ABSTRACT
Background: Undifferentiated pleomorphic sarcoma of the extremity and trunk (ET-UPS) presents a unique therapeutic challenge. Although immunotherapy has recently been employed in advanced soft tissue sarcoma, there is limited data characterizing the immune infiltrate in ET-UPS. Radiotherapy (RT) has been shown in other tumor types to promote tumor antigen release and enhance tumor-specific targeting by the adaptive immune system. The aim of this study was to 1) characterize the baseline immune infiltrate and 2) evaluate the effect of preoperative RT on the histologic appearance of and the immune infiltrate in ET-UPS. Methods: We identified 17 matched ET-UPS samples before and after RT. Immunohistochemistry was performed with CD8, CD4, PD-L1, PD1, CD3, CD163 and FoxP3 positive cells identified in all samples. Changes in the immune infiltrate following RT were examined. Results: There was a trend towards increased density of tumor infiltrating immune cells in ET-UPS following RT, with increases in median number of CD3 (158 vs 219 cells/mm2, p = 0.06), CD4 (3 vs 13 cells/mm2, p = 0.01), CD8 (55 vs 111 cells/mm2, p = 0.17), and FOXP3 (14 vs 25 cells/mm2, p = 0.23) positive cells. Interestingly, although PD-L1 was not expressed in any ET-UPS tumor at baseline, positive PD-L1 expression was observed in 21% (3/14) of tumors after RT (p = 0.07). Conclusion: An immune infiltrate is present in ET-UPS at the time of diagnosis, with a trend towards increased density of immune infiltrate and PD-L1 expression after RT. These data support prospectively evaluating immune checkpoint inhibitors with standard of care RT in the treatment of ET-UPS.
KEYWORDS: undifferentiated pleomorphic sarcoma, extremity sarcoma, immunotherapy, immune check point, radiation therapy
Introduction
Within the past decade, major advances have been made in cancer therapy through the use of immune checkpoint blockade – with FDA approval of therapies targeting the PD-1 pathway in the setting of stage IV disease across several different cancer types.1–5 These therapies are now being extended to different histologies, including soft tissue sarcoma (STS).
STS are a heterogeneous group of tumors originating from connective tissue with > 60 histologic subtypes.6,7 Biologically, STS consist of two distinct groups: genetically “simple” and “complex” tumors. Complex tumors, such as undifferentiated pleomorphic sarcoma (UPS), have mutational and copy number heterogeneity8 and express high levels of genes related to antigen presentation and T-cell infiltration.
The mainstay of treatment for primary localized STS of the extremities or trunk is surgical resection. Achieving complete surgical resection is of critical importance as surgical resection margins have been shown to influence both local recurrence-free survival (LRFS) and distant metastasis-free survival (DMFS) as well as disease specific survival (DSS).9 Additionally, radiation therapy (RT) has been shown to improve local disease control, whether given in the neoadjuvant or adjuvant setting and particularly in patients with large (>5 cm), intermediate-grade or high-grade tumors, or in patients with uncertain or positive surgical margins.10–14 There is currently, however, no standard approach for the assessment and reporting of the histologic appearance of STS following neoadjuvant RT.15
For patients with STS of the extremities or trunk, the 5-year overall survival (OS) is approximately 75%, with 20% developing local recurrence within that time frame across all STS histologies. The most common cause of death, however, is distant metastatic disease, which occurs in 30% of patients by 5 years.16–20 The most common STS histologic subtype occurring in the extremities or trunk is UPS, which is associated with inferior 5-year LRFS, DMFS, and DSS (81%, 56.8%, 60.1%) compared to the next most common subtypes arising in these locations (well differentiated liposarcoma 86.4%, 100%, 100%; myxoid liposarcoma 94%, 81.7%, 84.9%; myxofibrosarcoma 82.8%, 68.3%, 76.7%; and leiomyosarcoma 82.3%, 65.4%, 76.8%, respectively).20
Current therapies for the management of advanced sarcomas are primarily based on cytotoxic chemotherapy and have modest efficacy with considerable toxicity.21,22 With recent successes of immunotherapy across multiple solid tumors, there has been increasing interest in applying immunotherapy to the treatment of STS with a small but growing number of immunotherapy clinical trials in advanced sarcomas.22–26 However, a significant subpopulation of patients treated with immunotherapy will not respond. At baseline, both the tumor immune microenvironment and the poor antigenicity of the tumor allow it to escape immune recognition. Compared to immunologically active tumors such as melanoma, STS are relatively immunologically quiet or “cold.”27 However, highly mutated tumors, such as UPS, may provide multiple immunologic mutated protein targets. Indeed, one such immunotherapy trial, SARC028, an open-label, phase II trial (NCT02301039) of anti-PD-1 monotherapy utilizing pembrolizumab in patients with advanced bone and soft tissue sarcomas, recently reported promising activity in UPS with a 40% overall response rate (vs 18% in the overall STS cohort).28
Radiation therapy can induce increased antigenic expression, release pro-inflammatory cytokines that recruit immune cells, promote antigen cross-presentation, and induce tumor expression of death receptors.29,30 Used together, RT and immunotherapy may have synergistic effects, which are being explored in such cancers as lung cancer.31 Although immunotherapy has recently started to be employed in clinical trials of advanced STS including UPS, there is limited data characterizing the immune infiltrate in this histologic subtype. Our aim in this study is to characterize the baseline immune infiltrate in UPS of the extremities or trunk (ET-UPS) and evaluate the effect of preoperative RT on the histologic appearance and tumor immune microenvironment of these tumors.
Results
Patient characteristics
We identified 17 patients with primary, resectable UPS of the trunk or extremities who received neoadjuvant RT prior to surgical excision at The University of Texas MD Anderson Cancer Center and had tissue available prior to and following RT. All patients had unifocal disease and all patients underwent pretreatment biopsy followed by neoadjuvant RT and surgical resection. Patient demographics and tumor characteristics are listed in Table 1. Median age was 56.3 years (range 20.3 to 83.2 years). Median tumor size was 8.7 cm (range 5.3 to 21.7 cm). The majority of tumors were located in the extremity (58.8% lower extremity, 17.6% upper extremity); 23.5% were UPS of the trunk.
Table 1.
Clinico-Demographic and Treatment Characteristics of 17 Patients with Undifferentiated Pleomorphic Sarcoma of the Extremity and Trunk.
| Median (range, %) | |
|---|---|
| Age, years | 56.3 (20.3–83.2) |
| Gender | |
| Male | 11 (35%) |
| Female | 6 (65%) |
| Tumor size, cm | 8.7 (5.3–21.7) |
| Primary tumor location | |
| Trunk | 4 (23.5%) |
| Upper extremity | 3 (17.6%) |
| Lower extremity | 10 (58.8%) |
| Mitoses per 10 HPF | 7.5 (3–70) |
| Neoadjuvant therapy | |
| XRT only | 7 (41.2%) |
| XRT + chemotherapy | 10 (58.8%) |
HPF: high-power fields
XRT: radiation therapy
Treatment
Patients received preoperative RT to a dose of 5000 centigray (cGy) delivered over 25 fractions. Of the 17 patients, 10 (58.8%) additionally received neoadjuvant chemotherapy (Supplemental Table 1). A slight majority of patients (58.8%) demonstrated stable disease (41.2%) or partial response (17.6%) with neoadjuvant RT by RECIST (Table 2).
Table 2.
Outcomes of 17 Patients with Undifferentiated Pleomorphic Sarcoma of the Extremity and Trunk.
| Median (range, %) | |
|---|---|
| RECIST response to neoadjuvant XRT | |
| Partial response | 3 (17.6%) |
| Stable disease | 7 (41.2%) |
| Disease progression | 7 (41.2%) |
| Final resection margins | |
| R0 | 16 (94.1%) |
| R1 | 1 (5.9%) |
| Percent necrosis | 20 (0–90) |
| Percent hyalinization | 17.5 (0–95) |
| RFS, months | 36.6 (2.3–79.3) |
| 3-year RFS | 50% |
| OS, months | NR (10–90.8) |
| 3-year OS | 70% |
| Status at last follow-up | |
| No evidence of disease | 10 (60%) |
| Alive with disease | 2 (11.8%) |
| Dead of disease | 4 (23.5%) |
| Dead, cause unknown | 1 (5.9%) |
NR: not reached
OS: overall survival
R0: microscopic negative margins
R1: gross complete resection with positive microscopic margins
RFS: recurrence-free survival
XRT: radiation therapy
All patients underwent surgical resection of UPS following completion of neoadjuvant RT. All patients underwent a macroscopically complete (R0 or R1) resection, with the majority having negative pathologic resection margins (R0, 94.1%). Neoadjuvant treatment effect (tumor necrosis and hyalinization) was seen in 12 of 17 (70.5%) resected tumor specimens on pathologic review. Median percent tumor necrosis was 20% (range 0 to 90%) and median percent tumor hyalinization was 17.5% (range 5 to 95%).
Patient outcomes
Median follow-up was 56 months (range 10 to 90.8 months). Eight patients (47.1%) developed disease recurrence. All recurrences occurred at distant sites (8 pulmonary, 2 also developed brain metastases). Recurrence-free survival (RFS) at 3 years was 50.4%. Median RFS was 36.3 months (range 2.3 to 79.3 months). OS at 3 years was 70.1%. Median OS was not reached (range 10 to 90.8 months). At last follow-up, 10 patients (60%) had no evidence of disease, 2 (11.8%) were alive with disease, 4 (23.5%) were dead of disease, and 1 (5.9%) had died of unknown cause.
Histological assessment of treatment response
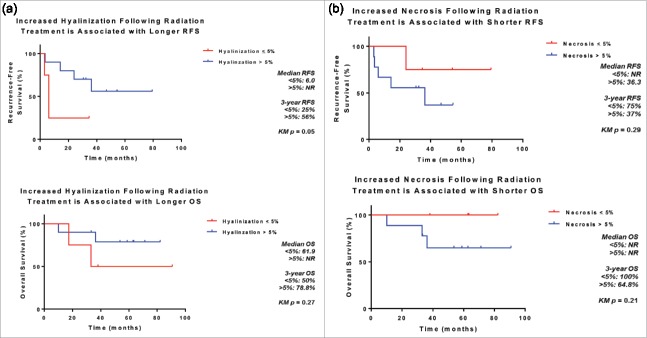
Extent of tumor necrosis and hyalinization/fibrosis were assessed in H&E sections of resected UPS treated with neoadjuvant RT (Fig. 1). We found significant rates of tumor necrosis and hyalinization following neoadjuvant RT (Table 2). Hyalinization was present in 93% of post-treatment samples, with a median extent of 17.5% of tumor (range 0%−95%). Necrosis was seen in 86% of tumors, with a median extent of 20% (range 0%−90%). Additionally, we found that patients whose tumors demonstrated >5% hyalinization following completion of neoadjuvant RT had better 3-year RFS (56% vs. 25%, p = 0.05) and a trend towards improved OS (79% vs. 50%, p = 0.27) (Fig. 2A). Increased necrosis, however, was associated with a trend toward worse 3-year RFS (37% vs 75%, p = 0.14) and OS (65% vs 100%, p = 0.21) (Fig. 2B), although small patient numbers limited the analysis and warrants further investigation.
Figure 1.
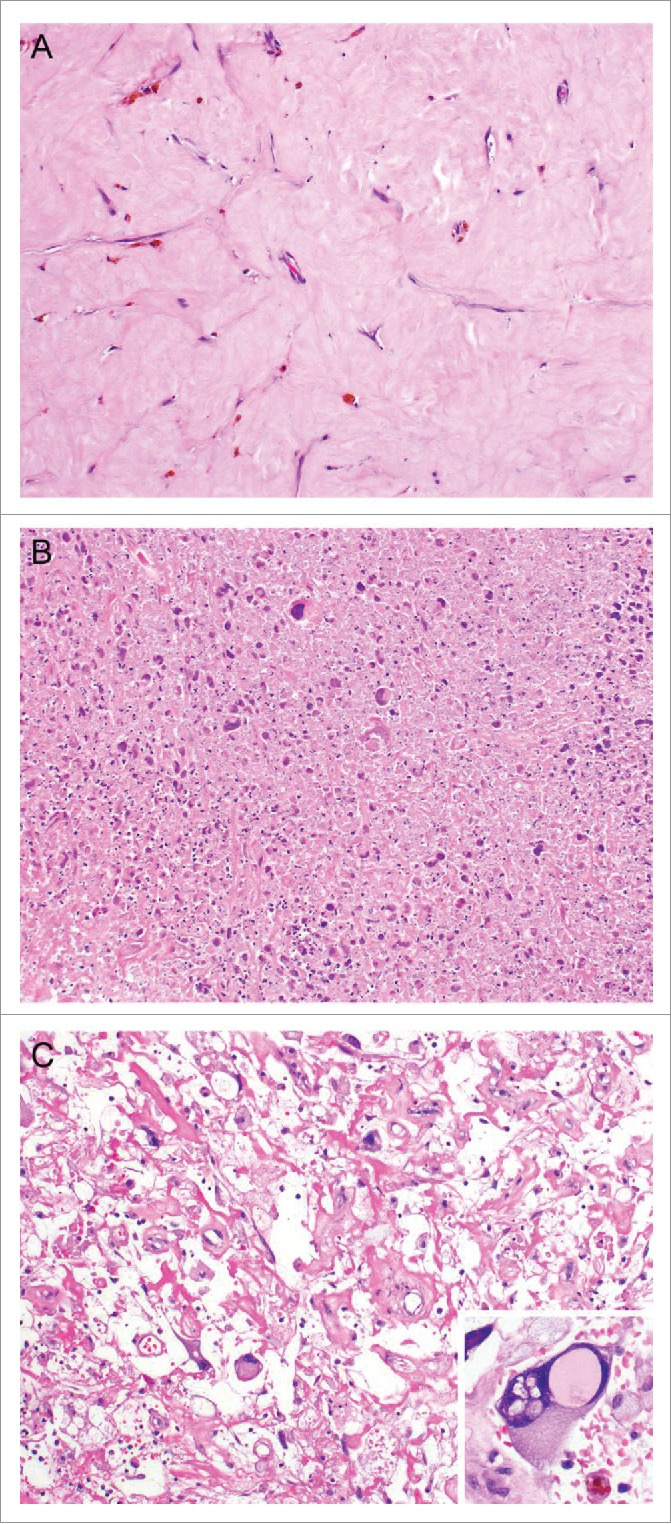
Examples of treatment effect following radiotherapy of undifferentiated pleomorphic sarcoma. Representative photographs of H+E stained undifferentiated pleomorphic sarcoma of the extremity and trunk demonstrating increased tumor necrosis and hyalinization following radiotherapy. (A) Tumor with extensive hyalinization (H&E, 100x). (B) Tumor with extensive necrosis with ghost and degenerative tumor cells (H&E, 100x). (C) Tumors could also have a mix of both necrosis and hyalinization (H&E, 100x). Cytological changes consistent with treatment effect (inset, H&E, 200x) was also present in this sample.
Figure 2.
Association between treatment effect following radiotherapy and survival. Increased hyalinization (A) and decreased necrosis (B) of undifferentiated pleomorphic sarcomas following radiotherapy are associated with improved recurrence-free and overall survival following surgical resection.
Characterizing the immune infiltrate
We next sought to characterize the immune infiltrate in ET-UPS by quantifying the density of tumor-infiltrating immune cells such as CD4 and CD8 positive T cells, regulatory T cells (Tregs), and monocytes/macrophages by staining for expression of CD4, CD8, FoxP3, and CD163, respectively, in biopsies of UPS obtained prior to initiation of neoadjuvant treatment (Fig. 3). Additionally, we evaluated for expression of PD-1, a T cell surface expressed negative regulator of tumor infiltrating T cells, as well as expression of its ligand, PD-L1, by UPS tumors. To gain insight into the effect of RT on the immune infiltrate in UPS, we compared the density of tumor-infiltrating immune cells expressing the above described immune markers in surgically resected specimens to pre-treatment biopsies (Fig. 4).
Figure 3.
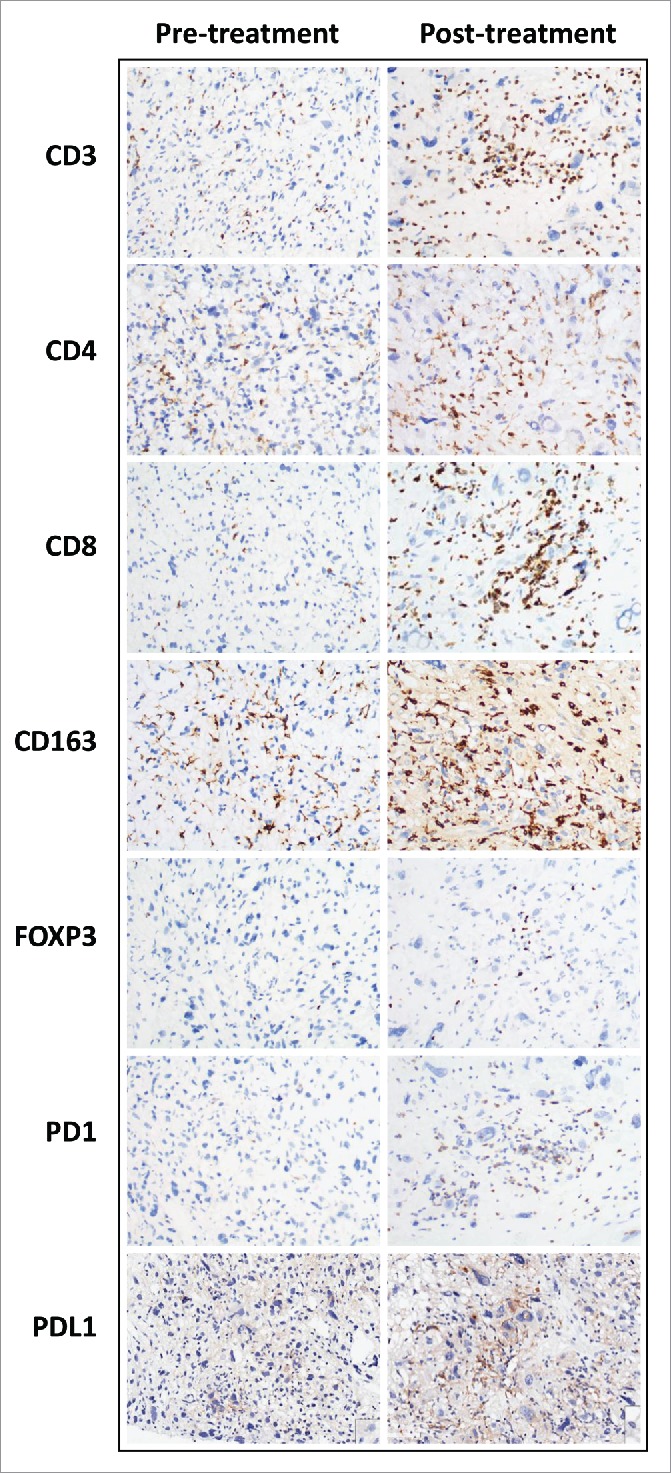
Comparison of immune infiltrate immunohistochemical studies in a pre- and post-treatment undifferentiated pleomorphic sarcoma. Representative photographs of immunostained undifferentiated pleomorphic sarcoma of the extremity and trunk. Increased CD3, CD4, CD8, CD163, FOXP3 and PD1 positive cells were seen in the post treatment specimen. Tumoral PD-L1 expression was increased.
Figure 4.
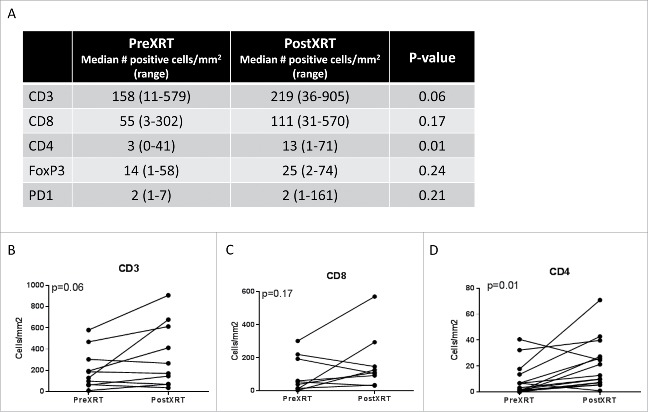
Quantification of the change in the immune infiltrate following radiotherapy of undifferentiated pleomorphic sarcomas of the extremity and trunk. (A) Median CD3, CD8, CD4, FoxP3, and PD-1 positive cells prior to and following radiotherapy; (B) change in the number of CD3 positive tumor infiltrating cells, paired samples by patient; (C) change in the number of CD8 positive tumor infiltrating cells, paired samples by patient; (D) change in the number of CD4 positive tumor infiltrating cells, paired samples by patient.
Overall, there was a trend towards increased density of tumor infiltrating immune cells in ET-UPS with RT. Following neoadjuvant RT, UPS tumors demonstrated an increased median number of tumor infiltrating immune cells expressing CD3 (158 vs 219 cells/mm2, p = 0.06), CD4 (3 vs 13 cells/mm2, p = 0.01), CD8 (55 vs 111 cells/mm2, p = 0.17), and FOXP3 (14 vs 25 cells/mm2, p = 0.23) positive cells. While there was an increase in CD163 (1363 vs 2011 cells/mm2, p = 0.76) between pre and post-treatment specimens, this was not statistically significant. Additionally, although PD-L1 was not expressed in any UPS tumor at baseline prior to RT, following RT PD-L1 expression was observed in 21% (3/14) of tumors (p = 0.07).
To evaluate whether neoadjuvant chemotherapy may impact the immune infiltrate in UPS, we compared the density of tumor-infiltrating immune cells between patients who received neoadjuvant RT alone (7/17, 41%) and those who received neoadjuvant chemotherapy and RT (10/17, 59%) (Supplemental Table 2). Despite small cohort size and heterogenous neoadjuvant chemotherapy regimens administered (Supplemental Table 1), there was no statistical difference between patients who received neoadjuvant RT alone versus neoadjuvant chemotherapy and RT both with respect to the immune infiltrate at baseline and after neoadjuvant therapy. The exception may be CD163 where the median number of positive staining cells following neoadjuvant therapy appears to be lower among patients who received neoadjuvant chemotherapy and RT compared to neoadjuvant RT alone. Further studies are needed to validate and investigate this finding given lower sample numbers. Additionally, despite small sample number, there overall appears to remain a trend towards increased density of tumor infiltrating immune cells in UPS following neoadjuvant RT, regardless of whether neoadjuvant chemotherapy had been administered.
Discussion
In this study, we profiled the immune infiltrate in UPS of the extremity or trunk acquired from 17 patients at baseline and following completion of RT. We show that tumor infiltrating immune cells are present at baseline and that following RT there is a trend towards increased density of the immune infiltrate, as represented by CD3, CD4, CD8, FOXP3 and PD-1 immunohistochemical staining. In addition, we demonstrate that although PD-L1 is not expressed in UPS at baseline, expression was induced with RT in a significant minority (21%) of patients. These data suggest that RT may change the tumor immune microenvironment and potentially enhance the efficacy of immunotherapies in UPS. Therefore, prospective evaluation of immune checkpoint inhibitors with standard of care RT in the treatment UPS and other STS histologic subtypes is warranted.
While the primary aim of this study was to profile the immune infiltrate of ET-UPS and to characterize the impact of RT on tumor infiltrating immune cells, we also wished to characterize the histologic appearance of ET-UPS following neoadjuvant RT, as there is currently no standard approach for the assessment and reporting of STS histologic appearance after neoadjuvant therapy.15 Indeed, prior to undertaking a planned prospective clinical trial to explore the potential role of immune checkpoint blockade with standard of care RT for ET-UPS, it is critical for us to understand the histologic appearance of ET-UPS after neoadjuvant RT alone. As reported in other STS subtypes, we found that RT was associated with significant rates of both tumor necrosis as well as treatment effect characterized by hyalinization and fibrosis in ET-UPS.32,32,33 Despite the small cohort of patients examined in this study, tumor necrosis following neoadjuvant RT was associated with shorter RFS and worse OS. Whether this is due to necrosis from treatment effect or tumor necrosis that could be associated with higher FNCLCC grade cannot be determined in these post-treatment samples. Past studies have shown various associations, both inferior and superior, between outcome after RT and tumor necrosis, perhaps partially due to imprecise distinctions of hyalinization and active necrosis.15,34–38 Conversely, we found that hyalinization/fibrosis following RT was associated with improved RFS and OS. These are similar to results recently reported by Schaefer et al in their single institution experience of 100 patients with primary localized STS of the extremity or trunk treated with preoperative RT followed by surgical resection.15 UPS represented a minority of tumors in this cohort. Similar to our results, the authors found a significant association between presence of radiation treatment effect and improved RFS (HR 0.48, p = 0.006) and OS (HR 0.36, p = 0.02) on multivariate analysis, with increasing extent of hyalinization and/or fibrosis associated with increasing 5-year RFS and 5-year OS.15 They did not detect an association between outcome and necrosis.
STS are generally not considered to be highly immunogenic malignancies and thus it is encouraging that we and others have identified the presence of tumor infiltrating immune cells in STS. They tend to have low total mutational burdens and high copy number alterations relative to most other solid tumors. In the present study, we demonstrate the presence of tumor infiltrating immune cells specifically in UPS of the extremity and trunk. We show that a variety of T cells (CD3+), including cytotoxic (CD8+), helper (CD4+) and regulatory (FOXP3+) T cells, as well as those bearing the checkpoint surface receptor PD-1, are present as components of the immune infiltrate in UPS of the extremity and trunk. These results are concordant with those recently published by others in various sarcoma subtypes, including osteosarcoma,39 synovial sarcoma,40 and chondrosarcoma,41 as well as across mixed histologic subtypes.8,42,43
Interestingly, there have been mixed reports regarding expression of PD-L1, one of the ligands for PD-1, by sarcomas.8,42,44,45 Pollack et al recently reported that UPS exhibited the highest levels of PD-L1 and PD-1 expression on immunohistochemistry among their cohort of STS,8 while conversely, D'Angelo et al reported PD-L1 tumor expression to be uncommon in sarcoma.42 Some groups have reported an association of PD-L1 expression with poorer prognosis and more aggressive disease.22,43 In this study, we found that none of the UPS tumors expressed PD-L1 at baseline, however PD-L1 expression was detected and increased following RT (in 21% of cases). Furthermore, published literature in other tumor types would suggest that lack of PD-L1 expression does not predict failure of response to anti-PD-1 therapy.46 Thus, the role of the PD-1/PD-L1 axis and potential efficacy of checkpoint blockade in STS, and UPS specifically, remains to be elucidated.
We observed an overall trend towards increased density of tumor infiltrating immune cells in UPS following RT compared to matched pretreatment tumors. Additionally, RT is correlated with increased tumor expression of PD-L1 in 21% of patients. Indeed, there have been numerous reports in the preclinical and clinical literature to suggest potential synergy between RT and immunotherapy.29,30,47–55 In 2011, Sharma et al reported increased expression of cancer testis antigens in human carcinoma cell lines as well as human tumor specimens upon RT and additionally found increased immune cell infiltrate in radiation treated human tumors.48 The same authors in 2013 compared RNA and protein expression of a panel of immune-associated markers in 38 matched pairs of sarcoma specimens obtained from patients at baseline and following RT and found similar findings in STS.49 These studies as well as the current study suggest that RT may be an effective immunoadjuvant.56
Our study is not without limitations. The sample size in the current study is small, however similar findings have been observed in other tumor types and efforts to expand this cohort are ongoing. Additionally, all post-RT samples were obtained after the completion of neoadjuvant RT at the time of definitive surgical resection. The influx or increase in tumor infiltrating immune cells may occur earlier following initiation of RT and the optimal time to obtain tumor biopsies to capture a change in the tumor immune microenvironment may be earlier during the time course of RT.
In conclusion, our current analysis demonstrates that tumor infiltrating immune cells are present in the UPS microenvironment and suggest that RT may further increase the density of these cells. Although we found that PD-L1 was not expressed in UPS at baseline presentation, PD-L1 expression was induced following RT in a significant subset of patients. These data support evaluating the role of immunotherapy for the treatment of UPS in conjunction with standard of care RT.
Materials and methods
Patient selection
Patients with primary UPS of the trunk or extremities who had received neoadjuvant RT prior to surgical resection between 2009 and 2012 at The University of Texas MD Anderson Cancer Center were identified from the Department of Radiation Oncology database. Patients were included if both pre-RT biopsy and post-RT surgical specimen were available for immunohistochemical stains and analysis. Preoperative RT was performed to a median dose of 50 Gy in all patients administered over 25 fractions.
Demographic, clinical, treatment, and outcome variables
Patient and tumor-related variables were collected, including age at diagnosis, patient gender, tumor size and location, mitoses per high power field, neoadjuvant treatment regimens, and surgical resection margins. Clinical response to neoadjuvant treatment was assessed radiographically using RECIST 1.1 criteria. Pathologic response to neoadjuvant treatment (RT alone or RT plus chemotherapy) was assessed by quantifying the percent tumor necrosis and hyalinization observed in surgically resected UPS tumors.
Data regarding last follow-up visit, disease recurrence, and death were recorded. Length of follow-up was defined as the time from data of biopsy to date of last follow-up. Patients whose cause of death was clearly attributed to their disease were classified as “dead of disease.” Patients for whom cause of death was either unknown or known but not attributable to their disease were classified as “dead of other cause or cause unknown.” RFS was defined as the time from date of surgical resection to date of first recurrence. For patients alive and without documentation of recurrence or progression, follow-up was censored at the date of last disease assessment. OS was defined as the time from date of biopsy to death (event), or censored at last known date of survival.
Pathologic review
H&E slides of pre-treatment and post-treatment specimens were reviewed by pathologists who specialize in bone and soft tissue tumors (WLW, AJL, JWT). Diagnosis of undifferentiated pleomorphic sarcoma (formerly known as malignant fibrous histiocytoma) was confirmed using criteria established as a diagnosis of exclusion per the criteria of the 2013 WHO Classification of Tumours of Soft Tissue and Bone. In post treatment specimens, the percentage present of certain features related to treatment effect was assessed. Histological features of treatment effect include decreased cellularity, hyalinization (dense collagen deposition), tumor necrosis (though this is impossible to distinguish from tumor necrosis associated with higher grade tumors in many post treatment cases), and cytological changes (enlarged cytoplasm with enlarged bizarre nuclei with smudgy chromatin and sometimes pseudo-nuclear vacuoles). Many cases had variable hyalinization and necrosis. The percentage of how much treatment effect was hyalinization or necrosis was estimated.
Immunohistochemistry
Four micron unstained slides were prepared from representative whole section formalin-fixed paraffin-embedded tumor blocks (both pre-treatment and post treatment samples). Immunohistochemical staining was performed using an autostainer (Bond Max, Leica BioSystems) for CD3 (Dako, 1:100), CD4 (Leica, clone 4B12, 1:80), CD8 (Thermo Scientific, clone C8/144B, 1:25), CD163 (Leica, clone 10D6, 1:100), FOXP3(BioLegend, clone 206D), PD1(Abcam, clone EPR4877(2), 1:250) and PD-L1 (Dako, clone 22C3, FDA-approved assay).
Image analysis
Image analysis was performed using Aperio analytic image software (Lecia Biosystems). Lymphocytic infiltrates (CD3, CD4, CD8, FOXP3, PD-1) and histiocytic infiltrate (CD163) were quantitated as density (Number/mm2). Five representative 1 mm2 fields were assessed and averaged for final density. PD-L1 expression was scored as percentage of membranous tumoral labeling (of any intensity).
Statistical analysis
Statistical tests were performed with SPSS Statistics 23 (IMB SPSS, Chicago, IL, USA). For all analyses, the Student's t test was used and a p-value <0.05 was considered significant. Survival analyses were performed by Kaplan-Meier method.
Supplementary Material
Acknowledgments
The authors would like to thank Davis R. Ingram, Khalida Wani, and Samia Khan (Department of Pathology, The University of Texas MD Anderson Cancer Center, Houston, TX). This work was supported in part by K12 Paul Calabresi Career Development Award for Clinical Oncology (CLR), the Amschwand Sarcoma Cancer Foundation (WLW, AJL), as well as a cancer center support grant for The University of Texas MD Anderson Cancer Center (P30CA016672).
Disclosures
None
Synopsis
An immune infiltrate is present at baseline in undifferentiated pleomorphic sarcoma (UPS). Following radiotherapy, there is a trend towards increased density of immune infiltrate and PD-L1 expression. These data support evaluating immune checkpoint inhibitors in combination with radiotherapy in UPS.
References
- 1.Hodi FS, O'Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, Gonzalez R, Robert C, Schadendorf D, Hassel JC, et al.. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363(8):711–723. doi: 10.1056/NEJMoa1003466. PMID:20525992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brahmer J, Reckamp KL, Baas P, Crinò L, Eberhardt WE, Poddubskaya E, Antonia S, Pluzanski A, Vokes EE, Holgado E, et al.. Nivolumab versus Docetaxel in Advanced Squamous-Cell Non-Small-Cell Lung Cancer. N Engl J Med. 2015;373(2):123–135. doi: 10.1056/NEJMoa1504627. PMID:26028407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Garon EB, Rizvi NA, Hui R, Leighl N, Balmanoukian AS, Eder JP, Patnaik A, Aggarwal C, Gubens M, Horn L, et al.. Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med. 2015;372(21):2018–2028. doi: 10.1056/NEJMoa1501824. PMID:25891174. [DOI] [PubMed] [Google Scholar]
- 4.Robert C, Thomas L, Bondarenko I. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med. 2011;364(26):2517–2526. doi: 10.1056/NEJMoa1104621. PMID:21639810. [DOI] [PubMed] [Google Scholar]
- 5.Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD, Sosman JA, Atkins MB, et al.. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366(26):2443–2454. doi: 10.1056/NEJMoa1200690. PMID:22658127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brennan MF, Antonescu CR, Moraco N, Singer S. Lessons learned from the study of 10,000 patients with soft tissue sarcoma. Ann Surg. 2014;260(3):416–421. doi: 10.1097/SLA.0000000000000869. PMID:25115417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cormier JN, Pollock RE. Soft tissue sarcomas. CA Cancer J Clin. 54(2):94–109. doi: 10.3322/canjclin.54.2.94. PMID:15061599. [DOI] [PubMed] [Google Scholar]
- 8.Pollack SM, He Q, Yearley JH, et al.. T-cell infiltration and clonality correlate with programmed cell death protein 1 and programmed death-ligand 1 expression in patients with soft tissue sarcomas. Cancer. May 2017:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu C-YC-L, Yen C, Chen W-M, Chen TH, Chen PC, Wu HT, Shiau CY, Wu YC, Liu CL, Tzeng CH. Soft tissue sarcoma of extremities: the prognostic significance of adequate surgical margins in primary operation and reoperation after recurrence. Ann Surg Oncol. 2010;17(8):2102–2111. doi: 10.1245/s10434-010-0997-0. PMID:20217247. [DOI] [PubMed] [Google Scholar]
- 10.Alektiar KM, Velasco J, Zelefsky MJ, Woodruff JM, Lewis JJ, Brennan MF. Adjuvant radiotherapy for margin-positive high-grade soft tissue sarcoma of the extremity. Int J Radiat Oncol Biol Phys. 2000;48(4):1051–1058. doi: 10.1016/S0360-3016(00)00753-7. PMID:11072162. [DOI] [PubMed] [Google Scholar]
- 11.Delaney TF, Kepka L, Goldberg SI, Hornicek FJ, Gebhardt MC, Yoon SS, Springfield DS, Raskin KA, Harmon DC, Kirsch DG, et al.. Radiation therapy for control of soft-tissue sarcomas resected with positive margins. Int J Radiat Oncol Biol Phys. 2007;67(5):1460–1469. doi: 10.1016/j.ijrobp.2006.11.035. PMID:17394945. [DOI] [PubMed] [Google Scholar]
- 12.Tiong SS, Dickie C, Haas RL, O'Sullivan B. The role of radiotherapy in the management of localized soft tissue sarcomas. Cancer Biol Med. 2016;13(3):373–383. doi: 10.20892/j.issn.2095-3941.2016.0028. PMID:27807504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Larrier NA, Czito BG, Kirsch DG. Radiation Therapy for Soft Tissue Sarcoma: Indications and Controversies for Neoadjuvant Therapy, Adjuvant Therapy, Intraoperative Radiation Therapy, and Brachytherapy. Surg Oncol Clin N Am. 2016;25(4):841–860. doi: 10.1016/j.soc.2016.05.012. PMID:27591502. [DOI] [PubMed] [Google Scholar]
- 14.O'Sullivan B, Davis AM, Turcotte R, Bell R, Catton C, Chabot P, Wunder J, Kandel R, Goddard K, Sadura A, et al.. Preoperative versus postoperative radiotherapy in soft-tissue sarcoma of the limbs: a randomised trial. Lancet. 2002;359(9325):2235–2241. doi: 10.1016/S0140-6736(02)09292-9. PMID:12103287. [DOI] [PubMed] [Google Scholar]
- 15.Schaefer I-M, Hornick JL, Barysauskas CM, Raut CP, Patel SA, Royce TJ, Fletcher CDM, Baldini EH. Histologic Appearance After Preoperative Radiation Therapy for Soft Tissue Sarcoma: Assessment of the European Organization for Research and Treatment of Cancer-Soft Tissue and Bone Sarcoma Group Response Score. Int J Radiat Oncol Biol Phys. 2017;98(2):375–383. doi: 10.1016/j.ijrobp.2017.02.087. PMID:28463157. [DOI] [PubMed] [Google Scholar]
- 16.Coindre JM, Terrier P, Bui NB, Bonichon F, Collin F, Le Doussal V, Mandard AM, Vilain MO, Jacquemier J, Duplay H, et al.. Prognostic factors in adult patients with locally controlled soft tissue sarcoma. A study of 546 patients from the French Federation of Cancer Centers Sarcoma Group. J Clin Oncol. 1996;14(3):869–877. doi: 10.1200/JCO.1996.14.3.869. PMID:8622035. [DOI] [PubMed] [Google Scholar]
- 17.Gronchi A, Lo Vullo S, Colombo C, Collini P, Stacchiotti S, Mariani L, Fiore M, Casali PG. Extremity soft tissue sarcoma in a series of patients treated at a single institution: local control directly impacts survival. Ann Surg. 2010;251(3):506–511. doi: 10.1097/SLA.0b013e3181cf87fa. PMID:20130465. [DOI] [PubMed] [Google Scholar]
- 18.Italiano A, Le Cesne A, Mendiboure J, Blay JY, Piperno-Neumann S, Chevreau C, Delcambre C, Penel N, Terrier P, Ranchere-Vince D, et al.. Prognostic factors and impact of adjuvant treatments on local and metastatic relapse of soft-tissue sarcoma patients in the competing risks setting. Cancer. 2014;120(21):3361–3369. doi: 10.1002/cncr.28885. PMID:25042799. [DOI] [PubMed] [Google Scholar]
- 19.Pisters PW, Leung DH, Woodruff J, Shi W, Brennan MF. Analysis of prognostic factors in 1,041 patients with localized soft tissue sarcomas of the extremities. J Clin Oncol. 1996;14(5):1679–1689. doi: 10.1200/JCO.1996.14.5.1679. PMID:8622088. [DOI] [PubMed] [Google Scholar]
- 20.Smith HG, Memos N, Thomas JM, Smith MJF, Strauss DC, Hayes AJ. Patterns of disease relapse in primary extremity soft-tissue sarcoma. Br J Surg. 2016;103(11):1487–1496. doi: 10.1002/bjs.10227. PMID:27503444. [DOI] [PubMed] [Google Scholar]
- 21.Ratan R, Patel SR. Chemotherapy for soft tissue sarcoma. Cancer. 2016;18(6):604–610. [DOI] [PubMed] [Google Scholar]
- 22.Burgess M, Tawbi H. Immunotherapeutic approaches to sarcoma. Curr Treat Options Oncol. 2015;16(6):26. doi: 10.1007/s11864-015-0345-5. PMID:25975445. [DOI] [PubMed] [Google Scholar]
- 23.Lim J, Poulin NM, Nielsen TO. New strategies in sarcoma: Linking genomic and immunotherapy approaches to molecular subtype. Clin Cancer Res. 2015;21(21):4753–4759. doi: 10.1158/1078-0432.CCR-15-0831. PMID:26330427. [DOI] [PubMed] [Google Scholar]
- 24.Mitsis D, Francescutti V, Skitzki J. Current Immunotherapies for Sarcoma: Clinical Trials and Rationale. Sarcoma. 2016;2016:9757219. doi: 10.1155/2016/9757219. doi: PMID:27703409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Angelo SPD. Manipulating the Immune System With Checkpoint Inhibitors for Patients With Metastatic Sarcoma. ASCO Educ B. 2016:e558–564. [DOI] [PubMed] [Google Scholar]
- 26.Paoluzzi L, Cacavio A, Ghesani M, Karambelkar A, Rapkiewicz A, Weber J, Rosen G. Response to anti-PD1 therapy with nivolumab in metastatic sarcomas. Clin Sarcoma Res. 2016;6(1):24. doi: 10.1186/s13569-016-0064-0. PMID:28042471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lawrence MS, Stojanov P, Polak P, Kryukov GV, Cibulskis K, Sivachenko A, Carter SL, Stewart C, Mermel CH, Roberts SA, et al.. Mutational heterogeneity in cancer and the search for new cancer-associated genes. Nature. 2013;499(7457):214–218. doi: 10.1038/nature12213. PMID:23770567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Melissa Amber Burgess, Vanessa Bolejack, Brian Andrew, Van Tine, Scott Schuetze, James Hu, Sandra P. D'Angelo, Steven Attia, Dennis A. Priebat, Scott H. Okuno, et al.. Multicenter phase II study of pembrolizumab (P) in advanced soft tissue (STS) and bone sarcomas (BS): Final results of SARC028 and biomarker analyses. J Clin Oncol. 2017;35(suppl; abstr 11008). [Google Scholar]
- 29.Kalbasi A, June C, Haas N, Vapiwala N. Radiation and immunotherapy: a synergistic combination. J Clin Invest. 2013;123(7):2756–2763. doi: 10.1172/JCI69219. PMID:23863633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tang C, Wang X, Soh H. Combining radiation and immunotherapy: a new systemic therapy for solid tumors? Cancer Immunol Res. 2014;2(9):831–838. doi: 10.1158/2326-6066.CIR-14-0069. PMID:25187273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schoenhals JE, Seyedin SN, Tang C, Cortez MA, Niknam S, Tsouko E, Chang JY, Hahn SM, Welsh JW. Preclinical Rationale and Clinical Considerations for Radiotherapy Plus Immunotherapy: Going Beyond Local Control. Cancer J. 22(2):130–137. doi: 10.1097/PPO.0000000000000181. PMID:27111909. [DOI] [PubMed] [Google Scholar]
- 32.Wardelmann E, Haas RL, Bovée JVMG, Terrier P, Lazar A, Messiou C, LePechoux C, Hartmann W, Collin F, Fisher C, et al.. Evaluation of response after neoadjuvant treatment in soft tissue sarcomas; the European Organization for Research and Treatment of Cancer-Soft Tissue and Bone Sarcoma Group (EORTC-STBSG) recommendations for pathological examination and reporting. Eur J Cancer. 2016;53:84–95. doi: 10.1016/j.ejca.2015.09.021. PMID:26700077. [DOI] [PubMed] [Google Scholar]
- 33.Messiou C, Bonvalot S, Gronchi A, Vanel D, Meyer M, Robinson P, Morosi C, Bloem JL, Terrier PH, Lazar A, et al.. Evaluation of response after pre-operative radiotherapy in soft tissue sarcomas; the European Organisation for Research and Treatment of Cancer-Soft Tissue and Bone Sarcoma Group (EORTC-STBSG) and Imaging Group recommendations for radiological examination. Eur J Cancer. 2016;56:37–44. doi: 10.1016/j.ejca.2015.12.008. PMID:26802529. [DOI] [PubMed] [Google Scholar]
- 34.Picci P, Böhling T, Bacci G, Ferrari S, Sangiorgi L, Mercuri M, Ruggieri P, Manfrini M, Ferraro A, Casadei R, et al.. Chemotherapy-induced tumor necrosis as a prognostic factor in localized Ewing's sarcoma of the extremities. J Clin Oncol. 1997;15(4):1553–1559. doi: 10.1200/JCO.1997.15.4.1553. PMID:9193352. [DOI] [PubMed] [Google Scholar]
- 35.Mullen JT, Hornicek FJ, Harmon DC, Raskin KA, Chen YL, Szymonifka J, Yeap BY, Choy E, DeLaney TF, Nielsen GP. Prognostic significance of treatment-induced pathologic necrosis in extremity and truncal soft tissue sarcoma after neoadjuvant chemoradiotherapy. Cancer. 2014;120(23):3676–3682. doi: 10.1002/cncr.28945. PMID:25081640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Menendez LR, Ahlmann ER, Savage K, Cluck M, Fedenko AN. Tumor necrosis has no prognostic value in neoadjuvant chemotherapy for soft tissue sarcoma. Clin Orthop Relat Res. 2007;455:219–224. doi: 10.1097/01.blo.0000238864.69486.59. PMID:17016226. [DOI] [PubMed] [Google Scholar]
- 37.Eilber FC, Rosen G, Eckardt J, Forscher C, Nelson SD, Selch M, Dorey F, Eilber FR. Treatment-induced pathologic necrosis: a predictor of local recurrence and survival in patients receiving neoadjuvant therapy for high-grade extremity soft tissue sarcomas. J Clin Oncol. 2001;19(13):3203–3209. doi: 10.1200/JCO.2001.19.13.3203. PMID:11432887. [DOI] [PubMed] [Google Scholar]
- 38.MacDermed DM, Miller LL, Peabody TD, Simon MA, Luu HH, Haydon RC, Montag AG, Undevia SD, Connell PP. Primary tumor necrosis predicts distant control in locally advanced soft-tissue sarcomas after preoperative concurrent chemoradiotherapy. Int J Radiat Oncol Biol Phys. 2010;76(4):1147–1153. doi: 10.1016/j.ijrobp.2009.03.015. PMID:19577863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schilham MW, Marie A, Jansen C. Increased PD-L1 and T-cell infiltration in the presence of HLA class I expression in metastatic high-grade osteosarcoma: a rationale for T-cell-based immunotherapy. Cancer Immunol Immunother. 2017;66(1):119–128. doi: 10.1007/s00262-016-1925-3. PMID:27853827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nowicki TS, Akiyama R, Huang RR, Shintaku IP, Wang X, Tumeh PC, Singh A, Chmielowski B, Denny C, Federman N, et al.. Infiltration of CD8 T Cells and Expression of PD-1 and PD-L1 in Synovial Sarcoma. Cancer Immunol Res. 2017;5(2):118–126. doi: 10.1158/2326-6066.CIR-16-0148. PMID:28039162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kostine M, Cleven AHG, Miranda NFCC De, Italiano A, Bovée JVMG. Analysis of PD-L1, T-cell infiltrate and HLA expression in chondrosarcoma indicates potential for response to immunotherapy specifically in the dedifferentiated subtype. Mod Pathol. 2016;29(9):1028–1037. doi: 10.1038/modpathol.2016.108. PMID:27312065. [DOI] [PubMed] [Google Scholar]
- 42.Angelo SPD, Shoushtari AN, Agaram NP, Kuk D, Qin LX, Carvajal RD, Dickson MA, Gounder M, Keohan ML, Schwartz GK, et al.. Prevalence of tumor-infiltrating lymphocytes and PD-L1 expression in the soft tissue sarcoma. Hum Pathol. 2015;46(3):357–365. doi: 10.1016/j.humpath.2014.11.001. PMID:25540867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kim JR, Moon YJ, Kwon KS, Bae JS, Wagle S, Kim KM, Park HS, Lee H, Moon WS, Chung MJ, et al.. Tumor Infiltrating PD1-Positive Lymphocytes and the Expression of PD-L1 Predict Poor Prognosis of Soft Tissue Sarcomas. PLoS One. 2013;8(12):1–9. doi: 10.1371/journal.pone.0082870.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Budczies J, Mechtersheimer G, Denkert C, Klauschen F, Mughal SS, Chudasama P, Bockmayr M, Jöhrens K, Endris V, Lier A, et al.. PD-L1 (CD274) copy number gain, expression, and immune cell infiltration as candidate predictors for response to immune checkpoint inhibitors in soft-tissue sarcoma. Oncoimmunology. 2017;6(3):1–9. doi: 10.1080/2162402X.2017.1279777.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kim C, Kim EK, Jung H, Chon HJ, Han JW, Shin KH, Hu H, Kim KS, Choi YD, Kim S, et al.. Prognostic implications of PD-L1 expression in patients with soft tissue sarcoma. BMC Cancer. 2016;16(1):434. doi: 10.1186/s12885-016-2451-6. PMID:27393385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wolchok J, Kluger H, Callahan M. Nivolumab plus Ipilimumab in Advanced Melanoma. N Engl J Med. 2013;369(2):122–133. doi: 10.1056/NEJMoa1302369. PMID:23724867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Filatenkov A, Baker J, Mueller AMS, Kenkel J, Ahn GO, Dutt S, Zhang N, Kohrt H, Jensen K, Dejbakhsh-Jones S, Shizuru JA, et al.. Ablative tumor radiation can change the tumor immune cell microenvironment to induce durable complete remissions. Clin Cancer Res. 2015;21(16):3727–3739. doi: 10.1158/1078-0432.CCR-14-2824. PMID:25869387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sharma A, Bode B, Wenger RH, Lehmann K, Sartori AA, Moch H, Knuth A, Boehmer Lv, Broek Mv. у-Radiation promotes immunological recognition of cancer cells through increased expression of cancer-testis antigens in vitro and in vivo. PLoS One. 2011;6(11):e28217. doi: 10.1371/journal.pone.0028217. PMID:22140550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sharma A, Bode B, Studer G, Moch H, Okoniewski M, Knuth A, von Boehmer L, van den Broek M. Radiotherapy of human sarcoma promotes an intratumoral immune effector signature. Clin Cancer Res. 2013;19(17):4843–4853. doi: 10.1158/1078-0432.CCR-13-0352. PMID:23861514. [DOI] [PubMed] [Google Scholar]
- 50.Deng L, Liang H, Burnette B, Beckett M, Darga T, Weichselbaum RR, Fu YX. Irradiation and anti-PD-L1 treatment synergistically promote antitumor immunity in mice. J Clin Invest. 2014;124(2):687–695. doi: 10.1172/JCI67313. PMID:24382348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dewan MZ, Galloway AE, Kawashima N, et al.. Fractionated but not single-dose radiotherapy induces an immune-mediated abscopal effect when combined with anti-CTLA-4 antibody. Clin Cancer Res. 2009;15(17):5379–5388. doi: 10.1158/1078-0432.CCR-09-0265. PMID:19706802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Postow MA, Callahan MK, Barker CA, Yamada Y, Yuan J, Kitano S, Mu Z, Rasalan T, Adamow M, Ritter E, et al.. Immunologic correlates of the abscopal effect in a patient with melanoma. N Engl J Med. 2012;366(10):925–931. doi: 10.1056/NEJMoa1112824. PMID:22397654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hiniker SM, Chen DS, Reddy S, Chang DT, Jones JC, Mollick JA, Swetter SM, Knox SJ. A systemic complete response of metastatic melanoma to local radiation and immunotherapy. Transl Oncol. 2012;5(6):404–407. doi: 10.1593/tlo.12280. PMID:23323154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Golden EB, Demaria S, Schiff PB, Chachoua A, Formenti SC. An abscopal response to radiation and ipilimumab in a patient with metastatic non-small cell lung cancer. Cancer Immunol Res. 2013;1(6):365–372. doi: 10.1158/2326-6066.CIR-13-0115. PMID:24563870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Grimaldi AM, Simeone E, Giannarelli D, Muto P, Falivene S, Borzillo V, Giugliano FM, Sandomenico F, Petrillo A, Curvietto M, et al.. Abscopal effects of radiotherapy on advanced melanoma patients who progressed after ipilimumab immunotherapy. Oncoimmunology. 2014;3:e28780. doi: 10.4161/onci.28780. PMID:25083318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Demaria S, Golden EB, Formenti SC. Role of Local Radiation Therapy in Cancer Immunotherapy. JAMA Oncol. 2015;1(9):1325. doi: 10.1001/jamaoncol.2015.2756. PMID:26270858. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.