ABSTRACT
Although neoadjuvant radiochemotherapy (nRCTx) is an established oncological treatment in patients with advanced rectal cancer, little is known about its effects on the tumor microenvironment. Quantity and composition of tumor infiltrating lymphocytes (TILs) are known to influence patients' prognosis but nRCTx-induced modifications are still unclear. We determined the composition of the immune cell infiltrate in rectal cancer after nRCTx and its influence on tumor regression, local recurrence rate and survival.
We investigated density and composition of tumor infiltrating CD3+ and CD8+ T-cells and the quantity and ratio of CD8+/GrzB+ T-cells to CD8+ T-cells in 130 rectal cancers after nRCTx compared to a cohort of 30 primarily resected rectal cancers. Furthermore, we analyzed 22 pretherapeutic rectal cancer biopsies, later treated with nRCTx and surgery to evaluate nRCTx-induced modifications of the tumor microenvironment.
The total numbers of CD3+ and CD8+ T-cells in tumor stroma (p < 0.001) and tumor epithelium (p < 0.001 CD3; 0.002 CD8) were significantly lower in rectal cancers after nRCTx compared to primarily resected cases, while the ratio of CD8+/GrzB+ T-cells to CD8+ T-cells was significantly increased in the nRCTx cohort (p < 0.001). In multivariate analyses, CD8+/GrzB+ T-cells in the tumor stroma were significantly associated with high regression grade and a lower likelihood of local recurrence (p = 0.029).
nRCTx modifies the tumor microenvironment of rectal cancer leading to a total decrease of TILs, but a relative increase in CD8+/GrzB+ T-cells in the tumor stroma. CD8+/GrzB+ T-cells may contribute to local tumor control and the better outcome.
KEYWORDS: rectal cancer, histological tumor regression, granzyme B (GrzB), neoadjuvant radiochemotherapy, tumor microenvironment
Introduction
Colorectal cancer (CRC) is the third most common cancer in men and the second most common in women in western countries. Worldwide an estimated 1.4 million new cases and about 693.300 deaths occurred in 2012,1 about a third were located in the rectum.2,3 Neoadjuvant radiochemotherapy (nRCTx) followed by total mesorectal excision (TME) constitutes the current standard of care for locally advanced rectal cancers (UICC II/III). Both, radiation (RTx) and chemotherapy (CTx) diminish the risk of local relapse. Furthermore, nRCTx leads to a decrease of the tumor mass, allowing for a higher rate of sphincter sparing surgical methods and a higher rate of R0-resections.4,5
The histological tumor regression (TRG) grade according to Dworak6 serves as a system to categorize therapy-induced effects within rectal cancers after nRCTx.7 Studies demonstrated the clinical relevance as a prognostic factor showing improved disease-free survival after pathologic response.8,9
The high mortality rate of nearly 33% in developed countries10 and the certainly not satisfactory five-year survival rate for CRC of still less than 60% demonstrate the need for optimized stage adapted therapy regimens in this type of cancer.
Over the last decade, significant progress has been made in the research of tumor immunology. Hanahan and Markman defined the term “tumor microenvironment”, which involves a range of recruited, non-neoplastic cells in addition to cancer cells.11 Tumor-infiltrating lymphocytes (TILs) are often found in epithelial tumors and apparently reflect an immune response against the tumor.12–15 In CRC, CD8+ cytotoxic T-cells but not CD3+ T-cells are associated with increased overall survival.16 Moreover, it is known that the composition of the immune cells within the tumor microenvironment of CRC is a well-defined predictor of patients' survival. Galon et al. introduced the evaluation of quantities of distinct immune cells in different regions of a tumor (center of the tumor (CT) and invasive margin (IM)) as the so-called “immunoscore”.17 The immunoscore has since then proven its value as a powerful prognostic marker in addition to the established TNM-System.18
While it is an established fact that the lack of effector enzymes and CD8+ T-cells in CRC is associated with adverse prognosis19 and that the extracellular secreted serine protease granzyme B (GrzB) plays an important role in the killing of tumor cells,20 little is known about the effector molecules within the cytotoxic CD8+-anti-tumor immune response.
Next to the unsolved identification of the tumorsuppressive effector molecules within CD8+-anti-tumor immune response, it is yet not fully understood which effects and alterations are triggered by neoadjuvant therapies.21,22
In this study, we determined the total number of T-lymphocytes (CD3+) and the number of cytotoxic T-cells (CD8+) in the tumor epithelium and the peritumoral stroma after nRCTx in 130 locally advanced rectal cancers. In addition, we determined the ratio of CD8+/GrzB+ to CD8+ T-cells in these tumors. Finally, the influence of the immune cell infiltrate on tumor regression, local recurrence rate and survival was determined.
In addition, we compared the results in neoadjuvantly treated rectal cancers to a cohort of 30 rectal cancers treated with surgery alone to elucidate changes in the tumor microenvironment induced by nRCTx.
Furthermore, we compared the T-cell density in pretherapeutic biopsies of 22 patients to the resection specimen after nRCTx to determine a possible predictive value of the immune cell infiltrate in preoperative biopsies.
Microsatellite instability was considered in both cohorts, since a high level of microsatellite instability (MSI-H) is associated with a significantly increased number of lymphocytes23 and a favorable prognosis.24
Material and methods
Patients
Reporting of the present study is in accordance with the REMARK guidelines.25 This is a retrospective study including 130 patients treated in the tertiary care University Hospital Carl Gustav Carus, Dresden, Germany with neoadjuvant radiochemotherapy (nRCTx) followed by surgery for rectal cancer between 2001 and 2013. From 22 of these cases pretherapeutic tumor biopsies were available. Additionally, a cohort of 30 primarily resected rectal cancer patients without nRCTx were matched according to TNM-stage and sex. The study was approved by the local institutional review board of the Faculty of Medicine of the Technische Universität Dresden. It was conducted in agreement with the Declaration of Helsinki, according to the ICH Harmonized Tripartite Guideline for Good Clinical Practice.
Table 1 lists the clinical characteristics of the study population for both the neoadjuvantly treated and the control cohort. Within the nRCTx-cohort were 88 men (67.7%) and 42 women (32.3%) with an average age of 61.0 (range 36 – 82) years. Gender distribution was similar in the control cohort with n = 20 male (66.7%) and n = 10 female (33.3%) patients but age was higher (median 67.6 years; p = 0.005) in control cohort.
Table 1.
Patients' characteristics.
| nRCTx (n = 130) |
control group (n = 30) |
||||
|---|---|---|---|---|---|
| n/median (range) | % | n/median (range) | % | p | |
| Sex | |||||
| male | 96 | 73.8 | 20 | 66.7 | n.s. |
| female | 34 | 26.2 | 10 | 33.3 | |
| Age (y) | 61.0 (36–82) | 67.6 (22–76) | |||
| ≤ 65 | 88 | 67.7 | 12 | 40.0 | 0.005 |
| > 65 | 42 | 32.3 | 18 | 60.0 | |
| NCTx | |||||
| 5-FU | 117 | 90.0 | — | — | — |
| others | 13 | 10.0 | — | — | — |
| Accumulated radiation dose [in Gy] | |||||
| 46.8 | 1 | 0.8 | — | — | — |
| 50.4 | 125 | 96.2 | — | — | — |
| 55.8 | 4 | 3 | — | — | — |
| Death | |||||
| Yes | 45 | 34.6 | 13 | 43.3 | n.s. |
| No | 85 | 65.4 | 17 | 56.7 | |
| Metastases in follow up | |||||
| Yes | 39 | 30.0 | 7 | 23.3 | n.s. |
| liver | 5 | 12.8 | 1 | 14.3 | |
| lung | 7 | 17.9 | 0 | 0.0 | |
| others | 6 | 15.4 | 0 | 0.0 | |
| No | 91 | 70.0 | 23 | 76.7 | |
| Local recurrence | |||||
| Yes | 10 | 7.7 | 2 | 6.7 | n.s. |
| No | 120 | 92.3 | 28 | 93.3 | |
| Follow up [months] | 59.8 (0.3–155.5) | 106.9 (12.9–163.4) | |||
Abbreviations: n.s.: not significant (P>0.05); 5-FU: 5-fluorouracil; nCTx: neoadjuvant chemotherapy; Gy: Gray.
Radiation was most frequently conducted with a fractioned schedule of 1.8 Gray (Gy) per fraction to a total radiation dose of 50.4 Gy. Four patients received a total radiation dose of 55.8 Gy and one patient 46.8 Gy. Chemotherapy regimens were based on either oral or intravenous 5-fluorouracil/leucovorin (5-FU). n = 117 patients received 5-FU alone, n = 12 patients additionally received Oxaliplatin, one patient was given 5-FU, Leucovorin (LV) and CPT-11, a combination also known as FOLFIRI plus Cetuximab.
The average follow up time was 59.8 (range 0.3 – 155.5) months in the nRCTx-cohort and 106.9 (12.9 – 163.4) months respectively in the control cohort. During follow up time n = 39 (30.0%) patients developed distant metastases in the nRCTx-cohort and n = 7 (23.3%) patients in the control cohort. 45 (34.6%) patients died in the nRCTx-cohort compared to 13 (43.3%) patients in the control cohort. Overall survival (OS) time was 59.8 (median; range 0.3 – 155.5) months in the nRCTx-cohort and recurrence free survival (RFS) time was 41.8 (median; range 0.3 – 155.5) months. In the control group OS spanned 106.9 (median; range 12.9 – 163.4) and RFS 86.2 (median; range 12.9 – 149.2) months, respectively.
Metastases were located either isolated in one organ (brain n = 2, distant lymph nodes n = 2, bone and ileum n = 1) or were present in multiple locations (n = 21) in the nRCTx-cohort. The control cohort showed one isolated liver metastasis and 6 patients with multiple organ metastases. Local recurrence appeared in n = 10 (7.6%) in the nRCTx-cohort and in n = 2 (6.7%) in the control cohort.
Histopathology and tumor regression grading
Histopathological staging was done according to UICC 2017 and tumor regression was evaluated using the Dworak grading system6 (see Table 6). One representative tumor block was chosen for immunohistochemical analyses. Pathological data of the study population for both the neoadjuvantly treated and the control cohort are listed in Table 2. Regarding pT, pN, UICC, perineural invasion and microsatellite status there was no difference between the nRCTx and the control cohort. Patients in the control cohort showed slightly higher proportions of pM1 (p = 0.043), lymphatic (p = 0.016) and blood vessel invasion (p = 0.003).
Table 6.
Tumor regression grade (TRG) system according to (6).
| Tumor Regression Grade | Histopathological findings |
|---|---|
| Grade 0: | No regression. |
| Grade 1: | Minor regression, residual tumor outgrowing fibrosis. |
| Grade 2: | Moderate regression, low tumor cell fibrosis in 26% to 50% of the tumor mass. |
| Grade 3: | Major regression, very few tumor cells in fibrotic tissue with or without mucous substance. |
| Grade 4: | Total regression, no viable tumor cells, only fibrotic mass. |
Tumor regression grade (TRG) system according to Dworak et al.
Table 2.
Pathological characteristics.
| nRCTx (n = 130) |
control group (n = 30) |
||||
|---|---|---|---|---|---|
| n/median (range) | % | n/median (range) | % | p | |
| ypT | |||||
| 1 | 7 | 5.4 | 3 | 10.0 | n.s. |
| 2 | 36 | 27.7 | 9 | 30.0 | |
| 3 a | 47 | 36.2 | 12 | 40.0 | |
| 3b | 35 | 26.9 | 6 | 20.0 | |
| 4 | 5 | 3.8 | 0 | 0.0 | |
| ypN | |||||
| 0 | 83 | 63.8 | 22 | 73.3 | n.s. |
| 1 | 31 | 23.8 | 5 | 16.7 | |
| 2 | 16 | 12.4 | 3 | 10.0 | |
| lymph nodes investigated | 14.0 (3–35) | 14.0 (4–56) | n.s. | ||
| CM | |||||
| 0 | 120 | 92.3 | 24 | 80.0 | 0.043 |
| 1 | 10 | 7.7 | 6 | 20.0 | |
| Microsatellites* | |||||
| stable | 123 | 94.6 | 28 | 93.3 | n.s. |
| instable | 5 | 3.8 | 2 | 6.7 | |
| L | |||||
| 0 | 108 | 83.1 | 19 | 63.3 | 0.016 |
| 1 | 22 | 16.9 | 11 | 36.7 | |
| V | |||||
| 0 | 122 | 92.3 | 22 | 73.3 | 0.003 |
| 1 | 10 | 7.7 | 8 | 26.7 | |
| Pn | |||||
| 0 | 98 | 75.4 | 21 | 70.0 | n.s. |
| 1 | 32 | 24.6 | 9 | 30.0 | |
| Tumor regression grade (TRG) | |||||
| 0 | 1 | 0.8 | — | — | — |
| 1 | 33 | 25.4 | — | — | — |
| 2 | 77 | 59.2 | — | — | — |
| 3 | 19 | 14.6 | — | — | — |
Abbreviations: n.s.: not significant (P>0.05); ypT: size and extent of primary tumor (y: posttherapy stage; p: pathological stage); ypN: involvement of regional lymph node (y: posttherapy stage; p: pathological stage); cM: presence or absence of distant metastasis (clinical stage); L: lymphatic invasion; V: vessel invasion; Pn: perineural invasion;
two cases were not evaluable because of insufficient tumor cell content.
Immunohistochemical immune cell characterization
Blocks were cut in serial sections of 1–2 µm thickness; sections were deparaffinized with BenchMark XT (Ventana Medical Systems, Inc., Oro Valley, AZ, USA) and then exposed to an antigen retrieval system. Two double IHC reactions were used: CD3+/ ki67 and CD8+/GrzB+. For the first double reaction, the primary specific CD3 antibody (clone 2GV6, ready-to-use; Ventana Medical Systems, Inc.) and the specific ki67 antibody (clone Mib-1 (1:50), Dako), for the second double reaction, the primary CD8 antibody (clone C8/144B (1:10), Dako) and the primary granzyme B antibody (clone GrzB-7 (1:10), Dako) were blended, followed by counterstaining with hematoxylin, dehydration and mounting of the slides.
Immune cell quantification
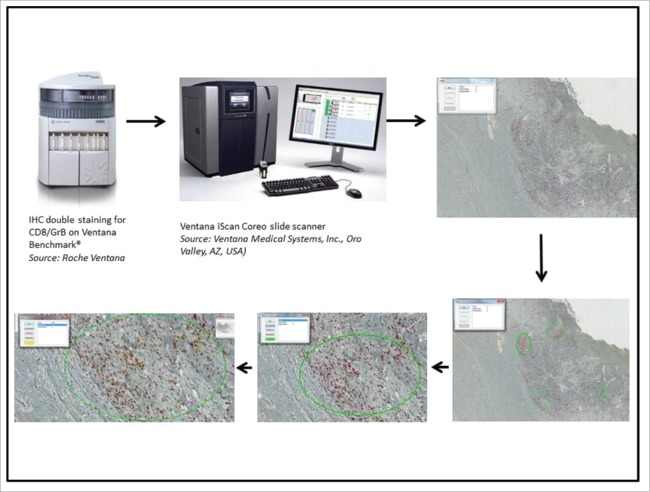
The immunoreactions for T-cell subpopulations were digitized with a Ventana iScan Coreo slide scanner (Ventana Medical Systems, Inc.) followed by a manual quantification of the different immune cells by a pathologist using Image viewer v. 3.1 (Ventana Medical Systems). Quantification was done on the whole tumor, differentiating intraepithelial lymphocytes from lymphocytes within the peritumoral stroma. For intraepithelial lymphocytes, number of TILs was counted per 100 cohesive tumor cells. To assess peritumoral stroma, the hot spot method was used. Three high power fields (0.237 µm2) with the highest density of immune cells within the peritumoral stroma were chosen and analyzed (see Fig. 1).
Figure 1.
Pathway of the immune cell quantification. IHC: immunohistochemistry.
Statistical analysis
Due to the retrospective and observational nature of the study, sample size was not chosen on the basis of power calculations. For univariate analyses categorical variables were compared using the χ2-test. Continuous variables were expressed as median and range and were compared – in accordance to their distribution – using the students't-test or Wilcoxon rank-sum test. Multivariate analyses were performed using a stepwise backward, logistic regression model adjusting for age, sex, and UICC. In univariate analysis, a p-value less than 0.05 was considered statistically significant and required to be entered into a multivariate model. No adjustment for multiple testing was applied. For survival analysis, dichotomous variables were compared using Kaplan-Meier curves and log rank-test. Continuous variables were dichotomized using the median as a cutoff value. All variables with a p-value < 0.1 were included in a stepwise backward, multivariate Cox-regression model adjusting for age, sex, ASA and TNM, testing every immune marker in a separate model. Overall survival, defined as time to death, and recurrence free survival (RFS), defined as time to metastasis or death, were investigated. Statistical analyses were carried out using IBM SPSS Statistics v23 (SPSS Inc.) and graphical illustration using GraphPad Prism v7 (Graph Pad Software Inc.).
Results
Immune cell characterization in the resection specimens
The number of CD3+ T-cells in tumor stroma and epithelium was significantly lower in the nRCTx-cohort than in the control cohort (p < 0.001). Almost equally, the number of CD8+ T-cells was lower after nRCTx in the tumor stroma (p < 0.001) and epithelium (p = 0.002). The number of CD8+/GrzB+ T-cells was also significantly lower in the tumor epithelium of the nRCTx-cohort (p = 0.028), whereas there was no significant difference in the tumor stroma between the two groups.
Consequently the ratio of stromal CD8+ T-cells with expression of granzyme B to all CD8+ T cells (CD8+/GrzB+//CD8+) was significantly higher in the nRCTx-cohort (p < 0.001) (Table 3 and Fig. 2).
Table 3.
Immune cell infiltrate.
| nRCTx (n = 130) | control group (n = 30) | ||
|---|---|---|---|
| n/median (range) | n/median (range) | p | |
| CD3+ stroma | 260.8 (51.3–719.3) | 526.3 (173.7–964.3) | <0.001 |
| CD3+ epithel | 3.0 (0–35) | 8.5 (1–55) | <0.001 |
| CD3+/MIB1+ stroma | 1.7 (0–37.7) | 0.7 (0–19.7) | n.s. |
| CD3+/MIB1+ epithel | 0.0 (0–4) | 0.0 (0–3) | n.s. |
| CD8+ stroma | 86.5 (0–451.3) | 154.0 (53.3–267.3) | <0.001 |
| CD8+ epithel | 2.0 (0–31) | 5.5 (0–48) | 0.002 |
| GrzB+/CD8+ stroma | 43.8 (0–240) | 48.8 (17.0–195.7) | n.s. |
| GrzB+/CD8+ epithel | 2.0 (0–29) | 5.0 (0–22) | 0.028 |
| CD8/GrzB//CD8 stroma | 0.52 (0–1) | 0.32 (0.21–0.78) | <0.001 |
| CD8/GrzB//CD8 epithel | 0.82 (0–1) | 0.57 (0–1) | n.s. |
Abbreviations: n.s.: not significant (P>0.05); CD: cluster of differentiation; MIB: mindbomb E3 ubiquitin protein ligase 1; GrzB: granzyme B.
Figure 2.

Boxplots of CD3+/CD8+ in tumor stroma (A) and in tumor epithel (B); Data distribution of CD8+/GrzB+ and CD8+ cells in tumor stroma (C) and tumor epithel (D). CD: cluster of differentiation; GrzB: granzyme B.
There was no significant difference between MSS and MSI tumors regarding the examined lymphocyte subpopulations.
Immune cell characterization in pretherapeutic biopsies
Differences in the immune expression were detected between pretherapeutic biopsies and tumor specimens after neoadjuvant therapy (see Table 4). In the epithelium and stroma of the tumor specimen a not significant decrease of CD3+ was detectable. Contradictory a significant increase in CD8+ (p = 0.036) and CD8+/GrzB+ (p = 0.011) in the tumor specimen was obvious only in the tumor stroma but not in the tumor epithelium. Since the increase in CD8+ T-cells was more pronounced than the increase in CD8+/GrzB+ T-cells, the ratio of CD8+/GrzB+ T-cells to CD8+ T-cells in the tumor stroma was lower after neoadjuvant therapy (p = 0.007).
Table 4.
Differences of immune expression in preoperative biopsy and tumor.
| Biopsy (median (range)) | Tumor (median (range)) | p | |
|---|---|---|---|
| CD3+ stroma | 387.3 (85.7–850.3) | 252.5 (94.7–719.3) | n.s. |
| CD3+ epithel | 9.0 (0.0–34.0) | 3.0 (0–23) | n.s. |
| CD3+/MIB1+ stroma | 4.8 (0.3–29.0) | 2.3 (0–19) | n.s. |
| CD3+/MIB1+ epithel | 0.5 (0.0–3.0) | 0.0 (0–4) | n.s. |
| CD8+ stroma | 65.0 (25.0–156.7) | 103.5 (21.7–253.7) | 0.036 |
| CD8+ epithel | 3.0 (0–17) | 2.0 (0–21) | n.s. |
| GrzB+/CD8+ stroma | 26.7 (12.7–79.7) | 38.5 (0–167.3) | 0.011 |
| GrzB+/CD8+ epithel | 2.0 (0–12) | 2.0 (0–19) | n.s. |
| GrzB/CD8//CD8 stroma | 0.60 (0–0.71) | 0.46 (0.27–0.59) | 0.007 |
| GrzB/CD8//CD8 epithel | 0.85 (0–1) | 0.77 (0–1) | n.s. |
Abbreviations: n.s.: not significant (P>0.05); CD: cluster of differentiation; MIB: mindbomb E3 ubiquitin protein ligase 1; GrzB: granzyme B.
Tumors showing good response to nRCTx (TRG3) showed a significantly higher number of intraepithelial CD8+ and CD8+/GrzB+ T-cells in their pretherapeutic biopsies than tumors with poor response (TRG 1) (p = 0.038 and p = 0.019, respectively). A statistically significant correlation between the number of circulating lymphocytes and the immune cell infiltrate was not detected in the serial blood counts measured at the time of biopsy or resection, respectively (Supplementary Table 1).
Immune cell infiltrate, tumor regression and local recurrence
In multivariate analyses, the total number of CD8+/GrzB+ T-cells as well as an increase in the ratio of CD8+/GrzB+ T-cells to CD8+ T-cells (OR: 18.189; 95% CI: 1.335–247.888; p = 0.030) in the tumoral stroma of the tumor specimen showed a significant association with higher TRG (TRG 2/3) (Odds ratio (OR): 1.012, 95% confidence interval (CI): 1.001–1.023; p = 0.029).
Higher tumor regression (TRG 2/3) resulted in a lower likelihood of local recurrence (OR 0.146; 95% CI 0.026–0.825; p = 0.029) in a multivariate logistic regression model.
Survival analyses
In univariate analyses of the neoadjuvantly treated patients, decreased overall survival (OS) was found to be significantly associated with age > 65y (p = 0.014), ypT3/4 (p = 0.039), ypN+ (p = 0.024), cM+ (p = 0.002), MSI-H (p = 0.015), perineural invasion (p = 0.005), and elevated CD3+ T-cells in tumor epithelium (p = 0.020).
Decreased recurrence free survival (RFS) was significantly associated with ypN+ (p = 0.008), cM+ (p < 0.001), lymphatic (p = 0.023) and perineural invasion (p = 0.019). Further analyses using Cox regression models showed that age > 65 years (OS HR: 2.18; 95% CI: 1.14–4.20; p = 0.019), positive nodal status (OS HR: 2.15; 95% CI: 1.05–4.40; p = 0.036) and presence of distant metastases (M1) (OS HR: 3.45; 95% CI: 1.40–8.48; p = 0.007; RFS HR: 4.54; 95% CI: 1.90–10.85; p = 0.001) were independent risk factors for poor OS or RFS (Table 5).
Table 5.
Survival analysis.
| OS |
RFS |
|||||
|---|---|---|---|---|---|---|
| univariate | multivariate |
univariate | multivariate |
|||
| p* | HR (95% CI) | p** | p* | HR (95% CI) | p** | |
| gender (m) | n.s. | n.s. | n.s. | n.s. | ||
| age (>65y) | 0.014 | 2.18 (1.14–4.20) | 0.019 | n.s. | n.s. | |
| ASA (3) | 0.005 | 3.37 (1.64–6.93) | 0.001 | n.s. | 1.84 (1.00–3.39) | 0.050 |
| ypT (3/4) | 0.039 | n.s. | n.s. | n.s. | ||
| ypN (+) | 0.024 | 2.15 (1.05–4.40) | 0.036 | 0.008 | n.s. | |
| cM (1) | 0.002 | 3.45 (1.40–8.48) | 0.007 | <0.001 | 4.54 (1.90–10.85) | 0.001 |
| MSI-H | 0.015 | n.s. | n.s. | n.s. | ||
| L (1) | n.s | n.s. | 0.023 | n.s. | ||
| V (1) | n.s. | – | n.s. | n.s. | ||
| Pn (1) | 0.005 | n.s. | 0.019 | n.s. | ||
| CD3+ stroma | n.s. | n.s. | n.s. | n.s. | ||
| CD3+ epithel | 0.020 | n.s. | n.s. | n.s. | ||
| CD3+/MIB1+ stroma | n.s. | n.s. | n.s. | n.s. | ||
| CD3+/MIB1+ epithel | n.s. | n.s. | n.s. | n.s. | ||
| CD8+ stroma | n.s. | n.s. | n.s. | n.s. | ||
| CD8+ epithel | n.s. | n.s. | n.s. | n.s. | ||
| GrzB+/CD8+ stroma | n.s. | n.s. | n.s. | n.s. | ||
| GrzB+/CD8+ epithel | n.s. | n.s. | n.s. | n.s. | ||
| GrzB+/CD8+//CD8+ stroma | n.s. | n.s. | n.s. | n.s. | ||
| GrzB+/CD8+//CD8+ epithel | n.s. | 0.51 (0.27–0.96) | 0.037 | n.s. | n.s. | |
Abbreviations: n.s.: not significant (P>0.05); OS: overall survival; RFS: recurrence free survival; HR: hazard ratio; CI: confidence interval; ASA: American Society of Anesthesiologists; ypT: size and extent of primary tumor (y: posttherapy stage; p: pathological stage); ypN: involvement of regional lymph node (y: posttherapy stage; p: pathological stage); cM (1): presence of distant metastasis (clinical stage); MSI-H: microsatellite instability – high; L: lymphatic invasion; V: vessel invasion; Pn: perineural invasion; CD: cluster of differentiation; MIB: mindbomb E3 ubiquitin protein ligase 1; GrzB: granzyme.
Finally, an elevated the ratio of CD8+/GrzB+ T-cells to CD8+ T-cells in tumor epithelium was found to be beneficial for OS in Cox regression model (HR: 0.051; 95% CI: 0.27 – 0.96; p = 0.037). The number of CD3+ T-cells in tumor epithelium failed to show its prognostic value in multivariate analyses.
Discussion
Several studies have shown that the immunological tumor microenvironment with its various cell components is of enormous importance for tumor prognosis. Lymphocytes in general and in particular CD8+/GrzB+ T-cells (CD8+/GrzB+) as cytotoxic effector T-cells are major players in tumor immunity and increased numbers of tumor-infiltrating and peritumoral CD8+/GrzB+ T-cells are associated with improved survival.12–15
In this study, we compared the immune cell infiltrate in neoadjuvantly treated rectal cancer to that in primarily resected rectal cancers to evaluate the modulation of the immune cell infiltrate – specifically regarding CD3+/ki67 and CD8+/GrzB+ T-cells – through neoadjuvant treatment.
For immune cell quantification, we chose the hot spot method and did not differentiate between different tumor areas. Previous studies showed a prognostic influence of tumor infiltrating lymphocytes in all areas enclosing tumor cells regardless of the specific tumor area.26 By using the hot spot method (see paragrah Immune cell quantification in pretherapeutic biopsies) we determined the areas with the highest immune cells densities as the relevant areas. Moreover, therapy-induced effects (e.g. fibrosis, necrosis and mucus lakes) hamper the determination of invasive margin vs center of tumor.
nRCTx of rectal adenocarcinoma alters significantly the peritumoral immune architecture, as it leads to a significant decrease of all CD3+ T-cells as well as to a decrease of CD8+ cytotoxic T-cells in tumor epithelium and tumor stroma. This finding is in line with the previous observation, that nRCTx has an immunosuppressive effect.27 However, in a comparative analysis of the total number of T-cells in the peritumoral stroma the decrease of the cytotoxic T-cell fraction (CD8) was less evident than the decrease of the whole T-cell population (CD3), indicating that CD8+ T-cells are more robust towards the nRCTx -induced cell stress.
As it is well known, that the antitumor immune response of CD8+ T-cells is mainly mediated by GrzB, the most abundant serine protease in human, we analyzed not only the number of CD8+ T-cells, but also the number of CD8+/GrzB+ T-cells.34
In the tumor stroma the ratio of CD8+/GrzB+ T-cells to CD8+ T-cells was significantly higher after nRCTx. This relative increase of potentially more effective immune cells indicates a modification of the peritumoral immune architecture through nRCTx towards a more potent capability in granzyme B- dependent tumor cell elimination.
Tumor regression grade (TRG) describes the histological effects of nRCTx on the tumor and its environment and is commonly used as a surrogate marker for local treatment response to nRCTx (Table 6). Several studies have shown that TRG acts as an independent prognostic factor.31 In our multivariate analyses we analyzed a significant association between a higher TRG (indicating a better pathologic response) and a higher number of CD8+/GrzB+ T-cells in the tumor stroma. In addition, there was also a significant increase in the ratio of CD8+GrzB+ to CD8+ T-cells in higher TRG. This finding is in line with studies describing the prominent role of granzyme B in local tumor suppression20 and supports the assumption, that nRCTx leads to a relatively up-regulation of highly effective cytotoxic T-cells.
Knowing the association between immune cell infiltrate and TRG in the resection specimen, the next question was whether we could predict the response to nRCTx of an individual tumor by investigating the immune cell infiltrate of the corresponding pretherapeutic biopsy.
In order to do that, we analyzed the correlation of the number CD8+/GrzB+ T-cells and the ratio of CD8+/GrzB+ T-cells to CD8+ T-cells in the preoperative biopsies with TRG and ypT-stage of the resection specimen. We found that tumors with small ypT stages (ypT1/2) after resection showed a significantly higher number of CD8+/GrzB+ T-cells in the tumor epithelium of the preoperative biopsies, which indicates a tumor-suppressive cytotoxic T-cell function and goes along with the assumption of granzyme B as a major effector molecule.
Furthermore, we could show a significant correlation between the number of intraepithelial CD8+ T-cells and CD8+/GrzB+ T-cells in the biopsy and the treatment response to nRCTx. High numbers of intraepithelial cytotoxic T-cells predicted a better treatment response to nRCTx using TRG as the surrogate marker. As this finding is based on only a small number of patients, it should certainly be re-examined in a prospective trial in order to optimize the treatment strategy for advanced rectal cancer as it may help to select patients for either nRCTx or a surgery first approach.
Regarding the tumor stroma, the comparison between pretherapeutic biopsies and resection specimens after nRCTx revealed a decrease of the CD3+-T-cell-infiltrate, and, interestingly, a significant increase of CD8+ T-cells and CD8+/GrzB+ T-cells in the resection specimens.
One reason for this finding might be the lower number of cytotoxic T-cells at the surface/apex of the tumors compared to the invasive margin and the tumor core of the resection specimen. Results of other studies showed, that CD8+ cytotoxic T-cells were primarily located around cancer cell nests and at the invasive margin28 but not at the luminal surface of a tumor. As tumor biopsies preferentially represent the luminal surface of a tumor, a lower number of CD8+ T-cells and CD8+/GrzB+ T-cells are represented, which may lead to our finding of a lower number of cytotoxic T-cells in the biopsies before nRCTx. Another reason might be the nRCTx-induced release of tumor antigens, leading to a positive up-regulation of cytotoxic T-cells.29 Conclusively, immune cells in tumor stroma of pretherapeutic biopsies do not qualify as predictive markers of tumor response, whereas intraepithelial T-cells might lend themselves as predictive markers of tumor response.
OS and PFS were associated with the known predictors such as high age, lymph node and distant metastases. Going beyond that, we showed in multivariate analyses that an elevated ratio of CD8+/GrzB+ T-cells in relation to all CD8+ T-cells in the tumor epithelium was significantly beneficial for OS (P = 0.037), which is in line with other malignancies.30 This result suggests that the modulation of the peritumoral immune cell architecture due to nRCTx has an enduring impact on the outcome of some patients. If our data on pretherapeutic biopsies prove to be reproducible in a prospective trial, these patients could be identified on the basis of the immune cell architecture in their therapy naive preoperative biopsy.
According to our data, nRCTx may not only lead to improved local tumor control31 but also to immune cell modulation. Antitumor activity induced by radiation can result in IFNγ production32 or in a CXCL16 release33 and therefore in antitumor effects. The results of our study suggest the immune cell modification and increase of granzyme B as a further antitumoral mechanism. The beneficial impact of neoadjuvant radiochemotherapy therefore seems not only to trigger the amount of tumor infiltrating lymphocytes but also modifies them to be more efficient in killing tumor cells.
In summary, nRCTx significantly decreases the absolute amount of the general CD3+ T-cell population and – less pronounced – also the amount of cytotoxic CD8+ T-cells, which indicates an immunosuppressive effect of this therapy. Interestingly, this “immunosuppressive” effect seems to be offset by a significant increase of the expression of granzyme B in the remaining CD8+ T-cells.
Supplementary Material
Disclosure of conflict of interest
The authors declare that they have no competing interests and all authors have read and approved the final manuscript.
References
- 1.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. eng. doi: 10.3322/caac.21262. PMID:25651787 [DOI] [PubMed] [Google Scholar]
- 2.Krebs in Deutschland 2011/2012. 10. Ausgabe Robert Koch-Institut und die Gesellschaft der epidemiologischen Krebsregister in Deutschland e.V. (Hrsg). Berlin, 2015. ISBN 978-3-89606-228-4. doi: 10.17886/rkipubl-2015-004 [DOI] [Google Scholar]
- 3.American Cancer Society Cancer Facts & Figures 2017. Atlanta, Ga 2017. [Available from: https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2017/cancer-facts-and-figures-2017.pdf]
- 4.Hafner MF, Debus J. Radiotherapy for Colorectal Cancer: Current Standards and Future Perspectives. Visc Med. 2016;32:172–177. eng. doi: 10.1159/000446486. doi: 10.1159/000446486. PMID:27493944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li Y, Wang J, Ma X, Tan L, Yan Y, Xue C, Hui B, Liu R, Ma H, Ren J. A Review of Neoadjuvant Chemoradiotherapy for Locally Advanced Rectal Cancer. Int J Biol Sci. 2016;12:1022–1031. eng. doi: 10.7150/ijbs.15438. doi: 10.7150/ijbs.15438. PMID:27489505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dworak O, Keilholz L, Hoffmann A. Pathological features of rectal cancer after preoperative radiochemotherapy. Int J Colorectal Dis. 1997;12:19–23. eng. doi: 10.1007/s003840050072. PMID:9112145 [DOI] [PubMed] [Google Scholar]
- 7.Thies S, Langer R. Tumor regression grading of gastrointestinal carcinomas after neoadjuvant treatment. Front Oncol. 2013;3:262. eng. doi: 10.3389/fonc.2013.00262. PMID:24109590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rodel C, Martus P, Papadoupolos T, Fuzesi L, Klimpfinger M, Fietkau R, Liersch T, Hohenberger W, Raab R, Sauer R, Wittekind C. Prognostic significance of tumor regression after preoperative chemoradiotherapy for rectal cancer. J Clin Oncol. 2005;23:8688–8696. eng. doi: 10.1200/JCO.2005.02.1329. PMID:16246976 [DOI] [PubMed] [Google Scholar]
- 9.Jakob C, Liersch T, Meyer W, Baretton GB, Schwabe W, Hausler P, Kulle B, Becker H, Aust DE. Prognostic value of histologic tumor regression, thymidylate synthase, thymidine phosphorylase, and dihydropyrimidine dehydrogenase in rectal cancer UICC Stage II/III after neoadjuvant chemoradiotherapy. Am J Surg Pathol. 2006;30:1169–1174. eng. doi: 10.1097/01.pas.0000213302.13435.6e. PMID:16931962 [DOI] [PubMed] [Google Scholar]
- 10.Cunningham D, Atkin W, Lenz H, Lynch HT, Minsky B, Nordlinger B, Starling N. 2010. Colorectal cancer. Lancet. 375:1030–1047. eng. doi: 10.1016/S0140-6736(10)60353-4. PMID:20304247 [DOI] [PubMed] [Google Scholar]
- 11.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. eng. doi: 10.1016/j.cell.2011.02.013. PMID:21376230 [DOI] [PubMed] [Google Scholar]
- 12.Gooden MJM, Bock GH de, Leffers N, Daemen T, Nijman HW. 2011. The prognostic influence of tumour-infiltrating lymphocytes in cancer: a systematic review with meta-analysis. Br J Cancer. 105:93–103. eng. doi: 10.1038/bjc.2011.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Denkert C, Loibl S, Noske A, Roller M, Muller BM, Komor M, Budczies J, Darb-Esfahani S, Kronenwett R, Hanusch C, et al.. Tumor-associated lymphocytes as an independent predictor of response to neoadjuvant chemotherapy in breast cancer. J Clin Oncol. 2010;28:105–113. eng. doi: 10.1200/JCO.2009.23.7370. PMID:19917869 [DOI] [PubMed] [Google Scholar]
- 14.Balermpas P, Rodel F, Rodel C, Krause M, Linge A, Lohaus F, Baumann M, Tinhofer I, Budach V, Gkika E, et al.. CD8+ tumour-infiltrating lymphocytes in relation to HPV status and clinical outcome in patients with head and neck cancer after postoperative chemoradiotherapy: A multicentre study of the German cancer consortium radiation oncology group (DKTK-ROG). Int J Cancer. 2016;138:171–181. eng. doi: 10.1002/ijc.29683. PMID:26178914 [DOI] [PubMed] [Google Scholar]
- 15.Leffers N, Gooden MJM, Jong RA de, Hoogeboom B-N, Hoor KA ten, Hollema H, Boezen HM, van der Zee AGJ, Daemen T, Nijman HW. Prognostic significance of tumor-infiltrating T-lymphocytes in primary and metastatic lesions of advanced stage ovarian cancer. Cancer Immunol Immunother. 2009;58:449–459. eng. doi: 10.1007/s00262-008-0583-5. PMID:18791714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mei Z, Liu Y, Liu C, Cui A, Liang Z, Wang G, Peng H, Cui L, Li C. Tumour-infiltrating inflammation and prognosis in colorectal cancer: systematic review and meta-analysis. Br J Cancer. 2014;110:1595–1605. eng. doi: 10.1038/bjc.2014.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Galon J, Costes A, Sanchez-Cabo F, Kirilovsky A, Mlecnik B, Lagorce-Pages C, Tosolini M, Camus M, Berger A, Wind P, et al.. 2006. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science. 313:1960–1964. eng. doi: 10.1126/science.1129139. PMID:17008531 [DOI] [PubMed] [Google Scholar]
- 18.Wittekind C, editor TNM-Klassifikation maligner Tumoren [Internet]. Achte Auflage. Weinheim: Wiley-VCH Verlag GmbH & Co. KGaA; 2017. 313 p. ISBN: 9783527807598. ger. [Google Scholar]
- 19.Daster S, Eppenberger-Castori S, Hirt C, Soysal SD, Delko T, Nebiker CA, Weixler B, Amicarella F, Iezzi G, Governa V, et al.. 2015. Absence of myeloperoxidase and CD8 positive cells in colorectal cancer infiltrates identifies patients with severe prognosis. Oncoimmunology. 4:e1050574. eng. doi: 10.1080/2162402X.2015.1050574. PMID:26587320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bots M, Medema JP. Granzymes at a glance. J Cell Sci. 2006;119:5011–5014. eng. doi: 10.1242/jcs.03239. PMID:17158907 [DOI] [PubMed] [Google Scholar]
- 21.Kuerer HM, Newman LA, Smith TL, Ames FC, Hunt KK, Dhingra K, Theriault RL, Singh G, Binkley SM, Sneige N, et al.. Clinical course of breast cancer patients with complete pathologic primary tumor and axillary lymph node response to doxorubicin-based neoadjuvant chemotherapy. J Clin Oncol. 1999;17:460–469. eng. doi: 10.1200/JCO.1999.17.2.460. PMID:10080586 [DOI] [PubMed] [Google Scholar]
- 22.Teng F, Meng X, Kong L, Mu D, Zhu H, Liu S, Zhang J, Yu J. Tumor-infiltrating lymphocytes, forkhead box P3, programmed death ligand-1, and cytotoxic T lymphocyte-associated antigen-4 expressions before and after neoadjuvant chemoradiation in rectal cancer. Transl Res. 2015;166:721–732.e1. eng. doi: 10.1016/j.trsl.2015.06.019. PMID:26209749 [DOI] [PubMed] [Google Scholar]
- 23.Kim JH, Kang GH. Molecular and prognostic heterogeneity of microsatellite-unstable colorectal cancer. World J Gastroenterol. 2014;20:4230–4243. eng. doi: 10.3748/wjg.v20.i15.4230. PMID:24764661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shia J, Ellis NA, Paty PB, Nash GM, Qin J, Offit K, Zhang X, Markowitz AJ, Nafa K, Guillem JG, et al.. Value of histopathology in predicting microsatellite instability in hereditary nonpolyposis colorectal cancer and sporadic colorectal cancer. Am J Surg Pathol. 2003;27:1407–1417. eng. doi: 10.1097/00000478-200311000-00002. PMID:14576473 [DOI] [PubMed] [Google Scholar]
- 25.McShane LM, Altman DG, Sauerbrei W, Taube SE, Gion M, Clark GM. REporting recommendations for tumour MARKer prognostic studies (REMARK). Br J Cancer. 2005;93:387–391. doi: 10.1038/sj.bjc.6602678. PMID:16106245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Roxburgh CSD, McMillan DC. The role of the in situ local inflammatory response in predicting recurrence and survival in patients with primary operable colorectal cancer. Cancer Treat Rev. 2012;38:451–466. eng. doi: 10.1016/j.ctrv.2011.09.001. PMID:21945823 [DOI] [PubMed] [Google Scholar]
- 27.Order SE. The effects of therapeutic irradiation on lymphocytes and immunity. Cancer. 1977;39:737–743. eng. doi: 10.1002/1097-0142(197702)39:2+%3c737::AID-CNCR2820390708%3e3.0.CO;2-M. PMID:300040 [DOI] [PubMed] [Google Scholar]
- 28.Richards CH, Roxburgh CSD, Powell AG, Foulis AK, Horgan PG, McMillan DC. The clinical utility of the local inflammatory response in colorectal cancer. Eur J Cancer. 2014;50:309–319. eng. doi: 10.1016/j.ejca.2013.09.008. PMID:24103145 [DOI] [PubMed] [Google Scholar]
- 29.Teng F, Meng X, Kong L, Mu D, Zhu H, Liu S, Zhang J, Yu J. Tumor-infiltrating lymphocytes, forkhead box P3, programmed death ligand-1, and cytotoxic T lymphocyte-associated antigen-4 expressions before and after neoadjuvant chemoradiation in rectal cancer. Transl Res. 2015;166:721. eng. doi: 10.1016/j.trsl.2015.06.019. PMID:26209749 [DOI] [PubMed] [Google Scholar]
- 30.Sato E, Olson SH, Ahn J, Bundy B, Nishikawa H, Qian F, Jungbluth AA, Frosina D, Gnjatic S, Ambrosone C, et al.. Intraepithelial CD8+ tumor-infiltrating lymphocytes and a high CD8+/regulatory T cell ratio are associated with favorable prognosis in ovarian cancer. Proc Natl Acad Sci U S A. 2005;102:18538–18543. eng. doi: 10.1073/pnas.0509182102. PMID:16344461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McCoy MJ, Hemmings C, Hillery S, Penter C, Bulsara MK, Zeps N, Platell CF. Neoadjuvant chemoradiotherapy for rectal cancer: how important is tumour regression? ANZ J Surg. 2015; eng. doi: 10.1111/ans.13394. PMID:26631340 [DOI] [PubMed] [Google Scholar]
- 32.Sauer R, Becker H, Hohenberger W, Rodel C, Wittekind C, Fietkau R, Martus P, Tschmelitsch J, Hager E, Hess CF, et al.. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N engl J Med. 2004;351:1731–1740. eng. doi: 10.1056/NEJMoa040694. PMID:15496622 [DOI] [PubMed] [Google Scholar]
- 33.Matsumura S, Wang B, Kawashima N, Braunstein S, Badura M, Cameron TO, Babb JS, Schneider RJ, Formenti SC, Dustin ML, Demaria S. Radiation-induced CXCL16 release by breast cancer cells attracts effector T-cells. J Immunol. 2008;181:3099–3107. eng. doi: 10.4049/jimmunol.181.5.3099. PMID:18713980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rousalova I, Krepela E, Granzyme B-induced apoptosis in cancer cells and its regulation (review). Int J Oncol. 2010;37(6):1361–78. eng. PMID:21042704 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.