Abstract
Background
While many groups use experimental autoimmune encephalomyelitis (EAE) as a model to uncover therapeutic targets and understand the pathology underlying multiple sclerosis (MS), EAE protocol variability introduces discrepancies in central nervous system (CNS) pathogenesis and clinical disease, limiting the comparability between studies and slowing much-needed translational research.
Optimized method
Here we describe a detailed, reliable protocol for chronic EAE induction in C57BL/6 mice utilizing two injections of myelin oligodendrocyte glycoprotein (35–55) peptide mixed with Complete Freund’s Adjuvant and paired with pertussis toxin.
Results
The active MOG35–55 EAE protocol presented here induces ascending paralysis in 80–100% of induced mice. We observe: (1) consistent T cell immune activation, (2) robust CNS infiltration by peripheral immune cells, and (3) perivascular demyelinating lesions concurrent with axon damage in the spinal cord and various brain regions, including the optic nerve, cortex, hippocampus, internal capsules, and the cerebellum.
Comparison with existing method(s)
Lack of detailed protocols, combined with variability between laboratories, make EAE results difficult to compare and hinder the use of this model for therapeutic development. We provide the most detailed active MOG35–55-EAE protocol to date. With this protocol, we observe high disease incidence and a consistent, reliable disease course. The resulting pathology is MS-like and includes optic neuritis, perivascular mononuclear infiltration, CNS axon demyelination, and axon damage in both infiltrating lesions and otherwise normal-appearing white matter.
Conclusions
By providing a detailed active MOG35–55-EAE protocol that yields consistent and robust pathology, we aim to foster comparability between pre-clinical studies and facilitate the discovery of MS therapeutics.
Introduction
Multiple sclerosis (MS) is an autoimmune, demyelinating, neurodegenerative disease of the central nervous system (CNS) that presents with varied clinical and pathological manifestations. The hallmark of MS is the demyelinated lesion, which is characterized by demyelination, axon damage, mononuclear cell infiltrates, and astrocytic scar formation (Mahad et al., 2015). While the etiology of MS is currently unknown, a prevailing hypothesis of MS pathogenesis involve immune cells, including macrophages, B cells, and T cells, gaining access to the CNS, where they release an array of pro-inflammatory mediators (Friese and Fugger, 2009; Hollenbach and Oksenberg, 2015). This results in regions of demyelination and axon degeneration, called “plaques.” As the disease progresses, debilitating motor symptoms develop, eventually leading to complete paralysis and death (Lassmann, 2007b; Lucchinetti and Bruck, 2004; Trapp et al., 1999). The devastating and complex nature of MS, combined with the lack of an effective cure and difficulties in recapitulating a human disease, has led to the employment of animal models to aid in elucidating the mechanisms of MS progression and the development of novel therapeutics.
EAE protocol development
Many experimental animal models have been developed to study MS pathology. Currently, these include: (1) demyelination models, such as cuprizone, ethidium bromide, or lysolecithin administration; (Fernandes et al., 1997; Woodruff and Franklin, 1999) (2) viral models, such as infection with Theiler murine encephalomyelitis virus or murine hepatitis viru ((Rodriguez, 1988; Sorensen et al., 1980); and (3) transgenic models, which can be used to knock-out or overexpress chemokines or and cytokines in a specific cell types (e.g. overexpression of IFNγ in astrocytes as well as conditional and targeted ablation of oligodendrocytes (OLs) (Kipp et al., 2012; Mecha et al., 2012). However, the oldest and most studied animal model of MS, experimental autoimmune encephalomyelitis (EAE), has been shown to most closely recapitulate MS pathogenesis (Baxter, 2007; Mangiardi et al., 2011; Sternberger et al., 1984; Wekerle et al., 1994).
The first reported cases of EAE-like symptoms were described in human patients following rabies inoculations by Louis Pasteur. Pasteur’s early rabies vaccine was administered by drying infected rabbit spinal cords for up to two weeks, homogenizing the cords in an emulsion, and administering the emulsion in a series of injections. Initially, the treatments were successful and had no detrimental side effects. However, the use of more virulent (i.e., Less dried) spinal cords resulted in cases of muscle weakness, paralysis, and, sometimes, death. Interestingly, these side effects were not directly associated with the rabies virus itself, as the pathology was histologically distinct (reviewed in (Baxter, 2007)).
Intrigued by Pasteur’s results, Thomas Rivers investigated the cause of these complications. This led to the first comprehensive description of EAE in 1933 (Baxter, 2007; Rivers et al., 1933). Rivers’ initial studies involved injecting Rhesus macaques with an emulsion of brain tissue from healthy rabbits. This induced inflammatory peripheral immune cell infiltration and demyelination, similar to Pasteur’s observations as well as observations in MS patients, and demonstrated that injection with foreign CNS tissue devoid of pathogens was sufficient to initiate acute CNS disease. Since these methods were extremely inefficient (Baxter, 2007; Rivers et al., 1933), extensive development of the method continued in the following decades. This has resulted in increasingly specific reagents for efficient and controlled induction in a variety of species, with mice being most widely employed (Denic et al., 2011).
Currently, EAE can be induced in mice by immunization with specific myelin peptides (i.e., Antigens), such as myelin basic protein (MBP), proteolipid protein (PLP), or myelin oligodendrocyte glycoprotein (MOG), emulsified with adjuvant (i.e., immunopotentiator), which initiates an autoimmune response T cell response against specific myelin proteins. When specific myelin peptides are paired with particular strains of mice, chronic non-relapsing, monophasic, or relapsing-remitting (RR) disease courses are observed, mimicking the clinical forms of MS (Robinson et al., 2014). For example, immunization of B10.PL mice with MBP84–104 peptide produces a monophasic disease course (McCarthy et al., 2012), immunization of SJL mice with PLP139–151 peptide produces a RR disease course (McRae et al., 1992), and immunization of C57BL/6 mice with MOG35–55 peptide produces a chronic, non-relapsing disease course (Mendel et al., 1995). Along with the similarities in disease pathology, this adaptability makes murine EAE the most germane model of MS.
MOG peptides have autoimmune reactivity in more than 50% of MS patients (Kerlero de Rosbo et al., 1997; Kerlero de Rosbo et al., 1995). With this is mind, the MOG-EAE model in mice was pioneered by Mendel and colleagues in the mid-1990s (Mendel et al., 1995) when they performed experiments focused on immunizing female C57BL/6 mice with multiple synthetic MOG peptides: 1–21, 35–55, and 104–117. All MOG peptide-immunized mice developed a T cell response; however, more severe neurological impairment was observed in mice immunized with MOG35–55. More specifically, MOG35–55-immunized mice showed persistent neuropathy paired with ascending paralysis, as well as CNS inflammation, demyelination, axonal loss, and gliosis (Mendel et al., 1995; Stromnes and Goverman, 2006a). These clinical and pathological features are similar to those observed in MS patients, supporting the use of MOG35–55-EAE as a model of MS (Crawford et al., 2010b; Mangiardi et al., 2011; Tompkins et al., 2002).
EAE Protocol application
The chronic MOG35–55-EAE model is capable of recapitulating aspects of all three subtypes of MS. RR-MS is the most common form of the disease, accounting for 85% of MS patients, and is marked by acute episodes of disability followed by recovery (Lassmann, 2007a). The onset stage of MOG35–55-EAE can serve as a model of these early relapses and offers the opportunity to monitor possible key effectors in MS progression and test interventions prior to permanent CNS damage. Typically, RR-MS patients progress to a chronic disease known as secondary progressive (SP) MS, during which they develop permanent motor and cognitive impairments (Lassmann, 2009). A third subtype, primary progressive (PP) MS, affects 15% of patients (Lassmann, 2009) and presents with a chronic disease course at onset, devoid of remittances. As such, the chronic nature of MOG35–55-EAE recapitulates the permanent damage observed in both SP-MS and PP-MS. Thus, the development of MOG35–55-EAE has provided an invaluable model for studying both the progression and treatment of multiple clinical forms of MS.
Examples of how EAE has played a critical role in elucidating MS pathology include the identification of the aryl hydrocarbon receptor (AHR) as a ligand-dependent transcription factor needed for the development of Th17 and T regulatory (Treg) responses, and the discovery of retinoid related orphan receptor gamma (RORγ) as a critical transcription factor for Th17 cell development (Veldhoen et al., 2008); (Ivanov et al., 2006). Additionally, multiple therapeutics, including glatiramer acetate, an amino acid copolymer (Teitelbaum et al., 1999), and natalizumab, an antibody against the adhesion molecule α4β1-integrin (very late antigen 4 (VLA-4), CD49d/CD29) (Miller et al., 2003; Yednock et al., 1992), demonstrated efficacy in EAE models prior to proceeding to clinical trials. It has also been reported that all currently-approved MS treatments reduce EAE symptoms to a certain extent (Robinson et al., 2014).
Limitations
Despite the many parallels between EAE and MS, it is important to note that EAE differs from MS in a number of ways. First, active EAE induction requires peripheral activation of T cells using a known antigen, typically a myelin peptide, whereas the cause of autoimmune activation in MS is currently unknown (Bittner et al., 2014; Friese and Fugger, 2009). Second, EAE is mediated by Th1 and Th17 CD4+ T cells, whereas MS pathology is attributed to both CD4+ and CD8+ T cells (Babbe et al., 2000; Friese and Fugger, 2009; Wekerle et al., 1994). Additionally, administration of pertussis toxin (PTx), which is not involved in MS pathogenesis, promotes EAE induction by increasing blood-brain barrier permeability and clonal expansion and differentiation of T cells (Friese and Fugger, 2009; Wekerle et al., 1994). While this is by no means a comprehensive list of the variations between EAE and MS, it does serve as an important reminder that, although EAE resembles MS, it is not capable of recapitulating the disease in all respects.
There have also been a number of potential MS treatments that decreased EAE symptoms, but failed in clinical trials for MS (Arnason, 1999; van Oosten et al., 1997); (Wolinsky et al., 2000). This may be attributable to dissimilar disease timelines or any combination of the aforementioned differences between EAE and MS, but it is also possible that EAE protocol discrepancies contribute to these pre-clinical and clinical incongruences. For example, while the EAE clinical disease may appear similar between studies, differences in protocols lead to dramatically different cellular and subcellular disease profiles (Boullerne et al., 2014; Dias et al., 2015; Hofstetter et al., 2002; Jee and Matsumoto, 2001) potentially biasing pre-clinical drug study results. Irrespective of the origin of these incongruities, these findings support the notion that consistent EAE induction is imperative and that all therapeutics should be verified in multiple MS models prior to progressing to clinical applications.
Comparison with other methods
Protocol optimization for investigating various aspects of EAE disease and repair has led to significant variation in MOG35–55-EAE induction. A sampling of studies employing MOG35–55-EAE (Lo et al., 2003; Tompkins et al., 2002; Tseveleki et al., 2004) reveals significant differences in: (1) the quality and concentrations of MOG35–55 and, M. tuberculosis (TB), (2) PTx, injection number and placement, and (3) clinical disease scoring criteria. This makes, it extremely challenging to meaningfully compare MOG35–55-EAE results obtained by independent labs. This discordance is further exacerbated by the fact that induction with different MOG35–55 peptide doses directly influence EAE pathology (Dias et al., 2015). A study by Dias and colleagues found that female C57BL/6 mice immunized using either 100 or 300 µg of MOG35–55 peptide presented with variable pathology. At both concentrations, mice succumbed to chronic disease. However, the 100 µg doses resulted in an earlier increase of inflammatory infiltrate and increased cytokine levels in the CNS after disease induction compared to 300 µg MOG (Dias et al., 2015). Furthermore, active MOG35–55 peptide fraction purity can influence disease onset and incidence (Boullerne et al., 2014) and it has been reported that PTx dosage has a direct effect on disease incidence, progression, and immune cell profiles (Hofstetter et al., 2002; Jee and Matsumoto, 2001) [Hooke Laboratories, hookelabs.com/protocols/eae AI_C57BL6.html]. These results demonstrate that protocol variation, specifically in MOG35–55 peptide concentration and purity as well as PTx dose, have direct effects on EAE incidence, progression, and pathology.
Further discrepancies arise upon reviewing other published active MOG35–55-EAE protocols (Bittner et al., 2014; McCarthy et al., 2012; Stromnes and Goverman, 2006a; Stromnes and Goverman, 2006b), in which all fail to report sufficient methodological details or reagent concentrations and purities. This lack of detail combined with unavoidable variability between independent labs makes data verification extremely difficult and strongly hinders therapeutic development. To combat this, we provide the most detailed active MOG35–55-EAE protocol to date with which we observe high incidence and a consistent, reliable disease course. Pathologically, we observe numerous MS-like symptoms, including optic neuritis (Ghezzi et al., 1999; Shams and Plant, 2009), ascending paralysis (Batoulis et al., 2011); perivascular mononuclear infiltration, CNS axon demyelination (Sun and Wekerle, 1986), and axon damage in both infiltrating lesions and otherwise normal-appearing white matter (Gruppe et al., 2012; Mangiardi et al., 2011; Trapp et al., 1999). Furthermore, we have demonstrated reliable results in a variety of mouse lines on the C57BL/6 background: (1) consistent induction regardless of gender, (2) controlled symptom severity, and (3) robust peripheral immune infiltration in both the brain and spinal cord, culminating in a reliable and established model of MS (Kumar et al., 2013; Mangiardi et al., 2011; Moore et al., 2014a; Moore et al., 2013b; Ziehn et al., 2010).
Experiment overview
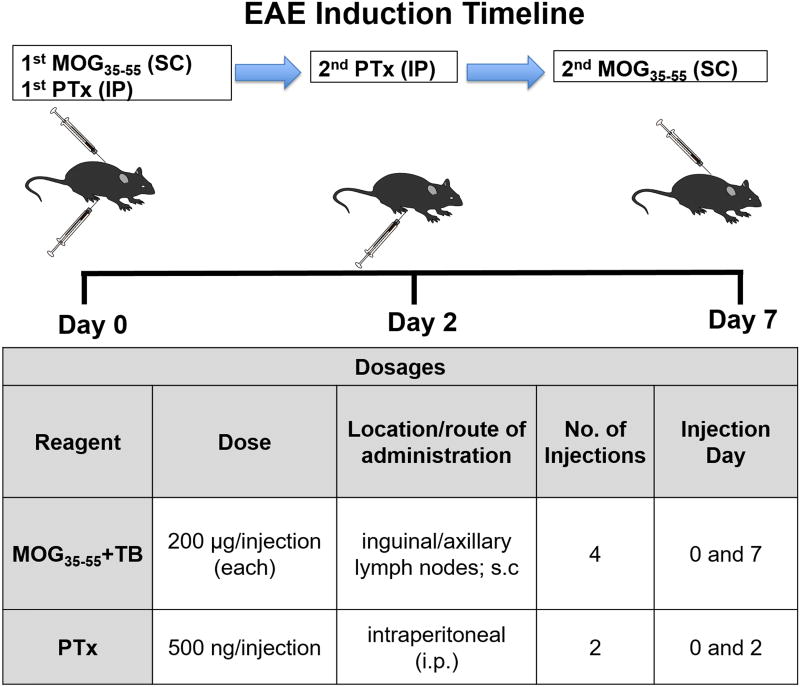
Figure 1 provides an overview of the induction procedure. In brief, the induction protocol takes place over seven days. On post-induction Days 0 and 7, mice receive two subcutaneous (s.c.) injections, each of which consists of 200 µg MOG35–55 peptide emulsified with Complete Freund’s Adjuvant (CFA). Specifically, the MOG35–55/CFA emulsion injections are performed proximal to the inguinal and axillary lymph nodes, facilitating immune recognition of the antigen and subsequent dispersion into the CNS. On Days 0 and 2, mice also receive an intraperitoneal (i.p.) injection of 500 ng PTx. This facilitates the expansion of immune cell populations and compromises the integrity of the blood brain barrier (Friese and Fugger, 2009; Wekerle et al., 1994). This protocol induces a chronic disease course, with clinical disease onset occurring 7–15 day post-immunization.
Figure 1. Experimental autoimmune encephalomyelitis (EAE) induction timeline.
To induce chronic EAE, C57BL/6 mice are immunized with MOG35–55 (200 µg/mouse) and M. tuberculosis (TB; 200 µg/mouse) in Complete Freund’s Adjuvant (CFA), as well as Pertussis toxin (PTx). On post-induction Day 0, mice receive two MOG35–55 emulsion injections [subcutaneous (s.c.); 0.05 mL/injection], one near the left axillary lymph nodes and one near the left inguinal lymph nodes. PTx (500 ng/mouse) is also administered [intraperitoneal (i.p.); 0.30 mL]. On Day 2, mice receive another PTx injection. Finally, on Day 7 each mouse receives two MOG35–55 emulsion injections (s.c.; 0.05 mL each), this time near the right axillary and inguinal lymph nodes.
Experimental planning
Groups
For each experiment, the following three groups should be included. Note that all groups should be matched with respect to:
Sex
Age
Litter (when possible)
Genetic modifications (i.e., transgene, knock-in, knock-out)
Any other pertinent conditions (e.g., gonadectomy, housing conditions)
Normal control group: these mice do not receive any injections and should remain asymptomatic.
No MOG35–55 control group*: to verify that the effects observed in MOG35–55-induced EAE mice are attributable to an immune response mounted against MOG35–55 peptide, a group of animals injected with all EAE reagents except MOG35–55 should be included.
MOG35–55-EAE group: receives all complete EAE injections
* Include this group while establishing the protocol and any time that a parameter (reagent batch, mouse line, animal housing, etc.) is altered to verify that the mice are responding as expected.
Group size and statistical power
Upon deciding on the required number of groups, group sizes should be determined by utilizing a power analysis to ensure proper statistical strength. We have provided various definitions and tried to explain how to calculate group numbers using power analysis. In addition, we have provided a table of typical group sizes over a variety of effect sizes for an experiment consisting of 3 groups and a desired power of 80%, which is the typically recommended minimum value (Supplementary Table 1 (Cohen, 1992)). It is important to note that the values listed in Table 1 will not fit all experimental designs and each experiment should include its own power analysis. We have to caution the readers that as the requirements for sufficient group size vary based on a variety of experimental factors, the experimenter should be able to understand the complexities of the correct use of different statistical tests and consult “A Power Primer” by Jacob Cohen (Cohen, 1992) and a statistician for clarity.
Table 1.
Clinical EAE scores
| Score | Scoring Method | Observation |
|---|---|---|
| 0 | Hold mouse by the base of the tail | Unaffected; mouse can “helicopter” tail |
| 0.5 | Hold mouse by the base of the tail | Some loss of tail tone |
| 1.0 | Hold mouse by the base of the tail | Complete tail limpness, with no evidence of limb weakness |
| 1.5 | Hold mouse at base of the tail between index finger and thumb, resting the heel of your palm flat on a surface. Attempt to roll the mouse once by rolling its tail between your fingers. | Can roll mouse, but with some struggling |
| 2.0 | Hold mouse at base of the tail between index finger and thumb, resting the heel of your palm flat on a surface. Attempt to roll the mouse once by rolling its tail between your fingers. | No hind limb paralysis upon ambulation, but mouse fails to remain upright when the examiner attempts to roll the mouse |
| 2.5 | Hold mouse at base of tail and place its forepaws on edge of the cage. Be prepared to catch the mouse should it fall. | Climbs into cage with difficulty; normal ambulation |
| 3.0 | Hold mouse at base of tail and place forepaws on edge of cage. Be prepared to catch the mouse should it fall. | Inability to climb over cage edge; partial paralysis of hind limbs; waddles upon ambulation (but does not drag limbs) |
| 3.5 | Observe ambulation | Partial paralysis of hind limbs as evidenced by dragging one limb upon ambulation |
| 4.0 | Observe ambulation | Complete paralysis of both hind limbs; dragging body by forearms; still capable of moving around the cage |
| 4.5 | Observe mouse behavior | Responsive but not moving/stationary, listless, rapid breathing (consult institutional veterinarian; consider euthanasia) |
| 5.0 | Observe mouse behavior | Immobile and unresponsive; Moribund (immediate euthanasia recommended) |
Reagent dosage
During the initial optimization of this protocol, the average mouse weight was 20 g, and dosages of 10 mg/kg MOG35–55 (i.e., 200 µg/mouse) and 25 µg/kg PTx (i.e., 500 ng/mouse) were sufficient for consistent induction. Since that time, 200 µg/mouse MOG35–55 and 500 ng/mouse PTx have been used regardless of exact mouse weight to maintain accuracy and reproducibility. See “Supplemental Equations” for calculations.
Materials and Methods
Caution: All procedures must be performed in accordance with the regulations set forth by the local animal ethics committee.
Reagents (Figure 2A)
Figure 2. EAE solution preparation.
(A) Reagents used, including DPBS, Complete Freund’s Adjuvant, MOG35–55 peptide, M. Tuberculosis, and Pertussis toxin (left to right). (B) Typical EAE solution preparation setup. Appearance of MOG35–55+TB solutions before (C) and after (D) emulsification. Properly emulsified solution is white, viscous, and uniform.
Critical: We do not encourage deviation from the specified reagents. Alterations in reagent purity have been shown to affect the outcome of this protocol.
Dulbecco’s phosphate buffer saline (DPBS) without calcium or magnesium (Life Technologies, Carlsbad, CA; Cat. No.14190)
- Lyophilized MOG35–55 peptide (Sequence: MEVGWYRSPFSRVVHLYRNGK; >95% purity) (Mimotopes, Clayton, Victoria, Australia; custom order)Critical: Store MOG35–55 peptide at −20°C with desiccant.
- Pertussis toxin (PTx), lyophilized in pure water, salt-free (List Biological Laboratories, Campbell, CA; Cat. No. 181)Caution: PTx is an active agent and can cause irritation if inhaled or contacted directly with skin or eyes. PTx should only be handled in a Biosafety Cabinet (BSC) with appropriate personal protective equipment (PPE).
- Heat-killed Mycobacterium tuberculosis H37 RA (Becton Dickinson (BD) Difco, Franklin Lakes, NJ; Cat. No. 231141)Caution: M. tuberculosis can stimulate an immune response. Use proper PPE to limit direct contact or inhalation.
- Complete Freund’s Adjuvant (BD Difco, Franklin Lakes, NJ; Cat. No. 263810)Caution: CFA can stimulate an immune response and cause skin lesions. Use proper PPE to limit direct contact.
Ethanol, 70% (vol/vol) (VWR, Radnor, PA; Cat. No. 89125–188)
Reagent setup
We have successfully used the following previously-opened reagents when stored at 4°C for the lengths of time specified below. Dispose of reagents in the manner specified by your environmental health and safety committee. Note that MOG35–55 solution (i.e., MOG35–55 peptide in DPBS) must be made fresh:
PTx: 14 days
CFA: 3 weeks
TB: 1 week
Equipment
Analytical balance (readability = 0.1 mg) (RadWag AS220/C/2)
Square anti-static weighing dishes, small (VWR, Radnor, PA; Cat. No. 89106–704)
Disposable anti-static microspatulas (VWR, Radnor, PA; Cat. No. 80081–194)
50 mL Nunc conical centrifuge cubes (Thermo Fisher Scientific, Waltham, MA; Cat. No. 339652)
2.0 mL Corning, orange round bottom cryogenic vial (Thermo Fisher Scientific, Waltham, MA; Cat. No. 430489)
PrecisionGlide 27 × 1/2-gauge needle, gray (BD Difco, Franklin Lakes, NJ; Cat. No. 305109)
PrecisionGlide 25 × 5/8-gauge needle, blue (BD Difco, Franklin Lakes, NJ; Cat. No. 305122)
Tuberculin slip tip 1 mL syringe (BD, Franklin Lakes, NJ; Cat. No. 309659)
3 cc Popper & Sons Perfektum glass matching numbered syringes, cleaned and autoclaved (Cadence, Cranston, RI; Cat. No. 5167)
Micro emulsifying needle, 20 gauge (Cadence, Cranston, RI; Cat. No. 7976)
Scintillation vials (VWR, Radnor, PA; Cat. No. 66022–081)
Sterile alcohol prep pads (Thermo Fisher Scientific, Waltham, MA; Cat. No.23–501–711)
Procedure
Caution: All procedures must be performed in accordance with the regulations set forth by the local animal ethics committee.
Caution: Mice should be housed in a pathogen-free facility. Differences between housing environments, specifically in the microbes present, can result in alterations to the mouse immune system (Beura et al., 2016).
Caution: Perform this procedure within a within a sterile biological safety cabinet, Class II (BSC) and sterilize all items using 70% ethanol (EtOH) before placing them in the BSC. Introducing additional pathogens can alter EAE incidence and disease course. Keep solutions sterile.
Calculations (Timing: 10 min)
-
1
Calculate the required quantities of reagents based on the number of mice being used in the experiment. Each mouse will receive 0.1 mL total of MOG35–55-CFA via two 0.05 mL injections on both Days 0 and Day 7. MOG35–55 and M. tuberculosis will be administered at 200 µg/mouse. See Supplementary Equations for details on proper concentrations, ratios, and caveats.
Critical step: Altering MOG35–55, M. tuberculosis, or PTx dosages results in different disease profiles. Even if disease course as measured by onset and clinical scoring is consistent, changes in cytokines, chemokines, immune cell population, and lesion location are dependent on the concentrations used (Dias et al., 2015); (Boullerne et al., 2014); (Hofstetter et al., 2002); (Jee and Matsumoto, 2001).
M. tuberculosis and Complete Freund’s Adjuvant storage (Timing: 20 min)
-
2
Within BSC, carefully open the M. tuberculosis H37 RA ampule per manufacturer’s specifications. Transfer the contents into a Corning cryotube, parafilm, and store at 4°C until use.
-
3
Vortex a CFA ampule to re-suspend the M. tuberculosis. Within BSC, open the CFA ampule per manufacturer’s specifications. Transfer the contents into a scintillation vial, parafilm, and store at 4°C until use.
Preparing MOG35–55-CFA emulsion (Timing: ∼1.5 h)
-
4
Attach glass emulsifying syringes to the emulsifying needles while pressing down on the plungers (Fig. 2B).
Check syringe, plunger, and needle for cleanliness. Ensure that syringes and plungers are correctly paired (i.e. matched by number). Syringe needles and reinforcing bars should not be bent.
Once assembled, for each assembly note which syringe unscrews more easily. Ensure that this syringe is on top. Verify that both syringes are tightly secured to the needle without over-tightening.
-
Remove the plunger from the top syringe and place it on a sterile surface within the BSC.
Critical step: Carefully clean and autoclave emulsifying syringes and needles prior to use to prevent introduction of additional immunogens.
-
5
Fill a 50 mL conical tube with ∼1 mL more than the calculated volume of DPBS.
-
6
Clean the balance with Kim wipe sprayed with 70% EtOH. Obtain a clean antistatic weigh boat and spatula.
-
7
Obtain MOG35–55 peptide from the −20°C freezer and allow it to come to room temperature prior to weighing (do not heat it by any means; exposing it to room temperature is sufficient). Weigh out MOG35–55 peptide using an anti-static weigh boat and spatula based on the calculations performed in step 1.
Critical step: Generate excess MOG35–55/DPBS solution to account for any loss during transfer to the emulsifying syringes.
-
8
After weighing out the MOG35–55 peptide and without removing the weigh boat from the balance, carefully add half of the final volume of DPBS to the weigh boat, expelling the buffer around the edges of the peptide. Once the peptide is covered by DPBS, carefully transfer the weigh boat into the BSC. Add the remaining volume of DPBS. Pipet up and down slowly without aspirating air (i.e., limit bubble formation) until the peptide is fully incorporated. The final solution should be clear.
Critical step: Do not expel DPBS directly onto MOG35–55 peptide, as this will cause the peptide to disperse and be lost. Slowly expel DPBS around the edges of the peptide. Allow the peptide to go into solution as it comes in contact with the DPBS.
Critical step: Do not vortex peptide.
-
9
Add the appropriate predetermined volume of MOG35–55 solution to the top emulsification syringe. Then, carefully take the assembled emulsification syringe in one hand without changing its orientation and use your other hand to slowly aspirate all of the solution into the bottom syringe by pulling the bottom syringe plunger. Next, slowly compress the bottom syringe plunger to expel any bubbles that may have formed within the bottom syringe. Ensure that the total MOG35–55 solution volume is equally distributed between the top and bottom syringes, and carefully place the assembly back onto the stand.
Critical step: The ratio of (MOG35–55-DPBS):(TB-CFA) should be 1:1.3. For effective emulsification, do not exceed the maximum specified syringe volume.
-
10
Obtain the scintillation vial containing CFA and the tube containing TB. Briefly vortex the CFA to ensure uniform suspension.
-
11
Obtain a clean weigh boat and spatula. Weigh out the predetermined amount of TB. Before transferring the weigh boat off the balance, carefully add half of the final CFA volume to the weigh boat. Be sure to pipet slowly, as the CFA is viscous. Once CFA covers the TB, carefully transfer the weigh boat into the BSC. Add the remaining volume of CFA. Pipet up and down slowly, without aspirating air, until the TB is uniformly suspended.
Critical step: Generate excess TB/CFA solution to account for any loss during transfer to the emulsifying syringes.
-
12
Add the appropriate predetermined volume of TB-CFA solution to the top of the assembled emulsification syringe. Then, carefully take the assembled emulsification syringe in one hand without changing its orientation and use your other hand to slowly aspirate all of the solution into the bottom syringe by pulling the bottom syringe plunger. Verify that the volume added is correct using the syringe markings. Then, unscrew the top syringe only (not the needle). Reinsert the plunger into the top syringe. Slowly compress the bottom syringe plunger to press the solution through the needle and remove any air bubbles that form by gently popping them with the tip of the disassembled top syringe. Once the solution reaches the top of the needle, reattach the top syringe while applying pressure to the top plunger. Be sure not to lose solution or introduce air.
-
13
Once the syringes are securely reassembled, begin emulsifying within the BSC. Hold the syringes horizontally and alternately press the plungers. Once emulsified, the solution will become white (Figure 2C). Upon continued mixing, the solution viscosity will increase. Once a uniform viscosity is achieved, continue emulsifying 25 times on each side (50 times total), then place the syringes at −20°C for 1 h.
Critical step: This solution must be thoroughly emulsified for the induction to be successful.
-
14
After 1 h at −20°C, place the syringes at 4°C until the emulsion is until the emulsion softens enough to pass through the needle. Re-emulsify 25 times on each side (50 times total) in a BSC. Return the syringes to −20°C for at least 1 h or until ready to immunize (up to 6 hrs).
Preparing PTx (Timing: 45 min)
Mice will be administered 0.3 mL of 500 ng PTx in DPBS on Days 0 and 2.
-
15
Obtain a vial of PTx, a bottle of DPBS, and a 50 mL conical tube. Place these items in a clean BSC. Aliquot 30 mL DPBS into the 50 mL conical tube.
-
16
Remove the metal sleeve from the PTx vial. Do not remove the rubber cap. Draw 1 mL of DPBS from the conical tube into a 1 mL tuberculin syringe attached to a 25-gauge needle. Carefully push the needle through the rubber cap. The DPBS will automatically be drawn into the vial. Carefully remove the needle and syringe from the rubber cap. Remove the rubber cap from the vial. Gently resuspend the PTx by pipetting up and down P200 pipette. Avoid introducing air.
Critical step: PTx does not dissolve; it will become uniformly suspended upon gentle mixing. Do not vortex.
-
17
Pipette the PTx vial contents into the 50 mL conical tube. Rinse the vial with 1 mL DPBS from the conical tube approximately three more times, or until PTx particles are no longer apparent in the vial. Ensure even suspension in conical tube.
-
18
Obtain the necessary number of 1 mL tuberculin syringes and 27-gauge needles. Fill each syringe, avoiding bubble formation. Store at 4°C.
MOG35–55-CFA emulsion syringe loading (Timing: 45 min)
-
19
Place the emulsion into the 4°C fridge until the emulsion softens enough to pass through the needle.
-
20
Obtain the necessary number of 1 mL tuberculin syringes and 25-gauge needles and place them in the BSC. Open each syringe package half way, remove the plunger from the syringe, and place the plunger into the package, next to the syringe (keep sterile). Place an ice pack in the BSC to keep the emulsification syringes cold.
-
21
When the tuberculin syringes are ready to be loaded, bring the emulsion syringes into the BSC and re-emulsify 25 times on each side (50 times total). When complete, push all of the emulsion into one of the emulsification syringes and detach the syringe from the needle.
-
22
Slowly load ∼0.6 mL of the emulsion into the top of each 1 mL tuberculin syringe. Hold the tuberculin syringe horizontally (this helps prevent the introduction of air bubbles) and confirm that the emulsion is thick, not runny. Insert the plunger slowly, compressing air bubbles as the solution moves through the tuberculin syringe. Attach a 25-gauge needle to the loaded tuberculin syringe and place it on the ice pack.
Critical step: The emulsion must stay cold or the pellet will disperse upon injection, negatively impacting disease incidence and progression. MOG35–55-CFA syringes should be kept on ice packs at all times.
Day 0 MOG35–55-CFA and PTx injections (Timing: 2–3 h, depending on number of animals)
-
23
Transfer the MOG35–55-CFA-containing syringes into the animal procedure area on an ice pack, along with PTx solution-containing syringes.
-
24
Prepare the MOG35–55-CFA-containing syringes for use by pressing the plunger to remove air bubbles. You may lose some solution, which is why it is encouraged to generate 1.5–2 times the amount of solution you need. Ensure that the plunger is stopped at a 0.1 or 0.05 mL mark. Pull back the plunger by 0.02 mL. Thoroughly clean the needle with an alcohol prep pad to remove any MOG35–55-CFA solution. This is essential to avoid development of any skin lesions.
-
25
CFA is a skin irritant and potentially causes pain and discomfort upon injection(Iadarola et al., 1988). As such, anesthesia is recommended to reduce the potential of any unnecessary pain and distress. We recommend using isoflurane delivered in oxygen (2–2.5% isoflurane: 2 L/min oxygen) to maintain anesthesia during the procedure.
-
26
Position the anesthetized mouse for injection by placing it on its ventral surface, with the head facing away from the experimenter. Gently extend the forelimbs and hind limbs laterally. First, clean the skin on the left side (near the axillary and inguinal lymph nodes) with a new alcohol prep pad.
-
27
Gently pinch the skin between the animal’s left thigh and the lower lumbar vertebrae and insert the needle subcutaneously. Slowly inject the 0.05 mL of the emulsion. Count ten seconds to ensure complete injection of the viscous emulsion, and then remove the needle. Clean the needle with an alcohol prep pad and pull back on the plunger by 0.02 mL to prepare for the next injection.
-
28
Immediately clean any excess emulsion off the animal’s skin with a clean alcohol prep pad. Check and note the position of the injection pellet placement.
Caution: CFA can cause skin lesions. It is important to clean the mouse’s skin and the syringe needle with alcohol prep pads before and after injection to reduce the risk of lesion formation.
-
29
Pinch the skin between the cervical vertebrae and the animal’s left shoulder and insert the needle subcutaneously. Slowly inject the 0.05 mL of the emulsion. Count to ten seconds to ensure complete injection of the viscous emulsion, and then remove the needle. Clean the needle with an alcohol prep pad and pull back on the plunger by 0.02 mL to prepare for the next injection.
-
30
Immediately clean any visible solution off the animal’s skin using a clean alcohol prep pad. Verify injection pellet location. If the pellet is not in the proper location, do not reinject, just take note of improper placement for future troubleshooting.
-
31
Administer an i.p. injection of 0.3 mL (500 ng) PTx. Do not disrupt injection pellets in the process.
Critical step: Administering PTx at different concentrations can cause alterations in disease severity and incidence.
-
32
After injection, put the animal back in its home cage and monitor it. Ensure that the animal wakes up from anesthesia.
-
33
Repeat steps 25–32 for each additional animal.
Pause point: Continue to step 34 on Day 2.
Day 2 PTx injections (Timing: ∼45 min)
-
34
Load the required number of PTx syringes (step 18).
-
35
Restrain the animal by grasping the dorsal skin, being sure to avoid squeezing the pellets on the animal’s left side, and perform an i.p. injection of 0.3 mL (500 ng) of PTx in a clean BSC.
Pause point: Continue with step 36 on Day 7.
Day 7 MOG35–55-CFA injections (Timing: 2–3 h, depending on number of animals)
-
36
Repeat steps 1–14, 19–30, and 32–33. PTx is not administered.
-
37
Perform the MOG35–55-CFA emulsion injections as described for Day 0, this time injecting on the animal’s right side.
Post-induction animal care
Symptom onset typically occurs between Days 7–15, with a rapid increase in clinical scores between Days 12–18. Beginning at Day 7, mice should be scored and checked for general health on a daily basis at a consistent time. As disease pathology progresses, the following precautions should be taken to provide the animals with the required care:
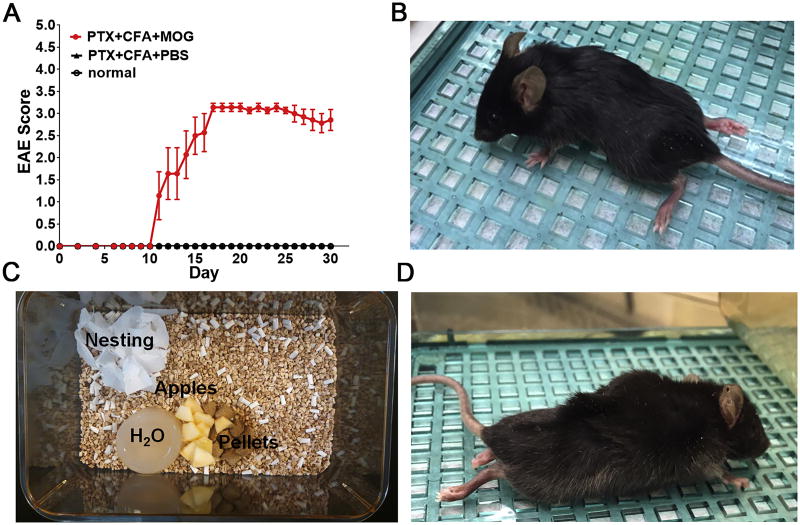
Attach long sippers to water bottles, add a non-wetting water replacement (e.g. hydrogel), and/or provide apple slices to prevent dehydration (Figure 3C). Additionally, saline injections must be given, per current animal use protocols or the institutional veterinarian, to any mouse displaying skin “tenting,” (Figure 3D) a sign of severe dehydration, for as long as symptoms persist. Additionally, easily accessible, moistened food pellets should be placed on the base of the cage to combat malnutrition (Figure 3C).
Single animal housing should be avoided and group housing which aids in thermoregulation should be encouraged. Exceptions are animals with an EAE score of 4 or more. These mice should be removed away from ambulatory cage mates that may tread on them. The single housed mice should have additional nesting material to aid in thermoregulation.
Mice with a score of ≥3.5 may lose control of their bladders. It is important to ensure that the bladder is completely emptied to decrease the chance of bladder infections or rupture. Affected mice require manual bladder expression at least once daily until normal bladder function is reestablished which may not occur in all cases.
Mice show a complete inability to move due to paralysis in all limbs are assigned a score of 5 and are the most severe end-stage assessment of EAE. For humane reasons mice that reach this stage and are moribund with EAE should be sacrificed within the day.
Figure 3. EAE expected outcome.
(A) Eight-week-old female C57BL/6 mice were injected with either PTx+CFA+MOG35–55 (EAE group-red circle) or PTx+CFA+DPBS (no MOG35–55 control group-black triangle), or not injected (normal control group-black circle). EAE scores are shown as mean±SEM; n = 8 mice/group. The EAE group displayed clinical symptoms beginning at post-induction Day ∼10. (B) A symptomatic mouse with a clinical score of 3.5. (C) Typical supplements for EAE mice. Food pellets wetted with water, apple slices, a non-wetting water replacement, and strips of nesting material are commonly added to cages. (D) A dehydrated mouse exhibiting “tenting” following the pinch test.
Clinical scoring
Clinical disease scoring should be performed on a daily basis, beginning on Day 7 and in accordance with the standard EAE clinical disease scoring scale modified from Pettinelli and McFarlin (Pettinelli and McFarlin, 1981) and shown in Table 1 (discussed in detail below). It is very important to note that the mice will become weak and/or paralyzed as symptoms progress. Always be prepared to catch the animal during clinical score evaluations as their righting reflex may be compromised. Furthermore, unless an animal is clearly a 4 or 5, daily evaluation of clinical score should start at the methodology described for a score of 0 and progress through the tests until the animal meets the described score criteria. It is also imperative that mice are scored at the same time each day to account for behavioral changes in the animal’s wake/sleep cycle as well as hormonal changes that occur throughout the day.
Data handling
Daily clinical scores should be recorded for each experimental group, including control groups. This data can then be plotted in a standard, grouped XY graph and displayed as the mean group score ± standard deviation (SD) or standard error of the mean (SEM) for each day that measurements are taken. We recommend displaying data as the mean score, as opposed to cumulative scores. Furthermore, the 2 way ANOVA, which is the recommended statistical analysis, considers the daily mean score and standard deviation to determine the differences/compare the variability between groups (how far apart are the means) to the variability within the groups (natural variation in measurements).
Additionally, combining clinical scores from multiple experiments is discouraged, since EAE induction can vary between experiments. Changes in reagent lots between experiments will also increase the possibility of reagent based variations. We suggest that results be confirmed in multiple, independent experiments, rather than grouping experimental data together, to account for these changes. Also, due to this natural variability, it is recommended that all experiments be performed and confirmed at least three times with group sizes that provide sufficient analytical power for the specific experimental parameters (see Experimental Planning).
There is also a possibility that some animals will succumb to the disease or will need to be euthanized for health reasons before experiment end. Fortunately, proper application of this protocol typically results in mouse scores between 3–4, and mice at this level do not typically die or require euthanasia. However, in the occasional cases when it is necessary to remove an animal, it is not advisable to completely exclude the animals scores from the data set, nor is it advisable to input scores of 5 in for the remaining days, as both options have the potential to skew the data and either fabricate or diminish between group and intra-group variance (discussed below).
Troubleshooting
Timing
Steps 1–22, calculations, reagent preparation, MOG35–55 emulsion syringe loading: ∼3 h
Steps 23–33, day 0 MOG35–55 emulsion and PTx injections: 2–3 h
Steps 34–35, day 2 PTx injections: ∼45 min
Steps 36–37, reagent preparation, MOG35–55 emulsion syringe loading, day 7 MOG35–55 emulsion injections: 5–6 h
Daily clinical scoring and animal care: 1–2 h per d
Results
Disease progression
Beginning on post-induction Day 7, mice must be monitored daily for development of EAE clinical disease. Normal and no MOG35–55 control groups are not expected to show clinical disease. In contrast, the EAE group(s) will begin to display symptoms between Days 7–15 (Figure 3–5). To accurately monitor disease onset and progression, a clinical disease score is assigned to each mouse utilizing a standard scoring procedure (Table 1, discussed below). Typical results are shown in Figure 3A. Additionally, mouse weights will fluctuate during the disease course and can serve as an additional indicator of disease severity. Generally, EAE mice lose ∼15–20% of their body weight just prior to disease onset, reach a minimum weight a few days before peak clinical severity, and continue to regain weight throughout the experiment (Moore et al., 2014b). Mice which fail to regain weight should be closely monitored to ensure proper nutrition and hydration.
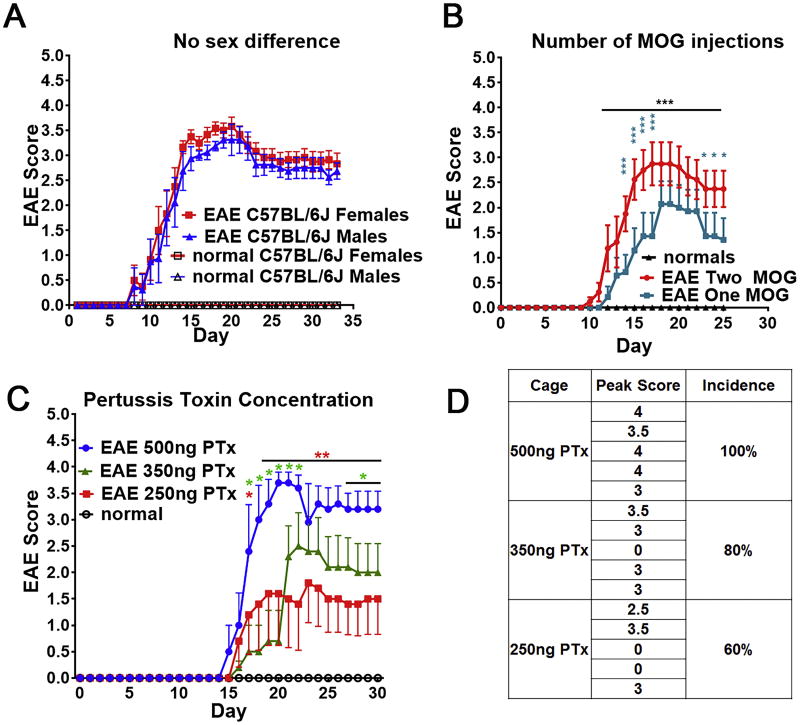
Figure 5. Effects of sex, number of MOG35–55-CFA injections, and PTx concentration on EAE clinical disease.
(A) Both male and female C57BL/6J mice displayed 100% EAE clinical disease incidence, with no difference in peak clinical score (∼3.5). Clinical EAE scores are represented as mean±SEM (B) EAE clinical scores for female C57BL/6 mice (n = 7–8/group) immunized with MOG35–55-CFA on post-induction Days 0 and 7 (Two MOG) or Day 0 only (One MOG). PTx was administered on Days 0 and 2 in both groups. The normal group does not display clinical disease. Mice receiving one or two days of MOG35–55-CFA injections developed EAE, with similar time of clinical disease onset. Mice receiving one day of MOG35–55-CFA injections, however, exhibited reduced clinical disease severity. (C) Clinical EAE scores represented as mean±SEM for female PLP-EGFP C57BL/6 mice (n = 5/group) receiving 500 ng, 350 ng, or 250 ng of PTx on Days 0 and 2. Clinical disease onset was delayed by one day in the 250 ng and 350 ng PTx groups relative to the 500 ng group. (D) Clinical disease severity was reduced in a dose-dependent manner, with a mean peak disease score of 3.7 for the 500 ng PTx group, 2.5 for the 350 ng PTx group, and 1.6 for the 250 ng PTx group. A dose-dependent reduction in EAE clinical disease incidence was observed in the 250 ng PTx and 350 ng groups, with only 60–80% of mice displaying clinical disease. *p ≤ 0.1, **p ≤ 0.01, ****p ≤ 0.0001; 2 Way ANOVA with Bonferroni multiple comparisons test.
Symptoms generally progress in a predictable manner and most EAE mice will display motor deficits (Table 1). Figure 3B depicts a mouse with symptoms representative of a clinical score of 3.5. Occasionally, atypical symptoms may be observed, such as a head tilt and ataxia. This may manifest as an inability to balance, with the affected mouse leaning against the cage or falling onto one side while ambulating. These mice must be monitored carefully to ensure that access to food and water; if not, immediate euthanasia should be considered.
As symptoms progress, mice with a clinical score of 3.0 or higher may also have difficulty accessing food and water and can become rapidly malnourished and dehydrated without proper care. Therefore, it is imperative to take steps to preemptively combat dehydration and malnutrition. Water bottles with long sippers or a non-wetting water replacement (e.g., hydrogel) should be added to all cages as a first line of defense. Apple slices and moistened food pellets should be added for nutrition and hydration (Figure 3C). Finally, the skin pinch test should be administered regularly to check for skin “tenting” (Figure 3D), which indicates severe dehydration. If this is observed, injections of 0.30 mL sterile saline should be administered subcutaneously every 6 hours or per approved protocols or institution guidelines. It is also important to note that mouse teeth grow continuously and mice may develop malocclusion if they are not feeding regularly. While we have not witnessed this outside of mice genetically predisposed to the condition, it is important to check for this condition.
Observed cellular pathology
Our protocol results in robust demyelination, immune infiltration and concomitant pathology throughout both the brain and spinal cord (Crawford et al., 2010a) (Kumar et al., 2013; Mangiardi et al., 2011; Moore et al., 2013a; Smith et al., 2007; Ziehn et al., 2010). As previously published, an increase in inflammatory CD45+ cell numbers into both white and grey matter of the CNS and a reduction in axon myelination in various brain regions is observed (Figure 4B–C) (Crawford et al., 2010a; Kumar et al., 2013; MacKenzie-Graham et al., 2009; Mangiardi et al., 2011; Moore et al., 2014b; Ziehn et al., 2010). For a detailed analysis of the CNS pathology please refer to the paper by Mangiardi et al., 2011.
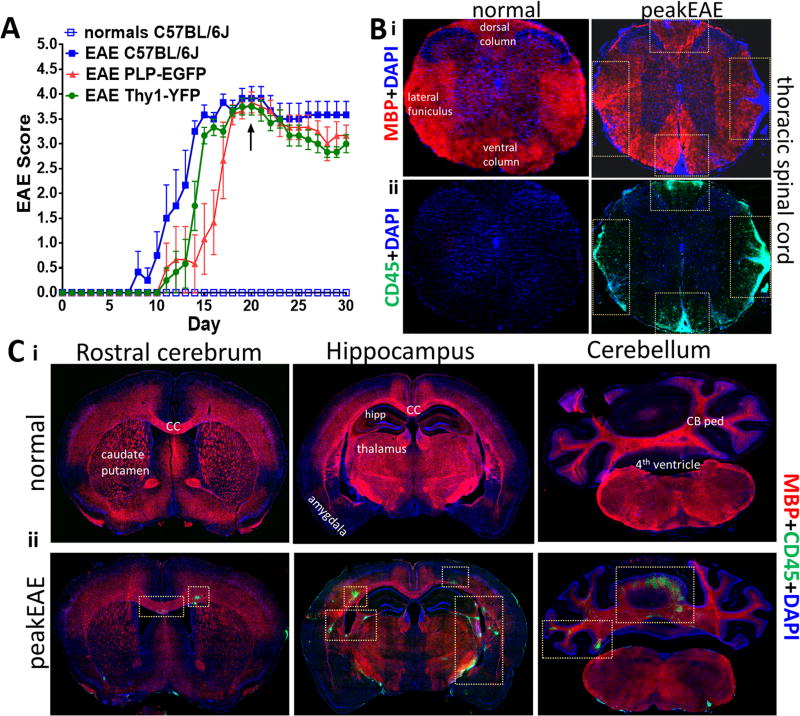
Figure 4. EAE pathology.
(A) C57BL/6J (closed blue square), PLP-EGFP (red), and Thy1-YFP (green) mice underwent EAE induction, and C57BL/6J (open blue square) were used as normal controls (n = 5–6 mice/group). Clinical scores were assessed daily and represented as mean±SEM. C57BL/6J, PLP-EGFP, and Thy1-YFP mice responded similarly to EAE induction, with clinical disease onset at Days 8–15 and peak disease at Days 15–20 (black arrow). (B) Thoracic spinal cord coronal sections and (C) Coronal brain sections from normal and peak clinical disease EAE (Day 21) mice were stained for myelin basic protein (MBP; red) to stain myelin and leukocyte antigen marker CD45+ to stain microglia/leukocytes (ii; green), as well as DAPI (blue). EAE mice exhibit decreased MBP+ and increased CD45+ cell reactivity. from normal and peak EAE mice. Increased Inflammatory lesions (CD45+; green) are observed in all brain sections depicted here at peak EAE similar to as seen in (Mangiardi et al., 2011). 10X magnification images.
Variations in application
We have demonstrated that EAE pathology and clinical disease incidence and severity are consistent across a variety of mice on the C57BL/6 background, including PLP-EGFP, Thy1-YFP, C57BL/6J, and C57BL/6N.Hsd mice (Figure 4A) (Crawford et al., 2010a; Kumar et al., 2013; Mangiardi et al., 2011; Moore et al., 2014b). In typical cases, an incidence of 80–100% is achieved with onset of symptoms at Days 7–15 and peak disease occurring between Days 18–21. While overall disease course is consistent, occasionally an earlier onset of disease (Days 7–10) in C57BL/6J and C57BL/6N.Hsd mice compared to the transgenic PLP_EGFP and Thy1-YFP lines is observed.
Our protocol has also been extensively applied to both male and female C57BL/6 mice, as previously described (Crawford et al., 2010a; Kumar et al., 2013; Mangiardi et al., 2011; Moore et al., 2014b). No sex differences in clinical disease onset, severity or incidence were observed, consistent with previous findings (Figure 5A) (Okuda et al., 2002; Papenfuss et al., 2004; Smith-Bouvier et al., 2008).
Some laboratories use only one MOG injection (Bailey et al., 2007) to induce EAE. We performed an EAE induction with either Day 0 and Day 7 MOG35–55 injections or only Day 0 injections (Figure 5B). Although both groups displayed similar clinical disease onset, the one MOG injection group exhibited reduced disease severity and a higher level of spontaneous recovery compared to the two MOG injection group. This result, paired with the results of Dias and colleagues (Dias et al., 2015), offer further support that disease course and severity are strongly dependent on the administered dosages and timing of MOG35–55 injections.
It has been reported that EAE onset and symptom severity can be altered by PTx dose [Hooke Laboratories, hookelabs.com/protocols/eaeAI_C57BL6.html]. We have demonstrated this by inducing EAE using either 500 ng, 350 ng, or 250 ng of PTx. In both the 250 ng and 350 ng PTx groups, clinical disease progression was delayed when compared to the 500 ng PTx group. In addition, overall clinical scores and percent incidence decreased in a dose-dependent manner (Figure 5C and D). It is also important to note that alterations in MOG35–55 dosages have been reported to modify disease course as well as infiltrating cell populations, cytokines, and chemokines (Baxter, 2007; Boullerne et al., 2014). For consistency, the authors do not recommend deviating from any of the proposed concentrations in this protocol, as it is expected to change the disease outcome and reduce consistency.
Discussion
Clinical scoring
A daily clinical score is typically reported to rate the animal’s clinical disease course progression. The most common EAE scoring system is a scale ranging from 0 to 5 (Chen et al., 2014; Dutra et al., 2013; Mangiardi et al., 2011; Martin et al., 2016; Saijo et al., 2011; Smith-Bouvier et al., 2008; Tiwari-Woodruff et al., 2007). Some studies use a 8, 10 or 16 point scale (Bittner et al., 2014; Chakrabarty et al., 2004; Deslauriers et al., 2011; Emerson et al., 2009; Gold et al., 2004). The traditional EAE scoring scale employed by our lab ranges from 0 to 5, with further demarcations every 0.5 points. Our method of scoring is simple and objective. Each full point represents an easily identifiable progression in clinical disease and each half-point increase denotes a distinct increase in clinical disease severity. These clear progressions reduce the amount of experimenter-introduced variation and, in the case of therapeutic studies, hold the experimental drugs to more stringent criteria, since notable improvements are required to report a decrease in clinical disease. Furthermore, this scale mirrors the major stages of disease progression outlined in the expanded disability status scale (EDSS), which is used to diagnose and monitor MS patients (Kurtzke, 2008). It should be noted that the proposed scale does have limitations when it comes to reporting atypical EAE symptoms (see discussion). We do not currently have a modified scoring system to account for this occurrence and have instead chosen to keep records of atypical symptoms.
Alternate clinical disease scoring scales currently in use provide both advantages and disadvantages. Expanded, non-linear scales increase the number of observations that are made, thereby increasing the sensitivity of the scale (Bittner et al., 2014). This, unfortunately, is accompanied by a reduction in objectivity, as the experimenter is required to discriminate between increasingly subtle behavioral deficiencies (Bittner et al., 2014) and allows for potentially minute improvements to be reported as significant. On the other hand, analysis of clinical disease by rotarod offers a linear measurement capable of discriminating between subtle changes in coordination and mobility and if combined with other electrophysiological and behavioral assay can be extremely useful in assessing therapeutic efficacy of various drugs (de Bruin et al., 2016; Mangiardi et al., 2011; Moore et al., 2014a; Moore et al., 2014b) in an objective manner. However, determination of clinical disease severity by rotarod becomes non-linear and less sensitive once animals begin displaying hind limb paralysis (Clinical score≥3) because many animals are unable to remain on the rod beyond the first rotation. Therefore, while rotarod can be a valid behavioral measurement, it should be used in conjunction with, not in lieu of, a traditional clinical scoring scale (Moore et al., 2013b).
Statistical analysis
In 2010, due to the limited internal validity of in vivo experiments using animal models, “The Animal Research: Reporting of In Vivo Experiments” (ARRIVE) was published in PLOS biology (Kilkenny et al., 2010) to guide authors on appropriate study design, and experimental procedures for correct and comprehensive reporting of in vivo experiments with animals. However, an investigation of the literature by Baker and colleagues (Baker et al., 2014) found that even after the availability of the ARRIVE guidelines, there were many shortcomings in reporting experimental design and the selection of appropriate statistical analyses. Baker et al. observed that 13% of EAE studies failed to report any statistical analyses and 55% of EAE studies used an inappropriate statistical analysis.
A number of publications have used the Mann Whitney U test (Goldmann et al., 2013; Su et al., 2012; Zhang et al., 2015), the Friedman test (the non-parametric alternative to the one-way ANOVA with repeated measures (all the papers published from our group and others(Du et al., 2011; Hu and Qin, 2013; Spence et al., 2013; Wisdom et al., 2013)), or a two-way ANOVA followed by a Bonferroni post hoc test (Lélu et al., 2010; Rothhammer et al., 2011; Yu et al., 2015) to analyze EAE clinical scores. Unfortunately, multiple issues arise from employing these types of analyses. Using a one-way ANOVA (or any equivalent) is incorrect since that analysis is only capable of analyzing data sets with one independent variable. Applying this test, in Prism for example, sums a group scores for all days and then calculates a single average value for each group. This eliminates the ability to properly analyze disease course by disregarding day-to-day intra-group variance, while inappropriately calculating the variance between groups, thus greatly increasing the odds of obtaining a false positive. This is also a problem in studies that utilize cumulative disease index (CDI) scoring(Becklund et al., 2009; Ochoa-Reparaz et al., 2010), in which EAE clinical scores for a group are summed over all days and then divided by the group size. The average score for each group is then plotted on a column graph and analyzed. This produces the same aggregation effect as applying a one-way ANOVA for daily EAE scores and should be avoided. The most appropriate statistical analysis is a repeated-measures (RM), 2-way ANOVA with a Dunnett’s (if only a set of comparisons are being made to one particular group) or Bonferroni multiple comparison analysis because:
A typical EAE experiment will consist of two independent variables (IV) or factors (e.g., day and treatment group) with multiple levels (e.g. Treatment group may consist of a drug and a vehicle group and one dependent variable (DV) (i.e. clinical score). This experimental design will require the use of a two-way ANOVA because the effect/interactions of the two IVs on the DV is being considered.
The repeated measures are necessary because the typical experiment is structured such that the same mice are measured every day, rather than new groups being measured at each time point.
The use of a multiple comparisons post-hoc test will allow the experimenter to compare differences in the clinical score (DV) for multiple treatment groups (first IV) at every time point (second IV) (McHugh, 2011). The recommended tests are among the most widely used and offer sufficient power the avoid Type I errors (i.e. false positives). However, ANOVA doesn’t provide analysis of pairwise differences (pairwise = subgroup differences). T- tests between groups are not recommended as it causes alpha inflation, and reports significant differences between pairs that do not actually have any differences (type I error). Multivariate analysis (multiple comparisons test) overcomes the limitations of the t test as well as decreases the probability of making a type I error (McHugh, 2011).
The Bonferroni method detects the significant differences between the interaction components, (specify the components, at the significance level alpha = 0.05). (McHugh, 2011)
In using this analysis, the ANOVA will allow for the determination of between-group differences in overall disease course and the multiple comparisons will determine significance between each group at every time point all while avoiding compounding error when comparing multiple groups.
Most EAE data sets are not ideal and the main shortfall to this method is that the RM 2-way ANOVA is incapable of analyzing data sets that have missing values. This becomes a problem if any mice need to be euthanized for health reasons or succumb to the disease over the course of the experiment. In this case, it is inappropriate to completely exclude the animal(s) or to put a score of 5 for the rest of the experiment as both approaches will undoubtedly skew the data set and increase the chance of introducing a Type I or Type II error It is possible, however, to run an ordinary 2-way ANOVA with multiple comparisons on data sets with missing data points. In some situations, this approach will introduce a significant amount of error into the ANOVA analysis, since the within-subject variance (formerly accounted for by the repeated-measures) is now being incorrectly attributed to one of the independent variables, but the multiple comparisons post-hoc test will be calculated identically. Therefore, it is our recommendation that, in the event of missing data points, an ordinary 2-way ANOVA with multiple comparisons and a Dunnett’s or Bonferroni post-hoc test be used to analyze the data followed by only using the results of the multiple comparisons to determine between-group significance.
Conclusion
We have optimized this MOG35–55 EAE induction protocol for maximal disease incidence and stable progression in both males and females, and in multiple transgenic lines (on a C57BL/6 background). We have also addressed previously overlooked details that may contribute to inter-experiment variability and reduced comparability between studies. Additionally, we have included an in-depth description of proper experimental design, data analysis, and statistical analysis in order to avoid currently pervasive design and analysis flaws. In sum, we provide a highly detailed MOG35–55 EAE protocol with the aim of increasing the comparability of pre-clinical studies using EAE, and with the hope of improving the translatability of pre-clinical MS research.
Supplementary Material
Table 2.
Troubleshooting
| Step | Problem | Possible Reason | Solution |
|---|---|---|---|
| Step 13 | The emulsification mixing step does not produce a viscous solution | Insufficient mixing | Allow emulsification syringes to sit until the CFA and DPBS phases separate. Attempt to emulsify again. Repeat (1–3 times) until there is a noticeable and consistent shift in viscosity |
| Step 22 | The emulsion is runny and loses its viscosity when loading 1mL syringes | The emulsion is too warm | Place the emulsion at −20°C for a minimum of 30 min. Keep the emulsion on an ice pack while loading. |
| Steps 28, 30 | The emulsion pellets disperse following injection | The emulsion is too warm | Keep the 1 mL syringes on an ice pack or place at −20°C until the solution has become more viscous. |
| Disease onset/Clinic al scoring | Symptoms do not occur or disease incidence is low | Improper preparation or faulty/expired reagents | Double-check all calculations, concentrations, sample preparations, and reagent expiration dates (especially PTx). |
| Improper MOG35–55 CFA pellet injection location | Euthanize and dissect mouse to verify that the pellets are in proximity to the inguinal and axial lymph nodes. Adjust future injections accordingly. |
Highlights.
Detailed protocol that yields consistent and robust pathology
Two injections of myelin oligodendrocyte glycoprotein peptide (MOG35–55)
CNS axon demyelination, and axon damage in both infiltrating lesions
Ascending paralysis in 80–100% of induced mice.
Aim to foster comparability between pre-clinical studies
Acknowledgments
This work was generously supported by NMSSRG 4853A3/2 and NIHR01 NS081141.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Contributions
SKTW conceived of and supervised the study and wrote the manuscript with the help of JPCH, HK, SG, and AJK.
JPCH, HK, and AJK performed experiments.
SKTW, SG, JPCH, HK, and AJK discussed and interpreted results.
Competing Financial Interests
The authors declare that they have no competing financial interests.
References
- Arnason BGW. TNF neutralization in MS: results of a randomized, placebo-controlled multicenter study. The Lenecept multiple sclerosis study group and the university of British Colombia MS/MRI analysis group. Neurology. 1999;53:457–65. [PubMed] [Google Scholar]
- Babbe H, Roers A, Waisman A, Lassmann H, Goebels N, Hohlfeld R, Friese M, Schroder R, Deckert M, Schmidt S, Ravid R, Rajewsky K. Clonal expansions of CD8(+) T cells dominate the T cell infiltrate in active multiple sclerosis lesions as shown by micromanipulation and single cell polymerase chain reaction. The Journal of experimental medicine. 2000;192:393–404. doi: 10.1084/jem.192.3.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey SL, Schreiner B, McMahon EJ, Miller SD. CNS myeloid DCs presenting endogenous myelin peptides ‘preferentially’ polarize CD4+ T(H)-17 cells in relapsing EAE. Nat Immunol. 2007;8:172–80. doi: 10.1038/ni1430. [DOI] [PubMed] [Google Scholar]
- Baker D, Lidster K, Sottomayor A, Amor S. Two years later: journals are not yet enforcing the ARRIVE guidelines on reporting standards for pre-clinical animal studies. PLoS Biol. 2014;12:e1001756. doi: 10.1371/journal.pbio.1001756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batoulis H, Recks MS, Addicks K, Kuerten S. Experimental autoimmune encephalomyelitis--achievements and prospective advances. APMIS. 2011;119:819–30. doi: 10.1111/j.1600-0463.2011.02794.x. [DOI] [PubMed] [Google Scholar]
- Baxter AG. The origin and application of experimental autoimmune encephalomyelitis. Nat Rev Immunol. 2007;7:904–12. doi: 10.1038/nri2190. [DOI] [PubMed] [Google Scholar]
- Becklund BR, Hansen DW, Jr, Deluca HF. Enhancement of 1,25-dihydroxyvitamin D3-mediated suppression of experimental autoimmune encephalomyelitis by calcitonin. Proc Natl Acad Sci U S A. 2009;106:5276–81. doi: 10.1073/pnas.0813312106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beura LK, Hamilton SE, Bi K, Schenkel JM, Odumade OA, Casey KA, Thompson EA, Fraser KA, Rosato PC, Filali-Mouhim A, Sekaly RP, Jenkins MK, Vezys V, Haining WN, Jameson SC, Masopust D. Normalizing the environment recapitulates adult human immune traits in laboratory mice. Nature. 2016;532:512–6. doi: 10.1038/nature17655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bittner S, Afzali AM, Wiendl H, Meuth SG. Myelin oligodendrocyte glycoprotein (MOG35–55) induced experimental autoimmune encephalomyelitis (EAE) in C57BL/6 mice. J Vis Exp. 2014 doi: 10.3791/51275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boullerne AI, Polak PE, Braun D, Sharp A, Pelligrino D, Feinstein DL. Effects of peptide fraction and counter ion on the development of clinical signs in experimental autoimmune encephalomyelitis. J. Neurochem. 2014;129:696–703. doi: 10.1111/jnc.12664. [DOI] [PubMed] [Google Scholar]
- Chakrabarty A, Danley MM, LeVine SM. Immunohistochemical localization of phosphorylated protein kinase R and phosphorylated eukaryotic initiation factor-2 alpha in the central nervous system of SJL mice with experimental allergic encephalomyelitis. J Neurosci Res. 2004;76:822–33. doi: 10.1002/jnr.20125. [DOI] [PubMed] [Google Scholar]
- Chen H, Assmann JC, Krenz A, Rahman M, Grimm M, Karsten CM, Kohl J, Offermanns S, Wettschureck N, Schwaninger M. Hydroxycarboxylic acid receptor 2 mediates dimethyl fumarate’s protective effect in EAE. J Clin Invest. 2014;124:2188–92. doi: 10.1172/JCI72151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J. A power primer. Psychol Bull. 1992;112:155–9. doi: 10.1037//0033-2909.112.1.155. [DOI] [PubMed] [Google Scholar]
- Crawford DK, Mangiardi M, Song B, Patel R, Du S, Sofroniew MV, Voskuhl RR, Tiwari-Woodruff SK. Oestrogen receptor beta ligand: a novel treatment to enhance endogenous functional remyelination. Brain: a journal of neurology. 2010a;133:2999–3016. doi: 10.1093/brain/awq237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford DK, Mangiardi M, Song BB, Patel R, Du SM, Sofroniew MV, Voskuhl RR, Tiwari-Woodruff SK. Oestrogen receptor beta ligand: a novel treatment to enhance endogenous functional remyelination. Brain. 2010b;133:2999–3016. doi: 10.1093/brain/awq237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Bruin NM, Schmitz K, Schiffmann S, Tafferner N, Schmidt M, Jordan H, Haussler A, Tegeder I, Geisslinger G, Parnham MJ. Multiple rodent models and behavioral measures reveal unexpected responses to FTY720 and DMF in experimental autoimmune encephalomyelitis. Behav Brain Res. 2016;300:160–74. doi: 10.1016/j.bbr.2015.12.006. [DOI] [PubMed] [Google Scholar]
- Denic A, Johnson AJ, Bieber AJ, Warrington AE, Rodriguez M, Pirko I. The relevance of animal models in multiple sclerosis research. Pathophysiology. 2011;18:21–9. doi: 10.1016/j.pathophys.2010.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deslauriers AM, Afkhami-Goli A, Paul AM, Bhat RK, Acharjee S, Ellestad KK, Noorbakhsh F, Michalak M, Power C. Neuroinflammation and endoplasmic reticulum stress are coregulated by crocin to prevent demyelination and neurodegeneration. J Immunol. 2011;187:4788–99. doi: 10.4049/jimmunol.1004111. [DOI] [PubMed] [Google Scholar]
- Dias AT, De Castro SB, Alves CC, Mesquita FP, De Figueiredo NS, Evangelista MG, Castanon MC, Juliano MA, Ferreira AP. Different MOG(35–55) concentrations induce distinguishable inflammation through early regulatory response by IL-10 and TGF-beta in mice CNS despite unchanged clinical course. Cell Immunol. 2015;293:87–94. doi: 10.1016/j.cellimm.2014.12.009. [DOI] [PubMed] [Google Scholar]
- Du S, Sandoval F, Trinh P, Umeda E, Voskuhl R. Estrogen receptor-beta ligand treatment modulates dendritic cells in the target organ during autoimmune demyelinating disease. Eur J Immunol. 2011;41:140–50. doi: 10.1002/eji.201040796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutra RC, Moreira EL, Alberti TB, Marcon R, Prediger RD, Calixto JB. Spatial reference memory deficits precede motor dysfunction in an experimental autoimmune encephalomyelitis model: the role of kallikrein-kinin system. Brain Behav Immun. 2013;33:90–101. doi: 10.1016/j.bbi.2013.06.002. [DOI] [PubMed] [Google Scholar]
- Emerson MR, Gallagher RJ, Marquis JG, LeVine SM. Enhancing the ability of experimental autoimmune encephalomyelitis to serve as a more rigorous model of multiple sclerosis through refinement of the experimental design. Comp Med. 2009;59:112–28. [PMC free article] [PubMed] [Google Scholar]
- Fernandes CG, Graça DL, Pereira LA. [Demyelination and remyelination after multiple intramedullary injections of ethidium bromide in Wistar rats] Arq Neuropsiquiatr. 1997;55:452–9. doi: 10.1590/s0004-282x1997000300017. [DOI] [PubMed] [Google Scholar]
- Friese MA, Fugger L. Pathogenic CD8(+) T cells in multiple sclerosis. Ann Neurol. 2009;66:132–41. doi: 10.1002/ana.21744. [DOI] [PubMed] [Google Scholar]
- Ghezzi A, Martinelli V, Torri V, Zaffaroni M, Rodegher M, Comi G, Zibetti A, Canal N. Long-term follow-up of isolated optic neuritis: the risk of developing multiple sclerosis, its outcome, and the prognostic role of paraclinical tests. J Neurol. 1999;246:770–5. doi: 10.1007/s004150050453. [DOI] [PubMed] [Google Scholar]
- Gold BG, Voda J, Yu X, McKeon G, Bourdette DN. FK506 and a nonimmunosuppressant derivative reduce axonal and myelin damage in experimental autoimmune encephalomyelitis: neuroimmunophilin ligand-mediated neuroprotection in a model of multiple sclerosis. J Neurosci Res. 2004;77:367–77. doi: 10.1002/jnr.20165. [DOI] [PubMed] [Google Scholar]
- Goldmann T, Wieghofer P, Muller PF, Wolf Y, Varol D, Yona S, Brendecke SM, Kierdorf K, Staszewski O, Datta M, Luedde T, Heikenwalder M, Jung S, Prinz M. A new type of microglia gene targeting shows TAK1 to be pivotal in CNS autoimmune inflammation. Nat Neurosci. 2013;16:1618–26. doi: 10.1038/nn.3531. [DOI] [PubMed] [Google Scholar]
- Gruppe TL, Recks MS, Addicks K, Kuerten S. The extent of ultrastructural spinal cord pathology reflects disease severity in experimental autoimmune encephalomyelitis. Histol Histopathol. 2012;27:1163–74. doi: 10.14670/HH-27.1163. [DOI] [PubMed] [Google Scholar]
- Hofstetter HH, Shive CL, Forsthuber TG. Pertussis Toxin Modoulates the immune response to neuroantigens injected in incomplete freund’s adjuvant: induction of Th1 cells and experimental autoimmune encephalomyelitis in the presence of high frequencies of Th2 cells. J. Immunol. 2002;169:117–25. doi: 10.4049/jimmunol.169.1.117. [DOI] [PubMed] [Google Scholar]
- Hollenbach JA, Oksenberg JR. The immunogenetics of multiple sclerosis: A comprehensive review. J Autoimmun. 2015;64:13–25. doi: 10.1016/j.jaut.2015.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu X, Qin X. Lentivirus-mediated estrogen receptor α overexpression in the central nervous system ameliorates experimental autoimmune encephalomyelitis in mice. Int J Mol Med. 2013;31:1209–21. doi: 10.3892/ijmm.2013.1306. [DOI] [PubMed] [Google Scholar]
- Iadarola MJ, Brady LS, Draisci G, Dubner R. Enhancement of dynorphin gene expression in spinal cord following experimental inflammation: stimulus specificity, behavioral parameters and opioid receptor binding. Pain. 1988;35:313–26. doi: 10.1016/0304-3959(88)90141-8. [DOI] [PubMed] [Google Scholar]
- Ivanov II, McKenzie BS, Zhou L, Tadokoro CE, Lepelley A, Lafaille JJ, Cua DJ, Littman DR. The orphan nuclear receptor RORγt directs the differentiation program of proimflammatory IL-17+ T helper cells. Cell. 2006;126:1121–33. doi: 10.1016/j.cell.2006.07.035. [DOI] [PubMed] [Google Scholar]
- Jee Y, Matsumoto Y. Two-step activation of T cells, clonal expansion and subsequent Th1 cytokine production, is essential for the development of clinical autoimmune encephalomyelitis. Eur. J. Immunol. 2001;31:1800–12. doi: 10.1002/1521-4141(200106)31:6<1800::aid-immu1800>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Kerlero de Rosbo N, Hoffman M, Mendel I, Yust I, Kaye J, Bakimer R, Flechter S, Abramsky O, Milo R, Karni A, Ben-Nun A. Predominance of the autoimmune response to myelin oligodendrocyte glycoprotein (MOG) in multiple sclerosis: reactivity to the extracellular domain of MOG is directed against three main regions. Eur. J. Immunol. 1997;27:3059–69. doi: 10.1002/eji.1830271144. [DOI] [PubMed] [Google Scholar]
- Kerlero de Rosbo N, Mendel I, Ben-Nun A. Chronic relapsing experimental autoimmune encephalomyelitis with a delayed onset and an atypical clinical course, induced in PL/J mice by myelin oligodendrocyte glycoprotein (MOG)-derived peptide: preliminary analysis of MOG T cell epitopes. Eur. J. Immunol. 1995;25:985–93. doi: 10.1002/eji.1830250419. [DOI] [PubMed] [Google Scholar]
- Kilkenny C, Browne W, Cuthill IC, Emerson M, Altman DG, Group NCRRGW. Animal research: reporting in vivo experiments: the ARRIVE guidelines. Br J Pharmacol. 2010;160:1577–9. doi: 10.1111/j.1476-5381.2010.00872.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kipp M, van der Star B, Vogel DY, Puentes F, van der Valk P, Baker D, Amor S. Experimental in vivo and in vitro models of multiple sclerosis: EAE and beyond. Mult Scler Relat Disord. 2012;1:15–28. doi: 10.1016/j.msard.2011.09.002. [DOI] [PubMed] [Google Scholar]
- Kumar S, Patel R, Moore S, Crawford DK, Suwanna N, Mangiardi M, Tiwari-Woodruff SK. Estrogen receptor beta ligand therapy activates PI3K/Akt/mTOR signaling in oligodendrocytes and promotes remyelination in a mouse model of multiple sclerosis. Neurobiology of disease. 2013;56:131–44. doi: 10.1016/j.nbd.2013.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurtzke JF. Historical and clinical perspectives of the expanded disability status scale. Neuroepidemiology. 2008;31:1–9. doi: 10.1159/000136645. [DOI] [PubMed] [Google Scholar]
- Lassmann H. Axonal and neuronal pathology in multiple sclerosis: What have we learnt from animal models. Exp Neurol. 2009 doi: 10.1016/j.expneurol.2009.10.009. [DOI] [PubMed] [Google Scholar]
- Lassmann H. Experimental models of multiple sclerosis. Rev Neurol (Paris) 2007a;163:651–5. doi: 10.1016/s0035-3787(07)90474-9. [DOI] [PubMed] [Google Scholar]
- Lassmann H. Multiple sclerosis: is there neurodegeneration independent from inflammation? J Neurol Sci. 2007b;259:3–6. doi: 10.1016/j.jns.2006.08.016. [DOI] [PubMed] [Google Scholar]
- Lélu K, Delpy L, Robert V, Foulon E, Laffont S, Pelletier L, Engelhardt B, Guéry JC. Endogenous estrogens, through estrogen receptor α, constrain autoimmune inflammation in female mice by limiting CD4+ T-cell homing into the CNS. Eur J Immunol. 2010;40:3489–98. doi: 10.1002/eji.201040678. [DOI] [PubMed] [Google Scholar]
- Lo AC, Saab CY, Black JA, Waxman SG. Phenytoin protects spinal cord axons and preserves axonal conduction and neurological function in a model of neuroinflammation in vivo. J Neurophysiol. 2003;90:3566–71. doi: 10.1152/jn.00434.2003. [DOI] [PubMed] [Google Scholar]
- Lucchinetti C, Bruck W. The pathology of primary progressive multiple sclerosis. Mult Scler. 2004;10(Suppl 1):S23–30. doi: 10.1191/1352458504ms1027oa. [DOI] [PubMed] [Google Scholar]
- MacKenzie-Graham A, Tiwari-Woodruff SK, Sharma G, Aguilar C, Vo KT, Strickland LV, Morales L, Fubara B, Martin M, Jacobs RE, Johnson GA, Toga AW, Voskuhl RR. Purkinje cell loss in experimental autoimmune encephalomyelitis. Neuroimage. 2009;48:637–51. doi: 10.1016/j.neuroimage.2009.06.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahad DH, Trapp BD, Lassmann H. Pathological mechanisms in progressive multiple sclerosis. Lancet Neurol. 2015;14:183–93. doi: 10.1016/S1474-4422(14)70256-X. [DOI] [PubMed] [Google Scholar]
- Mangiardi M, Crawford DK, Xia X, Du S, Simon-Freeman R, Voskuhl RR, Tiwari-Woodruff SK. An animal model of cortical and callosal pathology in multiple sclerosis. Brain Pathol. 2011;21:263–78. doi: 10.1111/j.1750-3639.2010.00444.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin BN, Wang C, Zhang CJ, Kang Z, Gulen MF, Zepp JA, Zhao J, Bian G, Do JS, Min B, Pavicic PG, Jr, El-Sanadi C, Fox PL, Akitsu A, Iwakura Y, Sarkar A, Wewers MD, Kaiser WJ, Mocarski ES, Rothenberg ME, Hise AG, Dubyak GR, Ransohoff RM, Li X. T cell-intrinsic ASC critically promotes T(H)17-mediated experimental autoimmune encephalomyelitis. Nat Immunol. 2016;17:583–92. doi: 10.1038/ni.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy DP, Richards MH, Miller SD. Mouse models of multiple sclerosis: experimental autoimmune encephalomyelitis and theiler’s virus-induced demyelinating disease. Methods Mol. Biol. 2012;900:381–401. doi: 10.1007/978-1-60761-720-4_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McHugh ML. Multiple comparison analysis testing in ANOVA. Biochem Med (Zagreb) 2011;21:203–9. doi: 10.11613/bm.2011.029. [DOI] [PubMed] [Google Scholar]
- McRae BL, Kennedy MK, Tan LJ, Dal Canto MC, Picha KS, Miller SD. Induction of active and adoptive relapsing experimental autoimmune encephalomyelitis (EAE) using an encephalitogenic epitope of proteolipid protein. J. Neuroimmunol. 1992;38:229–40. doi: 10.1016/0165-5728(92)90016-e. [DOI] [PubMed] [Google Scholar]
- Mecha M, Carrillo-Salinas FJ, Mestre L, Feliu A, Guaza C. Viral models of multiple sclerosis: neurodegeneration and demyelination in mice infected with Theiler’s virus. Prog Neurobiol. 2012;101–102:46–64. doi: 10.1016/j.pneurobio.2012.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendel I, Kerlero de Rosbo N, Ben-Nun A. A myelin oligodendrocyte glycoprotein peptide induces typical chronic experimental autoimmune encephalomyelitis in H-2b mice: fine specificity and T cell receptor V beta expression of encephalitogenic T cells. European journal of immunology. 1995;25:1951–9. doi: 10.1002/eji.1830250723. [DOI] [PubMed] [Google Scholar]
- Miller DH, Khan OA, Sheremata WA, Blumhardt LD, Rice GP, Libonati MA, Willmer-Hulme AJ, Dalton CM, Miszkiel KA, O’Connor PW, Group INMST. A controlled trial of natalizumab for relapsing multiple sclerosis. N. Engl. J. Med. 2003;348:15–23. doi: 10.1056/NEJMoa020696. [DOI] [PubMed] [Google Scholar]
- Moore S, Hannsun G, Yoon J, Patel R, Yoo T, Khalaj A, Tiwari-Woodruff S. Therapeutic Laquinimod Treatment Restores Axon Myelination, Callosal Conduction Motor Deficit in a Chronic Mouse Model of multiple Sclerosis (P05197) Neurology. 2013a;80 doi: 10.1002/brb3.174. P05. 197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore S, Khalaj AJ, Patel R, Yoon J, Ichwan D, Hayardeny L, Tiwari-Woodruff SK. Restoration of axon conduction and motor deficits by therapeutic treatment with glatiramer acetate. Journal of neuroscience research. 2014a doi: 10.1002/jnr.23440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore S, Khalaj AJ, Yoon J, Patel R, Hannsun G, Yoo T, Sasidhar M, Martinez-Torres L, Hayardeny L, Tiwari-Woodruff SK. Therapeutic laquinimod treatment decreases inflammation, initiates axon remyelination, and improves motor deficit in a mouse model of multiple sclerosis. Brain and behavior. 2013b;3:664–82. doi: 10.1002/brb3.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore SM, Khalaj AJ, Kumar S, Winchester Z, Yoon J, Yoo T, Martinez-Torres L, Yasui N, Katzenellenbogen JA, Tiwari-Woodruff SK. Multiple functional therapeutic effects of the estrogen receptor β agonist indazole-Cl in a mouse model of multiple sclerosis. Proc Natl Acad Sci U S A. 2014b;111:18061–6. doi: 10.1073/pnas.1411294111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochoa-Reparaz J, Mielcarz DW, Wang Y, Begum-Haque S, Dasgupta S, Kasper DL, Kasper LH. A polysaccharide from the human commensal Bacteroides fragilis protects against CNS demyelinating disease. Mucosal immunology. 2010;3:487–95. doi: 10.1038/mi.2010.29. [DOI] [PubMed] [Google Scholar]
- Okuda Y, Okuda M, Bernard CC. Gender does not influence the susceptibility of C57BL/6 mice to develop chronic experimental autoimmune encephalomyelitis induced by myelin oligodendrocyte glycoprotein. Immunol Lett. 2002;81:25–9. doi: 10.1016/s0165-2478(01)00339-x. [DOI] [PubMed] [Google Scholar]
- Papenfuss TL, Rogers CJ, Gienapp I, Yurrita M, McClain M, Damico N, Valo J, Song F, Whitacre CC. Sex differences in experimental autoimmune encephalomyelitis in multiple murine strains. J Neuroimmunol. 2004;150:59–69. doi: 10.1016/j.jneuroim.2004.01.018. [DOI] [PubMed] [Google Scholar]
- Pettinelli CB, McFarlin DE. Adoptive transfer of experimental allergic encephalomyelitis in SJL/J mice after in vitro activation of lymph node cells by myelin basic protein: requirement for Lyt 1+ 2- T lymphocytes. Journal of immunology. 1981;127:1420–3. [PubMed] [Google Scholar]
- Rivers TM, Sprunt DH, Berry GP. Observations on Attempts to Produce Acute Disseminated Encephalomyelitis in Monkeys. J Exp Med. 1933;58:39–53. doi: 10.1084/jem.58.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson AP, Harp CT, Noronha A, Miller SD. The experimental autoimmune encephalomyelitis (EAE) model of MS: utility for understanding disease pathophysiology and treatment. Handb Clin Neurol. 2014;122:173–89. doi: 10.1016/B978-0-444-52001-2.00008-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez M. Mechanisms of virus-induced demyelination and remyelination. Ann N Y Acad Sci. 1988;540:240–51. doi: 10.1111/j.1749-6632.1988.tb27066.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothhammer V, Heink S, Petermann F, Srivastava R, Claussen MC, Hemmer B, Korn T. Th17 lymphocytes traffic to the central nervous system independently of alpha4 integrin expression during EAE. J Exp Med. 2011;208:2465–76. doi: 10.1084/jem.20110434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saijo K, Collier JG, Li AC, Katzenellenbogen JA, Glass CK. An ADIOL-ERbeta-CtBP transrepression pathway negatively regulates microglia-mediated inflammation. Cell. 2011;145:584–95. doi: 10.1016/j.cell.2011.03.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shams PN, Plant GT. Optic neuritis: a review. Int MS J. 2009;16:82–9. [PubMed] [Google Scholar]
- Smith-Bouvier DL, Divekar AA, Sasidhar M, Du S, Tiwari-Woodruff SK, King JK, Arnold AP, Singh RR, Voskuhl RR. A role for sex chromosome complement in the female bias in autoimmune disease. The Journal of experimental medicine. 2008;205:1099–108. doi: 10.1084/jem.20070850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith D, Du S, Tiwari-Woodruff S, Arnold AP, King JK, Singh RR, Voskuhl RR. The XX Sex Chromosome Complement, as compared to the XY, Confers Greater Susceptibility to Experimental Autoimmune Diseases—EAE and SLE. Clinical Immunology. 2007;123:S178–S9. [Google Scholar]
- Sorensen O, Perry D, Dales S. In vivo and in vitro models of demyelinating diseases III. JHM virus infection of rats. Arch Neurol. 1980;37:478–84. doi: 10.1001/archneur.1980.00500570026003. [DOI] [PubMed] [Google Scholar]
- Spence RD, Wisdom AJ, Cao Y, Hill HM, Mongerson CR, Stapornkul B, Itoh N, Sofroniew MV, Voskuhl RR. Estrogen Mediates Neuroprotection and Anti-Inflammatory Effects during EAE through ERalpha Signaling on Astrocytes But Not through ERbeta Signaling on Astrocytes or Neurons. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2013;33:10924–33. doi: 10.1523/JNEUROSCI.0886-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sternberger NH, McFarlin DE, Traugott U, Raine CS. Myelin basic protein and myelin-associated glycoprotein in chronic, relapsing experimental allergic encephalomyelitis. J Neuroimmunol. 1984;6:217–29. doi: 10.1016/0165-5728(84)90010-9. [DOI] [PubMed] [Google Scholar]
- Stromnes IM, Goverman JM. Active induction of experimental allergic encephalomyelitis. Nat. Protoc. 2006a;1:1810–9. doi: 10.1038/nprot.2006.285. [DOI] [PubMed] [Google Scholar]
- Stromnes IM, Goverman JM. Passive induction of experimental allergic encephalomyelitis. Nature protocols. 2006b;1:1952–60. doi: 10.1038/nprot.2006.284. [DOI] [PubMed] [Google Scholar]
- Su KG, Savino C, Marracci G, Chaudhary P, Yu X, Morris B, Galipeau D, Giorgio M, Forte M, Bourdette D. Genetic inactivation of the p66 isoform of ShcA is neuroprotective in a murine model of multiple sclerosis. Eur J Neurosci. 2012;35:562–71. doi: 10.1111/j.1460-9568.2011.07972.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun D, Wekerle H. Ia-restricted encephalitogenic T lymphocytes mediating EAE lyse autoantigen-presenting astrocytes. Nature. 1986;320:70–2. doi: 10.1038/320070a0. [DOI] [PubMed] [Google Scholar]
- Teitelbaum D, Arnon R, Sela M. Immunomodulation of experimental autoimmune encephalomyelitis by oral administration of copolymer 1. Proc Natl Acad Sci U S A. 1999;96:3842–7. doi: 10.1073/pnas.96.7.3842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiwari-Woodruff S, Morales LB, Lee R, Voskuhl RR. Differential neuroprotective and antiinflammatory effects of estrogen receptor (ER)alpha and ERbeta ligand treatment. P Natl Acad Sci USA. 2007;104:14813–8. doi: 10.1073/pnas.0703783104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tompkins SM, Fuller KG, Miller SD. Theiler’s virus-mediated autoimmunity: local presentation of CNS antigens and epitope spreading. Annals of the New York Academy of Sciences. 2002;958:26–38. [PubMed] [Google Scholar]
- Trapp BD, Bo L, Mork S, Chang A. Pathogenesis of tissue injury in MS lesions. J Neuroimmunol. 1999;98:49–56. doi: 10.1016/s0165-5728(99)00081-8. [DOI] [PubMed] [Google Scholar]
- Tseveleki V, Bauer J, Taoufik E, Ruan C, Leondiadis L, Haralambous S, Lassmann H, Probert L. Cellular FLIP (long isoform) overexpression in T cells drives Th2 effector responses and promotes immunoregulation in experimental autoimmune encephalomyelitis. Journal of immunology. 2004;173:6619–26. doi: 10.4049/jimmunol.173.11.6619. [DOI] [PubMed] [Google Scholar]
- van Oosten BW, Lai M, Hodgkinson S, Barkhof F, Miller DH, Moseley IF, Thompson AJ, Rudge P, McDougall A, McLeod JG, Adèr HJ, Polman CH. Treatment of multiple sclerosis with the monoclonal anti-CD4 antibody cM-T412: results of a randomized, double-blind, placebo-controlled, MR-monitored phase II trial. Neurology. 1997;49:351–7. doi: 10.1212/wnl.49.2.351. [DOI] [PubMed] [Google Scholar]
- Veldhoen M, Hirota K, Westendorf AM, Buer J, Dumoutier L, Renauld JC, Stockinger B. The aryl hydrocarbon receptor links Th17-cell-mediated autoimmunity to environmental toxins. Nature. 2008;454:106–9. doi: 10.1038/nature06881. [DOI] [PubMed] [Google Scholar]
- Wekerle H, Kojima K, Lannes-Vieira J, Lassmann H, Linington C. Animal models. Annals of neurology. 1994;36(Suppl):S47–53. doi: 10.1002/ana.410360714. [DOI] [PubMed] [Google Scholar]
- Wisdom AJ, Cao Y, Itoh N, Spence RD, Voskuhl RR. Estrogen receptor-β ligand treatment after disease onset is neuroprotective in the multiple sclerosis model. J Neurosci Res. 2013;91:901–8. doi: 10.1002/jnr.23219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolinsky JS, Narayana PA, Noseworthy JH, Lublin FD, Whitaker JN, Linde A, Gjörstrup P, Sullivan HC. Linomide in relapsing and secondary progressive MS: part II: MRI results. MRI Analysis Center of the University of Texas-Houston, Health Science Center, and the North American Linomide Investigators. Neurology. 2000;54:1726–33. doi: 10.1212/wnl.54.9.1734. [DOI] [PubMed] [Google Scholar]
- Woodruff RH, Franklin RJ. The expression of myelin protein mRNAs during remyelination of lysolecithin-induced demyelination. Neuropathol Appl Neurobiol. 1999;25:226–35. doi: 10.1046/j.1365-2990.1999.00172.x. [DOI] [PubMed] [Google Scholar]
- Yednock TA, Cannon C, Fritz LC, Sanchez-Madrid F, Steinman L, Karin N. Prevention of experimental autoimmune encephalomyelitis by antibodies against alpha 4 beta 1 integrin. Nature. 1992;356:63–6. doi: 10.1038/356063a0. [DOI] [PubMed] [Google Scholar]
- Yu Z, Sun D, Feng J, Tan W, Fang X, Zhao M, Zhao X, Pu Y, Huang A, Xiang Z, Cao L, He C. MSX3 Switches Microglia Polarization and Protects from Inflammation-Induced Demyelination. J Neurosci. 2015;35:6350–65. doi: 10.1523/JNEUROSCI.2468-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Ray A, Miller NM, Hartwig D, Pritchard KA, Jr, Dittel BN. Inhibition of Myeloperoxidase at the Peak of Experimental Autoimmune Encephalomyelitis Restores Blood-Brain-Barrier Integrity and Ameliorates Disease Severity. J Neurochem. 2015 doi: 10.1111/jnc.13426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziehn MO, Avedisian AA, Tiwari-Woodruff S, Voskuhl RR. Hippocampal CA1 atrophy and synaptic loss during experimental autoimmune encephalomyelitis, EAE. Lab Invest. 2010;90:774–86. doi: 10.1038/labinvest.2010.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.