Abstract
The evidences on the association of Helicobacter pylori (H. pylori) to coronary heart diseases (CHD) are conflicting. In order to answer this important but yet unanswered clinical health issue, a large cohort study such as big data from the Taiwan National Health Insurance Research Database should be more convincing. Therefore, we aimed to make use of these big data source to analyze and clarify the relevance of H. pylori eradication and CHD risks. We looked through a total of 208196 patients with peptic ulcer diseases (PUD) from the years of 2000 to 2011. First, 3713 patients who received H. pylori eradication within 365 days of the index date were defined as the group A. We randomly selected the same number of patients as cohort A from 55249 non-eradication patients to be the comparison group B using propensity scores (including age, gender and comorbidity) so that we could control the confounding variables of CHD and mortality. Importantly, we perform sensitivity analysis for the time-dependent association between H. pylori eradication and risk of CHD, interactions between patient demographic characteristics and therapy by age (≥ or < 65 years old). The results showed that a trend of decreased association of CHD in patients with early eradication was observed compared to those without eradication (2.58% vs. 3.35%, p = 0.0905). The mortality rate was lower in early eradication subgroup compared to cohort B (2.86% vs. 4.43%, p = 0.0033). Interestingly, there was also significant difference observed in composite end-points for CHD and death in the early eradication subgroup (0.16% vs.0.57%, p = 0.0133). Further, the cumulative CHD rate was significantly lower in younger patients (< 65 years old) with H. pylori eradication therapy started < 1 year compared to those patients without eradication at all (p = 0.0384); the treatment did not appear to have an effect in older patients (≥ 65 years old) (p = 0.1963). Multivariate analysis showed that hypertension and renal diseases were risk factors for CHD in patients without eradication whilst younger age (< 65 years old) initiated with H. pylori therapy was a protective factor. In conclusion, the trend of decrease in CHD occurrence after early H. pylori eradication in addition to the significant decrease in composite end points for CHD and death, the significantly lower cumulative CHD rate in younger patients < 65 years old with H. pylori treated within 365 days suggested that there was positive association between H. pylori eradication and CHD.
Introduction
Coronary heart disease (CHD) is the most common type of heart disease and characterized by atherosclerosis in the epicardial coronary arteries. Atherosclerosis is considered as a chronic inflammatory disease of blood vessels. Many studies suggested that infection with microbes and inflammation at the site of vessel wall have effects on the formation of atherosclerotic plaque and fasten the process of atherosclerosis [1,2]. In recent years, more and more evidences have come to the literature proposing association between CHD and infectious microbes, including those intracellular pathogens such as Helicobacter pylori (H. pylori) [3]. H. pylori infection relates to the development of gastrointestinal diseases and extra-gastrointestinal disorders [4–8]. The effects of H. pylori in the pathogenesis and prognosis of CHD still remained controversial. Some previous studies had shown a positive correlation between H. pylori infection and CHD, whereas others demonstrated that the correlation was only because of confounding effects [9–11]. Moreover, several meta-analyses had also reported diverse results supporting or opposing the association between H. pylori infection and CHD [12–14]. In order to answer this important but yet unanswered clinical health issue, a large cohort study such as big data from the Taiwan National Health Insurance Research Database (NHIRD) should be more convincing. Therefore, we aimed to make use of these big data source to analyze and clarify the relevance of H. pylori eradication and CHD risks.
Materials and methods
Ethics statement
This retrospective cohort study was approved by both the institutional review board and the ethics committee of Chang Gung Memorial Hospital and Kaohsiung Medical University Hospital, Taiwan
Data source
The database used in this study included one million randomly selected patients from the Taiwan NHIRD claims data between the years of 2000 and 2011 which provided coverage for approximately 23 million residents (99% of the population) of Taiwan [15]. We used the inpatient and outpatient claims data as the datasets, and used International Classifications of Diseases, Revision 9, Clinical Modification (ICD-9-CM) to define diseases. All the data calculations in current study were performed by statistician from the center for medical informatics of Kaohsiung Medical Center, Taiwan.
Study subjects
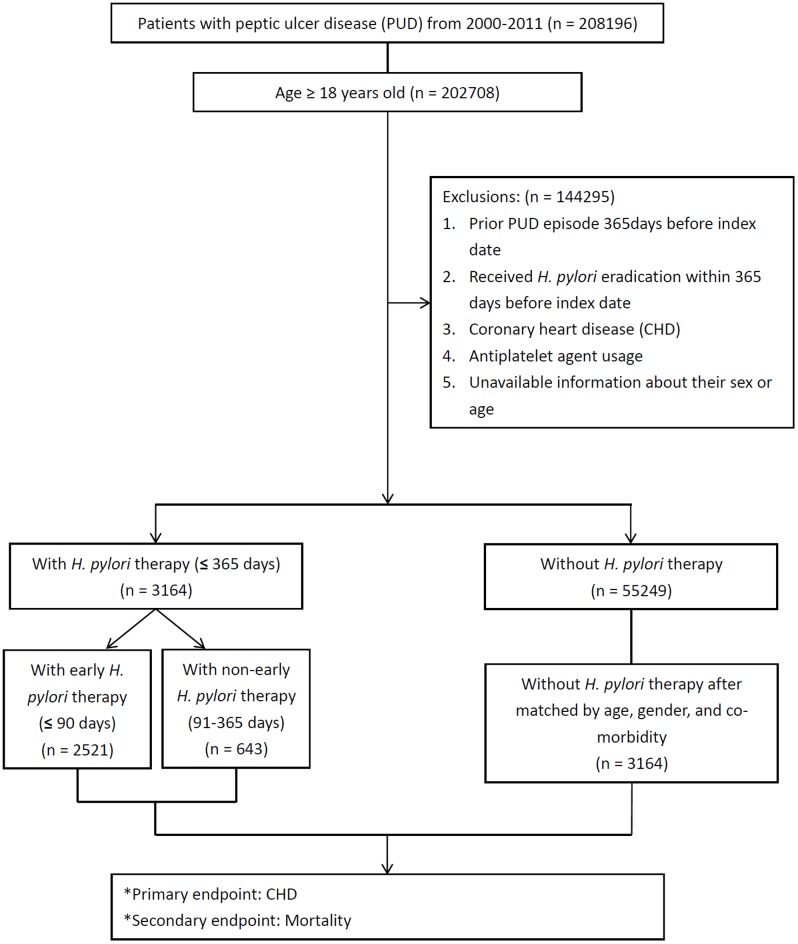
Fig 1 shows the schematic flowchart of the study design. We enrolled only eligible patients aged more than or equal to 18 years old. We used the date of diagnosis with PUD as index date instead of H. pylori infection as inclusion criteria because as high as 90% of PUD patients had H. pylori infection [16]. We identified patients with PUD by using ICD-9-CM codes 531–534 and identified those with CHD by using ICD-9-CM codes 410–414. We identify patients with CHD who had ≥ hospital admission records or ≥ two outpatient visits ≥ 84 days apart. We excluded 144295 patients with H. pylori eradication within 365 days before the index date, patients who were diagnosed with prior PUD, CHD, antiplatelet agent usage, or without sex or age information.
Fig 1. Schematic flowchart of study design.
The patients who received H. pylori eradication within 365 days of the index date were classified into cohort A (n = 3713). We randomly selected the same number of patients as group A from the non-eradication cohort (n = 55249) to form the comparison group B after matched by age, gender, and Charlson indexed comorbidity using propensity score matching to control potential confounding factors of CHD and all-cause mortality. Comorbid conditions, such as acute myocardial infarction, congestive heart failure, cerebrovascular vascular accident, diabetes mellitus and malignancy had no difference of frequency distribution between groups were excluded from the equation of propensity score.
In this study, we identify patients who received H. pylori eradication treatment by using drug prescription registry of the NHIRD database when a triple or quadruple therapy consisted of antacid with either a proton-pump inhibitor (PPI) or histamine type 2 receptor antagonists (H2RA) in combination with clarithromycin or metronidazole plus amoxicillin or tetracycline prescribed within the same prescription order for 7–14 days. Further subgroup analysis was performed for the time-dependent association between H. pylori eradication and risk of CHD, interactions between patient demographic characteristics and therapy by age (≥ or < 65 years old). Early H. pylori eradication was identified in 2521 patients who received treatment ≤ 90 days after the index date.
Comorbidities, other covariates and outcome+
We assessed general health status by Charlson comorbidity index (CCI), which was a method of predicting mortality by classifying or weighting comorbidities and widely utilized to control for confounding in epidemiological studies [17]. The outcomes of each patient was identified from the NHIRD claims files of CHD patient who had ≥ hospital admission records or ≥ two outpatient visits ≥ 84 days apart.
Statistical analysis
The number and percentage of patients were calculated for the categorical variables, including age, gender, comorbidities, and medication use. The differences between the two groups were compared by using the chi-square test. Multivariate Cox proportional hazard analysis was used to estimate the hazard ratio (HR) of CHD and mortality, and the 95% confidence interval (CI) among H. pylori eradication, non-H. pylori eradication, early H. pylori eradication and non-early H. pylori eradication groups. In the models, age, sex, and comorbidities were controlled. To further evaluate the time-dependent association between H. pylori eradication and risk of CHD, interactions between patient demographic characteristics and therapy were considered and a Cox proportional hazards regression was performed with time dependent covariates in relation to CHD occurrence. Kaplan-Meier curves were also used to display the association of H. pylori eradication to the occurrence of CHD and mortality over time. The statistical software used in this study was SAS (version 9.4; SAS Institute Inc., Cary, NC, USA). All tests were two-tailed and significance was set at p value < 0.05.
Results
Demographic data
During the years 2000 to 2011, there were a total of 58413 patients conforming to the inclusion and exclusion criteria. Table 1 demonstrated the demographic characteristics of the study population with and without HP therapy. There were no significant differences in comorbidities in both groups of patients meaning that they were well matched to avoid possible bias during the subsequent analysis.
Table 1. Demographic characteristics of the study population with and without HP therapy.
| Characteristics | Group A Patients with HP therapy (≤ 365 days) (n = 3164) | Group B Patients without HP therapy (n = 3164) | P value | ||
|---|---|---|---|---|---|
| N | % | N | % | ||
| HP therapy* | |||||
| First | 3141 | 99.27% | -- | -- | |
| HP4+HP3+HP1 | 3081 | 98.09% | -- | -- | |
| HP4+HP3+HP2 | 2 | 0.06% | -- | -- | |
| HP5+HP3+HP1 | 101 | 3.22% | -- | -- | |
| HP5+HP3+HP2 | 1 | 0.03% | -- | -- | |
| Second | 24 | 0.76% | -- | -- | |
| HP4+HP6+HP8+HP2 | 0 | 0.00% | -- | -- | |
| HP5+HP6+HP8+HP2 | 0 | 0.00% | -- | -- | |
| HP4+HP7+HP1 | 19 | 79.17% | -- | -- | |
| HP5+HP7+HP1 | 9 | 37.50% | -- | -- | |
| Age, years old (mean ± SD) | 47.73±14.24 | 47.73±14.24 | 1.0000 | ||
| Age_Class1 | |||||
| < 49 | 1821 | 57.55% | 1821 | 57.55% | 0.9951 |
| 50–59 | 713 | 22.53% | 713 | 22.53% | |
| 60–69 | 353 | 11.16% | 354 | 11.19% | |
| ≥ 70 | 277 | 8.75% | 276 | 8.72% | |
| Age_Class2 | |||||
| < 65 | 2741 | 86.63% | 2741 | 86.63% | 1.0000 |
| ≥ 65 | 423 | 13.37% | 423 | 13.37% | |
| Gender | |||||
| Male | 1895 | 59.89% | 1896 | 59.92% | 0.9795 |
| Female | 1269 | 40.11% | 1268 | 40.08% | |
| Charlson score | |||||
| 0 | 2441 | 77.15% | 2441 | 77.15% | 0.9998 |
| 1 | 649 | 20.51% | 648 | 20.48% | |
| 2 | 69 | 2.18% | 70 | 2.21% | |
| ≥ 3 | 5 | 0.16% | 5 | 0.16% | |
| Charlson score (mean ± SD) | 0.25±0.49 | 0.25±0.49 | 1.0000 | ||
| Charlson comorbidity | |||||
| Dementia | 5 | 0.16% | 5 | 0.16% | 1.0000 |
| Pulmonary disease | 95 | 3.00% | 95 | 3.00% | 1.0000 |
| Connective tissue disorder | 14 | 0.44% | 14 | 0.44% | 1.0000 |
| Peptic ulcer | 535 | 16.91% | 535 | 16.91% | 1.0000 |
| Liver disease | 131 | 4.14% | 130 | 4.11% | 1.0000 |
| Paraplegia | 0 | 0.00% | 1 | 0.03% | 0.3173 |
| Renal disease | 11 | 0.35% | 11 | 0.35% | 1.0000 |
| Comorbidity | |||||
| Hypertension | 286 | 9.04% | 287 | 9.07% | 0.9651 |
| Hyperlipidemia | 115 | 3.63% | 115 | 3.63% | 1.0000 |
Abbreviations: HP, Helicobacter pylori; HIV, human immunodificiency virus
*HP1 = Amoxicillin, HP2 = Metronidazole, HP3 = Clarithromycin, HP4 = PPI, HP5 = H2 blockers, HP6 = Bismuth, HP7 = Levofloxacin, HP8 = Tetracycline
Outcomes of the study population
The occurrences of CHD and the mortality rate in both cohorts were demonstrated in Table 2. A trend of decreased association of CHD in patients with early eradication compared to those without eradication (2.58% vs. 3.35%, p = 0.0905). The mortality rate was lower in early eradication subgroup compared to cohort B (2.86% vs. 4.43%, p = 0.0033). Multivariate analysis showed that hypertension and renal diseases were the risk factors for CHD in patients without eradication whilst younger age (< 65 years old) with H. pylori therapy was a protective factor (Table 3). Moreover, in those who did not received early eradication, age, male gender and PUD was the risk factors for all-cause mortality (Table 4).
Table 2. Outcomes of the study population.
| Characteristics | Patients with HP therapy (≤ 365 days) (n = 3164) | Patients without HP therapy (n = 3164) | P value | ||
| N | % | N | % | ||
| Endpoint | |||||
| Coronary heart disease | 90 | 2.84% | 106 | 3.35% | 0.2457 |
| Death | 109 | 3.45% | 137 | 4.33% | 0.0686 |
| Coronary heart disease and death | 10 | 0.32% | 18 | 0.57% | 0.1297 |
| Characteristics | Patients with early HP therapy (≤ 90 days) (n = 3164) | Patients without HP therapy (n = 3164) | P value | ||
| N | % | N | % | ||
| Endpoint | |||||
| Coronary heart disease | 65 | 2.58% | 106 | 3.35% | 0.0905 |
| Death | 72 | 2.86% | 137 | 4.33% | 0.0033 |
| Coronary heart disease and death | 4 | 0.16% | 18 | 0.57% | 0.0133 |
Abbreviations: HP: Helicobacter pylori
Table 3. Multivariate analysis of potential risk factors for coronary heart disease in patients with peptic ulcer disease (with versus without HP therapy among all ages, by age < and ≥ 65 years old).
| Variable | Multivariate analysis | |||
|---|---|---|---|---|
| HR | 95% CI | P value | ||
| Group (all ages) | ||||
| Patients without HP therapy | 1 | |||
| Patients with HP therapy (≤ 365 days) | 0.92 | 0.69 | 1.22 | 0.5581 |
| Gender (male is reference) | 0.76 | 0.56 | 1.03 | 0.0798 |
| Charlson comorbidity | ||||
| Pulmonary disease | 1.26 | 0.66 | 2.41 | 0.4795 |
| Connective tissue disorder | 1.76 | 0.25 | 12.64 | 0.5726 |
| Peptic ulcer | 0.80 | 0.55 | 1.17 | 0.2496 |
| Liver disease | 0.90 | 0.44 | 1.84 | 0.7655 |
| Renal disease | 7.86 | 2.88 | 21.42 | <0.0001 |
| Comorbidity | ||||
| Hypertension | 3.03 | 2.12 | 2.25 | 0.0195 |
| Hyperlipidemia | 1.30 | 0.69 | 2.43 | 0.4196 |
| Group (age < 65 years old) | ||||
| Patients without HP therapy | 1 | |||
| Patients with HP therapy (≤ 365 days) | 0.68 | 0.46 | 0.99 | 0.0464 |
| Gender (male is reference) | 0.88 | 0.59 | 1.31 | 0.5170 |
| Charlson comorbidity | ||||
| Pulmonary disease | 1.31 | 0.41 | 4.13 | 0.6507 |
| Connective tissue disorder | 3.25 | 0.45 | 23.43 | 0.2429 |
| Peptic ulcer | 0.68 | 0.40 | 1.15 | 0.1477 |
| Liver disease | 0.75 | 0.27 | 2.06 | 0.5745 |
| Renal disease | 8.07 | 1.11 | 58.50 | 0.5745 |
| Comorbidity | ||||
| Hypertension | 2.66 | 1.49 | 4.74 | 0.0009 |
| Hyperlipidemia | 1.70 | 0.71 | 4.11 | 0.2366 |
| Group (age ≥ 65 years old) | ||||
| Patients without HP therapy | 1 | |||
| Patients with HP therapy (≤ 365 days) | 1.4 | 0.91 | 2.15 | 0.1244 |
| Gender (male is reference) | 0.55 | 0.34 | 0.89 | 0.0145 |
| Charlson comorbidity | ||||
| Pulmonary disease | 0.74 | 0.34 | 1.65 | 0.4658 |
| Peptic ulcer | 0.93 | 0.54 | 1.60 | 0.7894 |
| Liver disease | 1.92 | 0.69 | 5.36 | 0.2151 |
| Renal disease | 4.67 | 1.43 | 15.19 | 0.0105 |
| Comorbidity | ||||
| Hypertension | 1.58 | 0.98 | 2.54 | 0.0585 |
| Hyperlipidemia | 1.21 | 0.48 | 3.05 | 0.6916 |
Abbreviations: HP: Helicobacter pylori; CI: confidence interval
Table 4. Multivariate analysis of potential risk factors for mortality in patients with PUD (with and without HP therapy).
| Variable | Multivariate analysis | |||
|---|---|---|---|---|
| HR | 95% CI | P value | ||
| Group | ||||
| Patients without HP therapy | 1 | |||
| Patients with HP therapy (≤ 365 days) | 0.86 | 0.67 | 1.11 | 0.2428 |
| Age | 1.08 | 1.07 | 1.09 | <.0001 |
| Gender (male is reference) | 0.58 | 0.44 | 0.77 | 0.0001 |
| Charlson comorbidity | ||||
| Dementia | 2.05 | 0.50 | 8.34 | 0.3178 |
| Pulmonary disease | 1.01 | 0.59 | 1.73 | 0.9610 |
| Connective tissue disorder | 1.19 | 0.17 | 8.49 | 0.8650 |
| Peptic ulcer | 0.69 | 0.48 | 0.99 | 0.0424 |
| Liver disease | 1.07 | 0.53 | 2.19 | 0.8474 |
| Paraplegia | 0 | -- | -- | -- |
| Renal disease | 1.78 | 0.44 | 7.29 | 0.4206 |
| Comorbidity | ||||
| Hypertension | 1.04 | 0.73 | 1.48 | 0.8266 |
| Hyperlipidemia | 0.70 | 0.32 | 1.49 | 0.3518 |
Abbreviations: HP: Helicobacter pylori; CI: confidence interval
Kaplan-Meier analysis
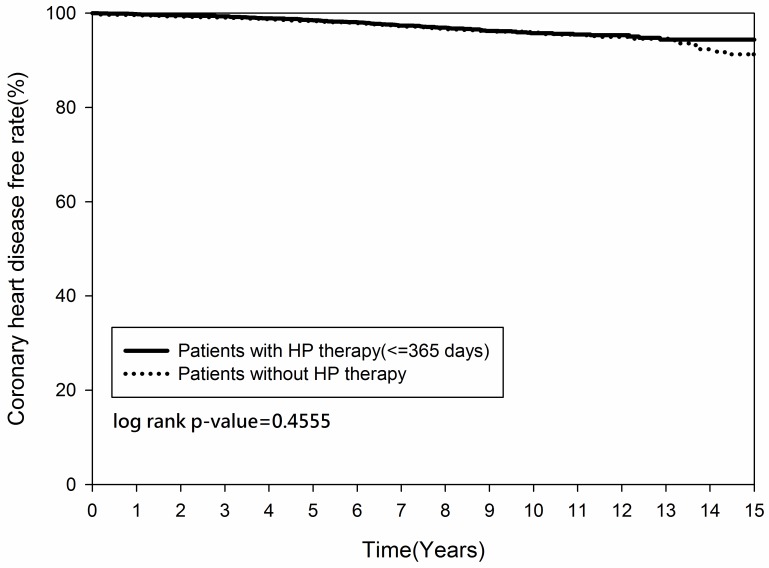
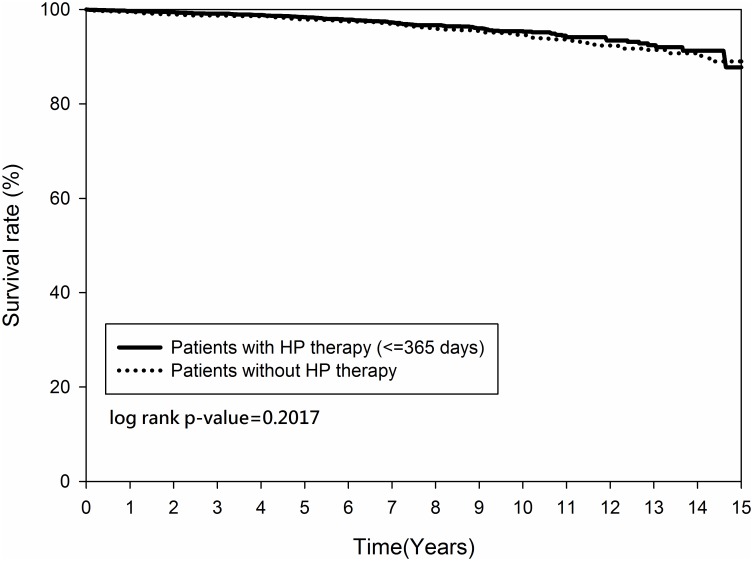
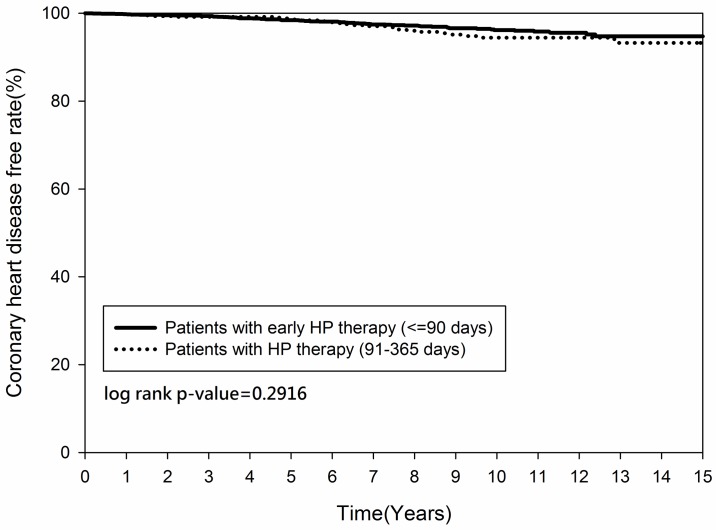
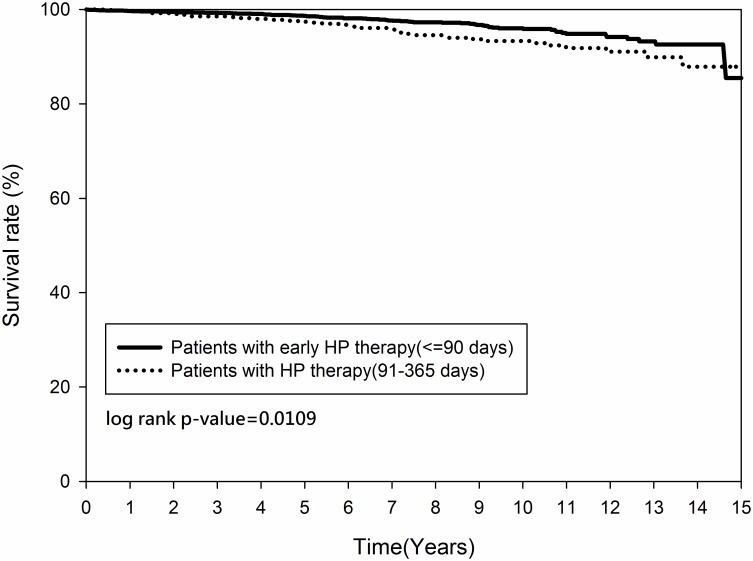
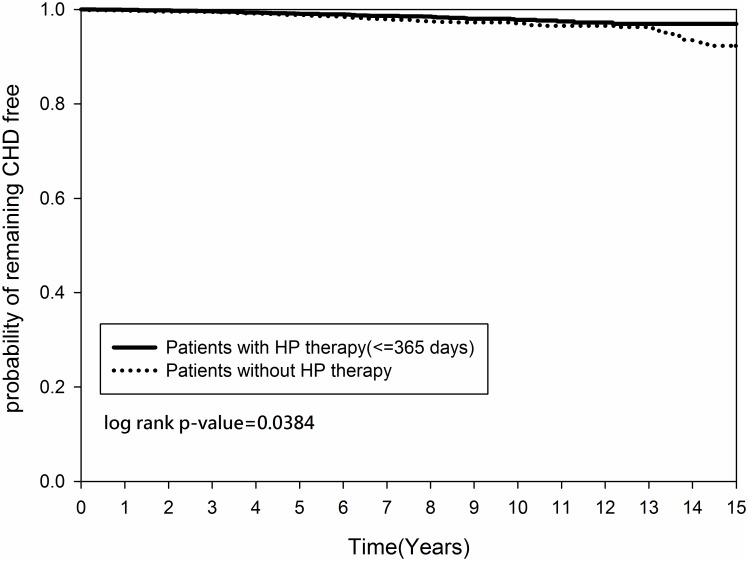
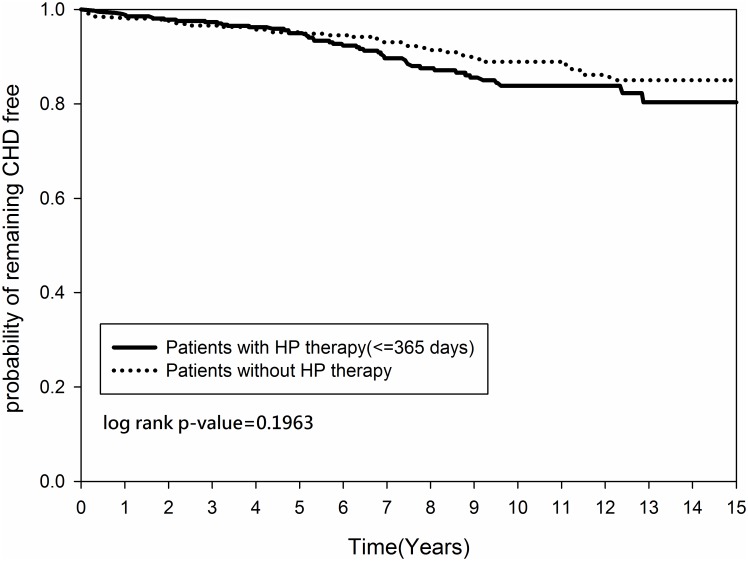
Figs 2 and 3 demonstrated that the cumulative occurrence of CHD and the mortality rate were not significantly different between the two groups since the index date. The cumulative occurrence of CHD between the early and non-early H. pylori eradication subgroups was similar (p = 0.2916) (Fig 4) but the mortality rate was higher in the non-early H. pylori eradication subgroup (p = 0.0109) (Fig 5). Further, the cumulative CHD rate was significantly lower in younger patients (< 65 years old) with H. pylori eradication therapy started < 1 year compared to those patients without eradication at all (p = 0.0384) (Fig 6); the treatment did not appear to have an effect in older patients (≥ 65 years old) (p = 0.1963) (Fig 7).
Fig 2. Kaplan-Meier curve for coronary heart disease rate between patients with and without Helicobacter pylori therapy.
Fig 3. Kaplan-Meier curve for mortality rate between patients with and without Helicobacter pylori therapy.
Fig 4. Kaplan-Meier curve for coronary heart disease rate between patients with and without Helicobacter pylori therapy, by time of initiation.
Fig 5. Kaplan-Meier curve for mortality rate between patients with early and non-early Helicobacter pylori therapy.
Fig 6. Kaplan-Meier curve for coronary heart disease rate by age between patients (age < 65 years old) with and without Helicobacter pylori therapy.
Fig 7. Kaplan-Meier curve for coronary heart disease rate by age between patients (age ≥ 65 years old) with and without Helicobacter pylori therapy.
Discussion
The attempt to demonstrate the association between H. pylori and CHD is always a challenging issue due to the conflicting reports in the literatures. In current study, we used large database and extracted data from Taiwan NHIRD (2000–2011) to clarify the relevance between H. pylori eradication to CHD in patients with PUD. We observed a trend of decreased association of CHD in patients with early eradication compared to those without eradication and a significant difference observed in composite end-points for CHD and mortality rate in the early eradication subgroup. In addition, the cumulative CHD rate was significantly lower in younger patients younger than 65 years old with H. pylori eradication therapy started within 365 days compared to those patients without eradication at all.
By searching the literature, these are the evidences we have found. Vafaeimanesh et al. reported that the prevalence of serologically detectable evidence of H. pylori infection was more in patients with angiographically documented CHD. The evidence of infection was found in more than 70% patients with single vessel disease and double vessel disease but only in 50% individuals without CHD [18]. The other studies also have shown that CHD patients have a higher prevalence of H. pylori infection [19–21]. However, Danesh et al. conducted a meta-analysis which included 18 epidemiological studies involving 10000 patients but did not find any positive association between H. pylori and CHD [22].
For those reports which supported H. pylori eradication could reduce the risk of CHD, it was believed that the timing of eradication mattered. Nozaki et al. found that H. pylori eradication at an early stage of inflammation (< 15 weeks) might be effective in preventing gastric carcinogenesis [23]. Kowalski et al. found that the patients with serological evidence of H. pylori infection had the higher loss of coronary lumen, and compared with the placebo group, eradication of H. pylori attenuated this reduction in lumen of the coronary artery [24]. However, there is by far no other study to further assess the long-term effect of H. pylori eradication on the incidence of new CHD. This could account for the results in our study that there was significantly lower cumulative CHD rate in patients younger than 65 years with H. pylori eradication therapy started within 365 days and mortality rate in the early eradication subgroup at the long-term follow-up.
The possible direct and indirect mechanisms of H. pylori related CHD included induction of inflammatory response secondary to chronic infectious state, endothelial damage, chronic low grade activation of coagulation cascade, dysregulation of lipid metabolism, and hyperhomocysteinaemia [25]. Another larger study showed that the eradication of H. pylori seemed to increase HDL levels and reduce the levels of C reactive protein (CRP) and those of fibrinogen [26]. Gen et al. demonstrated changes in lipid profile including an increase in HDL levels and a fall in low density lipoprotein (LDL) levels with H. pylori eradication [27]. Corrado et al. found that chronic H. pylori infection induces increase of level of the gastric juice and decrease of ascorbic acid levels, both of which cause folate absorption reduction. Low folate hampers the methionine synthase reaction, and it will increase blood hemocysteine concentration which results in the damage of the endothelial cells [28]. Therefore, we believe that early H. pylori eradication could decrease CHD risks especially in those aged < 65 years.
Our study has certain strengths. First, this was a big data study with large sample size from Taiwan NHIRD database which was a nationwide cohort. Second, the important confounding variables for CHD and mortality were available in detail from NHIRD, and were excluded to reduce the confounding effects. Importantly, as shown in Table, we successfully matched the two groups as there were no significant differences in comorbidities in both groups of patients to avoid possible bias during the subsequent analysis.
However, there are still some limitations in our study. First, several published meta-analysis studies reported positive association between cytotoxin-associated protein (Cag-A) positive strain of H. pylori infection and CHD [29, 30], but we couldn’t define Cag-A positive or Cag-A negative strain of H. pylori infection by ICD-9-CM codes. Second, the patient numbers of composite end points for CHD and mortality are rather small, which may have relatively low power in statistical analysis. Third, we were unable to evaluate the patients’ socio-economic disparities which could be associated to both CHD and H. pylori infection as these data were unavailable in the NHIRD database. Common limitations of the claims data include lack of information on body mass index, level of glucose and lifestyle, which could affect the interpretation of the present study. Finally, as high as > 90% of H. pylori were found in patients with duodenal ulcers and 70–90% in gastric ulcer patients [31]. In addition, true H. pylori infection may be underdiagnosed among patients with peptic ulcer patients. It is expected that a high-level of significant association between H. pylori eradication and CHD will be considered if more true H. pylori infections were identified in practice settings.
In conclusion, the trend of decrease in CHD occurrence after early H. pylori eradication in addition to the significant decrease in composite end points for CHD and death, the significantly lower cumulative CHD rate in younger patients < 65 years old with H. pylori treated within 365 days suggested that there was positive association between H. pylori eradication and CHD.
Supporting information
HP365 by age. This file provides data of the multivariate analysis of potential risk factors for coronary heart disease in patients with peptic ulcer disease (with versus without H. pylori therapy among all ages, by age < and ≥ 65 years old) in manuscript.
(XLS)
Acknowledgments
The authors thank Professor Yi-Hsin Yang and center for medical informatics and statistics for their suggestions and help on data analysis. This study was supported partially by grants from Kaohsiung Medical University Aim for the Top Universities Grant, (grant No. KMU-TP105G00, KMU-TP105G01) and Kaohsiung Municipal Ta-Tung Hospital (NHIRD-1050910). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Abbreviations
- CCI
Charlson comorbidity index
- CHD
coronary heart disease
- CI
confidence interval
- H. pylori
Helicobacter pylori
- H2RA
histamin-2 receptor antagonists
- HIV
human immunodificiency virus
- HR
hazard ratio
- ICD-9-CM
International Classifications of Diseases, Revision 9, Clinical Modification
- NHI
National Health Insurance
- NHIRD
National Health Research Institute database
- PPI
proton pump inhibitors
- PUD
peptic ulcer diseases
- SD
standard deviation
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This study was supported partially by grants from Kaohsiung Medical University Aim for the Top Universities Grant (grant no. KMU-TP105G00, KMU-TP105G01) to Deng-Chyang Wu and Kaohsiung Municipal Ta-Tung Hospital (NHIRD-1050910) to Jiunn-Wei Wang. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Stassen FR, Vainas T, Bruggeman CA. (2008) Infection and atherosclerosis. An alternative view on an outdated hypothesis. Pharmacol Rep 60: 85–92. [PubMed] [Google Scholar]
- 2.Campbell LA, Rosenfeld ME. (2015) Infection and Atherosclerosis Development. Arch Med Res 46: 339–350. doi: 10.1016/j.arcmed.2015.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Prasad A, Zhu J, Halcox JP, Waclawiw MA, Epstein SE, Quyyumi AA. (2002) Predisposition to atherosclerosis by infections: role of endothelial dysfunction. Circulation 106: 184–190. [DOI] [PubMed] [Google Scholar]
- 4.Uemura N, Okamoto S, Yamamoto S, Matsumura N, Yamaguchi S, Yamakido M et al. (2001) Helicobacter pylori infection and the development of gastric cancer. N Engl J Med 2001; 345: 784–789. doi: 10.1056/NEJMoa001999 [DOI] [PubMed] [Google Scholar]
- 5.Franceschi F, Zuccala G, Roccarina D, Gasbarrini A. (2014) Clinical effects of Helicobacter pylori outside the stomach. Nat Rev Gastroenterol Hepatol 11: 234–242. doi: 10.1038/nrgastro.2013.243 [DOI] [PubMed] [Google Scholar]
- 6.Tai WC, Lee CH, Chiou SS, Kuo CM, Kuo CH, Liang CM et al. (2014) The Clinical and Bacteriological Factors for Optimal Levofloxacin-Containing Triple Therapy in Second-Line Helicobacter pylori Eradication. PLoS One 9: e105822 doi: 10.1371/journal.pone.0105822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tai WC, Liang CM, Lee CH, Chiu CH, Hu ML, Lu LS, et al. (2015) Seven-day non-bismuth containing quadruple therapy could achieve a grade ‘‘A” success rate for first-Line Helicobacter pylori eradication. Biomed Res Int 2015: 623732 doi: 10.1155/2015/623732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chuah SK, Tai WC, Hsu PI, Wu DC, Wu KL, Kuo CM et al. (2012) The Efficacy of Second-line Anti-Helicobacter Pylori Therapy Using an Extended 14-days levofloxacin/amoxicillin/proton pump inhibitors -A Pilot Study. Helicobacter 17: 374–381. doi: 10.1111/j.1523-5378.2012.00960.x [DOI] [PubMed] [Google Scholar]
- 9.Koenig W, Rothenbacher D, Hoffmeister A, Miller M, Bode G, Adler G et al. (1999) Infection with Helicobacter pylori is not a major independent risk factor for stable coronary heart disease: lack of a role of cytotoxin-associated protein A-positive strains and absence of a systemic inflammatory response. Circulation 100: 2326–2331. [DOI] [PubMed] [Google Scholar]
- 10.Danesh J, Wong Y, Ward M, Muir J. (1999) Chronic infection with Helicobacter pylori, Chlamydia pneumoniae, or cytomegalovirus: population based study of coronary heart disease. Heart 81: 245–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rogha M, Dadkhah D, Pourmoghaddas Z, Shirneshan K, Nikvarz M, Pourmoghaddas M. (2012) Association of helicobacter pylori infection with severity of coronary heart disease. ARYA Atheroscler 7: 138–141. [PMC free article] [PubMed] [Google Scholar]
- 12.Danesh J, Wong Y, Ward M, Muir J. (1999) Risk factors for coronary heart disease and persistent infection with Chlamydia pneumoniae or cytomegalovirus: a population-based study. J Cardiovasc Risk 6: 387–390. [DOI] [PubMed] [Google Scholar]
- 13.Danesh J. (1999) Coronary heart disease, Helicobacter pylori, dental disease, Chlamydia pneumoniae, and cytomegalovirus: meta-analyses of prospective studies. Am Heart J 138: S434–437. [DOI] [PubMed] [Google Scholar]
- 14.Danesh J, Whincup P, Walker M, Lennon L, Thomson A, Appleby P et al. (2000) Low grade inflammation and coronary heart disease: prospective study and updated meta-analyses. BMJ 321: 199–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.National health insurance research database, NHRI. http://nhirdnhriorgtw/en/indexhtm accessed at 30 July, 2015.
- 16.Kurata JH, Nogawa AN. (1997) Meta-analysis of risk factors for peptic ulcer. Nonsteroidal antiinflammatory drugs, Helicobacter pylori, and smoking. J Clin Gastroenterol 24: 2–17. [DOI] [PubMed] [Google Scholar]
- 17.Deyo RA, Cherkin DC, Ciol MA. (1992) Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol 45: 613–619. [DOI] [PubMed] [Google Scholar]
- 18.Vafaeimanesh J, Hejazi SF, Damanpak V, Vahedian M, Sattari M, Seyyedmajidi M. (2014) Association of Helicobacter pylori infection with coronary artery disease: is Helicobacter pylori a risk factor? ScientificWorldJournal 2014: 516354 doi: 10.1155/2014/516354 24574896 [Google Scholar]
- 19.Vijayvergiya R, Agarwal N, Bahl A, Grover A, Singh M, Sharma M, et al. (2006) Association of Chlamydia pneumoniae and Helicobacter pylori infection with angiographically demonstrated coronary artery disease. Int J Cardiol 107: 428–429. doi: 10.1016/j.ijcard.2005.02.028 [DOI] [PubMed] [Google Scholar]
- 20.Mendall MA, Goggin PM, Molineaux N, Levy J, Toosy T, Strachan D et al. (1994) Relation of Helicobacter pylori infection and coronary heart disease. Br Heart J 71: 437–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Danesh J. (1998) Is there a link between chronic Helicobacter pylori infection and coronary heart disease? Eur J Surg Suppl 582: 27–31. [DOI] [PubMed] [Google Scholar]
- 22.Danesh J, Peto R. (1998) Risk factors for coronary heart disease and infection with Helicobacter pylori: meta-analysis of 18 studies. BMJ 316: 1130–1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nozaki K, Shimizu N, Ikehara Y, Inoue M, Tsukamoto T, Inada K et al. (2003) Effect of early eradication on Helicobacter pylori-related gastric carcinogenesis in Mongolian gerbils. Cancer Sci 94: 235–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kowalski M. (2001) Helicobacter pylori (H. pylori) infection in coronary artery disease: influence of H. pylori eradication on coronary artery lumen after percutaneous transluminal coronary angioplasty. The detection of H. pylori specific DNA in human coronary atherosclerotic plaque. J Physiol Pharmacol 52: 3–31. [PubMed] [Google Scholar]
- 25.Vijayvergiya R, Vadivelu R. (2015) Role of Helicobacter pylori infection in pathogenesis of atherosclerosis. World J Cardiol 7: 134–43. doi: 10.4330/wjc.v7.i3.134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pellicano R, Oliaro E, Fagoonee S, Astegiano M, Berrutti M, Saracco G et al. (2009) Clinical and biochemical parameters related to cardiovascular disease after Helicobacter pylori eradication. Int Angiol 28: 469–73. [PubMed] [Google Scholar]
- 27.Gen R, Demir M, Ataseven H. Effect of Helicobacter pylori eradication on insulin resistance, serum lipids and low-grade inflammation. South Med J 2010; 103: 190–196. [DOI] [PubMed] [Google Scholar]
- 28.Corrado E, Novo S. (2005) Role of inflammation and infection in vascular disease. Acta Chir Belg 105: 567–579. [DOI] [PubMed] [Google Scholar]
- 29.Pasceri V, Patti G, Cammarota G, Pristipino C, Richichi G, Di Sciascio G. (2006) Virulent strains of Helicobacter pylori and vascular diseases: a meta-analysis. Am Heart J 151: 1215–1222. doi: 10.1016/j.ahj.2005.06.041 [DOI] [PubMed] [Google Scholar]
- 30.Franceschi F, Niccoli G, Ferrante G, Gasbarrini A, Baldi A, Candelli M et al. (2009) CagA antigen of Helicobacter pylori and coronary instability: insight from a clinico-pathological study and a meta-analysis of 4241 cases. Atherosclerosis 202: 535–542. doi: 10.1016/j.atherosclerosis.2008.04.051 [DOI] [PubMed] [Google Scholar]
- 31.Kurata JH, Nogawa AN. (1997) Meta-analysis of risk factors for peptic ulcer: Nonsteroidal antiinflammatory drugs, Helicobacter pylori, and smoking. J Clinic Gastroenterol 24: 2–17. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
HP365 by age. This file provides data of the multivariate analysis of potential risk factors for coronary heart disease in patients with peptic ulcer disease (with versus without H. pylori therapy among all ages, by age < and ≥ 65 years old) in manuscript.
(XLS)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.