The graph that appears as Fig 2A is incorrectly duplicated in Fig 2B. Please see the corrected Fig 2 here.
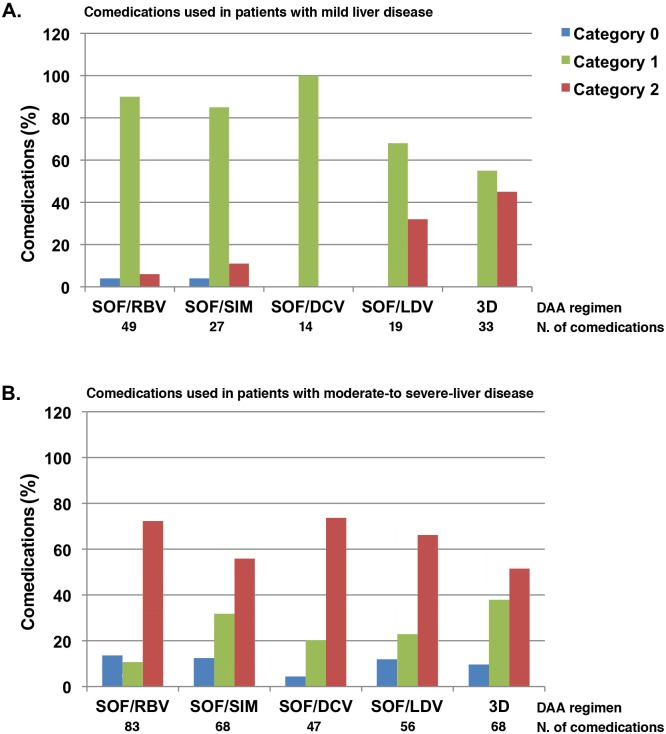
Fig 2. Category of potential DDIs, by DAA regimen and severity of liver disease, among HCV-infected patients.
Comedication used in patients with mild liver disease (A) or in (B) patients with moderate-to severe-liver disease (B). DAA regiments and number of comedications used are shown. SOF/RBV: sofosbuvir plus ribavirin, SOF/SIM: sofosbuvir plus simeprevir, SOF/DCV: sofosbuvir plus daclatasvir, SOF/LDV: sofosbuvir plus ledipasvir, 3D: paritaprevir/ritonavir, ombitasvir, dasabuvir. Category 0: Classification not possible due to lack of information; Category 1: No clinical interaction possible; Category 2: May require dose adjustment/closer monitoring.
Reference
- 1.Kondili LA, Gaeta GB, Ieluzzi D, Zignego AL, Monti M, Gori A, et al. (2017) Real-life data on potential drug-drug interactions in patients with chronic hepatitis C viral infection undergoing antiviral therapy with interferon-free DAAs in the PITER Cohort Study. PLoS ONE 12(2): e0172159 https://doi.org/10.1371/journal.pone.0172159 [DOI] [PMC free article] [PubMed] [Google Scholar]