Abstract
Lymphocytes participate in the early pathogenesis of ischemia-reperfusion injury (IRI) in kidney; however, their role during repair is largely unknown. Recent data have shown that Foxp3+ regulatory T cells (Tregs) traffic into kidney during healing from IRI and directly participate in repair. Since lymphocyte-targeting therapy is currently administered to prevent rejection during recovery from IRI in renal transplants, we hypothesized that mycophenolate mofetil (MMF) would alter Treg trafficking and kidney repair. C57BL/6J and T cell deficient mice underwent unilateral clamping of renal pedicle for 45 min, followed by reperfusion, and were sacrificed at day 10. Mice were treated with saline (C) or MMF (100 mg/kg) i.p. daily starting at day 2 until sacrifice (n=5–12/group). MMF worsened kidney tubular damage compared to C at 10 days (cortex and outer medulla: p< 0.05) in wild-type mice; tubular apoptotic index was increased in cortex in MMF group as well (p=0.01). MMF reduced the total number of kidney-infiltrating mononuclear cells (p<0.001 versus C) and the percentages of TCRβ+CD4+ and TCRβ+CD8+ T cells (p<0.01), but not natural killer (NK), NKT or B lymphocytes. MMF specifically reduced kidney Foxp3+ Tregs (0.82±0.11% versus 1.75±0.17%, p<0.05). Tubular proliferative index and tissue levels of bFGF were increased in MMF group (p< 0.05), IL-10 and IL-6 were decreased (p<0.05). To evaluate if MMF effect occurred through non-lymphocytic cells, T cell deficient mice were treated with MMF. Tubular injury in T cell deficient mice was not affected by MMF treatment, though MMF-treated animals had increased VEGF and decreased PDGF-BB protein tissue levels compared to controls (p<0.05). Thus, MMF modifies the structural, epithelial proliferative and inflammatory response during healing, likely through effects on T cells and possibly Tregs. Kidney repair after IRI can be altered by agents that target lymphocytes.
Keywords: Ischemia-reperfusion injury, Kidney, Repair, Lymphocyte, Mycophenolate mofetil
1. INTRODUCTION
1.1. Ischemia-reperfusion injury and delayed graft function
Ischemia-reperfusion injury (IRI) represents the major cause of delayed graft function (DGF) in kidney allograft recipients from deceased donors. DGF in turn is an independent risk factor for acute rejection at one year after transplantation and for short- and long-term graft survival [1]. Few studies have investigated the possible mechanisms and mediators involved in the healing phase of kidney IRI and there is no specific therapy available to promote repair from DGF. Current therapy is supportive and aimed at minimizing factors that can delay repair.
1.2. IRI and lymphocytes
Several studies have established that immune cells are involved early in the pathogenesis of IRI in kidneys and other organs [2–3]; in particular, the role of B, T and natural killer (NK) lymphocytes has been extensively studied [4–7]. Recent evidence from different model systems suggested that T lymphocytes could also be involved in protection from delayed damage and in repair promotion [8–9]. Consistent with these data, the transfer of kidney-infiltrating lymphocytes isolated from ischemic-reperfused (IR) kidneys partially protected from IRI [10].
As already demonstrated for the early phase of kidney IRI in other studies [11–12], we hypothesized that different lymphocyte subsets could act with distinct effects through the healing process, specifically worsening existing damage or promoting repair [13]. In recent years, considerable attention has been paid to small subpopulations of T cells that exhibit regulatory activity and can promote the acceptance of allografts. CD4+CD25+Foxp3+ regulatory T cells (Tregs) are major regulators in autoimmunity and transplant tolerance [14–15]. We recently demonstrated that Foxp3+ Tregs play an important role in repair form kidney IRI, possibly by down-regulating the pro-inflammatory cytokine production of effector T cells [13]. Other T subsets with regulatory activity could also be involved in modulation of repair from IRI. NKT cells have demonstrated a dual role in different experimental models; they promoted early damage in liver and kidney IRI [2, 16], while they have been shown to be pivotal in promoting tolerance in rat islet xenografts and murine cardiac transplantation [17–18].
1.3. IRI and immunosuppression
In the early post-transplant period, the occurrence of DGF remains a key problem in the management of allograft recipients. Ischemic acute kidney injury (AKI) influences the choice of immunosuppressive therapy. Calcineurin inhibitors are frequently delayed or used in lower doses, because they are known to retard recovery [19]; rapamycin can have a negative impact on tubular regeneration if given during DGF [20–21]. The lymphocyte-targeting immunosuppressive agent mycophenolate mofetil (MMF) is considered non-nephrotoxic and is usually administered during DGF. MMF acts by reversible inhibition of inosine monophosphate dehydrogenase and markedly reduces proliferation of lymphocytes that are strictly dependent of the enzyme function for purine synthesis [22]. Recently, however, MMF was also demonstrated to exert anti-proliferative effects on epithelial cells, in particular renal tubular cells [23]. Data regarding MMF effects on the repair phase of kidney IRI are not concordant [24–26]; moreover the relative influence of immune-modulatory and non-specific anti-proliferative effects has not been extensively studied.
2. OBJECTIVE
In the current study, we tested the hypothesis that MMF could alter repair from IRI via its effects on lymphocyte and particularly Treg trafficking. For this purpose, we studied repair from kidney IRI in a murine model of severe unilateral ischemia.
We initially found that MMF treatment worsened tubular damage during repair. Subsequently, we wanted to evaluate MMF effects on lymphocyte trafficking into IR kidneys and the potential link between immune-modulatory activity of the drug and delay of kidney repair. We observed that MMF markedly reduced trafficking of T lymphocytes, in particular regulatory subsets. We also correlated this observation with kidney production of growth factors and cytokines.
Since MMF could also be working independent of T cells, we administered the drug to T cell deficient mice during repair from IRI. Absence of T cells abrogated MMF effects on histology and differently affected growth factor and cytokine levels, suggesting that MMF modulation of the repair phase is likely due to its immunosuppressive effects on T cells.
3. MATERIALS AND METHODS
3.1. Mice
Male T cell deficient mice (B6.Cg-Foxn1nu/J) and wild-type C57Bl/6J mice, 6–12 weeks of age, were purchased from The Jackson Laboratory (Bar Harbor, ME) and housed under specific pathogen-free conditions. All experiments were performed in accordance with the Animal Care and Use Committee guidelines.
3.2. Renal IRI model
An established model of renal IRI was used, as previously described [10]; during the surgical procedure, the left renal pedicle was clamped for 45 min, followed by reperfusion. The animals were sacrificed at 10 days after IRI. The unilateral model was chosen to prevent the high short-term mortality of mice which occurs if both pedicles are clamped for 45 min.
3.3. Immunosuppressive treatment
Two days after IRI, mice were divided in two intervention groups: MMF 100 mg/kg (CellCept, Roche, Nutley, NJ) or, as control, 0.9% saline solution 10 ml/kg (C), were administered by daily i.p. injection until sacrifice. Doses were based on previous studies in mice [27]. MMF was started 48 hours after initial IRI in order not to alter the early injury response and focus on repair effects. At the time of sacrifice, kidneys were processed for histology, immunohistochemistry, lymphocyte isolation and bio-plex protein array.
3.4. Histology
At 10 days after IRI, kidneys were removed and tissue slices were fixed in 10% formalin. Subsequently, formalin-fixed tissue was embedded in paraffin and 4-μm sections were stained with H&E and Masson’s Trichrome. Damaged tubules were identified by the presence of diffuse tubular dilatation, intraluminal casts and/or tubular cell blebbing, vacuolization and detachment, in cortex and outer medulla in 6–10 HPF (400× magnification) per H&E section. The number of damaged tubules was divided by the number of the total tubules in the same field to obtain the “% damaged tubules”. The pathologist was blinded to the groups. No histological damage was observed in the papilla. The presence of fibrosis was evaluated on Masson’s Trichrome stained slides.
3.5. Immunohistochemistry
Ki67 immunohistochemical detection is a commonly used tool to assess tubular proliferation in kidneys [28–29]. Cleaved p17 caspase 3 is the active fragment of pro-caspase-3 which targets key modulators of the apoptotic pathway and therefore represents a good marker for apoptosis [30]. The staining was performed on formalin-fixed kidney sections (4 μm), after deparaffinization and rehydration. Ki67 was stained using rat anti-mouse monoclonal antibody (mAb) (Clone TEC-3, DAKO Carpinteria, CA) and cleaved caspase-3 (17/19 KDa) using rabbit anti-mouse polyclonal Ab (Cell Signaling Technology, Inc., Danvers, MA), following the manufacturer’s protocol, with biotinylated secondary Ab to rat and rabbit IgG respectively (Jackson ImmunoResearch, West Grove, PA). As an additional step, slides were incubated in Hoechst Dye solution (Invitrogen, Carlsbad, CA) for nuclear counterstaining. Light and fluorescence microscopy of the same field was performed in three areas of cortex and outer medulla for each animal (200x, Eclipse E600 microscope, Nikon, Melville, NY); images were captured and digitized by Digital Still Camera DXM1200 and ACT-1 software (Nikon) and analyzed using ImageJ software (NIH, Bethesda, MD). Tubular proliferative index in cortex and outer medulla was expressed as ratio between tubular Ki67 positive cells and total tubular cell nuclei of the examined fields [29]; in a similar fashion, tubular apoptotic index was calculated.
3.6. Lymphocyte isolation from mouse kidneys
At the time of sacrifice, mice were exsanguinated and kidneys collected and decapsulated. Experiments were performed by pooling two IR kidneys together, for each analysis. Viable kidney-infiltrating mononuclear cells (KMNCs) were isolated as previously described [31] and counted on a hemocytometer by trypan blue exclusion. The percentage of infiltrating lymphocyte subpopulations into IR kidneys was determined by flow cytometry analysis.
3.7. Antibodies
The following mAbs were obtained from BD Biosciences (San José, CA): anti-CD16/CD32 (clone 2.4G2), anti-CD19 Fluorescein isothiocyanate (FITC) (1D3), anti-CD69 Phycoerythrin (PE) (H1.2F3), anti-TCRβ PE and Allophycocyanin (APC) (H57-597), anti-CD4 Peridinin chlorophyll protein (PerCP) (RM4-5), anti-CD8α PerCP (53-6.7). Anti-CD25 FITC (PC 61.5), anti-NK1.1 APC (PK136) mAbs and anti-Foxp3 APC (FJK-16s) mAb, along with its staining kit, were purchased from eBioscience (San Diego, CA).
3.8. Flow cytometry analysis
KMNCs were incubated with anti-CD16/CD32 mAb for 10 min, followed by various combinations of mAbs for 20 min at 4°C [10]; Foxp3 intracellular staining was performed according to the manufacturer’s instructions. Four-color immunofluorescence staining was analyzed using FACSCalibur instrument and CellQuest software (BD Biosciences). Lymphocytes were gated using forward and side scatter and 10,000 events were acquired in each assay.
3.9. Kidney Bio-plex Protein Array
Levels of basic FGF, PDGF-BB, VEGF, IL-2, IL-6 and IL-10 were measured in IR kidneys from the different intervention groups, using a Bio-Plex multiple cytokines array technique (Bio-Rad Laboratories Inc., Hercules, CA), as previously described [32].
3.10. Statistical analysis
Statistical comparisons between groups were performed by Student’s t test or, for non-parametric data sets, Mann-Whitney U test. A p value ≤ 0.05 was considered statistically significant.
4. RESULTS
4.1 MMF affects tubular damage in the healing phase of renal IRI
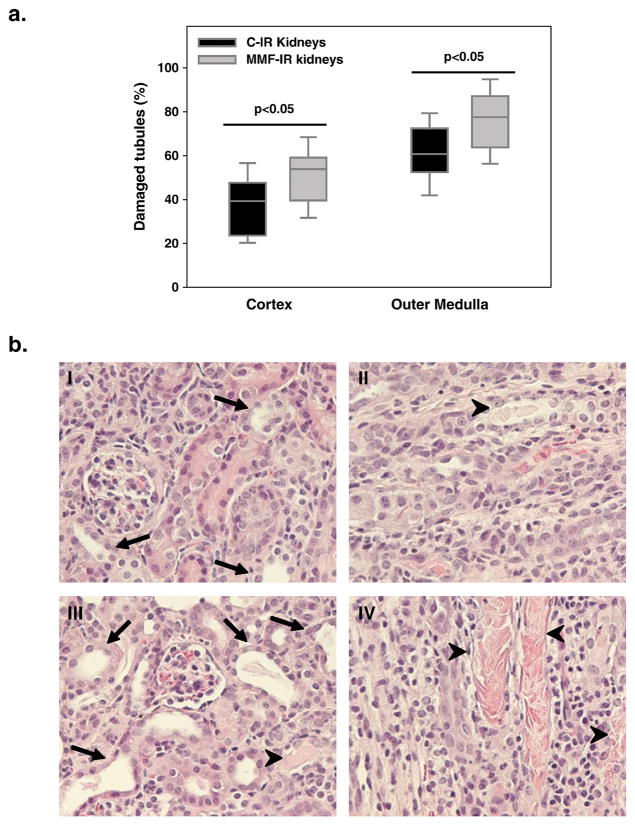
C57BL/6J mice, that had undergone unilateral clamping of renal pedicle for 45 min, were injected daily i.p. with MMF or isotonic saline solution (C), starting from day 2, and were sacrificed at day 10. At the time of sacrifice, IR kidneys from mice treated with MMF (MMF-IR) had increased tubular damage compared to IR kidneys from the control group (C-IR) both in cortex and outer medulla (p<0.05) (Figure 1a–b). Increased cortical tubular damage was also associated with increased tubular apoptotic index in MMF group (% tubular apoptotic cells: C-IR kidneys 0.92± 0.05, MMF-IR kidneys 1.19±0.06, p=0.01); no difference in the percentage of apoptotic cells was observed in the outer medulla between the groups (C-IR kidneys 1.22±0.05, MMF-IR kidneys 1.35±0.05, p=0.26). Fibrosis was minimal in IR kidneys from both the groups (data not shown).
Figure 1. MMF effects on tubular damage in the healing phase of kidney IRI.
a. Box plots show the 5th, 25th, 50th (median), 75th and 95th percentile values for tubular damage score at 10 days in IR kidneys obtained from control mice treated with saline solution 10 ml/kg (C-IR) and mice treated with MMF 100 mg/kg (MMF-IR), from day 2 to day 9 by daily i.p. injection. IR kidneys from MMF-treated mice showed increased tubular damage compared to controls in cortex and outer medulla (p<0.05). n=9 to 12/group.
b. Representative histological images of H&E stained renal sections at 10 days after IRI for control IR kidneys (cortex (I), outer medulla (II)) and MMF-treated IR kidneys (cortex (III), outer medulla (IV)). Tubular injury is identified by diffuse tubular dilatation (arrows), intraluminal casts (arrow-heads) and tubular cell detachment (original magnification: 200x).
4.2. MMF reduces T lymphocyte trafficking in IR kidneys during repair
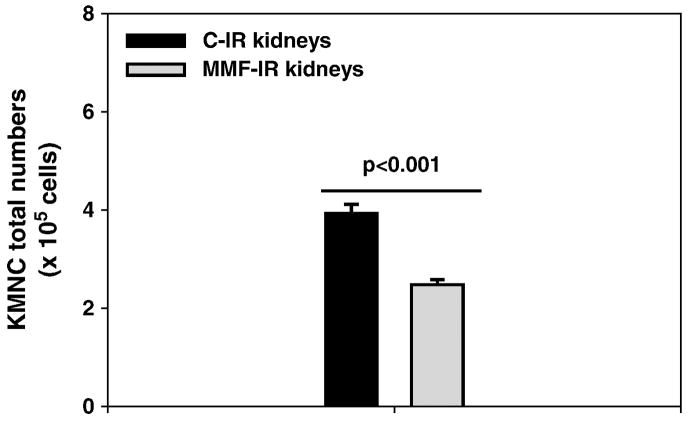
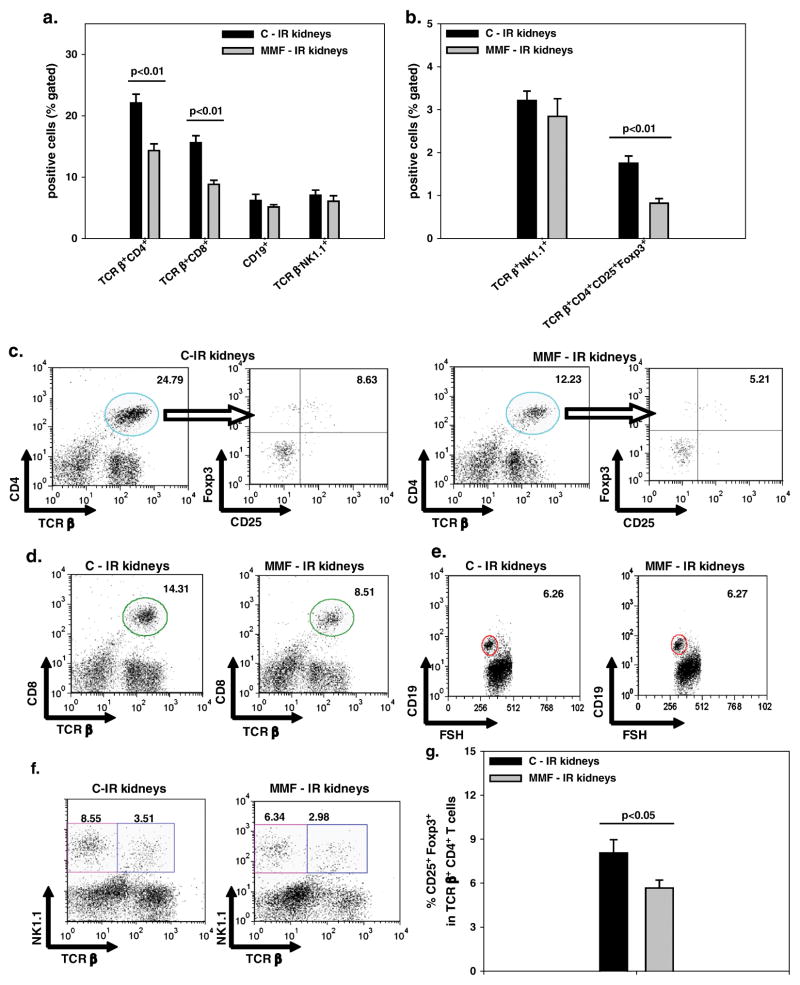
MMF-treated mice showed decreased KMNC infiltration in IR kidneys compared to the control group, at 10 days after IRI (p<0.001) (Figure 2). When we phenotyped KMNCs, we observed that IR kidneys from MMF-treated mice had reduced infiltration of TCRβ+CD4+ and TCRβ+CD8+ T cells, compared to mice injected with saline solution (p<0.01 for both subpopulations); no difference, was observed for the percentage of CD19+ B or TCRβ−NK1.1+ NK cells (Figure 3a, c–f).
Figure 2. MMF reduces the renal infiltration of mononuclear cells.
Reduced numbers of mononuclear cells infiltrating the kidney (KMNCs) were observed in IR kidneys from mice treated with MMF (MMF-IR), compared to mice treated with saline solution (C-IR) (p<0.001). Means ± SE. n=6/group.
Figure 3. MMF effects on renal lymphocyte subset trafficking during repair from kidney IRI.
a. The percentages of TCRβ+CD4+ and TCRβ+CD8+ T lymphocytes were reduced in IR kidneys from mice treated with MMF (MMF-IR), compared to saline controls (C-IR), at 10 days after IRI, as assessed by flow cytometry analysis. No difference was observed in the percentages of CD19+ and TCRβ−NK1.1+ NK cells between the two groups. b. The percentage of TCRβ+CD4+CD25+Foxp3+ Tregs was reduced in IR kidneys from MMF-treated mice (p<0.01), while no difference was observed for TCRβ+NK1.1+ NKT cells. Means ± SE. n=6/group.
c–f. Representative dot plots for the different lymphocyte subsets, from control and MMF-treated IR kidneys at 10 days. All the subsets were initially gated from the lymphocyte area. For the identification of TCRβ+CD4+CD25+Foxp3+ Tregs, TCRβ+CD4+ T lymphocytes were initially gated from the lymphocyte area (and here identified by the circle in the dot plots on the left); subsequently the expression of CD25 and Foxp3 was examined in this population. Finally the percentage of TCRβ+CD4+CD25+Foxp3+ Tregs was obtained by multiplying the percentage of TCRβ+CD4+ T lymphocytes by the percentage of CD25+Foxp3+ cells (upper right quadrant in the dot plots on the right for each group).
g. The proportion of cells expressing both CD25 and Foxp3 among total TCRβ+CD4+ T lymphocytes was decreased in IR kidneys from MMF-treated mice, at 10 days (p<0.05). Means ± SE. n= 6/group.
When we analyzed the degree of T cell immune activation, we observed that the expression of CD69, marker of early activation, on TCRβ+CD4+ and TCRβ+CD8+ T cells was not different between the groups (% TCRβ+CD4+: C-IR kidneys 58.31±2.07, MMF-IR kidneys 55.53±2.19, p=0.26; % TCRβ+CD8+: C-IR kidneys 74.80±2.43, MMF-IR kidneys 67.06±2.90, p=0.09).
4.3. MMF inhibits Treg subset infiltration in IR kidneys during repair
We focused on T cell subsets known for their regulatory properties and observed that the percentage of TCRβ+CD4+CD25+Foxp3+ Tregs was decreased in IR kidneys of MMF-treated mice (p<0.01); no difference was observed for TCRβ+NK1.1+ NKT cells (Figure 3b, c, f). Moreover, in IR kidneys from mice injected with MMF, the percentage of cells expressing both CD25 and Foxp3 among total TCRβ+CD4+ T lymphocytes was decreased (p<0.05) (Figure 3g).
4.4. Effects of MMF treatment on tubular proliferation and kidney cytokine and growth factor levels
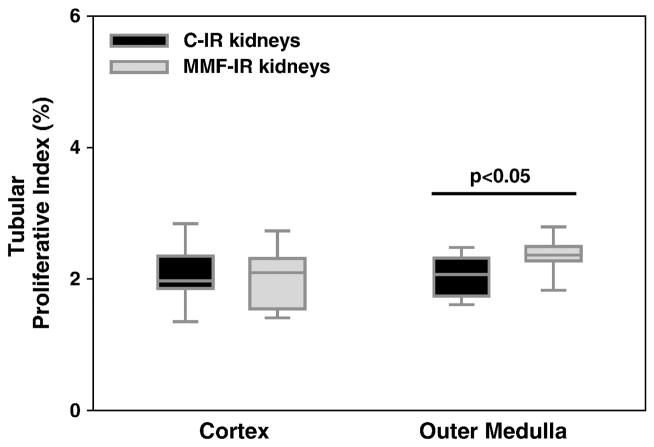
We then evaluated MMF effects on the proliferation of tubular cells. At 10 days after IRI, no difference in the proliferative index was observed between the groups in renal cortex, despite the increased tubular damage present in IR kidneys of MMF-treated animals. Interestingly, MMF-treated mice showed increased tubular proliferation, compared to the control group, in the outer medulla, a vulnerable location for kidney damage after IRI (Figure 4).
Figure 4. MMF increases kidney tubular proliferation during repair from kidney IRI.
Box plots show the 5th, 25th, 50th (median), 75th and 95th percentiles for tubular proliferative index at 10 days, in IR kidneys from control and MMF-treated mice. MMF treatment was associated with increased tubular proliferation in outer medulla in IR kidneys (p<0.05). n= 9–12/group.
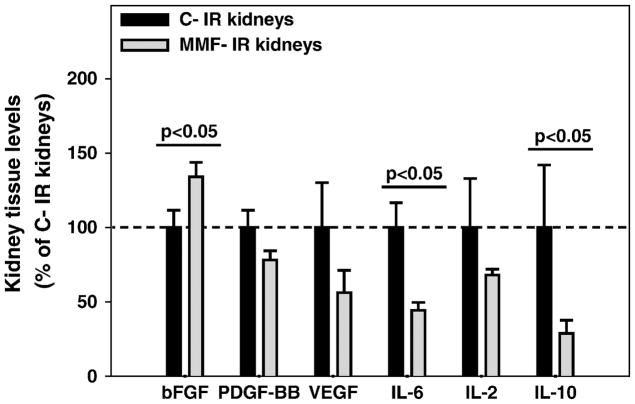
We investigated MMF effects on renal cytokines and growth factors after ischemia in an effort to explain the impact of MMF on kidney histology and lymphocyte trafficking. IR kidneys from MMF-treated mice presented increased basic FGF levels and reduced levels of IL-6 and IL-10 compared to the control group (p<0.05 for each protein); no difference was observed for tissue levels of PDGF-BB, VEGF and IL-2 (Figure 5).
Figure 5. MMF effects on kidney cytokine and growth factor levels in the healing phase of kidney IRI.
Bar charts represent tissue levels of different cytokines and growth factors measured in IR kidneys from control mice (C-IR) or MMF-treated mice (MMF-IR) at 10 days. Tissue levels in MMF-IR kidneys are expressed as percentages of the values in C-IR kidneys (normalized to 100%).
Basic FGF levels were increased in IR kidneys from MMF-treated mice, while the levels of IL-6 and IL-10 were decreased (p<0.05 for all the proteins). Means ± SE. n=5–6/group.
4.5. MMF-induced modulation of the repair phase is absent in T cell deficient mice
Though MMF is primarily used to target T cells therapeutically, it can alter the proliferation of non-T cell populations. To test if MMF effects on healing from IRI were mediated by T cells, we performed the same experiments as detailed above in T cell deficient (nu/nu) mice.
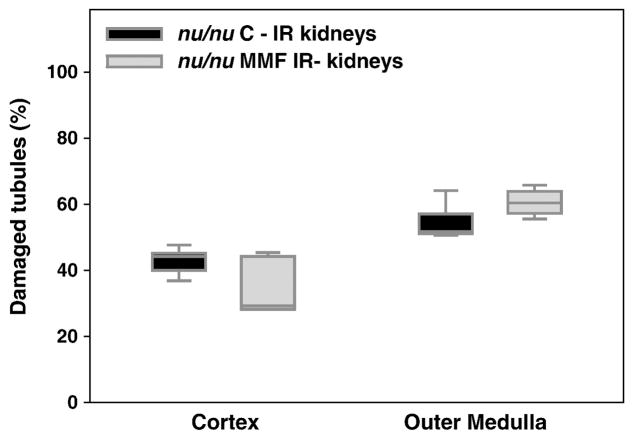
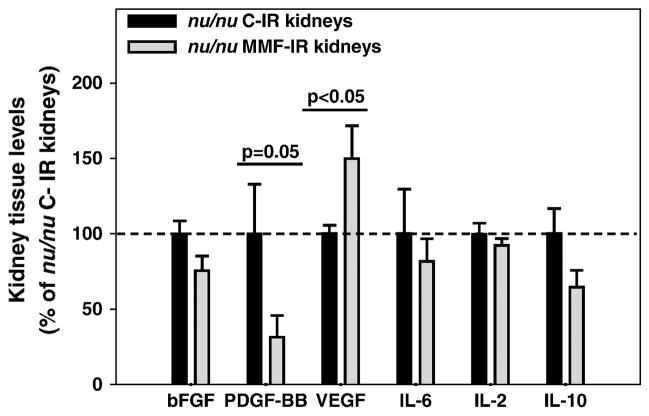
In T cell deficient mice, MMF treatment did not induce changes in the severity of IR kidney tubular damage at 10 days, either in cortex or outer medulla in comparison with nu/nu control mice (Figure 6). However, IR kidneys from MMF-treated nu/nu mice had reduced levels of PDGF-BB and increased levels of VEGF (p<0.05 for both growth factors) compared to the nu/nu control group. No difference was observed for basic FGF, IL-6, IL-2 or IL-10 tissue levels (Figure 7).
Figure 6. MMF treatment effects on tubular damage during repair from kidney IRI in T cell deficient mice.
We performed the same experimental model in T cell deficient (nu/nu) mice. Box plots show the 5th, 25th, 50th (median), 75th and 95th percentile values for tubular damage score at 10 days in IR kidneys obtained from control nu/nu mice treated with isotonic saline solution 10 ml/kg (nu/nu C-IR) and nu/nu mice treated with MMF 100 mg/kg (nu/nu MMF-IR), from day 2 to day 9 by daily i.p. injection. IR kidneys from MMF-treated nu/nu mice did not show differences in tubular damage compared to controls. n=5/group.
Figure 7. MMF effects on kidney cytokine and growth factor levels in the healing phase from renal IRI in T cell deficient mice.
Bar charts represent tissue levels of different cytokines and growth factors measured in IR kidneys from control nu/nu mice (nu/nu C-IR) or MMF-treated nu/nu mice (nu/nu MMF-IR) at 10 days. Tissue levels in nu/nu MMF-IR kidneys are expressed as percentages of the values in nu/nu C-IR kidneys (normalized to 100%). PDGF-BB levels were decreased in IR kidneys from MMF-treated nu/nu mice, while the VEGF levels were increased (p<0.05 for both the proteins). Means ± SE. n=4–5/group.
5. DISCUSSION
These data demonstrate that MMF, administered during the repair phase of kidney IRI, modifies tubular injury, proliferation, lymphocyte trafficking and growth factor/cytokine production. These effects are largely due to MMF modulation of T cells, and Foxp3+ Tregs are a possible candidate target. Given the frequent use of MMF during DGF, our observations have important clinical implications.
Different lymphocyte subsets have been shown to be pathogenic in the early phase of kidney IRI [4, 7, 12]. A few reports have suggested the presence of lymphocytes, and T cells in particular, in the healing phase after renal ischemia. Ysebaert et al. [24] demonstrated, by immunohistochemistry, that CD4+ and CD8+ T lymphocytes infiltrate IR kidneys also during the extension phase of severe kidney IRI in a rat model. Ascon et al. [33] observed activation and expansion of effector-memory T cells long-term after IRI in mice, by cell isolation from the kidney and flow cytometry phenotyping. We recently confirmed the increased infiltration of activated T subset and NK lymphocytes in the murine kidney, healing from severe IRI [13].
In the current study, we hypothesized that modulation of kidney-infiltrating lymphocytes by MMF could alter kidney healing responses. We designed our experiments in order to simulate current clinical practice when patients with transplanted kidney IRI are treated with MMF during DGF to prevent rejection [34–35]. We therefore performed 45 minutes unilateral ischemia and initiated MMF treatment on day 2 after IRI, in order to study repair, without altering early injury.
At the time of sacrifice on day 10 after IRI, we observed that MMF worsened tubular injury in cortex and outer medulla in IR kidneys compared to controls. Histological changes were associated with increased tissue levels of basic FGF, a cytokine involved in the process of scarring and interstitial fibrosis, by acting through TGF-β1 [36]. In order to begin to analyze the cellular mechanisms of tubular injury, we observed that IR kidneys from MMF-treated mice presented increased apoptotic index in cortex but not in outer medulla. Tubular cell apoptosis has been suggested to play a role in mediating the short- and long-term effects of IRI [37–38]. In our model, the more severe kidney IRI observed in MMF group was only partially explained by the increased apoptotic death of tubular cells; these data suggest that necrosis probably plays an additional important role in mediating renal IRI, especially in the more vulnerable outer medulla compartment.
In MMF-treated IR kidneys, overall mononuclear cell trafficking was reduced. When we analyzed the specific effects of the drug on lymphocyte subpopulations we observed that the T lymphocytes were the main target of MMF action; in particular both the percentages of infiltrating TCRβ+CD4+ and TCRβ+CD8+ T cells were reduced. However, the level of activation of these cells was not affected by MMF treatment and the percentages of B and NK cells did not change.
Regulatory T cells are specific subsets of T lymphocytes that control the activity and responses of several subsets of immune cells. CD4+CD25+ Tregs, the most studied subpopulation, modulate the proliferative and cytokine responses of effector T lymphocytes, as well as B lymphocytes and cells of the innate immune system. Foxp3, a forkhead winged helix family transcriptional regulator, is a critical molecular switch for the genetic programming of Treg cell development and function [14] and nowadays is considered a marker for CD4+CD25+ Tregs. In the kidney, these cells have shown to be protective in models of anti-glomerular basement membrane glomerulonephritis and adriamycin nephropathy [39–41]. We recently demonstrated that Foxp3+ Tregs infiltrated the ischemic kidney during repair and could modulate healing. When Tregs were depleted during healing, IR kidneys presented delayed repair and infiltration of effector T cells with increased capacity of TNF-α and INF-γ production; accelerated healing was seen when ischemic mice were injected with Tregs during the repair phase [13]. In the field of transplantation, the relative expansion of Tregs in total CD4+ T compartment has been proposed as an important mechanism in allograft tolerance promoted by the immunosuppressive drug rapamycin [42–43]. In different transplant models, Tregs can inhibit allo-reactive CD4+ and CD8+ T cells by IL-10 involvement [15, 44]. In the current experimental model of warm unilateral IRI, MMF treatment was associated with reduced infiltration of Tregs in the ischemic kidney. Moreover, we observed a decreased proportion of the Treg subset in the TCRβ+CD4+ T compartment, suggesting a preferential inhibition on the recruitment of these cells by MMF. This was consistent with our previous observations and further supports the importance of this small population in the process of kidney repair. On the contrary MMF did not affect the percentage of NKT cells.
IL-10 is one of the key cytokines produced by Tregs (and also other immune cells) and is known to have immunosuppressive and anti-inflammatory properties (reviewed in [45]). The role for IL-10 in promoting healing has been suggested by previous reports; IL-10 reduced kidney damage from IRI and blunted renal cisplatin toxicity, even when administered to mice after the induction of damage [46]. In our model, reduced tissue levels of IL-10 were observed in MMF-treated mice. This observation could contribute to explain the different healing rate and could find explanation in the different ratio of IL-10 producing cells.
Even though our data demonstrated that MMF alters post-ischemic lymphocyte trafficking and tubular epithelial cell injury, we were aware that MMF could decrease cellular proliferation by reversible inhibition of inosine monophosphate dehydrogenase in tubular cells as well as lymphocytes in vitro [23]. MMF also reduces the ability of stimulated tubular cells to produce increased amount of IL-6 [47], involved in tubular regeneration following acute kidney damage [48]. In vivo, the direct role of MMF in repair from renal IRI is controversial. Worsening of histological damage and reduced tubular regeneration have been reported in the kidneys of MMF-treated animals, along with decreased T lymphocyte infiltration [24–25, 49]. However, MMF effects on tubular proliferation and lymphocyte presence changed according to storage time in rat transplanted kidneys [49]. Reduced tubular proliferation, consequence of attenuated damage, has been reported in MMF-treated rats, 2 days after severe IRI [26]. In our current study, IL-6 levels were lower in IR kidneys from MMF treated wild type mice, but proliferation rate was increased, at least in the outer medulla. Therefore, MMF didn’t appear to impair directly epithelial proliferation and the worse histological picture was more likely due to immunosuppressive effects.
To test if MMF effect on kidney repair was actually due to its T lymphocyte-modulating properties, we performed the same experimental model in T-cell deficient mice. We found that histological damage did not change in presence of MMF treatment. However, MMF was still able to increase VEGF and reduce PDGF-BB tissue levels, a growth factor pattern compatible with reduced fibrosis and repair promotion [50–51]. MMF did not change IL-6 and IL-10 tissue levels in T cell deficient mice post-ischemia.
In summary, we demonstrated that MMF partially inhibited kidney repair after IRI, likely through affecting T cell and particularly Foxp3+ Treg renal trafficking. Our observations have direct implications in the management of patients with DGF, and future studies are important to examine if our observations are translatable to humans.
Acknowledgments
We thank Priya Kesari for assistance with kidney bioplex protein array, Lee Blosser and Ada Kam for assistance with flow cytometry analysis, Klaus B. Piontek and Luis Menezes for suggestions about Ki67 immunostaining and Prof. Giacomo Garibotto for support and helpful critical comments throughout the project.
ROLE OF FUNDING SOURCE
This study was supported by grant RO1DK054770.
Footnotes
DISCLOSURE STATEMENT
The authors have no financial conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Yarlagadda SG, Coca SG, Formica RN, Jr, Poggio ED, Parikh CR. Association between delayed graft function and allograft and patient survival: a systematic review and meta-analysis. Nephrol Dial Transplant. 2009;24:1039–47. doi: 10.1093/ndt/gfn667. [DOI] [PubMed] [Google Scholar]
- 2.Li L, Huang L, Sung SS, Lobo PI, Brown MG, Gregg RK, et al. NKT cell activation mediates neutrophil IFN-gamma production and renal ischemia-reperfusion injury. J Immunol. 2007;178:5899–911. doi: 10.4049/jimmunol.178.9.5899. [DOI] [PubMed] [Google Scholar]
- 3.Tsuchihashi S, Ke B, Kaldas F, Flynn E, Busuttil RW, Briscoe DM, et al. Vascular endothelial growth factor antagonist modulates leukocyte trafficking and protects mouse livers against ischemia/reperfusion injury. Am J Pathol. 2006;168:695–705. doi: 10.2353/ajpath.2006.050759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rabb H, Daniels F, O’Donnell M, Haq M, Saba SR, Keane W, et al. Pathophysiological role of T lymphocytes in renal ischemia-reperfusion injury in mice. Am J Physiol Renal Physiol. 2000;279:F525–31. doi: 10.1152/ajprenal.2000.279.3.F525. [DOI] [PubMed] [Google Scholar]
- 5.Zwacka RM, Zhang Y, Halldorson J, Schlossberg H, Dudus L, Engelhardt JF. CD4(+) T-lymphocytes mediate ischemia/reperfusion-induced inflammatory responses in mouse liver. J Clin Invest. 1997;100:279–89. doi: 10.1172/JCI119533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burne-Taney MJ, Ascon DB, Daniels F, Racusen L, Baldwin W, Rabb H. B cell deficiency confers protection from renal ischemia reperfusion injury. J Immunol. 2003;171:3210–5. doi: 10.4049/jimmunol.171.6.3210. [DOI] [PubMed] [Google Scholar]
- 7.Zhang ZX, Wang S, Huang X, Min WP, Sun H, Liu W, et al. NK cells induce apoptosis in tubular epithelial cells and contribute to renal ischemia-reperfusion injury. J Immunol. 2008;181:7489–98. doi: 10.4049/jimmunol.181.11.7489. [DOI] [PubMed] [Google Scholar]
- 8.Davis PA, Corless DJ, Aspinall R, Wastell C. Effect of CD4(+) and CD8(+) cell depletion on wound healing. Br J Surg. 2001;88:298–304. doi: 10.1046/j.1365-2168.2001.01665.x. [DOI] [PubMed] [Google Scholar]
- 9.Hofstetter HH, Sewell DL, Liu F, Sandor M, Forsthuber T, Lehmann PV, et al. Autoreactive T cells promote post-traumatic healing in the central nervous system. J Neuroimmunol. 2003;134:25–34. doi: 10.1016/s0165-5728(02)00358-2. [DOI] [PubMed] [Google Scholar]
- 10.Ascon DB, Lopez-Briones S, Liu M, Ascon M, Savransky V, Colvin RB, et al. Phenotypic and functional characterization of kidney-infiltrating lymphocytes in renal ischemia reperfusion injury. J Immunol. 2006;177:3380–7. doi: 10.4049/jimmunol.177.5.3380. [DOI] [PubMed] [Google Scholar]
- 11.Monteiro RM, Camara NO, Rodrigues MM, Tzelepis F, Damiao MJ, Cenedeze MA, et al. A role for regulatory T cells in renal acute kidney injury. Transpl Immunol. 2009;21:50–5. doi: 10.1016/j.trim.2009.02.003. [DOI] [PubMed] [Google Scholar]
- 12.Lai LW, Yong KC, Igarashi S, Lien YH. A sphingosine-1-phosphate type 1 receptor agonist inhibits the early T-cell transient following renal ischemia-reperfusion injury. Kidney Int. 2007;71:1223–31. doi: 10.1038/sj.ki.5002203. [DOI] [PubMed] [Google Scholar]
- 13.Gandolfo MT, Jang HR, Bagnasco SM, Ko GJ, Agreda P, Satpute SR, et al. Foxp3(+) regulatory T cells participate in repair of ischemic acute kidney injury. Kidney Int. 2009 doi: 10.1038/ki.2009.259. [DOI] [PubMed] [Google Scholar]
- 14.Piccirillo CA. Regulatory T cells in health and disease. Cytokine. 2008;43:395–401. doi: 10.1016/j.cyto.2008.07.469. [DOI] [PubMed] [Google Scholar]
- 15.Jiang S, Lechler RI, He XS, Huang JF. Regulatory T cells and transplantation tolerance. Hum Immunol. 2006;67:765–76. doi: 10.1016/j.humimm.2006.07.013. [DOI] [PubMed] [Google Scholar]
- 16.Kuboki S, Sakai N, Tschop J, Edwards MJ, Lentsch AB, Caldwell CC. Distinct contributions of CD4+ T cell subsets in hepatic ischemia/reperfusion injury. Am J Physiol Gastrointest Liver Physiol. 2009;296:G1054–G9. doi: 10.1152/ajpgi.90464.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ikehara Y, Yasunami Y, Kodama S, Maki T, Nakano M, Nakayama T, et al. CD4(+) Valpha14 natural killer T cells are essential for acceptance of rat islet xenografts in mice. J Clin Invest. 2000;105:1761–7. doi: 10.1172/JCI8922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Seino KI, Fukao K, Muramoto K, Yanagisawa K, Takada Y, Kakuta S, et al. Requirement for natural killer T (NKT) cells in the induction of allograft tolerance. Proc Natl Acad Sci U S A. 2001;98:2577–81. doi: 10.1073/pnas.041608298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cravedi P, Codreanu I, Satta A, Turturro M, Sghirlanzoni M, Remuzzi G, et al. Cyclosporine prolongs delayed graft function in kidney transplantation: are rabbit anti-human thymocyte globulins the answer? Nephron Clin Pract. 2005;101:c65–71. doi: 10.1159/000086224. [DOI] [PubMed] [Google Scholar]
- 20.Fuller TF, Freise CE, Serkova N, Niemann CU, Olson JL, Feng S. Sirolimus delays recovery of rat kidney transplants after ischemia-reperfusion injury. Transplantation. 2003;76:1594–9. doi: 10.1097/01.TP.0000095897.38634.30. [DOI] [PubMed] [Google Scholar]
- 21.Lui SL, Chan KW, Tsang R, Yung S, Lai KN, Chan TM. Effect of rapamycin on renal ischemia-reperfusion injury in mice. Transpl Int. 2006;19:834–9. doi: 10.1111/j.1432-2277.2006.00361.x. [DOI] [PubMed] [Google Scholar]
- 22.Allison AC, Eugui EM. Purine metabolism and immunosuppressive effects of mycophenolate mofetil (MMF) Clin Transplant. 1996;10:77–84. [PubMed] [Google Scholar]
- 23.Baer PC, Gauer S, Hauser IA, Scherberich JE, Geiger H. Effects of mycophenolic acid on human renal proximal and distal tubular cells in vitro. Nephrol Dial Transplant. 2000;15:184–90. doi: 10.1093/ndt/15.2.184. [DOI] [PubMed] [Google Scholar]
- 24.Ysebaert DK, De Greef KE, Vercauteren SR, Verhulst A, Kockx M, Verpooten GA, et al. Effect of immunosuppression on damage, leukocyte infiltration, and regeneration after severe warm ischemia/reperfusion renal injury. Kidney Int. 2003;64:864–73. doi: 10.1046/j.1523-1755.2003.00150.x. [DOI] [PubMed] [Google Scholar]
- 25.Gonzalez N, Alvarez V, Pons H, Parra G, Quiroz Y, Rodriguez-Iturbe B. Mycophenolate mofetil aggravates postischemic acute renal failure in rats. Transplant Proc. 2002;34:43–4. doi: 10.1016/s0041-1345(01)02658-6. [DOI] [PubMed] [Google Scholar]
- 26.Ventura CG, Coimbra TM, de Campos SB, de Castro I, Yu L, Seguro AC. Mycophenolate mofetil attenuates renal ischemia/reperfusion injury. J Am Soc Nephrol. 2002;13:2524–33. doi: 10.1097/01.asn.0000030143.73830.3c. [DOI] [PubMed] [Google Scholar]
- 27.Birck R, Newman M, Braun C, Neumann I, Nemoto K, Yard B, et al. 15-Deoxyspergualin and cyclophosphamide, but not mycophenolate mofetil, prolong survival and attenuate renal disease in a murine model of ANCA-associated crescentic nephritis. Nephrol Dial Transplant. 2006;21:58–63. doi: 10.1093/ndt/gfi070. [DOI] [PubMed] [Google Scholar]
- 28.Hall PA, Greenwood RA, d’Ardenne AJ, Levison DA. In situ demonstration of renal tubular regeneration using the monoclonal antibody Ki67. Nephron. 1988;49:122–5. doi: 10.1159/000185037. [DOI] [PubMed] [Google Scholar]
- 29.Nadasdy T, Laszik Z, Blick KE, Johnson LD, Silva FG. Proliferative activity of intrinsic cell populations in the normal human kidney. J Am Soc Nephrol. 1994;4:2032–9. doi: 10.1681/ASN.V4122032. [DOI] [PubMed] [Google Scholar]
- 30.Hughes J, Gobe G. Identification and quantification of apoptosis in the kidney using morphology, biochemical and molecular markers. Nephrology (Carlton) 2007;12:452–8. doi: 10.1111/j.1440-1797.2007.00854.x. [DOI] [PubMed] [Google Scholar]
- 31.Ascon DB, Ascon M, Satpute S, Lopez-Briones S, Racusen L, Colvin RB, et al. Normal mouse kidneys contain activated and CD3+CD4− CD8− double-negative T lymphocytes with a distinct TCR repertoire. J Leukoc Biol. 2008;84:1400–9. doi: 10.1189/jlb.0907651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Molls RR, Savransky V, Liu M, Bevans S, Mehta T, Tuder RM, et al. Keratinocyte-derived chemokine is an early biomarker of ischemic acute kidney injury. Am J Physiol Renal Physiol. 2006;290:F1187–93. doi: 10.1152/ajprenal.00342.2005. [DOI] [PubMed] [Google Scholar]
- 33.Ascon M, Ascon DB, Liu M, Cheadle C, Sarkar C, Racusen L, et al. Renal ischemia-reperfusion leads to long term infiltration of activated and effector-memory T lymphocytes. Kidney Int. 2009;75:526–35. doi: 10.1038/ki.2008.602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Grinyo JM, Gil-Vernet S, Cruzado JM, Caldes A, Riera L, Seron D, et al. Calcineurin inhibitor-free immunosuppression based on antithymocyte globulin and mycophenolate mofetil in cadaveric kidney transplantation: results after 5 years. Transpl Int. 2003;16:820–7. doi: 10.1007/s00147-003-0638-7. [DOI] [PubMed] [Google Scholar]
- 35.Allison AC. Mechanisms of action of mycophenolate mofetil. Lupus. 2005;14(Suppl 1):s2–8. doi: 10.1191/0961203305lu2109oa. [DOI] [PubMed] [Google Scholar]
- 36.Strutz F, Zeisberg M, Ziyadeh FN, Yang CQ, Kalluri R, Muller GA, et al. Role of basic fibroblast growth factor-2 in epithelial-mesenchymal transformation. Kidney Int. 2002;61:1714–28. doi: 10.1046/j.1523-1755.2002.00333.x. [DOI] [PubMed] [Google Scholar]
- 37.Yang B, Jain S, Ashra SY, Furness PN, Nicholson ML. Apoptosis and caspase-3 in long-term renal ischemia/reperfusion injury in rats and divergent effects of immunosuppressants. Transplantation. 2006;81:1442–50. doi: 10.1097/01.tp.0000209412.77312.69. [DOI] [PubMed] [Google Scholar]
- 38.Kunduzova OR, Escourrou G, Seguelas MH, Delagrange P, De La Farge F, Cambon C, et al. Prevention of apoptotic and necrotic cell death, caspase-3 activation, and renal dysfunction by melatonin after ischemia/reperfusion. FASEB J. 2003;17:872–4. doi: 10.1096/fj.02-0504fje. [DOI] [PubMed] [Google Scholar]
- 39.Wolf D, Hochegger K, Wolf AM, Rumpold HF, Gastl G, Tilg H, et al. CD4+CD25+ regulatory T cells inhibit experimental anti-glomerular basement membrane glomerulonephritis in mice. J Am Soc Nephrol. 2005;16:1360–70. doi: 10.1681/ASN.2004100837. [DOI] [PubMed] [Google Scholar]
- 40.Mahajan D, Wang Y, Qin X, Zheng G, Wang YM, Alexander SI, et al. CD4+CD25+ regulatory T cells protect against injury in an innate murine model of chronic kidney disease. J Am Soc Nephrol. 2006;17:2731–41. doi: 10.1681/ASN.2005080842. [DOI] [PubMed] [Google Scholar]
- 41.Wang YM, Zhang GY, Wang Y, Hu M, Wu H, Watson D, et al. Foxp3-transduced polyclonal regulatory T cells protect against chronic renal injury from adriamycin. J Am Soc Nephrol. 2006;17:697–706. doi: 10.1681/ASN.2005090978. [DOI] [PubMed] [Google Scholar]
- 42.Battaglia M, Stabilini A, Roncarolo MG. Rapamycin selectively expands CD4+CD25+FoxP3+ regulatory T cells. Blood. 2005;105:4743–8. doi: 10.1182/blood-2004-10-3932. [DOI] [PubMed] [Google Scholar]
- 43.Noris M, Casiraghi F, Todeschini M, Cravedi P, Cugini D, Monteferrante G, et al. Regulatory T cells and T cell depletion: role of immunosuppressive drugs. J Am Soc Nephrol. 2007;18:1007–18. doi: 10.1681/ASN.2006101143. [DOI] [PubMed] [Google Scholar]
- 44.Hara M, Kingsley CI, Niimi M, Read S, Turvey SE, Bushell AR, et al. IL-10 is required for regulatory T cells to mediate tolerance to alloantigens in vivo. J Immunol. 2001;166:3789–96. doi: 10.4049/jimmunol.166.6.3789. [DOI] [PubMed] [Google Scholar]
- 45.Moore KW, O’Garra A, de Waal Malefyt R, Vieira P, Mosmann TR. Interleukin-10. Annu Rev Immunol. 1993;11:165–90. doi: 10.1146/annurev.iy.11.040193.001121. [DOI] [PubMed] [Google Scholar]
- 46.Deng J, Kohda Y, Chiao H, Wang Y, Hu X, Hewitt SM, et al. Interleukin-10 inhibits ischemic and cisplatin-induced acute renal injury. Kidney Int. 2001;60:2118–28. doi: 10.1046/j.1523-1755.2001.00043.x. [DOI] [PubMed] [Google Scholar]
- 47.Baer PC, Wegner B, Geiger H. Effects of mycophenolic acid on IL-6 expression of human renal proximal and distal tubular cells in vitro. Nephrol Dial Transplant. 2004;19:47–52. doi: 10.1093/ndt/gfg429. [DOI] [PubMed] [Google Scholar]
- 48.Homsi E, Ribeiro-Alves MA, Lopes de Faria JB, Dias EP. Interleukin-6 stimulates tubular regeneration in rats with glycerol-induced acute renal failure. Nephron. 2002;92:192–9. doi: 10.1159/000064478. [DOI] [PubMed] [Google Scholar]
- 49.Fuller TF, Hoff U, Rose F, Linde Y, Freise CE, Dragun D, et al. Effect of mycophenolate mofetil on rat kidney grafts with prolonged cold preservation. Kidney Int. 2006;70:570–7. doi: 10.1038/sj.ki.5001591. [DOI] [PubMed] [Google Scholar]
- 50.Leonard EC, Friedrich JL, Basile DP. VEGF-121 preserves renal microvessel structure and ameliorates secondary renal disease following acute kidney injury. Am J Physiol Renal Physiol. 2008;295:F1648–57. doi: 10.1152/ajprenal.00099.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Taneda S, Hudkins KL, Topouzis S, Gilbertson DG, Ophascharoensuk V, Truong L, et al. Obstructive uropathy in mice and humans: potential role for PDGF-D in the progression of tubulointerstitial injury. J Am Soc Nephrol. 2003;14:2544–55. doi: 10.1097/01.asn.0000089828.73014.c8. [DOI] [PubMed] [Google Scholar]