Abstract
Natural pathogen transmission of Rickettsia prowazekii, the etiologic agent of epidemic typhus, to humans is associated with arthropods, including human body lice, ticks, and ectoparasites of eastern flying squirrel. Recently, we have documented the presence of small RNAs in Rickettsia species and expression of R. prowazekii sRNAs during infection of cultured human microvascular endothelial cells (HMECs), which represent the primary target cells during human infections. Bacterial noncoding transcripts are now well established as critical post-transcriptional regulators of virulence and adaptation mechanisms in varying host environments. Despite their importance, little is known about the expression profile and regulatory activities of R. prowazekii sRNAs (Rp_sRs) in different host cells encountered as part of the natural life-cycle. To investigate the sRNA expression profile of R. prowazekii during infection of arthropod host cells, we employed an approach combining in vitro infection, bioinformatics, and PCR-based quantitation. Global analysis of R. prowazekii transcriptome by strand-specific RNA sequencing enabled us to identify 67 cis-acting (antisense) and 26 trans-acting (intergenic) Rp_sRs expressed during the infection of Amblyomma americanum (AAE2) cells. Comparative evaluation of expression during R. prowazekii infection of HMECs and AAE2 cells by quantitative RT-PCR demonstrated significantly higher expression of four selected Rp_sRs in tick AAE2 cells. Examination of the coding transcriptome revealed differential up-regulation of >150 rickettsial genes in either HMECs or AAE2 cells and yielded evidence for host cell-dependent utilization of alternative transcription start sites by 18 rickettsial genes. Our results thus suggest noticeable differences in the expression of both Rp_sRs as well as the coding transcriptome and the exploitation of multiple transcription initiation sites for select genes during the infection of human endothelium and tick vector cells as the host and yield new insights into rickettsial virulence and transmission mechanisms.
Keywords: Rickettsia prowazekii, Small RNAs, RNA Sequencing, Vascular Endothelium, and Epidemic typhus
1. Introduction
Pathogenic bacteria in the Genus Rickettsia belong to two major groups, namely spotted fever and typhus, which continue to pose significant health threats to humans across the globe. Rickettsia prowazekii, the causative agent of epidemic typhus, is an obligate intracellular, Gram-negative bacterium transmitted primarily by the body lice (Pediculus humanus corporis). Consequently, outbreaks of epidemic typhus tend to occur with conditions of crowding in close quarters and compromised hygiene during the times of war, famine, or natural disasters and the disease is also known as camp/famine/jail fever. During human infections, vascular endothelial cells lining the small and medium-sized blood vessels are the primary targets of infection and salient features of disease pathogenesis include vascular inflammation/dysfunction and perturbation of the vasculature’s barrier function manifesting as altered permeability and fluid leakage from the intravascular compartment to the interstitium (Bechah et al., 2008b; Walker and Ismail, 2008). Considered to be one of the most severe forms of human rickettsioses, epidemic typhus due to R. prowazekii is associated with high mortality rates, in particular during the absence of appropriate sanitation and timely intervention with antibiotics-based therapies (Bechah et al., 2008a; Raoult et al., 2004; Uchiyama, 2012). Also, the recrudescent form of epidemic typhus or Brill-Zinsser disease can manifest in patients years after the primary infection and clinical recovery (Bechah et al., 2008a; Uchiyama, 2012), and such a reoccurence can lead to new cases or outbreaks of epidemic typhus. Although human body lice are the established principal vectors, ectoparasites (fleas and lice) of the flying squirrel maintain R. prowazekii in the sylvatic cycle. The presence of R. prowazekii in Amblyomma ticks from Mexico and Ixodes ticks in the Netherlands has also been demonstrated recently, suggesting the possibility of tick transmission in natural infections (Bozeman et al., 1975; Medina-Sanchez et al., 2005; Philip et al., 1966).
Once thought to be junk DNA, bacteria encode small regulatory RNAs (sRNAs) that act as critical post-transcriptional regulators of gene expression. These sRNAs typically range from 50 to 500 nucleotides and regulate a variety of processes such as environmental sensing, metabolism, stress responses, and virulence in pathogenic bacteria. The major families of sRNAs include true antisense RNAs originating from the ‘opposite’ complementary strand to the mRNA (cis-acting), sRNAs that act by limited complementarity base pairing with their targets (trans-acting), and sRNAs that exhibit binding interactions with proteins affecting their activity. Trans-acting sRNAs are encoded within the intergenic regions and act on target RNAs located elsewhere in the genome. In essence, such sRNAs are akin to eukaryotic microRNAs in their ability to modulate the activity and stability of multiple mRNAs (Gottesman and Storz, 2011; Liu and Camilli, 2010). Unlike cis-acting sRNAs, trans-acting sRNAs display only partial nucleotide complementarity and generally require an RNA chaperone to facilitate interactions with their targets (Waters and Storz, 2009).
Using a bioinformatics-based approach, we recently predicted the presence of over 1,700 trans-acting sRNAs in the genomes of 16 different strains encompassing 13 rickettsial species (Schroeder et al., 2015). Using infection of cultured human microvascular endothelial cells (HMECs) with R. prowazekii, we further identified expression of 35 novel trans-acting and 23 cis-acting sRNAs through Next Generation Sequencing and confirmed expression of four novel R. prowazekii sRNAs (named Rp_sRs) along with the highly conserved and known bacterial sRNAs, namely α-tmRNA, RNaseP_bact_a, ffs, and 6S RNA (Schroeder et al., 2016). The present study was undertaken to conduct a compare and contrast analysis of the expression of R. prowazekii transcriptome during infection of human and tick host cells. Our results enable identification of additional Rp_sR candidates uniquely expressed during infection of tick cells and suggest differential expression of select Rp_sRs in tick vis-à-vis human host cells. In addition, a comprehensive analysis of encoding rickettsial transcriptome during infection of human and tick vector host cells yields the first evidence for the utilization of multiple transcription start sites depending on the host niche.
2. Materials and Methods
2.1. Rickettsia prowazekii and Cell Culture
Stocks of R. prowazekii strain Breinl were prepared by infecting Vero cells cultured in DMEM supplemented with 2% fetal bovine serum in an atmosphere of 95%O2: 5% CO2 at 35°C following standard protocols (Rydkina et al., 2005). Rickettsiae were purified by differential centrifugation, titered by a quantitative PCR-based assay, and stored at −80°C as aliquots until use (Labruna et al., 2004). Considering that repeat freeze-thaw cycles may alter rickettsial viability and transcriptome, all experiments were performed using R. prowazekii stocks gently thawed on ice for the first time. Human dermal microvascular endothelial cells (HMECs) were cultured at 37°C with 5% CO2 in MCDB131 medium supplemented with 10% fetal bovine serum, 10mM L-glutamine, 1μg/ml hydrocortisone, and 10ng/ml epidermal growth factor as previously described (Schroeder et al., 2015; Schroeder et al., 2016). The use of human cell lines in our study was exempt by the University of Texas Medical Branch (UTMB) Institutional Review Board (IRB), but approved by the UTMB Institutional Biosafety Committee (IBC). Amblyomma americanum tick cells (AAE2) were grown in L-15B complete medium (pH 7.5) at 34°C to ~90% confluence. Approximately 24h prior to infection, the medium in each flask was replaced with L-15B infection medium (pH 7.5) containing 25mM sodium bicarbonate and HEPES (Munderloh and Kurtti, 1989). In vitro infection of HMECs with R. prowazekii stocks was carried out at 37°C or 34°C according to our standard protocols and procedures (Rydkina et al., 2005; Narra et. al., 2016). To achieve a comparable level of infection, AAE2 cells were infected with different stocks of R. prowazekii and incubated at 34°C. At 24 h, the cells were gently scraped and pelleted by centrifugation at 400 g for 5 minutes. The cell pellet was washed twice with sterile phosphate buffered saline (PBS) and processed for total DNA extraction using DNeasy Blood & Tissue kit (Qiagen). The MOI was estimated by absolute quantification using gene specific primers (Supplementary table 1) targeting tick calreticulin and R. prowazekii citrate synthase (gltA) genes. For RNA-Seq experiments, HMECs were infected with R. prowazekii at an MOI of 5:1 (~6×104 pfu of rickettsiae per cm2) in minimal volume of MCDB131 medium and incubated at room temperature for 15 minutes with gentle, intermittent rocking to enhance adhesion and invasion. The inoculation medium was then replaced with fresh medium and the cells were incubated for 24 h at 37°C, 5% CO2 (Schroeder et al., 2015; Schroeder et al., 2016). For AAE2 cells, the L-15B medium (containing viable, semi-adherent cells) from the culture flask was collected to pellet the non-adherent cells by centrifugation at 400 g for 5 minutes. The pellet was then suspended in 1 ml of L-15B infection medium and added back to the adherent cells within the same flask. The cells were then infected with R. prowazekii at an MOI of 5:1 based on the estimation of infectivity titers as described above and gently rocked at room temperature for 15 minutes, at which time the medium containing rickettsiae was replaced with fresh medium as described above and the flasks were placed in an incubator at 34°C for 24 h. At the end of incubation, the medium was completely removed and total RNA was extracted by the Tri-Reagent method detailed below. Each RNA-Seq experiment was performed on two independent biological replicates.
2.2. RNA Isolation and Sequencing
Isolation of total RNA from HMECs and AAE2 cells infected with R. prowazekii was carried out at 24h using our standard Tri-Reagent (Molecular Research Center) protocol for deep sequencing. Total RNA was treated with DNaseI (Zymo Research) to remove any contaminating genomic DNA, and the samples were further processed using Dynabeads® Oligo (dT)25 (ThermoFisher Scientific) and Ribo-Zero (Epicentre) to remove any interfering eukaryotic mRNAs and ribosomal RNAs, respectively. The enriched total RNA preparations thus obtained were quantified using the MultiSkan Go Microplate Spectrophotometer (ThermoScientific) and assessed for their quality on an Agilent 2100 Bioanalyzer (Agilent Technologies).
Subsequently, the total RNA from each biological replicate was divided into two equal aliquots. One aliquot was treated with Terminator 5′-Phosphate-Dependent Exonuclease (TEX) (Epicentre) resulting in the degradation of processed RNA transcripts containing the 5′ monophosphate (+TEX) but not the primary transcripts with 5′ triphosphate. The other aliquot served as untreated control and contained both processed and primary transcripts (−TEX). Independent cDNA libraries for each aliquot were generated using the TruSeq RNA Sample Prep Kit (Illumina) as per manufacturer’s directions. Strand-specific sequencing on non-size selected cDNA libraries was performed on an Illumina HiSeq 1500 at our institutional Next Generation Sequencing Core facility. The sequencing libraries were comprised of 100 base long reads in a FASTQ format. The quality of each read was assessed and any base with a PHRED < 15 was excluded from the analysis. The first 14 bases of each read were trimmed and the remaining 86 base long reads were mapped onto the R. prowazekii Breinl genome (NC_020993) allowing up to four base mismatches using Bowtie2 (Langmead and Salzberg, 2012). Reads that did not map to the R. prowazekii Breinl genome or that mapped to more than one genomic location were discarded from the analysis. ‘Transcripts per million’ (TPM) is computed as:
where the sum is over the ‘reads per kilobase per mapped reads’ (RKPM) values of all genes/transcripts (Li et al., 2010). The RKPM is measured as shown below and described earlier (Mortazavi et al., 2008)
2.3. Quantitative Real-Time Reverse Transcriptase PCR (q-RT-PCR)
To validate the expression profile of differentially expressed Rp_sRs and R. prowazekii genes, q-RT-PCR was performed on total RNA extracted from HMECs and AAE2 cells infected with R. prowazekii. Because temperature shifts are known to alter the rickettsial transcriptome (Dreher-Lesnick et al., 2008; Ellison et al., 2009; Galletti et al., 2013), we performed q-RT-PCR on R. prowazekii infected HMECs at both 37°C and 34°C and on infected AAE2 cells grown at 34°C. HMECs and AAE2 cells were infected with R. prowazekii as described in Section 2.1 above. Briefly, after gentle rocking for 15 minutes at room temperature, the medium in the flasks was replaced with fresh medium and infected HMECs were incubated at either 37°C or 34°C with 5% CO2 and AAE2 cells infected with R. prowazekii were incubated at 34°C. Quantitative RT-PCR was performed on RNA extracted at 3h and 24h post-infection to capture the expression profiles of R. prowazekii small RNAs and transcripts at early (3h) and late (24h) stages of infection. For comparative analysis, HMECs and AAE2 cells infected for 30 minutes at the respective temperatures were designated as the ‘base line’. Total RNA was extracted by Tri-reagent method following our standard protocol. One microgram (1μg) of DNaseI treated total RNA was reverse transcribed using High Capacity Reverse Transcription Kit (Applied Biosystems) with random primers following the manufacturer’s instructions. q-RT PCR was performed using SYBR® Green PCR Master Mix (Life Technologies). Each 20 μL reaction contained 1X SYBR® Green PCR Master Mix (contains DNA polymerase and dNTPs), 0.5 μM forward primer, 0.5 μM reverse primer, and 100 ng cDNA template. Thermal cycler conditions were: stage 1 at 95°C for 10 minutes, stage 2 (40 cycles) at 95°C for 30 seconds and 60°C for 30 seconds, followed by melt curve. R. prowazekii 16S rRNA was used as internal control and the expression of Rp_sR and rickettsial transcripts was analyzed by 2−ΔΔCt method (Schmittgen and Livak, 2008). The data are presented as the mean±SEM from a minimum of three biological replicates processed as two technical replicates. All primers used in this study are listed in Supplementary table 1. Statistical analysis was performed using GraphPad Prism using Mann-Whitney t-test with statistical significance set to a threshold P-value of ≤ 0.05.
2.4. Computational Identification of Target Genes Regulated by Rp_sRs
To gain insight into the post-transcriptional regulation by Rp_sRs, targets genes potentially regulated by Rp_sR76, 83, 86 and 159 were predicted using IntaRNA algorithm (Busch et al., 2008) using default parameters with the only exception that the region of interrogation for identification of seed region was set to +150 and −100 bases with respective to the translational start codon of an ORF (Narra et. al., 2016). Only target genes exhibiting a significance of p<0.05 for the Rp_sR-target mRNA seed region interaction were considered for the analysis.
3. Results
3.1. R. prowazekii Coding Sequence (CDS) Expression in HMECs and AAE2 Cells
Global transcriptional profiling is a comprehensive approach to better understand the expression profile of coding and non-coding transcripts in a given condition. To gain functional insights into the transcriptional landscape of R. prowazekii during its interaction with host vis-à-vis vector cells in vitro, we compared the expression profiles of R. prowazekii coding genes (ORFs) in infected HMECs and A. americanum tick (AAE2) cells via normalization of our RNA-seq data by calculating the transcripts per kilobase million (TPM) for each rickettsial ORF (Supplementary table 2). This method was chosen over the “reads per kilobase per million” (RPKM), because TPM eliminates an intrinsic statistical bias attributed to an inconsistent measure of molar concentrations (Wagner et al., 2012). The comparative analysis of the expression levels of R. prowazekii ORFs in HMECs versus AAE2 cells is presented in Supplementary table 2. Employing a cut-off of 3-fold or higher, we determined that 34 rickettsial genes were expressed at significantly higher levels in HMECs (Table 1). Among these, a majority (82%) were found to be expressed at levels 3 to 7 times higher than in AAE2 cells. The most striking change of 11 to 23 times higher expression levels in relation to AAE2 cells was noticed for three genes namely, H375_9040 (Heat shock protein 60), H375_7520 (Tol-Pal system peptidoglycan lipoprotein), and H375_6910 (hypothetical protein). Conversely, about 72% of rickettsial genes were found to be expressed in AAE2 cells at levels 3 to 7 times greater than in HMECs and there was very high expression of another 16 rickettsial genes in AAE2 cells as evidenced by an increase of 10 to 29-fold over the same during the infection of HMECs (Table 2). Intriguingly, the steady-state levels of transcripts for a total of 47 rickettsial genes were below the limit of detection in HMECs despite their transcription during infection of AAE2 cells, whereas transcripts for only four genes could not be detected in AAE2 cells despite their expression in HMECs. Furthermore, expression levels of a total of 11 genes were below the limit of detection in either host cell type (Supplementary table 2). Of these, 10 genes were annotated as the hypothetical proteins with as yet unassigned functions and the remaining gene, H375_8360, encodes for a lipid A export ATP-binding/permease protein.
Table 1.
List of R. prowazekii ORFs upregulated during the infection of HMECs when compared to AAE2 cells in vitro.
| Gene | TPM | Annotation |
|---|---|---|
| H375_9040 | 22.93 | Heat shock protein 60 family co-chaperone GroES |
| H375_7520 | 12.67 | Tol-Pal system peptidoglycan-associated lipoprotein PAL |
| H375_6910 | 11.08 | Putative adhesion (homolog of Adr1) |
| H375_3980 | 9.81 | Threonyl-tRNA synthetase |
| H375_450 | 8.23 | Cell division protein MraZ |
| H375_7750 | 7.84 | Twin-arginine translocation protein TatA |
| H375_6090 | 6.64 | ATP synthase F0 sector subunit c (EC 3.6.3.14) |
| H375_0400 | 5.66 | Uncharacterized protein RT0563 |
| H375_3470 | 5.66 | rickettsial conserved |
| H375_5060 | 3.92 | HflC protein |
| H375_9050 | 3.79 | Heat shock protein 60 family chaperone GroEL |
| H375_4550 | 3.77 | DNA-binding protein HU |
| H375_1770 | 3.76 | Succinyl-CoA ligase [ADP-forming] alpha chain |
| H375_8520 | 3.62 | 2-methoxy-6-polyprenyl-1,4-benzoquinol methylase |
| H375_4120 | 3.45 | Peptide deformylase |
| H375_4630 | 3.45 | rickettsial conserved |
| H375_5960 | 3.45 | Acetoacetyl-CoA reductase |
| H375_1100 | 3.02 | SSU ribosomal protein S15p |
| H375_3430 | 4.66 | Small heat shock protein C1 |
| H375_9200 | 4.61 | SSU ribosomal protein S21p |
| H375_6920 | 4.58 | hypothetical protein |
| H375_9250 | 4.53 | hypothetical protein |
| H375_8350 | 4.39 | rickettsial conserved |
| H375_1700 | 4.32 | rickettsial conserved |
| H375_6600 | 4.02 | hypothetical protein |
| H375_7550 | 3.28 | Rod shape-determining protein MreC |
| H375_8110 | 3.15 | hypothetical protein |
| H375_2030 | 3.02 | Cytochrome c oxidase polypeptide II |
| H375_3520 | 3.02 | Outer membrane protein H precursor |
| H375_7430 | 3.02 | rickettsial conserved |
| H375_7600 | 3.02 | Acyl carrier protein |
| H375_7730 | 3.02 | LSU ribosomal protein L21p |
| VBIRicPro269054_0891 | 3.02 | hypothetical protein |
| H375_1740 | 3.02 | Ribosome-binding factor A |
Table 2.
List of R. prowazekii ORFs upregulated during the infection of AAE2 cells when compared to HMECs in vitro.
| Gene | TPM | Annotation |
|---|---|---|
| H375_8480 | 28.84 | Thymidylate kinase |
| H375_1050 | 23.2 | Lipopolysaccharide ABC transporter |
| H375_2780 | 22.87 | dTDP-4-dehydrorhamnose reductase |
| H375_6110 | 22.21 | ATP synthase F0 sector subunit b |
| H375_2300 | 18.23 | 3-deoxy-manno-octulosonate cytidylyltransferase |
| H375_7500 | 15.25 | LSU ribosomal protein L32p |
| H375_7840 | 14.91 | Metal-dependent hydrolase YbeY |
| H375_1210 | 12.76 | rickettsial conserved |
| H375_4640 | 12.37 | hypothetical protein |
| H375_9060 | 12.35 | guanosine-3,5-bis(diphosphate) 3-pyrophosphohydrolase SpoTc |
| H375_2110 | 12.26 | Signal peptide peptidase SppA, 36K type |
| H375_0670 | 11.93 | Citrate synthase |
| H375_8910 | 11.77 | LSU ribosomal protein L30p |
| H375_1070 | 10.94 | Lipopolysaccharide-assembly protein LptC |
| H375_0860 | 10.77 | Fumarate hydratase class II |
| H375_5480 | 10.27 | hypothetical protein |
| H375_7110 | 9.69 | YrbA protein |
| H375_1170 | 9.61 | Multicopper polyphenol oxidase |
| H375_0490 | 9.53 | LSU ribosomal protein L10p |
| H375_4210 | 9.28 | rickettsial conserved |
| H375_3780 | 8.95 | Inositol-1-monophosphatase |
| H375_6030 | 8.78 | DNA recombination and repair protein RecF |
| H375_5500 | 8.12 | hypothetical protein |
| H375_0500 | 8.07 | Succinate dehydrogenase hydrophobic membrane anchor protein |
| H375_1840 | 7.79 | Undecaprenyl diphosphate synthase |
| H375_6230 | 7.79 | 3-hydroxyacyl-[acyl-carrier-protein] dehydratase |
| H375_6070 | 7.73 | ATP synthase protein I |
| H375_7490 | 7.62 | Outer membrane lipoprotein OmlA |
| H375_4230 | 7.46 | Universal stress protein family 3 |
| H375_6390 | 7.46 | Octanoate-[acyl-carrier-protein]-protein-N-octanoyltransferase |
| H375_7130 | 7.29 | Putative glutathione-regulated potassium-efflux system protein KefB |
| H375_8740 | 6.96 | LSU ribosomal protein L4p |
| H375_2600 | 4.64 | Exodeoxyribonuclease VII small subunit |
| H375_0780 | 4.47 | hemolysin C |
| H375_5450 | 4.47 | SSU ribosomal protein S2p (SAe) |
| H375_2690 | 4.4 | RND efflux system |
| H375_0850 | 4.39 | Outer membrane protein Imp |
| H375_6020 | 4.38 | hypothetical protein |
| H375_3220 | 4.37 | Guanosine pentaphosphate phosphohydrolase |
| H375_4250 | 4.27 | Multimodular transpeptidase-transglycosylase |
| H375_8940 | 4.14 | Adenylate kinase |
| H375_1780 | 4.14 | Succinyl-CoA ligase |
| H375_2760 | 4.08 | UDP-N-acetyl-L-fucosamine synthase |
| H375_7700 | 4.02 | hypothetical protein |
| H375_4520 | 4.01 | Signal recognition particle protein Ffh |
| H375_5310 | 4 | LSU ribosomal protein L28p |
| H375_5490 | 3.98 | hypothetical protein |
| H375_6300 | 3.98 | Uroporphyrinogen III decarboxylase |
| H375_2020 | 3.95 | hypothetical protein |
| H375_2480 | 3.93 | hypothetical protein |
| H375_6800 | 3.92 | hypothetical protein |
| H375_2010 | 3.91 | Lipoprotein signal peptidase |
| H375_5900 | 3.87 | LSU ribosomal protein L9p |
| H375_0960 | 3.81 | carbonic anhydrase, family 3 |
| H375_7970 | 3.79 | NADH:ubiquinone oxidoreductase 17.2 kD subunit |
| H375_5050 | 3.76 | HtrA protease/chaperone protein |
| H375_3930 | 3.74 | hypothetical protein |
| H375_2360 | 3.73 | RalF protein |
| H375_2200 | 3.72 | rare lipoprotein A precursor |
| H375_8000 | 3.71 | tRNA-guanine transglycosylase |
| H375_1890 | 3.71 | hypothetical protein |
| H375_7280 | 3.65 | NADH-ubiquinone oxidoreductase chain I |
| H375_4740 | 3.65 | Aspartyl-tRNA(Asn) amidotransferase subunit C |
| H375_4180 | 3.65 | Chaperone protein HscB |
| H375_7380 | 6.96 | hypothetical protein |
| H375_6130 | 6.52 | cell surface antigen |
| H375_0060 | 6.46 | RNA binding methyltransferase FtsJ like |
| H375_0950 | 6.46 | Ribosome hibernation protein YhbH |
| H375_6190 | 6.46 | hypothetical protein |
| H375_1730 | 6.37 | rickettsial conserved |
| H375_8460 | 6.3 | 4-hydroxybenzoate polyprenyltransferase |
| H375_4360 | 6.3 | serine protease, HtrA/DegQ/DegS family |
| H375_6010 | 5.83 | Cytochrome oxidase biogenesis protein |
| H375_5370 | 5.83 | Phospholipid ABC transporter shuttle protein MlaC |
| H375_0020 | 5.8 | hypothetical protein |
| H375_6040 | 5.72 | Methyltransferase |
| H375_3940 | 5.66 | Type I secretion outer membrane protein, TolC precursor |
| H375_3010 | 5.63 | hypothetical protein |
| H375_2720 | 5.5 | Membrane protein, putative |
| H375_1830 | 5.47 | Osmolarity sensory histidine kinase EnvZ |
| H375_3830 | 5.44 | SSU ribosomal protein S9p (S16e) |
| H375_0160 | 5.44 | ATP-dependent protease La (EC 3.4.21.53) Type I |
| H375_3020 | 5.37 | TolA protein |
| H375_1940 | 5.36 | Diaminopimelate epimerase |
| H375_2490 | 5.3 | rickettsial conserved |
| H375_1920 | 5.3 | Phenylalanyl-tRNA synthetase alpha chain |
| H375_2550 | 5 | NADH-ubiquinone oxidoreductase chain C |
| H375_9240 | 4.97 | hypothetical protein |
| H375_7670 | 4.97 | hypothetical protein |
| H375_5710 | 4.97 | Chromosome (plasmid) partitioning protein ParB/Stage 0 sporulation protein J |
| H375_5660 | 4.91 | dNTP triphosphohydrolase, broad substrate specificity |
| H375_2140 | 4.81 | rickettsial conserved |
| H375_1870 | 4.79 | Ribonuclease D related protein |
| H375_3390 | 4.72 | rickettsial conserved |
| H375_0470 | 4.72 | Ribosome recycling factor |
| H375_7940 | 4.64 | UDP-2,3-diacylglucosamine pyrophosphatase |
| H375_2710 | 3.6 | minor teichoic acids biosynthesis protein ggaB |
| H375_4420 | 3.56 | Thermostable carboxypeptidase 1 |
| H375_2700 | 3.55 | hypothetical protein |
| H375_6310 | 3.42 | Ferrochelatase |
| H375_3910 | 3.42 | Topoisomerase IV subunit B |
| H375_7170 | 3.4 | Ferroxidase |
| H375_7530 | 3.37 | hypothetical protein |
| H375_1990 | 3.37 | UDP-N-acetylmuramoylalanine |
| H375_8240 | 3.35 | Integration host factor alpha subunit |
| H375_8610 | 3.31 | rickettsial conserved |
| H375_0140 | 3.31 | Rhodanese-related sulfurtransferase |
| H375_1010 | 3.31 | rickettsial conserved |
| H375_4140 | 3.28 | Multidrug resistance protein Atm1 |
| H375_3250 | 3.27 | VirB10 |
| H375_7190 | 3.26 | ATP synthase delta chain |
| H375_3180 | 3.26 | rickettsial conserved |
| H375_0600 | 3.2 | Bacterial ribosome SSU maturation protein RimP |
| H375_3130 | 3.2 | Thymidylate synthase ThyX |
| H375_6290 | 3.2 | Phosphoenolpyruvate synthase regulatory protein |
| H375_5890 | 3.2 | tRNA(Ile)-lysidine synthetase |
| H375_5040 | 3.2 | Rhodanese domain protein |
| H375_8080 | 3.15 | Mobile element protein |
| H375_4990 | 3.15 | SSU ribosomal protein S12p (S23e) |
| H375_5840 | 3.15 | Small-conductance mechanosensitive channel |
| H375_7680 | 3.13 | hypothetical protein |
| H375_0540 | 3.12 | 2-octaprenyl-6-methoxyphenol hydroxylase |
| H375_4400 | 3.08 | lipoprotein ComL |
| H375_4050 | 3.03 | rickettsial conserved |
| H375_7420 | 3.01 | rickettsial conserved |
| H375_0520 | 3.01 | VirB6 |
3.2. q-RT-PCR based quantitation of Novel Differentially Expressed R. prowazekii genes in Human and Tick cells
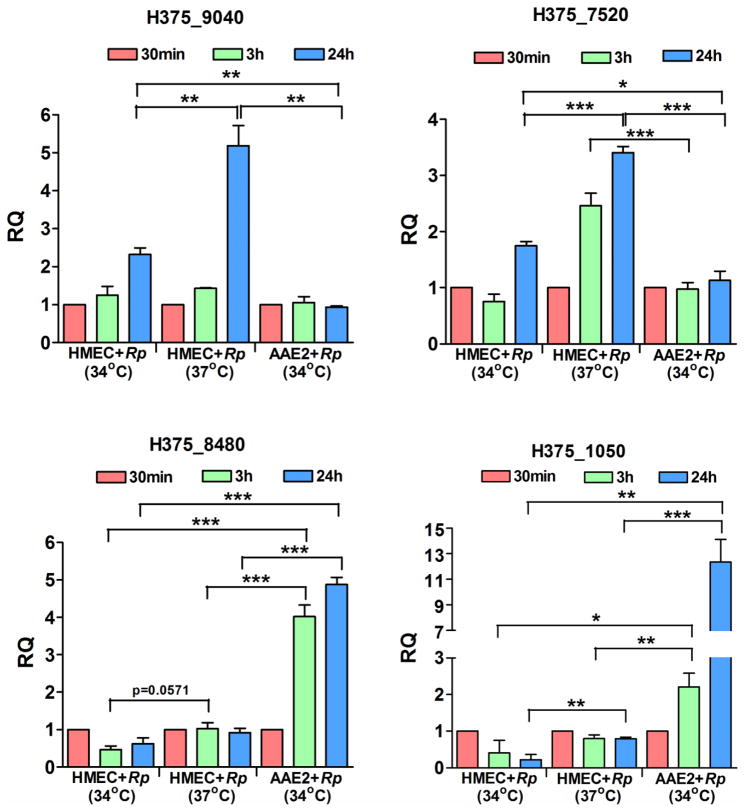
To validate the differential expression of R. prowazekii transcripts observed in our RNA Seq, q-RT PCR was performed on four genes, namely, H375_9040 and H375_7520 (upregulated during infection of HMECs), and H375_8480 and H375_1050 (highly expressed during infection of AAE2 cells). As temperature shifts are known to alter rickettsial gene expression, total RNA preparations from HMECs infected with R. prowazekii at either 37°C or 34°C and AAE2 cells infected at 34°C were used for comparative quantitative analysis using gene-specific primers and 16S rRNA as an internal control. Interestingly, H375_9040 and H375_7520 were highly expressed during the infection of HMECs, whereas H375_8480 and H375_1050 were up-regulated during tick cell infection (Figure 1). Quantitative RT-PCR based analysis of these genes exhibited an overall pattern of expression consistent with the RNA-Seq data, although some variation in fold-change values were noted as expected. Notably, all genes exhibited highest transcript abundance at 24 h post-infection in both cell types. Although temperature (37°C vs. 34°C) had an influence on the expression of H375_1050 during the infection of HMECs, significant differences were also evident in the transcript abundance depending on the host niche, thus confirming the results from global transcriptomic profiling of R. prowazekii transcripts during host-pathogen and vector-pathogen interactions in vitro.
Figure 1. Quantitative PCR based validation of R. prowazekii genes differentially expressed during host and tick cell infection in vitro.
Confluent monolayers of human microvascular endothelial cells (HMECs) grown at 37°C or 34°C or Amblyomma americanum cells (AAE2) grown at 34°C were infected with R. prowazekii for 0.5h, 3h, and 24h. Total RNA was extracted using Trizol, treated with DNaseI, and reverse transcribed for RT-PCR (n≥3). Expression profile of four genes namely, H375_9040, H375_7520, H375_8480 and H375_1050 was quantified at early (3h) and late (24h) stages of infection of HMECs and AAE2 cells using gene specific primers and 16S rRNA as endogenous control. The data is calculated using expression levels at 0.5h as the baseline and presented as mean±SEM. Asterisks indicate *p<0.05, **p<0.01 and ***p<0.001.
3.3. Alternate ORF Transcription Start Sites during the Infection of Human and Tick Cells
Regulation of transcription initiation during mRNA biogenesis represents the first layer in the control of gene expression and alternative transcription initiation results in the generation of transcripts differing in the length of the 5′-untranslated region (5′-UTR). Identification of mRNA transcription start sites (TSSs) is, therefore, critical for characterization of promoter regions, which is essential for understanding gene expression and regulation patterns in both prokaryotes as well as eukaryotes. Application of differential RNA-seq relying on the tri-phosphorylation of primary, but not processed, transcripts at their 5′ ends has led to the mapping of TSSs in E. coli grown under different conditions (Wade, 2015). To examine possible differences in rickettsial TSS during infection of human and tick cells as the host, we subjected our RNA samples to treatment with terminator 5′-phosphate-dependent exonuclease (TEX) prior to sequencing. Under these experimental conditions, an average of 333,431 and 256,084 reads belonging to primary transcripts mapped to R. prowazekii genome in HMECs and AAE2 cells, respectively, and allowed us to identify TSSs for 97 rickettsial genes expressed during the infection of host and tick cells (Supplementary table 3). Further in-depth examination of sequencing data from R. prowazekii-infected HMECs and AAE2 cells revealed utilization of an alternative TSS by 18 rickettsial genes involved in diverse set of rickettsial pathways, such as transcription, metabolism, signaling and type I secretion system (Table 3). A strand bias was also apparent in that 12 of 18 genes were located on the positive strand. Interestingly, the transcript length was longer for a majority of these genes (12 of 18) during their expression in AAE2 cells (Table 3).
Table 3.
List of R. prowazekii ORFs exhibiting different transcription start sites during their in vitro expression in HMECs and AAE2 cells
| Locus Tag | HMEC-T | AAE2-T | Strand | Annotation |
|---|---|---|---|---|
| H375_0890 | 119283 | 119025 | + | Transcription termination factor Rho |
| H375_0950 | 128486 | 128393 | + | Ribosome hibernation protein YhbH |
| H375_2140 | 285600 | 285557 | + | Rickettsial conserved |
| H375_3140 | 400166 | 400204 | − | Membrane-bound metallopeptidase |
| H375_3150 | 400514 | 400453 | + | Ribose 5-phosphate isomerase B |
| H375_3390 | 427957 | 427919 | + | Hypothetical protein |
| H375_3530 | 443893 | 443878 | + | GTP-binding protein TypA |
| H375_3540 | 447237 | 447188 | − | Pyruvate dehydrogenase E1 subunit |
| H375_3630 | 457043 | 457059 | + | UDP-3-O-[3-hydroxymyristoyl] N-Acetylglucosamine deacetylase |
| H375_3640 | 458837 | 458779 | + | Membrane c-type cytochrome cy |
| H375_3710 | 466907 | 466938 | − | Hypothetical protein |
| H375_3800 | 472515 | 472467 | + | Pole remodelling regulatory diguanylate cyclase |
| H375_3940 | 488473 | 488445 | − | Type I secretion outer membrane protein, TolC |
| H375_4120 | 513479 | 513553 | − | Peptide deformylase |
| H375_4800 | 591148 | 591116 | + | Integral membrane protein |
| H375_5400 | 666077 | 666043 | + | Aspartate aminotransferase |
| H375_8580 | 1041290 | 1041233 | + | Outer membrane protein Imp |
| H375_9020 | 1076523 | 1076555 | − | Heat shock protein GrpE |
3.4. Identification of R. prowazekii small RNAs expressed during the infection of AAE2 tick cells
We have recently demonstrated that R. prowazekii encodes and expresses a number of Rp_sR’s during in vitro infection of human microvascular endothelial cells (Schroeder et al., 2015). To investigate the possibility of divergence in sRNA transcriptome of R. prowazekii in different host environments, we infected AAE2 cells for in-depth analysis by next generation sequencing to identify and catalogue Rp_sR’s specifically expressed during vector-pathogen interactions. To achieve a direct comparison of Rp_sR profiles during the infection of human and tick cells as the host, we carried out a side-by-side analysis of infected HMECs as well. RNA sequencing of cDNA libraries prepared from AAE2 cells and HMECs infected with R. prowazekii and enriched for bacterial transcripts resulted in approximately an average of 91 million and 46 million total reads, respectively. Despite enrichment of total RNA preparations for bacterial transcripts, a significant percentage of reads corresponded to the eukaryotic genome and mapped to non-polyadenylated transcripts originating from mitochondrial genes, rRNAs, tRNAs, and long and small non-coding RNAs. In correlation with our previous study (Schroeder et al., 2016), our sequencing analysis resulted in approximately an average of 2.9 million and 1.3 million reads mapping to the bacterial genome in libraries originating from total RNA isolated from AAE2 cells and HMECs infected with R. prowazekii, respectively. Overall, the eukaryotic and bacterial contributions in our RNA sequencing is in general agreement with the finding that obligately intracellular bacterial genomes only constitute 2–5% of extracted total RNA despite application of efficient enrichment protocols and that only 5% of the bacterial RNA represents mRNAs and sRNAs, with the remaining 95% mapping to rRNAs and tRNAs (Westermann et al., 2012). Thus, application of genome-wide identification of sRNA candidate-containing regions within the RNA-Seq datasets revealed an additional 93 novel candidate sRNA-encoding regions in intergenic regions (Figure 2, Supplementary table 4) and transcripts from regions antisense to open reading frames bearing the characteristics of cis-acting antisense sRNAs at 24h post-infection (Figure, Supplementary table 4). Of these, cis-acting accounted for approximately 72% of newly identified sRNAs in AAE2 cells, among which Rp_sR152, a cis-acting sRNA antisense to H375_8370, was the smallest with a length of 148 bp. Interestingly, 11 other sRNAs (Rp_sR79, Rp_sR92, Rp_sR93, Rp_sR94, Rp_sR114, Rp_sR120, Rp_sR128, Rp_sR130, Rp_sR135, Rp_sR143, and Rp_sR154) ranged from 516 bp to 844 bp in length and 8 of these were classified as cis-acting. No strand bias in regards to their origin was evident based on the expression of 47% of Rp_sRs from the leading strand and remaining 53% on the lagging strand. There were no significant differences in strand-specificity as well based on the location of 38% trans-acting and 50% cis-acting Rp_sRs on the leading strand. Further, 6 (Rp_sR71, Rp_sR74, Rp_sR75, Rp_sR129, Rp_sR139, and Rp_sR155) of the 26 trans-acting Rp_sRs were encoded in the same orientation as their upstream genes. As expected, 66 cis-acting sRNAs were found to be directly anti-sense to an open reading frame on the opposite strand. Interestingly, Rp_sR124 was expressed in a manner such that it overlapped with the 3′ end of a 3-polyprenyl-4-hydroxybenzoate carboxylase (H375_7030) and the 5′ end of H375_7040 coding for a polyhydroxyalkanoic acid synthase. Collectively, our results reveal the expression of several novel Rp_sRs during R. prowazekii infection of tick cells.
Figure 2. Expression profile of novel trans-Acting AAE2 specific Rp_sRs.
Shown are the coverage plots for selected trans-acting Rp_sRs observed during the infection of AAE2 cells. Nucleotide positions within the genome are indicated on X-axis and the Y-axis displays the number of reads for that particular nucleotide position. The light grey arrow represents the small RNA. The dark grey arrows represent the orientation of upstream and downstream ORFs, respectively.
3.5. q-RT-PCR based quantitation of Novel Differentially Expressed sRNAs in Human and Tick cells
As previously reported, bacterial sRNA expression can not only differ among tissues and organ systems within an infected host, but also between genders of a particular host species (Woolfit et al., 2015) and evidence from our laboratory has suggested differential regulation of R. conorii sRNAs in tick and human host cells (Narra et al., 2016). To further investigate whether R. prowazekii selectively and/or differentially express sRNAs in different host cells, we measured the expression levels of five novel candidate sRNAs in HMECs infected at either 37°C or 34°C and AAE2 cells infected at 34°C. The steady state levels of Rp_sRs in cells infected for only 0.5h served as the baseline for comparative analysis at 3 and 24h post-infection using 16S rRNA as the endogenous control. Both Rp_sR76 and Rp_sR83 demonstrated significantly higher levels of expression in AAE2 cells at 3h post-infection when compared to HMECs grown at 37°C (p≤0.001), but failed to show any significance differences at the later time of 24h. Interestingly, significant difference (p<0.05) in Rp_sR76 and Rp_sR83 expression was seen at 24h between HMECs and AAE2 cells grown at 34°C (Figure 4). Consistent with our RNA Seq data, Rp_sR86 was significantly highly expressed during AAE2 cell infection at both early (3h) and late (24h) time points when compared to HMECs grown at either 37°C or 34°C. Rp_sR159 also displayed significantly higher expression in AAE2 cells at 3h (p≤0.01) when compared to the respective expression levels in HMECs at both 37°C and 34°C. The highest level of Rp_sR159 expression was, however, noticed in HMECs at 34°C during late stage of infection (24h), suggesting the potential effects of temperature on its expression (Figure 4). Also, there was no evidence of any significant differences in the pattern of expression of Rp_sR74 during R. prowazekii infection of HMECs and AAE2 cells (data not shown).
Figure 4. qPCR of novel differentially expressed Rp_sRs.
Confluent monolayers of human microvascular endothelial cells (HMECs) cultured at 37°C or 34°C or Amblyomma americanum cells (AAE2) cultured at 34°C were infected with R. prowazekii for 0.5h, 3h, and 24h. Total RNA was extracted using Trizol, DNaseI treated, and reverse transcribed for RT-PCR (n≥3). AAE2 and HMEC expression were baselined to 0.5h and normalized to 16S rRNA. The data is presented as mean±SEM for four trans-acting Rp_sRs, namely, Rp_sR76, Rp_sR83, Rp_sR86, and Rp_sR159. Asterisks indicate *p<0.05, **p<0.01 and ***p<0.001.
3.6. Prediction of target genes regulated by Rp_sR’s
Using IntaRNA, we have predicted a total of 45, 27, 38 and 36 genes as the potential targets of regulation by Rp_sR76, 83, 86 and 159, respectively. Of these, a number of important genes such as diguanylate cyclase (H375_RS01895), protease modulator hflC (H375_RS02485), ferredoxin-NADP(+) reductase (H375_RS00510), resolvase (H375_RS03980), and two hypothetical proteins (H375_RS02580 and H375_RS03250) may be regulated by at least two sRNAs. In addition, several other key genes, including multidrug transporter (H375_RS00115), two component sensor histidine kinase (H375_RS04485), DNA repair (H375_RS02190), and cold shock protein cspA (H375_RS04205) emerged as the potential regulatory targets of at least one Rp_sR (Supplementary table 5).
4. Discussion
In-depth transcriptomic analysis offers a comprehensive mapping and enhanced understanding of the systems biology of host-pathogen interactions. In the present study, we have employed an RNA-sequencing based approach to perform comparative evaluation of the coding as well as non-coding transcriptome of R. prowazekii during the infection of human endothelial cells as the preferred, primary target cell type in the mammalian host and tick vector cells as the arthropod stage of the pathogen’s life-cycle. Although body lice are the major established natural vectors responsible for epidemic typhus outbreaks, the rationale for our comparative analysis derives from recent reports documenting the presence of R. prowazekii in circulating tick vectors and easy availability as well as infectivity of cultured tick cells. Because RNA-seq studies using deep sequencing technologies have unequivocally enhanced our understanding of the extent and complexity of both prokaryotic and eukaryotic transcriptomes, we focused our investigation of rickettsial transcriptome on all transcript species, including mRNAs and non-coding RNAs, with the ultimate goal of determining the characteristics of genes in terms of their usage of transcriptional start sites and quantifying the changes in expression levels of each transcript during pathogen interplay with human versus tick host cells (Sharma and Vogel, 2014). Based on the genomic location and transcript orientation in relation to that of adjoining upstream and downstream genes and the number of mapped reads, we report on the presence of 93 sRNA candidates abundantly expressed in AAE2 cells in addition to 70 small RNAs (Rp_sR’s) previously identified during the infection of HMECs. Subsequent analysis of a few selected candidates, known to be selectively and differentially expressed in AAE2 cells and potentially regulating target genes involved in signaling, transport, cold shock and DNA repair (Supplementary table 5), by quantitative PCR further ascertains that Rp_sR76, Rp_sR83, Rp_sR86, and Rp_sR159 are expressed at significantly higher levels in arthropod cells, suggesting the possibility of an important role for these newly-identified candidate RNAs in pathogen interactions with and survival in arthropod cells. Because temperature shifts are known to alter the rickettsial transcriptome (Dreher-Lesnick et al., 2008; Ellison et al., 2009; Galletti et al., 2013), we assessed the expression of Rp_sRs in HMECs grown at either 37°C or 34°C and AAE2 cells grown at 34°C to delineate the impact of temperature versus host niche on sRNA expression. Interestingly, temperature variation had little effect on the transcript abundance of three Rp_sR’s (Rp_sR76, 83, and 86), while only Rp_sR159 showed significant differences in its expression dependent on the temperature and host niche (Figure 4). These results are in congruence to recently emerging evidence implicating differential regulation of sRNAs in different strains of a vertically transmitted, endosymbiotic α-proteobacterium Wolbachaia pipientis and under specific environmental conditions such as the infection of different host tissues and sexes. Specifically, a conserved intergenic sRNA ncrwmel02 is reportedly expressed at two and seven times higher levels, respectively, in strains wMel and wAu of W. pipientis, when compared to strains wMelCs and wMelPop. Furthermore, ncrwmel02 is present at significantly higher levels in the abdomens of male Drosophila melanogaster as their natural hosts than in the abdomens of female flies and there is more than 10-fold higher expression in testes compared to ovaries, suggesting both tissue-specific and host sex-specific regulation (Woolfit et al., 2015). Similarly, a broad analysis of Burkholderia thailandensis sRNA expression profiles via microarrays covering intergenic regions of more than 90 bases suggests differential expression of 38 novel and 2 conserved small RNAs in response to varying stressors (Stubben et al., 2014) and a comprehensive transcriptomic analysis of Burkholderia pseudomallei exposed to diverse physical, chemical, and biological conditions also reveals context-dependent expression of non-coding sRNAs, including a number of cis-regulatory motifs (Ooi et al., 2013).
Although a number of bacterial regulatory RNAs have been classified as trans-encoded sRNAs which require the RNA binding chaperone protein Hfq to facilitate base pairing with their target mRNAs (Khandige et al., 2015), about half of all Gram-positive and Gram-negative bacteria, including Rickettsia species, do not encode for Hfq (Dugar et al., 2013; Östberg et al., 2004). Recently, we have shown R. conorii trans-acting sRNA Rc_sR42 to directly bind to cydA mRNA in vitro, implicating the possibility of a chaperone-independent mechanism of sRNA-mRNA interaction in Rickettsia species (Narra et al., 2016). Using bioinformatics based approach, this study further identified several target genes involved in signaling, DNA repair, and cold shock response to be regulated by Rp_sR’s selectively and differentially expressed during tick cell infection. Further experimental investigations to validate the computational target gene predictions of rickettsial sRNAs are necessary and currently in progress.
It is believed that Gram-positive bacteria, in general, rely more on cis-acting regulatory mechanisms such as riboswitches than Gram-negative organisms known to utilize trans-acting sRNAs much more extensively (Lasa et al., 2012; Waters and Storz, 2009). Recent evidence suggests that approximately 10% of the genes in the environmental bacterium B. pseudomallei are subject to regulatory control by antisense transcription (Ooi et al., 2013). Similarly, a global transcriptomic analysis of Helicobacter pylori, a Gram-negative, ε-proteobacterium, has revealed at least one antisense transcription start site for nearly 46% of all ORFs and 28% of tRNAs (Sharma et al., 2010), whereas antisense transcription for nearly 50% of the coding ORFs has also been reported for Staphylococcus aureus as a Gram-positive bacterial pathogen (Lasa et al., 2011). In this regard, an intriguing aspect of our findings is that about 25% of R. prowazekii sRNAs expressed in AAE2 cells are categorized as trans-acting. Thus, combining the repertoire of novel sRNA candidates in AAE2 cells with those previously identified during the infection of HMECs allows for the classification of approximately 44% as trans-acting and 55% as cis-acting, representing a ratio closer to other reported organisms (Supplementary table 4).
Cis-encoded sRNAs overlapping functionally defined genes are pervasive through the genomes of prokaryotes and such antisense RNAs have been proposed to play a role analogous to that of transcription factors in transcription regulation in adaptive transition between distinct states. In the present study, we have identified 67 cis-acting sRNAs antisense to key ORFs encoding for structural proteins, transporters, membrane lipoproteins, and pathways of metabolism. For example, Rp_sR101 and Rp_sR102 are antisense to a VirB6 paralog (H375_5210) and the ATPase VirB4 (H375_5270) of the vir-induced type IV secretion system (T4SS), respectively. Spanning multiple membranes, T4SS is a complex multi-protein transporter encoded in many Gram-negative bacteria, which forms a syringe-like apparatus that functions to deliver a variety of virulence factors into host cells (Gillespie et al., 2009; Gillespie et al., 2015). The T4SS is composed of at least 12 Vir proteins with multiple paralogs performing unique functions as part of the overall complex (Gillespie et al., 2016). The VirB6 component, which composes the inner channel of T4SS essential for substrate transfer, is among the most divergent of the VirB proteins with at least five paralogs (Gillespie et al., 2009). Likewise, the VirB4 protein is an integral part of the T4SS due to its ATPase activity, which provides the required energy for operation. Similarly, cis-acting Rp_sR148 is present antisense to the gene coding for Outer membrane protein B (H375_8270) and an ATP-dependent helicase encoding UvrD (H375_1620) may hypothetically be regulated by Rp_sR77 as an antisense sRNA. With regards to their function(s), as an abundantly expressed protein expressed on the surface of all Rickettsia species, Outer membrane protein B (also known as Sca5) is involved in rickettsial binding and invasion of eukaryotic host cell and antibodies directed against OmpB have been reported to protect mice from lethal doses of rickettsial infection (Chan et al., 2009). As a component of the nucleotide excision repair and the transcription coupled repair machinery, UvrD acts in concert with UvrC to excise dimerized nucleotides for final repair by DNA polymerase I (Van Houten and Kad, 2014).
Bacteria are known to respond to environmental cues through a network of regulatory RNAs and proteins as the determinants of genome-wide transcription patterns. Many of such regulatory mechanisms depend on the initiation of messenger RNA synthesis by RNA polymerase at the transcription start sites. Accordingly, location of TSS and quantitative determination of changes in TSS usage is an important step to understand bacterial gene regulation. Although our fundamental understanding of basic mechanisms of transcription activation has arisen from the investigations of simple promoters such as lac and gal in E. coli, a majority of activator-dependent promoters are much more complex due to co-regulation either by another activator or repressor or possibly by both (Barnard et al., 2004). Such naturally occurring promoters allow bacteria to respond rapidly to specific environmental conditions. As an example, Salmonella regulates flagellar transcription through multiple promoters based on specific environmental conditions (Wozniak et al., 2010) and global examination of transcription start sites in Caulobacter crescentus, an α-proteobacterium closely related to Rickettsia, reveals the origin of transcription of 53 of its 769 genes from multiple start sites (McGrath et al., 2007). In Orientia (formerly Rickettsia) tsutsugamushi, there is evidence for the use of tandem promoters for the production of 56-kDa type-specific antigen as an abundant surface protein and two independent promoter-like sequences upstream from transcription start points for citrate synthase (gltA) transcripts are encoded in R. prowazekii (Cai et al., 1995; Ohashi et al., 1992). Identified in this study, the genes utilizing multiple TSSs encode for proteins essential to rickettsial metabolism, secretion, and other housekeeping functions. Upon examination, four genes with a >3 TPM difference between AAE2 or HMECs exhibit multiple TSSs, of which H375_2140, H375_3390, and H375_3940 show higher expression in AAE2 cells and H375_4210 displays higher expression in HMECs. Interestingly, two genes namely H375_2140 and H375_3390 are annotated as a conserved rickettsial protein and hypothetical protein, respectively. Nearly 14% of Methanolobus psychrophilus R15 genes are shown to have an alternate TSS, resulting in the generation of unique transcript isoforms under cold responsive conditions (Li et al., 2015). We propose that alternate TSSs identified in this study may have an impact on the translational efficiency and mRNA stability of the coding gene. Further investigations into these genes should yield new clues pertaining to transcriptional regulation during pathogen interactions with the human and vector host cells as the supportive intracellular milieu for rickettsiae.
Microarray-based screens have identified several rickettsial genes to be differentially expressed upon a shift in temperature and during natural blood feeding (Dreher-Lesnick et al., 2008; Ellison et al., 2009; Galletti et al., 2013). In this study, we decipher the global transcriptional landscape of R. prowazekii during host-pathogen and vector-pathogen interactions in vitro. Follow up quantitative analysis of the expression of four rickettsial genes encoding for heat shock protein 60 family co-chaperone GroES (H375_9040), Tol-Pal system peptidoglycan-associated lipoprotein PAL (H375_7520), thymidylate kinase (H375_8480), and lipopolysaccharide ABC transporter (H375_1050) reveal identical trends of changes in their expression as observed in our RNA-Seq (Figure 1). Importantly, although expression profiles of all four genes varied depending on the growth temperature of HMECs (37°C versus 34°C), host niche (HMECs versus AAE2) also had a profound impact on the abundance of these transcripts.
The field of bacterial small RNAs has been evolving quite rapidly over the past few years, yet the potential contributions of rickettsial sRNAs during pathogen-host and pathogen-vector interactions remain poorly understood. To the best of our knowledge, this is the first differential transcriptomics study of R. prowazekii in human and vector cells as the host. Precise identification and selective use of transcriptional start sites for rickettsial genes in a particular host furthers our understanding of genome organization and plasticity and discovery of hitherto unknown highly abundant sRNAs unique to arthropod host cells poses new queries related to their functions in R. prowazekii. Transcriptome analyses of other pathogenic rickettsiae, which exploit ticks as their predominant natural vectors, are currently ongoing and should reveal further insights into the roles of ribo-regulatory mechanisms among members of Rickettsiales.
Supplementary Material
Figure 3. Expression profile of novel Cis-Acting AAE2 Specific Rp_sRs.
Shown are the coverage plots for selected cis-acting Rp_sRs observed during the infection of AAE2 cells. Nucleotide positions within the genome are indicated on X-axis and the Y-axis displays the number of reads for that particular nucleotide position. The light grey arrow represents the small RNA. The dark grey arrows represent the orientation of the respective ORF.
Acknowledgments
Funding
This work was supported by National Institute of Allergy and Infectious Diseases at the National Institutes of Health [grant 5R21 AI115231-02], and in part by a pilot project grant from the Institute for Human Infections and Immunity, the James McLaughlin Fellowship Program, and institutional support funds from the University of Texas Medical Branch.
We thank Drs. Thomas Wood and Steve Widen for their help with library preparation and RNA sequencing.
Abbreviations
- AAE2
Amblyomma americanum cells
- CDS
coding sequence
- gltA
citrate synthase gene
- HMECs
human microvascular endothelial cells
- MEV
mean expression value
- Rp_sR
Rickettsia prowazekii small ribonucleic acid
- RPKM
reads per kilobase per million
- TEX
terminator 5′-phosphate-dependent exonuclease
- TPM
transcripts per kilobase million
- TSS
transcription start site
Footnotes
Conflict of interests
The authors declare no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Barnard A, Wolfe A, Busby S. Regulation at complex bacterial promoters: how bacteria use different promoter organizations to produce different regulatory outcomes. Curr Opin Microbiol. 2004;7(2):102–108. doi: 10.1016/j.mib.2004.02.011. [DOI] [PubMed] [Google Scholar]
- Bechah Y, Capo C, Mege JL, Raoult D. Epidemic typhus. Lancet Infect Dis. 2008a;8(7):417–426. doi: 10.1016/S1473-3099(08)70150-6. [DOI] [PubMed] [Google Scholar]
- Bechah Y, Capo C, Mege JL, Raoult D. Rickettsial diseases: from Rickettsia-arthropod relationships to pathophysiology and animal models. Future Microbiol. 2008b;3(2):223–236. doi: 10.2217/17460913.3.2.223. [DOI] [PubMed] [Google Scholar]
- Bozeman FM, Masiello SA, Williams MS, Elisberg BL. Epidemic typhus rickettsiae isolated from flying squirrels. Nature. 1975;255(5509):545–547. doi: 10.1038/255545a0. [DOI] [PubMed] [Google Scholar]
- Busch A, Richter AS, Backofen R. IntaRNA: efficient prediction of bacterial sRNA targets incorporating target site accessibility and seed regions. Bioinformatics. 2008;24:2849–2856. doi: 10.1093/bioinformatics/btn544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai J, Pang H, Wood DO, Winkler HH. The citrate synthase-encoding gene of Rickettsia prowazekii is controlled by two promoters. Gene. 1995;163(1):115–119. doi: 10.1016/0378-1119(95)00365-d. [DOI] [PubMed] [Google Scholar]
- Chan YG, Cardwell MM, Hermanas TM, Uchiyama T, Martinez JJ. Rickettsial outer-membrane protein B (rOmpB) mediates bacterial invasion through Ku70 in an actin, c-Cbl, clathrin and caveolin 2-dependent manner. Cell Microbiol. 2009;11(4):629–644. doi: 10.1111/j.1462-5822.2008.01279.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreher-Lesnick SM, Ceraul SM, Rahman MS, Azad AF. Genome-wide screen for temperature-regulated genes of the obligate intracellular bacterium, Rickettsia typhi. BMC Microbiol. 2008;8:61. doi: 10.1186/1471-2180-8-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dugar G, Herbig A, Förstner KU, Heidrich N, Reinhardt R, Nieselt K, Sharma CM. High-resolution transcriptome maps reveal strain-specific regulatory features of multiple Campylobacter jejuni isolates. PLoS Genet. 2013;9(5):e1003495. doi: 10.1371/journal.pgen.1003495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellison DW, Clark TR, Sturdevant DE, Virtaneva K, Hackstadt T. Limited transcriptional responses of Rickettsia rickettsii exposed to environmental stimuli. PLoS One. 2009;4(5):e5612. doi: 10.1371/journal.pone.0005612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galletti MFBM, Fujita A, Nishiyama MY, Jr, Malossi CD, Pinter A, Soares JF, Daffre S, Labruna MB, Fogaca AC. Natural blood feeding and temperature shift modulate the global transcriptional profile of Rickettsia rickettsii infecting its tick vector. PLoS One. 2013;8(10):e77388. doi: 10.1371/journal.pone.0077388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie JJ, Ammerman NC, Dreher-Lesnick SM, Rahman MS, Worley MJ, Setubal JC, Sobral BS, Azad AF. An anomalous type IV secretion system in Rickettsia is evolutionarily conserved. PloS one. 2009;4(3):e4833. doi: 10.1371/journal.pone.0004833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie JJ, Kaur SJ, Rahman MS, Rennoll-Bankert K, Sears KT, Beier-Sexton M, Azad AF. Secretome of obligate intracellular Rickettsia. FEMS Microbiol Rev. 2015;39(1):47–80. doi: 10.1111/1574-6976.12084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie JJ, Phan IQ, Driscoll TP, Guillotte ML, Lehman SS, Rennoll-Bankert KE, Subramanian S, Beier-Sexton M, Myler PJ, Rahman MS, Azad AF. The Rickettsia type IV secretion system: unrealized complexity mired by gene family expansion. Pathogens and disease. 2016;74(6) doi: 10.1093/femspd/ftw058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottesman S, Storz G. Bacterial small RNA regulators: versatile roles and rapidly evolving variations. Cold Spring Harb Perspect Biol. 2011;3(12):a003798–a003798. doi: 10.1101/cshperspect.a003798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khandige S, Kronborg T, Uhlin BE, Møller-Jensen J. sRNA-mediated regulation of P-fimbriae phase variation in uropathogenic Escherichia coli. PLoS Path. 2015;11(8):e1005109. doi: 10.1371/journal.ppat.1005109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labruna MB, Whitworth T, Horta MC, Bouyer DH, McBride JW, Pinter A, Popov V, Gennari SM, Walker DH. Rickettsia species infecting Amblyomma cooperi ticks from an area in the state of Sao Paulo, Brazil, where Brazilian spotted fever is endemic. J Clin Microbiol. 2004;42(1):90–98. doi: 10.1128/JCM.42.1.90-98.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langmead B, Salzberg SL. Fast gapped-read alignment with Bowtie 2. Nat Methods. 2012;9(4):357–359. doi: 10.1038/nmeth.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasa I, Toledo-Arana A, Dobin A, Villanueva M, de los Mozos IR, Vergara-Irigaray M, Segura V, Fagegaltier D, Penadés JR, Valle J, Solano C, Gingeras TR. Genome-wide antisense transcription drives mRNA processing in bacteria. Proc Natl Acad Sci U S A. 2011;108(50):20172–20177. doi: 10.1073/pnas.1113521108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasa I, Toledo-Arana A, Gingeras TR. An effort to make sense of antisense transcription in bacteria. RNA Biol. 2012;9(8):1039–1044. doi: 10.4161/rna.21167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B, Ruotti V, Stewart RM, Thomson JA, Dewey CN. RNA-Seq gene expression estimation with read mapping uncertainty. Bioinformatics. 2010;26(4):493–500. doi: 10.1093/bioinformatics/btp692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Qi L, Guo Y, Yue L, Li Y, Ge W, Wu J, Shi W, Dong X. Global mapping transcriptional start sites revealed both transcriptional and post-transcriptional regulation of cold adaptation in the methanogenic archaeon Methanolobus psychrophilus. Scientific reports. 2015;5:9209. doi: 10.1038/srep09209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu JM, Camilli A. A broadening world of bacterial small RNAs. Curr Opin Microbiol. 2010;13(1):18–23. doi: 10.1016/j.mib.2009.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrath PT, Lee H, Zhang L, Iniesta AA, Hottes AK, Tan MH, Hillson NJ, Hu P, Shapiro L, McAdams HH. High-throughput identification of transcription start sites, conserved promoter motifs and predicted regulons. Nat Biotechnol. 2007;25(5):584–592. doi: 10.1038/nbt1294. [DOI] [PubMed] [Google Scholar]
- Medina-Sanchez A, Bouyer DH, Alcantara-Rodriguez V, Mafra C, Zavala-Castro J, Whitworth T, Popov VL, Fernandez-Salas I, Walker DH. Detection of a typhus group Rickettsia in Amblyomma ticks in the state of Nuevo Leon, Mexico. Ann N Y Acad Sci. 2005;1063:327–332. doi: 10.1196/annals.1355.052. [DOI] [PubMed] [Google Scholar]
- Mortazavi A, Williams BA, McCue K, Schaeffer L, Wold B. Mapping and quantifying mammalian transcriptomes by RNA-Seq. Nature methods. 2008;5(7):621–628. doi: 10.1038/nmeth.1226. [DOI] [PubMed] [Google Scholar]
- Munderloh UG, Kurtti TJ. Formulation of medium for tick cell culture. Exp Appl Acarol. 1989;7(3):219–229. doi: 10.1007/BF01194061. [DOI] [PubMed] [Google Scholar]
- Narra HP, Schroeder CL, Sahni A, Rojas M, Khanipov K, Fofanov Y, Sahni SK. Small Regulatory RNAs of Rickettsia conorii. Scientific reports. 2016;6:36728. doi: 10.1038/srep36728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohashi N, Nashimoto H, Ikeda H, Tamura A. Diversity of immunodominant 56-kDa type-specific antigen (TSA) of Rickettsia tsutsugamushi. Sequence and comparative analyses of the genes encoding TSA homologues from four antigenic variants. J Biol Chem. 1992;267(18):12728–12735. [PubMed] [Google Scholar]
- Ooi WF, Ong C, Nandi T, Kreisberg JF, Chua HH, Sun G, Chen Y, Mueller C, Conejero L, Eshaghi M, Ang RML, Liu J, Sobral BW, Korbsrisate S, Gan YH, Titball RW, Bancroft GJ, Valade E, Tan P. The condition-dependent transcriptional landscape of Burkholderia pseudomallei. PLoS Genet. 2013;9(9):e1003795. doi: 10.1371/journal.pgen.1003795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Östberg Y, Bunikis I, Bergström S, Johansson J. The etiological agent of Lyme Disease, Borrelia burgdorferi, appears to contain only a few small RNA molecules. J Bacteriol. 2004;186(24):8472–8477. doi: 10.1128/JB.186.24.8472-8477.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philip CB, Hoogstraal H, Reiss-Gutfreund R, Clifford CM. Evidence of rickettsial disease agents in ticks from Ethiopian cattle. Bull WHO. 1966;35(2):127–131. [PMC free article] [PubMed] [Google Scholar]
- Raoult D, Woodward T, Dumler JS. The history of epidemic typhus. Infect Dis Clin North Am. 2004;18(1):127–140. doi: 10.1016/S0891-5520(03)00093-X. [DOI] [PubMed] [Google Scholar]
- Rydkina E, Silverman DJ, Sahni SK. Activation of p38 stress-activated protein kinase during Rickettsia rickettsii infection of human endothelial cells: role in the induction of chemokine response. Cell Microbiol. 2005;7:1519–1530. doi: 10.1111/j.1462-5822.2005.00574.x. [DOI] [PubMed] [Google Scholar]
- Schroeder CL, Narra HP, Rojas M, Sahni A, Patel J, Khanipov K, Wood TG, Fofanov Y, Sahni SK. Bacterial small RNAs in the Genus Rickettsia. BMC genomics. 2015;16(1):1075. doi: 10.1186/s12864-015-2293-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder CLC, Narra HP, Sahni A, Rojas M, Khanipov K, Patel J, Shah R, Fofanov Y, Sahni SK. Identification and characterization of novel small RNAs in Rickettsia prowazekii. Front Microbiol. 2016;7(859) doi: 10.3389/fmicb.2016.00859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma CM, Hoffmann S, Darfeuille F, Reignier J, Findeiss S, Sittka A, Chabas S, Reiche K, Hackermuller J, Reinhardt R, Stadler PF, Vogel J. The primary transcriptome of the major human pathogen Helicobacter pylori. Nature. 2010;464(7286):250–255. doi: 10.1038/nature08756. [DOI] [PubMed] [Google Scholar]
- Sharma CM, Vogel J. Differential RNA-seq: the approach behind and the biological insight gained. Curr Opin Microbiol. 2014;19:97–105. doi: 10.1016/j.mib.2014.06.010. [DOI] [PubMed] [Google Scholar]
- Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative CT method. Nature Protocols. 2008;3:1101–1108. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- Stubben CJ, Micheva-Viteva SN, Shou Y, Buddenborg SK, Dunbar JM, Hong-Geller E. Differential expression of small RNAs from Burkholderia thailandensis in response to varying environmental and stress conditions. BMC Genomics. 2014;15(1):385. doi: 10.1186/1471-2164-15-385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchiyama T. Tropism and pathogenicity of rickettsiae. Front Microbiol. 2012;3:230. doi: 10.3389/fmicb.2012.00230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Houten B, Kad N. Investigation of bacterial nucleotide excision repair using single-molecule techniques. DNA Repair. 2014;20:41–48. doi: 10.1016/j.dnarep.2013.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wade JT. Where to begin? Mapping transcription start sites genome-wide in Escherichia coli. J Bacteriol. 2015;197(1):4–6. doi: 10.1128/JB.02410-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner GP, Kin K, Lynch VJ. Measurement of mRNA abundance using RNA-seq data: RPKM measure is inconsistent among samples. Theory in Biosciences. 2012;131(4):281–285. doi: 10.1007/s12064-012-0162-3. [DOI] [PubMed] [Google Scholar]
- Walker DH, Ismail N. Emerging and re-emerging rickettsioses: endothelial cell infection and early disease events. Nat Rev Microbiol. 2008;6(5):375–386. doi: 10.1038/nrmicro1866. [DOI] [PubMed] [Google Scholar]
- Waters LS, Storz G. Regulatory RNAs in bacteria. Cell. 2009;136(4):615–628. doi: 10.1016/j.cell.2009.01.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westermann AJ, Gorski SA, Vogel J. Dual RNA-seq of pathogen and host. Nat Rev Microbiol. 2012;10(9):618–630. doi: 10.1038/nrmicro2852. [DOI] [PubMed] [Google Scholar]
- Woolfit M, Algama M, Keith JM, McGraw EA, Popovici J. Discovery of putative small non-coding RNAs from the obligate intracellular bacterium Wolbachia pipientis. PLoS One. 2015;10(3):e0118595. doi: 10.1371/journal.pone.0118595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wozniak CE, Chevance FFV, Hughes KT. Multiple promoters contribute to swarming and the coordination of transcription with flagellar assembly in Salmonella. J Bacteriol. 2010;192(18):4752–4762. doi: 10.1128/JB.00093-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.