Abstract
Use of multiple prescription medications is common among individuals with chronic obstructive pulmonary disease (COPD) because of coexisting inflammatory-related conditions. Specifically, the use of antidepressants, inhaled corticosteroids (ICSs), and statins may place individuals with COPD at high risk for new-onset diabetes. The objective was to examine the relationship between the use of antidepressants, ICSs, and statins and new-onset diabetes among Medicaid beneficiaries with COPD. This study used a retrospective longitudinal cohort design using multiple years (2005–2008) of Medicaid claims for beneficiaries with newly diagnosed COPD (n = 15,287), who were diabetes free at baseline. National Drug Codes were used to determine the receipt of antidepressants, ICSs, and statins, and International Classification of Diseases, Ninth Revision, Clinical Modification codes were used to define new-onset diabetes (250.x2). Multivariable logistic regression was used to examine the adjusted relationship between medication use and new-onset diabetes. Overall, 6.3% of the study population was diagnosed with new-onset diabetes. After controlling for baseline characteristics, individuals using ICSs (adjusted odds ratio [AOR]: 1.23; 95% confidence interval [CI]: 1.07, 1.47) or statins (AOR: 1.48; 95% CI: 1.27, 1.72) had a greater risk of new-onset diabetes compared to those not given ICSs, statins, or antidepressants. Analyses using combined medication categories revealed that adults using statins in combination with both antidepressants and ICSs, or when combined with ICS, were more likely to have new-onset diabetes. These findings indicate that multiple medication use (ICSs and statins) was associated with increased rates of new-onset diabetes. Further research is warranted to understand this association.
Introduction
Chronic obstructive pulmonary disease (COPD) is an inflammatory disease linked to persistent and progressive airflow limitation.1,2 Because COPD is no longer considered a disease exclusive to the lungs, it is increasingly being recognized as a “chronic systemic inflammatory syndrome.”3 The use of medications to treat inflammation-related multimorbidity among individuals with COPD has been increasing.4 A cross-sectional study using data on 126,283 individuals with COPD reported that 98% of the individuals received at least 1 prescription for “nonrespiratory drugs,” with 64% cardiovascular medication and 8% depression medication use reported.5
Although generally regarded as safe, the adverse effects of some of these medications have raised concerns about their safety, especially for patients with comorbid conditions. Antidepressants, inhaled corticosteroids (ICSs), and statins have been linked to new-onset diabetes.6–9 Several lines of evidence indicate that these medications can increase insulin resistance, decrease insulin secretion, and impact overall glucose metabolism.10–12 One recent literature review11 concluded that an insufficient number of clinical trial investigations and observational studies have evaluated the relationship between the use of antidepressants, statins, and ICSs on new-onset diabetes in the general population.
Investigators from a large randomized controlled trial have reported that antidepressant users had a higher likelihood of developing diabetes compared to nonusers.13 However, further observational studies have not conclusively determined the role of antidepressants in causing new-onset diabetes. In addition, recent evidence indicates that depression symptoms, rather than antidepressant use, are associated with new-onset diabetes.14
ICSs also have been evaluated with regard to the development of type 2 diabetes among individuals with COPD.7,9,15 The available data indicate that ICS levels in the systemic circulation are low and, thus, may not play a major role in the risk of incident diabetes. However, evidence regarding the risk of new-onset diabetes associated with ICS use is inconclusive. In a nested case–control study using data from Quebec Health Insurance databases, Suissa et al reported that among 388,584 individuals with respiratory disease, current ICS use was associated with a 34% higher risk of incident diabetes compared to individuals who were not currently taking ICS (relative risk: 1.34; 95% confidence interval [CI]: 1.29–1.39).9 However, a major limitation of this study was the combined evaluation of asthma and COPD patients, because it has been established that the risk of incident diabetes is different among individuals with COPD compared to those with asthma.16 Recently, a retrospective analysis of randomized controlled trials of the ICS budesonide indicated no statistically significant difference between users and nonusers in terms of new-onset diabetes.7 Therefore, the relationship between ICS use and new-onset diabetes remains uncertain.
The relationship between statin use and new-onset diabetes also has not been well established. Analysis of data from the Justification for the Use of Statins in Prevention: an Intervention Trial Evaluating Rosuvastatin (JUPITER) trial revealed that among 17,802 individuals randomly assigned to rosuvastatin or placebo, those in the rosuvastatin group had significantly higher rates of physician-reported incident diabetes cases compared to those in the placebo group (3.0% vs. 2.4%; P = 0.01).17 This trial included individuals with low cardiovascular risk, but high levels of inflammatory mediator C-reactive protein, a characteristic of smokers with COPD.17 On the contrary, findings from a large-scale randomized trial of pravastatin (West of Scotland Coronary Prevention Study [WOSCOPS]) suggested protective effects of pravastatin in reducing the risk of new-onset diabetes, because men aged between 45 and 64 years on pravastatin therapy had a 20% reduced hazard of developing new-onset diabetes compared to individuals on placebo (hazard ratio: 0.70; 95% CI: 0.50–0.99; P = 0.042).18
Although inconclusive, these studies indicate plausible risks associated with medication use and new-onset diabetes. With the increasing prevalence of comorbidities among individuals with COPD and the potential use of multiple medications, it is important to assess the risk of new-onset diabetes in a real-world setting. Furthermore, it is especially important to assess the risk of new-onset diabetes among patients with COPD, as the risk of new-onset diabetes has been found to be twice as high among individuals with COPD compared to those without COPD.16 Therefore, the primary objective of the current study was to evaluate the association between commonly used medications (antidepressants, ICSs, and statins) and new-onset diabetes among adults with newly diagnosed COPD. This study also evaluated the relationship between medication use and new-onset diabetes by statin type and determined whether the long-term use of antidepressants and statins was associated with new-onset diabetes as these medications are generally recommended for chronic condition management.
Methods
Study design
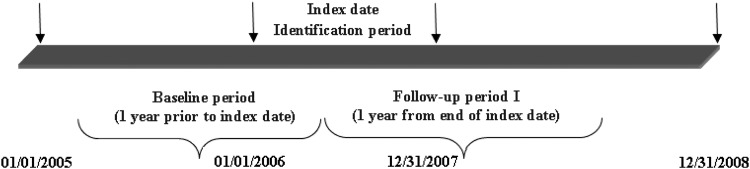
A retrospective longitudinal cohort design was used to determine the relationship between multiple medication use (antidepressants, ICSs, and statins) and new-onset diabetes. For the purpose of this study, data from administrative claims of Medicaid enrollees obtained from the Medicaid analytic extract (MAX) (2005–2008) provided by the Centers for Medicare & Medicaid Services (CMS) were used. Medicaid beneficiaries with newly-diagnosed COPD were identified between January 1, 2006 and December 31, 2007. The first date with an inpatient or outpatient claim of COPD diagnosis was considered as the index date. One year prior to the index date was considered the baseline period, and beneficiaries diagnosed with COPD or diabetes during this period were excluded from the study. New-onset diabetes was identified during the follow-up period, which was defined as 1 year after the index date. A schematic representation of the study periods is presented in Figure 1.
FIG. 1.
Schematic representation of the study design.
Data source: MAX (2005–2008)
Data from MAX from years 2005–2008 were used for this study. The files provided in the MAX administrative claim data are prepared and produced by CMS with the help of the Research and Data Assistance Center. The primary purpose of these files is to supply information regarding Medicaid beneficiary health care utilization to researchers and policy makers.
The MAX files contain person-level data, which are obtained using the Medicaid Statistical Information System through which all states administering Medicaid are required to supply information for Medicaid beneficiaries to CMS. These files provide information about beneficiaries' eligibility and their health care utilization and payment information. Several initiatives have been taken by the agencies to maintain and improve the quality of these data sets.19 CMS provided separate files that were linked based on a unique identification number given to each beneficiary. These files included enrollments (“personal summaries”), inpatient and outpatient medical claims, and pharmacy claims for beneficiaries.
The personal summary files provided information on Medicaid eligibility, patient demographics (eg, age, sex, race), managed care enrollment, utilization summaries, and Medicaid payments. Three additional files (ie, Other Therapy, Inpatient, and Prescription Drug files) were used to capture information regarding beneficiaries' fee-for-service claims. The Other Therapy file contained information regarding claims for Medicaid services provided at the outpatient level such as clinic services, physician services, home health care, and laboratory services. Enrollees' information regarding services provided during hospitalizations was obtained from the Inpatient file. Information regarding pharmacy or drug services was captured from the Prescription Drug file, which included date of prescription filled, days of supply, and national drug code (NDC).
Data for beneficiaries residing in the following states were used for the study: New York, Texas, Illinois, and California. These states were chosen to capture the diverse geographic and racial/ethnic populations represented by Medicaid.
Area resource file (ARF)
In addition to the demographic characteristics and utilization variables obtained from the MAX files, each Medicaid beneficiary's contextual county level variables were determined using ARF, which is a comprehensive county-level data set compiled by the Health Resources Service Administration's Bureau of Health Professions. ARF contains more than 6000 variables that provide county information, including health facility type, number and type of health professions, resource scarcity measures, health status, economic activity, health training programs, and socioeconomic and environmental characteristics. ARF files also include county codes and descriptors that allow data linkage with several secondary data sets such as MAX. For example, the density of pulmonologists in a beneficiary's residing county was a variable obtained from ARF data. This study used county codes and state information to link MAX files with the 2005 ARF file.
Study population
Medicaid beneficiaries with newly-diagnosed COPD were identified using physician office visit claims from MAX files. Individuals with at least 1 inpatient visit or 2 outpatient visits at least 14 days apart (obtained using type of service codes) for COPD based on International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) codes for chronic bronchitis (491.xx), emphysema (492.xx), or unspecified chronic airway obstruction (496.xx) were considered to have diagnosed COPD. These diagnosis codes have been utilized in prior published research to identify COPD and to evaluate medical treatments and health care outcomes among individuals with COPD.20–23 Indeed, the sensitivity and specificity of using ICD-9-CM codes to identify patients with COPD have been established.24,25 A study conducted by Gershon et al reported that identifying COPD using 1 or more ambulatory claims and/or hospitalizations for COPD resulted in a sensitivity of 85.0% (95% CI: 77.0 to 91.0) and a specificity of 78.4% (95% CI: 73.6 to 82.7). However, the present study requirement of 1 inpatient or 2 outpatient claims to identify individuals with COPD increased its specificity.
Other inclusion criteria included: (1) no COPD diagnoses during the baseline period; (2) no diabetes diagnoses (ICD-9-CM: 250.x2) during the baseline period; (3) 40–64 years of age (among young adults, this age group is at highest risk of COPD); (4) continuous eligibility during the baseline and follow-up periods; (5) no dual Medicaid/Medicare coverage; (6) enrolled in fee-for-service plans throughout the study observation period; (7) alive during the study observation period; and (8) use of services (inpatient or outpatient).
Dependent variable (new-onset diabetes)
A diagnosis of new-onset diabetes was determined during the follow-up period when a beneficiary had at least 1 inpatient or 2 outpatient claims for diabetes based on ICD-9-CM diagnosis (250.x2).
Independent variables
Multiple medication use
Antidepressants, ICSs, and statins were identified using NDCs recorded in pharmacy claims. Receipt of these medications was considered to be use of the medications. Statin users were defined as beneficiaries with at least 1 statin prescription during the baseline period. Antidepressant users were defined as individuals with at least 1 antidepressant prescription during the baseline period. ICS use was identified during the follow-up period because ICSs are usually prescribed to patients who have COPD, and the study population consisted of individuals with newly-diagnosed COPD who most likely had not received ICS medications before the diagnosis. Indeed, few cases with ICS use were identified during the baseline period. Medicaid beneficiaries with newly-diagnosed COPD having >120 days of supply for these medications during the baseline period were considered long-term users, while individuals with <120 days of supply were categorized as short-term users, and those with no receipt of the drugs during the baseline period were considered to be nonusers.
Other independent variables
These variables included demographic characteristics such as sex (male/female), race/ethnicity (white, African American, and other), age groups (40–49, 50–59, and 60–64 years of age), state (California, Illinois, New York, or Texas), Medicaid eligibility (income eligibility vs. medical eligibility), and clinical characteristics such as the presence of inflammation-related multimorbidity (eg, physical conditions including arthritis, cardiovascular disease, and osteoporosis; mental conditions such as depression), number of other clinical conditions, presence of serious mental illness (eg, bipolar disorder, schizophrenia), alcohol abuse, tobacco abuse, and substance abuse.
In addition, variables for access to care and socioeconomic characteristics were constructed using ARF data. These variables included quartiles for the density of the population having more than a high school education, unemployment, and poverty. The density of each county-level characteristic was calculated by dividing the total number of individuals in the county having the characteristic by the total county population. Metro status was ascertained for each Medicaid beneficiary. Medicaid beneficiaries with COPD were categorized into metropolitan residents or nonmetropolitan residents based on county-level information obtained from ARF. Other access to care variables included the density of primary care providers in the county (quartiles), density of specialist providers (quartiles), primary care shortage area (yes/no), mental health shortage area (yes/no), hospital bed density (quartiles), pulmonologist density (high/low), and cardiologist density (high/low).
Statistical analysis
Bivariate analysis
Chi-square tests of independence were used to determine subgroup differences in antidepressant, ICS, and statin use and new-onset diabetes. The results from unadjusted logistic regressions are also presented for a better understanding of the bivariate relationships between specific medication use categories and new-onset diabetes.
Multivariate analysis
Multivariable logistic regression was used to examine the relationship between antidepressant, ICS, and statin use and new-onset diabetes after controlling for the comprehensive set of independent variables as described. SAS v 9.3 (SAS Institute, Inc., Cary, NC) was used for the analyses.
Results
Table 1 presents a description of the study cohort and personal, as well as county-level, characteristics. The table also presents bivariate differences in medication use by beneficiary characteristics. Overall, 6.3% of the Medicaid beneficiaries (967 out of 15,287 individuals) with newly-diagnosed COPD were diagnosed with new-onset diabetes during the follow-up period. In the study population, a total of 7313 individuals (47.8%) received antidepressants, 3720 (24.3%) received statins, and 6554 (41.4%) received ICS. Chi-square tests showed that women were more likely to have medication use for all 3 medication groups (antidepressant, statin, and ICS). Similarly, white people were more likely to have medications than African Americans. A higher number of other clinical conditions and the presence of serious mental illness also were significantly associated with higher medication use.
Table 1.
Numbers & Percentages of Medication Use Among Medicaid Beneficiaries with Newly-Diagnosed Chronic Obstructive Pulmonary Disease, Medicaid Analytic Extract, 2005–2008
| Any AD use | Any statin use | Any ICS use | |||||||
|---|---|---|---|---|---|---|---|---|---|
| n | Row % | Sig. | n | Row % | Sig. | n | Row % | Sig. | |
| Total | 7313 | 47.8 | 3720 | 24.3 | 6554 | 41.4 | |||
| Cohort | * | ||||||||
| 2005–2007 | 4148 | 48.0 | 2041 | 23.6 | 3746 | 43.4 | |||
| 2006–2008 | 3165 | 47.6 | 1679 | 25.3 | 2808 | 42.2 | |||
| Sex | *** | *** | *** | ||||||
| Women | 4853 | 54.6 | 2299 | 25.9 | 4209 | 47.4 | |||
| Men | 2460 | 38.4 | 1421 | 22.2 | 2345 | 36.6 | |||
| Race/ethnicity | *** | *** | *** | ||||||
| White | 4121 | 53.9 | 2005 | 26.2 | 3372 | 44.1 | |||
| African American | 1527 | 37.6 | 755 | 18.6 | 1645 | 40.5 | |||
| Others | 1665 | 46.6 | 960 | 26.9 | 1537 | 43.0 | |||
| Age (in years) | *** | *** | |||||||
| 40–49 | 2787 | 51.9 | 934 | 17.4 | 2262 | 42.1 | |||
| 50–59 | 3470 | 47.6 | 1883 | 25.8 | 3129 | 42.9 | |||
| 60–64 | 1056 | 40.3 | 903 | 34.4 | 1163 | 44.4 | |||
| State | *** | *** | *** | ||||||
| California | 4055 | 48.0 | 2100 | 24.9 | 3481 | 41.2 | |||
| Illinois | 1548 | 48.0 | 764 | 23.7 | 1455 | 45.1 | |||
| New York | 1104 | 51.8 | 566 | 26.5 | 1188 | 55.7 | |||
| Texas | 606 | 40.8 | 290 | 19.5 | 430 | 29.0 | |||
| Income eligibility | ** | *** | * | ||||||
| Yes | 6753 | 48.2 | 3471 | 24.8 | 5976 | 42.7 | |||
| No | 560 | 43.7 | 249 | 19.4 | 578 | 45.1 | |||
| Medical eligibility | *** | ||||||||
| Yes | 814 | 48.1 | 338 | 20.0 | 740 | 43.7 | |||
| No | 6499 | 47.8 | 3382 | 24.9 | 5814 | 42.8 | |||
| Inflammation-related multimorbidity | *** | *** | |||||||
| Physical only | 2601 | 40.0 | 2087 | 32.1 | 2787 | 42.8 | |||
| Mental only | 1011 | 78.8 | 184 | 14.3 | 567 | 44.2 | |||
| Both | 2245 | 81.4 | 846 | 30.7 | 1216 | 44.1 | |||
| None | 1456 | 30.7 | 603 | 12.7 | 1984 | 41.9 | |||
| Number of other clinical conditions | *** | *** | *** | ||||||
| None | 135 | 30.6 | 61 | 13.8 | 143 | 32.4 | |||
| 1–3 | 1209 | 37.9 | 641 | 20.1 | 1244 | 39.0 | |||
| 4–6 | 1618 | 42.8 | 894 | 23.6 | 1579 | 41.7 | |||
| >6 | 4351 | 55.3 | 2124 | 27.0 | 3588 | 45.6 | |||
| Serious mental illness | *** | *** | *** | ||||||
| Yes | 2514 | 62.2 | 856 | 21.2 | 1403 | 34.7 | |||
| No | 4799 | 42.7 | 2864 | 25.5 | 5151 | 45.8 | |||
| Alcohol abuse | *** | *** | *** | ||||||
| Yes | 828 | 53.4 | 217 | 14.0 | 569 | 36.7 | |||
| No | 6485 | 47.2 | 3503 | 25.5 | 5985 | 43.6 | |||
| Substance abuse | *** | *** | |||||||
| Yes | 1286 | 57.3 | 295 | 13.1 | 950 | 42.3 | |||
| No | 6027 | 46.2 | 3425 | 26.3 | 5604 | 43.0 | |||
| Tobacco use | *** | *** | |||||||
| Yes | 1176 | 56.7 | 439 | 21.2 | 922 | 44.5 | |||
| No | 6137 | 46.4 | 3281 | 24.8 | 5632 | 42.6 | |||
| Polypharmacy | *** | *** | *** | ||||||
| Yes | 4559 | 65.2 | 2629 | 37.6 | 3720 | 53.2 | |||
| No | 2754 | 33.2 | 1091 | 13.1 | 2834 | 34.2 | |||
| ARF variables (county level) | |||||||||
| Above HS education density | *** | *** | *** | ||||||
| Q1 | 3621 | 45.6 | 1999 | 25.2 | 3239 | 40.8 | |||
| Q2 | 1107 | 50.2 | 552 | 25.0 | 943 | 42.7 | |||
| Q3 | 1593 | 50.1 | 767 | 24.1 | 1449 | 45.6 | |||
| Q4 | 992 | 50.5 | 402 | 20.5 | 923 | 47.0 | |||
| Unemployment density | *** | * | *** | ||||||
| Q1 | 5263 | 46.5 | 2734 | 24.1 | 4683 | 41.3 | |||
| Q2 | 1068 | 51.1 | 541 | 25.9 | 964 | 46.1 | |||
| Q3 | 741 | 52.1 | 318 | 22.4 | 686 | 48.3 | |||
| Q4 | 241 | 53.8 | 127 | 28.3 | 221 | 49.3 | |||
| Poverty density | ** | ** | *** | ||||||
| Q1 | 647 | 50.5 | 270 | 21.1 | 585 | 45.7 | |||
| Q2 | 1080 | 48.9 | 501 | 22.7 | 953 | 43.2 | |||
| Q3 | 1409 | 49.8 | 689 | 24.4 | 1286 | 45.5 | |||
| Q4 | 4177 | 46.6 | 2260 | 25.2 | 3730 | 41.6 | |||
| Metro | *** | ||||||||
| Yes | 6222 | 47.0 | 3202 | 24.2 | 5673 | 42.9 | |||
| No | 1091 | 53.3 | 518 | 25.3 | 881 | 43.0 | |||
| PCP shortage | * | ||||||||
| Yes | 7003 | 47.8 | 3586 | 24.5 | 6288 | 42.9 | |||
| No | 310 | 49.5 | 134 | 21.4 | 266 | 42.5 | |||
| Mental health specialist shortage | *** | *** | |||||||
| Yes | 6457 | 47.3 | 3313 | 24.3 | 5750 | 42.1 | |||
| No | 856 | 52.1 | 407 | 24.8 | 804 | 48.9 | |||
| PCP density | *** | *** | |||||||
| Q1 | 321 | 50.8 | 161 | 25.5 | 326 | 51.6 | |||
| Q2 | 1335 | 51.7 | 674 | 26.1 | 1147 | 44.4 | |||
| Q3 | 1136 | 51.8 | 527 | 24.0 | 1025 | 46.7 | |||
| Q4 | 4521 | 45.8 | 2358 | 23.9 | 4056 | 41.1 | |||
| Hospital bed density | *** | ** | *** | ||||||
| Q1 | 432 | 53.9 | 203 | 25.3 | 401 | 50.1 | |||
| Q2 | 1230 | 49.2 | 560 | 22.4 | 1092 | 43.6 | |||
| Q3 | 3983 | 46.4 | 2168 | 25.2 | 3440 | 40.0 | |||
| Q4 | 1668 | 49.2 | 789 | 23.3 | 1621 | 47.8 | |||
| Psychiatric hospital | *** | ||||||||
| Yes | 4497 | 46.2 | 2395 | 24.6 | 4132 | 42.5 | |||
| No | 2816 | 50.7 | 1325 | 23.9 | 2422 | 43.6 | |||
| Pulmonologist density | *** | * | *** | ||||||
| High | 4913 | 45.9 | 2546 | 23.8 | 4346 | 40.6 | |||
| Low | 2400 | 52.3 | 1174 | 25.6 | 2208 | 48.1 | |||
| Cardiologist density | *** | *** | |||||||
| High | 4601 | 46.1 | 2401 | 24.1 | 4094 | 41.0 | |||
| Low | 2712 | 51.1 | 1319 | 24.9 | 2460 | 46.4 | |||
Based on 15,287 Medicaid beneficiaries with newly diagnosed COPD and who were diabetes free during the baseline period obtained from Medicaid analytic extract files observed during 2005–2008. Asterisks represent significant group differences in beneficiary characteristics by medication use obtained from chi-square test.
P < 0.001; **0.001 ≤ P < 0.01; *0.01 ≤ P < 0.05.
AD, antidepressant; ARF, area resource file; COPD, chronic obstructive pulmonary disease; HS, high school; ICS, inhaled corticosteroid; PCP, primary care provider; Q, quarter; Sig., significance.
Table 2 describes the unadjusted and adjusted relationships between antidepressant, statin, or ICS use and new-onset diabetes in individuals with newly-diagnosed COPD. Rates of new-onset diabetes were not significantly different between antidepressant users and nonusers (6.5% vs. 6.2%). However, beneficiaries with statin use had significantly higher rates of new-onset diabetes compared to those without statin use (P < 0.001). Similarly, individuals with ICS use had significantly higher rates of new-onset diabetes compared to those without ICS use (P < 0.001). After controlling for baseline characteristics (eg, age, inflammation-related multimorbidity, and primary care provider density), beneficiaries who received statins or ICSs were 48% and 23% more likely to have new-onset diabetes compared with their counterparts who did not receive these medications.
Table 2.
Numbers, Percentages, Unadjusted & Adjusted Odds Ratio, and 95% Confidence Intervals from Logistic Regressions on New-Onset Diabetes Among Medicaid Beneficiaries with Newly-Diagnosed Chronic Obstructive Pulmonary Disease, Medicaid Analytic Extract, 2005–2008
| New-onset diabetes | ||||||||
|---|---|---|---|---|---|---|---|---|
| n | Row % | OR | 95% CI | Sig. | AOR | 95% CI | Sig. | |
| Total | 967 | 6.3 | ||||||
| Any antidepressant use | ||||||||
| Yes | 475 | 6.5 | 1.06 | 0.93, 1.20 | 0.91 | 0.78, 1.06 | ||
| No | 492 | 6.2 | ||||||
| Any ICS use | ||||||||
| Yes | 488 | 7.4 | 1.39 | 1.22, 1.58 | *** | 1.23 | 1.07, 1.47 | ** |
| No | 479 | 5.5 | ||||||
| Any statin use | ||||||||
| Yes | 344 | 9.2 | 1.79 | 1.56, 2.05 | *** | 1.48 | 1.27, 1.72 | *** |
| No | 623 | 5.4 | ||||||
Based on 15,287 Medicaid beneficiaries with newly diagnosed COPD who were diabetes free during the baseline period obtained from Medicaid analytic extract files observed during 2005–2008. Asterisks represent significant group differences in likelihood of new-onset diabetes by antidepressant, ICS, and statin use compared to the reference group (none) obtained from unadjusted and adjusted logistic regression analyses.
Adjusted logistic regressions controlled for cohort year, sex, race, age, state, poverty eligibility, inflammation-related multimorbidity, number of other clinical conditions, serious mental illness, alcohol abuse, substance abuse, tobacco use, polypharmacy, and county-level variables, including: above high school education density (quartiles), unemployment density (quartiles), poverty density (quartiles), metro status, primary care shortage area, mental health shortage area, primary care provider density (quartiles), hospital bed density (quartiles), psychiatric hospital, pulmonologist density, and cardiologist density in addition to medication use.
P < 0.001; **0.001 ≤ P < 0.01; *0.01 ≤ P < 0.05.
AOR, adjusted odds ratio; CI, confidence interval; COPD, chronic obstructive pulmonary disease; ICS, inhaled corticosteroid; OR, odds ratio; Sig., significance.
To assess the relationship between combined medication categories and new-onset diabetes, variables were created with the following categories: (1) all 3 medications; (2) antidepressants and statins; (3) antidepressants and ICSs; (4) statins and ICSs; (5) statins only; (6) ICSs only; and (7) none of these medications. Findings from bivariate and multivariate analyses with combined medication categories are presented in Table 3. Individuals who were given all 3 medications were significantly more likely to have new-onset diabetes (adjusted odds ratio: 1.56; 95% CI: 1.18, 2.05) compared to those who were not given any of these 3 medications. Similarly, individuals who used statins in combination with ICSs were more likely to have new-onset diabetes compared with those without any medications.
Table 3.
Numbers, Percentages, Unadjusted & Adjusted Odds Ratios, and 95% Confidence Intervals from Logistic Regressions on New-Onset Diabetes Medicaid Beneficiaries with Newly-Diagnosed Chronic Obstructive Pulmonary Disease (Combined Medication Use Categories), Medicaid Analytic Extract, 2005–2008
| New-onset diabetes | ||||||||
|---|---|---|---|---|---|---|---|---|
| n | Row% | OR | 95% CI | Sig. | AOR | 95% CI | Sig. | |
| Total | 967 | 6.3 | ||||||
| Medication use categories | ||||||||
| All 3 | 107 | 10.0 | 2.17 | 1.69, 2.77 | *** | 1.56 | 1.18, 2.05 | ** |
| AD/statin | 79 | 7.9 | 1.67 | 1.27, 2.19 | *** | 1.29 | 0.96, 1.74 | |
| AD/ICS | 149 | 6.3 | 1.31 | 1.05, 1.63 | * | 1.09 | 0.86, 1.39 | |
| Statin/ICS | 90 | 12.4 | 2.74 | 2.11, 3.57 | *** | 2.06 | 1.56, 2.72 | *** |
| AD only | 140 | 4.9 | 0.99 | 0.79, 1.24 | 0.90 | 0.71, 1.14 | ||
| Statin only | 68 | 7.3 | 1.54 | 1.15, 2.04 | ** | 1.26 | 0.94, 1.70 | |
| ICS only | 142 | 5.9 | 1.22 | 0.98, 1.53 | 1.11 | 0.88, 1.39 | ||
| None | 192 | 4.9 | Reference | Reference | ||||
Based on 15,287 Medicaid beneficiaries with newly diagnosed COPD who were diabetes free during the baseline period obtained from Medicaid analytic extract files observed during 2005–2008. Asterisks represent significant group differences in likelihood of new-onset diabetes by multiple medication use categories compared to the reference group (none) obtained from unadjusted and adjusted logistic regression analyses.
Adjusted logistic regressions controlled for cohort year, sex, race, age, state, poverty eligibility, inflammation-related multimorbidity, number of other clinical conditions, serious mental illness, alcohol abuse, substance abuse, tobacco use, polypharmacy, and county-level variables, including: above high school education density (quartiles), unemployment density (quartiles), poverty density (quartiles), metro status, primary care shortage area, mental health shortage area, primary care provider density (quartiles), hospital bed density (quartiles), psychiatric hospital, pulmonologist density, and cardiologist density in addition to medication use.
P < 0.001; **0.001 ≤ P < 0.01; *0.01 ≤ P < 0.05.
AD, antidepressant; AOR, adjusted odds ratio; CI, confidence interval; COPD, chronic obstructive pulmonary disease; ICS, inhaled corticosteroid; OR, odds ratio; Sig., significance.
As use of statins was associated with risk of developing new-onset diabetes, we further compared the certain baseline characteristics of patients with and without statin use that may have confounded the relationship between statin use, and new-onset diabetes. Results from this analyses are presented in Supplementary Table S1 (supplementary material is available in the online article at www.liebertpub.com/pop).
Discussion
This study used a retrospective longitudinal dynamic cohort design to examine the relationship between commonly used medications such as antidepressants, ICSs, and statins and new-onset diabetes among Medicaid beneficiaries with newly-diagnosed COPD. Medicaid claim data from multiple years (2005–2008) were used for the analysis. To the best of the authors' knowledge, this is the first observational study to examine this relationship using Medicaid claim data for nonelderly adults with newly-diagnosed COPD. Therefore, it is not feasible to compare these results with those of prior studies given the differences in patient demographic and clinical data.
Because statins are being investigated as novel therapeutic agents among individuals with COPD, this study examined the relationship between statin use and new-onset diabetes. The data revealed that adults who had used statins were more likely to have new-onset diabetes compared with those not taking statins. Previous randomized controlled trials and meta-analyses have yielded conflicting results on statin use and new-onset diabetes. A meta-analysis performed with data from the WOSCOPS study has shown that statin use did not increase the risk of new-onset diabetes.26 However, other studies have reported that statin use is associated with a greater risk of new-onset diabetes.8,27,28 Therefore, additional research is required to determine the safety of statin use in high-risk populations, such as in individuals with COPD.
The significant association between the use of ICS and new-onset diabetes found is consistent with the results from a retrospective cohort study using the Quebec Health Insurance database, which included patients with any respiratory disease.9 The present analyses also showed that when considering multiple combinations of medications, the combined use of statins and ICSs with or without antidepressants was significantly associated with a higher likelihood of new-onset diabetes.
After controlling for baseline characteristics, no statistically significant association was observed between only antidepressant use and new-onset diabetes. These results are consistent with studies that used data from the general population (ie, not specific to individuals with COPD). For example, no significant association between antidepressant use and new-onset diabetes was found in several previous studies.14,29,30 Recently, a review on glucose metabolism and antidepressant medications suggested that the relationship between antidepressant use and new-onset diabetes may vary by type of antidepressant. The deleterious effects of nonadrenergic antidepressants are thought to be negated by the beneficial effects of monoamine oxidase inhibitors with regard to glucose metabolism.31 However, monoamine oxidase inhibitors are an older class of antidepressants and are not commonly prescribed. Therefore, further analyses are required to determine whether the type of antidepressant used influences new-onset diabetes. Moreover, randomized controlled trials among individuals with COPD are warranted to determine whether treatment with antidepressants is a factor that affects new-onset diabetes.
Overall, the findings of the present study indicate that, after controlling for baseline characteristics and selection bias, the use of statins and ICSs was associated with increased risk of new-onset diabetes compared to individuals who did not receive any of the medications considered (statins, ICSs, and antidepressants). With the prevalence of diabetes increasing, it is important to recognize the greater risk of developing new-onset diabetes among individuals with COPD who take medications. Continuous glucose monitoring for COPD patients may be needed. Long-term observational studies and randomized controlled trials should be conducted in the future to evaluate the safety of the aforementioned medications in individuals with COPD and other specialized patient populations susceptible to new-onset diabetes.
Strengths and limitations
This study has several strengths, including the use of large data sets to gain a comprehensive understanding of whether multiple medication types were associated with new-onset diabetes in COPD patients. The use of Medicaid claim, data enabled the tracking of individuals for more than 2 years and the adoption of a longitudinal design. Clinical diagnoses with ICD-9-CM codes provided information on medical conditions. Prescription drug claims facilitated the identification of specific drugs and the precise time during which they were prescribed, enabling the calculation of the duration of medication use. This study also used statistical tools that controlled for selection bias related to unobserved characteristics.
A limitation of this study is a potential lack of generalizability, as only fee-for-service claims of Medicaid beneficiaries living in 4 states were included. However, these states were among the largest states in terms of the number of Medicaid enrollees and include very diverse geographic and racial/ethnic populations. There have been many studies using Medicaid data from 1 or more states.32–34 In terms of medication use, the study observations were restricted to specific classes of medications because taking into account all medication classes would be an impracticable task. In addition, the use of prescription drug claims has an inherent limitation of not being able to determine the actual use of these medications. However, using claims to identify the use of medications has been very well accepted in the literature. Finally, certain clinical data, such as cholesterol and baseline blood glucose levels, as well as body mass index, were unavailable and limited the ability to control for other important factors that may have been useful predictors of new-onset diabetes.
Conclusions
Despite these limitations, the current study is the first real-world observational study to examine the relationship between commonly used medications, such as antidepressants, ICSs, and statins, and new-onset diabetes among Medicaid beneficiaries with newly-diagnosed COPD. Although there has been documented evidence of increased risk of new-onset diabetes related to obesity, physical inactivity, unhealthy diet, and smoking,16,35 the current study highlighted additional risk factors—namely, statins and ICSs that are prescribed for treatment of COPD and allied comorbid conditions. The study finding suggests that patient education and counseling regarding the risk and benefits of statins and ICSs have to be provided for patients with COPD. In addition, health care providers must reinforce the importance of maintaining normal weight, healthy diet, physical activity, and smoking cessation in minimizing the risk of new-onset diabetes among individuals with COPD who are on statins for the management of their cardiovascular health.
Supplementary Material
Author Disclosure Statement
Drs. Ajmera, Shen, and Sambamoorthi declared that they have no conflicts of interest. The authors received no financial support for the research, authorship, and/or publication of this article.
References
- 1.Górska K, Maskey-Warzechowska M, Krenke R. Airway inflammation in chronic obstructive pulmonary disease. Curr Opin Pulm Med 2010;16:89–96 [DOI] [PubMed] [Google Scholar]
- 2.Rabe KF, Hurd S, Anzueto A, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med 2007;176:532–555 [DOI] [PubMed] [Google Scholar]
- 3.Fabbri LM, Rabe KF. From COPD to chronic systemic inflammatory syndrome? Lancet 2007;370:797–799 [DOI] [PubMed] [Google Scholar]
- 4.Lehr PS. Global markets for asthma and COPD drugs. Wellesley, MA: BCC Research LLC, 2012 [Google Scholar]
- 5.Anecchino C, Rossi E, Fanizza C, De Rosa M, Tognoni G, Romero M. Prevalence of chronic obstructive pulmonary disease and pattern of comorbidities in a general population. Int J Chron Obstruct Pulmon Dis 2007;2:567–574 [PMC free article] [PubMed] [Google Scholar]
- 6.Bhattacharjee S, Bhattacharya R, Kelley GA, Sambamoorthi U. Antidepressant use and new-onset diabetes: a systematic review and meta-analysis. Diabetes Metab Res Rev 2013;29:273–284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.O'Byrne PM, Rennard S, Gerstein H, et al. Risk of new onset diabetes mellitus in patients with asthma or COPD taking inhaled corticosteroids. Respir Med 2012;106:1487–1493 [DOI] [PubMed] [Google Scholar]
- 8.Sattar N, Preiss D, Murray HM, et al. Statins and risk of incident diabetes: a collaborative meta-analysis of randomised statin trials. Lancet 2010;375:735–742 [DOI] [PubMed] [Google Scholar]
- 9.Suissa S, Kezouh A, Ernst P. Inhaled corticosteroids and the risks of diabetes onset and progression. Am J Med 2010;123:1001–1006 [DOI] [PubMed] [Google Scholar]
- 10.Carvalho F, Barros D, Silva J, et al. Hyperglycemia induced by acute central fluoxetine administration: role of the central CRH system and 5-HT3 receptors. Neuropeptides 2004;38:98–105 [DOI] [PubMed] [Google Scholar]
- 11.Colbert JD, Stone JA. Statin use and the risk of incident diabetes mellitus: a review of the literature. Can J Cardiol 2012;28:581–589 [DOI] [PubMed] [Google Scholar]
- 12.Nielsen MF, Caumo A, Chandramouli V, et al. Impaired basal glucose effectiveness but unaltered fasting glucose release and gluconeogenesis during short-term hypercortisolemia in healthy subjects. Am J Physiol Endocrinol Metab 2004;286:E102–E110 [DOI] [PubMed] [Google Scholar]
- 13.Rubin RR, Ma Y, Marrero DG, et al. Elevated depression symptoms, antidepressant medicine use, and risk of developing diabetes during the diabetes prevention program. Diabetes Care 2008;31:420–426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sambamoorthi U, Ma Y, Findley PA, Rust G. Antidepressant use, depression, and new onset diabetes among elderly medicare beneficiaries. J Diabetes 2013;5:327–335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Blackburn D, Hux J, Mamdani M. Quantification of the risk of corticosteroid-induced diabetes mellitus among the elderly. J Gen Intern Med 2002;17:717–720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rana JS, Mittleman MA, Sheikh J, et al. Chronic obstructive pulmonary disease, asthma, and risk of type 2 diabetes in women. Diabetes Care 2004;27:2478–2484 [DOI] [PubMed] [Google Scholar]
- 17.Ridker PM, Danielson E, Fonseca FA, et al. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med 2008;359:2195–2207 [DOI] [PubMed] [Google Scholar]
- 18.Freeman DJ, Norrie J, Sattar N, et al. Pravastatin and the development of diabetes mellitus: evidence for a protective treatment effect in the West of Scotland Coronary Prevention Study. Circulation 2001;103:357–362 [DOI] [PubMed] [Google Scholar]
- 19.Hennessy S, Leonard CE, Palumbo CM, Newcomb C, Bilker WB. Quality of Medicaid and Medicare data obtained through Centers for Medicare and Medicaid Services (CMS). Med Care 2007;45:1216–1220 [DOI] [PubMed] [Google Scholar]
- 20.Ajmera M, Raval AD, Shen C, Sambamoorthi U. Explaining the increased health care expenditures associated with gastroesophageal reflux disease among elderly Medicare beneficiaries with chronic obstructive pulmonary disease: a cost-decomposition analysis. Int J Chron Obstruct Pulmon Dis 2014;9:339–348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ajmera M, Shen C, Pan X, Findley PA, Rust G, Sambamoorthi U. Inhaled anticholinergic use and all-cause mortality among elderly Medicare beneficiaries with chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis 2013;8:287–294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Halpern R, Baker CL, Su J, et al. Outcomes associated with initiation of tiotropium or fluticasone/salmeterol in patients with chronic obstructive pulmonary disease. Patient Prefer Adherence 2011;5:375–388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Make B, Dutro MP, Paulose-Ram R, Marton JP, Mapel DW. Undertreatment of COPD: a retrospective analysis of US managed care and Medicare patients. Int J Chron Obstruct Pulmon Dis 2012;7:1–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cooke CR, Joo MJ, Anderson SM, et al. The validity of using ICD-9 codes and pharmacy records to identify patients with chronic obstructive pulmonary disease. BMC Health Serv Res 2011;11:37–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gershon AS, Wang C, Guan J, Vasilevska-Ristovska J, Cicutto L, To T. Identifying individuals with physcian diagnosed COPD in health administrative databases. COPD 2009;6:388–394 [DOI] [PubMed] [Google Scholar]
- 26.Rajpathak SN, Kumbhani DJ, Crandall J, Barzilai N, Alderman M, Ridker PM. Statin therapy and risk of developing type 2 diabetes: a meta-analysis. Diabetes Care 2009;32:1924–1929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hennings JM, Schaaf L, Fulda S. Glucose metabolism and antidepressant medication. Curr Pharm Des 2012;18:5900–5919 [DOI] [PubMed] [Google Scholar]
- 28.Mills EJ, Wu P, Chong G, et al. Efficacy and safety of statin treatment for cardiovascular disease: a network meta-analysis of 170,255 patients from 76 randomized trials. QJM 2011;104:109–124 [DOI] [PubMed] [Google Scholar]
- 29.Wilkins TL, Sambamoorthi U. Antidepressant use, depression, lifestyle factors, and new-onset diabetes. Int Clin Psychopharmacol 2011;26:159–168 [DOI] [PubMed] [Google Scholar]
- 30.Bhattacharya R, Ajmera M, Bhattacharjee S, Sambamoorthi U. Use of antidepressants and statins and short-term risk of new-onset diabetes among high risk adults. Diabetes Res Clin Pract 2014;105:251–260 [DOI] [PubMed] [Google Scholar]
- 31.Preiss D, Seshasai SRK, Welsh P, et al. Risk of incident diabetes with intensive-dose compared with moderate-dose statin therapy: a meta-analysis. JAMA 2011;305:2556–2564 [DOI] [PubMed] [Google Scholar]
- 32.Prince JD, Akincigil A, Hoover DR, Walkup JT, Bilder S, Crystal S. Substance abuse and hospitalization for mood disorder among Medicaid beneficiaries. Am J Public Health 2009;99:160–167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Iezzoni LI. Using administrative data to study persons with disabilities. Milbank Q 2002;80:347–379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Riley GF. Administrative and claims records as sources of health care cost data. Med Care 2009;47 suppl 1:S51–S55 [DOI] [PubMed] [Google Scholar]
- 35.National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK). Risk factors for type 2 diabetes. www.niddk.nih.gov/health-information/health-communication-programs/ndep/am-i-at-risk/diabetes-risk-factors/Pages/diabetesriskfactors.aspx Accessed June23, 2016
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.