Abstract
The MID1 ubiquitin ligase activates mTOR signaling and regulates mRNA translation. Misregulation of MID1 expression is associated with various diseases including midline malformation syndromes, cancer and neurodegenerative diseases. While this indicates that MID1 expression must be tightly regulated to prevent disease states specific mechanisms involved have not been identified. We examined miRNAs to determine mechanisms that regulate MID1 expression. MicroRNAs (miRNA) are small non-coding RNAs that recognize specific sequences in their target mRNAs. Upon binding, miRNAs typically downregulate expression of these targets. Here, we identified four miRNAs, miR-19, miR-340, miR-374 and miR-542 that bind to the 3’-UTR of the MID1 mRNA. These miRNAs not only regulate MID1 expression but also mTOR signaling and translation of disease associated mRNAs and could therefore serve as potential drugs for future therapy development.
Introduction
The RING finger protein MID1 is involved in fundamental cellular processes including somatic cell growth and proliferation as well as neuron function (reviewed in [1]). Acting as E3 ubiquitin ligase MID1 marks the mTOR antagonist protein phosphatase 2A (PP2A) for degradation by the proteasome and thereby enhances mTOR activity [2]. Furthermore, MID1 assembles a ribonucleoprotein complex and regulates translation [3–6].
Germline mutations in MID1 cause Opitz BBB/G syndrome (OS), a rare monogenic disorder involving malformations of the ventral midline including hypertelorism and hypospadias among others. Besides its role in OS MID1 function has been associated with the development and progression of various other diseases including cancer and neurodegenerative diseases. MID1 is overexpressed in certain cancer types and promotes cancer growth [7, 8]. In the brain, MID1 binds to and induces translation of pathologically expanded CAG repeat mRNAs, which are the cause for neurodegenerative diseases such as Huntington’s disease and spinocerebellar ataxias [5, 6]. Reducing the expression of MID1 is a promising new option to treat these diseases.
MiRNAs are endogenously expressed short (~20 nucleotide long) non-coding RNAs that base-pair their mRNA targets with imperfect complementarity (reviewed in [9]). The so-called “seed region” comprising nucleotides 2–8 of the miRNA, however, shows perfect complementarity and is important for target recognition. MiRNA binding sites are often located in the 3’-untranslated region (3'-UTR) of their target mRNAs [10–12]. Binding of a miRNA to its target mRNA can either cause degradation or inhibit translation.
Mimics of miRNAs that target MID1 could be promising miRNA therapeutics to treat cancer as well as neurodegenerative diseases. Whether MID1 is subject to miRNA targeting was, however, unknown. Here, we identified four miRNAs, miR-19, miR-340, miR-374 and miR-542 that bind the 3’-UTR of MID1 mRNA and inhibit MID1 protein production.
Materials and methods
Human brain tissue was collected and stored as previously described [13]. Tissue was obtained with the families’ full consent and with the approval of the Leiden University Medical Center institutional Ethics Committee.
Prediction of miRNA binding sites
MiRNA binding sites in the MID1 mRNA (Human MID1 ENST00000453318.2) were predicted using TargetScanHuman 6.2 (http://www.targetscan.org).
Constructs
The first 1352 nucleotides of the MID1 3'-UTR containing the predicted binding sites of the above-mentioned miRNAs were cloned into the psiCHECK-2 vector (Promega) downstream of the renilla luciferase gene using the restriction enzymes NotI and XhoI.
MiRNA mimics and inhibitors transfections and luciferase reporter assays
Chemically synthesized double-stranded RNAs mimicking mature endogenous miRNAs were transfected into HEK293T cells (ATCC) or HEK293T cells stably expressing HTT-exon1 with 51 CAG repeats [14] or into SH-SY5Y cells (ATCC). 105 cells per well of a 24-well plate were seeded one day prior transfection. 2.5 μl per well of a 20 μM stock of miRNA mimics (hsa-miR-374a-5p, hsa-miR-542-3p, hsa-miR-216a-5p, hsa-miR-19b-3p, and hsa-miR-340-5p miScript miRNA Mimics, Qiagen) or miRNA inhibitors (hsa-miR-216a-5p, hsa-miR-340-3p, hsa-miR-374a, and hsa-miR-542-3p inhibitors from Qiagen, hsa-miR-19b-3p inhibitor from Sigma Aldrich (HSTUD0344)) were transfected using Oligofectamine (Invitrogen/Thermo Fisher Scientific) according to the manufacturer’s instructions. Twenty-four hours after transfection with mimics or inhibitors cells were transfected with plasmid DNA using Lipofectamine 2000 (Invitrogen/Thermo Fisher Scientific) according to the manufacturer’s instructions. Twenty-four hours later cells were lysed in 1x PLB (Promega) and protein concentration was measured by following the Qubit™ Protein Assay kit (Thermo Fisher Scientific). Samples were diluted to a concentration of 1 μg/μl. 10 μl of the diluted lysate was pipetted into a 96-well plate and luciferase assay was performed using the Dual-Luciferase® Reporter Assay System kit (Promega) following the manufacturer’s instructions. Measurement was performed in a FLUOstar Omega plate reader (BMG Labtech).
Western blot
Cells were transfected with miRNA mimics, inhibitors, MID1 siRNAs (pool 5 siRNAs: TTGAGTGAGCGCTATGACAAA, AAGGTGATGAGGCTTCGCAAA, TAGAACGTGATGAGTCATCAT, CACCGCAUCCUAGUAUCACACTT, CAGGAUUACAACUUUUAGGAATT) or non-silencing control siRNAs (AATTCTCCGAACGTGTCACGT) and lysed as described above. After addition of 4x SDS PAGE buffer (EDTA 50 mM, Tris 200 mM, glycerol 40%, SDS 8%, β-mercaptoethanol 4%, bromophenol blue 0.008%) samples were boiled for 10 min at 95°C and proteins were analyzed on 10% SDS gels and blotted onto PVDF membranes (Roche). Blots were blocked in milk and incubated with the following antibodies: anti-actin (Abcam, rabbit), HRP-anti-rabbit (Cell signaling). For production of polyclonal MID1 antibodies MID1-peptides were synthesized (amino acids 84–113) and used for immunisation of rabbits (PINEDA). Eight weeks after immunisation high-titre sera were collected and affinity purified using the peptide coupled to SulfoLink Coupling Resin (Thermo Scientific) following the manufacturer’s instructions. The purified antibodies were validated on western blots of lysates from MID1 knockdown cells, as well as in western blot experiments in which peptide-blocking was performed (S1 Fig). The resulting bands were quantified using ImageJ software. Statistical analyses were performed using one-way ANOVA with post hoc Dunnett’s test to accommodate for multiple comparisons or Student’s t-test for two-group comparisons, as appropriate.
Quantitative real-time PCR (qPCR)
Total RNA was isolated using the RNeasy Plus Mini Kit (Qiagen) according to the manufacturer’s instructions. cDNA was synthesized using the TaqMan reverse transcription reagents kit (Applied Biosystems) and qPCR was carried out using the SYBRGreen PCR master mix (Applied Biosystems). Primers used: GAPDH forward: CCACCCATGGCAAATTCC, GAPDH reverse: TGGGATTTCCATTGATGACAAG, MID1 forward: CTGCCAGGTCTGGTGTCATG, MID1 reverse: AATCAGGCTTAGGGCCCTTCT.
Reverse transcription PCR (RT-PCR)
To detect miRNAs in HEK293T cells, a miRNA-enriched fraction was purified using the miRNeasy Mini and MinElute Cleanup Kits (Qiagen) according to the manufacturer’s instructions. MiRNAs were subjected to poly(T) adaptor reverse transcription as described (Shi et al., 2012) using E. coli poly(A) polymerase (New England Biolabs) and the TaqMan reverse transcription kit (Applied Biosystems) in a combined reaction. MiRNA sequences were amplified by PCR using miRNA-specific forward primers and the described universal poly(T) adaptor reverse primer. Primers used: hsa-miR-19b-3p-fwd: TGTGCAAATCCATGCAAAACTGA, hsa-miR-216a-5p-fwd: TAATCTCAGCTGGCAACTGTGA, hsa-miR-340-5p-fwd: GCTTATAAAGCAATGAGACTGATT, hsa-miR-374a-5p-fwd: CGTTATAATACAACCTGATAAGTG, hsa-miR-542-3p-fwd: GCTGTGACAGATTGATAACTGAAA.
Results
MiRNAs hsa-miR-374a-5p, hsa-miR-542-3p, hsa-miR-216a-5p, hsa-miR-19b-3p, and hsa-miR-340-5p target the MID1 3’-UTR at specific sites
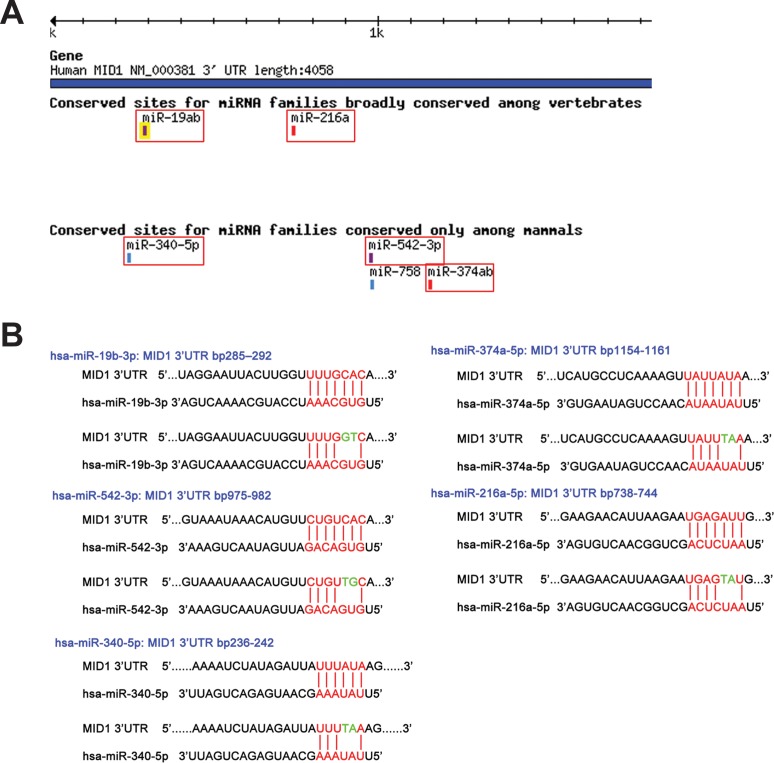
The software Targetscan predicted several miRNAs to target the MID1 3'-UTR (Fig 1A). Because the MID1 3'-UTR is subject to several alternative polyadenylation events we only picked miRNAs that were predicted to target sequences in the 5'-part of the MID1 3'-UTR for further analysis. Furthermore, we restricted our analysis to miRNAs whose predicted binding sites in the MID1 3'-UTR were highly conserved in vertebrates (has-miR-19b-3p, has-miR216a-5p) or mammals (hsa-miR-374a-5p, hsa-miR-542-3p, hsa-miR-340-5p).
Fig 1. MiRNA binding sites in the 3’-UTR of MID1.
(A) Targetscan prediction of miRNAs targeting within the first 1800bp of the MID1 3’-UTR. (B) Schematic drawing of the mutations that were inserted into the MID1 3’-UTR luciferase reporter constructs to mutate the seed region of the specific miRNAs.
To test for binding of the miRNAs to the MID1 3'-UTR a luciferase reporter vector containing the first 1352 nucleotides of the MID1 3'-UTR cloned downstream of the renilla luciferase together with a pool of miRNA mimics or miRNA inhibitors was transfected into HEK293T cells and luciferase activity was measured. The pools were comprised of mimics or inhibitors of all five miRNAs. Firefly luciferase expressed from the same plasmid as renilla luciferase was used for normalization. Endogenous expression of these five miRNAs in HEK293T cells has been reported previously (miRmine Human miRNA Expression Database, [15]) and we validated this finding by reverse transcription PCR (S2 Fig).
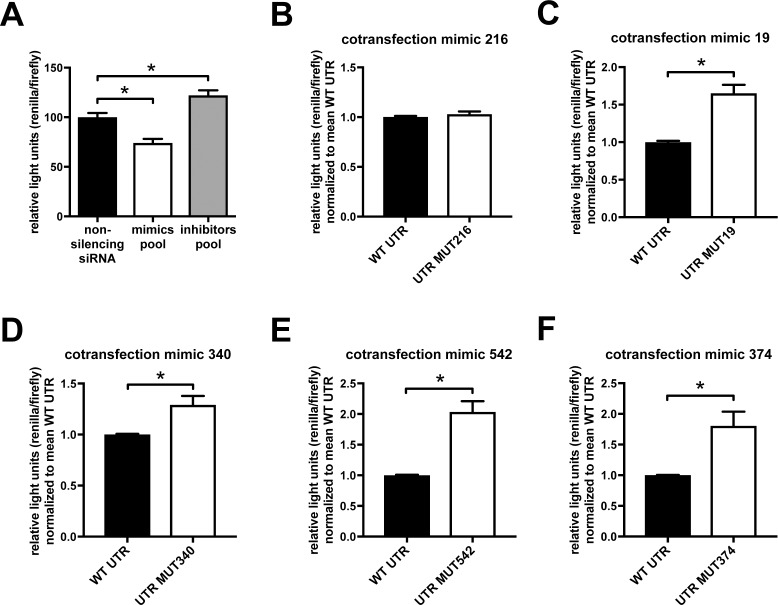
Transfecting the pool of miRNA mimics and inhibitors significantly decreased or increased, respectively, the activity of the MID1 luciferase reporter (Fig 2A), suggesting that miRNAs hsa-miR-374a-5p, hsa-miR-542-3p, hsa-miR-216a-5p, hsa-miR-19b-3p, and hsa-miR-340-5p indeed target the MID1 3’-UTR. To test each miRNA individually for its capacity to target the MID1 3’-UTR we mutated the respective miRNA binding sites in the MID1 luciferase reporter constructs (Fig 1B). These mutant constructs were co-transfected with the corresponding miRNA mimics and compared to transfections of the non-mutated constructs. A significant increase in luciferase activity could be observed upon transfection of all mutant constructs except for the construct containing the mutated miR-216 binding site (Fig 2B). These data show that miRNAs hsa-miR-374a-5p, hsa-miR-542-3p, hsa-miR-19b-3p, and hsa-miR-340-5p but not miR-216a-5p target the MID1 3’-UTR at the predicted sites.
Fig 2. MiRNAs hsa-miR-374a-5p, hsa-miR-542-3p, hsa-miR-216a-5p, hsa-miR-19b-3p, and hsa-miR-340-5p target the MID1 3’-UTR.
(A) HEK293T cells were transfected with a pool of miRNA mimics (hsa-miR-374a-5p, hsa-miR-542-3p, hsa-miR-216a-5p, hsa-miR-19b-3p, and hsa-miR-340-5p), a pool of miRNA inhibitors (hsa-miR-374a-5p, hsa-miR-542-3p, hsa-miR-216a-5p, hsa-miR-19b-3p, and hsa-miR-340-5p), or a non-specific control siRNA. 24 hours later the same cells were transfected with a reporter construct carrying renilla luciferase fused to the MID1 3’-UTR sequence (bp 1–1352) as well as firefly luciferase, which is used for normalization. Relative light units of renilla normalized to firefly luciferase are shown. Columns represent mean values +/- SEM (* p < 0.001, n = 13). (B-F) Cotransfection of HEK293T cells with miRNA mimics in combination with reporter constructs carrying renilla luciferase fused to the MID1 3’-UTR sequence (bp 1–1352), in which the seed regions of the specific miRNAs have been mutated (MUT). As in (A) firefly luciferase was used for normalization. The resulting data were normalized to the mean value for a control using the non-mutant (WT) MID1 reporter construct described in (A). Columns represent mean values +/- SEM (* p < 0.01, n = 18).
MiRNAs hsa-miR-374a-5p, hsa-miR-542-3p, hsa-miR-19b-3p, and hsa-miR-340-5p target endogenous MID1
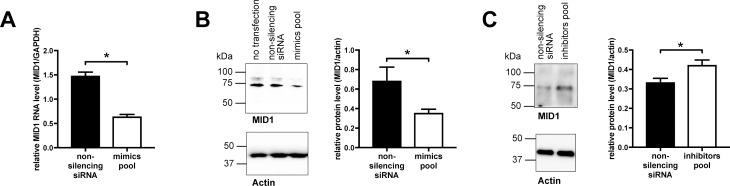
To test if miRNAs hsa-miR-374a-5p, hsa-miR-542-3p, hsa-miR-19b-3p, and hsa-miR-340-5p target endogenous MID1, we transfected a pool of the corresponding miRNA mimics into HEK293T cells and analyzed the expression level of MID1 by qPCR and on western blots. In line with the above-mentioned luciferase reporter experiments, transfection of the miRNA mimics pool led to a significant reduction of MID1 expression at both RNA (Fig 3A) and protein level (Fig 3B). Consistently, transfection of miRNA inhibitors had the opposite effect and let to a significant increase in endogenous MID1 protein level (Fig 3C, S3 Fig, S4 Fig). Together these data indicate that miRNAs hsa-miR-374a-5p, hsa-miR-542-3p, hsa-miR-19b-3p, and hsa-miR-340-5p regulate MID1 expression.
Fig 3. Micro RNAs hsa-miR-374a-5p, hsa-miR-542-3p, hsa-miR-19b-3p, and hsa-miR-340-5p target endogenous MID1.
(A) HEK293T cells were transfected with a pool of miRNA mimics (hsa-miR-374a-5p, hsa-miR-542-3p, hsa-miR-19b-3p, and hsa-miR-340-5p), or a non-specific control siRNA. Relative MID1 mRNA expression was analyzed in a qPCR using MID1 specific primers and GAPDH-specific primers. Columns represent mean values +/- SEM (* p < 0.0001, n = 12). (B) HEK293T cells were transfected with a pool of miRNA mimics (hsa-miR-374a-5p, hsa-miR-542-3p, hsa-miR-19b-3p, and hsa-miR-340-5p), or a non-specific control siRNA. Left: MID1 protein levels were analyzed on a western blot using MID1 specific antibodies (upper blot) or Actin-specific antibodies (lower blot). A representative blot of n = 6 is shown. Right: quantification of blots. Columns represent mean values +/- SEM (* p < 0.05). (C) HEK293T cells were transfected with a pool of miRNA inhibitors (hsa-miR-374a-5p, hsa-miR-542-3p, hsa-miR-19b-3p, and hsa-miR-340-5p), or a non-specific control siRNA. Left: MID1 protein levels were analyzed on a western blot using MID1 specific antibodies (upper blot) or Actin-specific antibodies (lower blot). A representative blot of n = 6 is shown. Right: quantification of blots. Columns represent mean values +/- SEM (* p < 0.05).
Targeting of MID1 by miRNAs hsa-miR-374a-5p, hsa-miR-542-3p, hsa-miR-19b-3p, and hsa-miR-340-5p reduces translation of MID1-target mRNAs
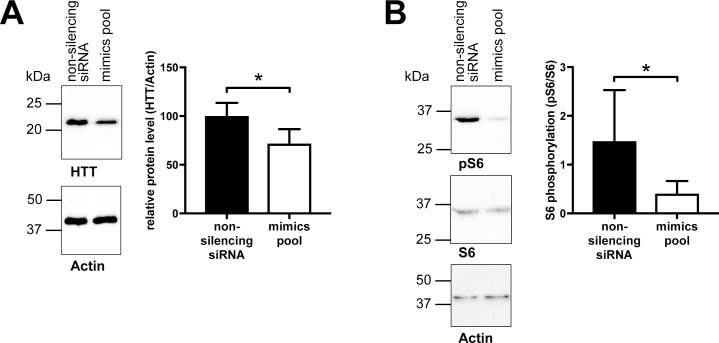
One previously described cellular function of the MID1 protein is the regulation of translation of its target mRNAs. MID1 induces translation of pathologically expanded CAG repeat mRNAs, such as huntingtin (HTT) mRNA [5, 6]. A miRNA-mediated reduction in MID1 should decrease protein levels of mutant HTT accordingly. To test this hypothesis, we transfected a previously described cell culture model for Huntington’s disease [14] with a pool of miRNA mimics (hsa-miR-374a-5p, hsa-miR-542-3p, hsa-miR-19b-3p, and hsa-miR-340-5p). These cells, a derivative of HEK293T stably expressing HTT-exon 1 with 51 CAG-repeats, were transfected either with the pool of MID1-targeting miRNA mimics or non-silencing control siRNA oligonucleotides. Cells were analyzed for HTT-exon 1 protein expression by western blot analysis. As expected, depletion of MID1 by miRNA mimics resulted in a significant reduction of HTT protein (Fig 4A, S5 Fig, S6 Fig).
Fig 4. Targeting of endogenous MID1 by miRNAs hsa-miR-374a-5p, hsa-miR-542-3p, hsa-miR-19b-3p, and hsa-miR-340-5p leads to a reduction of HTT protein.
HEK293T cells stably expressing HTT-exon 1 with 51 CAG-repeats were transfected with a pool of miRNA mimics (hsa-miR-374a-5p, hsa-miR-542-3p, hsa-miR-19b-3p, and hsa-miR-340-5p), or a non-specific control siRNA. Upper panel: Left: HTT-exon 1 protein levels were analyzed on a western blot using HTT specific antibodies or Actin-specific antibodies. A representative blot of n = 7 is shown. Right: quantification of blots. Columns represent mean values +/- SEM (* p < 0.05). Lower panel: phospho-S6 (pS6) as well as total S6 protein levels were analyzed on a western blot using specific antibodies. Actin was detected on the same blots as a loading control. A representative blot of n = 3 is shown. Right: quantification of blots. Columns represent mean values +/- SEM (* p < 0.05).
MID1 inhibits PP2A, which in turn affects the phosphorylation of the translational regulator S6K and its target protein, the ribosomal subunit S6 [2, 5]. A decrease in MID1 expression leads to an upregulation of PP2A and dephosphorylation of S6K and S6. To test whether MID1-regulating miRNAs have an effect on S6 phosphorylation we measured S6 phosphorylation by western blot analysis upon transfection of the miRNA mimics pool containing mimics of hsa-miR-374a-5p, hsa-miR-542-3p, hsa-miR-19b-3p, and hsa-miR-340-5p. As expected expressing the miRNA mimic pool caused a strong reduction in S6 phosphorylation (Fig 4B, S7 Fig, S8 Fig).
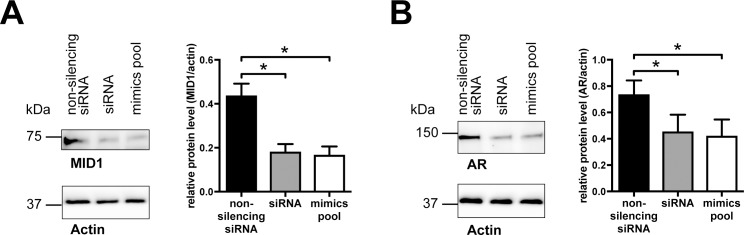
The androgen receptor (AR) mRNA is another important MID1 target [7]. To test if depletion of MID1 by miRNAs hsa-miR-374a-5p, hsa-miR-542-3p, hsa-miR-19b-3p, and hsa-miR-340-5p results in decreased AR protein levels, we transfected SH-SY5Y cells with the same pool of miRNA mimics as before. As positive control MID1-specific siRNAs and as a negative control non-silencing siRNAs were used. Cells were analyzed for MID1 and AR protein expression by western blot analysis. As expected, depletion of MID1 by miRNA mimics resulted in a significant reduction of AR protein (Fig 5, S9 Fig, S10 Fig, S11 Fig, S12 Fig). Taken together, these data show that depletion of MID1 by miRNAs hsa-miR-374a-5p, hsa-miR-542-3p, hsa-miR-19b-3p, and hsa-miR-340-5p leads to a reduction of several known MID1-targets: mutant HTT, S6 and AR.
Fig 5. Targeting of endogenous MID1 by miRNAs hsa-miR-374a-5p, hsa-miR-542-3p, hsa-miR-19b-3p, and hsa-miR-340-5p leads to a reduction of AR protein.
SH-SY5Y were transfected with a pool of miRNA mimics (hsa-miR-374a-5p, hsa-miR-542-3p, hsa-miR-19b-3p, and hsa-miR-340-5p), or MID1-specific siRNAs as positive control or a non-specific control siRNA as negative control. Upper panel: Left: MID1 as well as AR protein levels were analyzed on a western blot using specific antibodies. Actin was detected on the same membranes as loading control. A representative blot of n = 3 is shown. Right: quantification of blots. Columns represent mean values +/- SEM (* p < 0.05).
Discussion
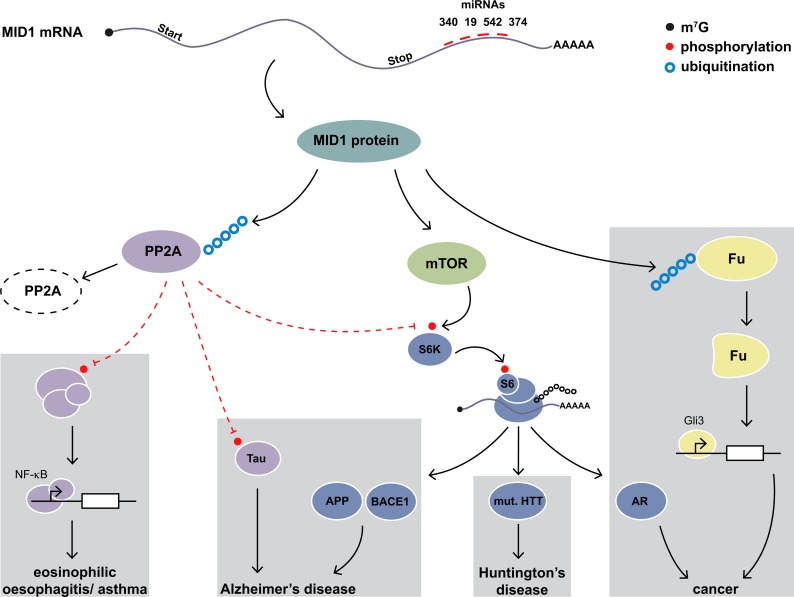
In the embryo, MID1 is a dosage sensitive gene and is important for the development of ventral midline structures. In the adult, however, abnormal MID1 function has been associated with neurodegenerative diseases as well as cancer. Reducing MID1 expression in patients suffering from these diseases may be a promising therapeutic option. MiRNA-based therapeutics such as miRNA mimics could potentially be used to achieve such a downregulation of MID1 in disease state. Only one miRNA, miR-135b, has been shown previously to bind the MID1 3’-UTR and regulate MID1 expression in breast cancer cells [16]. Here we identified four additional miRNAs, hsa-miR-19b-3p, hsa-miR-340-5p, hsa-miR-374a-5p and hsa-miR-542-3p, that target the MID1 3'-UTR and regulate MID1 expression (Fig 6).
Fig 6. Model showing the role of MID1 in diverse diseases.
We identified miRNAs hsa-miR-374a-5p, hsa-miR-542-3p, hsa-miR-19b-3p, and hsa-miR-340-5p as regulators of MID1. By controlling the expression levels of MID1 these miRNAs may affect several MID1-dependent processes. Functionally, MID1 acts as E3 ubiquitin ligase. Known targets of MID1’s ubiquitin ligase activity include PP2A and Fu. By catalyzing the ubiquitination PP2A MID1 induces the proteasomal degradation of PP2A, thereby reducing PP2A activity towards its target proteins. These include proteins involved in transcription regulation of inflammatory genes via NF-κB signaling, which are essentially involved in chronic inflammatory diseases such as asthma or eosinophilic oesophagitis, as well as the Tau protein, that is important in Alzheimer’s disease. Besides inhibiting PP2A MID1 stimulates the activity of mTOR. PP2A and mTOR regulate phosphorylation and thereby activity of the translational regulator S6K. Via PP2A and mTOR, MID1 controls translation of its target mRNAs. These include APP and BACE1, which play an important role in Alzheimer’s disease, mutant HTT, which causes Huntington’s disease, as we as that androgen receptor (AR), which is involved in prostate cancer. The second known target of MID1’s ubiquitin ligase activity is the kinase Fu. Upon MID1-dependent ubiquitination, this protein gets cleaved, which produces an active truncated protein that regulates the transcription factor GLI3.
MID1 enhances the translation of mutant CAG repeat mRNAs and is therefore contributing to the neuropathology of polyglutamine diseases such as Huntington’s disease [5, 6]. Additionally, MID1 regulates processes that are linked to carcinogenesis and an abnormal activity of MID1 has been shown in different cancer cell types [7, 8]. Furthermore, MID1 expression is increased in certain types of cancer for example in prostate cancer cells [7]. Mechanistically, a carcinogenic action of MID1 can be explained by its interaction with proteins that play a role in cell cycle regulation. For example, MID1 regulates expression of CyclinD1 via the transcription factor GLI3 [8, 17]. Furthermore, MID1 regulates activity of PP2A [2], a well established regulator of the cell cycle (reviewed in [18]). Whereas the four MID1-targeting miRNAs that we have identified have not been linked to Huntington’s disease so far, all of them have previously been linked to carcinogenesis [19–25]. Interestingly, expression of hsa-miR-19b-3p is down-regulated in gastric cancer [19] and hsa-miR-542-3p is decreased in several cancer types including oral squamous cell carcinoma [26], serous ovarian tumor [22], esophageal cancer [24] and colorectal cancer [25] and acts as a tumor suppressor miRNA. Future studies should investigate whether a downregulation of these miRNAs is functionally connected to an upregulation of MID1 in these cancers.
MicroRNAs have oftentimes more than one target and can regulate the expression of several genes. In addition to MID1, miRNAs hsa-miR-19b-3p, hsa-miR-340-5p, hsa-miR-374a-5p and hsa-miR-542-3p have other target mRNAs (Table 1). Several of these known target mRNAs are associated with carcinogenesis. For example, hsa-miR-19b-3p regulates PTEN, a tumor suppressor that is mutated in a large number of cancers [27], and ESR1, mutations in which are associated with breast cancer [28].
Table 1. Targets of miRNAs hsa-miR-19b-3p, hsa-miR-340-5p, hsa-miR-374a-5p and hsa-miR-542-3p.
| Experimentally validated miRNA target mRNAs | ||||
|---|---|---|---|---|
| miRNA: | hsa-miR-19b-3p | hsa-miR-340-5p | hsa-miR-374a-5p | hsa-miR-542-3p |
| target mRNA | ATXN1 [33] | ABCB5 [34] | CEBPB [35] | BIRC5 [36] |
| BCL2L11 [37] | HNRNPA2B1 [38] | MID1 | ILK [39] | |
| CUL5 [40] | KRAS [41] | PTEN [42] | MTDH [43] | |
| CYP19A1 [44] | LPAATβ [45] | SRCIN1 [46] | BMP7 [47] | |
| ESR1 [48] | MECP2 [41] | WIF1 [42] | RPS23 [23] | |
| GCM1 [44] | MET [49] | WNT5A [50] | ANGPT2 [51] | |
| MID1 | MID1 | FZD7 [52] | ||
| MXD1 [53] | PTBP1 [38] | PIM1 [54] | ||
| MYCN [55] | RHOA [56] | cortactin [57] | ||
| PPP2R5E [37] | ROCK1 [58] | AKT1 [59] | ||
| PRKAA1 [37] | SOX2 [60] | integrin-linked kinase [59] | ||
| PTEN [61] | STAT3 [62] | PIK3R1 [59] | ||
| ring finger protein 11 [63] | sphingosine-1-phosphate receptor 1 [64] | |||
| SMAD4 [65] | UBE3C [66] | |||
| SOCS1 [67] | MID1 | |||
| TGF-β R II [68] | ||||
| TLR2 [69] | ||||
| TNFAIP3 [70] | ||||
| TP53 [71] | ||||
Hsa-miR-340-5p regulates expression of oncogenic target mRNAs. For example, MET is a proto-oncogene [45], KRAS is a proto-oncogene [46], and overexpression of RHOA leads to tumor formation [47]. Hsa-miR-542-3p acts as tumor suppressor by targeting survivin (BIRC5) [29, 30], the oncogene astrocyte-elevated gene-1 (MTDH) [38], or angiopoietin-2 (ANGPT2) that plays a role in tumor angiogenesis [41]. Hsa-miR-374a-5p interferes with carcinogenesis by regulating Srcin1, which contributes to the growth and metastasis of colorectal cancer [31], or by regulating WIF1 that acts as tumor suppressor [32]. Interestingly, we identified MID1 as the first common target of these four miRNAs.
Another interesting observation is that a significantly decreased expression of miR-19b-3p has been reported to coincide with increased protein expression of BACE1 in Alzheimer’s disease, although miR-19b-3p does not directly target BACE1 [72]. We show here that miR-19b-3p targets MID1, a protein that we have previously shown to induce protein expression of two mRNAs that are crucial for the development of amyloid plaques in Alzheimer’s disease, namely BACE1 [4] and APP [73]. Furthermore, we have observed increased expression of MID1 in Alzheimer’s disease brains [74]. Our data showing that miR-19b-3p targets MID1 provide one possible explanation for increased expression of MID1 in Alzheimer’s disease tissue, that may be caused by reduced levels of miR-19b-3p [72, 75]. Future studies should address if miRNA based therapeutics such as miRNA mimics of the four MID1-targeting miRNAs hsa-miR-19b-3p, hsa-miR-340-5p, hsa-miR-374a-5p and hsa-miR-542-3p could be used to downregulate MID1 in Alzheimer’s disease models and thereby counteract formation of amyloid plaques.
Supporting information
(a) HEK293T cells were transfected with non-targeting (“control”) or MID1-specific siRNAs, lysed, and subjected to western blotting using the MID1 antibody for detection, either in absence (left) or presence (right) of the immunizing peptide (1 μg/ml). The antibody detects a specific band at ~75 kDa, that is reduced in MID1 knockout samples. Blocking with the immunizing peptide results in total loss of the specific signal. (b) Human temporal cortex lysate was subjected to western blotting using the MID1 antibody in absence or presence of the immunizing peptide. The antibody detects two specific bands.
(TIF)
From HEK293T total RNA a miRNA-enriched fraction was prepared. RNAs were extended by a poly(A) tailing reaction followed by reverse transcription using a poly(T) adaptor primer. MiRNA sequences were amplified by PCR using miRNA-specific forward primers and a universal poly(T) adaptor reverse primer. Samples in which reverse transcriptase was omitted (No RT) were used as controls.
(TIF)
(TIF)
(TIF)
(TIF)
(TIF)
(TIF)
(TIF)
(TIF)
(TIF)
(TIF)
(TIF)
Acknowledgments
We thank Willeke van Roon-Mom (Leiden University Medical Center) for providing human brain lysates for validation of the MID1-anitbody on western blots.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The authors received no specific funding for this work.
References
- 1.Winter J, Basilicata MF, Stemmler MP, Krauss S. The MID1 protein is a central player during development and in disease. Front Biosci (Landmark Ed). 2016;21:664–82. . [DOI] [PubMed] [Google Scholar]
- 2.Trockenbacher A, Suckow V, Foerster J, Winter J, Krauss S, Ropers HH, et al. MID1, mutated in Opitz syndrome, encodes an ubiquitin ligase that targets phosphatase 2A for degradation. Nat Genet. 2001;29(3):287–94. doi: 10.1038/ng762 . [DOI] [PubMed] [Google Scholar]
- 3.Aranda-Orgilles B, Rutschow D, Zeller R, Karagiannidis AI, Kohler A, Chen C, et al. Protein phosphatase 2A (PP2A)-specific ubiquitin ligase MID1 is a sequence-dependent regulator of translation efficiency controlling 3-phosphoinositide-dependent protein kinase-1 (PDPK-1). J Biol Chem. 2011;286(46):39945–57. Epub 2011/09/21. doi: M111.224451 [pii] doi: 10.1074/jbc.M111.224451 ; PubMed Central PMCID: PMC3220588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hettich MM, Matthes F, Ryan DP, Griesche N, Schroder S, Dorn S, et al. The anti-diabetic drug metformin reduces BACE1 protein level by interfering with the MID1 complex. PLoS One. 2014;9(7):e102420 doi: 10.1371/journal.pone.0102420 ; PubMed Central PMCID: PMC4099345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Krauss S, Griesche N, Jastrzebska E, Chen C, Rutschow D, Achmuller C, et al. Translation of HTT mRNA with expanded CAG repeats is regulated by the MID1-PP2A protein complex. Nat Commun. 2013;4:1511 doi: 10.1038/ncomms2514 . [DOI] [PubMed] [Google Scholar]
- 6.Griesche N, Schilling J, Weber S, Rohm M, Pesch V, Matthes F, et al. Regulation of mRNA translation by MID1: a common mechanism of expanded CAG repeat RNAs. Front Cell Neurosci. 2016. doi: 10.3389/fncel.2016.00226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kohler A, Demir U, Kickstein E, Krauss S, Aigner J, Aranda-Orgilles B, et al. A hormone-dependent feedback-loop controls androgen receptor levels by limiting MID1, a novel translation enhancer and promoter of oncogenic signaling. Mol Cancer. 2014;13(1):146 doi: 10.1186/1476-4598-13-146 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Krauss S, Foerster J, Schneider R, Schweiger S. Protein phosphatase 2A and rapamycin regulate the nuclear localization and activity of the transcription factor GLI3. Cancer Res. 2008;68(12):4658–65. doi: 10.1158/0008-5472.CAN-07-6174 . [DOI] [PubMed] [Google Scholar]
- 9.Goodall EF, Heath PR, Bandmann O, Kirby J, Shaw PJ. Neuronal dark matter: the emerging role of microRNAs in neurodegeneration. Front Cell Neurosci. 2013;7:178 doi: 10.3389/fncel.2013.00178 ; PubMed Central PMCID: PMC3794211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell. 2009;136(2):215–33. doi: 10.1016/j.cell.2009.01.002 ; PubMed Central PMCID: PMC3794896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lewis BP, Burge CB, Bartel DP. Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets. Cell. 2005;120(1):15–20. doi: 10.1016/j.cell.2004.12.035 . [DOI] [PubMed] [Google Scholar]
- 12.Lewis BP, Shih IH, Jones-Rhoades MW, Bartel DP, Burge CB. Prediction of mammalian microRNA targets. Cell. 2003;115(7):787–98. . [DOI] [PubMed] [Google Scholar]
- 13.Waldvogel HJ, Bullock JY, Synek BJ, Curtis MA, van Roon-Mom WM, Faull RL. The collection and processing of human brain tissue for research. Cell Tissue Bank. 2008;9(3):169–79. doi: 10.1007/s10561-008-9068-1 . [DOI] [PubMed] [Google Scholar]
- 14.Scherzinger E, Lurz R, Turmaine M, Mangiarini L, Hollenbach B, Hasenbank R, et al. Huntingtin-encoded polyglutamine expansions form amyloid-like protein aggregates in vitro and in vivo. Cell. 1997;90(3):549–58. . [DOI] [PubMed] [Google Scholar]
- 15.Panwar B, Omenn GS, Guan Y. miRmine: a database of human miRNA expression profiles. Bioinformatics. 2017;33(10):1554–60. doi: 10.1093/bioinformatics/btx019 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Arigoni M, Barutello G, Riccardo F, Ercole E, Cantarella D, Orso F, et al. miR-135b coordinates progression of ErbB2-driven mammary carcinomas through suppression of MID1 and MTCH2. Am J Pathol. 2013;182(6):2058–70. doi: 10.1016/j.ajpath.2013.02.046 . [DOI] [PubMed] [Google Scholar]
- 17.Schweiger S, Dorn S, Fuchs M, Kohler A, Matthes F, Muller EC, et al. The E3 Ubiquitin Ligase MID1 Catalyzes Ubiquitination and Cleavage of Fu. J Biol Chem. 2014;289(46):31805–17. Epub 2014/10/04. doi: M113.541219 [pii] doi: 10.1074/jbc.M113.541219 ; PubMed Central PMCID: PMC4231658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wlodarchak N, Xing Y. PP2A as a master regulator of the cell cycle. Crit Rev Biochem Mol Biol. 2016;51(3):162–84. doi: 10.3109/10409238.2016.1143913 ; PubMed Central PMCID: PMCPMC4905575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang J, Song Y, Zhang C, Zhi X, Fu H, Ma Y, et al. Circulating MiR-16-5p and MiR-19b-3p as Two Novel Potential Biomarkers to Indicate Progression of Gastric Cancer. Theranostics. 2015;5(7):733–45. doi: 10.7150/thno.10305 ; PubMed Central PMCID: PMCPMC4402497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goswami S, Tarapore RS, Poenitzsch Strong AM, TeSlaa JJ, Grinblat Y, Setaluri V, et al. MicroRNA-340-mediated degradation of microphthalmia-associated transcription factor (MITF) mRNA is inhibited by coding region determinant-binding protein (CRD-BP). J Biol Chem. 2015;290(1):384–95. doi: 10.1074/jbc.M114.590158 ; PubMed Central PMCID: PMCPMC4281741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xie J, Tan ZH, Tang X, Mo MS, Liu YP, Gan RL, et al. MiR-374b-5p suppresses RECK expression and promotes gastric cancer cell invasion and metastasis. World J Gastroenterol. 2014;20(46):17439–47. doi: 10.3748/wjg.v20.i46.17439 ; PubMed Central PMCID: PMCPMC4265603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li Y, Yao L, Liu F, Hong J, Chen L, Zhang B, et al. Characterization of microRNA expression in serous ovarian carcinoma. Int J Mol Med. 2014;34(2):491–8. doi: 10.3892/ijmm.2014.1813 . [DOI] [PubMed] [Google Scholar]
- 23.Wang Y, Huang JW, Castella M, Huntsman DG, Taniguchi T. p53 is positively regulated by miR-542-3p. Cancer Res. 2014;74(12):3218–27. doi: 10.1158/0008-5472.CAN-13-1706 ; PubMed Central PMCID: PMCPMC4058365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen BB, Chen XB, Bie LY, Mu Y, Wang HL, Lv HF, et al. Decreased expression of miR-542-3p exerts growth inhibitory functions in esophageal cancer. J Cancer Res Ther. 2015;11 Suppl 1:C24–8. doi: 10.4103/0973-1482.163834 . [DOI] [PubMed] [Google Scholar]
- 25.Ye C, Yue G, Shen Z, Wang B, Yang Y, Li T, et al. miR-542-3p suppresses colorectal cancer progression through targeting survivin. Translational Cancer Research. 2016;5(6):817–26. [Google Scholar]
- 26.Wu N, Lu Y, Liang JZ. [Expression and correlation of survivin and hsa-miR-542-3p in patients with oral squamous cell carcinoma]. Shanghai Kou Qiang Yi Xue. 2016;25(6):720–4. . [PubMed] [Google Scholar]
- 27.Chu EC, Tarnawski AS. PTEN regulatory functions in tumor suppression and cell biology. Med Sci Monit. 2004;10(10):RA235–41. . [PubMed] [Google Scholar]
- 28.Jeselsohn R, De Angelis C, Brown M, Schiff R. The Evolving Role of the Estrogen Receptor Mutations in Endocrine Therapy-Resistant Breast Cancer. Curr Oncol Rep. 2017;19(5):35 doi: 10.1007/s11912-017-0591-8 . [DOI] [PubMed] [Google Scholar]
- 29.Zhang J, Wang S, Han F, Li J, Yu L, Zhou P, et al. MicroRNA-542-3p suppresses cellular proliferation of bladder cancer cells through post-transcriptionally regulating survivin. Gene. 2016;579(2):146–52. doi: 10.1016/j.gene.2015.12.048 . [DOI] [PubMed] [Google Scholar]
- 30.Althoff K, Lindner S, Odersky A, Mestdagh P, Beckers A, Karczewski S, et al. miR-542-3p exerts tumor suppressive functions in neuroblastoma by downregulating Survivin. Int J Cancer. 2015;136(6):1308–20. doi: 10.1002/ijc.29091 . [DOI] [PubMed] [Google Scholar]
- 31.Zhang M, Ma F, Xie R, Wu Y, Wu M, Zhang P, et al. Overexpression of Srcin1 contributes to the growth and metastasis of colorectal cancer. Int J Oncol. 2017;50(5):1555–66. doi: 10.3892/ijo.2017.3952 ; PubMed Central PMCID: PMC5403293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang R, Geng N, Zhou Y, Zhang D, Li L, Li J, et al. Aberrant Wnt-1/beta-catenin signaling and WIF-1 deficiency are important events which promote tumor cell invasion and metastasis in salivary gland adenoid cystic carcinoma. Biomed Mater Eng. 2015;26 Suppl 1:S2145–53. doi: 10.3233/BME-151520 . [DOI] [PubMed] [Google Scholar]
- 33.Lee Y, Samaco RC, Gatchel JR, Thaller C, Orr HT, Zoghbi HY. miR-19, miR-101 and miR-130 co-regulate ATXN1 levels to potentially modulate SCA1 pathogenesis. Nat Neurosci. 2008;11(10):1137–9. doi: 10.1038/nn.2183 ; PubMed Central PMCID: PMCPMC2574629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wozniak M, Sztiller-Sikorska M, Czyz M. Diminution of miR-340-5p levels is responsible for increased expression of ABCB5 in melanoma cells under oxygen-deprived conditions. Exp Mol Pathol. 2015;99(3):707–16. doi: 10.1016/j.yexmp.2015.11.014 . [DOI] [PubMed] [Google Scholar]
- 35.Pan S, Zheng Y, Zhao R, Yang X. miRNA-374 regulates dexamethasone-induced differentiation of primary cultures of porcine adipocytes. Horm Metab Res. 2013;45(7):518–25. doi: 10.1055/s-0033-1334896 . [DOI] [PubMed] [Google Scholar]
- 36.Yoon S, Choi YC, Lee S, Jeong Y, Yoon J, Baek K. Induction of growth arrest by miR-542-3p that targets survivin. FEBS Lett. 2010;584(18):4048–52. doi: 10.1016/j.febslet.2010.08.025 . [DOI] [PubMed] [Google Scholar]
- 37.Mavrakis KJ, Wolfe AL, Oricchio E, Palomero T, de Keersmaecker K, McJunkin K, et al. Genome-wide RNA-mediated interference screen identifies miR-19 targets in Notch-induced T-cell acute lymphoblastic leukaemia. Nat Cell Biol. 2010;12(4):372–9. doi: 10.1038/ncb2037 ; PubMed Central PMCID: PMCPMC2989719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sun Y, Zhao X, Zhou Y, Hu Y. miR-124, miR-137 and miR-340 regulate colorectal cancer growth via inhibition of the Warburg effect. Oncol Rep. 2012;28(4):1346–52. doi: 10.3892/or.2012.1958 . [DOI] [PubMed] [Google Scholar]
- 39.Oneyama C, Morii E, Okuzaki D, Takahashi Y, Ikeda J, Wakabayashi N, et al. MicroRNA-mediated upregulation of integrin-linked kinase promotes Src-induced tumor progression. Oncogene. 2012;31(13):1623–35. doi: 10.1038/onc.2011.367 . [DOI] [PubMed] [Google Scholar]
- 40.Xu XM, Wang XB, Chen MM, Liu T, Li YX, Jia WH, et al. MicroRNA-19a and -19b regulate cervical carcinoma cell proliferation and invasion by targeting CUL5. Cancer Lett. 2012;322(2):148–58. doi: 10.1016/j.canlet.2012.02.038 . [DOI] [PubMed] [Google Scholar]
- 41.Hashimoto Y, Akiyama Y, Yuasa Y. Multiple-to-multiple relationships between microRNAs and target genes in gastric cancer. PLoS One. 2013;8(5):e62589 doi: 10.1371/journal.pone.0062589 ; PubMed Central PMCID: PMCPMC3648557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cai J, Guan H, Fang L, Yang Y, Zhu X, Yuan J, et al. MicroRNA-374a activates Wnt/beta-catenin signaling to promote breast cancer metastasis. J Clin Invest. 2013;123(2):566–79. doi: 10.1172/JCI65871 ; PubMed Central PMCID: PMCPMC3561816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shen X, Si Y, Yang Z, Wang Q, Yuan J, Zhang X. MicroRNA-542-3p suppresses cell growth of gastric cancer cells via targeting oncogene astrocyte-elevated gene-1. Med Oncol. 2015;32(1):361 doi: 10.1007/s12032-014-0361-5 . [DOI] [PubMed] [Google Scholar]
- 44.Kumar P, Luo Y, Tudela C, Alexander JM, Mendelson CR. The c-Myc-regulated microRNA-17~92 (miR-17~92) and miR-106a~363 clusters target hCYP19A1 and hGCM1 to inhibit human trophoblast differentiation. Mol Cell Biol. 2013;33(9):1782–96. doi: 10.1128/MCB.01228-12 ; PubMed Central PMCID: PMCPMC3624183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Song L, Duan P, Gan Y, Li P, Zhao C, Xu J, et al. MicroRNA-340-5p modulates cisplatin resistance by targeting LPAATbeta in osteosarcoma. Braz J Med Biol Res. 2017;50(5):e6359 doi: 10.1590/1414-431X20176359 ; PubMed Central PMCID: PMCPMC5441287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Xu X, Wang W, Su N, Zhu X, Yao J, Gao W, et al. miR-374a promotes cell proliferation, migration and invasion by targeting SRCIN1 in gastric cancer. FEBS Lett. 2015;589(3):407–13. doi: 10.1016/j.febslet.2014.12.027 . [DOI] [PubMed] [Google Scholar]
- 47.Kureel J, Dixit M, Tyagi AM, Mansoori MN, Srivastava K, Raghuvanshi A, et al. miR-542-3p suppresses osteoblast cell proliferation and differentiation, targets BMP-7 signaling and inhibits bone formation. Cell death & disease. 2014;5:e1050 doi: 10.1038/cddis.2014.4 ; PubMed Central PMCID: PMCPMC3944264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Castellano L, Giamas G, Jacob J, Coombes RC, Lucchesi W, Thiruchelvam P, et al. The estrogen receptor-alpha-induced microRNA signature regulates itself and its transcriptional response. Proc Natl Acad Sci U S A. 2009;106(37):15732–7. doi: 10.1073/pnas.0906947106 ; PubMed Central PMCID: PMCPMC2747188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wu ZS, Wu Q, Wang CQ, Wang XN, Huang J, Zhao JJ, et al. miR-340 inhibition of breast cancer cell migration and invasion through targeting of oncoprotein c-Met. Cancer. 2011;117(13):2842–52. doi: 10.1002/cncr.25860 . [DOI] [PubMed] [Google Scholar]
- 50.Wang Y, Xia H, Zhuang Z, Miao L, Chen X, Cai H. Axl-altered microRNAs regulate tumorigenicity and gefitinib resistance in lung cancer. Cell death & disease. 2014;5:e1227 doi: 10.1038/cddis.2014.186 ; PubMed Central PMCID: PMCPMC4047906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.He T, Qi F, Jia L, Wang S, Song N, Guo L, et al. MicroRNA-542-3p inhibits tumour angiogenesis by targeting angiopoietin-2. J Pathol. 2014;232(5):499–508. doi: 10.1002/path.4324 . [DOI] [PubMed] [Google Scholar]
- 52.Wu W, Dang S, Feng Q, Liang J, Wang Y, Fan N. MicroRNA-542-3p inhibits the growth of hepatocellular carcinoma cells by targeting FZD7/Wnt signaling pathway. Biochem Biophys Res Commun. 2017;482(1):100–5. doi: 10.1016/j.bbrc.2016.10.136 . [DOI] [PubMed] [Google Scholar]
- 53.Wu Q, Yang Z, An Y, Hu H, Yin J, Zhang P, et al. MiR-19a/b modulate the metastasis of gastric cancer cells by targeting the tumour suppressor MXD1. Cell death & disease. 2014;5:e1144 doi: 10.1038/cddis.2014.110 ; PubMed Central PMCID: PMCPMC3973221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rang Z, Yang G, Wang YW, Cui F. miR-542-3p suppresses invasion and metastasis by targeting the proto-oncogene serine/threonine protein kinase, PIM1, in melanoma. Biochem Biophys Res Commun. 2016;474(2):315–20. doi: 10.1016/j.bbrc.2016.04.093 . [DOI] [PubMed] [Google Scholar]
- 55.Buechner J, Tomte E, Haug BH, Henriksen JR, Lokke C, Flaegstad T, et al. Tumour-suppressor microRNAs let-7 and mir-101 target the proto-oncogene MYCN and inhibit cell proliferation in MYCN-amplified neuroblastoma. Br J Cancer. 2011;105(2):296–303. doi: 10.1038/bjc.2011.220 ; PubMed Central PMCID: PMCPMC3142803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jian Q, An Q, Zhu D, Hui K, Liu Y, Chi S, et al. MicroRNA 340 is involved in UVB-induced dendrite formation through the regulation of RhoA expression in melanocytes. Mol Cell Biol. 2014;34(18):3407–20. doi: 10.1128/MCB.00106-14 ; PubMed Central PMCID: PMCPMC4135612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Long HC, Gao X, Lei CJ, Zhu B, Li L, Zeng C, et al. miR-542-3p inhibits the growth and invasion of colorectal cancer cells through targeted regulation of cortactin. Int J Mol Med. 2016;37(4):1112–8. doi: 10.3892/ijmm.2016.2505 . [DOI] [PubMed] [Google Scholar]
- 58.Zhou X, Wei M, Wang W. MicroRNA-340 suppresses osteosarcoma tumor growth and metastasis by directly targeting ROCK1. Biochem Biophys Res Commun. 2013;437(4):653–8. doi: 10.1016/j.bbrc.2013.07.033 . [DOI] [PubMed] [Google Scholar]
- 59.Cai J, Zhao J, Zhang N, Xu X, Li R, Yi Y, et al. MicroRNA-542-3p Suppresses Tumor Cell Invasion via Targeting AKT Pathway in Human Astrocytoma. J Biol Chem. 2015;290(41):24678–88. doi: 10.1074/jbc.M115.649004 ; PubMed Central PMCID: PMCPMC4598981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Das S, Bryan K, Buckley PG, Piskareva O, Bray IM, Foley N, et al. Modulation of neuroblastoma disease pathogenesis by an extensive network of epigenetically regulated microRNAs. Oncogene. 2013;32(24):2927–36. doi: 10.1038/onc.2012.311 ; PubMed Central PMCID: PMCPMC3477279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Olive V, Bennett MJ, Walker JC, Ma C, Jiang I, Cordon-Cardo C, et al. miR-19 is a key oncogenic component of mir-17-92. Genes Dev. 2009;23(24):2839–49. doi: 10.1101/gad.1861409 ; PubMed Central PMCID: PMCPMC2800084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Xiong Q, Wu S, Wang J, Zeng X, Chen J, Wei M, et al. Hepatitis B virus promotes cancer cell migration by downregulating miR-340-5p expression to induce STAT3 overexpression. Cell Biosci. 2017;7:16 doi: 10.1186/s13578-017-0144-8 ; PubMed Central PMCID: PMCPMC5389182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ashraf U, Zhu B, Ye J, Wan S, Nie Y, Chen Z, et al. MicroRNA-19b-3p Modulates Japanese Encephalitis Virus-Mediated Inflammation via Targeting RNF11. J Virol. 2016;90(9):4780–95. doi: 10.1128/JVI.02586-15 ; PubMed Central PMCID: PMCPMC4836334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wu HX, Wang GM, Lu X, Zhang L. miR-542-3p targets sphingosine-1-phosphate receptor 1 and regulates cell proliferation and invasion of breast cancer cells. Eur Rev Med Pharmacol Sci. 2017;21(1):108–14. . [PubMed] [Google Scholar]
- 65.Fuziwara CS, Kimura ET. High iodine blocks a Notch/miR-19 loop activated by the BRAF(V600E) oncoprotein and restores the response to TGFbeta in thyroid follicular cells. Thyroid. 2014;24(3):453–62. doi: 10.1089/thy.2013.0398 ; PubMed Central PMCID: PMCPMC3949441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tao J, Liu Z, Wang Y, Wang L, Yao B, Li Q, et al. MiR-542-3p inhibits metastasis and epithelial-mesenchymal transition of hepatocellular carcinoma by targeting UBE3C. Biomed Pharmacother. 2017;93:420–8. doi: 10.1016/j.biopha.2017.06.070 . [DOI] [PubMed] [Google Scholar]
- 67.Pichiorri F, Suh SS, Ladetto M, Kuehl M, Palumbo T, Drandi D, et al. MicroRNAs regulate critical genes associated with multiple myeloma pathogenesis. Proc Natl Acad Sci U S A. 2008;105(35):12885–90. doi: 10.1073/pnas.0806202105 ; PubMed Central PMCID: PMCPMC2529070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zou M, Wang F, Gao R, Wu J, Ou Y, Chen X, et al. Autophagy inhibition of hsa-miR-19a-3p/19b-3p by targeting TGF-beta R II during TGF-beta1-induced fibrogenesis in human cardiac fibroblasts. Sci Rep. 2016;6:24747 doi: 10.1038/srep24747 ; PubMed Central PMCID: PMCPMC4838850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Philippe L, Alsaleh G, Suffert G, Meyer A, Georgel P, Sibilia J, et al. TLR2 expression is regulated by microRNA miR-19 in rheumatoid fibroblast-like synoviocytes. J Immunol. 2012;188(1):454–61. doi: 10.4049/jimmunol.1102348 . [DOI] [PubMed] [Google Scholar]
- 70.Huang T, Yin L, Wu J, Gu JJ, Wu JZ, Chen D, et al. MicroRNA-19b-3p regulates nasopharyngeal carcinoma radiosensitivity by targeting TNFAIP3/NF-kappaB axis. J Exp Clin Cancer Res. 2016;35(1):188 doi: 10.1186/s13046-016-0465-1 ; PubMed Central PMCID: PMCPMC5139034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Fan Y, Yin S, Hao Y, Yang J, Zhang H, Sun C, et al. miR-19b promotes tumor growth and metastasis via targeting TP53. RNA. 2014;20(6):765–72. doi: 10.1261/rna.043026.113 ; PubMed Central PMCID: PMCPMC4024631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hebert SS, Horre K, Nicolai L, Papadopoulou AS, Mandemakers W, Silahtaroglu AN, et al. Loss of microRNA cluster miR-29a/b-1 in sporadic Alzheimer's disease correlates with increased BACE1/beta-secretase expression. Proc Natl Acad Sci U S A. 2008;105(17):6415–20. doi: 10.1073/pnas.0710263105 ; PubMed Central PMCID: PMCPMC2359789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Matthes F, Hettich MM, Schilling J, Flores-Dominguez D, Blank N, Wiglenda T, et al. Inhibition of the MID1 protein complex: a novel approach targeting APP protein synthesis Cell Death Discovery. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Schweiger S, Matthes F, Posey K, Kickstein E, Weber S, Hettich MM, et al. Resveratrol induces dephosphorylation of Tau by interfering with the MID1-PP2A complex. Sci Rep. 2017;7(1):13753 doi: 10.1038/s41598-017-12974-4 ; PubMed Central PMCID: PMCPMC5653760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wu Y, Xu J, Xu J, J, Jiao D, Zhou C, et al. Lower Serum Levels of miR-29c-3p and miR-19b-3p as Biomarkers for Alzheimer's Disease. Tohoku J Exp Med. 2017;242(2):129–36. doi: 10.1620/tjem.242.129 . [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(a) HEK293T cells were transfected with non-targeting (“control”) or MID1-specific siRNAs, lysed, and subjected to western blotting using the MID1 antibody for detection, either in absence (left) or presence (right) of the immunizing peptide (1 μg/ml). The antibody detects a specific band at ~75 kDa, that is reduced in MID1 knockout samples. Blocking with the immunizing peptide results in total loss of the specific signal. (b) Human temporal cortex lysate was subjected to western blotting using the MID1 antibody in absence or presence of the immunizing peptide. The antibody detects two specific bands.
(TIF)
From HEK293T total RNA a miRNA-enriched fraction was prepared. RNAs were extended by a poly(A) tailing reaction followed by reverse transcription using a poly(T) adaptor primer. MiRNA sequences were amplified by PCR using miRNA-specific forward primers and a universal poly(T) adaptor reverse primer. Samples in which reverse transcriptase was omitted (No RT) were used as controls.
(TIF)
(TIF)
(TIF)
(TIF)
(TIF)
(TIF)
(TIF)
(TIF)
(TIF)
(TIF)
(TIF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.