Abstract
Reduction in metabolic rate and body temperature is a common strategy for small endotherms to save energy. The daily reduction in metabolic rate and heterothermy, or torpor, is particularly pronounced in regions with a large variation in daily ambient temperature. This applies most strongly in temperate bat species (order Chiroptera), but it is less clear how tropical bats save energy if ambient temperatures remain high. However, many subtropical and tropical species use some daily heterothermy on cool days. We recorded the heart rate and the body temperature of free-ranging Pallas' mastiff bats (Molossus molossus) in Gamboa, Panamá, and showed that these individuals have low field metabolic rates across a wide range of body temperatures that conform to high ambient temperature. Importantly, low metabolic rates in controlled respirometry trials were best predicted by heart rate, and not body temperature. Molossus molossus enter torpor-like states characterized by low metabolic rate and heart rates at body temperatures of 32°C, and thermoconform across a range of temperatures. Flexible metabolic strategies may be far more common in tropical endotherms than currently known.
Keywords: heterothermy, endothermy, body temperature, energetics, flight, tropics
1. Introduction
Maintaining body temperature (Tb) is energetically costly, particularly when ambient temperatures (Ta) are cold and food resources scarce [1]. This problem would appear to be minimal in the tropics, where Ta is generally high. However, there can be strong resource ephemerality in tropical systems, making energy conservation a priority [2–4]. As seasonality becomes more intense with climate change [5], understanding individual-level physiological mechanisms and energetic limitations will be essential to understand population-level adaptation [6]. This is particularly true for tropical species, which may be limited in their energetic flexibility due to current adaptation to high Ta [7–11].
One physiological strategy to minimize energetic expenditure is the controlled reduction of the metabolic rate (MR) and a subsequent reduction in body temperature (Tb). Often described as daily heterothermy or torpor [1,12,13], this phenomenon is widespread among mammals and birds, and is typically perceived as a response to mitigate low food availability and low Ta [13]. It is particularly pronounced in the large and diverse order of bats (Chiroptera), which need effective energy-saving strategies due to their small size, high metabolic requirements, and loss of large amounts of heat and water through large, naked wing membranes [14]. Although most common in temperate regions, subtropical and tropical species of bats also enter torpor and many species exhibit heterothermy when Ta falls below a threshold (e.g. 24°C: [15–17]) or at night [18,19]. However, desert-dwelling species and others in hot climates may enter torpor at Ta greater than 30°C [18,20]. The high Tas at which these bats begin to thermoconform approach the normal homoeothermic body temperature of most mammals (ca 36–38°C). In much of the lowland tropics, Ta remains high throughout the year despite large variation in rainfall and subsequent food availability, and this low variation in Ta could make measuring low energy states via Tb a challenge.
These small differences in Ta and Tb may mask that tropical mammals are more metabolically labile than we have been able to appreciate [21,22]. The estimates of energetic expenditure that skin or Tb give may not indicate reduced metabolic states [1,7], and it is increasingly apparent that Tb alone is not wholly representative of the energetic expenditure of tropical animals [7,11,18,22–25]. We need additional ways to measure how tropical mammals minimize energetic expenditure in environments with high Ta, such as the tropics. One such measure would be variation in heart rate (fH) and delivery of oxygen throughout the body. Adjustment of heart rate at high Ta would allow individuals to enter low metabolic states at high Tb, thus saving energy while staying alert and avoiding predation [26]. In addition, mostly due to methodological restrictions, most work has focused on measuring physiological responses to controlled variation in Ta [16] instead of under natural conditions, which might not reveal the full range of animals' physiological capacity [22,27–29].
Heart rate provides a measure of energy expenditure independent of Tb measures and can help clarify the energetic strategies used by animals in areas with low variation in Ta [30–32]. Because fH is proportional to oxygen consumption, and therefore the MR, it is a more direct measure of instantaneous individual energy consumption [32–34]. Reduction in fH is one of the first measurable aspects of a torpid state, with the metabolic rate falling at the same rate as fH. As Tb is a consequence of the MR, it subsequently falls at a slower rate to a controlled set point [35–37]. This lagging relationship between fH and Tb in torpor provides the opportunity for independence of these two aspects of metabolism that can be exploited by tropical animals at high Ta [36,38]. Reduced MR and torpor at high Tb in hibernating subtropical marsupials, lemurs and arid-adapted golden spiny mice and bats [12,18,25,39,40], all indicate that fH may be a better predictor of low energy states than Tb or the difference Tb − Ta (Tdiff), particularly when Tdiff is small, such as in the tropics.
Our goal was to test if Pallas' mastiff bats (Molossus molossus) enter a state of low MR, measured through fH, even though there is little room for its Tb to be depressed in a typical torpor state. In captivity M. molossus modulate their body temperature from ca 28°C during the day (2°C higher than Ta) to 34–35°C during the night when not flying [41], but there are no measures of field Tb. In our field site in Gamboa, Panamá, these 10–12 g bats forage in social groups to maximize the probability of locating ephemeral insect swarms in open air. They forage for 30–40 min per night, but return to their roosts 20% heavier [42–44]. In addition to this short window of food availability, inclement weather can disperse insect clouds and prevent animals from flying. This makes M. molossus susceptible to unpredictable food shortages and the use of low energy states (torpor) may be particularly advantageous in these scenarios. Molossus molossus spend 23 h in their roost with fH as low as 40–50 bpm and resting rates of 156 ± 71 bpm at night [43]. These resting rates are 50% lower than expected and indicate that the bats may employ torpor at high Ta, and presumed high Tb.
We hypothesized that M. molossus would thermoconform at high Ta, with similar low MRs across a wide range of Ta. Subsequently, low fH will be used by M. molossus across a wide range of Ta. We established relationships among fH, Tb, Ta and energy consumption in captive individuals, and then applied these relationships to free-ranging bats roosting in their natural social groups.
2. Material and methods
2.1. Animal capture and marking
We captured 11 M. molossus (10.5 ± 0.7 g) with mist nets as they emerged from their roosts in holes and crevices underneath houses in Gamboa, Panamá. We marked each bat with a subcutaneous temperature-sensitive PIT-tag (BioThermo13, Biomark Inc., Boise ID, USA) [26,41] and fitted it with an external heart rate transmitter (ca 0.8 g; SP2000 HR Sparrow Systems, Fisher, IL USA) that emits a continuous long-wave signal, interrupted by cardiac muscle potentials [34,45]. To attach the heart rate transmitter, we trimmed the fur in the middle of the back below the shoulder blades, applied a topical analgesic (Xylocaine gel, Astra Zeneca, Wedel Germany), and disinfected the skin and electrodes with 70% EtOH [43]. We inserted the transmitter's two disinfected gold electrodes ca 3 mm dorsally through a puncture made with a 23 ga sterile needle. The transmitters were mounted on thin, flexible cloth and then glued over the electrode insertion points using a silicone-based skin adhesive (Sauer Hautkleber, Manfred Sauer, Germany). The electrodes were flexible and did not appear to disturb the animals. Transmitters represented 7.0 ± 0.7% (s.d.) of body mass [46]. We removed transmitters immediately after respirometry experiments or after 2–3 days of deployment on free-ranging bats. We saw no signs of infection at the lead insertion sites, and fH and Tb records did not indicate elevation in the MR consistent with an immune challenge. Bats either maintained body mass or gained up to 1 g of mass (0.6 ± 0.3 g), revealing no measurable negative impact of the short-term deployment of the additional mass of the transmitter.
2.2. Laboratory measurements of heart rate, body temperature and metabolic rate
We used an open-flow, push-through respirometry system [47] to measure rates of oxygen consumption (), carbon dioxide production () and Tb of six bats for 10–20 h continuously [48]. Owing to logistical constraints, these were not the same bats that were tracked in the field. We dried incurrent air (greater than 75% relative humidity, approx. 26°C) with Drierite (WH Hammond Drierite Co, Ltd, Xenia, OH, USA) and pumped it through a mass flow controller (FB8, Sable Systems International, Las Vegas, NV, USA) into a 1.97 l respirometry chamber fitted with a thermocouple within a 20 l insulated cooler that was dark and temperature-controlled (PELT5, Sable Systems). An additional empty chamber served as a reference (baseline) to the animal chamber. The flow rate was 300 ml min−1 and relative humidity and vapour production were measured with an RH-300 (Sable Systems). After drying the excurrent air again with Drierite, we measured CO2 concentration (FOXBOX, Sable Systems), and after scrubbing the air of CO2 with Ascarite (Thomas Scientific, Swedesboro NJ, USA), we determined O2 concentrations (FOXBOX, Sable Systems). Chamber temperature, CO2, O2 and relative humidity were recorded at 1 Hz directly with Expedata via the UI-2 data acquisition interface (Sable Systems). Bats had the option to roost on vertical or horizontal mesh platforms above a layer of mineral oil used to trap excrement. We kept the bats at 28 and 32°C for at least two hours at a time, which is equal to or lower than the lower critical threshold of the thermoneutral zone (TNZ) for this species [49]. We measured bat Tb via PIT tag (BioThermo13, Biomark, Inc.) every minute using an antenna (HPR Plus, Biomark) in the insulated chamber [41], and we recorded fH as a sound file (see below). PIT tag calibrations against a thermometer traceable to the U.S. National Bureau of Standards showed a mean measurement error of 0.21 ± 0.2°C. fH was averaged over the 1 min preceding each Tb measurement. This gave five Tb and fH measures for each measurement of and . We used carbon dioxide () production to estimate metabolic rates using Equation 10.5 from Lighton [50]: , where FiCO2 is the incurrent CO2 content, FeCO2 is the excurrent CO2 content, FR is the flow rate and RER is the respiratory exchange ratio (:), which we calculated to be 0.8 from empirical measurements of CO2 and O2. We converted (ml min−1) to metabolic rates [51,52] that would be comparable to field rates using the standard conversion of 25.0 J ml−1 CO2. After conclusion of the experiment, the heart rate transmitters were removed, bats were offered water via a transfer pipette and placed in the entrance to their roost.
2.3. Field heart rate and body temperature telemetry
We recorded fH and Tb of five bats that were not part of the respirometry experiments during 1–3 days and nights in their natural roosts. We used telemetry receivers (AR8000, AOR Ltd) connected to 3-element Yagi antennae (Sparrow Systems) to detect the signal of the heart rate transmitter. This was then recorded via the mini-dv output to a wave file on a digital recorder (Tascam DR-05). The maximum recording distance for Tb was 10 cm, and 100 m for fH transmitters; therefore, no flying data were recorded. Three bats (1646, 1721, 1732) roosted in the walls of a wooden house and had both fH and Tb sampled every 10 min. The heart rate was recorded continuously for two bats 2253 and 2289, and Tb was recorded once per minute. Bat 2253 roosted in the roof of a wooden structure under a metal roof and bat 2289 in the brick ground floor of a house. To temporally synchronize Tb and fH, a smoothed fH of the previous 60 s was matched to each Tb. Ambient temperature data were recorded at 15 min intervals by the Autoridad del Canal de Panamá (ACP) for Gamboa and provided by the Smithsonian Tropical Research Institute's Physical Monitoring Program. The daytime mean ambient temperature was 25.87 ± 1.21°C (mean daytime minimum to mean maximum: 23.38–28.24), and the mean nightly ambient temperature was 23.74 ± 0.50°C (mean nightly minimum to mean maximum: 22.74–24.78°C). The ACP monitoring station was located along the banks of the Panama Canal 400–800 m from the observation roosts. While these temperatures do not directly measure the more insulated and stable microhabitats the bats experience in their roosts, they show the potential Ta that bats experience across the day.
2.4. Heart rate scoring
The heart rate from radio transmitters has been visually scored at sampling intervals of 5–10 min [33,34,45,53]. However, complete sampling can show novel energy-saving strategies like those in tent-making bats that depress fH several times per hour [26], a pattern that would not have been detected by sampling every five to ten minutes. We therefore fully sampled the recorded data using an automated approach in R 3.3.2 [54] to identify and count heartbeats [26]. We applied a finite impulse response filter in seewave [55] with a window length of 1500–2000 samples to select the carrier frequency of the transmitter. We counted individual heartbeats by applying a timer function in seewave that ran over non-overlapping windows of 500 samples. This created a resolution of 88–96 sampling windows per second. We then applied a kernel density filter in KernSmooth [56] to further eliminate noise that was outside of the 90% quantile. This approach is conservative and may have eliminated some heart rate outliers, but the autocorrelated nature of heart rate allowed us to filter out errors probably induced by static or other interference in the recordings. Automated samples were visually inspected periodically to validate the filtering method, particularly when there was high variation.
We could not apply all the automated methods to the respirometry data due to large amounts of interference from the PIT tag reader within the respirometry chamber. Here, we hand-scored fH by counting all heartbeats within the first 10 s of every minute. Sampling at regular intervals may underestimate short-term changes in fH [26], but the consistent low fH of the bats throughout our captive and field experiments did not warrant finer-scale sampling. This was the same time resolution as Tb measures and provided five fH measures per unit of MR sampling. We then averaged these measures to create a single value for each 5 min respirometry sample.
2.5. Analysis
We tested the fit of heart rate (fH), body temperature (Tb), and the difference between body temperature and ambient temperature (Tdiff = Tb − Ta) on the metabolic rate (MR) using generalized linear mixed-effects models (GLMMs) with individual as a random intercept in lme4 after inspecting the data for normality and equal variance. We used both the field metabolic rate (FMR, kJ h−1) and mass-specific metabolic rates (W g−1) to facilitate broader comparisons with published data, particularly those collected in field experiments. We compared the minimum metabolic rates of M. molossus, identified as the lowest 10% quantile of the MR for each individual, to minimum torpor metabolic rates for species found across a variety of temperature regimes (temperate, subtropical and tropical) that undergo daily torpor from three speciose mammalian orders (Chiroptera, Dasyuromorphia and Rodentia; data from Ruf and Geiser [13]). A nested model approach revealed that all three of our predictors (fH, Tb, Tdiff) contributed significantly to explaining the variation in both measures of energy consumption (FMR and mass-specific MR). We therefore took a model selection approach to evaluate which factors were the most efficient at predicting energy consumption in respirometry. We calculated the Akaike information criterion corrected for small sample sizes (AICc) for each model, as well as the confidence intervals for each model parameter in lme4, and both the marginal (, fixed effects alone) and conditional (, full model) R2 values in MuMIn [57] using the approach outlined by Nakagawa & Schielzeth [58]. All analyses were performed in R 3.3.2 [54], and we present means ± s.d. for all variables unless otherwise noted.
3. Results
3.1. Respirometry, captive heart rate, body temperature and energy consumption
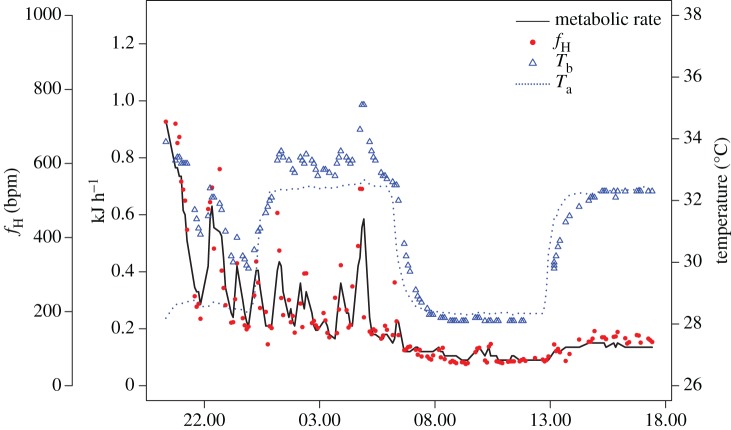
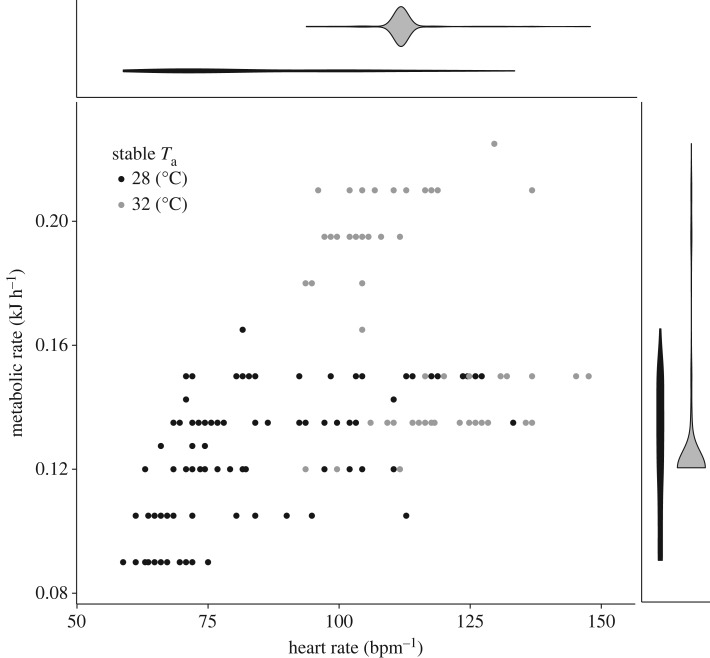
All bats reduced their MR and Tb in the respirometry chamber. We recorded a large range of MR (0.075–1.245 kJ h−1 or 0.0021–0.325 W g−1), fH (59–999 bpm) and Tb (27.9–37.6°C) across the 10–20 h of continuous sampling. In general, Tb followed Ta when the MR was reduced across the small range of Ta that we measured, and fH was not dependent on Tb (figure 1 and electronic supplementary material, figure S1). After physiological arousal generated by observers tapping on chamber walls, fH dropped rapidly into a low metabolic state, and Tb eventually followed at a slower rate. Bats showed a low MR of 0.1218 ± 0.021 (mean ± s.d.) kJ h−1 (0.00314 ± 0.00051 W g−1). When we further examined the stable MR at our two Ta, we found that metabolic rates were lower at 28°C (0.131 ± 0.014 s.e. kJ h−1) than at 32°C (0.160 ± 0.001 s.e. kJ h−1; , p < 0.001; figure 2). These lower MR at 28°C are consistent with lower fH at 28°C (82.4 ± 6.3 bpm) than at 32°C (115.8 ± 1.0 bpm; , p < 0.001; figure 2).
Figure 1.
Metabolic rate (black line), fH (red circles), Tb (blue triangles) and Ta (dotted line) of an exemplary M. molossus measured across 20 h in open-flow respirometry.
Figure 2.
Metabolic rate (kJ h−1) and heart rate (bpm) of bats during steady-state minimum measures at Ta of 28 and 32°C during respirometry experiments. Violin plots show the distribution and density of heart rate (top) and metabolic rate (right) at each temperature. Note that all heart rates remained below 150 bpm.
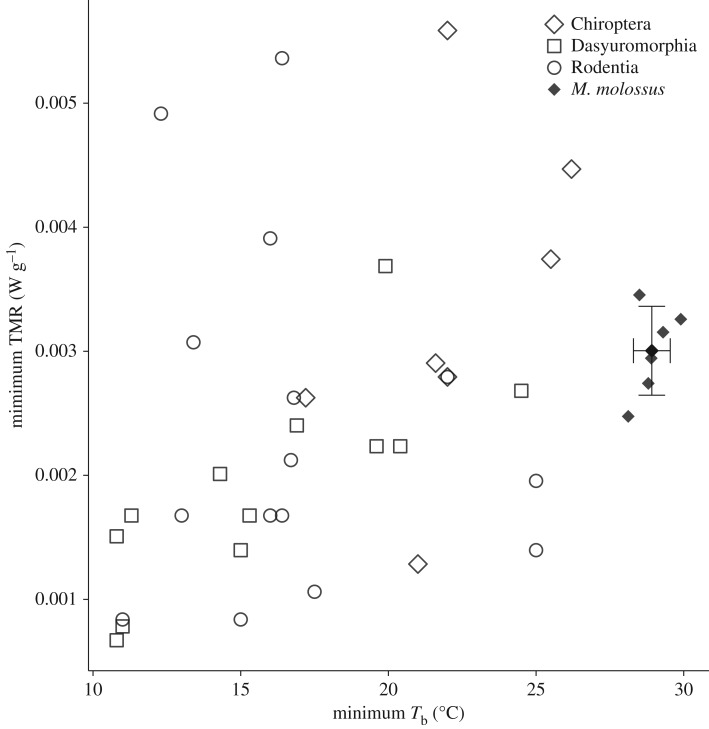
Molossus molossus generally used low mass-specific MR in the respirometry chamber. Bats showed a low minimum MR of 0.1138 ± 0.0167 kJ h−1 (0.00294 ± 0.00034 W g−1) and this did not differ from the minimum metabolic rates for other mammalian orders that use daily heterothermy (, p = 0.154; figure 3). Molossus molossus enter low metabolic states at substantially higher Tb than these other mammals (, p < 0.001; figure 3).
Figure 3.
Minimum torpor metabolic rates (TMRs) and minimum Tb for Chiroptera, Dasyuromorphia and Rodentia that use daily heterothermy (adapted from Ruf & Geiser [13]), and the mean 10% quantile values (± s.d.) for individual M. molossus. Molossus molossus use the same low range of torpor metabolic rates at higher Tb as other mammals that use daily heterothermy.
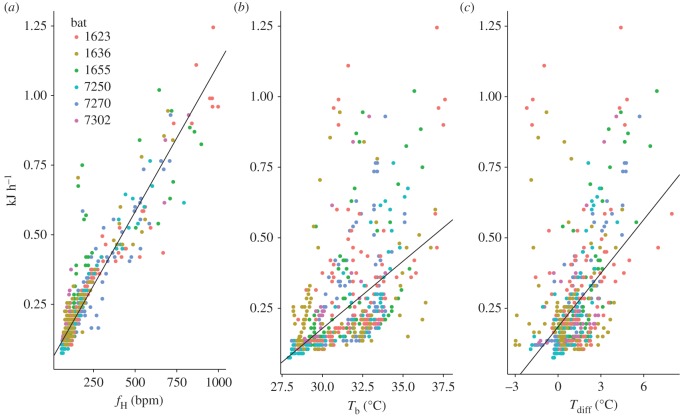
All three predictor variables (fH, Tb, Tdiff) explained substantial variation in the MR, but fH alone was the best model regardless of any temperature interaction (figure 4; electronic supplementary material, table S1). All models that included fH had low AICc values and high R2. Models including either Tb or Tdiff resulted in better fits, but the increase in model fit provided by adding in a temperature measure came with a high penalty of AICc. The wide range of Tb at any given MR and between the two temperature regimes illustrates the low predictive ability of both Tb and Tdiff (figures 2 and 4). The best-fit model predicted energetic expenditure as daily energetic expenditure (kJ d−1) = 0.00106fH + 0.0527 (). Ta had minor effects on fH, showing a small but significant increase in fH with rising Ta (, p < 0.001; fH = 7.41 × Ta − 115.67; ; electronic supplementary material, figure S2 and table S2). There was a stronger relationship between Tb and fH (, p < 0.001; fH = 14.985 × Tb − 353.237; ; electronic supplementary material, table S2).
Figure 4.
The relationship between M. molossus metabolic rate (kJ h−1) and (a) fH, (b) Tb and (c) Tdiff. In all models evaluated, fH has the best predictive fit for energy consumption (electronic supplementary material, table S1).
3.2. Free-ranging heart rate and body-temperature telemetry
The mean fH of free-ranging M. molossus in their roosts were generally low across the 24 h period but were in the range of 58–1068 bpm. Heart rate varied most in the early evening period when bats returned from foraging or were returned to their roost after instrumentation (figure 5). The heart rate was elevated to over 1000 bpm during those times. Mean roosting fH were below 200 bpm for all periods of the day and night (147 ± 57 bpm) and remained in stable low-level states (electronic supplementary material, figure S3).
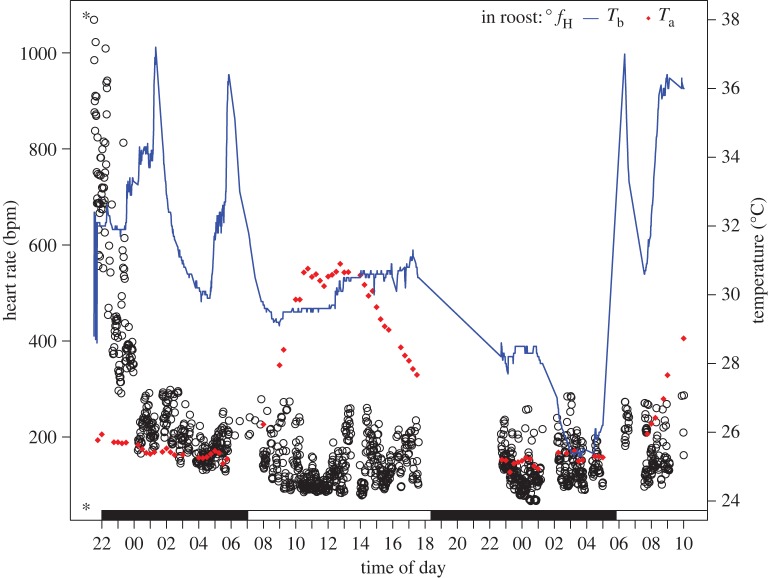
Figure 5.
In-roost fH (open circles), Tb (blue line) and Ta (red diamonds) measured for bat 2289 across a 36 h period. The asterisk (*) indicates where the bat was released back to its roost. Missing fH and Tb data coincide with the bat's foraging period and equipment adjustment. Scotophase is indicated by the filled bar along the bottom.
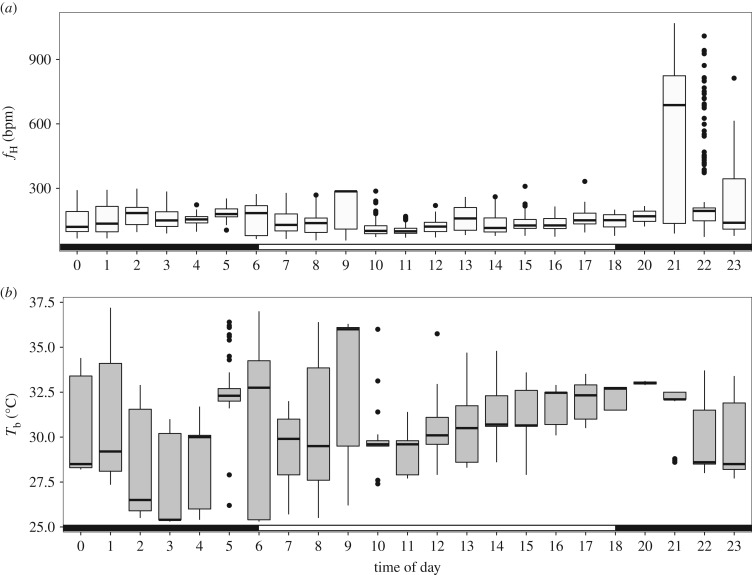
Tb of roosting bats were in the range of 25.3–37.2°C and varied both within an hour and across the day (figure 6). Tb increased with daily Ta (, p < 0.001; slope = 0.481, ). fH in the lowest 10% of observed values (i.e. lower than 90 bpm) were observed in all hours of the day except 2, 5, 19 and 20 h. These low rates were observed at Tb up to 33°C, and fH of 100 bpm was observed at up to 36°C (electronic supplementary material, figure S3).
Figure 6.
Hourly means of fH (a) and Tb (b) simultaneously measured from bats in their natural roosts. Scotophase is indicated by the filled bar along the bottom of each panel.
Our in-roost Tb measures indicated that M. molossus used heterothermy, with minimum Tb occurring during 02.00–04.00 in the coolest portion of the night. As daily Ta increased during the diurnal period, Tb became less variable, with individuals maintaining a constant Tb that was close to Ta. When individual variation was accounted for, Tb was mildly related to fH (, p < 0.001; slope = 10.12 ± 0.37 (s.e.m.); ; electronic supplementary material, figure S3). The Ta and Tb differential was also related to fH (, p < 0.001; slope = 6.97 ± 1.05, ) across a range of Tdiff (fH ∼ Tb AIC = 21 720, N = 2210; fH ∼ Tdiff AIC = 2230, N = 224; electronic supplementary material, table S2 and figure S3).
Tdiff explained 34% of the variation in fH when inter-individual variation was accounted for via random effects in our models. This is best illustrated by individual daily variation in these variables (figure 5). In the example we illustrate, the bat roosted between a wooden ceiling and a metal roof approximately 2 m above the ground. We could not measure the interior temperature of the roost, but Tb measured through the PIT tag reader ranged from 25.3 to 37.2°C and heart rate was generally low.
4. Discussion
We found that Molossus molossus entered low energy states independent of Ta, which results in substantial energy savings across much of the Ta they experienced. In respirometry chambers, fH was the best predictor of metabolic rate. These results extended to free-ranging M. molossus that used low fH across nearly the full range of Tb measured. Minimizing energy expenditure in tropical settings with low variation in high Ta may then be possible through modifications of heart rate and oxygen consumption independent of Tb. Such physiological adaptations are perhaps vital for tropical lowland endotherms because they free them from some of the energetic constraints of high Ta, but these effects remain understudied like many aspects of tropical ecosystems [59].
A range of mammals achieve low metabolic rates at relatively high Tb, described as potential ‘hyperthermic daily torpor’ by Lovegrove et al. [8]. For example, in gerbils (32–35°C) [40], fat-tailed dwarf lemurs (30°C) [25] and some desert dwelling bats (31–33°C, [18,19]), Tb thermoconforms at high Ta while the animals remain in low metabolic states, and the MR then increases with rising Ta. Bats appear to be particularly flexible in their thermal profiles, and some are even able to thermoconform to Ta > 45°C for extended periods of time, which would be lethal in many other mammals [19,60]. This may be related to the high Tb generated during flight [14], or to the high TNZ upper critical temperature (e.g. 38°C) found in many species [49], although the thermoneutral dynamics for most bat species are underexplored. In previous work on tropical and subtropical bats, the finding of a reduction in Tb associated with heterothermy is variable, and they only show heterothermy when Ta is below 20–25°C [29,61–64]. However, many of these species, particularly those studied in free-ranging field conditions, have only had Tb measured. It is possible that like M. molossus, they enter low energy states at relatively high Tb and maintain low heart rates across a wide range of Ta and Tb. Entering a low energy state at high Tb, or a Tb within the thermoneutral zone, would allow animals to lower their FMR without the costs of rewarming that are incurred as they leave torpid states [65], while taking advantage of the benefits of heterothermy with minimal costs [7,8]. Passive warming would be particularly advantageous for nocturnal animals that enter their active periods as Ta lowers and is less likely to provide much passive thermal support in their transition towards active states.
Our respirometry measures did not capture the full metabolic potential of M. molossus observed in the wild roosts. The Ta that we used in our respirometry measures did not extend far beyond the lower critical temperature (ca 30°C) of the thermoneutral zone reported for M. molossus [49], but energy consumption at these temperatures was far lower than the basal metabolic rate (BMR) reported for this species, and steady-state MRs were lower at 28°C than at 32°C, with a mean difference of 0.029 kJ h−1. The mean of the 30% quantile of respirometry MR (0.00314 ± 0.0005 W g−1) was only 39% of the previously measured BMR for M. molossus (0.008044 W g−1 [49]). We did not observe this value until the 90% quantile of our data, placing it among the highest MRs we recorded for our bats. The high temporal variability in metabolic rate indicates that bats did not employ a stable BMR in our respirometry measurements, despite extended measurement periods and Ta that should be within a neutral range for this species. Accurately measuring the BMR and the thermoneutral zone is difficult when species thermoconform across a wide range of Ta [7,66]. Our data show that measuring this type of energetic and thermal flexibility is important, and that we may find that this energetic flexibility is common in tropical bats.
Heart rate is an accurate measure of the metabolic rate during steady-state conditions and when transitioning between resting states. However, torpor with strong heterothermy is not just an extrapolation of a resting state as regressions of torpid bats would underestimate resting by up to 75% [67]. The low metabolic states in M. molossus do not show such a curvilinear relationship, with linear models providing the best fit to our respirometry data. We are careful not to infer a continuous linear relationship once animals are exercising because this probably underestimates the metabolic rate during exercise [30]. Through careful calibration and the use of nonlinear exponential estimates of energy consumption from fH based on body mass and heart mass, it may be possible to even more accurately estimate the energetic expenditure of exercising free-ranging animals.
The regular low metabolic states used by M. molossus in both respirometry and natural roosts may be an overall energetic conservation strategy to compensate for their ephemeral food resources. These small bats typically forage over water bodies for less than an hour per night on unpredictable, but rich patches of insects at dusk and dawn [42]. When nights are particularly windy or rainy, these bats tend to forgo foraging (DKN Dechmann, unpublished data), and there is a positive relationship between the duration of foraging and fH in the roost [43]. Furthermore, we observed Tb reductions across a wide range of Ta, with free-ranging bats using Tb of 25.3–37.2°C. Multi-day torpor bouts have not been observed in M. molossus and we did not attempt to measure this in our experiment. While M. molossus are capable of maintaining stable blood glucose levels for up to 48 h of fasting [68], total reduction of the MR through extended torpor-like states may allow them to cope with multiple nights of inclement weather. In some cases, Tb was lower than the Ta recorded at the Panama Canal (electronic supplementary material, figure S2). Bats then select cool, stable roost microhabitats regardless of their ability to maintain a low MR at high Tb. The use of torpor by tropical and subtropical mammals [13,16,22,25,69,70] illustrates the utility of reductions in metabolic rates and Tb during periods of low food availability. However, starvation risk is not the only driver of torpor, as well-fed bats will also use torpor to minimize time outside the roost [17]. These torpor and torpor-like states at high Tb may be particularly important to reduce the evaporative water loss incurred by bats via the large naked membrane of their wings [14], which can be reduced by 90% during torpid states [71,72]. The possibility of low energy states at relatively high Tb allows M. molossus to remain active and alert and move away from observers. This means that unlike the lethargic torpid bats with low Tb in the temperate zone, bats at higher Tb can escape from predators at any given time [71,73]. Daily torpor-like states in M. molossus allow them to minimize exposure to risks outside of their roost (such as predators and water loss), while maximizing their energy savings.
5. Conclusion
We suggest that the low energy state that we measure in M. molossus is torpor with shallow heterothermy. In other small mammals, minimum fH during daily torpor at low Tb is near 70 bpm [36,37,74–76]. This is similar to the minimum stable rates (58–75 bpm) of M. molossus both during respirometry and in their natural roosts, but much higher than the fH of 8 bpm for hibernating bats [67]. The metabolic rates used by our bats were well within the ranges reported for other mammalian orders that use daily heterothermy (figure 3), but occur at higher Tb. Bats evolved in the tropics and our findings in M. molossus are probably an example of the basal form of heterothermy that evolved near the root of the mammalian radiation [77]. The ability to maintain low metabolic rates and subsequently low Tb in deep torpor or hibernation would build upon this set of regulatory networks driven by cellular requirements for oxygen diffusion via fH, which reduced total metabolic rates while in thermoneutral conditions or above. Tropical heterothermy or torpor may allow individuals to flexibly adjust energetic expenditure to rapid environmental changes at fine timescales, as well as minimize energetic expenditure during pregnancy and lactation when Tb reductions may be constrained [7,17,64,78].
Low fH in response to lowered oxygen demands is an effective, flexible strategy. Work integrating energetic expenditure via fH in birds repeatedly shows lower energetic expenditures than would be predicted [33,34,45,79,80], indicating that a diversity of adaptations may be possible by manipulating one of the primary drivers of energy delivery to metabolism. The widespread nature of heterothermy or torpor-like states in tropical species leads to challenges when using traditional cut-off Tb or frequency distributions of Tb. Heart rate studies in tropical bats, including this study, have shown surprising metabolic strategies to cope with life in warm ambient temperatures [21,26]. This direct information on the flow of energy through an individual allows us further insight into the variability of energetic strategies in tropical systems, particularly as ambient conditions become more unpredictable.
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Acknowledgements
We would like to thank Rachel Page and the Gamboa Bat Lab, Marion Muturi, Lara Keicher, Inge Müller, Sebastian Stockmaier, Yann Gager, Bart Kranstauber and Anna Nele Herdina as well as the Smithsonian Tropical Research Institute and the Autoridad del Canal de Panamá for their support during this project. We also thank the residents of Gamboa for their patience and cooperation. Steve Paton (STRI) provided the environmental data for Gamboa.
Ethics
All methods were reviewed and approved by the Ministerio de Ambiente de Panamá (SE/A-68-13; SE/A 73-14; SE/A 16-5) and by the Animal Care and Use Committee of the Smithsonian Tropical Research Institute (2012.0505.2015; 2014-0701-2017).
Data accessibility
Data are available for download from Dryad http://dx.doi.org/10.5061/dryad.6620j [81].
Authors' contributions
M.T.O., S.R., M.W. and D.K.N.D. designed experiments. M.T.O., S.R. and D.K.N.D. collected field data. M.T.O., A.T. and H.S.P. analysed data. M.T.O. and D.K.N.D. interpreted the results and wrote the first draft of the manuscript. All authors revised the drafted manuscript and gave their final approval for the manuscript, and agree to be accountable for all aspects of the work.
Competing interests
The authors declare no competing interests.
Funding
Financial support came from the Deutsche Forschungsgemeinschaft (DE 1807/3-1 to D.K.N.D.), National Geographic Northern Explorers Fund (GEFNE124-14 to D.K.N.D., M.T.O., M.W.), the Deutscher Akademischer Austauschdienst (S.R.), the University of Illinois at Urbana-Champaign (H.S.P.), the Max Planck Society (M.T.O., D.K.N.D., A.T., M.W.) and the University of Konstanz (M.T.O.).
References
- 1.Geiser F, Ruf T. 1995. Hibernation versus daily torpor in mammals and birds: physiological variables and classification of torpor patterns. Physiol. Zool. 68, 935–966. (doi:10.1086/physzool.68.6.30163788) [Google Scholar]
- 2.Lovegrove BG. 2003. The influence of climate on the basal metabolic rate of small mammals: a slow-fast metabolic continuum. J. Comp. Physiol. B 173, 87–112. (doi:10.1007/s00360-002-0309-5) [DOI] [PubMed] [Google Scholar]
- 3.Withers PC, Cooper CE, Larcombe AN. 2006. Environmental correlates of physiological variables in marsupials. Physiol. Biochem. Zool. 79, 437–453. (doi:10.1086/501063) [DOI] [PubMed] [Google Scholar]
- 4.Wright S, Muller-Landau H, Schipper J. 2009. The future of tropical species on a warmer planet. Conserv. Biol. 23, 1418–1426. (doi:10.1111/j.1523-1739.2009.01337.x) [DOI] [PubMed] [Google Scholar]
- 5.Touchon JC. 2012. A treefrog with reproductive mode plasticity reveals a changing balance of selection for nonaquatic egg laying. Am. Nat. 180, 733–743. (doi:10.1086/668079) [DOI] [PubMed] [Google Scholar]
- 6.Ricklefs R, Wikelski M. 2002. The physiology/life-history nexus. Trends Ecol. Evol. 17, 462–468. (doi:10.1016/S0169-5347(02)02578-8) [Google Scholar]
- 7.Canale CI, Levesque DL, Lovegrove BG. 2012. Tropical heterothermy: does the exception prove the rule or force a re-definition? In Living in a seasonal world: thermoregulatory and metabolic adaptations (eds Ruf T, Bieber C, Arnold W, Millesi E), pp. 29–40. Berlin, Germany: Springer. [Google Scholar]
- 8.Lovegrove BG, Canale C, Levesque D, Fluch G, Rehakova-Petru M, Ruf T. 2014. Are tropical small mammals physiologically vulnerable to Arrhenius effects and climate change? Physiol. Biochem. Zool. 87, 30–45. (doi:10.1086/673313) [DOI] [PubMed] [Google Scholar]
- 9.Khaliq I, Hof C, Prinzinger R, Bohning-Gaese K, Pfenninger M. 2014. Global variation in thermal tolerances and vulnerability of endotherms to climate change. Proc. R. Soc. B 281, 20141097 (doi:10.1098/rspb.2014.1097) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huey RB, Kearney MR, Krockenberger A, Holtum JAM, Jess M, Williams SE. 2012. Predicting organismal vulnerability to climate warming: roles of behaviour, physiology and adaptation. Phil. Trans. R. Soc. B 367, 1665–1679. (doi:10.1098/rstb.2012.0005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Levesque DL, Menzies AK, Landry-Cuerrier M, Larocque G, Humphries MM. 2017. Embracing heterothermic diversity: non-stationary waveform analysis of temperature variation in endotherms. J. Comp. Physiol. B 187, 749–757. (doi:10.1007/s00360-017-1074-9) [DOI] [PubMed] [Google Scholar]
- 12.Geiser F. 2004. Metabolic rate and body temperature reduction during hibernation and daily torpor. Annu. Rev. Physiol. 66, 239–274. (doi:10.1146/annurev.physiol.66.032102.115105) [DOI] [PubMed] [Google Scholar]
- 13.Ruf T, Geiser F. 2015. Daily torpor and hibernation in birds and mammals. Biol. Rev. 90, 891–926. (doi:10.1111/brv.12137) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Speakman JR, Thomas DW. 2003. Physiological ecology and energetics of bats. In Bat ecology (eds Kunz T, Fenton M), pp. 430–490. Chicago, IL: University of Chicago Press. [Google Scholar]
- 15.Stawski C. 2012. Comparison of variables of torpor between populations of a hibernating subtropical/tropical bat at different latitudes. In Living in a seasonal world: thermoregulatory and metabolic adaptations (eds Ruf T, Bieber C, Arnold W, Millesi E), pp. 99–108. Berlin, Germany: Springer. [Google Scholar]
- 16.Geiser F, Stawski C. 2011. Hibernation and torpor in tropical and subtropical bats in relation to energetics, extinctions, and the evolution of endothermy. Integr. Comp. Biol. 51, 337–348. (doi:10.1093/icb/icr042) [DOI] [PubMed] [Google Scholar]
- 17.Stawski C, Geiser F. 2010. Fat and fed: frequent use of summer torpor in a subtropical bat. Naturwissenschaften 97, 29–35. (doi:10.1007/s00114-009-0606-x) [DOI] [PubMed] [Google Scholar]
- 18.Marom S, Korine C, Wojciechowski MS, Tracy CR, Pinshow B. 2006. Energy metabolism and evaporative water loss in the European free-tailed bat and Hemprich's long-eared bat (Microchiroptera): species sympatric in the Negev Desert. Physiol. Biochem. Zool. 79, 944–956. (doi:10.1086/505999) [DOI] [PubMed] [Google Scholar]
- 19.Maloney SK, Bronner GN, Buffenstein R. 1999. Thermoregulation in the Angolan free-tailed bat Mops condylurus: a small mammal that uses hot roosts. Physiol. Biochem. Zool. 72, 385–396. (doi:10.1086/316677) [DOI] [PubMed] [Google Scholar]
- 20.Bondarenco A, Kortner G, Geiser F. 2016. How to keep cool in a hot desert: torpor in two species of free-ranging bats in summer. Temperature 3, 476–483. (doi:10.1080/23328940.2016.1214334) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tomlinson S, Arnall SG, Munn A, Bradshaw SD, Maloney SK, Dixon KW, Didham RK. 2014. Applications and implications of ecological energetics. Trends Ecol. Evol. 29, 280–290. (doi:10.1016/j.tree.2014.03.003) [DOI] [PubMed] [Google Scholar]
- 22.Kobbe S, Nowack J, Dausmann KH. 2014. Torpor is not the only option: seasonal variations of the thermoneutral zone in a small primate. J. Comp. Physiol. B 184, 789–797. (doi:10.1007/s00360-014-0834-z) [DOI] [PubMed] [Google Scholar]
- 23.Gray DA, Marais M, Maloney SK. 2013. A review of the physiology of fever in birds. J. Comp. Physiol. B 183, 297–312. (doi:10.1007/s00360-012-0718-z) [DOI] [PubMed] [Google Scholar]
- 24.Boyles J, Smit B, McKechnie A. 2011. A new comparative metric for estimating heterothermy in endotherms. Physiol. Biochem. Zool. 84, 115–123. (doi:10.1086/656724) [DOI] [PubMed] [Google Scholar]
- 25.Dausmann KH, Glos J, Heldmaier G. 2009. Energetics of tropical hibernation. J. Comp. Physiol. B 179, 345–357. (doi:10.1007/s00360-008-0318-0) [DOI] [PubMed] [Google Scholar]
- 26.O'Mara MT, Wikelski M, Voigt CC, Ter Maat A, Pollock HS, Burness G, Desantis LM, Dechmann DK. 2017. Cyclic bouts of extreme bradycardia counteract the high metabolism of frugivorous bats. elife 6, e26686 (doi:10.7554/eLife.26686) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Geiser F, Holloway JC, Kortner G. 2007. Thermal biology, torpor and behaviour in sugar gliders: a laboratory-field comparison. J. Comp. Physiol. B 177, 495–501. (doi:10.1007/s00360-007-0147-6) [DOI] [PubMed] [Google Scholar]
- 28.Geiser F, Holloway JC, Körtner G, Maddocks TA, Turbill C, Brigham RM. 2000. Do patterns of torpor differ between free-ranging and captive mammals and birds? In Life in the cold (eds Heldmaier G, Klingenspor M), pp. 95–102. Berlin, Germany: Springer. [Google Scholar]
- 29.Audet D, Thomas D. 1997. Facultative hypothermia as a thermoregulatory strategy in the phyllostomid bats, Carollia perspicillata and Sturnira lilium. J. Comp. Physiol. B 167, 146–152. (doi:10.1007/s003600050058) [DOI] [PubMed] [Google Scholar]
- 30.Bishop C, Spivey R. 2013. Integration of exercise response and allometric scaling in endotherms. J. Theor. Biol. 323, 11–19. (doi:10.1016/j.jtbi.2013.01.002) [DOI] [PubMed] [Google Scholar]
- 31.Butler PJ, Green JA, Boyd I, Speakman J. 2004. Measuring metabolic rate in the field: the pros and cons of the doubly labelled water and heart rate methods. Funct. Ecol. 18, 168–183. (doi:10.1111/j.0269-8463.2004.00821.x) [Google Scholar]
- 32.Green JA. 2011. The heart rate method for estimating metabolic rate: review and recommendations. Comp. Biochem. Physiol. A 158, 287–304. (doi:10.1016/j.cbpa.2010.09.011) [DOI] [PubMed] [Google Scholar]
- 33.Barske J, Fusani L, Wikelski M. 2014. Energetics of the acrobatic courtship in male golden-collared manakins (Manacus vitellinus). Proc. R. Soc. B 281, 20132482 (doi:10.1098/rspb.2013.2482) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Steiger S, Kelley J, Cochran W, Wikelski M. 2009. Low metabolism and inactive lifestyle of a tropical rain forest bird investigated via heart-rate telemetry. Physiol. Biochem. Zool. 82, 580–589. (doi:10.1086/605336) [DOI] [PubMed] [Google Scholar]
- 35.Milsom WK, Zimmer MB, Harris MB. 1999. Regulation of cardiac rhythm in hibernating mammals. Comp. Biochem. Physiol. A 124, 383–391. (doi:10.1016/S1095-6433(99)00130-0) [DOI] [PubMed] [Google Scholar]
- 36.Elvert R, Heldmaier G. 2005. Cardiorespiratory and metabolic reactions during entrance into torpor in dormice, Glis glis. J. Exp. Biol. 208, 1373–1383. (doi:10.1242/jeb.01546) [DOI] [PubMed] [Google Scholar]
- 37.Swoap SJ, Gutilla MJ. 2009. Cardiovascular changes during daily torpor in the laboratory mouse. Am. J. Physiol. Regul. Integr. Comp. Physiol. 297, R769–R774. (doi:10.1152/ajpregu.00131.2009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Heldmaier G, Ruf T. 1992. Body temperature and metabolic rate during natural hypothermia in endotherms. J. Comp. Physiol. B 162, 696–706. (doi:10.1007/Bf00301619) [DOI] [PubMed] [Google Scholar]
- 39.Busse S, Lutter D, Heldmaier G, Jastroch M, Meyer CW. 2014. Torpor at high ambient temperature in a neotropical didelphid, the grey short-tailed opossum (Monodelphis domestica). Naturwissenschaften 101, 1003–1006. (doi:10.1007/s00114-014-1226-7) [DOI] [PubMed] [Google Scholar]
- 40.Grimpo K, Legler K, Heldmaier G, Exner C. 2013. That's hot: golden spiny mice display torpor even at high ambient temperatures. J. Comp. Physiol. B 183, 567–581. (doi:10.1007/s00360-012-0721-4) [DOI] [PubMed] [Google Scholar]
- 41.Stockmaier S, Dechmann DK, Page RA, O'Mara MT. 2015. No fever and leucocytosis in response to a lipopolysaccharide challenge in an insectivorous bat. Biol. Lett. 11, 20150576 (doi:10.1098/rsbl.2015.0576) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dechmann D, Kranstauber B, Gibbs D, Wikelski M. 2010. Group hunting: a reason for sociality in molossid bats? PLoS ONE 5, e9012 (doi:10.1371/journal.pone.0009012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dechmann DKN, Ehret S, Gaub A, Kranstauber B, Wikelski M. 2011. Low metabolism in a tropical bat from lowland Panama measured using heart rate telemetry: an unexpected life in the slow lane. J. Exp. Biol. 214, 3605–3612. (doi:10.1242/jeb.056010) [DOI] [PubMed] [Google Scholar]
- 44.Gager Y, Gimenez O, O'Mara MT, Dechmann DK. 2016. Group size, survival and surprisingly short lifespan in socially foraging bats. BMC Ecol. 16, 2 (doi:10.1186/s12898-016-0056-1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bowlin M, Cochran W, Wikelski M. 2005. Biotelemetry of new world thrushes during migration: physiology, energetics and orientation in the wild. Integr. Comp. Biol. 45, 295–304. (doi:10.1093/icb/45.2.295) [DOI] [PubMed] [Google Scholar]
- 46.O'Mara M, Wikelski M, Dechmann D. 2014. 50 years of bat tracking: a review of device attachment and future directions. Methods Ecol. Evol. 5, 311–319. (doi:10.1111/2041-210X.12172) [Google Scholar]
- 47.Lighton JR, Halsey LG. 2011. Flow-through respirometry applied to chamber systems: pros and cons, hints and tips. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 158, 265–275. (doi:10.1016/j.cbpa.2010.11.026) [DOI] [PubMed] [Google Scholar]
- 48.Wikelski M, Spinney L, Schelsky W, Scheuerlein A, Gwinner E. 2003. Slow pace of life in tropical sedentary birds: a common-garden experiment on four stonechat populations from different latitudes. Proc. R. Soc. B 270, 2383–2388. (doi:10.1098/rspb.2003.2500) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.McNab BK. 1969. The economics of temperature regulation in neotropical bats. Comp. Biochem. Physiol. 31, 227–268. (doi:10.1016/0010-406X(69)91651-X) [DOI] [PubMed] [Google Scholar]
- 50.Lighton JR. 2008. Measuring metabolic rates: a manual for scientists. New York, NY: Oxford University Press. [Google Scholar]
- 51.Munoz-Garcia A, Larrain P, Ben-Hamo M, Cruz-Neto A, Williams JB, Pinshow B, Korine C. 2016. Metabolic rate, evaporative water loss and thermoregulatory state in four species of bats in the Negev desert. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 191, 156–165. (doi:10.1016/j.cbpa.2015.10.010) [DOI] [PubMed] [Google Scholar]
- 52.Ben-Hamo M, Munoz-Garcia A, Williams JB, Korine C, Pinshow B. 2013. Waking to drink: rates of evaporative water loss determine arousal frequency in hibernating bats. J. Exp. Biol. 216, 573–577. (doi:10.1242/jeb.078790) [DOI] [PubMed] [Google Scholar]
- 53.Wagner DN, Mineo PM, Sgueo C, Wikelski M, Schaeffer PJ. 2013. Does low daily energy expenditure drive low metabolic capacity in the tropical robin, Turdus grayi? J. Comp. Physiol. B 183, 833–841. (doi:10.1007/s00360-013-0747-2) [DOI] [PubMed] [Google Scholar]
- 54.R Core Team. 2016. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- 55.Sueur J, Simonis C. 2008. Seewave: a free modular tool for sound analysis and synthesis. Bioacoustics 18, 213–226. (doi:10.1080/09524622.2008.9753600) [Google Scholar]
- 56.Wand M.2015. KernSmooth: functions for kernel smoothing supporting Wand & Jones (1995). (R package version 2.23-15 ed.
- 57.Bartón K.2016. MuMIn: Multi-Model Inference. R package version 1.15.6. ( https://CRAN.R-project.org/package=MuMIn)
- 58.Nakagawa S, Schielzeth H. 2013. A general and simple method for obtaining R2 from generalized linear mixed-effects models. Methods Ecol. Evol. 4, 133–142. (doi:10.1111/j.2041-210x.2012.00261.x) [Google Scholar]
- 59.Stroud JT, Feeley KJ. 2017. Neglect of the tropics is widespread in ecology and evolution: a comment on Clarke et al. Trends Ecol. Evol. 32, 626–628. (doi:10.1016/j.tree.2017.06.006) [DOI] [PubMed] [Google Scholar]
- 60.Bondarenco A, Kortner G, Geiser F. 2014. Hot bats: extreme thermal tolerance in a desert heat wave. Naturwissenschaften 101, 679–685. (doi:10.1007/s00114-014-1202-2) [DOI] [PubMed] [Google Scholar]
- 61.Bartels W, Law BS, Geiser F. 1998. Daily torpor and energetics in a tropical mammal, the northern blossom-bat Macroglossus minimus (Megachiroptera). J. Comp. Physiol. B 168, 233–239. (doi:10.1007/s003600050141) [DOI] [PubMed] [Google Scholar]
- 62.Bondarenco A, Kortner G, Geiser F. 2013. Some like it cold: summer torpor by freetail bats in the Australian arid zone. J. Comp. Physiol. B 183, 1113–1122. (doi:10.1007/s00360-013-0779-7) [DOI] [PubMed] [Google Scholar]
- 63.Turbill C, Law B, Geiser F. 2003. Summer torpor in a free-ranging bat from subtropical Australia. J. Therm. Biol 28, 223–226. (doi:10.1016/S0306-4565(02)00067-0) [Google Scholar]
- 64.Stawski C, Geiser F. 2010. Seasonality of torpor patterns and physiological variables of a free-ranging subtropical bat. J. Exp. Biol. 213, 393–399. (doi:10.1242/jeb.038224) [DOI] [PubMed] [Google Scholar]
- 65.Currie SE, Noy K, Geiser F. 2015. Passive rewarming from torpor in hibernating bats: minimizing metabolic costs and cardiac demands. Am. J. Physiol. Regul. Integr. Comp. Physiol. 308, R34–R41. (doi:10.1152/ajpregu.00341.2014) [DOI] [PubMed] [Google Scholar]
- 66.Stephenson PJ, Racey PA. 1994. Seasonal variation in resting metabolic rate and body temperature of streaked tenrecs, Hemicentetes nigriceps and H. semispinosus (Insectivora: Tenrecidae). J. Zool 232, 285–294. (doi:10.1111/j.1469-7998.1994.tb01573.x) [Google Scholar]
- 67.Currie S, Kortner G, Geiser F. 2014. Heart rate as a predictor of metabolic rate in heterothermic bats. J. Exp. Biol. 217, 1519–1524. (doi:10.1242/jeb.098970) [DOI] [PubMed] [Google Scholar]
- 68.Freitas M, Goulart L, Barros M, Morais D, Amaral T, Matta S. 2010. Energy metabolism and fasting in male and female insectivorous bats Molossus molossus (Chiroptera: Molossidae). Braz. J. Biol. 70, 617–621. (doi:10.1590/S1519-69842010000300019) [DOI] [PubMed] [Google Scholar]
- 69.Stawski C, Willis CKR, Geiser F. 2014. The importance of temporal heterothermy in bats. J. Zool. 292, 86–100. (doi:10.1111/jzo.12105) [Google Scholar]
- 70.Ruf T, Streicher U, Stalder GL, Nadler T, Walzer C. 2015. Hibernation in the pygmy slow loris (Nycticebus pygmaeus): multiday torpor in primates is not restricted to Madagascar. Sci. Rep. 5, 17392 (doi:10.1038/srep17392) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Levin E, Plotnik B, Amichai E, Braulke LJ, Landau S, Yom-Tov Y, Kronfeld-Schor N. 2015. Subtropical mouse-tailed bats use geothermally heated caves for winter hibernation. Proc. R. Soc. B 282, 20142781 (doi:10.1098/rspb.2014.2781) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hosken DJ, Withers PC. 1997. Temperature regulation and metabolism of an Australian bat, Chalinolobus gouldii (Chiroptera: Vespertilionidae) when euthermic and torpid. J. Comp. Physiol. B 167, 71–80. (doi:10.1007/s003600050049) [DOI] [PubMed] [Google Scholar]
- 73.Willis C, Brigham R. 2003. Defining torpor in free-ranging bats: experimental evaluation of external temperature-sensitive radiotransmitters and the concept of active temperature. J. Comp. Physiol. B 173, 379–389. (doi:10.1007/s00360-003-0343-y) [DOI] [PubMed] [Google Scholar]
- 74.Harris MB, Milsom WK. 1995. Parasympathetic influence on heart rate in euthermic and hibernating ground squirrels. J. Exp. Biol. 198, 931–937. [DOI] [PubMed] [Google Scholar]
- 75.Chatfield PO, Lyman CP. 1950. Circulatory changes during process of arousal in the hibernating hamster. Am. J. Physiol. 163, 566–574. [DOI] [PubMed] [Google Scholar]
- 76.Zosky GR, Larcombe AN. 2003. The parasympathetic nervous system and its influence on heart rate in torpid western pygmy possums, Cercatetus concinnus (Marsupialia: Burramyidae). Zoology 106, 143–150. (doi:10.1078/0944-2006-00108) [DOI] [PubMed] [Google Scholar]
- 77.Lovegrove BG. 2012. The evolution of endothermy in Cenozoic mammals: a plesiomorphic-apomorphic continuum. Biol. Rev 87, 128–162. (doi:10.1111/j.1469-185X.2011.00188.x) [DOI] [PubMed] [Google Scholar]
- 78.Canale CI, Perret M, Henry P-Y. 2012. Torpor use during gestation and lactation in a primate. Naturwissenschaften 99, 159–163. (doi:10.1007/s00114-011-0872-2) [DOI] [PubMed] [Google Scholar]
- 79.Bisson I, Butler L, Hayden T, Romero L, Wikelski M. 2009. No energetic cost of anthropogenic disturbance in a songbird. Proc. R. Soc. B 276, 961–969. (doi:10.1098/rspb.2008.1277) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bishop CM, et al. 2015. The roller coaster flight strategy of bar-headed geese conserves energy during Himalayan migrations. Science 347, 250–254. (doi:10.1126/science.1258732) [DOI] [PubMed] [Google Scholar]
- 81.O'Mara MT, Rikker S, Wikelski M, Ter Maat A, Pollock HS, Dechmann DKN. 2017. Data from: Heart rate reveals torpor at high body temperatures in lowland tropical free-tailed bats Dryad Digital Repository. (http://dx.doi.org/10.5061/dryad.6620j) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- O'Mara MT, Rikker S, Wikelski M, Ter Maat A, Pollock HS, Dechmann DKN. 2017. Data from: Heart rate reveals torpor at high body temperatures in lowland tropical free-tailed bats Dryad Digital Repository. (http://dx.doi.org/10.5061/dryad.6620j) [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
Data are available for download from Dryad http://dx.doi.org/10.5061/dryad.6620j [81].