Abstract
Acne vulgaris is a ubiquitous problem affecting 80 percent of people ages 11 to 30 years, with many patients experiencing some degree of scarring. This review focuses on atrophic scars, the most common type of acne scar. We briefly address the cellular sequelae that lead to scar formation and the initial evaluation of patients with acne scars. We then discuss an algorithmic approach to the treatment of acne scarring based on the classification of scars into erythematous and atrophic types. Lastly, we discuss the future treatment of acne scars and ongoing clinical trials.
Keywords: Acne scarring, acne vulgaris, acne, laser, light devices, resurfacing agents
ACNE VULGARIS IS A UBIQUITOUS PROBLEM, affecting up to 80 percent of people ages 11–30 and five percent of adults 30 years and older in the United States.1 While epidemiologic data on acne scarring vary, many patients experience some degree of scarring. Here, we address the most common type of acne scar— the atrophic scar— and discuss options for amelioration in a comprehensive manner. Heterogeneity in study design, assessment scales, and level of evidence regarding efficacy for various treatment options make comparative conclusions difficult. In clinical practice, a tailored combination approach using multiple modalities is optimal.
PATHOGENESIS
Acne vulgaris is an inflammatory process localized to the pilosebaceous units of the face, chest, upper arms, and back.2 The presumed pathophysiology involves alteration of keratinization within the pilosebaceous unit resulting in comedone formation, increased sebum production, proliferation of Propionibacterium acnes (P. acnes), and production of perifollicular inflammation.3
The early preclinical inflammation in acne persists throughout the acne lesion’s life cycle, from micro-comedones to closed comedones to inflammatory lesions and eventually to postinflammatory erythema (PIE), post-inflammatory hyperpigmentation (PIH), and scarring.4 PIE is typically persistent in individuals with fair skin and PIH is more typical in individuals with dark skin. Both sequelae represent grossly visible and histologically notable inflammation5 that may be partially related to slow degradation of non-viable P. acnes within the follicle.6 PIE results from wound healing-related microvascular dilatation that is perceived as general redness, not visible telangiectasia, which is exacerbated by repair-related epidermal thinning.7
Acne affects the face in a majority of cases, with many patients experiencing some degree of scarring, the severity of which correlates to acne grade.8 Acne scars result from an altered wound healing response to cutaneous inflammation, with inflammatory cell infiltrates found in 77 percent of atrophic scars.11 Different P. acnes phylotypes differentially activate epidermal innate immunity, contributing to variations in acne severity.12 In patients not prone to scarring, early lesions have a large, nonspecific immune response that subsides in resolving lesions.13 In contrast, in patients prone to scarring, early lesions are characterized by a smaller number of skin-homing CD4+ T-cells compared to non-scarring patients, a response that becomes more active in resolving lesions.13,14
Atrophic scars. Aberrant production and degradation of collagen during the healing process leads to various types of acne scars. In 80 to 90 percent of cases, there is a net destruction of collagen in the dermis that results in atrophic scars. Less commonly, there is a net gain of collagen that results in hypertrophic or keloid scars.
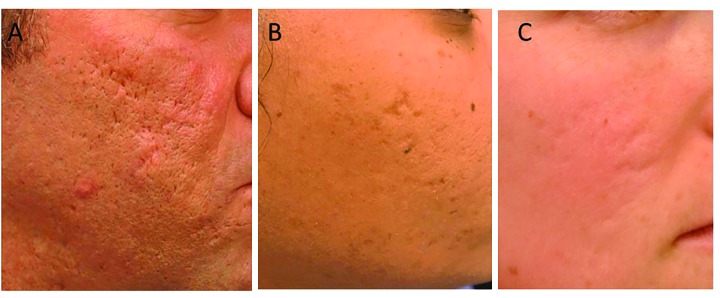
Atrophic scars are classified according to the depth and size of destruction; however, different scar types are typically seen on the same person, making differentiation difficult (Figure 1).1 In addition to the variations in collagen, the appearance of acne scars may be accentuated by PIE in individuals with light skin, making PIE treatment an important initial component of therapy.
FIGURE 1.
The three types of atrophic acne scars: A) icepick, B) boxcar, and C) rolling
Icepick scars comprise 60 to 70 percent of atrophic scars. These narrow, less-than-2mm, V-shaped epithelial tracts have a sharp margin that extends vertically to the deep dermis or subcutaneous tissue. Their depth of involvement makes icepick scars resistant to conventional skin resurfacing options. Boxcar scars comprise 20 to 30 percent of atrophic scars. These scars are wider, 1.5-to 4.0mm, round-to-oval depressions with sharply demarcated vertical edges. Shallow boxcar scars (0.1–0.5mm) are amenable to skin resurfacing treatments, whereas deep boxcar scars (≥0.5mm) are resistant. Rolling scars comprise 15 to 25 percent of atrophic scars. These scars are the widest and may reach up to 5mm in diameter. Fibrous anchoring of the dermis to the subcutis results in superficial shadowing and an undulating appearance of the scars. Treatment must focus on correction of the subdermal component.
EVALUATION
The approach to treatment of acne scarring involves both a comprehensive physical exam of the patient and a discussion regarding patient goals, concerns, and tolerance of various treatment options. Considerations include the presence of erythema and the type, depth, and location of scarring as well as the patient’s baseline skin phototype (SPT).
Expectation management is important in approaching the discussion of treatment options. Complete resolution of acne scarring is the exception rather than the rule. Patients should be well-informed about potential risks, including post-procedure erythema, infection, poor wound healing, hyperpigmentation, and paradoxically, scarring. Finally, interventions often entail a significant cost, and financial considerations should be addressed.15
TREATMENT OF ACNE SCARRING
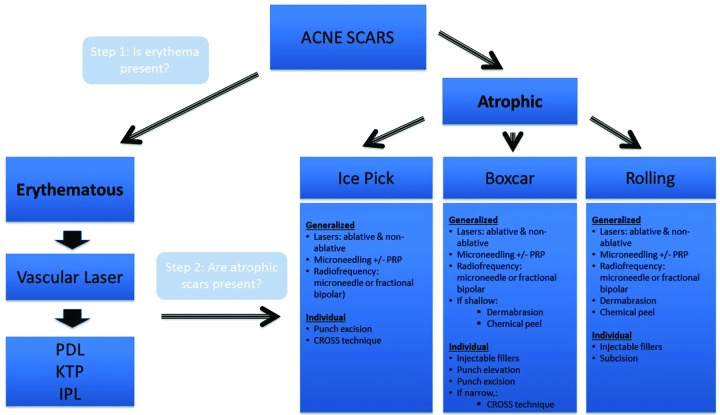
The management of acne scars should follow an algorithmic approach that targets each component of scarring (Figure 2). Treatment should begin with targeting erythema, if present. Once scar-associated erythema has been addressed, treatment should focus on addressing atrophic scarring, with the approach determined by the types of scar present and whether generalized or individual scars predominate. Combination treatment in a patient-specific way can offer the best chance of significant improvement. Early treatment of active acne remains the best way to prevent or limit acne-related scarring.16 It is also imperative to ensure active acne has been treated before approaching scar treatment so as not to create a cycle where active lesions continue to scar in areas already addressed.
FIGURE 2.
Acne scarring treatment algorithm
Scar-associated erythema. For patients with significant scar-associated erythema (SAE), treatment to address the redness can be an initial and dramatic step toward improving the overall appearance of acne scarring. Pulse dye laser (PDL) and other laser and light devices have been successfully utilized in treating SAE.
Pulsed dye laser (PDL). PDL is the gold-standard for treating SAE.17 PDL utilizes selective thermolysis to destroy vascular components of the dermis leading to clinical improvement of erythema. The major chomophore of PDL is oxyhemoglobin within cutaneous vessels, which absorbs light in the yellow and green range, with peaks at 418, 542, and 577nm.18 The long-pulsed PDL (595–600nm) slowly heats target vessels with less risk of post-procedure purpura.19
In a split-face, observer-blinded study of 22 patients with erythematous and/or hypertrophic facial acne scars, one or two treatment sessions with a 585nm flashlamp-pumped PDL (0.45ms pulse, average fluence 6.5J/cm2, 7mm spot size) decreased clinical erythema/scarring by 68 percent compared to untreated skin six weeks post-treatment.17 Complete clearing of PIE often requires multiple treatments. Purpura is a relatively PDL-specific side effect.20
In addition to treating SAE, PDL also induces collagen remodeling. Heat energy created by the laser diffuses from targeted vessels into the surrounding dermis and results in increased transforming growth factor beta (TGF-β), which ultimately stimulates fibroblasts.21,22 In a small study of 10 patients (SPT I-IV) with atrophic acne scarring, PDL improved the depressed appearance of scars, with one patient experiencing a two-day episode of transient purpura.20
PDL performance was shown to be comparable to the 1,064nm Nd:YAG with regard to atrophic scar improvement. A split-face, single-blinded comparative study of 18 patients with darker SPTs (IV and V) demonstrated that the mean improvement in scarring between 585nm PDL and the long-pulsed Nd:YAG (fluence of 50–70J/cm2, 50-to 100ms pulse duration, 7mm spot size) were nearly equivalent (18.3 vs. 18.7% improvement, respectively). Subgroup analysis revealed that icepick scars responded better to PDL and deep boxcar scars responded better to Nd:YAG laser.21 A small study of 12 Korean patients found no significant difference in efficacy between 595nm PDL and 1,550nm erbium-doped fractional laser (EDL) in treating PIE but more patients in the fractional laser group were satisfied (91.7 vs.75%).23 In patients with dark skin, PDL should be used cautiously, as purpura or blistering can result in PIH or hypopigmentation.
Other vascular laser and light devices. Other laser and light devices have been successfully utilized in treating SAE. These devices include the potassium titanyl phosphate (KTP, also known as frequency-doubled Nd:YAG), EDL, and intense pulsed-light (IPL).24–26
Like PDL, the wavelength of KTP (532nm) has a target specificity for the first peak of the oxyhemoglobin absorption curve. A single-blinded, split-scar study comparing the efficacy of KTP to 595nm PDL in reduction of erythema in surgical scars found no significant difference in blinded photographic scar assessments, investigator and subject treatment/satisfaction assessments, and intraoperative pain scores.26 The KTP laser did show significant improvement in the vascularity component on the Vancouver Scar Scale, which was originally developed to assess burn scars and also measure height/thickness, pliability, and pigmentation of scars. The thermal energy delivered by KTP extends only to the papillary dermis, making it useful for PIE without significant effects on collagen remodeling.27 The KTP laser is especially successful for SAE with pulse durations of 20 to 30ms, fluences of 6 to 9J/cm2, and spot sizes of 4–5mm.28
The 1,550nm wavelength emitted by EDL penetrates to approximately 1,000μm into the skin to target tissue water, allowing for improvement of erythema through microvascular destruction of vessels deeper in the dermis.24 Although fractional 1,550nm EDL is considered a front-line agent for atrophic scars, a case study of two patients revealed improvement in scar erythema after one treatment.24 Microsecond-pulsed Nd:YAG lasers (Laser Genesis™, Cutera Inc., Brisbane, California) have shown a greater reduction of SAE by delivering energy to the papillary dermis through small a spot size (5mm), short pulse durations (0.3ms), low fluence (13–16J/cm2), and quick (5–10Hz) laser bursts.28,29
IPL emits incoherent light across a range of wavelengths (500–1,200nm), with the application of filters to achieve the preferred effect.30 With the IPL, one has the ability to manipulate settings, including pulse duration, fluence, and the application of filters, allowing treatment of various conditions, sometimes simultaneously (e.g., hyperpigmentation and erythema). However, adjacent, competing chromophore absorption peaks and poor specificity may prevent optimal reduction in scar erythema if settings are not chosen properly. In one study of 35 patients (SPTs I–III) with diffuse facial erythema, a broad-filtered IPL (555–950nm) achieved clinical improvement of at least 25 to 50 percent in 72.7 percent of patients after 1 to 2 treatments, compared to a 35-percent improvement in those treated with the 530 to 750nm filter.31 While both PDL and IPL effectively treat SAE, IPL does not typically produce purpura, and larger spot sizes allow for greater surface area to be treated deeper and more quickly. However, given the range of wavelengths that may be used, drawing conclusions regarding efficacy in treating scar erythema with IPL is difficult. Care must also be taken to avoid post-inflammatory hypopigmentation and PIH in dark skin types.28
Generalized atrophic scars. Atrophic scars are seen in nearly 80 to 90 percent of patients, and they are typically numerous.8 Treatment of generalized atrophic acne scars involves a field approach, including lasers, chemical peels, dermabrasion, microneedling and radiofrequency.
Laser resurfacing. The options for laser treatment of acne scarring have expanded in recent years and have gained in popularity given their impressive results. Lasers for acne scarring fall under two main categories: ablative (traditional and fractional) and non-ablative (traditional and fractional) (Table 1). Laser resurfacing for acne scarring uses monochromatic light to deliver thermal energy, which ultimately stimulates dermal fibroblasts to replace lost collagen and elastin.32 As newer technologies develop, expanding options allow physicians to tailor the balance between efficacy, tolerability, and side effects for each patient. Traditional ablative lasers offer impressive clinical results but are associated with significant peri-procedural discomfort, prolonged recovery, and a significant risk of side effects. Alternatively, non-ablative lasers are more tolerable with shorter recovery times; however, multiple sessions are required and results are often less clinically impressive.
TABLE 1.
Lasers for acne scarring
| LASER CATEGORY | TRADITIONAL | FRACTIONATED |
|---|---|---|
| 10,6000nm CO2 | Fractional 10,6000nm CO2 | |
| Ablative | 2,940nm ER:YAG | Fractional 2940nm ER: YAG |
| Fractional 2,790nm YSGG | ||
| 1,064nm Nd:YAG | ||
| 1,320nm Nd:YAG | ||
| 1,450nm diode | Fractional 1,550nm Er-doped | |
| Non-ablative | 755nm picosecond pulse duration laser | Fractional 1,540nm Er:glass |
| 585nm PDL | ||
| 595nm PDL | ||
| 532nm KTP |
PDL: pulsed dye laser; KTP: potassium titanyl phosphate
Traditional ablative lasers. Considered the gold standard in acne scarring treatment, ablative lasers offer significant improvement in scar appearance with collagen contraction, remodeling, and skin tightening. Improvement is significant after one treatment session, compared to multiple sessions required with non-ablative lasers. The marked clinical improvement comes at a cost, namely significant procedural discomfort or pain, increased risk of dyspigmentation, scarring, and infections with prolonged healing when compared to non-ablative lasers. Due to the extensive injury to the skin, pre-operative prophylaxis (e.g., antiviral therapy) is typically administered, as the rate of herpes virus infection post-treatment has been reported to be as high as seven percent for traditional ablative lasers.33 Pre-procedure antibiotic therapy is also commonly administered. As collagen remodeling continues after 12 months, a waiting period of up to 18 months prior to evaluating the need for retreatment is warranted.34 Traditional ablative lasers used for acne scarring include 10,600nm carbon dioxide (CO2) lasers and 2,940nm pulsed Er:YAG lasers. The target chromophore of these lasers is water in the skin. Despite the higher wavelength of these lasers, the emitted light is readily absorbed by water, so these lasers have less dermal penetration than their fractionated counterparts.35
Traditional 10,600 nm CO2 laser. The ablative CO2 laser emits light in the far infrared spectrum. An 18-month prospective, uncontrolled study of 60 patients with moderate-to-severe atrophic facial acne scars demonstrated significant immediate and prolonged improvement in skin tone, texture, and appearance after one treatment session of the high-energy CO2 laser.32 Clinical improvement scores were 69 percent at one month and 75 percent at 18 months. Persistent collagen formation was noted on histology 18 months post-procedure. Post-procedure erythema lasted 14 weeks and one-third of patients experienced temporary hyperpigmentation. The use of a pulsed, single-pass CO2 non-overlapping laser (300mJ, 60 watts, CPG density of 5) has comparable efficacy with decreased recovery time (3.5 weeks) but increased transient hyperpigmentation (46 vs. 36%).36
Traditional 2,940nm Er:YAG laser. The traditional 2,940nm Er:YAG laser was developed as a less aggressive alternative to the traditional CO2 laser.37 The emitted wavelength is also in the infrared spectrum but the thermal energy is more circumscribed and precise. The light from the Er:YAG laser is more efficiently absorbed by water within the skin by an order of magnitude of 12 to 18.38 With a pulse duration of 250sec, a short-pulsed Er:YAG laser ablates 10 to 20μm of tissue per pass with a residual zone of thermal damage of up to 15μm, compared to 20 to 60μm of tissue ablation and up to 150μm of residual thermal damage with each pass of the CO2 laser.39 This allows for increased absorption of energy higher in the dermis and decreased non-specific damage to surrounding structures.37,40 The decreased dermal damage translates into shorter recovery times and decreased intraoperative pain. However, the ER:YAG laser does not have hemostatic properties akin to the CO2 laser and thus treatment with the laser confers and increased bleeding risk.37,40
The Er:YAG laser has comparable efficacy to the CO2 laser for treatment of acne scarring.37,40–45 In a study of 35 patients with pitted facial scars and dark skin types (SPT III–V), multiple passes with the long-pulsed Er:YAG laser resulted in excellent (36%) and good (57%) results.40 All patients had erythema after treatment, and this lasted longer than three months in 54 percent of the subjects. PIH occurred in 29 percent of the subjects and lasted longer than three months in six percent. A non-randomized prospective study of 158 darkly pigmented patients (SPTs III–V) comparing the efficacy of short-pulsed, variable-pulsed, and dual-mode Er:YAG lasers found that all three modes resulted in significant improvement in acne scar appearance. The dual-mode showed the most consistent results across the varying types of atrophic scars. Deep boxcar scars showed only a "poor to fair" response with the short-pulsed Er:YAG laser treatment.43 Er:YAG and CO2 lasers show comparable rates of re-epithelialization and transient hyperpigmentation but post-procedure erythema is decreased with Er:YAG.44
Ablative fractional lasers. Ablative fractional lasers were developed to combine the milder side effects profile of fractional technology (as discussed below) with the efficacy of ablative lasers. Although a single treatment can produce noticeable results, multiple treatments create greater clinical improvement.61 Adverse effects of fractional ablative lasers include erythema that lasts for 3 to 14 days and resolves by 12 weeks, PIH that lasts for approximately one month, and procedural discomfort that typically necessitates full-face anesthesia akin to traditional ablative lasers.61
Fractional 10,600 nm CO2 laser. Multiple studies support the efficacy of fractional CO2 lasers on acne scars, albeit with less marked clinical improvement than traditional ablative lasers. In a single-blind study of 13 patients, treatment with fractional CO2 showed only modest improvement in scar texture and scar atrophy.62 The authors purported that low pulse energies of 48 to 56mJ accounted for the modest results. A study of 13 patients (SPTs I–IV) with moderate-to-severe acne scars treated with fractional CO2 laser with higher pulse and larger microscopic treatment zones showed significant improvements of 26 to 50 percent on a quartile-scale and improved scar depths of 66.6 percent.69 All patients experienced erythema, which resolved within one month in most patients. A high-fluence, low-density setting has been shown to be more efficacious than a low-fluence, high density setting.70
Fractional CO2 laser has shown better clinical efficacy compared to non-ablative lasers, but increased rates of PIH and a more protracted side effect profile were reported.71,72 Fife et al121 reported four cases complicated either by scarring located on the neck or ectropion, two of which involved preceding bacterial infections in the area. The deeper penetration of the laser might lead to contraction of the underlying muscle, so lower energy and density should be used on the neck, chest and periocular region.
Fractional 2,940nm Er:YAG and 2,790 Eryttrium scandium gallium garnet (Er:YSGG) lasers. Similar to the fractional CO2 laser, the fractional 2,940nm Er:YAG and the 2,790nm YSGG have been shown to produce comparable rates of improvement in atrophic acne scars after multiple treatments.73,74 Mild erythema has been reported, but no serious adverse events, such as scarring or long-lasting dyschromia, were seen.
Traditional non-ablative lasers. With newer therapies, the adverse effects and a long recovery period of traditional ablative lasers have made them less popular.46,47 Non-ablative lasers, such as the short- and long-pulsed and Q-switched Nd:YAG lasers, induce collagen remodeling by targeting water as their primary chromophore, albeit less specifically than ablative lasers. They also demonstrate variable amounts of absorption by hemoglobin and melanin.28 These lasers deliver photothermal energy to the dermis without ablating the overlying epidermis, thereby minimizing epidermal damage and decreasing postprocedure downtime. However, results are accordingly modest, and multiple treatment sessions are required to achieve typically less impressive results.48
1,064nm Nd:YAG laser. The 1,064nm Nd:YAG induces collagen remodeling in the papillary and reticular dermis, confirmed by post-treatment histologic examination that revealed thickening of papillary dermal collagen49 and increased expression of heat-shock protein 70 and Type I procollagen by dermal dendritic cells.50 Due to its decreased scatter and increased depth of penetration, the Nd:YAG can be used to treat dark skin types with minimal risk of pigment alteration.51 Undesired consequences of epidermal injury can be avoided, since thermal damage is limited to the dermis; cooling systems may be employed to reduced heat injury.52
Variations on the 1,064nm Nd:YAG laser include short-pulsed (0.2–0.5ms, 13–18J/cm2), long-pulsed (50–100ms, 50–70J/cm2), and Q-switched (4–6ns, 5–15J/cm2).48 Improvement in appearance of atrophic scars typically ranges from 20 to 30 percent.37 The short-pulsed 1,064nm Nd:YAG targets oxyhemoglobin and produces gradual dermal heating through cumulative absorption within the dermal microvasculature. Since the heat has time to diffuse into the surrounding dermis, cooling systems are not required to protect the skin. An uncontrolled study of 10 patients with atrophic scars showed a 29.4-percent mean cumulative improvement after eight sessions with the short pulsed 1,064nm Nd:YAG laser.51 Post-procedure side effects were minimal, with erythema lasting less than two hours and with no reports of pain, swelling, oozing, or scarring.
The long-pulsed 1,064nm Nd:YAG laser has also shown efficacy in atrophic acne scars. A study comparing long-pulsed Nd:YAG laser to combined 585nm/1,064nm laser in 19 patients (SPTs IV–V) who underwent four treatment sessions at two-week intervals with atrophic facial acne scars found the combination to have a slightly greater clinical improvement (27% vs. 32%, respectively), which was most significant in deep scars.29
The 1,064nm Q-switched Nd:YAG delivers nanosecond rapid pulses that target pigmented structures, such as melanin, hemosiderin, and tattoo ink. Data regarding the efficacy of the Q-switched laser for acne scarring are contradictory. A non-blinded study of 11 patients (SPTs I–III) with atrophic acne scars showed an improvement in skin smoothness of 39.2 percent and skin roughness of 23.3 percent.53 In contrast, a study of 10 Iraqi patients (SPT III) showed no significant improvement in scar appearance.48
1,320nm Nd:YAG and 1,450nm diode lasers. The 1,320nm Nd:YAG laser has historically been used for facial rejuvenation and rhytides, while the 1,450nm diode laser has been used for active acne.32 While a few studies have shown the lasers to have similar efficacy to other non-ablative lasers,45,52–56 other studies have shown less impressive clinical results compared to the 1,064nm laser.57,58 There is also a considerable risk of PIH with the 1,450nm diode laser, with occurrence rates from 18 to 39 percent.45,58,59,60
Non-ablative fractionated lasers. Newer fractional lasers improve acne scarring by inducing photothermal damage that stimulates collagen remodeling with variable absorption by melanin and hemoglobin in addition to water.27 Ablative lasers uniformly treat the skin in horizontal planes, leaving no reservoir of intact skin for re-epithelialization. In contrast, fractional lasers emit microscopic columns of light or microthermal zones (MTZs), leaving the intervening skin unaffected and minimizing damage to the epidermis. The skin adjacent to sites of laser injury remains intact, allowing for rapid post-procedural re-epithelialization due to migration of intact cells into the damaged microcolumns. The stratum corneum contains little water and remains intact after treatment, decreasing average recovery time to three days.61 Similar to traditional non-ablative lasers, this comes at the expense of efficacy with decreased collagen remodeling,62 more treatment sessions, and decreased clinical improvement.48
Fractional 1,540nm Er:glass laser. The Er:glass laser has been shown to improve atrophic acne scars in multiple trials. An examination of 10 patients (SPTs I–IV) revealed an improvement in smoothness with transient erythema and no reported scarring or dyspigmentation.62 A larger study of 87 Italian patients (SPTs I–V) revealed greater than 50-percent improvement in atrophic scars compared to baseline in 92 percent of patients after six months.63 Confocal microscopy revealed increased collagen production and bundle arrangement that was closer to normal skin. Another study of 16 Asian patients (SPTs II–IV) revealed a greater than 50-percent improvement in atrophic scars in 62.5 percent of patients, with only transient erythema and edema reported.64 Twelve weeks post-procedure skin samples showed an increase in collagen and elastin content within the papillary dermis. The efficacy of the fractionated Er:glass laser on different scar types was examined in 35 Asian patients.65 Boxcar scars showed a 52.9-percent improvement in appearance, followed by 43.1-percent for rolling, and 25.9-percent for icepick.
Fractional 1,550nm Erbium-doped laser (EDL). The 1,550m EDL was the first available fractional laser. Compared to its ablative counterparts, multiple studies have shown decreased bleeding and post-procedure erythema, edema, infection and scarring; however, multiple therapy sessions are required are required and less impressive results are seen. A study of 53 patients (SPTs I–V) with atrophic acne scarring showed a scar improvement within the 51 to 75-percent quartile in 87 percent of patients.66 Improvement in scars did not significantly differ depending on SPT. Side effects included transient erythema, edema, and skin dryness but no dyspigmentation, ulceration, or scarring was reported. In a study of 29 patients (SPTs I–VI) with atrophic facial and truncal acne scars treated with the EDL, 79 percent of patients showed at least 50-percent improvement. Erythema resolved within 2 to 5 days.67 This laser is safe in dark skin types, with less dyschromia than ablative lasers. Lower densities have been associated with less risk for hyperpigmentation.68
Emerging laser technologies.
Picosecond 755nm Alexandrite laser. Compared to the traditional nanosecond lasers, picosecond lasers deliver shorter pulse durations with lower fluences of energy, and therefore may lead to fewer adverse effects.116 The picosecond 755nm Alexandrite laser Picosure® (Cynosure, West Hartford, Massachusetts) received FDA-approval to treat tattoos and pigmented lesions in 2014. It has also been evaluated for the treatment of acne scars and, with the aid of a diffractive lens array, which delivers pulses 500μm apart, permitting treatment of a greater surface area and pattern density per pulse. The Picosure® has been shown to improve the appearance and texture of atrophic rolling scars similar to fractional ablative lasers.116 Histologic examination after treatment showed evidence of laser-induced optical breakdown consistent with a localized plasma formation in the epidermis. This breakdown is initiated by absorption of the picosecond light by melanin.117 These changes led to dermal wound healing with formation of new collagen, elastic tissue, and mucin.117 Notably, this technology has a favorable safety profile that is reproducible across SPTs I to V. The mean pain score was mild, and downtime was minimal, with transient erythema and edema. No exfoliation, vesiculation, crusting, scarring, hypopigmentation, or PIH was noted. PicoWay® (Syneron-Candela, Irvine, California) is a 532nm and/or 1064nm Alexandrite picosecond laser that is currently being investigated for the treatment of acne scars (clinicaltrials.gov NCT02592993).
Other resurfacing agents.
Dermabrasion. Dermabrasion utilizes a manual hand or machine-driven source (e.g., high-speed brush, fraise, silicon carbide sandpaper, diamond cylinder) to remove the epidermis with or without part of the dermis. This is in contrast to the crystal-based dermabrasion offered at many medispas, which does not represent a true dermabrasion. The procedure allows the clinician to precisely define scar edges. It is best used for well-defined scars with distinct borders or broad-based scars with indistinct borders,75 but not for icepick or deep boxcar scars.76 Dermabrasion seeks to reorganize the papillary dermal collagen without injury to the reticular dermis. Improvement correlates with histological evidence of new collagen formation in the dermis.77 Adverse effects include significant pain, scarring, pigment alterations, and milia formation.15 After treatment, patients will experience increased sun sensitivity for several months, and unprotected skin can often develop hyperpigmentation.75
Chemical peels. Chemical peels are used to treat small, depressed scars but not icepick or deep boxcar scars.79 They induce injury to the skin that stimulates collagen remodeling and are categorized as superficial, medium, and deep based on the depth of the injury.78 Superficial peels, such as lactic acid, salicylic acid, glycolic acid, Jessner solution, and 10 to 25% trichloroacetic acid, only affect the epidermis. Medium depth peels, such as combined Jessner solution with 35 to 50% trichloroacetic acid affect the epidermis and papillary dermis. In most patients, medium-depth peels result in moderate clinical improvement (51–75% clearance), with transient PIH resolving within three months.80 Deep peels, such as phenol, injure skin to the mid-reticular dermis. A thicker zone of collagen was induced by a deep phenol peel than by a pulsed CO2 laser treatment three months postprocedure in one study.78
Adequate control of the peeling depth may be difficult to achieve. Complications, including prolonged erythema, infection, PIH, and scarring, are more common in dark SPTs, deeper peels, and sun exposure.76,81 Phenol has been associated with cardiac toxicity related to systemic absorption.82 While phenol peels produce a thicker zone of collagen than a pulsed CO2 laser, it is rarely used due to its risk of cardiotoxicity.
The chemical reconstruction of skin scars (CROSS) technique. The CROSS technique is indicated for icepick and narrow boxcar scars. It involves a high-strength trichloroacetic acid (TCA) peel (65–100%) applied to the base of the scar to ablate the epithelial wall and to promote dermal remodeling. The degree of clinical improvement is proportional to the number of courses of CROSS treatment, with good improvement after 3 to 6 courses reported in more than 90 percent of cases.104 Another study of 53 patients (SPTs IV and V) treated with 70% TCA CROSS found that treatment of boxcar scars and higher pretreatment scar severity were associated with better treatment outcomes.105 PIH of the treated areas was noted in 34.0 percent and more likely to occur in SPT V. Fractional 1,550nm erbium-doped laser has been shown to outperform CROSS for the treatment of rolling scars, but no difference has been observed for icepick scars.84
Microneedling. Microneedling (collagen induction therapy) utilizes tiny needles to puncture the skin multiple times, creating micro-clefts that penetrate into the dermis. The trauma in the dermis initiates wound healing and growth factor release, leading to collagen production and deposition in the upper dermis.83 Skin needling renders facial skin smoother and improves rolling acne scars.83 Similar to subcision, the tethered rolling scars can be overcome by greater collagen and elastin deposition induced by needling.84 The full result may take 8 to 12 months as the deposition of new collagen takes place slowly.83 One important advantage is that the epidermis remains intact, eliminating most of the risks of chemical peeling or laser resurfacing. In a study of 36 patients (SPTs IV–V) who underwent five microneedling sessions under topical anesthesia, there was a decrease in mean acne scar assessment score from 11.73 at baseline to 6.5, and a 50- to 75-percent improvement in the majority of patients on a quartile scale.118 Furthermore, microneedling provides a clear channel for the efficient absorption of topical agents, including platelet-rich plasma (PRP), which can improve cosmetic results.85 In a split-face study that investigated the use of microneedling plus PRP on one side of the face versus microneedling plus distilled water on the contralateral side, the PRP-treated face showed greater improvement in acne scarring after three monthly sessions (62.20% and 45.84% improvement, respectively).119 Results of microneedling treatment were comparable to the non-ablative fractional laser in one study. Forty-six patients with atrophic facial acne scars were randomized to three monthly treatments with microneedling (2mm microneedles, 20 passes in four directions) or the 1,340nm non-ablative fractional laser (5ms, 120J/cm2). Blinded assessments at six months post-treatment showed a significant, but not significantly different, improvement in acne scars in the microneedling and in the laser groups (4.05 and 3.41, respectively) on the Quantitative Global Grading System for Postacne Scarring.120
Radiofrequency. Non-ablative radiofrequency (RF) treatments can be used as a monotherapy or adjuvant therapy with fractional lasers. Radiofrequency delivers a current through the dermis that stimulates dermal remodeling, producing new collagen and softening scar defects.86 With traditional unipolar or monopolar RF, a single electrode allows for penetration deep into the dermis, but this is associated with increased pain and discomfort.87 New developments have allowed for more precision in the delivery of RF energy to deeper tissues, with decreased injury to the overlying epidermis. Bipolar RF allows for delivery of a more focused current to the dermis. Fractional RF uses an array of electrodes to create zones of thermal wounds that stimulate dermal remodeling. Micro-needles can be used to deliver the electrical current to a particular depth within the dermis. Microneedle bipolar RF and fractional bipolar RF treatments offer the best results for acne scarring, particularly icepick and boxcar scars.88 An improvement of 25 to 75 percent can be expected after 3 to 4 treatment sessions.86 The results are optimal three months after the final treatment due to the time required for fibroblast activation and upregulation of collagen production.86 The adverse reactions associated with RF include transient pain, erythema, and scabbing that resolve within 3 to 5 days.86
Individual atrophic scars. For isolated acne scars or for scars that remain after treatment of generalized atrophic scars, the following techniques may offer cosmetic improvement.
Fillers. Injectable fillers can be used to augment soft tissue, particularly in soft atrophic rolling or boxcar scars. Modes of injection include serial punctures, linear threading, fanning and cross-hatching, deep bolus, and superficial micro-droplet injections. The common adverse effects include infection, pain, erythema, lumps, swelling, and abscess formation. Fillers can be classified as temporary, semi-permanent, and permanent.
Temporary fillers typically last for a few months, making repeated treatments necessary, which increases cost. The injection of hyaluronic acid fillers (HAF) stimulates collagen production by fibroblasts, augmenting soft tissue and improving the quality of the overlying skin.89–91 Biphasic HAFs comprise cross-linked HA particles suspended in a lubricating non-cross-linked HA gel, which allows passage through a fine needle.
Semi-permanent fillers can last up to two years and are biostimulatory, stimulating fibrous tissue formation. Poly-I-lactic acid (PLL) is commercially available as Sculptra® (Dermik Laboratories, Berwyn, Pennsylvania) and New Fill® (Valeant US, Sinclair Pharma, Paris, France), which have both shown significant improvement in acne scars, particularly rolling scars.92,93 Calcium hydroxylapatite (CaHA) is a synthetic filler, available as Radiesse® (Merz North America, Raleigh, North Carolina), that has shown improvement in boxcar scars as a monotherapy94 and when used one week after subcision.95
Permanent fillers comprise larger particles that cannot be phagocytosed. They can last several years to lifelong and can be displaced over time due to changes in the adjacent connective tissue.89 Adverse effects can be permanent and might require their complete removal. Silicone is relatively cheap and is stable for 10 to 20 years.96 Despite widespread use, there have been no controlled studies on scar improvement. Polymethylmethacrylate (PMMA) is a synthetic permanent filler suspended in bovine collagen and lidocaine. Commercial products, including Artecoll® (Canderm Pharma, Saint Lorent, QC, Canada), Artefill® (Canderm Pharma, Saint Lorent, QC, Canada), and Bellafill® (Suneva Medical, San Diego, California, USA), improve acne scars when compared with controls.97,98 Bellafill®, the first FDA-approved medical device for the correction of acne scars, is indicated for moderate-to-severe atrophic, distensible acne scars on the cheeks in adults. The safety and efficacy of Bellafill® in acne scars is being further evaluated as a monotherapy (clinicaltrials.gov, NCT02642627) and following microneedling (clinicaltrials.gov, NCT02643628)
Punch excision and punch elevation. Punch excision is indicated for icepick and boxcar scars.32 A punch instrument approximately the size of the scar should be selected, followed by excision to the subcutaneous layer. The defect should be closed by sutures along relaxed skin tension lines.32 Placing a single non-absorbable suture for punch holes 2.5mm or larger might facilitate wound healing and minimize spreading.1 Some authors espouse punch excision followed by secondary intention healing, in which a scar is created but is less noticeable because of change at the depth of the base.75 For scars larger than 3.5mm, elliptical excision may be more favorable than punch excision.99 Punch excision also works well in conjunction with laser resurfacing.
Punch elevation is best suited for treatment of broad boxcar scars without underlying fibrosis.100 The scar is excised by punch instrument equal in size to the depressed scar down to the subcutaneous tissue. The tissue is then elevated and sutured in place at a level slightly higher than the surrounding skin to account for contraction during wound healing1.
Subcision. Subcision is best suited for rolling acne scars, with less efficacy for icepick and boxcar scars.101 The procedure involves inserting a needle under the acne scar to sever the fibrous components that anchor the scar below the dermis.102 The release of the fibrous tether elevates the scar and, when successful, produces new collagen formation through normal physiological healing, without recreating a depression.101,103 Needle choices include an 18- or 20-gauge tri-beveled hypodermic needle or an 18-gauge Nokor™ needle (Becton Dickinson, Franklin Lakes, New Jersey), whose triangular tip allows for the smooth and thorough separation of the fibrous tethers. Multiple treatments may be required to achieve an optimal outcome. Adverse events include depression recurrence, swelling, bruising, bleeding, and infection.101
FUTURE TREATMENT OF ACNE SCARRING
Given the strong relationship between severity and duration of inflammation in scar development, early treatment of acne lesions is the best approach to prevent acne scarring. Therapy should be maintained until resolution of persistent inflammation and control of new lesion emergence.106 Determining at-risk patients will be enhanced with a better understanding of risk factors for severe acne and acne scar formation. Recently, two new susceptibility loci, 1 q24.2 and 11p11.2, have been shown to confer risk of severe acne in the Chinese Han population.107
Autologous PRP can enhance wound healing by accelerating tissue repair and reducing postoperative pain.108,109 Platelets are the first cells to arrive at the sites of tissue damage, and they mediate tissue repair through the release of growth factors, cytokines, and chemokines from their α-granules.110 Intradermal injections of PRP were first noted to improve acne scarring when used for skin rejuvenation.111 Topical PRP has a synergistic effect with skin needling in atrophic acne scars, as the skin needling creates a way for PRP absorption to occure and allows the additional platelets to contribute to wound healing.83,85 PRP as both an intradermal injection and topical application after fractional ablative CO2 therapy enhanced the recovery of laser damaged skin and improved the clinical appearance of acne scars compared to control.112,113
In addition to PRP, human-derived cellular components are being evaluated for the treatment of acne scars. Multipotential mesenchymal stem cells (MSC) are capable of differentiation into various cell lineages and have been shown to promote wound healing.114 MSCs can be isolated from umbilical cord blood and expanded.115 The safety of umbilical cord MSC and HA as filler agents is currently under investigation (clinicaltrials.gov, NCT02698813). In contrast to umbilical cord MSC, adipose-derived MSC are relatively easy to obtain. The safety of adipose-derived autologous MSC associated with HA as a filler agent is currently being investigated (clinicaltrials.gov NCT02034786).
CONCLUSION
Acne scarring is a common problem facing a significant number of patients with acne vulgaris, leading many to seek treatment options for improved cosmesis. The first step in treating acne scarring involves addressing residual erythema, if present. This should be followed by addressing generalized atrophic scars, tailoring the treatment method to the predominant scar type present (Figure 2, Table 2). Finally, any remaining scars should be treated according to the most suitable method for the individual scar. Surgical techniques and injectable fillers may suffice for solitary scars; however, a majority of patients require field treatment for broad areas of scarring, for which lasers and other resurfacing agents remain the mainstay of treatment. A patient-centered, multi-step approach that takes into account the type of acne scarring and patient goals will yield the best cosmetic results and highest patient satisfaction.
TABLE 2.
Effectiveness of treatments for acne scarring
| TREATMENT TYPE | ICE PICK SCARS | ROLLING SCARS | BOX PICK SCARS |
|---|---|---|---|
| Ablative fractionated resurfacing | + | ++ | ++ |
| Non-ablative fractionated Resurfacing | ++ | ++ | |
| Needling | - | ++ | ++ |
| TCA/CROSS | ++ | ++ | |
| Fillers | - | ++ | - |
| Subcision | - | ++ | - |
| Excision | |||
| Punch Excision | +++ | - | ++ |
- no role/evidence; + fair; ++ good; +++ great efficacy
TCA/CROSS: trichloroacetic acid/chemical reconstruction of skin scars
REFERENCES
- 1.Jacob CI, Dover JS, Kaminer MS. Acne scarring: a classification system and review of treatment options. J Am Acad Dermatol. 2001;45:109–117. doi: 10.1067/mjd.2001.113451. [DOI] [PubMed] [Google Scholar]
- 2.Kang S, Cho S, Chung JH, et al. Inflammation and extracellular matrix degradation mediated by activated transcription factors nuclear factor-kappaB and activator protein-1 in inflammatory acne lesions in vivo. Am J Pathol. 2005;166:1691–1699. doi: 10.1016/s0002-9440(10)62479-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Layton AM. Optimal management of acne to prevent scarring and psychological sequelae. Am J Clin Dermotol. 2001;2:135–141. doi: 10.2165/00128071-200102030-00002. [DOI] [PubMed] [Google Scholar]
- 4.Kircik LH. Re-evaluating treatment targets in acne vulgaris: adapting to a new understanding of pathophysiology. J Drugs Dermatol. 2014;13:s57–60. [PubMed] [Google Scholar]
- 5.Davis EC, Callender VD. Postinflammatory hyperpigmentation: a review of the epidemiology, clinical features, and treatment options in skin of color. J Clin Aesthet Dermatol. 2010;3:20–31. Jul. [PMC free article] [PubMed] [Google Scholar]
- 6.Vowels BR, Yang S, Leyden JJ. Induction of proinflammatory cytokines by a soluble factor of Propionibacterium acnes: implications for chronic inflammatory acne. InfectImmun. 1995;63:3158–3165. doi: 10.1128/iai.63.8.3158-3165.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bae-Harboe YS, Graber EM. Easy as PIE (Postinflammatory Erythema) J Clin Aesthet. Dermatol. 2013;6:46–47. [PMC free article] [PubMed] [Google Scholar]
- 8.Layton AM, Henderson CA, Cunliffe WJ. A clinical evaluation of acne scarring and its incidence. Clin Exp Dermatol. 1994;19:303–308. doi: 10.1111/j.1365-2230.1994.tb01200.x. [DOI] [PubMed] [Google Scholar]
- 9.Layton AM, Seukeran D, Cunliffe WJ. Scarred for life? Dermatology. 1997;195(Suppl 1):15–21. doi: 10.1159/000246015. [DOI] [PubMed] [Google Scholar]
- 10.Goodman GJ. Management of post-acne scarring. What are the options for treatment? Am J Clin Dermatol. 2000;1:3–17. doi: 10.2165/00128071-200001010-00001. [DOI] [PubMed] [Google Scholar]
- 11.Lee WJ, Jung HJ, Lim HJ, et al. Serial sections of atrophic acne scars help in the interpretation of microscopic findings and the selection of good therapeutic modalities. J Eur Acad Dermatol. Venereol. 2013;27:643–646. doi: 10.1111/j.1468-3083.2011.04330.x. [DOI] [PubMed] [Google Scholar]
- 12.Jasson F, Nagy I, Knol AC, et al. Different strains of Propionibacterium acnes modulate differently the cutaneous innate immunity. Exp Dermatol. 2013;22:587–592. doi: 10.1111/exd.12206. [DOI] [PubMed] [Google Scholar]
- 13.Holland DB, Jeremy AH, Roberts SG, et al. Inflammation in acne scarring: a comparison of the responses in lesions from patients prone and not prone to scar. Br J Dermatol. 2004;150:72–81. doi: 10.1111/j.1365-2133.2004.05749.x. [DOI] [PubMed] [Google Scholar]
- 14.Saint-Jean M, Khammari A, Jasson F, et al. Different cutaneous innate immunity profiles in acne patients with and without atrophic scars. Eur J Dermatol. 2016;26:68–74. doi: 10.1684/ejd.2015.2713. [DOI] [PubMed] [Google Scholar]
- 15.Abdel HR, Shalaby K, Zaber H, et al. Interventions for acne scars. Coch Data Systemat Rev. 2016;4:CD011946. doi: 10.1002/14651858.CD011946.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Williams HC, Dellavalle RP, Garner S. Acne vulgaris. Lancet. 2012;379:361–372. doi: 10.1016/S0140-6736(11)60321-8. [DOI] [PubMed] [Google Scholar]
- 17.Alster TS, McMeekin TO. Improvement of facial acne scars by the 585 nm flashlamp-pumped pulsed dye laser. J Am Acad Dermatol. 1996;35:79–81. doi: 10.1016/S0190-9622(96)90501-0. [DOI] [PubMed] [Google Scholar]
- 18.Anderson RR, Parrish JA. Selective photothermolysis: precise microsurgery by selective absorption of pulsed radiation. Science. 1983;220:524–527. doi: 10.1126/science.6836297. [DOI] [PubMed] [Google Scholar]
- 19.Iyer S, Fitzpatrick RE. Long-pulsed dye laser treatment for facial telangiectasias and erythema: evaluation of a single purpuric pass versus multiple subpurpuric passes. Dermatol Surg. 2005;31:898–903. doi: 10.1097/00042728-200508000-00002. [DOI] [PubMed] [Google Scholar]
- 20.Patel N, Clement M. Selective nonablative treatment of acne scarring with 585 nm flashlamp pulsed dye laser. Dermatol Surg. 2002;28:942–945. doi: 10.1046/j.1524-4725.2002.02062.x. [DOI] [PubMed] [Google Scholar]
- 21.Lee DH, Choi YS, Min SU, et al. Comparison of a 585-nm pulsed dye laser and a 1064-nm Nd:YAG laser for the treatment of acne scars: a randomized split-face clinical study. J Am Acad Dermatol. 2009;60:801–807. doi: 10.1016/j.jaad.2008.11.883. [DOI] [PubMed] [Google Scholar]
- 22.Seaton ED, Mouser PE, Charakida A, et al. Investigation of the mechanism of action of nonablative pulsed-dye laser therapyin photorejuvenation and inflammatory acne vulgaris. Br J Dermatol. 2006;155:748–755. doi: 10.1111/j.1365-2133.2006.07429.x. [DOI] [PubMed] [Google Scholar]
- 23.Park KY, Ko EJ, Seo SJ, Hong CK. Comparison of fractional, nonablative, 1550-nm laser and 595-nm pulsed dye laser for the treatment of facial erythema resulting from acne: a split-face, evaluator-blinded, randomized pilot study. J Cosmet Laser Ther. 2014;16:120–123. doi: 10.3109/14764172.2013.854626. [DOI] [PubMed] [Google Scholar]
- 24.Glaich AS, Goldberg LH, Friedman RH, Friedman PM. Fractional photothermolysis for the treatment of postinflammatory erythema resulting from acne vulgaris. Dermatol Surg. 2007;33:842–846. doi: 10.1111/j.1524-4725.2007.33180.x. [DOI] [PubMed] [Google Scholar]
- 25.Cartier H. Use of intense pulsed light in the treatment of scars. J Cosmet Dermatol. 2005;4:34–40. doi: 10.1111/j.1473-2165.2005.00157.x. [DOI] [PubMed] [Google Scholar]
- 26.Keaney TC, Tanzi E, Alster T. Comparison of 532 nm potassium titanyl phosphate laser and 595 nm pulsed dye laser in the treatment of erythematous surgical scars: a randomized, controlled, open-label study. Dermatol Surg. 2016;42:70–76. doi: 10.1097/DSS.0000000000000582. [DOI] [PubMed] [Google Scholar]
- 27.Cohen BE, Brauer JA, Geronemus RG. Acne scarring: A review of available therapeutic lasers. Lasers Surg Med. 2016;48:95–115. doi: 10.1002/lsm.22410. [DOI] [PubMed] [Google Scholar]
- 28.Rao J. Treatment of acne scarring. Facial Plast Surg Clin North Am. 2011;19:275–291. doi: 10.1016/j.fsc.2011.04.004. [DOI] [PubMed] [Google Scholar]
- 29.Min SU, Choi YS, Lee DH, et al. Comparison of a long-pulse Nd:YAG laser and a combined 585/1,064-nm laser for the treatment of acne scars: a randomized split-face clinical study. Dermatol Surg. 2009;35:1720–1727. doi: 10.1111/j.1524-4725.2009.01086.x. [DOI] [PubMed] [Google Scholar]
- 30.Sadick NS, Weiss R. Intense pulsed-light photorejuvenation. Semin Cutan Med Surg. 2002;21:280–287. doi: 10.1053/sder.2002.36902. [DOI] [PubMed] [Google Scholar]
- 31.Bjerring P, Christiansen K, Troilius A, Dierickx C. Facial photo rejuvenation using two different intense pulsed light (IPL) wavelength bands. Lasers Surg Med. 2004;34:120–126. doi: 10.1002/lsm.20000. [DOI] [PubMed] [Google Scholar]
- 32.Rivera AE. Acne scarring: a review and current treatment modalities. J Am Acad Dermatol. 2008;59:659–676. doi: 10.1016/j.jaad.2008.05.029. [DOI] [PubMed] [Google Scholar]
- 33.Metelitsa Al, Alster TS. Fractionated laser skin resurfacing treatment complications: a review. Dermatol Surg. 2010;36:299–306. doi: 10.1111/j.1524-4725.2009.01434.x. [DOI] [PubMed] [Google Scholar]
- 34.Walia S, Alster TS. Prolonged clinical and histologic effects from CO2 laser resurfacing of atrophic acne scars. Dermatol Surg. 1999;25:926–930. doi: 10.1046/j.1524-4725.1999.99115.x. [DOI] [PubMed] [Google Scholar]
- 35.Brightman LA, Brauer JA, Anolik R, et al. Ablative and fractional ablative lasers. Dermatol Clin. 2009;27:479–489. doi: 10.1016/j.det.2009.08.009. [DOI] [PubMed] [Google Scholar]
- 36.Alster T, Hirsch R. Single-pass CO2 laser skin resurfacing of light and dark skin: extended experience with 52 patients. J Cosmet Laser Ther. 2003;5:39–42. [PubMed] [Google Scholar]
- 37.Tanzi EL, Alster TS. Treatment of atrophic facial acne scars with a dual-mode Er:YAG laser. Dermatol Surg. 2002;28:551–555. doi: 10.1046/j.1524-4725.2002.01319.x. [DOI] [PubMed] [Google Scholar]
- 38.Walsh JT, Jr, Flotte TJ, Deutsch TF. Er:YAG laser ablation of tissue: effect of pulse duration and tissue type on thermal damage. Lasers Surg Med. 1989;9:314–326. doi: 10.1002/lsm.1900090403. [DOI] [PubMed] [Google Scholar]
- 39.Alster TS. Cutaneous resurfacing with CO2 and erbium: YAG lasers: preoperative, intraoperative, and postoperative considerations. Plast Reconstr Surg. 1999;103:619–634. doi: 10.1097/00006534-199902000-00040. [DOI] [PubMed] [Google Scholar]
- 40.Jeong JT, Kye YC. Resurfacing of pitted facial acne scars with a long-pulsed Er:YAG laser. Dermatol Surg. 2001;27:107–110. doi: 10.1046/j.1524-4725.2001.00201.x. [DOI] [PubMed] [Google Scholar]
- 41.Hu S, Gold MH. Treatment of facial acne scars in Asian skin with the single-spot, 2940-nm Er:YAG dual-mode laser. J Drugs Dermatol. 2010;9:1341–1344. [PubMed] [Google Scholar]
- 42.Wanitphakdeedecha R, Manuskiatti W, Siriphukpong S, Chen TM. Treatment of punched-out atrophic and rolling acne scars in skin phototypes III, IV, and V with variable square pulse erbium:yttrium-aluminum-garnet laser resurfacing. Dermatol Surg. 2009;35:1376–1383. doi: 10.1111/j.1524-4725.2009.01244.x. [DOI] [PubMed] [Google Scholar]
- 43.Woo SH, Park JH, Kye YC. Resurfacing of different types of facial acne scar with short-pulsed, variable-pulsed, and dual-mode Er:YAG laser. Dermatol Surg. 2004;30:488–493. doi: 10.1111/j.1524-4725.2004.30161.x. [DOI] [PubMed] [Google Scholar]
- 44.Tanzi EL, Alster TS. Single-pass carbon dioxide versus multiple-pass Er:YAG laser skin resurfacing: a comparison of postoperative wound healing and side-effect rates. Dermatol Surg. 2003;29:80–84. doi: 10.1046/j.1524-4725.2003.29012.x. [DOI] [PubMed] [Google Scholar]
- 45.Tanzi EL, Alster TS. Comparison of a 1450-nm diode laser and a 1320-nm Nd:YAG laser in the treatment of atrophic facial scars: a prospective clinical and histologic study. Dermatol Surg. 2004;30:152–157. doi: 10.1111/j.1524-4725.2004.30078.x. [DOI] [PubMed] [Google Scholar]
- 46.Abraham MT, Vic Ross E. Current concepts in nonablative radiofrequency rejuvenation of the lower face and neck. Facial Plast Surg. 2005;21:65–73. doi: 10.1055/s-2005-871765. [DOI] [PubMed] [Google Scholar]
- 47.Tierney EP, Eisen RF, Hanke CW. Fractionated CO2 laser skin rejuvenation. Dermatol Ther. 2011;24:41–53. doi: 10.1111/j.1529-8019.2010.01377.x. [DOI] [PubMed] [Google Scholar]
- 48.Maluki AH, Mohammad FH. Treatment of atrophic facial scars of acne vulgaris by Q-Switched Nd:YAG (Neodymium: Yttrium-Aluminum-Garnet) laser 1064nm wavelength. J Cosmet Laser Ther. 2012;14:224–233. doi: 10.3109/14764172.2012.723807. [DOI] [PubMed] [Google Scholar]
- 49.Goldberg DJ, Silapunt S. Q-switched Nd:YAG laser: rhytid improvement by non-ablative dermal remodeling. J Cutan Laser Ther. 2000;2:157–160. doi: 10.1080/14628830050516425. [DOI] [PubMed] [Google Scholar]
- 50.Prieto VG, Zhang PS, Sadick NS. Evaluation of pulsed light and radiofrequency combined for the treatment of acne vulgaris with histologic analysis of facial skin biopsies. J Cosmet Laser Ther. 2005;7:63–68. doi: 10.1080/14764170500231848. [DOI] [PubMed] [Google Scholar]
- 51.Upper GM, Perez M. Nonablative acne scar reduction after a series of treatments with a short-pulsed 1,064-nm neodymium:YAG laser. Dermatol Surg. 2006;32:998–1006. doi: 10.1111/j.1524-4725.2006.32222.x. [DOI] [PubMed] [Google Scholar]
- 52.Alam M, Hsu TS, Dover JS, et al. Nonablative laser and light treatments: histology and tissue effects—a review. Lasers Surg Med. 2003;33:30–39. doi: 10.1002/lsm.10195. [DOI] [PubMed] [Google Scholar]
- 53.Friedman PM, Jih MH, Skover GR, et al. Treatment of atrophic facial acne scars with the 1064-nm Q-switched Nd:YAG laser: six-month follow-up smdy. Arch Dermatol. 2004;140:1337–1341. doi: 10.1001/archderm.140.11.1337. [DOI] [PubMed] [Google Scholar]
- 54.Bellew SG, Lee C, Weiss MA, Weiss RA. Improvement of atrophic acne scars with a 1,320 nm Nd:YAG laser: retrospective study. Dermatol Surg. 2005;31:1218–1222. doi: 10.1111/j.1524-4725.2005.31929. [DOI] [PubMed] [Google Scholar]
- 55.Bhatia AC, Dover JS, Arndt KA, et al. Patient satisfaction and reported long-term therapeutic efficacy associated with 1,320 m Nd:YAG laser treatment of acne scarring and photoaging. Dermotol Surg. 2006;32:346–352. doi: 10.1111/j.1524-4725.2006.32071.x. [DOI] [PubMed] [Google Scholar]
- 56.Wada T, Kawada A, Hirao A, et al. Efficacy and safety of a low-energy double-pass 1450-nm diode laser for the treatment of acne scars. Photomed Laser Surg. 2012;30:107–111. doi: 10.1089/pho.2011.3063. [DOI] [PubMed] [Google Scholar]
- 57.Yaghmai D, Garden JM, Bakus AD, Massa MC. Comparison of a 1,064 nm laser and a 1,320 nm laser for the nonablative treatment of acne scars. Dermatol Surg. 2005;31:903–909. doi: 10.1097/00042728-200508000-00003. [DOI] [PubMed] [Google Scholar]
- 58.Chua SH, Ang P, Khoo LS, Goh CL. Nonablative 1450-nm diode laser in the treatment of facial atrophic acne scars in type IV to V Asian skin: a prospective clinical study. Dermatol Surg. 2004;30:1287–1291. doi: 10.1111/j.1524-4725.2004.30402.x. [DOI] [PubMed] [Google Scholar]
- 59.Rogachefsky AS, Hussain M, Goldberg DJ. Atrophic and a mixed pattern of acne scars improved with a 1320-nm Nd:YAG laser. Dermatol Surg. 2003;29:904–908. doi: 10.1046/j.1524-4725.2003.29256.x. [DOI] [PubMed] [Google Scholar]
- 60.Tanzi EL, Williams CM, Alster TS. Treatment of facial rhytides with a nonablative 1,450-nm diode laser: a controlled clinical and histologic study. Dermatol Surg. 2003;29:124–128. doi: 10.1046/j.1524-4725.2003.29046.x. [DOI] [PubMed] [Google Scholar]
- 61.Ong MW, Bashir SJ. Fractional laser resurfacing for acne scars: a review. Br J Dermatol. 2012;166:1160–1169. doi: 10.1111/j.1365-2133.2012.10870.x. [DOI] [PubMed] [Google Scholar]
- 62.Hedelund L, Moreau KE, Beyer DM, et al. Fractional nonablative 1,540-nm laser resurfacing of atrophic acne scars, a randomized controlled trial with blinded response evaluation. Lasers Med Sci. 2010;25:749–754. doi: 10.1007/s10103-010-0801-1. [DOI] [PubMed] [Google Scholar]
- 63.Bencini PL, Jourlaki A, Galimberti M, et al. Nonablative fractional photothermolysis for acne scars: clinical and in vivo microscopic documentation of treatment efficacy. Dermatol Ther. 2012;25:463–467. doi: 10.1111/j.1529-8019.2012.01478.x. [DOI] [PubMed] [Google Scholar]
- 64.Yoo KH, Ahn JY, Kim JY, et al. The use of 1540 nm fractional photothermolysis for the treatment of acne scars in Asian skin: a pilot study. Photodermatol Photoimmunol Photomed. 2009;25:138–142. doi: 10.1111/j.1600-0781.2009.00430.x. [DOI] [PubMed] [Google Scholar]
- 65.Sardana K, Manjhi M, Garg VK, Sagar V. Which type of atrophic acne scar (ice-pick, boxcar, or rolling) responds to nonablative fractional laser therapy? Dermatol Surg. 2014;40:288–300. doi: 10.1111/dsu.12428. [DOI] [PubMed] [Google Scholar]
- 66.Alster TS, Tanzi EL, Lazarus M. The use of fractional laser photothermolysis for the treatment of atrophic scars. Dermatol Surg. 2007;33:295–299. doi: 10.1111/j.1524-4725.2007.33059.x. [DOI] [PubMed] [Google Scholar]
- 67.Chrastil B, Glaich AS, Goldberg LH, Friedman PM. Second-generation 1,550-nm fractional photothermolysis for the treatment of acne scars. Dermatol Surg. 2008;34:1327–1332. doi: 10.1111/j.1524-4725.2008.34284.x. [DOI] [PubMed] [Google Scholar]
- 68.Chan NP, Ho SG, Yeung CK, et al. The use of nonablative fractional resurfacing in Asian acne scar patients. Lasers Surg Med. 2010;42:710–715. doi: 10.1002/lsm.20976. [DOI] [PubMed] [Google Scholar]
- 69.Chapas AM, Brightman L, Sukal S, et al. Successful treatment of acneiform scarring with C2 ablative fractional resurfacing. Lasers Surg. Med. 2008;40:381–386. doi: 10.1002/lsm.20659. [DOI] [PubMed] [Google Scholar]
- 70.Jung JY, Lee JH, Ryu DJ, et al. Lower-fluence, higher-density versus higher-fluence, lower-density treatment with a 10,600-nm carbon dioxide fractional laser system: a split-face, evaluator-blinded study. Dermatol Surg. 2010;36:2022–2029. doi: 10.1111/j.1524-4725.2010.01803.x. [DOI] [PubMed] [Google Scholar]
- 71.Asilian A, Salimi E, Faghihi G, et al. Comparison of Q-Switched 1064-nm Nd:YAG laser and fractional CO2 laser efficacies on improvement of atrophic facial acne scar. J Res Med Sci. 2011;16:1189–1195. [PMC free article] [PubMed] [Google Scholar]
- 72.Cho SB, Lee SJ, Kang JM, et al. The efficacy and safety of 10,600-nm carbon dioxide fractional laser for acne scars in Asian patients. Dermatol Surg. 2009;35:1955–1961. doi: 10.1111/j.1524-4725.2009.01316.x. [DOI] [PubMed] [Google Scholar]
- 73.Kim S. Treatment of acne scars in Asian patients using a 2,790-nm fractional yttrium scandium gallium garnet laser. Dermatol Surg. 2011;37:1464–1469. doi: 10.1111/j.1524-4725.2011.02050.x. [DOI] [PubMed] [Google Scholar]
- 74.Zhu JT, Xuan M, Zhang YN, et al. The efficacy of autologous platelet-rich plasma combined with erbium fractional laser therapy for facial acne scars or acne. Mol Med Rep. 2013;8:233–237. doi: 10.3892/mmr.2013.1455. [DOI] [PubMed] [Google Scholar]
- 75.Hirsch RJ, Lewis AB. Treatment of acne scarring. Semin Cutan Med Surg. 2001;20:190–198. doi: 10.1053/sder.2001.27557. [DOI] [PubMed] [Google Scholar]
- 76.You HJ, Kim DW, Yoon ES, Park SH. Comparison of four different lasers for acne scars: Resurfacing and fractional lasers. J Plast Reconstr Aesthet Surg. 2016;69:e87–95. doi: 10.1016/j.bjps.2015.12.012. [DOI] [PubMed] [Google Scholar]
- 77.Frank W. Therapeutic dermabrasion, back to the future. Arch Dermatol. 1994;130:1187–1189. doi: 10.1001/archderm.130.9.1187. [DOI] [PubMed] [Google Scholar]
- 78.Moy LS, Kotler R, Lesser T. The histologic evaluation of pulsed carbon dioxide laser resurfacing versus phenol chemical peels in vivo. Dermatol Surg. 1999;25:597–600. doi: 10.1046/j.1524-4725.1999.98246.x. [DOI] [PubMed] [Google Scholar]
- 79.Garg VK, Sinha S, Sarkar R. Glycolicacid peels versus salicylic-mandelic acid peels in active acne vulgaris and post-acne scarring and hyperpigmentation: a comparative study. Dermatol Surg. 2009;35:59–65. doi: 10.1111/j.1524-4725.2008.34383.x. [DOI] [PubMed] [Google Scholar]
- 80.Al-Waiz MM, Al-Sharqi Al. Medium-depth chemical peels in the treatment of acne scars in dark-skinned individuals. Dermatol Surg. 2002;28:383–387. doi: 10.1046/j.1524-4725.2002.01081.x. [DOI] [PubMed] [Google Scholar]
- 81.Nikalji N, Godse K, Sakhiya J, et al. Complications of medium depth and deep chemical peels. J Cutan Aesthet Surg. 2012;5:254–260. doi: 10.4103/0974-2077.104913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Landau M. Cardiac complications in deep chemical peels. Dermatol Surg. 2007;33:190–193. doi: 10.1111/j.1524-4725.2006.33037.x. [DOI] [PubMed] [Google Scholar]
- 83.Fabbrocini G, Annunziata MC, D’Arco V, et al. Acne scars: pathogenesis, classification and treatment. Dermatol Res Pract. 2010;2010:893080. doi: 10.1155/2010/893080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kim HJ, Kim TG, Kwon YS, et al. Comparison of a 1,550 nm Erbium: glass fractional laser and a chemical reconstruction of skin scars (CROSS) method in the treatment of acne scars: a simultaneous split-face trial. Lasers Surg Med. 2009;41:545–549. doi: 10.1002/lsm.20796. [DOI] [PubMed] [Google Scholar]
- 85.Nofal E, Helmy A, Nofal A, et al. Platelet-rich plasma versus CROSS technique with 100% trichloroacetic acid versus combined skin needling and platelet rich plasma in the treatment of atrophic acne scars: a comparative study. Dermatol Surg. 2014;40:864–873. doi: 10.1111/dsu.0000000000000091. [DOI] [PubMed] [Google Scholar]
- 86.Simmons BJ, Griffith RD, Falto-Aizpurua LA, Nouri K. Use of radiofrequency in cosmetic dermatology: focus on nonablative treatment of acne scars. Clin Cosmet Investig Dermatol. 2014;7:335–339. doi: 10.2147/CCID.S74411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lolis MS, Goldberg DJ. Radiofrequency in cosmetic dermatology: a review. Dermatol Surg. 2012;38:1765–1776. doi: 10.1111/j.1524-4725.2012.02547.x. [DOI] [PubMed] [Google Scholar]
- 88.Min S, Park SY, Yoon JY, Suh DH. Comparison of fractional microneedling radiofrequency and bipolar radiofrequency on acne and acne scar and investigation of mechanism: comparative randomized controlled clinical trial. Arch Dermatol Res. 2015;307:897–904. doi: 10.1007/s00403-015-1601-z. [DOI] [PubMed] [Google Scholar]
- 89.Wollina U, Goldman A. Fillers for the improvement in acne scars. Clin Cosmet Investig Dermatol. 2015;8:493–499. doi: 10.2147/CCID.S86478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hasson A, Romero WA. Treatment of facial atrophic scars with Esthelis, a hyaluronic acid filler with polydense cohesive matrix (CPM) J Drugs Dermatol. 2010;9:1507–1509. [PubMed] [Google Scholar]
- 91.Halachmi S, Ben Amitai D, Lapidoth M. Treatment of acne scars with hyaluronic acid: an improved approach. J Drugs Dermatol. 2013;12:e121–123. [PubMed] [Google Scholar]
- 92.Kalantar-Hormozi A, Mozafari N, Rasti M. Adverse effects after use of Polyacrylamide gel as a facial soft tissue filler. Aesthet Surg J. 2008;28:139–142. doi: 10.1016/j.asj.2007.12.005. [DOI] [PubMed] [Google Scholar]
- 93.Sadove R. Injectable poly-L: -lactic acid: a novel sculpting agent for the treatment of dermal fat atrophy after severe acne. Aesthetic Plast Surg. 2009;33:113–116. doi: 10.1007/s00266-008-9242-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Jacovella PF. Use of calcium hydroxylapatite (Radiesse) for facial augmentation. Clin Interv Aging. 2008;3:161–174. doi: 10.2147/cia.s2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Goldberg DJ, Amin S, Hussain M. Acne scar correction using calcium hydroxylapatite in a carrier-based gel. J Cosmet Laser Ther. 2006;8:134–136. doi: 10.1080/14764170600891632. [DOI] [PubMed] [Google Scholar]
- 96.De Boulle K, Heydenrych I. Patient factors influencing dermal filler complications: prevention, assessment, and treatment. Clin Cosmet Investig Dermatol. 2015;8:205–214. doi: 10.2147/CCID.S80446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Epstein RE, Spencer JM. Correction of atrophic scars with artefill: an open-label pilot study. J Drugs Dermatol. 2010;9:1062–1064. [PubMed] [Google Scholar]
- 98.Karnik J, Baumann L, Bruce S, et al. A double-blind, randomized, multicenter, controlled trial of suspended polymethylmethacrylate microspheres for the correction of atrophic facial acne scars. J Am Acad Dermatol. 2014;71:77–83. doi: 10.1016/j.jaad.2014.02.034. [DOI] [PubMed] [Google Scholar]
- 99.Fife D. Practical evaluation and management of atrophic acne scars: tips for the general dermatologist. J Clin Aesthet Dermatol. 2011;4:50–57. [PMC free article] [PubMed] [Google Scholar]
- 100.Goodman GJ, Baron JA. The management of postacne scarring. Dermatol Surg. 2007;33:1175–1188. doi: 10.1111/j.1524-4725.2007.33252.x. [DOI] [PubMed] [Google Scholar]
- 101.Alam M, Omura N, Kaminer MS. Subcision for acne scarring: technique and outcomes in 40 patients. Dermatol Surg. 2005;31:310–317. doi: 10.1111/j.1524-4725.2005.31080. [DOI] [PubMed] [Google Scholar]
- 102.Orentreich DS, Orentreich N. Subcutaneous incisionless (subcision) surgery for the correction of depressed scars and wrinkles. Dermatol Surg. 1995;21:543–549. doi: 10.1111/j.1524-4725.1995.tb00259.x. [DOI] [PubMed] [Google Scholar]
- 103.Nilforoushzadeh M, Lotfi E, Nickkholgh E, et al. Can subcision with the cannula be an acceptable alternative method in treatment of acne scars? Med Arch. 2015;69:384–386. doi: 10.5455/medarh.2015.69.384-386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Lee JB, Chung WG, Kwahck H, Lee KH. Focal treatment of acne scars with trichloroacetic acid: chemical reconstruction of skin scars method. Dermatol Surg. 2002;28:1017–1021. doi: 10.1046/j.1524-4725.2002.02095.x. [DOI] [PubMed] [Google Scholar]
- 105.AgarwaI N, Gupta LK, Khare AK, et al. Therapeutic response of 70% trichloroacetic acid CROSS in atrophic acne scars. Dermatol Surg. 2015;41:597–604. doi: 10.1097/DSS.0000000000000355. [DOI] [PubMed] [Google Scholar]
- 106.Del Rosso JQ, Kircik LH. The sequence of inflammation, relevant biomarkers, and the pathogenesis of acne vulgaris: what does recent research show and what does it mean to the clinician? J Drugs Dermatol. 2013;12:s109–115. [PubMed] [Google Scholar]
- 107.He L, Wu WJ, Yang JK, et al. Two new susceptibility loci 1 q24.2 and 11 p 11.2 confer risk to severe acne. Nat Commun. 2014;5:2870. doi: 10.1038/ncomms3870. [DOI] [PubMed] [Google Scholar]
- 108.Carter CA, Jolly DG, Worden CES, et al. Platelet-rich plasma gel promotes differentiation and regeneration during equine wound healing. Exp Mol Pathol. 2003;74:244–255. doi: 10.1016/s0014-4800(03)00017-0. [DOI] [PubMed] [Google Scholar]
- 109.Gardner MJ, Demetrakopoulos D, Klepchick PR, Mooar PA. The efficacy of autologous platelet gel in pain control and blood loss in total knee arthroplasty. an analysis of the haemoglobin, narcotic requirement and range of motion. Int Orthop. 2007;31:309–313. doi: 10.1007/s00264-006-0174-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Eppley BL, Pietrzak WS, Blanton M. Platelet-rich plasma: a review of biology and applications in plastic surgery. Plast Reconstr Surg. 2006;118:147e–159e. doi: 10.1097/01.prs.0000239606.92676.cf. [DOI] [PubMed] [Google Scholar]
- 111.Redaelli A, Romano D, Marciano A. Face and neck revitalization with platelet-rich plasma (PRP): clinical outcome in a series of 23 consecutively treated patients. J Drugs Dermatol. 2010;9:466–472. [PubMed] [Google Scholar]
- 112.Lee JW, Kim BJ, Kim MN, Mun SK. The efficacy of autologous platelet rich plasma combined with ablative carbon dioxide fractional resurfacing for acne scars: a simultaneous split-face trial. Dermatol Surg. 2011;37:931–938. doi: 10.1111/j.1524-4725.2011.01999.x. [DOI] [PubMed] [Google Scholar]
- 113.Gawdat HI, Hegazy RA, Fawzy MM, Fathy M. Autologous platelet rich plasma: topical versus intradermal after fractional ablative carbon dioxide laser treatment of atrophic acne scars. Dermatol Surg. 2014;40:152–161. doi: 10.1111/dsu.12392. [DOI] [PubMed] [Google Scholar]
- 114.Nie C, Yang D, Xu J, et al. Locally administered adipose-derived stem cells accelerate wound healing through differentiation and vasculogenesis. Cell Transplant. 2011;20:205–216. doi: 10.3727/096368910X520065. [DOI] [PubMed] [Google Scholar]
- 115.Doi H, Kitajima Y, Luo L, et al. Potency of umbilical cord blood-and Wharton’s jelly-derived mesenchymal stem cells for scarless wound healing. Sci Rep. 2016;6:18844. doi: 10.1038/srep18844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Brauer JA, Kazlouskaya V, Alabdulrazzaq H, et al. Use of a picosecond pulse duration laser with specialized optic for treatment of facial acne scarring. JAMA Dermatol. 2015;151:278–284. doi: 10.1001/jamadermatol.2014.3045. [DOI] [PubMed] [Google Scholar]
- 117.Tanghetti EA. The histology of skin treated with a picosecond alexandrite laser and a fractional lens array. Lasers Surg Med. 2016;48:646–652. doi: 10.1002/lsm.22540. [DOI] [PubMed] [Google Scholar]
- 118.Dogra S, Yadav S, Sarangal R. Microneedling for acne scars in Asian skin type: an effective low cost treatment modality. J Cosmet Dermatol. 2014;13:180–187. doi: 10.1111/jocd.12095. [DOI] [PubMed] [Google Scholar]
- 119.Asif M, Kanodia S, Singh K. Combined autologous platelet-rich plasma with microneedling verses microneedling with distilled water in the treatment of atrophic acne scars: a concurrent split-face study. J Cosmet Dermatol. 2016;15:434–443. doi: 10.1111/jocd.12207. [DOI] [PubMed] [Google Scholar]
- 120.Cachafeiro T, Escobar G, Maldonado G, et al. Comparison of nonablative fractional erbium laser 1,340 nm and microneedling for the treatment of atrophic acne scars: a randomized clinical trial. Dermatol Surg. 2016;42:232–241. doi: 10.1097/DSS.0000000000000597. [DOI] [PubMed] [Google Scholar]
- 121.Fife DJ1, Fitzpatrick RE, Zachary CB. Complications of fractional CO2 laser resurfacing: fourcases. Lasers Surg Med. 2009;41(3):179–184. doi: 10.1002/lsm.20753. [DOI] [PubMed] [Google Scholar]