Abstract
Background
Despite of numerous evidences that elevated serum lactate levels were associated with unfavorable outcomes, there have been no study demonstrated an optimal cutoff of serum lactate in unselected patients. This study was aimed to evaluate the prognostic property of lactate, and to identify a cutoff of serum lactate level for predicting 30-day in-hospital mortality among unselected patients presenting to the emergency department (ED).
Methods
We performed a retrospective observational study from January 2010 to December 2016. 61,151 patients were used for propensity score analysis after exclusion. 14,015 patients who underwent lactate test at ED arrival were enrolled for final analysis.
Results
The average treatment effect (ATE) of carrying out a lactate test on 30-day in-hospital mortality was 0.53% (adjusted odds ratio (OR) = 1.013, p = 0.19; 95% confidence interval (CI), 0.997–1.013). Adjusted OR of serum lactate calculated from multivariable analysis was 1.09 (p < 0.001; 95% CI, 1.07–1.10). The area under a ROC curve (AUC) of serum lactate was 0.711 (p < 0.001; 95% CI, 0.703–0.718). The sensitivity, specificity, and positive and negative predictive values for the cutoff > 2.6 mmol/L were 56.7%, 74.3%, 20.8%, and 93.5%, respectively. Mortality of the high-lactate group (> 2.6 mmol/L) was significantly higher than that of the low lactate group (≤ 2.6 mmol/L) (20.8% vs. 6.5%, difference = 14.3%, p < 0.01; 95% CI, 13.0% - 15.7%).
Conclusions
A serum lactate level > 2.6 mmol/L predicted 30-day in-hospital mortality in unselected patients who arrived to the ED and were admitted to the hospital. Additionally, serum lactate test in the ED could be an effective screening method for identifying low risk patients.
Introduction
Hyperlactatemia has been shown to be related to higher mortality in selected patients admitted in the intensive care units (ICUs) with specific conditions such as sepsis, trauma, or critical illness [1, 2]. In the emergency department (ED) setting, several studies reported similar results in patients with specific diagnoses [3, 4]. However, the limited study populations lead to narrow applicability of serum lactate. Many previous studies evaluated the relationship between serum lactate level and mortality in ED patients with specific diagnoses [5, 6], however, studies of unselected patients were few [7, 8]. Because serum lactate tests have not been established as part of a routine work up in unselected ED patients, selection bias was unavoidable in studies that evaluated the relationship between serum lactate level and mortality in patients in the ED setting. To overcome this limitation, a recent study included a control group of patients in whom lactate was not measured; the study showed hyperlactatemia was associated with higher mortality. However, this study also had some limitations such as a relatively small (n = 600) and restricted (ED patients with internal disease) study population [9].
Another issue regarding the feasibility of serum lactate is determination of the cutoff value. Despite a value of ≥ 4.0 mmol/L being recommended for prompt resuscitation in current international guidelines for the management of sepsis and septic shock [10], a recent study in patients with septic shock in ICU setting reported a value of 2.5 mmol/L as a cutoff in predicting mortality among patients with severe sepsis and septic shock [11]. Besides, other previous studies have shown that lactate levels lower than 4.0 mmol/L were associated with poor outcome [12, 13]. More recently, combination of the National Early Warning Score (NEWS) and lactate has been studied as a triage tool for predicting outcomes of ED patients [14]. The study showed lactate provided additional predictive value to the NEWS and NEWS plus lactate were superior to the NEWS alone in predicting unfavorable outcomes.
Despite these abundant studies suggested that lactate may play an important role in detection of patients with high risk for poor outcome in various clinical situations, there have been no studies demonstrating whether lactate can be used as a prognostic marker in truly unselected ED population.
The aim of this study was (1) to evaluate the prognostic property of lactate, (2) to identify a cutoff value of serum lactate in predicting in-hospital mortality among unselected patients presenting to the ED, and (3) to assess the outcomes in patients with hyperlactatemia defined by the determined cutoff value.
Materials and methods
Study design
This was a single center retrospective hospital-based cohort study that analyzed patients presenting to the ED of the Gyeongsang National University Hospital, which is a tertiary referral hospital located in the south-central region of the Republic of Korea. Gyeongsang National University institutional review board approved this study with the exemption of informed consent because of the retrospective nature of the analysis.
Study setting and participants
All patients arriving at the ED must be enrolled in the National Emergency Department Information System (NEDIS) of Korea. The input data are organized using the standard NEDIS registry format in the electronic medical records (EMR) of the hospital and sent to the NEDIS server. The NEDIS is a national database that is prepared by 146 emergency medical centers and managed by a government-funded national ED control agency [15, 16]. Initial data from patients are entered by triage nurses and duty doctors at ED arrival: physiologic parameters at ED arrival, symptoms, and diagnosis. Basic demographic and temporal information, treatment details including drugs and procedures, outcomes, and other information are created from the EMR and transferred to the registry. Validity of all data is checked by function modules within the EMR system before the data is saved.
Consecutive patients who presented to the ED between January 2010 and December 2016 were enrolled. Patients were included if they were ≥ 18 years of age, admitted to the hospital after ED management, and had lactate result in the first three hours after ED arrival. We excluded patients who were transferred to other facilities after admission, discharged with no hope of recovery, or left the hospital against medical advice, because the final outcomes of these patients could not be determined.
The annual ED census of the hospital during the study period ranged from 32,000 to 35,000 patients.
Study protocol and measurements
Data were extracted from the EMR system of the hospital. Demography (age and sex), patient′s categorization (disease stemming from medical illness or injury from external cause), physiologic parameters (mental status, systolic blood pressure, heart rate, respiratory rate, body temperature, and oxygen saturation (SpO2)), laboratory results (lactate, white blood cell count (WBC), hemoglobin, platelet, international normalized ratio of prothrombin time (PTinr), glucose, creatinine, bilirubin, c-reactive protein (CRP), and base deficit), time variables (date of ED arrival, death, and discharge), and final outcome (discharge, transfer, death, or other) were collected. The patients were classified into subgroups for further analysis: diseased or injured by patient category at ED arrival; patients with suspected infection (antibiotic use within 24 hours) or without infection.
We analyzed the first laboratory results within three hours after ED arrival. The serum lactate tests were embedded in the point-of-care testing (POCT) module in the ED, and the results were reported within 5 minutes after blood sampling. The POCT module included arterial blood gas, electrolytes, complete blood count, and lactate. Blood samples were mostly collected from the radial or femoral artery. Other blood tests were ordered as routine work-up. Final discharge results were extracted from the discharge summary in the EMR system. Primary outcome was 30-day in-hospital mortality.
Data analysis
Because of the retrospective nature of the study and concern of selection bias, we evaluated whether execution of the serum lactate test was associated with the outcome (30-day in-hospital mortality) using propensity score analysis. Serum lactate was not performed as a routine test in patients who presented to our ED. Physicians tended to order the test when patients appeared to be in serious condition, such as worsening physiological parameters, decreased mentality, lower SpO2, and significant results from other routine blood tests. Additionally, because our POCT module comprised blood gas, electrolytes, complete blood count, and lactate, physicians also tended to order the POCT (with the serum lactate test) as a follow-up test. The propensity of carrying out a lactate test were estimated using a logistic regression with all available information before the physician decided to order the test. Average treatment effect (ATE) of execution of lactate test on 30-day in-hospital mortality was calculated using propensity score matching analysis with the inverse probability weights method. We hypothesized that selection bias would be minimal if the ATE was less than statistically significant level.
We used multivariate imputation with chained equation (MICE) to impute all missing values [17]. The number of multiple imputations should be increased as the fraction of missing information increases. Most researchers accepted the rule that the number of imputations should reach the percentage of missing cases (e.g., at least 10 iterations for 10% missing data) [18, 19]. In our data set, base deficit was the most frequently missing value (6.4%), followed by the value of CRP (3.0%), SpO2 (1.3%), creatinine (1.2%), complete blood cell count (0.3%), and physiologic parameters (0.1~0.3%) (Table 1). To minimize additional variation due to the estimation of the missing data, we conducted 20 imputations considering the variable with the largest missing fraction (6.4%, base deficit). An important premise for multiple imputation analysis is that the missing data mechanism depends on the variables in the same dataset (missing at random). In our study, missing data mostly occurred in laboratory results. The physicians’ decision to order a specific blood test mainly depended on initial clinical findings such as demographic characteristics and physiologic parameters; therefore, we assumed that our data were missing at random. Multiple imputation with the MICE method by predictive mean matching was performed to create 20 multiply-imputed datasets. Univariable and multivariable logistic regression analysis were performed for all the demographic, physiological, and biochemical variables in each imputed dataset, and the results were combined. Every combination of steps for the estimated results from the imputed data followed the Rubin’s rule [20]. After verification of the adjusted odds ratio (OR) of serum lactate, receiver operating characteristics (ROC) curve was constructed. The lactate cutoff was chosen as the maximum value on Youden’s index calculated by following formula [21].
Table 1. Baseline characteristics of study population.
| Variable | Value | Missing, n (%) |
|---|---|---|
| Demography | ||
| Age, year | 68 (56–78) | 0 (0) |
| < 40, n (%) | 1013 (7.2) | 0 (0) |
| 40–70, n (%) | 6669 (47.6) | 0 (0) |
| > 70, n (%) | 6333 (45.2) | 0 (0) |
| Sex (male), n (%) | 8382 (59.8) | 0 (0) |
| Category (disease), n (%) | 12366 (88.2) | 0 (0) |
| Suspected infection, n (%) | 4054 (28.9) | 0 (0) |
| Physiologic parameters | ||
| Consciousness (alert), n (%) | 11919 (85.0) | 0 (0) |
| O2 supply, n (%) | 5558 (39.7) | 0 (0) |
| Systolic blood pressure, mmHg | 120 (100–140) | 45 (0.3) |
| Heart rate, per minute | 88 (78–106) | 41 (0.3) |
| Respiratory rate, per minute | 20 (20–22) | 41 (0.3) |
| Body temperature, °C | 36.6 (36.3–37.1) | 14 (0.1) |
| Oxyhemoglobin saturation, % | 97 (94–98) | 181 (1.3) |
| NEWS | 6 (4–9) | 209 (1.5) |
| Laboratory results | ||
| Lactate, mmol/L | 1.7 (1.1–3) | 0 (0) |
| White blood cell, x103/mm3 | 9.79 (6.89–13.86) | 46 (0.3) |
| Hemoglobin, g/dl | 12 (10.3–13.6) | 46 (0.3) |
| Platelet, x103/mm3 | 217 (159–281) | 46 (0.3) |
| PTinr | 1.09 (1.01–1.22) | 68 (0.5) |
| Glucose, mg/dl | 138 (112–189) | 90 (0.6) |
| Creatinine, mg/dl | 0.91 (0.69–1.31) | 171 (1.2) |
| Bilirubin, mg/dl | 0.61 (0.39–1.01) | 75 (0.5) |
| C-reactive protein, mg/L | 12.5 (1.7–74.1) | 427 (3) |
| Base deficit, mEq/L | 1.9 (-0.5–5.3) | 903 (6.4) |
| 30-day in hospital mortality, n (%) | 1487 (10.6) | 0 (0) |
Abbreviations: NEWS, National Early Warning Score; PTinr, international normalized ratio of prothrombin time. Values are presented as median and interquartile range when not stated otherwise
Sensitivity, specificity, and positive and negative predictive values for the cutoff level were calculated. The derived lactate cutoff were assessed in subgroups of patients with disease or injury and patients with or without suspected infection. We divided patients into two groups (low-lactate and high-lactate group), and survival analysis was performed using the Kaplan-Meier method to obtain the 30-day survival curves of the groups. Differences between the groups were examined using the log-rank test. Because predictive values of a test vary depending on the prevalence of disease, we performed a sensitivity analysis to estimate the positive and negative predictive value based on plausible mortality rates.
The χ2 test was used to test for differences in categorical data. Independent t-test and Mann-Whitney U test were used for continuous data with normal and skewed distribution, respectively. All p values were two-sided, and a value of < 0.05 was considered statistically significant. Analyses were performed using MedCalc 17 (MedCalc Software BVBA, Ostend, Belgium) and Stata version 13 (StataCorp, LP, College Station, TX).
Results
Characteristics of study subjects
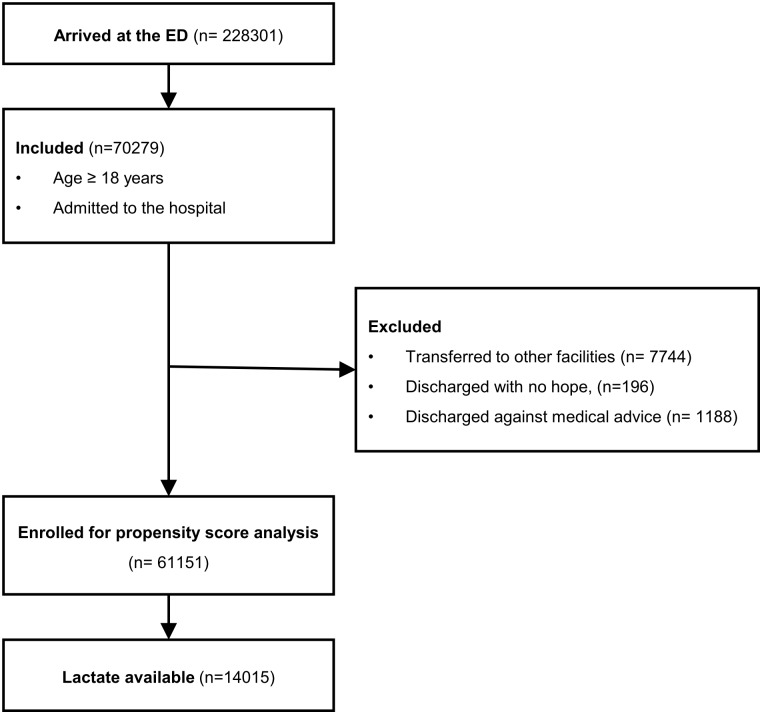
A total of 228,301 patients presented to the ED during the study period. 70,279 patients matched inclusion criteria (≥ 18 years of age, hospitalized after ED management). We excluded total 9,128 patients: 7,744 were transferred, 196 were discharged with no hope of recovery, and 1,188 were discharged against medical advice. After exclusion, 61,151 patients were used for propensity score analysis. Among them, serum lactate values were available in 14,015 patients that were used for final analysis (Fig 1). Age was categorized into three groups (<40, 40–70, and >70), and the NEWS was calculated in each patient. Basal characteristics of the patients are shown in Table 1.
Fig 1. Inclusion and exclusion flow chart.
Propensity score analysis
In a multivariable logistic regression analysis, we selected statistically significant variables (p < 0.01) which explained the propensity of execution of the lactate test: age, consciousness, systolic blood pressure, breath rate, SpO2, WBC, hemoglobin, platelet, glucose, creatinine, bilirubin, CRP, and base deficit (Table 2). The ATE of carrying out a lactate test on 30-day in-hospital mortality was 0.53% (95% confidence interval (CI), -0.27%–1.32%), and not statistically significant (adjusted OR = 1.013, p = 0.19; 95% confidence interval (CI), 0.997–1.013).
Table 2. Adjusted odds ratio of variables on execution of lactate test.
| Variable | Adjusted odds ratio (95% CI) | p value |
|---|---|---|
| Sex | 0.937 (0.887–0.908) | 0.011 |
| Age | 1.009 (1.008–1.031) | < 0.001 |
| Consciousness (not alert) | 2.376 (2.292–4.237) | < 0.001 |
| Systolic blood pressure | 0.987 (0.986–0.964) | < 0.001 |
| Heart rate | 1.002 (1.000–1.007) | 0.016 |
| Respiratory rate | 1.051 (1.044–1.165) | < 0.001 |
| Body temperature | 1.000 (0.972–1.053) | 0.992 |
| Oxyhemoglobin saturation | 0.983 (0.979–0.956) | < 0.001 |
| White blood cell | 1.005 (1.002–1.021) | 0.001 |
| Hemoglobin | 0.968 (0.957–0.925) | < 0.001 |
| Platelet | 0.999 (0.999–0.999) | < 0.001 |
| PTinr | 1.002 (0.974–1.063) | 0.871 |
| Glucose | 1.002 (1.001–1.006) | < 0.001 |
| Creatinine | 1.043 (1.028–1.156) | < 0.001 |
| Bilirubin | 0.958 (0.946–0.896) | < 0.001 |
| C-reactive protein | 0.998 (0.998–0.996) | < 0.001 |
| Base deficit | 1.010 (1.005–1.041) | < 0.001 |
Prognostic properties of serum lactate in unselected patients and subgroups
Univariable and multivariable logistic regression analyses were performed on multiply imputed datasets. All the variables (age, sex, NEWS, WBC, hemoglobin, platelet, PTinr, creatinine, bilirubin, CRP, base deficit, and lactate) were significant in univariable analysis (Table 3), and the adjusted OR of serum lactate calculated from multivariable analysis was 1.09 (p < 0.001; 95% CI, 1.07–1.10) (Table 4). The area under a ROC curve (AUC) of serum lactate was 0.711 (p < 0.001; 95% CI, 0.703–0.718). The sensitivity, specificity, and positive and negative predictive values for the cutoff > 2.6 mmol/L (the point of maximum Youden’s index) were 56.7%, 74.3%, 20.8%, and 93.5%, respectively (Table 5).
Table 3. Results of univariable analysis on multiply imputed datasets.
| Variable | Odds ratio (95% CI) | p value |
|---|---|---|
| Age | 1.0150 (1.0112–1.0188) | < 0.001 |
| <40 | - | - |
| 40–70 | 2.8415 (2.0424–3.9531) | < 0.001 |
| >70 | 3.4120 (2.4566–4.7389) | < 0.001 |
| Sex (female) | 0.7010 (0.6255–0.7856) | < 0.001 |
| Lactate | 1.1546 (1.1389–1.1704) | < 0.001 |
| White blood cell | 1.0318 (1.0246–1.0391) | < 0.001 |
| Hemoglobin | 0.9068 (0.8886–0.9254) | < 0.001 |
| Platelet | 0.9981 (0.9975–0.9986) | < 0.001 |
| PTinr | 1.2496 (1.1982–1.3032) | < 0.001 |
| Glucose | 1.0011 (1.0006–1.0015) | < 0.001 |
| Creatinine | 1.0869 (1.0619–1.1124) | < 0.001 |
| Bilirubin | 1.1433 (1.1226–1.1643) | < 0.001 |
| C-reactive protein | 1.0050 (1.0045–1.0056) | < 0.001 |
| Base deficit | 1.0853 (1.0771–1.0935) | < 0.001 |
| NEWS | 1.1877 (1.1703–1.2053) | < 0.001 |
Table 4. Adjusted odds ratios of serum lactate in multivariable analysis.
| Category | n | Odds ratio (95% CI) | p value |
|---|---|---|---|
| All patients | 14015 | 1.09 (1.07–1.10) | 0.000 |
| Patients with disease | 12366 | 1.10 (1.08–1.12) | 0.000 |
| Patients with injury | 1649 | 1.05 (1.03–1.08) | 0.000 |
| Patients with suspected infection | 9961 | 1.08 (1.06–1.10) | 0.000 |
| Patients without suspected infection | 4054 | 1.09 (1.06–1.12) | 0.000 |
Abbreviations: CI, confidence interval.
Table 5. Prognostic properties of serum lactate (cutoff > 2.6 mmol/L).
| Category | Sensitivity | Specificity | PPV | NPV |
|---|---|---|---|---|
| (95% CI) | (95% CI) | (95% CI) | (95% CI) | |
| All patients | 56.7 | 74.3 | 20.8 | 93.5 |
| (54.1–59.2) | (73.5–75.1) | (19.9–21.6) | (93.2–93.9) | |
| Patients with disease | 54.7 | 76.5 | 22.1 | 93.3 |
| (52.0–57.4) | (75.7–77.3) | (21.1–23.1) | (92.9–93.7) | |
| Patients with injury | 74.8 | 58.2 | 14.9 | 95.9 |
| (67.0–81.6) | (55.6–60.7) | (13.5–16.4) | (94.7–96.9) | |
| Patients with suspected infection | 58.2 | 76.6 | 19.9 | 94.8 |
| (54.9–61.4) | (75.7–77.5) | (18.8–21.0) | (94.5–95.2) | |
| Patients without suspected infection | 54.4 | 68.2 | 22.4 | 89.9 |
| (50.2–58.4) | (66.7–69.8) | (20.9–24.0) | (89.0–90.7) |
Abbreviations: CI, confidence interval; PPV, positive predictive value; NPV, negative predictive value
Adjusted OR of lactate in subgroups were all statistically significant (p < 0.001) (Table 4). We chose the cutoff > 2.6 mmol/L for subgroups analysis (patients with disease or injury, and patients with or without suspected infection). Prognostic properties of lactate in subgroups were summarized in Table 5.
Survival analysis
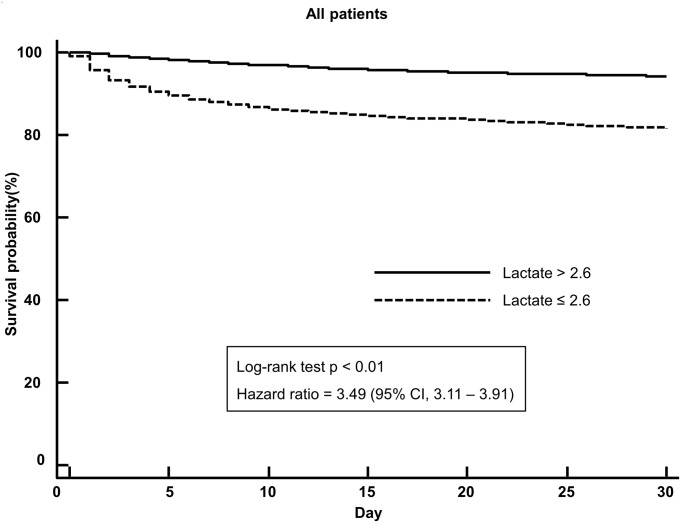
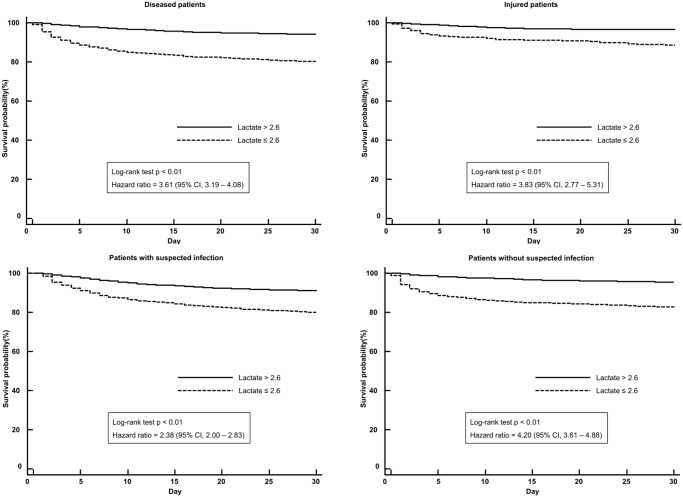
Mortality of the high-lactate group (> 2.6 mmol/L) was significantly higher than that of the low lactate group (≤ 2.6 mmol/L) (20.8% vs. 6.5%, difference = 14.3%, p < 0.01; 95% CI, 13.0%–15.7%). In patients with medical illness (n = 12,366), the high-lactate group showed significantly higher mortality than the low-lactate group (22.1% vs. 6.7%, difference = 15.4%, p < 0.01; 95% CI, 13.9%–16.9%). In patients with injury (n = 1,649), the high-lactate group showed significantly higher mortality than the low-lactate group (14.9% vs. 4.1%, difference = 10.8%, p < 0.01; 95% CI, 7.9%–13.8%). In patients with suspected infection (n = 4,054), the high-lactate group showed significantly higher mortality than the low-lactate group (22.4% vs. 10.1%, difference = 12.3%, p < 0.01; 95% CI, 9.8%–14.8%). In patients without suspected infection (n = 9,961), the high-lactate group showed significantly higher mortality than the low-lactate group (19.9% vs. 5.2%, difference = 14.7%, p < 0.01; 95% CI, 13.1%–16.4%). Kaplan-Meier curves for unselected patients and subgroups were illustrated in Figs 2 and 3.
Fig 2. Kaplan-Meier survival curves for 30-day survival according to the cutoff (>2.6 mmol/L) in all patients.
Fig 3. Kaplan-Meier survival curves for 30-day survival according to the cutoff (>2.6 mmol/L) in subpopulations.
Sensitivity analysis
Mortality rate of our study population was 10.6%, and those of subgroups were ranged from 8.9 to 14.4%. Because predictive values of serum lactate varied depending on mortality rate in our study, we performed a sensitivity analysis for the positive and negative predictive value based on plausible mortality rates ranged from 1 to 40% (Table 6).
Table 6. Sensitivity analysis for predictive values.
| Category | PPV (95% CI) | NPV (95% CI) | Mortality (%) |
|---|---|---|---|
| All patients | |||
| 59.5 (58.2–60.8) | 72.0 (70.8–73.2) | 40 | |
| 48.6 (47.3–49.9) | 80.0 (79.1–80.9) | 30 | |
| 35.6 (34.3–36.8) | 87.3 (86.6–87.9) | 20 | |
| 28.0 (27.0–29.1) | 90.7 (90.2–91.2) | 15 | |
| 20.8 (19.9–21.6) | 93.5 (93.2–93.9) | 10.6a | |
| 10.4 (9.9–10.9) | 97.0 (96.8–97.2) | 5 | |
| 6.4 (6.1–6.7) | 98.2 (98.1–98.3) | 3 | |
| 2.2 (2.1–2.3) | 99.4 (99.4–99.4) | 1 | |
| Patients with disease | |||
| 60.8 (59.4–62.2) | 71.7 (70.5–72.9) | 40 | |
| 49.9 (48.5–51.4) | 79.8 (78.8–80.7) | 30 | |
| 36.8 (35.4–38.2) | 87.1 (86.4–87.8) | 20 | |
| 29.1 (27.9–30.4) | 90.5 (90.0–91.0) | 15 | |
| 22.1 (21.1–23.1) | 93.3 (92.9–93.7) | 10.84a | |
| 10.9 (10.4–11.5) | 97.0 (96.8–97.1) | 5 | |
| 6.7 (6.4–7.1) | 98.2 (98.1–98.3) | 3 | |
| 2.3 (2.2–2.4) | 99.4 (99.4–99.4) | 1 | |
| Patients with injury | |||
| 54.4 (51.6–57.1) | 77.6 (72.3–82.1) | 40 | |
| 43.4 (40.7–46.2) | 84.4 (80.3–87.7) | 30 | |
| 30.9 (28.6–33.3) | 90.2 (87.5–92.5) | 20 | |
| 24.0 (22.0–26.1) | 92.9 (90.8–94.6) | 15 | |
| 14.9 (13.5–16.4) | 95.9 (94.7–96.9) | 8.9a | |
| 8.6 (7.8–9.5) | 97.8 (97.1–98.3) | 5 | |
| 5.2 (4.7–5.8) | 98.7 (98.3–99.0) | 3 | |
| 1.8 (1.6–2.0) | 99.6 (99.4–99.7) | 1 | |
| Patients with suspected infection | |||
| 53.3 (51.1–55.5) | 69.2 (67.2–71.1) | 40 | |
| 42.3 (40.2–44.5) | 77.7 (76.1–79.3) | 30 | |
| 30.0 (28.1–31.9) | 85.7 (84.5–86.8) | 20 | |
| 22.4 (20.9–24.0) | 89.9 (89.0–90.7) | 14.43a | |
| 16.0 (14.8–17.2) | 93.1 (92.5–93.6) | 10 | |
| 8.3 (7.6–9.0) | 96.6 (96.3–96.9) | 5 | |
| 5.0 (4.6–5.5) | 98.0 (97.8–98.1) | 3 | |
| 1.7 (1.6–1.9) | 99.3 (99.3–99.4) | 1 | |
| Patients without suspected infection | |||
| 62.4 (60.8–64.0) | 73.3 (71.8–74.8) | 40 | |
| 51.6 (50.0–53.3) | 81.1 (79.8–82.2) | 30 | |
| 38.4 (36.8–40.0) | 88.0 (87.2–88.8) | 20 | |
| 30.5 (29.1–32.0) | 91.2 (90.6–91.8) | 15 | |
| 19.9 (18.8–21.0) | 94.8 (94.5–95.2) | 9.06a | |
| 11.6 (10.9–12.3) | 97.2 (97.0–97.4) | 5 | |
| 7.2 (6.7–7.6) | 98.3 (98.2–98.5) | 3 | |
| 2.5 (2.3–2.6) | 99.5 (99.4–99.5) | 1 | |
a Observed mortality in study population
Discussion
In this study, the AUC of initial lactate level as a predictor of 30-day in-hospital mortality in unselected patients who presented at the ED was 0.711. The discrimination power was acceptable according to the standard suggested by Metz CE (0.9–1 excellent, 0.8–0.9 good, 0.7–0.8 fair, 0.6–0.7 poor, 0.5–0.6 fail) [22]. Statistical significance of the cutoff of lactate > 2.6 mmol/L was maintained even after categorization of the patients into groups according to etiology (disease and injury) and the presence of suspected infection. Higher mortality in ED patients with mild hyperlactatemia has been addressed in many previous studies. In a systematic review of the prognosis of ED patients with suspected infection, lower lactate levels (2.0–4.0 mmol/L) were associated with higher mortality [6]. Several studies showed consistent results in trauma patients [23, 24]. In these studies, cutoffs of serum lactate associated with higher mortality were 2.5–4.0 mmol/L. There have been very few studies in unselected ED patients on this issue. In a retrospective cohort study involving 5,360 ED patients, intermediate lactate levels (2.0–3.9 mmol/L) were shown to be associated with a worse outcome [7]. A prospective cohort study involving 747 ill ED patients showed that initial mild hyperlactatemia (2.0–4.0 mmol/L) was associated with higher mortality [8]. Despite numerous studies showed that mild hyperlactatemia (cutoffs ranged from 2.0 to 2.5) was associated with unfavorable outcome, there have been no studies demonstrating optimal cutoffs of serum lactate. To our best knowledge, this is the first study indicating a lactate cutoff value for detection of high risk patients among unselected ED population.
We performed a propensity score analysis in order to estimate selection bias and to justify that our study population was unselected. The ATE of carrying out lactate test on 30-day in-hospital mortality was only 0.53% and statistically insignificant. This means, in our study, execution of lactate test was not associated with patient outcome, and therefore selection bias from including patients who underwent lactate test only was minimal.
The sensitivity and specificity of serum lactate were 56.7% and 74.3. At the cutoff of lactate > 2.6 mmol/L, positive and negative predictive value were 20.8%, and 93.5%. This tendency of prognostic properties were consistently shown in the study by Filho et al. In the study, sensitivity, specificity, and positive and negative predictive values were 67.4%, 61.7%, 16.9%, and 94.2%, respectively, and AUC of serum lactate was 0.70 [11]. The authors demonstrated a cutoff of > 2.5 mmol/L which was similar to our results. The low positive predictive value and high negative predictive value indicate that serum lactate test might be an effective screening tool. We believe these consistent results from different population (ICU patients with sepsis and unselected ED patients) imply that serum lactate test should be more widely used for early detection of high risk patients in various clinical settings.
Overall mortality in this study (10.6%) was probably higher than general population presented in the ED because we only included patients admitted after ED management. Sensitivity analysis showed rule-out capacity of blood lactate test could be improved with mortality decrease in target population. For example, in a certain emergency department where an overall mortality rate is 1% (lower than our population, 10.6% in all patients), estimated positive and negative predictive value of lactate > 2.6 mmol/L are 2.2% and 99.4% (i.e., only 0.6% patients of ≤ lactate 2.6 mmol/L will die), lactate test in such population would be an excellent screening tool for discriminating low risk patients.
One limitation to the implementation of routine lactate test is the cost. Ward MJ et al. reported point-of-care lactate testing for screening ED patients with suspected sepsis is cost-effective to identify patients responsive to early resuscitation [25]. However, there have been no studies on the cost-effectiveness of lactate test for screening in unselected ED population. In addition, hyperlactatemia is not just caused by real serious conditions with sustained tissue hypoxia (type A hyperlactatemia) including type B hyperlactatemia such as brief seizure, asthmatic attack, drugs, various chronic diseases, and inborn errors of metabolism. Therefore, lactate level should be used as a part of decision making with consideration in clinical presentation and other diagnostic tools.
Neverthless, we believe that routine blood lactate test in unselected patients may play an important role as a screening tool in the ED. It could alleviate ED overcrowding by fast decision making on patients with lower risk of poor prognosis, and could contribute to reduce adverse events such as ED revisit and early death after discharge.
This study has several limitations. First, it is known that the propensity score analysis does not correct for all selection biases; thus, potential biases in this study existed. Second, information about comorbidity that could influence the propensity to execute a serum lactate test was not collected. However, the physician’s decision to order a lactate test in the ED was more dependent on the patients’ current condition, not the underlying status. Third, although arterial blood was collected for the lactate test in most cases, we could not identify whether a given result was from arterial or venous blood. However, current guidelines state that either collection is appropriate for the management of sepsis and septic shock [10]. Fourth, Vasilevskis et al. stated that 30-day in-hospital mortality could be biased by the proportion of transferred patients and early post-discharge mortality [26]. Transferred patients were excluded from our analysis, whereas we could not identify patients who died after discharge in our study.
Conclusion
In our retrospective cohort study, a serum lactate level > 2.6 mmol/L predicted a 30-day in-hospital mortality in unselected patients who arrived at the ED and were admitted to the hospital. Additionally, serum lactate test in the ED could be an effective screening method for identifying low risk patients.
Data Availability
Data are available from the Harvard Dataverse (https://dataverse.harvard.edu/) database (accession number: 10.7910/DVN/OUKREG).
Funding Statement
The authors received no specific funding for this work.
References
- 1.Casserly B, Phillips GS, Schorr C, Dellinger RP, Townsend SR, Osborn TM, et al. Lactate measurements in sepsis-induced tissue hypoperfusion: results from the Surviving Sepsis Campaign database. Critical care medicine. 2015;43(3):567–73. doi: 10.1097/CCM.0000000000000742 [DOI] [PubMed] [Google Scholar]
- 2.Ouellet J-F, Roberts DJ, Tiruta C, Kirkpatrick AW, Mercado M, Trottier V, et al. Admission base deficit and lactate levels in Canadian patients with blunt trauma: are they useful markers of mortality? Journal of Trauma and Acute Care Surgery. 2012;72(6):1532–5. doi: 10.1097/TA.0b013e318256dd5a [DOI] [PubMed] [Google Scholar]
- 3.Datta D, Walker C, Gray AJ, Graham C, Masson M, Coyle J. Arterial lactate levels in an emergency department are associated with mortality: a prospective observational cohort study. Emergency Medicine Journal. 2015. September;32(9):673–7. doi: 10.1136/emermed-2013-203541 [DOI] [PubMed] [Google Scholar]
- 4.Singer AJ, Taylor MB, Domingo AT, Ghazipura S, Khorasanchi A, Thode HC. Diagnostic Characteristics of a Clinical Screening Tool in Combination with Poct Lactates in Ed Patients with Suspected Sepsis. Academic Emergency Medicine. 2013;20:S277. [DOI] [PubMed] [Google Scholar]
- 5.Shapiro NI, Howell MD, Talmor D, Nathanson LA, Lisbon A, Wolfe RE, et al. Serum lactate as a predictor of mortality in emergency department patients with infection. Annals of emergency medicine. 2005;45(5):524–8. doi: 10.1016/j.annemergmed.2004.12.006 [DOI] [PubMed] [Google Scholar]
- 6.Puskarich MA, Illich BM, Jones AE. Prognosis of emergency department patients with suspected infection and intermediate lactate levels: a systematic review. Journal of critical care. 2014;29(3):334–9. doi: 10.1016/j.jcrc.2013.12.017 [DOI] [PubMed] [Google Scholar]
- 7.Pedersen M, Brandt VS, Holler JG, Lassen AT. Lactate level, aetiology and mortality of adult patients in an emergency department: a cohort study. Emergency Medicine Journal. 2015;32(9):678–84. doi: 10.1136/emermed-2014-204305 [DOI] [PubMed] [Google Scholar]
- 8.Datta D, Walker C, Gray AJ, Graham C, Masson M, Coyle J. Arterial lactate levels in an emergency department are associated with mortality: a prospective observational cohort study. Emergency Medicine Journal. 2015;32:673–677. doi: 10.1136/emermed-2013-203541 [DOI] [PubMed] [Google Scholar]
- 9.van den Nouland DP, Brouwers MC, Stassen PM. Prognostic value of plasma lactate levels in a retrospective cohort presenting at a university hospital emergency department. BMJ open. 2017;7(1):e011450 doi: 10.1136/bmjopen-2016-011450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rhodes A, Evans LE, Alhazzani W, Levy MM, Antonelli M, Ferrer R, et al. Surviving Sepsis Campaign: International guidelines for management of sepsis and septic shock: 2016. Intensive care medicine. 2017;43(3):304–77. doi: 10.1007/s00134-017-4683-6 [DOI] [PubMed] [Google Scholar]
- 11.Rabello Filho R, Rocha LL, Corrêa TD, Pessoa CMS, Colombo G, Assuncao MSC. Blood lactate levels cutoff and mortality prediction in sepsis—time for a reappraisal? A retrospective cohort study. Shock (Augusta, Ga). 2016;46(5):480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mikkelsen ME, Miltiades AN, Gaieski DF, Goyal M, Fuchs BD, Shah CV, et al. Serum lactate is associated with mortality in severe sepsis independent of organ failure and shock. Critical care medicine. 2009;37(5):1670–7. doi: 10.1097/CCM.0b013e31819fcf68 [DOI] [PubMed] [Google Scholar]
- 13.Wacharasint P, Nakada T-a, Boyd JH, Russell JA, Walley KR. Normal-range blood lactate concentration in septic shock is prognostic and predictive. Shock. 2012;38(1):4–10. doi: 10.1097/SHK.0b013e318254d41a [DOI] [PubMed] [Google Scholar]
- 14.Jo S, Yoon J, Lee JB, Jin Y, Jeong T, Park B. Predictive value of the National Early Warning Score–Lactate for mortality and the need for critical care among general emergency department patients. Journal of critical care. 2016;36:60–8. doi: 10.1016/j.jcrc.2016.06.016 [DOI] [PubMed] [Google Scholar]
- 15.Cha WC, Do Shin S, Cho JS, Song KJ, Singer AJ, Kwak YH. The association between crowding and mortality in admitted pediatric patients from mixed adult-pediatric emergency departments in Korea. Pediatric emergency care. 2011;27(12):1136–41. doi: 10.1097/PEC.0b013e31823ab90b [DOI] [PubMed] [Google Scholar]
- 16.Yang HJ, Kim GW, Kim H, Cho JS, Rho TH, Yoon HD, et al. Epidemiology and outcomes in out-of-hospital cardiac arrest: a report from the NEDIS-based cardiac arrest registry in Korea. Journal of Korean medical science. 2015;30(1):95–103. doi: 10.3346/jkms.2015.30.1.95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Van Buuren S. Multiple imputation of discrete and continuous data by fully conditional specification. Statistical methods in medical research. 2007;16(3):219–42. doi: 10.1177/0962280206074463 [DOI] [PubMed] [Google Scholar]
- 18.Graham JW, Olchowski AE, Gilreath TD. How many imputations are really needed? Some practical clarifications of multiple imputation theory. Prevention Science. 2007;8(3):206–13. doi: 10.1007/s11121-007-0070-9 [DOI] [PubMed] [Google Scholar]
- 19.White IR, Royston P, Wood AM. Multiple imputation using chained equations: issues and guidance for practice. Statistics in medicine. 2011;30(4):377–99. doi: 10.1002/sim.4067 [DOI] [PubMed] [Google Scholar]
- 20.Toutenburg H. Rubin DB: Multiple imputation for nonresponse in surveys. Springer; 1990. [Google Scholar]
- 21.Youden WJ. Index for rating diagnostic tests. Cancer. 1950;3(1):32–35 [DOI] [PubMed] [Google Scholar]
- 22.Metz CE. Basic principles of ROC analysis. Semin Nucl Med. 1978;8:283–298. [DOI] [PubMed] [Google Scholar]
- 23.Callaway DW, Shapiro NI, Donnino MW, Baker C, Rosen CL. Serum lactate and base deficit as predictors of mortality in normotensive elderly blunt trauma patients. Journal of Trauma and Acute Care Surgery. 2009;66(4):1040–4. [DOI] [PubMed] [Google Scholar]
- 24.Neville AL, Nemtsev D, Manasrah R, Bricker SD, Putnam BA. Mortality risk stratification in elderly trauma patients based on initial arterial lactate and base deficit levels. The American Surgeon. 2011;77(10):1337–41. [PubMed] [Google Scholar]
- 25.Ward MJ, Self WH, Singer A, Lazar D, Pines JM. Cost-effectiveness analysis of early point-of-care lactate testing in the emergency department. Journal of critical care. 2016;36:69–75. doi: 10.1016/j.jcrc.2016.06.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vasilevskis EE, Kuzniewicz MW, Dean ML, Clay T, Vittinghoff E, Rennie DJ, et al. Relationship between discharge practices and intensive care unit in-hospital mortality performance: evidence of a discharge bias. Med Care. 2009;47(7):803–12. doi: 10.1097/MLR.0b013e3181a39454 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available from the Harvard Dataverse (https://dataverse.harvard.edu/) database (accession number: 10.7910/DVN/OUKREG).