Abstract
Background
While outcomes for acute myeloid leukemia have improved over time, they remain poor overall, and toxicity from both disease and treatment can impact quality of life. One barrier to including quality of life endpoints into clinical trials is the lack of a disease-specific quality of life instrument that can efficiently capture the major quality of life deficits in this population.
Methods
We conducted a cross-sectional study to elicit concepts for inclusion in a new acute myeloid leukemia-specific quality of life instrument, the AML-QOL. Eighty-two patients at various stages of disease were interviewed about sources of support (positive concepts) and problems and symptoms (negative concepts) experienced over the past week and were asked to grade how much each impacted their quality of life. In addition, patients were asked to complete two validated instruments, the Functional Assessment of Cancer Therapy with leukemia and transplant modules and the Patient Reported Outcome Measurement Information System 29-item questionnaire.
Results
Using data from the open-ended and questionnaire-based portions of the interview, we elicited 7 positive and 64 negative concepts. Among these, we selected 5 positive and 21 negative concepts for inclusion in the preliminary AML-QOL on the basis of concept prevalence and impact on quality of life.
Conclusions
These concepts will form the basis of a new quality of life instrument specific to acute myeloid leukemia.
Keywords: Acute Myeloid Leukemia, Quality of life, Myelodysplastic syndrome, Hematopoietic cell transplantation, Qualitative methods
INTRODUCTION
Despite advances in supportive care, survival with acute myeloid leukemia (AML) and other high-risk myeloid neoplasms is often measured in months rather than years,1, 2 and long-term survival typically requires multi-agent chemotherapy with or without allogeneic hematopoietic cell transplantation (HCT).3 As these therapies carry a risk for substantial, potentially life-threatening toxicities, and survival for many patients is limited, there is an increasing interest in measuring quality of life (QOL) as well as quantity of life and in incorporating QOL metrics in clinical trials.4 In the United States, this interest has been encouraged, in part, by the fact that federal regulations support drug approval based on demonstrated clinical benefit, including improvements in QOL.4, 5
To date, however, relatively few AML studies have incorporated QOL measurement.6 Several perceived barriers exist, including the lack of an AML-specific instrument and low survey completion rates in this population, possibly due in part to patient fatigue and questionnaire length.7 Among the few AML studies to incorporate QOL endpoints, there is heterogeneity in which instrument is employed.6 Some use the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire (EORTC QLQ-C30)8, which was developed in lung cancer patients and has not been validated in patients with AML. More recently, studies have incorporated the Functional Assessment of Cancer Therapy (FACT), which can be supplemented with leukemia and transplant modules (FACT-LEU and FACT-BMT).9 The leukemia module, however, contains some questions more pertinent to chronic leukemias, and the transplant module contains items that would not be relevant in a non-transplant setting. There is an AML/MDS-specific symptom checklist developed by MD Anderson (the MDASI-AML/MDS), which has been published in abstract form, though this measures symptom burden rather than QOL.10 Non-cancer specific instruments may also be used, including the recently developed Patient Reported Outcome Measurement Information System (PROMIS) questionnaires, which include several item banks that can encompass multiple health dimensions.11 With all of these options, however, there is no consensus on which instrument to use in AML trials. Development of a disease-specific QOL instrument could help standardize QOL measurement in AML studies, and the resulting instrument may be more responsive and clinically useful than more generic measures.12 To address these concerns, we conducted a study that elicited concepts for inclusion in a new AML-specific QOL instrument, the AML-QOL, through interviews with patients before, during, and after therapy.
MATERIALS AND METHODS
Patients
English-speaking patients with AML or high-risk myelodysplastic syndrome (MDS) undergoing treatment at the University of Washington/Seattle Cancer Care Alliance (UW/SCCA) were identified through review of the inpatient census and outpatient clinics. The Fred Hutchinson Cancer Research Center Institutional Review Board (IRB) approved the study protocol, and all patients gave written informed consent in accordance with the Declaration of Helsinki.
Data Collection
Sociodemographic information was collected at baseline. Patients participated in semi-structured interviews to elicit symptoms or situations (“concepts”) that impacted their QOL either negatively or positively, with a focus on experiences relevant over the past 7 days: “Tell me about the symptoms that you’ve experienced over the past week,” and, “In addition to physical symptoms, people can have emotional symptoms or other sources of stress. Have you had anything like that over the past week?” Interviews were recorded with the participant’s permission. For each negative concept, patients were asked to quantify the degree to which each experience impacted their QOL on a 3-point scale (little impact, some impact, high impact).13 These impact scores were not elicited for positive concepts. Next, patients were asked to report their current Karnofsky performance status (KPS).14 They then completed English language FACT-G with the –LEU and –BMT subscales (67 total items) as well as the 29-item PROMIS questionnaire. They were asked to “think out loud” and to voice any areas of confusion or perceived ambiguity. Finally, questionnaire responses were reviewed, and responses indicating the presence of any other negative concepts were discussed with patients. Enrollment continued until no new concepts were elicited (“content saturation”). In order to ensure broad applicability of the AML-QOL, efforts were made to enroll in roughly equal distributions from the following groups: newly diagnosed, initial chemotherapy, relapsed/refractory, post-chemotherapy, within 1 year of HCT, and more than 1 year after HCT. Furthermore, efforts were made to enroll a roughly equal number of inpatients and outpatients.
All interviews were conducted by one physician (S.A.B.) and were transcribed verbatim. A codebook was developed based on initial review of the interviews, and two researchers independently coded all interviews (S.A.B. and D.J-S.). Disputed codes were resolved by a third party (S.J.L.). The percentage concordance was determined as the number of agreed upon codes divided by the number of agreed upon codes plus the number of codes coded by one researcher but not the other. The concordance in impact of each concept was calculated similarly among agreed-upon concepts.
Biostatistical Methods
Patient characteristics are reported descriptively. Positive concepts (e.g., sources of support) are reported based on prevalence, and their inclusion in the AML-QOL was determined on this basis. Negative concepts (e.g., problems, symptoms) were described both by overall prevalence and by percentage of cases leading to high impact on QOL. In order to select concepts for inclusion based on both prevalence and impact13, a prevalence-impact score was calculated by multiplying prevalence by impact, and concepts were included in the AML-QOL on the basis of this score with a goal of including ~20–25 concepts. Logistic regression was used to assess associations between concepts and patient characteristics. Interviews were coded using QDA Miner Lite (Provalis Research, Montreal, Canada), and analysis used STATA version 14 (Stata Corp, College Station, TX).
RESULTS
Patient Characteristics
A total of 100 eligible patients were approached between March and September 2016, and 82 consented to participate. Two patients were eligible but not approached because their providers felt that they were too ill for an interview. Among the 18 that declined enrollment, 4 reported feeling too ill to participate, while 12 declined for other reasons (including not wanting to fill out questionnaires, not enough time, not wanting further interaction with medical staff). Patient demographics are described in Table 1. Most participants (61%) were inpatient at the time of the interview, and most interviews (78%) were conducted one-on-one without family members present. All interviewees agreed to audio recording. The majority of patients were married or living with a partner. About half had a college or post-graduate degree. Only 10 (12%) were currently working at least part time, including 7 of the 22 patients (32%) who had completed all planned therapy. All patients had AML except for 4 (5%) with high-risk MDS; these patients were receiving intensive AML-like chemotherapy or were undergoing stem cell transplantation.
Table 1.
Patient characteristics
| Variable | N=82 |
|---|---|
|
| |
| Age, median (range) | 58 (21–84) |
|
| |
| Male, n (%) | 40 (49%) |
|
| |
| Race, n (%) | |
| White | 69 (84%) |
| Non-white | 13 (16%) |
|
| |
| Married or living with partner, n (%) | 52 (63%) |
|
| |
| College or post-graduate degree, n (%) | 40 (49%) |
|
| |
| Disease stage, n (%) | |
| Newly diagnosed | 11 (13%) |
| Receiving chemotherapy, non-relapsed | 16 (20%) |
| Post-chemotherapy, non-relapsed* | 13 (16%) |
| HCT year 0–1, non-relapsed | 11 (13%) |
| HCT year 1+, non-relapsed | 8 (10%) |
| Relapsed/refractory, no prior HCT | 15 (18%) |
| Relapsed/refractory after HCT | 8 (10%) |
Including pre-HCT
Abbreviations: HCT, hematopoietic cell transplantation
Interviews lasted a median of 35.6 (range: 11.3–67.7) minutes. All 82 patients completed the open-ended portion, while 72 completed the FACT instruments, and 68 completed PROMIS.
We coded 7 positive and 64 negative concepts with 75% concordance for concept coding and 84% concordance for impact coding. Content saturation was achieved after 67 patients, with no new concepts elicited thereafter. KPS was 80% to 100% in 67% of patients and 70% or less in 33%.
Positive Concepts
The most commonly described sources of support were family (n=70, 85%), having a positive attitude or hope for the future (n=52, 63%), friends or community (n=43, 52%), trust in the medical team (n=43, 52%), and participation in activities or exercise (n=29, 35%). Less commonly listed were religion or spirituality (n=14, 17%) and being informed about or actively seeking out information on AML (n=14, 17%). We hypothesized that older patients or those with relapsed/refractory disease might rely more heavily on religion or spirituality, that unmarried patients would rely more on friends/community, and that younger patients would rely more on being informed, but we did not find any of these associations (all odds ratios between 0.95–1, all p>0.05). Due to lower prevalence, we did not include concept questions about religion/spirituality or being informed about the disease in the AML-QOL.
Negative Concepts
As shown in Table 2, the most prevalent symptoms within a week prior to the interview were also often those deemed most impactful by patients, with several notable exceptions. For example, reduced sexual function and bruising were both reported relatively frequently, but few felt that these seriously impacted QOL. Conversely, fevers and taste changes were less often reported but were considered very impactful when they were present. Concepts with lower prevalence are described in Supplementary Table 1. Twenty-one negative concepts were selected for inclusion in the AML-QOL based on an apparent cut point on visual inspection (Figure 1). Different concepts took on greater importance depending on patient disease state. For example, while fear/anxiety was an important concept in all groups, it was most prevalent/impactful at initial diagnosis and in patients who had relapsed after HCT. Pain was most important among patients receiving chemotherapy, either for initial diagnosis or as salvage. Long-term survivors, particularly those having undergone HCT, were particularly concerned with the impact of their disease on their family (Table 3). Compared to outpatients, inpatients rated many concepts as having higher prevalence/impact, particularly concepts dealing with emotion, loss of control or function, and chemotherapy side effects (Supplementary Figure 1).
Table 2.
Negative concepts with prevalence ≥10%
| Concept | Prevalence | Very impactful |
|---|---|---|
| Fear/anxiety | 80% | 42% |
| Fatigue | 76% | 44% |
| Pain | 60% | 55% |
| Difficulty with day-to-day activity | 59% | 47% |
| Impact of disease on family members | 55% | 67% |
| Difficulty with hobbies or leisure activity | 52% | 56% |
| Bothered by time required for medical care | 46% | 48% |
| Nausea | 40% | 44% |
| Economic impact | 39% | 43% |
| Low moods, sadness, or depression | 39% | 52% |
| Loss of control or reliance on others | 37% | 55% |
| Impact on sexual function | 37% | 9% |
| Difficulty with sleep | 37% | 60% |
| Weakness or deconditioning | 37% | 67% |
| Bruising | 35% | 19% |
| Changes in appearance | 33% | 33% |
| Bowel trouble (constipation, diarrhea) | 33% | 38% |
| Confusion/memory impairment | 32% | 33% |
| Separation from family or friends | 29% | 21% |
| Weight loss | 29% | 20% |
| Difficulty with role functioning | 29% | 75% |
| Skin conditions or changes | 27% | 19% |
| Difficulty engaging in social activity | 27% | 20% |
| Bleeding | 24% | 41% |
| Inability to work | 24% | 22% |
| Decreased appetite | 23% | 29% |
| Difficulty with exercise | 23% | 29% |
| Changes in taste | 22% | 42% |
| Fevers | 21% | 67% |
| Dyspnea | 20% | 44% |
| Blurry vision | 16% | 22% |
| Chills | 15% | 17% |
| Hair loss | 15% | 9% |
| Malaise | 15% | 20% |
| Feeling emotionally isolated from others | 13% | 50% |
| Sweats | 13% | 8% |
| Lightheadedness | 12% | 38% |
| Feeling unsupported by friends or family | 12% | 29% |
| Urinary frequency | 11% | 60% |
Figure 1. Concepts with the highest prevalence-impact scores.
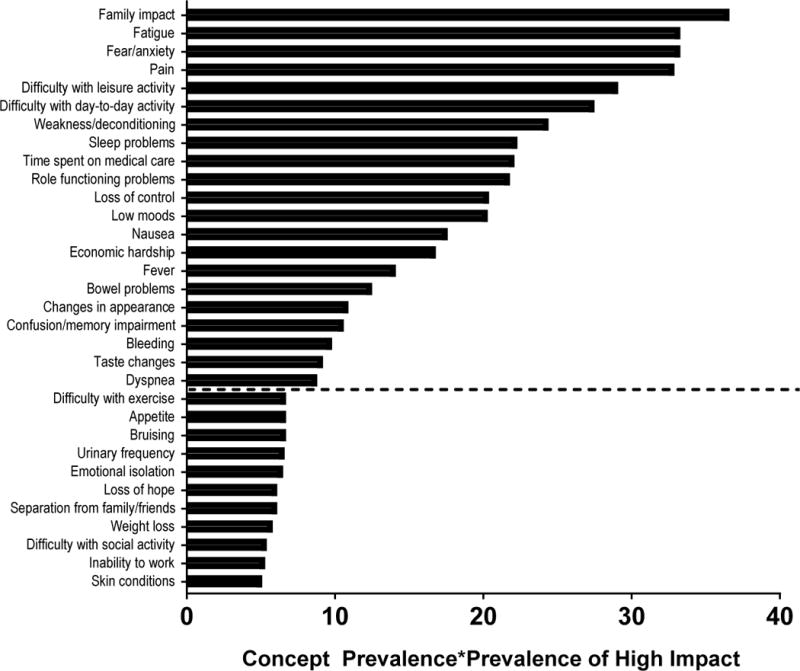
Dashed line indicates threshold for concept inclusion in the AML-QOL. Prevalence of concept and of high impact are treated as percentages, and their product is multiplied by 100 to arrive at the prevalence-impact score.
Table 3.
Negative concepts with highest prevalence-impact score for each treatment state
| Prevalence- Impact Score |
Newly diagnosed |
Getting chemo |
Post-chemo | Relapsed/ refractory |
HCT 0–1 year | HCT 1+ year | Relapsed after HCT |
|---|---|---|---|---|---|---|---|
| ≥ 60 |
|
|
|||||
| 50 to <60 |
|
|
|
|
|
|
|
| 40 to < 50 |
|
|
|
|
|
|
|
| 30 to <40 |
|
|
|
|
|
|
|
| 20 to < 30 |
|
|
|
|
|
|
|
Sources of Ambiguity and Confusion with Validated Instruments
Among the 72 patients who completed at least some of the FACT or PROMIS questionnaires, the most common source of confusion or ambiguity, as expressed by 16 patients, related to decrements in functional status as a result of temporary restrictions (e.g., inpatient stays) rather than true functional limitations. This issue arose both in the FACT and the PROMIS questionnaires, though they were more common in the latter, where patients were asked about ability to perform chores (“I’m not able to… because I can’t get out of the hospital.”), housework (“There’s nothing to do here.”), yard work (“I can’t do anything with dirt.”), and leisure/social activities (“They won’t let me through those [hospital] doors.”). Indeed, among patients who reported a KPS of 80% or above, indicating ability to carry on normal activity, 27% reported “much difficulty” or “inability” to do chores. Similarly, this level of difficulty was reported by 29% with leisure activity, 37% with work, and 43% with social activity with friends. In contrast, all reported that they could go for a walk of at least 15 minutes without significant difficulty. We hypothesized that circumstantial restrictions would have less impact on QOL than true functional limitations, and when possible during interview coding, we indicated whether functional limitations were due to physical inability (i.e., related to weakness, fatigue, pain, etc.) vs. temporary restrictions (i.e., staying in the hospital or clinic housing, environmental or social restrictions because of a weakened immune system). Indeed, patients were more likely to report significant QOL impact from inability to participate in day-to-day activities when the limitation was due to physical inability (10 of 15) vs. from temporary restrictions (1 of 8).
Another source of potential errors, described by 14 patients (19%), was directional changes in the Likert scale (e.g., from high numbers being “good” to high numbers being “bad”.) Most patients caught their own errors related to these changes, though in 4 cases errors were only noted after the survey was completed when patients were asked why they had scored a particular concept as problematic, and it turned out that they had not intended to do so.
Several patients expressed confusion about how to answer questions related to sexual function and satisfaction. While low libido was discussed in 30 interviews, only 5 patients brought this up unprompted during the initial open-ended concept elicitation portion of the interview. The majority of patients reported not being bothered at all by this change, and those who were generally reported that their biggest concern was the effect of this change on their partners rather than on themselves (and scored the impact of their disease on family as being very impactful accordingly). Along the same lines, some patients reported ambiguity over questions about concepts that predated and were unaffected by the leukemia diagnosis, including low libido, blurry vision, and satisfaction with physical appearance. As an example, 7 patients reported unchanged blurry vision due to longstanding issues, often corrected with glasses, and wondered how they should score a question on this concept. Three scored this low (0 or 1), one intermediate (2) and three high (3 or 4).
Finally, several words in the surveys were unclear to patients. Seven patients reported uncertainty over the concept of ‘acceptance’, indicating that neither they nor their families has “accepted” their illness, as this would indicate acquiescence, loss of hope, or stopping treatment: “to me, that almost means… just going along with it. It’s a negative connotation.” No patient reported confusion over the question about lymphadenopathy, phrased as “lumps or swelling”, although among the 11 who responded at least “Somewhat” to this question, only 2 were referring to lymphadenopathy; the others referred to soft tissue infections, edema, or bruises. Five patients reported that they were unsure what “tremors” were; when prompted, 2 guessed correctly.
Qualitative versus Quantitative Results
For some concepts, quantitative results from FACT and PROMIS seemed to under-estimate burden relative to the qualitative open-ended interview. For example, in the semi-structured interviews, 80% of patients expressed fear/anxiety, with 42% and 66% of those noting high or some/high impact respectively. In contrast, of participants responding to the 4 anxiety-related items on PROMIS-29, only 15–40% responded positively (score ≥3 on a 1–5 scale), and 4–12% responded strongly (score ≥4). Similarly, of those responding to the 3 related items on FACT-G, only 25–42% responded positively (score ≥2 on a 0–4 scale), and 15–24% responded strongly (score ≥3). Likewise for pain, PROMIS-29 has 4 questions on the impact that pain has on day-to-day activities, and only 32–34% responded positively with 18–22% responding strongly. In contrast, 60% of interviewees described having pain, among whom 55% and 85% reporting high or some/high impact on QOL. A similar trend exists for sadness/depression, particularly with the PROMIS items.
For other concepts, including fatigue (on FACT and PROMIS), family impact, economic impact, and nausea (on FACT), prevalence and impact based on interview statements correlated well with scores for corresponding survey items.
DISCUSSION
Our cross-sectional study elicited both positive and negative concepts for inclusion in the AML-QOL and provided guidance for avoiding confusion or ambiguity in drafting the instrument. Some of our results are similar to those reported by previous studies of newly diagnosed and recently relapsed patients with acute (mostly myeloid) leukemia using the Memorial Symptom Assessment Scale (MSAS) to describe symptom burden.15, 16 While those investigators noted more prevalent side effects of chemotherapy (as >90% of each cohort was receiving chemotherapy), their reported prevalence for emotional concepts, such as fear/anxiety, was much lower than ours, possibly reflecting differences in study technique with questionnaire- versus interview-elicited symptoms. Several chemotherapy-related side effects had low prevalence in our study, including decreased appetite (23%), taste changes (22%), and fevers (21%), likely because 29 of the 44 patients receiving chemotherapy (66%) were within 5 days of starting or had already recovered their counts and were less likely to be experiencing some of these toxicities.
Results from our study allowed us to draft a preliminary version of the AML-QOL, which includes 40 questions covering 5 positive and 21 negative concepts. Concepts with higher prevalence-impact scores were represented with more questions in order to include more wording choices for particularly important concepts. We did not incorporate items from PROMIS-29 that were identified as sources of ambiguity or confusion in the study population. Item wording is based on speech patterns and themes drawn from patient interviews.
We also took into account the sources of ambiguity and confusion expressed by patients about established instruments. In particular, we were attentive to the distinction between temporary functional restrictions and true functional limitations. For example, a question about leisure activity might read, “I’m not able to do things that I enjoy,” but we chose to include, “I don’t think I’ll be able to get back to doing all the things I enjoy.” The latter represents a fear or state of permanence, and patients who face temporary restrictions in their functional ability might answer differently than those with perhaps long-standing (and likely more troublesome) limitations.
While our study comprised a large number of interviews and extended well beyond content saturation, it is limited by having been conducted at a single academic institution. It is possible that patients in other geographic areas or in smaller community hospitals might experience their disease and their health-related QOL differently. Furthermore, of the 51 patients receiving AML-directed therapy at the time of their interview, 45 were receiving intensive cytotoxic chemotherapy, reflecting practice patterns at our center. While this distribution may be useful in construction of an instrument intended for use in clinical trials testing intensive regimens, it is a potential limitation for broader applications of the instrument.
Validation of the AML-QOL with patients, caregivers, and medical providers is ongoing to ensure that it is comprehensible, unambiguous, and contains appropriate content. The most updated version of the instrument is available upon request. We intend for the AML-QOL to undergo prospective validation in multiple centers and across a variety of treatment types in order to ensure that our instrument is not specific to patients undergoing intensive therapy at our institution. Our ultimate goal is to create a well-validated and concise instrument that can be used to document and assess the impact of AML and its treatment on patients’ QOL.
Supplementary Material
PRÉCIS.
Currently, there is no quality of life instrument that is specific to patients with acute myeloid leukemia, and available instruments may not be ideal for measuring quality of life in this population. Here, we describe initial development of the first such instrument, the AML-QOL.
Acknowledgments
Funding: This work was supported by a T32 training grant from the National Institutes of Health [HL007093 to S.A.B.]
Footnotes
Disclosure: The authors have declared no conflicts of interest.
Author contribution: S.A.B., R.B.W., and S.J.L. designed this study. Interviews were conducted by S.A.B. and transcribed by D.J-S. Data analysis was performed by S.A.B., D.J-S., and M.O. Manuscript was written by S.A.B. with edits from all co-authors.
References
- 1.Appelbaum FR, Gundacker H, Head DR, et al. Age and acute myeloid leukemia. Blood. 2006;107:3481–3485. doi: 10.1182/blood-2005-09-3724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alibhai SM, Leach M, Minden MD, Brandwein J. Outcomes and quality of care in acute myeloid leukemia over 40 years. Cancer. 2009;115:2903–2911. doi: 10.1002/cncr.24373. [DOI] [PubMed] [Google Scholar]
- 3.Juliusson G, Antunovic P, Derolf A, et al. Age and acute myeloid leukemia: real world data on decision to treat and outcomes from the Swedish Acute Leukemia Registry. Blood. 2009;113:4179–4187. doi: 10.1182/blood-2008-07-172007. [DOI] [PubMed] [Google Scholar]
- 4.Appelbaum FR, Rosenblum D, Arceci RJ, et al. End points to establish the efficacy of new agents in the treatment of acute leukemia. Blood. 2007;109:1810–1816. doi: 10.1182/blood-2006-08-041152. [DOI] [PubMed] [Google Scholar]
- 5.Acquadro C, Berzon R, Dubois D, et al. Incorporating the patient’s perspective into drug development and communication: an ad hoc task force report of the Patient-Reported Outcomes (PRO) Harmonization Group meeting at the Food and Drug Administration, February 16, 2001. Value Health. 2003;6:522–531. doi: 10.1046/j.1524-4733.2003.65309.x. [DOI] [PubMed] [Google Scholar]
- 6.Cannella L, Caocci G, Jacobs M, Vignetti M, Mandelli F, Efficace F. Health-related quality of life and symptom assessment in randomized controlled trials of patients with leukemia and myelodysplastic syndromes: What have we learned? Crit Rev Oncol Hematol. 2015;96:542–554. doi: 10.1016/j.critrevonc.2015.07.012. [DOI] [PubMed] [Google Scholar]
- 7.Buckley SA, Lee SJ, Walter RB. Measuring quality of life in acute myeloid leukemia: limitations and future directions. Expert Rev Hematol. 2016;9:821–823. doi: 10.1080/17474086.2016.1211006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aaronson NK, Ahmedzai S, Bergman B, et al. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993;85:365–376. doi: 10.1093/jnci/85.5.365. [DOI] [PubMed] [Google Scholar]
- 9.Cella D, Jensen SE, Webster K, et al. Measuring health-related quality of life in leukemia: the Functional Assessment of Cancer Therapy–Leukemia (FACT-Leu) questionnaire. Value Health. 2012;15:1051–1058. doi: 10.1016/j.jval.2012.08.2210. [DOI] [PubMed] [Google Scholar]
- 10.Williams LA, Garcia-Gonzalez A, Ahaneku HO, Cortes JE, Garcia-Manero G, Kantarjian HM, Mendoza TR, Shi Q, Borthakur G, Burger JA, Jabbour EJ, Takahashi K, Lin HK, Limaye A, Cleeland C. A Patient-Reported Outcome Measure for Symptoms and Symptom Burden of Acute Myeloid Leukemia (AML) and Myelodysplastic Syndrome (MDS); American Society of Hematology 57th Annual Meeting; 2015. [Google Scholar]
- 11.Hays RD, Bjorner JB, Revicki DA, Spritzer KL, Cella D. Development of physical and mental health summary scores from the patient-reported outcomes measurement information system (PROMIS) global items. Qual Life Res. 2009;18:873–880. doi: 10.1007/s11136-009-9496-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wiebe S, Guyatt G, Weaver B, Matijevic S, Sidwell C. Comparative responsiveness of generic and specific quality-of-life instruments. J Clin Epidemiol. 2003;56:52–60. doi: 10.1016/s0895-4356(02)00537-1. [DOI] [PubMed] [Google Scholar]
- 13.Johnson C, Aaronson NB, J M, Bottomley A, Fayers P, Koller M, Kulis D, Ramage J, Sprangers M, Velikova G, Young T, EORTC Quality of Life Group Guidelines for Developing Questionnaire Modules. 2011 [Google Scholar]
- 14.Schag CC, Heinrich RL, Ganz PA. Karnofsky performance status revisited: reliability, validity, and guidelines. J Clin Oncol. 1984;2:187–193. doi: 10.1200/JCO.1984.2.3.187. [DOI] [PubMed] [Google Scholar]
- 15.Zimmermann C, Yuen D, Mischitelle A, et al. Symptom burden and supportive care in patients with acute leukemia. Leuk Res. 2013;37:731–736. doi: 10.1016/j.leukres.2013.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rodin GYD, Mischitelle A, Minden MD, Brandwein J, Schimmer A, Marmar C, Gagliese L, Lo C, Rydall A, Zimmermann C. Tarumatic stress in acute leukemia. Psychooncology. 2013;22:299–307. doi: 10.1002/pon.2092. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


