Abstract
Objective: The human heart expresses the type 2 deiodinase (D2) that activates thyroxine (T4) to triiodothyronine (T3). At the same time, the inactivating type 3 deiodinase (D3) has been found in a rat model of right ventricular hypertrophy. It is not known whether the human myocardium metabolizes thyroid hormone. This study examined myocardial thyroid hormone metabolism in patients with aortic valve stenosis (AS) undergoing aortic valve replacement and in patients with coronary artery disease (CAD) undergoing coronary artery bypass grafting surgery.
Methods: Myocardial thyroid hormone metabolism was assessed by analyzing the difference in serum thyroid hormone levels between the aortic root (incoming blood) and the coronary sinus (outgoing blood) of patients undergoing cardiac surgery. A total of 23 patients with AS and 35 patients with CAD were included. Patients received a pre-surgical echocardiogram, and pre-, during and post-surgical thyroid hormone serum levels were collected in the myocardial and peripheral circulations.
Results: Patients with AS exhibited the expected left ventricle (LV) hypertrophy (i.e., 20–30% increase in LV posterior wall and interventricular septum thickness and ∼10% increase in AS in LV diastolic diameter). Immediately before cardiopulmonary bypass, blood flowing through the AS myocardium exhibited a 4.6% reduction in T3 and 6.9% increase in rT3 levels, decreasing the serum T3/rT3 ratio by 9.6%. T4 and thyrotropin serum levels remained similar between the aortic root and coronary sinus. In contrast, no myocardial thyroid hormone metabolism was observed in CAD patients. Notably, the AS myocardium lost the ability to inactivate thyroid hormone after cardiopulmonary bypass, possibly due to myocardial stunning.
Conclusions: There is accelerated thyroid hormone inactivation in the AS myocardium, which is likely the result of D3 expression. No evidence to suggest thyroid hormone activation in the myocardium was obtained in the present study.
Keywords: : euthyroid sick syndrome, deiodinases, thyroid hormones, aortic stenosis, heart surgery
Introduction
In contrast to other metabolically relevant hormones, thyroid hormone levels in the circulation are relatively stable in adults, only changing significantly during fasting or severe illness (1). This contrasts with rapid metabolism of thyroid hormone inside most tissues related to the action of deiodinases. In fact, thyroid hormone is secreted from the thyroid gland predominantly as thyroxine (T4) a pro-hormone that can be activated by conversion to 3,3′,5-triiodothyronine (T3) inside multiple cell types spread throughout the body. Two deiodinases, type 1 and type 2 (D1, D2), are responsible for >80% of T3 available in the circulation. Extrathyroidal deiodination can also inactivate thyroid hormone by converting T4 to 3,3′,5′-T3 (rT3) or T3 to 3,5-T2 via D3, a reaction that terminates thyroid hormone action. Thus, the intracellular concentration of T3 in a given tissue is not only affected by serum T3 levels but also by the types of deiodinases locally expressed and their activity (2). Of note, the human heart is a target of thyroid hormones and deiodinases expressed in the myocardium could potentially control local thyroid hormone concentration and action. This is relevant, as thyroid hormones are key regulators of myocardial contractility, heart rate, and cardiac output.
The healthy human myocardium expresses Dio2 and thus is potentially capable of generating T3 inside the muscle fiber (3,4). The commonly prescribed antiarrhythmic amiodarone and its active metabolite are noncompetitive inhibitors of D2 (5), which could contribute to the antiarrhythmic efficacy of amiodarone (6). Mouse models in which human Dio2 is ectopically expressed in the myocardium (α-MHC-D2) develop an increased T3 content of about 30% (7,8). These animals have normal thyroid function tests but their hearts exhibit greater capacity to generate cyclic adenosine monophosphate (9) and increased glucose uptake (10). Notably, such studies showed that D2 activity in the myocardium is protective against adverse myocardial remodeling caused by pressure overload (8) or doxorubicin-induced chemical injury (10). D2 also provides a host of mechanical improvements to the heart such as increased fractional shortening, velocity of circumferential fiber shortening, peak aortic outflow velocity, and aortic velocity acceleration (8).
Notably, a knock-in mouse model of inherited dilated cardiomyopathy with a deletion mutation (DeltaK210) in the cardiac troponin T gene exhibits an increase in myocardial Dio2 mRNA and activity likely as a result of generalized activation in cyclic adenosine monophosphate–dependent pathways (11). Similarly, post-myocardial infarction mice develop markedly enlarged hearts with left ventricular (LV) systolic dysfunction and marked up-regulation of Dio2 mRNA expression in the heart (11). While a direct involvement of D2-generated T3 in myocardial disease mechanisms has not been established, it is possible that chronic activation of D2 expression in the setting of a preexistent myocardial condition might not be beneficial and even be maladaptive.
Severe illnesses associated with tissue ischemia/hypoxia result in expression of D3 in skeletal muscle and liver (12), which could potentially dampen thyroid hormone signaling. In addition, increased myocardial Dio3 expression has been observed in animal models of myocardial infarction (13) and chronic pulmonary hypertension with right ventricular hypertrophy (RVH). Notably, D3 activity in the RVH model is associated with a decrease in T3 content only in the RV myocardium, underlying the role of tissue hypoxia in the regulation of thyroid metabolism in the heart (14,15).
The presence of Dio2 or Dio3 mRNA and/or the detection of in vitro D2 or D3 activity in the human or mouse myocardium is not conclusive evidence that these pathways are active and physiologically relevant in vivo or play a role in the setting of cardiac disease. Therefore, this study was conducted in patients with aortic stenosis (AS) and coronary artery disease (CAD) undergoing heart surgery as a way to characterize thyroid metabolism in the human heart as well as to test whether left ventricular (LV) hypertrophy and ischemia affect thyroid metabolism within the human myocardium.
Methods
Study population
The study included patients between 18 and 80 years of age undergoing open heart surgery due to severe AS or stable CAD at the Heart Institute of the University of Sao Paulo Medical School. Exclusion criteria included current or prior thyroid disease or use of thyroid hormones or amiodarone, previous head or neck irradiation, use of corticosteroids within the three weeks before surgery or during the procedure, moderate or severe myocardial dysfunction (LV ejection fraction <45%), chronic kidney disease (creatinine >1.5 mg/dL), acute coronary syndromes, emergency or combined aortic and coronary artery bypass grafting (CABG) surgeries. The protocol was approved by the local institutional review board and followed principles outlined in the Declaration of Helsinki. Informed consent form was signed before the inclusion of patients in the study.
Heart imaging
Enrolled patients underwent coronary angiography approximately 2–4 months before surgery. In addition, patients underwent pre-operative echocardiography (Table 1). LV ejection fraction was used to estimate ventricular function except in 8 CAD patients in which LV ejection fraction and function was estimated through myocardial scintigraphy. The interventricular septum and LV posterior wall thickness, as well as LV diastolic and systolic diameters were used to analyze ventricular mass and hypertrophy. Left atrium size was also analyzed.
Table 1.
Baseline Characteristics of Patients Enrolled in the Study
| Baseline characteristics | CAD (n = 35) | AS (n = 23) |
|---|---|---|
| Age (years) | 61.2 ± 8.3 | 60.0 ± 13.2 |
| Hypertension (%) | 82.9 | 65.2 |
| Diabetes (%) | 45.7 | 36.4 |
| Dyslipidemia (%) | 88.6 | 26.1 |
| Hemoglobin (g/dL) | 13.9 ± 1.8 | 13.9 ± 1.7 |
| Creatinine (mg/dL) | 1.11 ± 0.20 | 1.11 ± 0.23 |
| LVEF (%) | 58.9 ± 8.6 | 62.2 ± 8.3 |
| IVS (mm) | 10.3 ± 1.7 | 12.8 ± 2.5* |
| LVPW (mm) | 9.9 ± 1.6 | 12.0 ± 2.1* |
| LA (mm) | 38.2 ± 6.1 | 41.2 ± 4.1 |
| LVDD (mm) | 49.5 ± 5.1 | 53.7 ± 8.3** |
| LVSD (mm) | 33.4 ± 5.3 | 34.7 ± 7.3 |
Values are the mean ± standard deviation (SD); *p < 0.0001, **p = 0.0202 vs. CAD by unpaired two-tailed Student's t-test; eight CAD patients did not have the echo studies before surgery for operational reasons.
AS, aortic stenosis; CAD, coronary artery disease; IVS, interventricular septum thickness; LA, left atrium diameter; LVDD, left ventricular diastolic diameter; LVEF, left ventricular ejection fraction; LVPW, left ventricular posterior wall thickness; LVSD, left ventricular systolic diameter.
Study design
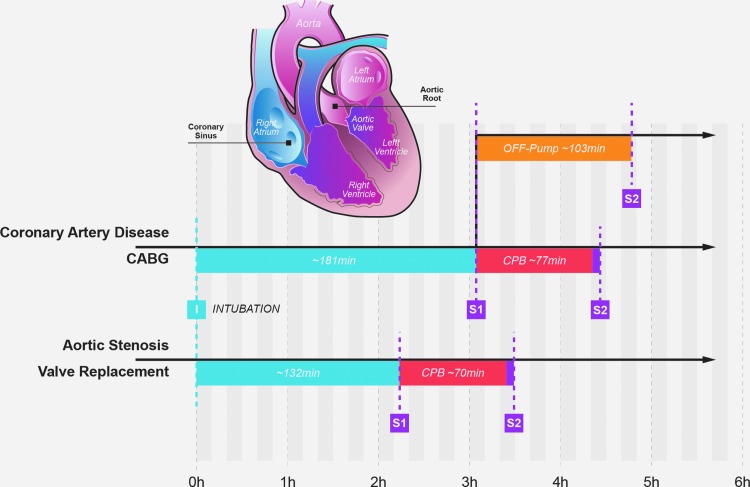
Patients with severe AS underwent valve replacement with a bioprosthesis (AS group) while those with CAD were treated with coronary artery bypass grafting (CABG). As the participants were recruited to the protocol one day before surgery, staging of the disease severity and surgical indication was left to the physician's discretion. It was up to the surgeon at the time of surgery to utilize cardiopulmonary bypass (CPB) or to operate off-pump; cardioplegia was also managed by the surgeon.
In patients undergoing CPB, myocardial thyroid hormone metabolism was assessed at two moments during surgery: at the baseline time-point, immediately before the aorta was clamped—about 2 and 3 h after intubation in AS and CAD patients respectively, and conclusion time-point, about 6 min after the aorta was unclamped (Fig. 1). In patients that were kept off-pump during CABG, myocardial thyroid hormone metabolism was assessed at baseline immediately before grafting was initiated, and at conclusion, immediately after the implants were in place (Fig. 1).
FIG. 1.
Graphical representation of a coronal section of the heart exhibiting the four chambers and main vessels as indicated. Note the location of the aortic root and coronary venous sinus where blood samples were obtained. Also shown is the time line observed in all three groups of patients. CABG, coronary artery bypass grafting; CPB, cardiopulmonary bypass—the period time between clamping and unclamping the aorta; I, intubation time; OFF-pump, period of time during which grafting took place; S1, baseline time point; S2, conclusion time point.
Heart blood samples were collected from the aortic root (incoming coronary blood) and coronary venous sinus (heart circulation drainage site). Samples of the arterial blood entering the coronary arteries were collected from the cardioplegia catheter inserted in the aortic root. Venous blood samples from the coronary sinus were collected through a central line inserted into the coronary sinus during surgery. In the off-pump patients, baseline and conclusion samples were collected before and after coronary revascularization and aortic root samples were collected from the radial artery as off-pump CABG does not require aortic root puncture.
Peripheral blood samples and analytical procedures
Peripheral blood samples were collected to evaluate changes in systemic thyroid hormone concentrations induced by cardiac surgery. Samples were collected from the proximal access of the central catheter at different time points during the procedure: (i) preoperative, just after intubation, (ii) before aortic clamping, (iii) 6 minutes after removal of aortic clamp, and (iv) 6 h postoperative and (v) 24 h postoperative time, defined by the time patients were admitted to the postoperative care unit. All blood samples were stored in test tubes containing ethylenediamine tetra-acetic acid. Total and free T4, total T3, and thyrotropin (TSH) were measured using a chemiluminescence immunoassay (Immulite 2000 Siemens Healthcare Diagnostics Products, Marburg, Germany). rT3 was measured using tandem mass spectrometry (API 3000 tandem mass spectrometer, SCIEX, Toronto, Canada). Interleukin-6 and tumor necrosis factor-alpha measurements were also performed by chemiluminescence (Immulite 1000, Siemens Medical Solutions Diagnostics, Los Angeles, CA). C-reactive protein (CRP) was quantified by immunophelometry (BN II Systems and CARDIO PHASE® hs-CRP kit, Siemens Healthcare Diagnostics Products, Marburg, Germany). Troponin I was measured by direct chemiluminescence (ADVIA Centaur TnI-Ultra kit and device, Siemens Healthcare Diagnostics, Tarrytown, NY). Mass creatine kinase myocardial b fraction (CK-MB) was also quantified by direct chemiluminometry (ADVIA Centaur CKMB kit and device, Siemens Healthcare Diagnostics, Tarrytown, NY). Free T4 was not considered in central blood samples because heparin infused at the time of CPB reacts with T4-binding proteins and leads to an overestimation of free T4 concentrations.
Cardiac troponin I and CK-MB were measured 6 and 24 h postoperative blood samples to estimate the ischemic burden during surgery. Interleukin 6, tumor necrosis factor-alpha, and CRP were measured at after removal of the aortic clamp and 24 h post-operative to evaluate the inflammatory response associated with each surgical procedure.
Statistical analysis
Peripheral blood sample comparisons were done with ANOVA followed by Bonferroni's test when statistical difference was initially detected. In central blood samples, one-tailed paired Student's t-tests were performed to analyze aortic root-to-coronary sinus differences within the same group. For baseline and procedure characteristics, Chi-square, Student's t-test or ANOVA were used as indicated. For time comparisons, Kruskal-Wallis and Mann-Whitney tests were performed. Data were analyzed using PRISM software. A p value <0.05 was considered statistically significant.
Results
Between April 2012 and May 2014, a total of 64 patients were enrolled in the study. Of these, six were excluded due to subclinical hypothyroidism (4), use of amiodarone during surgery (1) or technical limitations in the collection of central blood samples (1). Of the 58 subjects included in the study, 23 had AS and 35 had CAD. Age, incidence of comorbidities, hemoglobin, and serum creatinine levels were similar in both groups (Table 1). LV posterior wall and interventricular septum thickness were significantly increased in the AS versus CAD group (respectively, 12.0 ± 2.1 vs 9.9 ± 1.6 mm and 12.8 ± 2.5 vs 10.3 ± 1.7 mm, p < 0.0001 for both comparisons). Diastolic diameter was also greater in the AS hearts (53.7 ± 8.3 vs. 49.5 ± 5.1 mm, p = 0.02).
The AS, but not the CAD, myocardium consumes thyroid hormone
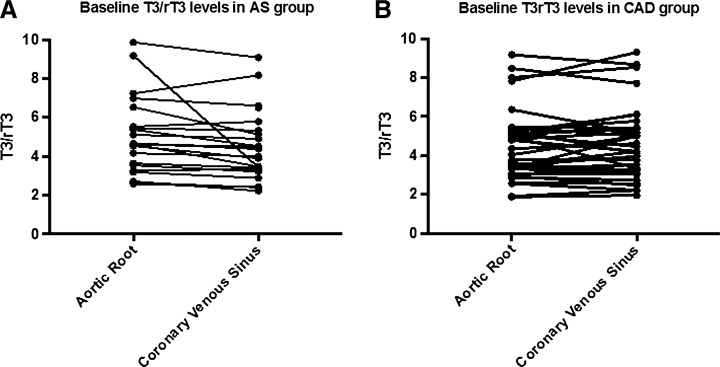
The baseline data in the AS patients indicate that thyroid hormone is consumed during the <5 s required for blood to flow through the AS myocardium. Serum T3 levels decreased by 4.6%, from 0.87 ± 0.26 in the aortic root to 0.83 ± 0.24 ng/mL in the coronary sinus (p = 0.022; Table 2). In contrast, rT3 levels increased by 6.9%, from 0.18 ± 0.07 in the aortic root to 0.20 ± 0.07 ng/mL in the coronary sinus (p = 0.040; Table 2). As a result, the serum T3/rT3 ratio decreased by 9.6%, from 5.0 in the aortic root to 4.5 in the coronary sinus (p = 0.02; Table 2, Fig. 2). In contrast, at baseline there was no evidence of thyroid hormone activation or consumption in CAD patients. No differences in thyroid hormone levels were observed as a result of blood flowing through the CAD myocardium (Table 2, Fig. 2).
Table 2.
Serum Thyroid Hormone Levels in the “Baseline” Blood Samples According to the Site Where Blood Was Collected and Type of Disease
| Baseline | Aortic root | Coronary venous sinus | p |
|---|---|---|---|
| AS | |||
| T3 (ng/mL) | 0.87 ± 0.26 | 0.83 ± 0.24 | 0.022 |
| rT3 (ng/mL) | 0.18 ± 0.07 | 0.20 ± 0.07 | 0.040 |
| T3/rT3 | 5.0 ± 1.97 | 4.52 ± 1.77 | 0.02 |
| T4 (ng/mL) | 86.2 ± 22.3 | 86.5 ± 20.5 | 0.412 |
| TSH (μU/mL) | 1.22 ± 0.86 | 1.23 ± 0.87 | 0.374 |
| CAD | |||
| T3 (ng/mL) | 0.83 ± 0.32 | 0.82 ± 0.30 | 0.446 |
| rT3 (ng/mL) | 0.20 ± 0.08 | 0.20 ± 0.08 | 0.964 |
| T3/rT3 | 4.46 ± 1.81 | 4.54 ± 1.88 | 0.402 |
| T4 (ng/mL) | 90.7 ± 25.7 | 88.8 ± 27.2 | 0.964 |
| TSH (μU/mL) | 1.38 ± 1.26 | 1.43 ± 1.34 | 0.067 |
Values are the mean ± SD.
Reference ranges for peripheral blood are: T3(0.7–1.8 ng/mL); rT3 (0.10–0.24 ng/mL); T4 (45–120 ng/mL); TSH (0.45–4.5 μU/mL).
Statistical analysis was by paired Student's t-test.
rT3, 3,3′,5′-T3; T3, triiodothyronine; T4, thyroxine; TSH, thyrotropin.
FIG. 2.
T3/rT3 ratio in blood obtained from aortic root and coronary venous sinus of each patient in the aortic stenosis (AS) (A) and coronary artery disease (CAD) (B) groups.
No differences in T4 levels were observed across the AS and CAD myocardial circulation (Table 2). Serum TSH levels were used as an internal control given that TSH is not known to be taken up by or undergo metabolic breakdown by the myocardium. No differences in serum TSH were observed as a result of AS or CAD myocardial blood flow (Table 2).
Given that consumption of T3 by the AS myocardium is likely the result of LV hypertrophy and D3 expression (14,15), we looked for a correlation between LV wall thickness and T3 consumption, rT3 production, or a decrease in T3/rT3 ratio, but none was detected at a statistically significant level (data not shown).
Heart surgery modifies myocardial thyroid hormone economy
The study of thyroid hormone levels performed after the surgery allowed us to examine how three modalities of heart surgery affected myocardial thyroid hormone metabolism: (i) aortic valve replacement, (ii) CABG with CPB (n = 23), or (iii) off-pump CABG (n = 12). In the three cases, blood from the aortic root and the coronary sinus was collected after minutes of unclamping the aorta (patients who underwent CPB) or immediately after grafting was finished (off-pump CABG). Remarkably, the differences in thyroid hormone levels seen after AS myocardium circulation were dissipated (Table 3). Specifically, T3 and rT3 levels were not affected by the myocardial circulation and the T3/rT3 ratio remained stable at 4.62 ± 1.96 in the aortic root versus 4.44 ± 1.70 in the coronary sinus (p = 0.277). At the same time, no significant myocardial thyroid hormone metabolism was observed in the CAD patients immediately after the aorta was unclamped or grafting was finished (Table 3).
Table 3.
Serum Thyroid Hormone Levels in the “Conclusion” Blood Sample According to the Site Where Blood Was Collected and Type of Surgical Procedure
| Conclusion sample | Aortic root | Coronary venous sinus | p |
|---|---|---|---|
| Valve replacement | |||
| T3 (ng/mL) | 0.75 ± 0.14 | 0.74 ± 0.13 | 0.335 |
| rT3 (ng/mL) | 0.18 ± 0.05 | 0.18 ± 0.04 | 0.421 |
| T3/rT3 | 4.62 ± 1.96 | 4.44 ± 1.70 | 0.277 |
| T4 (ng/mL) | 77.0 ± 13.6 | 78.9 ± 11.7 | 0.048 |
| TSH (μU/mL) | 2.09 ± 1.85 | 2.08 ± 1.96 | 0.432 |
| CABG off-pump | |||
| T3 (ng/mL) | 0.75 ± 0.29 | 0.83 ± 0.30 | 0,021 |
| rT3 (ng/mL) | 0.20 ± 0.11 | 0.23 ± 0.12 | 0,084 |
| T3/rT3 | 4.31 ± 2.24 | 4.43 ± 2.25 | 0.088 |
| T4 (ng/mL) | 80.9 ± 28.3 | 92.6 ± 38.0 | 0,010 |
| TSH (μU/mL) | 1.94 ± 3.09 | 1.68 ± 2.73 | 0,168 |
| CABG CPB | |||
| T3 (ng/mL) | 0.67 ± 0.22 | 0.68 ± 0.24 | 0.342 |
| rT3 (ng/mL) | 0.15 ± 0.03 | 0.15 ± 0.03 | 0.074 |
| T3/rT3 | 4.60 ± 1.99 | 4.86 ± 2.14 | 0.103 |
| T4 (ng/mL) | 64.2 ± 13.8 | 65.3 ± 13.6 | 0.093 |
| TSH (μU/mL) | 1.29 ± 0.65 | 1.27 ± 0.58 | 0.213 |
Values are the mean ± SD.
Reference ranges for peripheral blood are: T3 (0.7–1.8 ng/mL); rT3 (0.10–0.24 ng/mL); T4 (45–120 ng/mL); TSH (0.45–4.5 μU/mL).
Statistical analysis was by paired Student's t-test.
CABG, coronary artery bypass grafting; CPB, cardiopulmonary bypass.
A noticeable aspect was an approximately 14.5% increase in serum T4 levels and an approximately 10.7% increase in serum T3 levels due to myocardial circulation (Table 3; 8.09 ± 2.83 vs. 9.26 ± 3.80 μg/dL, p = 0.01) in patients undergoing off-pump CABG. Only a minimal trend (1.7–2.5% elevation in T4 levels after myocardial circulation) was observed in patients undergoing the other two types of surgeries (Table 3). Despite heart surgery, serum TSH levels remained stable while flowing through the myocardium (Table 3).
Systemic thyroid hormone levels
All patients exhibited systemic changes in serum thyroid hormone levels characteristic of nonthyroidal illness. Overall, there was a 40–50% drop in systemic T3 at 6 and 24 h after surgery (Table 4). The decline in serum T3 was detected early, prior to CPB, possibly due to fasting and/or anesthesia (Table 4). In contrast, surgery was associated with a 2- to 3-fold increase in serum rT3 levels (Table 4). A mild but significant reduction in serum T4 levels, in the absence of changes in free T4, was seen through the surgical procedure, whereas serum TSH levels dropped substantially in all groups (Table 4).
Table 4.
Serum Thyroid Hormone Levels in Peripheral Blood Samples
| Group | Pre-op | Pre-CPB | Post-CPB | Post-op 6 h | Post-op 24 h | p |
|---|---|---|---|---|---|---|
| Valve Replacement | ||||||
| T3 (ng/mL) | 1.13 ± 0.30 | 0.92 ± 0.26* | 0.73 ± 0.15* | 0.79 ± 0.20* | 0.71 ± 0.14* | <0.001 |
| rT3 (ng/mL) | 0.26 ± 0.08 | 0.21 ± 0.07 | 0.19 ± 0.05 | 0.38 ± 0.10* | 0.63 ± 0.21* | <0.001 |
| T4 (ng/mL) | 95.5 ± 14.5 | 90.2 ± 21.3 | 76.2 ± 10.8* | 84.4 ± 17.3 | 82.2 ± 19.1 | <0.001 |
| FT4 (ng/mL × 102) | 1.35 ± 0.23 | 2.12 ± 0.52 | 1.97 ± 0.42 | 1.31 ± 0.24 | 1.34 ± 0.26 | N.S. |
| TSH (μU/mL) | 1.83 ± 1.39 | 1.29 ± 0.97 | 2.04 ± 1.82 | 0.85 ± 1.09 | 0.62 ± 0.61* | <0.001 |
| CABG Off-pump | ||||||
| T3 (ng/mL) | 1.08 ± 0.24 | 0.84 ± 0.28 | 0.74 ± 0.28* | 0.60 ± 0.23* | 0.61 ± 0.20* | <0.001 |
| rT3 (ng/mL) | 0.25 ± 0.13** | 0.23 ± 0.10** | 0.22 ± 0.14** | 0.41 ± 0.33** | 0.68 ± 0.23 | <0.001 |
| T4 (ng/mL) | 76.8 ± 33.6 | 98.8 ± 26.0 | 80.0 ± 31.2 | 71.3 ± 26.0 | 75.7 ± 25.9 | 0.118 |
| FT4 (ng/mL × 102) | 1.29 ± 0.29 | 1.98 ± 0.39 | 1.72 ± 0.33 | 1.21 ± 0.22 | 1.32 ± 0.35 | N.S. |
| TSH (μU/mL) | 2.06 ± 1.61 | 1.80 ± 1.32 | 2.23 ± 3.49 | 0.73 ± 0.63 | 0.51 ± 0.27 | <0.001 |
| CABG CPB | ||||||
| T3 (ng/mL) | 1.08 ± 0.23 | 0.71 ± 0.26* | 0.66 ± 0.22* | 0.75 ± 0.20* | 0.71 ± 0.15* | <0.001 |
| rT3 (ng/mL) | 0.24 ± 0.05** | 0.18 ± 0.07** | 0.24 ± 0.35** | 0.35 ± 0.06** | 0.65 ± 0.33 | <0.001 |
| T4 (ng/mL) | 87.4 ± 19.2 | 79.1 ± 20.1* | 64.1 ± 13.3 | 77.2 ± 16.8 | 77.0 ± 19.1 | <0.001 |
| FT4 (ng/mL × 102) | 1.27 ± 0.17 | 1.80 ± 0.33 | 1.65 ± 0.29 | 1.27 ± 0.17 | 1.27 ± 0.23 | N.S. |
| TSH (μU/mL) | 1.69 ± 0.83** | 1.41 ± 1.51** | 1.26 ± 0.63 | 0.54 ± 0.23*,*** | 0.55 ± 0.45 | <0.001 |
Values are the mean ± SD.
Note that heparin, given at the time of CPB reacts with T4-binding proteins and leads to an overestimation of FT4 values.
Reference ranges for peripheral blood are: T3 (0.7–1.8 ng/mL); rT3 (0.10–0.24 ng/mL); T4 (45–120 ng/mL); FT4 (0.6–1.3 ng/mL × 102); TSH (0.45–4.5 μU/mL).
Statistical analysis was by ANOVA; post-test analysis was by Bonferroni's multiple comparison test.
p < 0.05 vs. pre-op; **p < 0.05 vs. post-op 24 h; ***p < 0.05 vs. post-CPB.
N.S., not significant.
Myocardial necrosis and inflammatory markers
Biomarkers reflecting myocardial necrosis were similar in all groups 6 and 24 h after surgery (Table 5). Although we expected off-pump CABG to be a less aggressive procedure, inflammatory and myocardial necrosis markers showed the opposite. Serum levels of inflammatory markers also behaved similarly among groups, except for CRP 24 h, which was higher in off-pump CABG patients compared with CPB CABG patients or AS patients (Table 5).
Table 5.
Serum Levels of Myocardial Necrosis and Inflammatory Markers
| Metabolites | Time point | CABG off pump | CABG CPB | Valve Replacement | p |
|---|---|---|---|---|---|
| CK-MB | Post-op 6h | 55.0 ± 65.3 | 55.9 ± 56.8 | 45.6 ± 33.7 | 0.722 |
| Post-op 24h | 60.6 ± 83.0 | 34.8 ± 42.6 | 26.7 ± 17.4 | 0.151 | |
| Tn | Post-op 6h | 9.1 ± 13.2 | 8.7 ± 11.0 | 8.8 ± 9.8 | 0.995 |
| Post-op 24h | 14.7 ± 20.3 | 7.5 ± 11.1 | 7.5 ± 7.1 | 0.227 | |
| IL-6 | Post-CPB 3h | 126.9 ± 307.0 | 84.1 ± 168.3 | 10.2 ± 6.8 | 0.314 |
| Post-op 24h | 103.2 ± 119.5 | 66.0 ± 79.9 | 55.5 ± 49.1 | 0.372 | |
| αTNF | Post-CPB 3min | 7.3 ± 2.4 | 9.3 ± 10.7 | 9.1 ± 4.5 | 0.787 |
| Post-op 24h | 8.6 ± 2.9 | 8.5 ± 5.2 | 10.4 ± 3.6 | 0.441 | |
| CRP | Post-CPB 3min | 9.6 ± 22.6 | 3.0 ± 2.9 | 3.34 ± 3.5 | 0.175 |
| Post-op 24h | 160.8 ± 58.8 | 107.4 ± 31.8 | 104.1 ± 35.6 | <0.001 |
Values are the mean ± SD.
Reference ranges for metabolites: CK-MB, <5 ng/mL; troponin, <0.04 ng/mL; IL-6, 5–15 pg/mL; and TNF-α, 5–27 pg/mL; CRP, <1.0 mg/dL.
Statistical analysis was by ANOVA.
CK-MB, creatine kinase myocardial b fraction; CRP, C-reactive protein; IL-6, interleukin-6; Tn, troponin; TNF-α, tumor necrosis factor alpha.
Discussion
To our knowledge the present study provides the first direct evidence that thyroid hormone is metabolized in the human heart. The results indicate that in patients with AS the human myocardium predominantly inactivates thyroid hormone. Based on animal studies (15–18), there is probably local dampening of thyroid hormone signaling in the AS myocardium. The fact that thyroid hormone inactivation was detected in the AS patients with cardiac hypertrophy is reminiscent of a rat model of D3 expression in the myocardium (15). In this model, Dio3 is induced by hypoxia and/or ischemia, dampening local thyroid hormone signaling. No significant thyroid hormone metabolism was observed in patients with CAD, which is unexpected given the reports of D2 expression in human hearts (3,4).
The heart is not the only tissue expressing Dio3 in response to hypoxia/ischemia. D3 activity was detected in ischemic human tissues such as liver and skeletal muscle removed from critically ill patients immediately after death (12). Given that thyroid hormone levels in these tissues are reduced (12,19), one could speculate that there is local acceleration in thyroid hormone inactivation and hypothyroidism. However, serum thyroid hormone levels are also low in these patients and thus the local role played by D3 remains undefined. In an animal model in which rats develop pulmonary hypertension and concentric RVH with congestive heart failure, there is a 9-fold increase in D3 activity only in the RV (15). This resulted in a 35% drop in local T3 concentration relative to the LV of the same hearts, whereas the T4 content was the same in both ventricles. Using a T3-reporter gene injected into the myocardium via thoracotomy, T3-dependent transcriptional activity was found to be reduced by 45% only in the Dio3-expressing RV (15). In light of these results, the present findings of thyroid hormone inactivation in patients with LV hypertrophy suggest the presence of an anatomically precise D3-mediated reduction of T3, which results in alterations in T3-dependent transcriptional activity.
While it is intuitive to assume that less thyroid hormone is a preferred condition in patients with ventricular hypertrophy, heart failure, or myocardial hypoxia/ischemia, much clinical data point toward the opposite hypothesis. For example, in pediatric patients undergoing cardiac surgery with CPB, the use of LT3 infusion in the postoperative period resulted in a marked decrease in requirement of inotropic support, spontaneous conversion to normal sinus rhythm, and improved clinical outcomes (20). In adult high-risk patients undergoing CABG, randomized postoperative administration of LT3 was associated with a higher mean cardiac index and lower systemic vascular resistance but did not change outcomes or alter the need for standard postoperative therapy (21). In addition, in patients with depressed LV function undergoing CABG, perioperative administration of LT3 resulted in a lower incidence of atrial fibrillation, a decreased need of cardioversion or anticoagulation during hospitalization, and lower requirements of antiarrhythmic therapy at discharge (22). Beneficial effects of short-term LT3 replacement therapy, such as improved ventricular performance, were also observed in stable patients with ischemic or non-ischemic dilated cardiomyopathy patients (23).
One could still argue that these clinical studies address the cardiac impact of systemic nonthyroidal illness but not the localized D3-mediated myocardial hypothyroidism. Indeed, all patients enrolled in the present studies exhibited an about 40% drop in serum T3 levels at the early stages of surgery, combined with a rT3 elevation, particularly at the end of follow-up. This is a typical finding that has been critically reviewed elsewhere (24). However, the novel aspect of the present investigation is the demonstration that the hypertrophic myocardium inactivates thyroid hormone and likely functions in a more hypothyroid environment than would be expected from the nonthyroidal illness–mediated drop in circulating T3 levels. To test whether myocardial Dio3 expression is adaptive or maladaptive, a mouse carrying one inactive Dio3 allele (HtzD3KO) has previously been used (16). Dio3 is a tissue-specific imprinted gene in the heart, and thus, HtzD3KO mice constitute a model of cardiac Dio3 inactivation in an otherwise systemically euthyroid animal. The HtzD3KO animals exhibit higher mortality when undergoing a 10-day treatment with isoproterenol, which may indicate an adaptive role played by myocardial D3 reactivation (16). However, this mouse model is not without limitations; HtzD3KO newborns have normal hearts but later develop restrictive cardiomyopathy, including myocardial fibrosis, impaired myocardial contractility, and diastolic dysfunction. Thus, at present, we have no basis to qualify the accelerated thyroid hormone inactivation observed in the AS heart as adaptive or maladaptive.
Given the inducibility of Dio3 to hypoxia/ischemia, we hypothesized that thyroid hormone inactivation would be accelerated after surgery and/or CPB. However, this was not the case and thyroid hormone metabolism could no longer be detected (Table 3). In addition, surgery had another unexpected effect, i.e. a net outflow of T4 and T3 from the myocardium into the coronary circulation. This was seen particularly in the CABG-off pump patients but similar trends were also seen in the patients undergoing CPB (Table 3). It is conceivable that the stress associated with heart surgery altered local thyroid hormone economy, suppressing thyroid hormone inactivation in the AS myocardium and depleting intracellular T4 and T3 levels. Such changes may have occurred as a result of “myocardium stunning.” During cardiac surgery there is evidence of several myocardial stresses, including ischemia, inflammatory response, operative trauma, cardioplegia and oxidative stress (25,26). In fact, the transient reversible myocardial ischemia in the setting of cardioplegia and CPB leads to prolonged depression of cardiac contractility after reperfusion, which might contribute to the modified thyroid hormone economy.
This study has important limitations. For example, we failed to obtain ventricular myocardium to study Dio2 or Dio3 expression. Left ventricular biopsies, however, would have increased the risk of the procedure. The failure to detect T3 production in CAD is unexpected in view of the presence of Dio2 mRNA in human cardiac tissue. While it is conceivable that study design or technique contributed to this negative result, it is also possible that the myocardium is simply not equipped with all the elements needed to produce T3 in vivo. In a previous study in which the human Dio2 was engineered to be expressed in the mouse myocardium, D2 was detected at extremely high levels when assayed in vitro (highest levels known to us) but the actual increase in myocardial T3 levels was only about 30% (7).
In conclusion, the present study indicates that the myocardium of AS patients preferentially inactivates thyroid hormone, most likely dampening local thyroid hormone signaling. It is conceivable that these changes are due to myocardial hypertrophy, thus explaining why they are restricted to patients with AS and not CAD patients. No significant myocardial thyroid hormone activation was observed in any of the groups. Whether myocardial thyroid hormone inactivation is beneficial or maladaptive in patients with AS syndrome remains to be determined. Prospective studies in which T3 is administered to these patients could test this hypothesis.
Acknowledgment
We thank Dr. Elizabeth McAninch for critically reviewing the manuscript.
Author Disclosure Statement
The authors have nothing to disclose.
References
- 1.Abdalla SM, Bianco AC. 2014. Defending plasma T3 is a biological priority. Clin Endocrinol 81:633–641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gereben B, Zavacki AM, Ribich S, Kim BW, Huang SA, Simonides WS, Zeold A, Bianco AC. 2008. Cellular and molecular basis of deiodinase-regulated thyroid hormone signaling. Endocr Rev 29:898–938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Croteau W, Davey JC, Galton VA, St Germain DL. 1996. Cloning of the mammalian type II iodothyronine deiodinase. A selenoprotein differentially expressed and regulated in human and rat brain and other tissues. J Clin Invest 98:405–417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Salvatore D, Bartha T, Harney JW, Larsen PR. 1996. Molecular biological and biochemical characterization of the human type 2 selenodeiodinase. Endocrinology 137:3308–3315 [DOI] [PubMed] [Google Scholar]
- 5.Rosene ML, Wittmann G, Arrojo e Drigo R, Singru PS, Lechan RM, Bianco AC. 2010. Inhibition of the type 2 iodothyronine deiodinase underlies the elevated plasma TSH associated with amiodarone treatment. Endocrinology 151:5961–5970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vassallo P, Trohman RG. 2007. Prescribing amiodarone: an evidence-based review of clinical indications. JAMA 298:1312–1322 [DOI] [PubMed] [Google Scholar]
- 7.Pachucki J, Hopkins J, Peeters R, Tu H, Carvalho SD, Kaulbach H, Abel ED, Wondisford FE, Ingwall JS, Larsen PR. 2001. Type 2 Iodothyronine Deiodinase Transgene Expression in the Mouse Heart Causes Cardiac-Specific Thyrotoxicosis. Endocrinology 142:13–20 [DOI] [PubMed] [Google Scholar]
- 8.Trivieri MG, Oudit GY, Sah R, Kerfant BG, Sun H, Gramolini AO, Pan Y, Wickenden AD, Croteau W, Morreale de Escobar G, Pekhletski R, St Germain D, Maclennan DH, Backx PH. 2006. Cardiac-specific elevations in thyroid hormone enhance contractility and prevent pressure overload-induced cardiac dysfunction. Proc Natl Acad Sci U S A 103:6043–6048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carvalho-Bianco SD, Kim BW, Zhang JX, Harney JW, Ribeiro RS, Gereben B, Bianco AC, Mende U, Larsen PR. 2004. Chronic cardiac-specific thyrotoxicosis increases myocardial beta-adrenergic responsiveness. Mol Endocrinol 18:1840–1849 [DOI] [PubMed] [Google Scholar]
- 10.Hong EG, Kim BW, Young Jung D, Hun Kim J, Yu T, Seixas Da Silva W, Friedline RH, Bianco SD, Seslar SP, Wakimoto H, Berul CI, Russell KS, Won Lee K, Larsen PR, Bianco AC, Kim JK. 2013. Cardiac expression of human type 2 iodothyronine deiodinase increases glucose metabolism and protects against doxorubicin-induced cardiac dysfunction in male mice. Endocrinology 154:3937–3946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang YY, Morimoto S, Du CK, Lu QW, Zhan DY, Tsutsumi T, Ide T, Miwa Y, Takahashi-Yanaga F, Sasaguri T. 2010. Up-regulation of type 2 iodothyronine deiodinase in dilated cardiomyopathy. Cardiovasc Res 87:636–646 [DOI] [PubMed] [Google Scholar]
- 12.Peeters RP, Wouters PJ, Kaptein E, van Toor H, Visser TJ, Van den Berghe G. 2003. Reduced activation and increased inactivation of thyroid hormone in tissues of critically ill patients. J Clin Endocrinol Metab 88:3202–3211 [DOI] [PubMed] [Google Scholar]
- 13.Olivares EL, Marassi MP, Fortunato RS, da Silva AC, Costa-e-Sousa RH, Araujo IG, Mattos EC, Masuda MO, Mulcahey MA, Huang SA, Bianco AC, Carvalho DP. 2007. Thyroid function disturbance and type 3 iodothyronine deiodinase induction after myocardial infarction in rats a time course study. Endocrinology 148:4786–4792 [DOI] [PubMed] [Google Scholar]
- 14.Wassen FW, Schiel AE, Kuiper GG, Kaptein E, Bakker O, Visser TJ, Simonides WS. 2002. Induction of thyroid hormone-degrading deiodinase in cardiac hypertrophy and failure. Endocrinology 143:2812–2815 [DOI] [PubMed] [Google Scholar]
- 15.Simonides WS, Mulcahey MA, Redout EM, Muller A, Zuidwijk MJ, Visser TJ, Wassen FW, Crescenzi A, da-Silva WS, Harney J, Engel FB, Obregon MJ, Larsen PR, Bianco AC, Huang SA. 2008. Hypoxia-inducible factor induces local thyroid hormone inactivation during hypoxic-ischemic disease in rats. J Clin Invest 118:975–983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ueta CB, Oskouei BN, Olivares EL, Pinto JR, Correa MM, Simovic G, Simonides WS, Hare JM, Bianco AC. 2012. Absence of myocardial thyroid hormone inactivating deiodinase results in restrictive cardiomyopathy in mice. Mol Endocrinol 26:809–818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pol CJ, Muller A, Zuidwijk MJ, van Deel ED, Kaptein E, Saba A, Marchini M, Zucchi R, Visser TJ, Paulus WJ, Duncker DJ, Simonides WS. 2011. Left-ventricular remodeling after myocardial infarction is associated with a cardiomyocyte-specific hypothyroid condition. Endocrinology 152:669–679 [DOI] [PubMed] [Google Scholar]
- 18.Freitas BC, Gereben B, Castillo M, Kallo I, Zeold A, Egri P, Liposits Z, Zavacki AM, Maciel RM, Jo S, Singru P, Sanchez E, Lechan RM, Bianco AC. 2010. Paracrine signaling by glial cell-derived triiodothyronine activates neuronal gene expression in the rodent brain and human cells. J Clin Invest 120:2206–2217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Peeters RP, van der Geyten S, Wouters PJ, Darras VM, van Toor H, Kaptein E, Visser TJ, Van den Berghe G. 2005. Tissue thyroid hormone levels in critical illness. J Clin Endocrinol Metab 90:6498–6507 [DOI] [PubMed] [Google Scholar]
- 20.Chowdhury D, Parnell VA, Ojamaa K, Boxer R, Cooper R, Klein I. 1999. Usefulness of triiodothyronine (T3) treatment after surgery for complex congenital heart disease in infants and children. Am J Cardiol 84:1107–1109, A1110 [DOI] [PubMed] [Google Scholar]
- 21.Klemperer JD, Klein I, Gomez M, Helm RE, Ojamaa K, Thomas SJ, Isom OW, Krieger K. 1995. Thyroid hormone treatment after coronary-artery bypass surgery. N Engl J Med 333:1522–1527 [DOI] [PubMed] [Google Scholar]
- 22.Klemperer JD, Klein IL, Ojamaa K, Helm RE, Gomez M, Isom OW, Krieger KH. 1996. Triiodothyronine therapy lowers the incidence of atrial fibrillation after cardiac operations. Ann Thorac Surg 61:1323–1327; discussion 1328–1329 [DOI] [PubMed] [Google Scholar]
- 23.Pingitore A, Galli E, Barison A, Iervasi A, Scarlattini M, Nucci D, L'Abbate A, Mariotti R, Iervasi G. 2008. Acute effects of triiodothyronine (T3) replacement therapy in patients with chronic heart failure and low-T3 syndrome: a randomized, placebo-controlled study. J Clin Endocrinol Metab 93:1351–1358 [DOI] [PubMed] [Google Scholar]
- 24.Klemperer JD. 2002. Thyroid hormone and cardiac surgery. Thyroid 12:517–521 [DOI] [PubMed] [Google Scholar]
- 25.Paparella D, Yau TM, Young E. 2002. Cardiopulmonary bypass induced inflammation: pathophysiology and treatment. An update. Eur J Cardio Thorac Surg 21:232–244 [DOI] [PubMed] [Google Scholar]
- 26.Zahler S, Massoudy P, Hartl H, Hahnel C, Meisner H, Becker BF. 1999. Acute cardiac inflammatory responses to postischemic reperfusion during cardiopulmonary bypass. Cardiovasc Res 41:722–730 [DOI] [PubMed] [Google Scholar]