis (n = 104). The Sample/Data theme referred to aspects of the biospecimen (e.g., samples of material, such as urine, blood, tissue, cells, DNA, RNA, and protein from humans, whether the sample is appropriate, such as healthy or unhealthy samples) and information/data that described the samples.
The Regulatory/Structure theme contained the second highest frequency of coded responses in the qualitative analysis (n = 82). The Regulatory/Structure theme referred to organizational aspects of a biorepository or the structure of a virtual biobank. For this section, the researchers coded opinions that included focus on the organization of the infrastructure and institutional protocols (including IRB and consent forms) that would be associated with the foundation and administration of the UC-wide virtual biobank. Respondents' concerns covered structural organization, including Committee on Human Research constraints, and assuring IRB approval. Respondents' suggestions indexed the consideration of such structural features as “global UC wide consent for all patients.” (R182). Respondents' willingness to participate in a virtual biobank was conditioned generally upon “policies and oversight” (R423), or the “build-up of more infrastructures” (R261). In addition, some repositories may receive funding from a program and would need approval to join the biobank. Regarding qualities/benefits, respondents felt that a UC-wide virtual biobank would lead to an ease of regulatory approval and enable studies that are usually “logistics-prohibitive” (R75).
The Financial/Resources theme contained the third highest frequency of coded responses in the qualitative analysis (n = 54). The Financial/Resources theme referred to budgetary, personnel, and other resource requirements needed to procure, process, store, and distribute biospecimens or to operate a biospecimen repository. Respondents described key issues involving cost and resources and some respondents questioned if there is any financial benefit at all. Respondent 416 was concerned about “who if anyone will benefit financially, and what services will be provided and at what cost.” For other respondents, while they anticipated costs associated with the creation of a biobank, this financial burden would be lightened since the creation of a virtual biobank network could potentially “decrease research costs by removing duplicative efforts” (R326). In addition, some departments “garner significant revenue by selling specimens” (R162), therefore participants felt there would have to be some sort of funding to replace this.
The Collaboration theme was tied with the Investigator/Researcher theme for the fourth highest frequency of coded responses (n = 38). Collaboration referred to working jointly with others and cooperating to develop a regional and/or virtual biobank. Respondents agreed that a shared program would promote collaboration, and this collaboration would also “go a long way in making [the program] work” (R22). Furthermore, these collaborations may lead to advancement of knowledge (R372). Respondents may also have specific criteria for collaboration, such as respondent 409 who was “cautious of having others exploit [their] samples without [their] permission outside of an accepted Collaboration.” Respondents had suggestions for collaboration, which included “identify potential collaborators for projects” and “either collaborators or recharge should be in place, depending on the extent of my involvement” (R294 and R434).
Investigator/Researcher was tied with Collaboration for the fourth highest frequency of coded responses in the qualitative analysis (n = 38). The theme included responses focusing on the intellectual rights, authorship, publication, and research of the respondent as the investigator in particular or these issues for researchers in general. Some respondents had concerns surrounding acknowledgment and authorship while others wanted to know at what level intellectual property would be acknowledged. Some were concerned if other investigators would “steal [their] idea” or [“exploit”] their samples (R195 and R215). Others agreed that the program will personally benefit investigators or researchers and went on to state that this program will make it possible for them to be further involved and contribute further to human health. For instance, Respondent 4 stated that, “creating a usable biobank would have the potential to help many investigators.”
The Research/Science theme contained the lowest frequency of coded responses in the qualitative analysis (n = 31). Research/Science referred to biobanking as an important resource in biomedical research supporting many types of translational research such as genomics, cell therapy, tissue engineering clinical trials, and other forms of personalized medicine.
Many respondents saw the creation of a virtual biobank network as an opportunity to “catch up” with other universities (R16). Second, respondents felt that the shared biobank would benefit, advance, and propel, and said that it is critical for the scientific community, for exploratory research, and research that contributes to public health policy (R59, R142, and R415).
Discussion
Implications of results
The results presented above show high levels of support among survey respondents for both requesting material from and voluntarily contributing material to a UC-wide virtual biobank. In addition, a considerable number of researchers on our campuses are conducting biospecimen research, that is, n = 435 respondents. We believe there are many more researchers on our campuses collecting biosamples who either did not respond to the survey or who were not correctly identified in the IRB dataset used to develop the sampling frame.
Several key ideas have been captured in this study, and the implications stemming from them are important to state. First, this study has clearly demonstrated a substantial scope of biobanking research that is being conducted across the five UC medical campuses and a significant potential for collaboration and coordination. There was an overwhelming eagerness from respondents to participate in such a biobank and additionally, a perceived lost opportunity in not creating a shared virtual biobank. Some of the costs and losses associated with not sharing specimens included potential redundancy and duplication of effort, as well as the loss of data in the event that grant funding is depleted and/or not renewed. In the case of a UC-wide virtual biobank, even after funding for a particular study has expired, the samples and data could be maintained within the network and be put to good scientific use.
Issues with formation and initiating sample sharing
As many of the respondents in our study noted, success of these virtual biobanks depends heavily on their “ability to process, store, and provide well-characterized specimens to researchers worldwide”5 A recent publication echoes the sentiment that it is important to implement both technical standards, as well as mechanisms to ensure buy-in, such as stakeholder involvement and embedding biosample sharing in institutional infrastructure.7 In some institutions, there are protocols that govern the sample and data requirements that are necessary to create a new biobank network community (e.g., The Global Genome Biodiversity Network [GGBN], Data Portal).8
Somiari and Somiari recently introduced a new conceptual model of federated biobank structure, as well as general strategies for implementing this model through a shift toward public–private partnerships in biobanking administration.9 To leverage a geographically disbursed set of biosamples and data, the authors recommend establishing and utilizing a network of what they call “Research Ready Hospitals”—hospitals that would be able to align both the everyday aspects of hospital administration with the specialized resources needed to collect biospecimens concurrently.9 This, especially with the inclusion of local community engagement, would take the oversight and administration of virtual biobanks out of the control of purely academic or corporate organizations and into a collaborative intersection of the public and private sectors.9
The UC system is well positioned to align a virtual biobank with its affiliated hospitals that are research ready and could collect and share biospecimens in alignment with this model.
Economic considerations for sharing biosamples
The focus of sharing biosamples has primarily remained in the domain of ethical concerns and regulatory issues, yet financial issues remain important to consider with the creation of a virtual biobank.10 Financial issues were a primary concern for many respondents in our study, and researchers discussed both benefits and concerns they had with the financial implications of such an endeavor: including who would provide resources or pay for the costs of the virtual biobanks, how the original owners would be compensated, and how much those accessing the specimens would pay for them.
Mitchell and Waldby present an argument for the economic benefit of biobanks, focusing specifically on the way in which national biobanks create unique biovalue.11 They call attention to the clinical labor—that is, the “regularized, embodied work that members of the populous perform in their role as biobank participants”—which creates biovalue through biobanks.11 Accordingly, researchers must continue to be mindful of the value that participants provide to each biobank and the fiscal and moral responsibility that we have to individuals whose samples we obtain.11
Virtual biobanks have a variety of funding sources depending on their characteristics, including government, public/advocacy groups, and charitable contributions.12 How these banks are funded have significant effects on the function and purpose of the biobank, as UC survey participants indicated, and this should be considered. In addition, funding sources also play a large role in determining the level of public support for such endeavors. Building on a literature base focused on public reaction to the commercialization of biobanks and their associated resources, conclude that while there is a shared belief that people are hesitant to support commercialization of biobanks, public support can be garnered through transparency and independent governance of biobanks and resources.13
Regulatory and ethical concerns regarding the development of virtual biobanks
Regulatory and ethical issues are pervasive in biobank literature and also constitute a significant portion of the qualitative responses in this study: they are extremely important to acknowledge for the preservation of patient health and the protection of patient identity. Respondents from the UC system noted that their participation in the virtual biobank would be dependent on regulatory and structural issues, including having appropriate guidelines in place and having appropriate oversight.
Regulatory guidance for biobanks comes from several sources, most notably the International Society for Biological and Environmental Repositories (ISBER, 2012) guidelines, the Organization for Economic Cooperation and Development (OECD) guidelines, and the National Cancer Institute's Best Practices for Biospecimen Resources.10,14,15 Different groups have developed a variety of data standards and documents, for example, the “National Mesothelioma Virtual Bank” group, The GGBN, and the Biobank Ireland Trust16 all have different guiding principles.
Despite the variety of available standards and guiding documents for regulating virtual biobanking, there is still a need for further guidance and policies as new virtual biobanks are being created. For example, Henderson et al. conducted the first national survey of biobanks in the United States, noting that “the complex organizational landscape of biobanking requires policies as nuanced as the biobanks themselves”.17 In a follow-up article, Henderson et al. also discuss stewardship practices for biospecimens that are collected, processed, stored, and transported, which are critical to the integrity of the science and the need for respect among the contributors.18
There needs to be some form of standard operating procedures or terms of reference in place, and this practice should ultimately involve the understanding of the variety of biobanks and their complex organizational structures.17
In addition, scholars in the field are currently discussing ethical issues such as sponsorship and benefit sharing, ethics committees, public engagement, consent, and data protection–as did our study participants. In general, a consensus is emerging in the international guidelines that the human genome is the property of all humanity, that benefit-sharing should include releasing relevant findings to the project participants, and that profit sharing may be appropriate.19,20 Similarly, there is agreement about the need for independent ethics committees, the requirement of voluntary informed consent, and the importance of protecting the privacy and confidentiality of patient information.
However, the guidelines are less uniform with regard to consultation and education of the lay population, withdrawal of consent, data coding methods, and future use of data.20 The use of biospecimens collected during routine clinical care for secondary research also has raised concerns.
In 2011, the U.S. Department of Health and Human Services (DHHS) released an Advance Notice of Proposed Rulemaking (ANPRM), and subsequently, a Notice of Proposed Rulemaking (NPRM), which included proposals for changes to federal regulation of research involving human subjects within the Common Rule.21,22 One section of the proposed changes specifically sets out to address consent issues surrounding secondary research by requiring informed consent for research use of remnant clinical samples.21 In addition, the ANPRM proposed that a short standardized form be used for obtaining consent for “future open-ended use of biospecimens in research”.21 While no changes to the Common Rule have been enacted, the discussion around this issue shows a wide variety of opinions and demonstrates why potential participants would want a virtual biobank to have transparent policies on legal and regulatory issues.23–25
Safeguards for participating investigators' best interests
Some of the qualitative responses brought up issues surrounding intellectual property safeguards and concerns for the best interests of the investigators who participate in the collection and sharing of biospecimens. Our qualitative report revealed that investigators are interested in a UC-wide biobank, but need to have an understanding of the added value, associated costs they may be asked to shoulder, and general affordability. In addition, investigators were concerned about whether there would be compensations and cost sharing in place for sharing biospecimens, resources, and time, such as reviewing/approving requests. These concerns, however, did not deter investigators from being eager to share their opinions about managing a biobank and did not dissuade many from being willing to participate in the biobank.
Collaboration and coordination across the UC system
Several investigators from our study agree that, while the initial development of a UC-wide virtual biobank would be time and resource intensive, the collaborations across the UC system that would emerge from such an endeavor are worthwhile and would open up possibilities for further benefits, such as an expanded use of individual biospecimens between a wider group of investigators. Beyond the obvious benefits of network leveraging and capacity building, such a biobank could bring a source of diverse data, boost current coordination efforts across the UC system, and move researchers forward in their careers. However, investigators are aware that the terms of collaboration and potential value exchanges need to be made clear as there is otherwise potential for exploitation of their samples. Investigators who support the UC-wide biobank appear to agree that the collaborative effort should benefit all investigators.
Significance of a virtual biobank network to research and science
The scientific literature, as well as the qualitative findings, demonstrates the significance, invaluable resources, benefits of integrating biosamples into a network, including advancement of knowledge and research programs, and increased collaborations and communication which help facilitate access to, and the sharing of, specimens and data that may otherwise be difficult to obtain. Specifically, efficient biobanks both facilitate and expedite research (especially translational research) on cancer, dementia, and other ubiquitous diseases, while also serving as a foundational resource for the investigation of new proteomics and genomic scientific ventures.5
Limitations
Each of the five UC BRAID CTSAs assisted their local IRB in generating a sampling frame for their respective campus. The IRB datasets varied in their sophistication for generating data for research and analysis. Some campuses were able to generate more specificity than others, meaning that some IRB's were able to provide lists that contained only biobankers and a few other researchers, while other campuses provided dataset lists that included a large number of biomedical researchers, many of whom were not necessarily involved in biobanking. We sought to mitigate IRB-related limitations in identification of biobankers by also having the biobanking leads at each campus review the IRB-generated list to identify biobankers who had not been included. While some biobankers at our campuses may have been excluded by these methods, we were able to identify a large cohort, and results of our survey indicate that most of these individuals were actively procuring biosamples, which suggest that our methods were successful.
Another limitation of this study is its reliance on hypothetical scenarios. Previous research suggests that people do not always act according to their predictions of how they will act;26 however, this was an unavoidable limitation given the purpose of the survey. Finally, the findings of this study are limited to the perceptions of researchers within the UC system, but may not generalize to the broader population of researchers outside of the UC system.
Conclusions
The UC system provides some unique advantages to exploring the concept of a virtual biobank. First, all campuses are governed by policy set by the UC Office of the President and the Regents of the University of California. Second, several years ago, the five UC campuses with CTSA grants established the UC BRAID program to encourage intercampus research collaborations. BRAID supported the development of the UC intercampus medical record search system (UC Rex) and the UC IRB Reliance (central IRB), both of which are essential to intercampus research using biosamples. Many successful UC researchers, similar to their counterparts across the nation, have developed careers by creating research patient databases, biobanks, and research infrastructure. These research “silos” may make some researchers less interested in participating in a virtual biobank.
Across UC, collaborations are occurring with increasing frequency, likely encouraged by the creation of UC BRAID and UC REX, as well as by the UC IRB Reliance Registry. Furthermore, the evolution of research toward genomic projects that require larger numbers of patients and data points and the NIH emphasis on including diverse communities in translational research incentivize UC researchers to engage in more collaborative work across the UC system. Federal requirements for data sharing have been in place for years and many of our most productive researchers have deposited data into these databases. The enthusiasm demonstrated in the responses to this survey indicates that UC biobankers and biomedical researchers see clear tangible value in sharing samples and data.
Acknowledgments
Funding for the study was provided by the University of California, Biomedical Research, Acceleration, Integration and Development (BRAID), Executive Committee and the National Center for Accelerating Clinical and Translational Science (NCATS).
NCATS Clinical and Translation Science Award (CTSA) numbers for the five UC BRAID CTSAs are provided below. The content is solely the responsibility of the coauthors and does not necessarily represent the official views of the National Institutes of Health (NIH).
UC Davis: Clinical and Translational Science Center funded through NCATS grant number TR000002 (http://ucdmc.ucdavis.edu/ctsc/aboutus/); UC Irvine: Institute for Clinical and Translational Science funded through NCATS grant number UL1 TR001414 (http://www.icts.uci.edu/citing-the-icts.asp); UC Los Angeles: Clinical and Translational Science Institute funded through NCATS grant number UL1TR000124 (https://ctsi.ucla.edu/pages/nih-acknowledgement); UC San Diego: Clinical and Translational Research Institute funded through NCATS grant number UL1TR001442 (https://ctri.ucsd.edu/about/Grant-support/Pages/about-publications.aspx); UC San Francisco: Clinical and Translational Science Institute funded through NCATS grant number UL1 TR000004 (http://accelerate.ucsf.edu/cite).
UC BRAID Program participating campuses: University of California, Davis Health System Clinical and Translational Science Center, University of California, Irvine Health System Institute for Clinical and Translational Science, University of California, Los Angeles Health System Clinical and Translational Science Center, University of California, San Diego Health System Clinical and Translational Research Institute, University of California, San Francisco Health System Clinical and Translational Science Institute.
The authors gratefully acknowledge the support and advice from members of the UC BRAID Executive Committee (Lars Berglund, Dan Cooper, Steven Dubinett, Gary Firestein, and Jennifer Grandis) and the UC BRAID Director Rachael Sak. Additional evaluation and analytic support were provided by the UCLA Clinical and Translational Science-Evaluation Program.
Author Disclosure Statement
No conflicting financial interests exist.
References
- 1.Schmitz G, Aslanidis C, Liebisch G, et al. . The Danubian Biobank project. Stud Health Technol Inform 2008;134:143–159 [PubMed] [Google Scholar]
- 2.Quinlan PR, Mistry G, Bullbeck H, et al. . A data standard for sourcing fit-for-purpose biological samples in an integrated virtual network of biobanks. Biopreserv Biobank 2014;12:184–191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kang B, Park J, Cho S, et al. . Current status, challenges, policies, and bioethics of biobanks. Genomics Inform 2013;11:211–217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Minamikumo M. Current status and future of biobanks. Policy Inst News 2012;36:15–21 [Google Scholar]
- 5.De Souza YG, Greenspan JS. Biobanking past, present and future: responsibilities and benefits. AIDS 2013;27:303–312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wells KJ, Arevalo M, Meade CD, et al. . Development and validation of the biobanking attitudes and knowledge survey (BANKS). Cancer Epidemiol Biomarkers Prev 2014;23:374–382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Riegman PH, de Jong B, Daidone MG, et al. . Optimizing sharing of hospital biobank samples. Sci Transl Med 2015;7:297fs31. [DOI] [PubMed] [Google Scholar]
- 8.International Society for Biological and Environmental Repositories. Best practices for repositories: collection, storage, retrieval, and distribution of biological materials for research. Biopreserv Biobank 2012;10:79–161 [DOI] [PubMed] [Google Scholar]
- 9.Somiari SB, Somiari RI. The future of biobanking: A conceptual look at how biobanks can respond to the growing human biospecimen needs of researchers. Adv Exp Med Biol 2015;864:11–27 [DOI] [PubMed] [Google Scholar]
- 10.Chalmers D, Nicol D, Kaye J, et al. . Has the biobank bubble burst? Withstanding the challenges for sustainable biobanking in the digital era. BMC Med Ethics 2016;17:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mitchell R, Waldby C. National biobanks: Clinical labor, risk production, and the creation of biovalue. Sci Technol Human Values 2010;35:330–355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vaught J, Kelly A, Hewitt R. A review of international biobanks and networks: success factors and key benchmarks. Biopreserv Biobank 2009;7:143–150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nicol D, et al. . Understanding public reactions to commercialization of biobanks and use of biobank resources. Soc Sci Med 2016;162:9–87 [DOI] [PubMed] [Google Scholar]
- 14.Organisation for Economic Co-operation and Development. OECD Guidelines on Human Biobanks and Genetic Research Databases. Paris: OECD; 2009 [Google Scholar]
- 15.NCI Best practices for biospecimen resources. Bethesda: National Cancer Institue, National Institutes of Health, U.S. Department of Health and Human Services; Available at: http://biospecimens.cancer.gov/bestpractices/2011-NCIBestPractices.pdf (Accessed October4, 2013) [Google Scholar]
- 16.Mee B, Gaffney E, Glynn SA, et al. . Development and progress of Ireland's Biobank Network: Ethical, legal, and social implications (ELSI), standardized documentation, sample and data release, and international perspective. Biopreserv Biobank 2013;11:3–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Henderson GE, Cadigan RJ, Edwards TP, et al. . Characterizing biobank organizations in the U.S.: Results from a national survey. Genome Med 2013;5:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Henderson GE, Edwards TP, Cadigan RJ, et al. . Stewardship practices of US biobanks. Sci Transl Med 2013;5:215cm7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Austin MA, Crouch J, DiGiacomo A. Applying international guidelines on ethical, legal, and social issues to new international genebanks. Jurimetrics 2005;45:115 [Google Scholar]
- 20.Astrin J, Baker S, Barr TJ, et al. . Best practices for repositories: collection, storage, retrieval, and distribution of biological materials for research. Biopreserv Biobank 2012;10:79–161 [DOI] [PubMed] [Google Scholar]
- 21.Emanuel EJ, Menikoff J. Reforming the regulations governing research with human subjects. N Engl J Med 2011;365:1145–1150 [DOI] [PubMed] [Google Scholar]
- 22.United States Government Publishing Office. Federal Registrar. Notice of Proposed Rulemaking. 2015. Available at: https://www.gpo.gov/fdsys/pkg/FR-2015-09-08/pdf/2015-21756.pdf (Accessed August19, 2016)
- 23.Hudson KL, Collins FS. Bringing the common rule into the 21st century. N Engl J Med 373:2293–2296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Allyse M, Karkazis K, Lee SSJ, et al. . Informational risk, institutional review, and autonomy in the proposed changes to the common rule. IRB 2012;34:17. [PMC free article] [PubMed] [Google Scholar]
- 25.Cargill SS. Biobanking and the abandonment of informed consent: An ethical imperative. Public Health Ethics 2016. [Epub ahead of print]; DOI: 10.1093/phe/phw001 [DOI] [Google Scholar]
- 26.Tourangeau R, Rips LJ, Rasinski K. The Psychology of Survey Response. New York, NY: Cambridge University Press; 2000 [Google Scholar]