Abstract
Since the initial discovery of hydrothermal vents in 1977, these ‘extreme’ chemosynthetic systems have been a focus of interdisciplinary research. The Okinawa Trough (OT), located in the semi-enclosed East China Sea between the Eurasian continent and the Ryukyu arc, hosts more than 20 known vent sites but all within a relatively narrow depth range (600–1880 m). Depth is a significant factor in determining fluid temperature and chemistry, as well as biological composition. However, due to the narrow depth range of known sites, the actual influence of depth here has been poorly resolved. Here, the Yokosuka site (2190 m), the first OT vent exceeding 2000 m depth is reported. A highly active hydrothermal vent site centred around four active vent chimneys reaching 364°C in temperature, it is the hottest in the OT. Notable Cl depletion (130 mM) and both high H2 and CH4 concentrations (approx. 10 mM) probably result from subcritical phase separation and thermal decomposition of sedimentary organic matter. Microbiota and fauna were generally similar to other sites in the OT, although with some different characteristics. In terms of microbiota, the H2-rich vent fluids in Neuschwanstein chimney resulted in the dominance of hydrogenotrophic chemolithoautotrophs such as Thioreductor and Desulfobacterium. For fauna, the dominance of the deep-sea mussel Bathymodiolus aduloides is surprising given other nearby vent sites are usually dominated by B. platifrons and/or B. japonicus, and a sponge field in the periphery dominated by Poecilosclerida is unusual for OT vents. Our insights from the Yokosuka site implies that although the distribution of animal species may be linked to depth, the constraint is perhaps not water pressure and resulting chemical properties of the vent fluid but instead physical properties of the surrounding seawater. The potential significance of these preliminary results and prospect for future research on this unique site are discussed.
Keywords: biodiversity, chemosynthetic ecosystem, fluid chemistry, hydrothermal vent, microbial composition, sulfide deposit
1. Introduction
The discovery of deep-sea hydrothermal vent [1,2] brought to light various unique processes occurring under the vast seawater mass. Four decades of cruise observations, in addition to theoretical and experimental approaches, led to the accumulation of a large dataset and knowledge with regards to global distribution of vent sites [3,4], heat and elemental flux between solid earth and ocean [5,6] including sulfide ore generation [7,8], physiological and phylogenetic characteristics of (hyper)thermophilic chemolithotrophic microbes [9,10] and vent-endemic fauna [11,12], as well as biogeography [13]. Based on this knowledge and from the primal perspective of energetics that bridges them [14], a generalized model predicting the relationships between underlying geology, fluid chemistry and microbial chemosynthetic primary production [15], also applicable to presume habitability of ancient Earth [16,17] and extraterrestrial bodies [18,19], has been built. Despite such significant progress in the understanding of each process and links among vent rock–fluid life, one obvious key question that has been raised since the initial discovery of vents [1] is yet poorly resolved today—that is the mechanisms and processes behind dispersal and thus realized distributions of vent-endemic organisms [20,21].
Understanding the mechanisms and factors regulating biodiversity and biogeography on the Earth is one of the most basic yet unresolved issues in biogeoscience. Findings from hydrothermal vent sites led to the confirmation of deep-sea chemosynthetic ecosystems, fuelled by chemical reactions between oxidizing components in seawater (e.g. O2, SO4) and reducing components originating at/beneath the seafloor (e.g. H2, CH4, H2S), often in the form of symbiosis [1,13,15]. Although it is now known that such ecosystems occur in other forms such as cold seeps and organic falls [22,23], hydrothermal vents and the surrounding ecosystems are most widely and systematically investigated at the global seafloor [13]. To date, more than 600 confirmed or inferred active vent sites are known [24], generally around plate boundaries. Vents on mid-oceanic ridges are distributed like stepping stones with intervals of a few to thousand kilometres between the neighbouring sites. Such systems pose significant difficulties in studying how biodiversity is formed within a biogeographic province [25], as the sites are distant and the dispersal is influenced by various factors from being in the open ocean. Indeed, such systems tend to have geographical barriers to dispersal and distribution of dominant animal species (e.g. [26]) within a region, making studying the influence of particular factors difficult. Vent sites within back-arc basins, on the other hand, tend to have well-mixed gene pools within basins and are well suited for investigating various factors governing biodiversity [21]. The East China Sea (ECS) is a semi-closed marginal sea between the Eurasian continent and the Ryukyu arc (figure 1a). The southeastern part of the ECS is characterized by an elongated, fault-controlled depression, that is called the Okinawa Trough (OT). The OT is an ideal study area for how the biodiversity within a site and biogeography of a species are regulated because of its topographic and oceanographic constraints. The ECS opens to the Pacific Ocean only via several straits shallower than 1100 m while its internal basin in the south, the southern OT (SOT), reaches depths greater than 2000 m (figure 1a). Despite only approximately 1300 km of the long axis (NE–SW direction), more than 20 hydrothermal vent sites have been detected here [27–29]. This close proximity among vent sites, in addition to the semi-enclosed oceanographic setting (e.g. [30]), provides high connectivity among each vent site within the OT [21].
Figure 1.
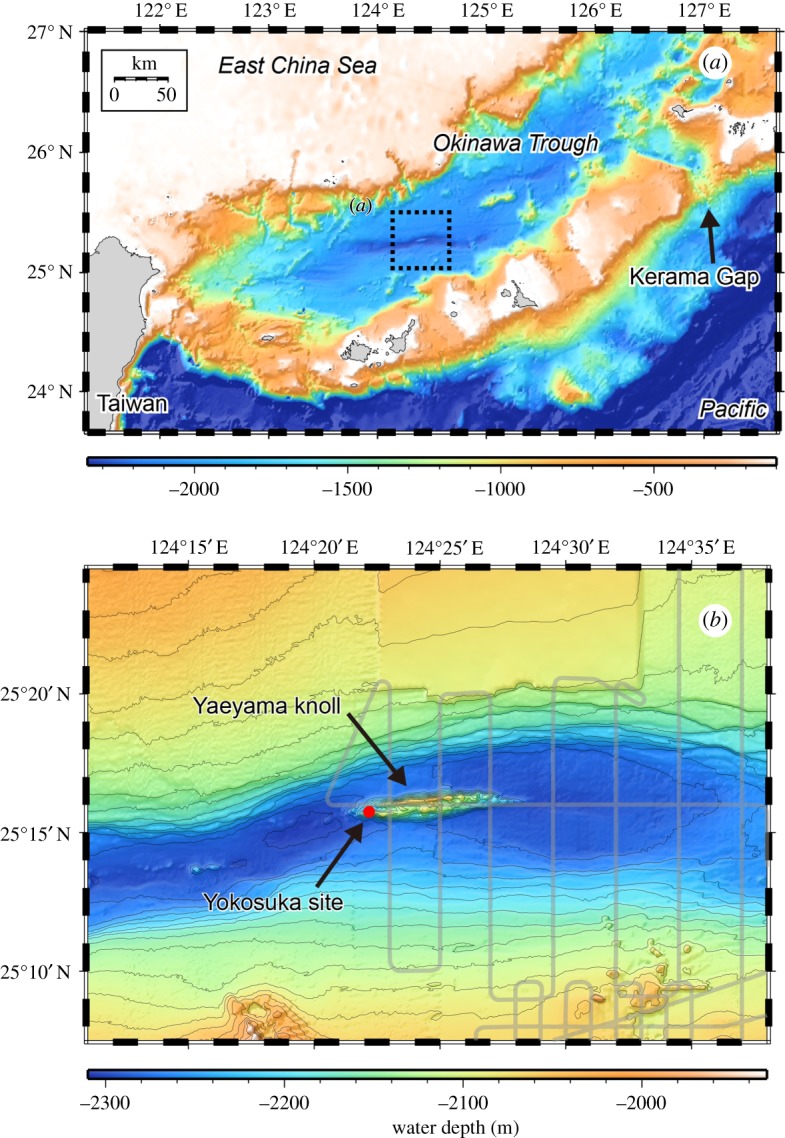
Seafloor topography of (a) entire southern Okinawa Trough and (b) Yaeyama Graben. Grey lines and a filled red circle in (b), respectively, represent surface ship track for geophysical observation including MBES-based hydrothermal site survey and the suggested location of hydrothermal activity. Seafloor topography in (b) was obtained by YK14-16, KR15-16 and YK16-07 cruises while partly sourced from a public database provided by Japan Coast Guard.
Most of the OT vent sites investigated so far are located within a narrow range of water depth between 950 and 1880 m [27,31]. The predominant factor determining the fluid temperature is pressure, which depends on the seawater depth, and the temperature is, in turn, the significant factor determining the fluid chemistry [6]. Fluid chemistry is indeed similar among the OT sites, in general [32]. The narrow range of depths also allows prosperity of similar species which have physiological advantage against the pressure range around 10–15 MPa. In fact, research of microbes and vent fauna inhabiting OT hydrothermal sites in the past decades [33–35] have revealed a well-mixed genetic pool among the OT sites, except the two sites shallower than 900 m [36]. If a hydrothermal vent site is discovered at another distinct water depth range within the OT, it would provide insights to understand and evaluate the significance of depth, temperature, pressure and fluid chemistry in determining the biodiversity and biogeography. Such information is truly valuable towards effective conservation and management of vent resources and biodiversity in the light of possible upcoming anthropogenic exploitation of OT hydrothermal vents [37,38].
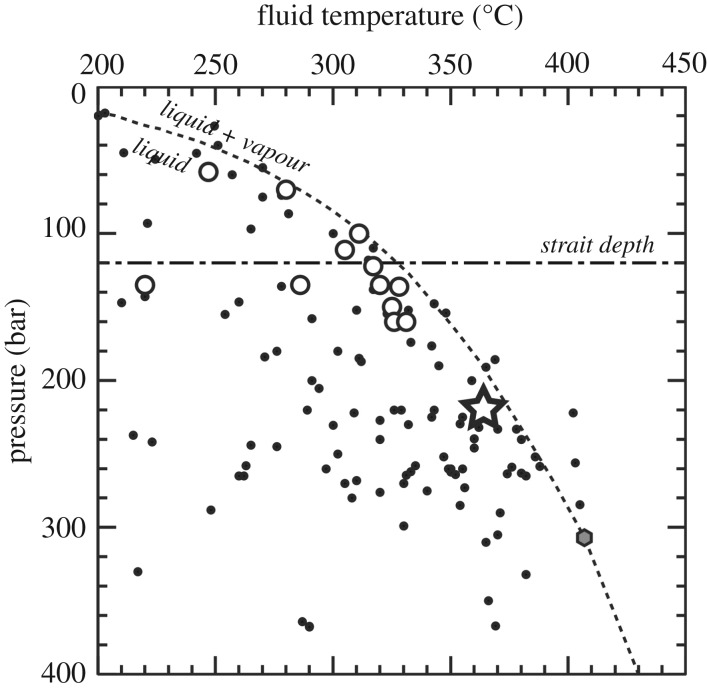
In this study, we surveyed the seafloor deeper than 2000 m in the SOT and discovered a hydrothermal fluid vent site at 2190 m depth, much deeper than the other known sites (approx. 1880 m) [39]. The existence of the present site was initially detected as an anomalous acoustic reflection of water column at the western end of the Yaeyama Knoll (figures 1b and 2); dives using the Remotely Operated Vehicle (ROV) KAIKO successfully located and surveyed the new vent site (figure 3). This new vent site is named ‘Yokosuka’ to honour the R/V Yokosuka which has contributed greatly to exploration of the global geofluid vent sites (e.g. [40–43]), including finding the initial signatures of the present site. The fluid of the Yokosuka site exhibited temperature up to 364°C, the highest recorded among all known OT sites (figure 4), the previous high temperature being 331°C recorded from the pNoho site (table 1). In the following, we outline the general picture of this new, hydrogeographically distinct, hydrothermal site in the OT with preliminary results from interdisciplinary studies across geochemistry, mineralogy, as well as microbiota and faunal composition.
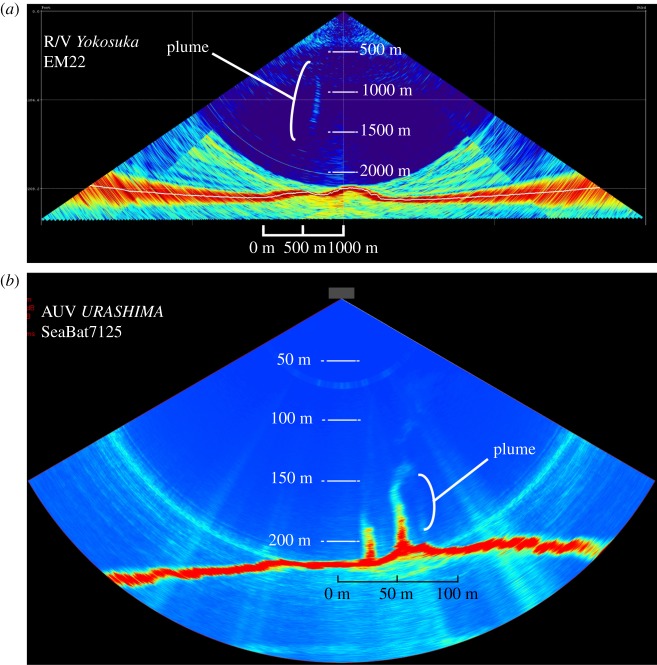
Figure 2.
Images acquired by MBES equipped on (a) R/V Yokosuka and (b) AUV URASHIMA. Anomalous reflections elongating vertically in water column over the western end of the Yaeyama Knoll suggest some compounds which have physical properties distinct from seawater such as CO2 bubbles/hydrates and hydrothermal fluid.
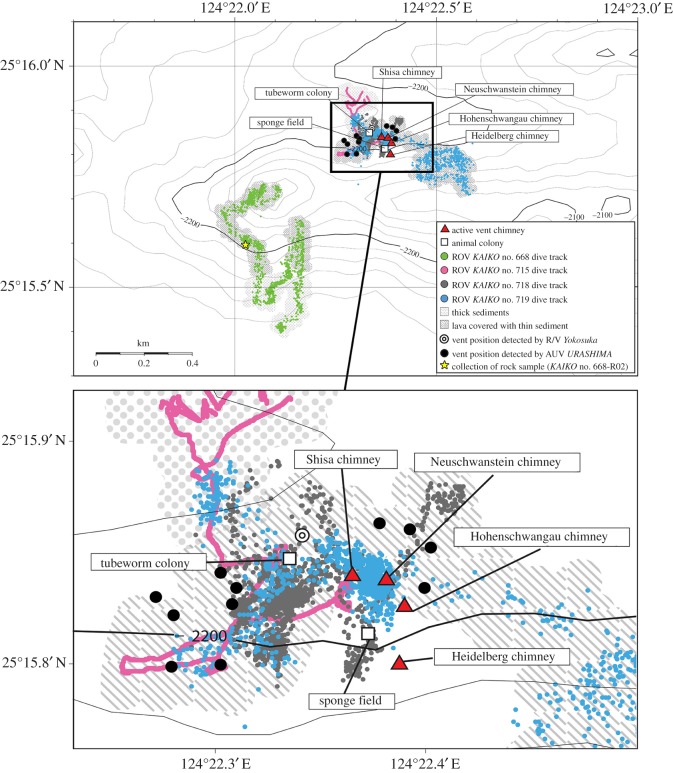
Figure 3.
Event map for the Yokosuka site, large area map showing all relevant dive tracks is presented on the top with an expanded map of the hydrothermally active area (indicated by a solid box) on the bottom. Indications of each symbol are shown in the legend.
Figure 4.
Pressure–temperature plot for the global hydrothermal sites (dataset used from [15,32]). An open star and open circles, respectively, represent the Yokosuka site and other OT sites, whereas black dots represent global hydrothermal sites. A broken curve and the grey hexagon, respectively, represent the two-phase boundary and critical point for 3.5% NaCl solution [44]. A horizontal broken-dot line represents the deepest strait in the East China Sea.
Table 1.
Endmember fluid chemistry of the Yokosuka site. Data from other Okinawa Trough sites and ambient seawater measured in this study are also presented for comparison.
| region | southern OT | southern OT | southern OT | mid OT | mid OT | mid OT | southern OT |
|---|---|---|---|---|---|---|---|
| field | Yokosuka | Daiyon Yonaguni | Hatoma | Sakai | Izena | Iheya North | Yokosuka |
| site—vent | Hohenschwangau | Lion | C2 | pNoho | Hakurei | Aki | ambient seawater |
| depth (m) | 2182 | 1385 | 1600 | 1101 | ∼2180 | ||
| MaxT (°C) | 364 | 328 | 325 | 331 | 326 | 316 | 2 |
| pH | 5.4 | 5.7 | 5.4 | 4.7 | 4.7 | 4.45 | 7.4 |
| Cl (mM) | 153 | 614 | 303 | 678 | 608 | 594 | 563 |
| Na (mM) | 126 | 416 | 205 | 509 | 458 | 451 | 470 |
| Na/Cl | 0.82 | 0.68 | 0.68 | 0.75 | 0.75 | 0.76 | 0.83 |
| K (mM) | 8.6 | 86 | 40.8 | 99 | 75 | 82 | 9.6 |
| K/Cl | 0.06 | 0.140 | 0.135 | 0.146 | 0.123 | 0.138 | 0.017 |
| Ca (mM) | 4.3 | 23 | 11.4 | 27.0 | 23 | 22.3 | 9.6 |
| Li (mM) | 0.17 | 1.23 | 3.7 | 2.8 | 1.44 | — | |
| Si (mM) | 10.2 | 11.3 | 8.63 | 13.7 | 11.3 | 14.1 | — |
| B (mM) | 4.05 | 3.9 | 2.33 | 4.4 | 3.7 | 2.09 | 0.54 |
| Sr (mM) | 0.024 | 0.046 | 0.111 | 0.13 | 0.086 | 0.087 | |
| Fe (mM) | <0.25 | 0.41 | 0.51 | <0.3 | — | ||
| Mn (mM) | 0.6 | 1.25 | 417 | 1.01 | 0.76 | — | |
| NH4 (mM) | 7.9 | 14.7 | 7.42 | 7.6 | 4.4 | 1.97 | — |
| H2S (mM) | 4.7 | 40.9 | 0.6 | 1.9 | — | ||
| CO2 (mM) | 294 | 22–329 | (1770) | 116 | 151 | 43–63 | — |
| δ13C–CO2 (‰) | −8.2 | −7.6 | −8.2 | −4.7 | −6.2 | −9 | — |
| CH4 (mM) | 9.6 | 1.2–13.5 | 10 | 3.2 | 6.8 | 0.4–0.9 | — |
| δ13C–CH4 (‰) | −24.8 | −26 | −49.1 | −27.8 | −32.1 | −48.4 | — |
| δ2H–CH4 (‰) | −104 | −111 | −113 | −112 | — | ||
| C2H6 (µM) | 69 | 40 | <0.5 | 2.5 | <0.5 | — | |
| C1/C2 | 139 | 250 | >6400 | 2720 | >800 | — | |
| H2 (mM) | 9.43 | 0.8–5.5 | 1.2 | 0.35 | 1.4 | 0.03 | — |
| δ2H–H2 (‰) | −369 | −359 | −379 | −373 | — | ||
| CO (µM) | 71 | <3 | 63 | <0.5 | — | ||
| δ2H–H2O (‰) | +2.4 | −4.8 | −0.6 | −1.4 | −0.43 | ||
| δ18O–H2O (‰) | +1.7 | 1.1 | 1.6 | 1.3 | −0.25 | ||
| ref. | this study | Suzuki [45] | Toki [46] | Miyazaki [31] | Ishibashi [47] | Miyazaki [31] | this study |
2. Material and methods
2.1. Yaeyama Knoll at the southern Okinawa Trough
2.1.1. Geological background
The SOT is characterized by active back-arc rifting of the eastern end of the Eurasian continental margin which forms a typical topographic feature, the Yaeyama Graben (figure 1a). The Yaeyama Knoll is a young volcanic ridge (less than 1 Ma) [48] located in association with the rifting at the center of the graben (figure 1b). The knoll is elongated in east–west direction, parallel to the graben, so volcanism is considered to be constrained by normal faults forming the rift structure. The knoll is about 200 m high, and the shallowest water depth is 2050 m. The western end of the knoll is branched, forming two minor ridges.
2.1.2. Hydrological background
The water depth of the Yaeyama Graben reaches 2300 m, far deeper than the deepest surrounding strait of ECS, the Kerama Gap (approx. 1100 m). Seawater deeper than 1100 m in the SOT is upwelled into the lower thermocline below the overlaying Kuroshio Current [30] with turnovers around 4.7–9.4 years [49]. The deep-water ventilation may allow larvae of vent-endemic animals to migrate from/into shallower hydrothermal vent sites beyond hydrogeographical barriers.
2.1.3. Cruises and dives
Water column observation using acoustic devices such as multibeam echo sounder (MBES) and side scan sonar systems have been successfully applied to exploration of seafloor hydrothermal vents in the last decade [28,29,49–52]. Particularly, the MBES survey from the sea-surface is far more effective in detecting and roughly locating undiscovered hydrothermal fluid venting sites because of extensive seafloor coverage [28] when compared to conventional hydrocast survey [4,53]. During the R/V Yokosuka cruise YK14–16 in 2014, the MBES system (EM122, 12 kHz) detected a reflection anomaly in its water column records that arose vertically from the seafloor, suggesting hydrothermal activity, at the southern minor ridge at the western end of the Yaeyama Knoll (figures 1b and 2a). During the YK16–07 cruise and a dive of the Autonomous Underwater Vehicle (AUV) URASHIMA (dive no. 252) in 2016, acoustic anomalies were also detected by the AUV-based MBES (SeaBat7125, 200 kHz) (figure 2b) and side scan sonar (EdgeTech2200, 120 kHz) above the seafloor of the northern slope of the minor ridge (figure 3). Based on these localization data, deep dives using the ROV KAIKO (with vehicle KAIKO Mk-IV) were conducted during R/V Kairei cruises KR15-16 (dive no. 668) in 2015 and KR16-16 (dive nos. 715, 718, 719) in 2016 (figure 3). The Yokosuka site was successfully discovered on dive no. 715.
2.2. Sampling and analyses
2.2.1. Fluid and rock chemistry
High-temperature hydrothermal fluids emerging from the chimneys were collected during ROV dives by a gas-tight fluid sampler, the WHATS-3 [31], in addition to ambient seawater collected by Niskin samplers as references. The full-fluid sample list is provided in electronic supplementary material, table S1. Fluid sampling and chemical analyses of the fluids collected followed procedures previously published (e.g. [31,54]). Fluid pH, alkalinity, NH4 concentrations and H2S concentrations were determined by a pH meter with titration and colorimetry [55]. Concentrations of Mg, Na, K, Ca, Sr, Si, B, Li, Fe, Mn, Zn, Cl and SO4 were determined with inductively coupled plasma optical emission spectrometry (ICP-OES: SPS5510; Hitachi High-Tech Science Corporation) and/or ion chromatography (IC: Dionex ICS-2100 and ICS-1600; Thermo Fisher Scientific) after appropriate sample dilution using Milli-Q deionized water (typically 200- to 2000-fold). Dissolved gases in the fluid samples were extracted on-board with a vacuum line, and concentrations of H2, CH4, CO, CO2 and C2H6 were determined onshore by gas chromatography with a helium ionization detector (GC-HID: GC4000, GL Sciences) and a flame ionization detector. Stable isotope ratios of CO2, CH4, CO, H2 and H2O were determined by continuous-flow isotope ratio mass spectrometry and a liquid water isotope analyser (Los Gatos Research, Inc.) and reported with delta (δ) notation to express the relative difference between minor/major isotope ratio (R) of a sample and the international standards (VSMOW for hydrogen and oxygen or VPDB for carbon) (δ = [(Rsample/Rstandard) − 1]) in the permil scale [56].
A massive volcanic rock collected at non-hydrothermal seafloor was crushed to obtain unaltered inner-part fragments for subsequent chemical analyses. The fragments were washed with Milli-Q water in an ultrasonic bath and then further crushed to powder form using tungsten carbide and agate mills. Abundances of major and trace elements were determined by X-ray fluorescence analysis and ICP-quadruple mass spectrometry, respectively, using procedures reported previously [57].
2.2.2. Microbiological analyses
A single chimney structure (1300–11 600 g) was collected from each of the three vents, the Neuschwanstein, Hohenschwangau and Heidelberg vents (figure 3), for microbiology. Immediately after the recovery on-board, samples were stored at −80°C until use. To estimate the abundance of microbial cells, chimney samples (0.8–1.6 g) were fixed with 3.7% formaldehyde-phosphate buffered saline (PBS) for 2 h at 4°C. After washing with PBS three times, samples were sonicated on ice with eight 5 s pulses with VP-050 ultrasonic homogenizer (power, 20%: TAITEC, Koshigaya, Japan) with 5 s pauses between power pulses. Suspended cells were filtered on 0.2 µm pore size Isopore filters (Merck Millipore, Schwalbach, Germany), and stained with SYBR Gold (Thermo Fisher Scientific, Waltham, MA, USA) for 10 min. After washing with PBS, microbial cells were counted in triplicate with an ECLIPSE Ni epifluorescence microscope (Nikon, Tokyo, Japan).
For DNA extractions, chimney samples were pulverized with a mortar and pestle in liquid nitrogen. DNA was extracted from each chimney sample (2.7–4.6 g) using the TRIzol reagent (Thermo Fisher Scientific) as described elsewhere [58]. V4-V5 regions of the 16S rRNA gene were amplified and analysed with a MiSeq sequencer (Illumina, San Diego, CA, USA) as previously described [59]. Sequences were processed using the QIIME software package [60]. Operational taxonomic units (OTUs) were selected based on 97% similarity level using the UCLUST [61] and were assigned to a reference taxonomic classification using the SILVA 119 [62] and the RDP classifier (SSU ref NR 119; http://www.arb-silva.de/no_cache/download/ archive/release_119/Exports). The raw sequence data have been deposited in GenBank/EMBL/DDBJ with the accession no. DRA005734.
2.2.3. Identification and relative abundance of megabenthos
High-quality video and images were taken using the camera system of ROV KAIKO during the deep dives, which allowed identification of megabenthos present in various areas of the Yokosuka site. Where possible, faunal specimens were collected using the manipulator or a slurp gun with a single-chambered sample chamber, equipped on the ROV. Upon recovery on-board R/V Kairei, the specimens were sorted and identified morphologically to ground-truth the video/photo-based identifications. Occurrences of each animal species were noted for various areas visited throughout the dives by analysing all available video and images for the areas of interest (i.e. active chimneys and peripheral faunal assemblages), and their relative abundances were estimated using dominant–abundant–common–occasional categories (as employed in [63]).
To investigate the extent of similarity in megabenthos species composition among different habitats within the Yokosuka site, Jaccard's index of similarity was calculated based on presence/absence data of the taxa identified. Nonmetric multidimensional scaling (nMDS) was used to visualize the data, and similarity contours based on a group-average clustering analysis was overlaid on the nMDS plot to indicate the extent of faunal resemblance among the closely clustering habitats (after [36]).
3. Results and discussion
3.1. Overview of the Yokosuka site
Figure 3 is a summary of all dive tracks, mapped against the newly discovered chimneys, and other indications of hydrothermal activity. During ROV KAIKO dive no. 668, the southern slope of the southern minor ridge was surveyed for hydrothermal signatures such as increases in water column turbidity and benthic animal density, but none was detected. A rock sample (KAIKO no. 668-R02; figure 3) was collected to characterize the volcanic body, the chemical analysis of which revealed K-medium basaltic andesite (island arc tholeiite; electronic supplementary material, table S1) standing on a mafic endmember of the bimodal composition of OT volcanic rocks [64]. Some other OT hydrothermal activities, on the other hand, are hosted by another endmember, dacite to rhyolite (e.g. [29,45,65]).
On KAIKO dive no. 715, the ROV landed at a depth of 2178 m on the northern slope of the minor ridge, where the seafloor was covered with thick sediment. After travelling southwards for about 150 m we arrived at a rocky field with thin sediment cover and occasionally with whip corals and sponges growing. As we travelled further south, water became increasingly turbid and corals disappeared, being replaced by rossellid sponges, commonly seen around other OT hydrothermal sites [66], and we soon encountered a colony of tubeworms sustained by diffuse flow venting (depth 2205 m). After travelling about 40 m eastwards, we then encountered rusty microbial mats, a field of small pieces of broken sulfide, and further animals typical of vents, such as the squat lobster Munidopsis spp. and vent shrimps Alvinocaris. Another 10 m eastwards, we discovered a very active black-smoker chimney with large flanges (approx. 12 m tall, depth 2190 m at base; figures 5a and 6a). We named this chimney ‘Neuschwanstein’ and collected animals, chimney and hydrothermal fluid. The highest fluid temperature measured was 347.3°C, the highest ever recorded at this point in all of OT vents [32]. However, we were unable to collect sufficient numbers of hydrothermal fluid sample (only one of four available bottles of WHATS-3 sampler [31]) because fragile flanges filled with high-temperature fluids prevented us from approaching close to the venting orifices, for safety reasons. With poor visibility and unstable positioning signals, we had difficulty identifying the absolute position of the ROV during this dive. In particular, the position shown by INS (Inertia Navigation System) and SSBL (Super Short Base-Line) were in disagreement.
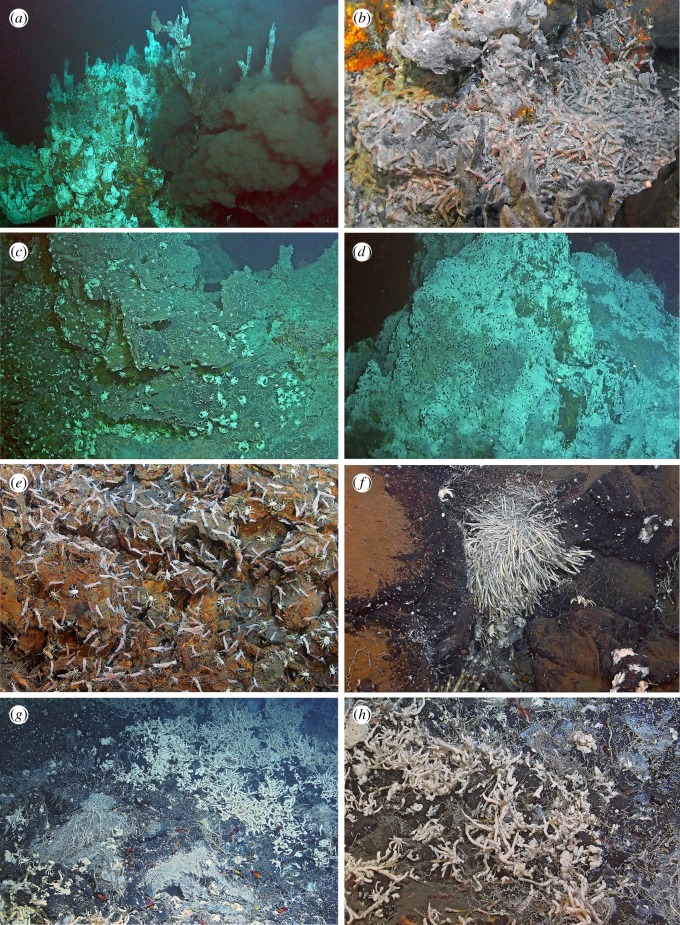
Figure 5.
Major vent chimneys in the Yokosuka site. (a) Neuschwanstein chimney (max temp. = 356.9°C), (b) Hohenschwangau chimney (max temp. = 364.1°C), (c) Heidelberg chimney (max temp. = 349.9°C) and (d) Shisa chimney.
Figure 6.
Megafaunal communities of the Yokosuka site. (a) Overview of shrimp aggregations on the top of the Neuschwanstein chimney. (b) Close-up of a Shinkaicaris leurokolos alvinocaridid shrimp aggregation on the Hohenschwangau chimney. (c) Shinkaia crosnieri squat lobsters near the base of the Hohenschwangau chimney. (d) Large aggregations of scale worms (black dots) on the surface of the Shisa chimney. (e) A typical animal colony around the base of chimneys of the Yokosuka vent field dominated by Alvinocaris longirostris shrimps, Munidopsis ryukyuensis squat lobsters, and Provanna clathrata snails. (f) Peripheral tubeworm (Lamellibrachia sp. and Alaysia sp.) bush 50 m east of the Neuschwanstein chimney. (g) Overview of a peripheral community visually dominated by poecilosclerid sponges near the Heidelberg chimney. (h) Close-up of the sponge-dominated peripheral community, most notable animals being Lamellibrachia sp. and Alaysia sp. tubeworms and B. aduloides mussels.
On dive no. 718, the ROV landed northeast of the best-estimated position of the Neuschwanstein chimney (figure 3). The seafloor was mostly sediment-covered, with occasional rossellid sponges. After exploring the vicinity against poor visibility for about an hour, we encountered a field of broken sulfide pieces inhabited by squat lobsters, similar to that seen near Neuschwanstein. Climbing a mound nearby the sulfide field revealed a large, mushroom-like black-smoker chimney with many flange structures. This chimney was obviously distinct from Neuschwanstein, and we named it ‘Hohenschwangau’ (approx. 7 m tall, depth 2190 m at base, figure 5b). Sampling of animals, chimney fragments and hydrothermal fluid was carried out; the highest temperature recorded was 364.1°C, even higher than Neuschwanstein and close to the boiling point at its depth (figure 4) [44]. After sampling and travelling northwest, we located a spot of shimmering water about 20 m away from Hohenschwangau. Further exploring the area, we travelled through an area of brown discoloration, apparently old dead chimneys covered by small sponges (as is typical for OT vent periphery areas), and finally, back to rocky seafloor. Then, to the southwest of Hohenschwangau, a very large area of diffuse flow venting appeared (figure 6g). This area was visually dominated by a branching demosponge in the order Poecilosclerida (similar to previously reported from the Minami-Ensei vent site) [66]. The occurrence of this type of sponge field here is intriguing as the Minami-Ensei site is a very shallow site (Depression B where poecilosclerid sponges were common is around 700 m deep [67]). We then moved southeast for about 30 m, where we discovered a further large mound-like chimney with large flange structures. This was clearly different from either Neuschwanstein or Hohenschwangau, and we named it ‘Heidelberg’ (approx. 10 m tall, depth 2165 m at base, figure 5c). Again we carried out sampling of animals, chimney pieces and hydrothermal fluids; the highest recorded temperature was 349.9°C.
For the final dive at the Yokosuka site (no. 719), the ROV landed on sediment-covered seafloor southwest of Neuschwanstein. After travelling for about 100 m northeast, we encountered sediments with brown discoloured mat followed shortly by white microbial mat and broken sulfide pieces. Continuing along the same direction, we soon found a large mound-like chimney that only had concentrated venting at a bulge-like flange structure at the side. We gave it a name, ‘Shisa’ (approx. 10 m tall, depth at base 2188 m, figure 5d), but no sampling was carried out. Carrying on the same direction past Shisa, we at last rediscovered Neuschwanstein chimney and commenced hydrothermal fluid sampling (maximum temperature 356.9°C). Although we further travelled eastward up to 400 m away from the four discovered chimneys, the seafloor was consistently rocky with occasional sponges after leaving Neuschwanstein and no further signs of hydrothermal activity were found.
3.2. Fluid chemistry
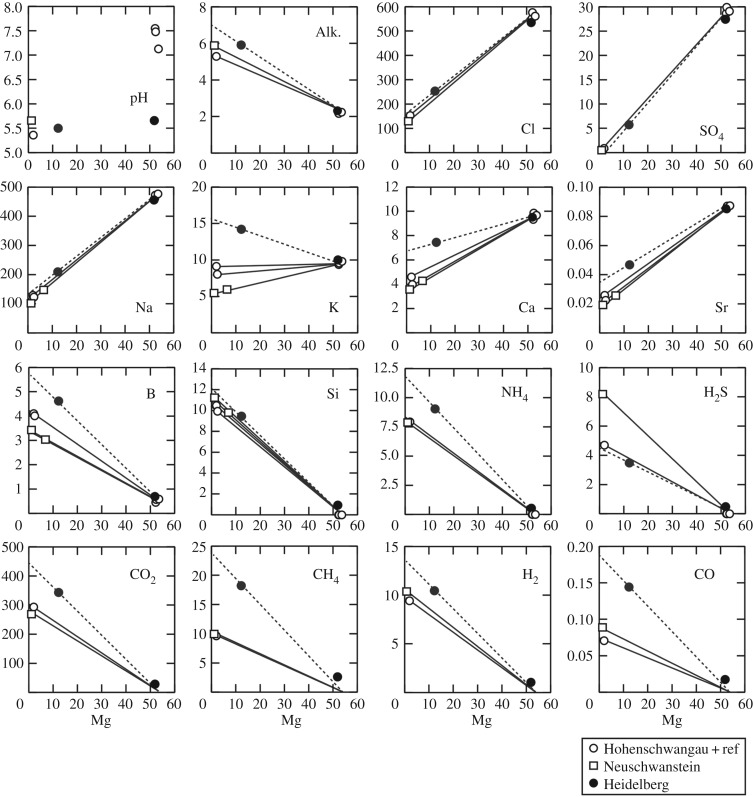
Concentrations of each chemical species dissolved in the fluid samples are shown in the magnesium diagram (figure 7) and all analytical results are listed in electronic supplementary material, table S2. The fluid pH of samples, with low magnesium concentrations, ranged between 5.3 and 5.7. Cl and Na concentrations of the low-Mg fluids were significantly lower than those of the ambient seawater (figure 7), being approximately one-third of their seawater values. As Cl has few removal processes during subseafloor fluid circulation, the result is interpreted to be due to subcritical phase separation (boiling) (figure 4) and preferential emergence of the resulting vapour-rich phase fluid (e.g. [68,69]). Fluids showing Cl enrichment compared with the seawater level were not found within the Yokosuka site, although simultaneous venting of vapour-rich and -depleted phases at distinct localities within a hydrothermal field has been found (e.g. [45,47,70]).
Figure 7.
Mg diagrams for Yokosuka site fluids. Open circles represent the chemical composition of the Hohenschwangau fluids and ambient seawater. The Neuschwanstein and Heidelberg fluids are shown by open squares and filled circles, respectively. Connecting lines from ambient seawater to each low-Mg fluid (dotted line for Neuschwanstein fluid) represent their extrapolation to Mg = 0 for estimating endmember fluid composition. All the concentrations are presented in the unit of millimolar.
Mg diagrams of each chemical species did not display a single mixing line from Mg-rich ambient seawater to Mg-depleted endmember fluid composition (figure 7), suggesting intra-field (inter-chimney) variation of the venting fluid chemistry. The estimated endmember fluid of the Neuschwanstein chimney contains relatively abundant ions, approximately 1.5 times higher than those of Heidelberg and Hohenschwangau chimneys. Inter-chimney differences in fluid chemistry probably result from differences in magnitudes and patterns of the two-phase segregation, due to uniform Na/Cl and K/Cl ratios among chimneys (table 1). Nevertheless, even in the Neuschwanstein fluid, estimated endmember concentrations of ion species (Cl, Na, K, Ca, Sr, Li, etc.) were the lowest values among those observed so far among all OT hydrothermal sites (table 1).
The endmember K/Cl value, which is a representation of the K content in the primitive upwelling fluid without influence from the phase separation, is significantly lower in the Yokosuka site fluids (0.055) than fluids of other OT sites (greater than 0.09) [32,46]. As the fluid K content is dominated by that of the host rock, which interacts with fluid flowing in the subseafloor, the low K/Cl value of the Yokosuka fluid points to a low K2O in the host rock. This seems to be consistent with basaltic andesite and its low K2O content (0.9%) exhibited by the Yaeyama Knoll body, which is probably identical to the subseafloor host rock of the Yokosuka site.
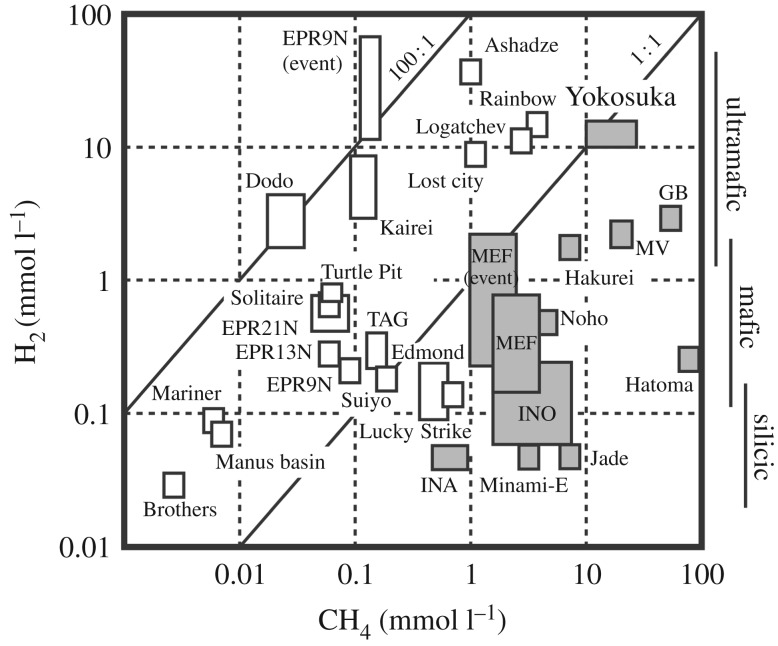
Each of the volatile species dissolved in the endmember fluid was notably abundant (table 1). The endmember fluid of the Neuschwanstein chimney contained much more volatiles, except H2S, than the other two chimneys (figure 7). Though the high volatile concentrations are mostly attributable to preferential venting of the vapour-enriched phase through subseafloor fluid boiling under subcritical condition [71] (suggested by the depletion of Cl), fluid–sediment interaction is also expected to generate and contribute some volatiles. The Yokosuka site hydrothermal fluids contained CH4 and H2 both as high as 10 mM, the first example among all global hydrothermal sites investigated so far [54,72] (figure 8). The high concentrations of CH4 and H2, comparable to H2S (up to 8.2 mM), potentially provide large yields of bioavailable energy for hydrogenotrophic and methanotrophic metabolisms in the mixing zone [15]. Relative abundance of methane against ethane (C1/C2 ratio) ranges 75–140, lower than typical range in the OT fields (approx. 103 [73]).
Figure 8.
A plot of H2 and CH4 concentrations in high-temperature hydrothermal fluids. Both axes are shown with a logarithmic scale. Grey-coloured and open squares, respectively, represent sediment-involved and sediment-not-involved hydrothermal sites. After Kawagucci et al. [54] with modification and additional data shown in this study (table 1).
The abundance of CH4 in sediment-associated hydrothermal sites, including the Yokosuka site, is thought to result from the thermal decomposition of sedimentary organic matter and microbial methanogenesis in the anoxic sediment [73,74]. The δ13C–CH4 value of −25.7‰ of the Yokosuka fluid is the uppermost value in the δ13C–CH4 range observed in the sediment-associated sites (−58 to −25‰) and suggests a predominance of thermogenic CH4 rather than biogenic one. The C1/C2 ratio supports the thermogenic origin of methane. As abundant H2 exceeding 1 mM is only seen with ultramafic-rock- and fresh basalt-hosted hydrothermal activities [54,68,72], the basaltic andesite-hosted activity of the Yokosuka site probably constrains H2 and CH4 concentrations to as low as 0.1 mM in the subseafloor fluid reservoir (figure 8). As thermal interaction of sediment beneath the vent can provide additional H2 to the upwelling fluid [47], thermogenic H2 input prior to phase separation would be able to account for the abundant H2 observed. The δ2H values of CH4 (−110‰) and H2 (−356‰) are in accordance with the hydrogen isotope equilibrium with H2O having δ2H–H2O of +0‰ at the endmember fluid temperature of 364°C [75]. The lower δ2H–H2 value in the Neuschwanstein chimney fluid, containing significant sulfate (no. 719-W1), may be attributable to microbial H2 oxidation after fluid cooling in the sampler bottle. Previously, hydrogenotrophic sulfate reducers, in particular, were reported to decrease the δ2H–H2 value approximately below −600‰ [76,77]. Indeed, microbiological analysis revealed hydrogenotrophic sulfate-reducing microbes inhabiting the Neuschwanstein chimney (see the next section), supporting this hypothesis.
The CO2 concentration of Yokosuka site fluids (approx. 300 mM) was as high as vent fluids from other SOT hydrothermal sites (table 1). Although magma degassing is presumed to be the initial source of abundant CO2 in the subseafloor fluid reservoir of back-arc hydrothermal sites (e.g. [70,78]), the δ13C–CO2 values varied among the chimneys (−11 to −6‰). Inputs of thermogenic CO2 with δ13C–CO2 of approximately 25‰ after branching of the upwelling fluid into each chimney, in addition to the primitive CO2 having δ13C–CO2 of −6 to 0‰ [79], may be accountable for the inter-chimney δ13C–CO2 variation in the Yokosuka site. The isotope effect on phase separation is another possible source of this variation, but it seems inconsistent with small variations in isotope ratios exhibited by other volatiles. Microbial production/consumption is not considered to be an attributable source due to the lack of carbon species comparable with CO2, from a stoichiometric viewpoint.
3.3. Microbial composition
The total microbial cell counts (mean ± standard deviation) in chimneys from Neuschwanstein, Hohenschwangau and Heidelberg vents were 2.3 × 107 ± 2.9 × 106 cells g−1, 1.2 × 106 ± 2.0 × 105 cells g−1 and 1.4 × 107 ± 3.3 × 106 cells g−1, respectively. These cell abundances were similar with those previously reported from other deep-sea vents (105–108 cells g−1 [80–83]), despite the notable enrichment of energetic molecules in the fluid.
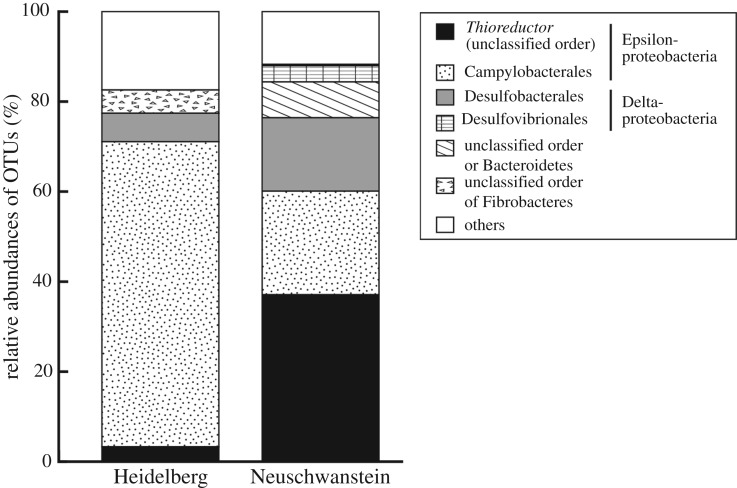
Microbial community structures were assessed via 16S rRNA gene amplicon sequencing. The PCR amplicon was not obtained from Hohenschwangau chimney, which had the lowest cell density. A total of 1169 different OTUs were identified from Neuschwanstein and Heidelberg chimneys on the basis of classification with greater than or equal to 97% of identity. Although the proportions of archaeal reads were low (1.3% and 1.2% in Neuschwanstein and Heidelberg, respectively), Methanococcales and Thermoplasmatales members were the most frequently detected archaea in Neuschwanstein and Heidelberg, respectively. Members of the order Methanococcales are hydrogenotrophic methanogens widely distributed in the OT hydrothermal fields [81,84,85]. The most abundant phylum was Proteobacteria in both chimney structures; 81.1% and 80.3% of reads in Neuschwanstein and Heidelberg, respectively. At the class level, the microbial communities were dominated by the phylotypes of Epsilonproteobacteria (60.1% and 71.1% in Neuschwanstein and Heidelberg, respectively), and were followed by the phylotypes of Deltaproteobacteria (16.3% and 6.3% in Neuschwanstein and Heidelberg, respectively) (figure 9). Members of the class Epsilonproteobacteria represent common and prevalent microorganisms in deep-sea vents of various depths [86]. Most of the known deep-sea vent Epsilonproteobacteria are strict chemoautotrophs using hydrogen and/or sulfur compounds as electron donors and nitrate, oxygen and sulfur compounds as electron acceptors [87]. Among Epsilonproteobacteria, members of the genus Thioreductor were frequently detected in the Neuschwanstein chimney (figure 9). In contrast, members of the genera Sulfurovum and Sulfurimonas (order Campylobacterales of the class Epsilonproteobacteria) were abundant in the Heidelberg chimney. All of these Epsilonproteobacteria are mesophilic chemoautotrophs [87]; however, Thioreductor species lack the ability to use sulfur compounds as the energy source and instead uses H2 as the primary energy source [80]. In previous studies in the OT hydrothermal fields, members of Thioreductor had a relatively limited distribution compared with those of Sulfurovum and Sulfurimonas [35,81,84,85]. For example, in the Iheya North Original hydrothermal site (water depth = approximately 1000 m; maximum fluid temperature = 311°C), members of Campylobacterales were frequently detected in the vicinity of deep-sea hydrothermal vents. By contrast, members of the genus Thioreductor were dominantly detected only in the in situ cultivation device deployed into the gas-rich fluid flow [84]. Similarly, hydrogenotrophic sulfate-reducing bacteria, e.g. Desulfobacterium species of the class Deltaproteobacteria, were abundantly detected only in Neuschwanstein chimney. These suggested that more H2-rich vent fluids in Neuschwanstein resulted in the dominance of hydrogenotrophic chemolithoautotrophs within the associated chimney structure.
Figure 9.
Composition of the microbial community based on taxonomic grouping (order level) of 16S rRNA gene amplicon reads. DNA was extracted from a single pulverized chimney sample from each vent. OTUs with greater than 3% frequency in either sample are presented, and the rest and unassigned taxa are indicated as ‘others’.
3.4. Megabenthos composition
In the hydrothermally active areas of the Yokosuka vent site, 21 species of megabenthos were identified (table 2), including two demosponges common in the periphery, four gastropods, one bivalve, six annelids including two tubeworms, and eight crustaceans including three alvinocaridid shrimps. All were species already known to be present in OT hydrothermal vents [36], although some could not be identified to species level (such as the poecilosclerid sponge) and a few still await formal taxonomic description (such as Alvinocaris sp. sensu [34]). The vent field could be divided into a few visually distinct regions differing in the dominant taxa and species present, including surface of active chimneys, base of active chimneys, diffuse flow sites dominated by tubeworm bushes, and periphery area dominated by poecilosclerid sponges. The species richness of the regions increased from focused vigorous venting towards weakly hydrothermally influenced peripheral areas (table 2).
Table 2.
List of megafauna species inhabiting the Yokosuka site and variation in occurrence and relative abundance among various habitats according to dominant–abundant–common–occasional categories as employed previously [63].
| chimney surface |
||||||||
|---|---|---|---|---|---|---|---|---|
| group | taxa | Neuschwanstein | Hohenschwangau | Heidelberg | Shisa | chimney base | diffuse flow (tubeworm bush) | periphery (sponge field) |
| Porifera | Poecilosclerida indet. | − | − | − | − | − | − | ++++ |
| Demospongiae indet. | − | − | − | − | + | + | ++ | |
| Mollusca: Gastropoda | Bathyacmaea sp. | − | − | + | − | + | ++ | + |
| Lepetodrilus nux | − | − | − | − | − | ++ | +++ | |
| Provanna clathrata | − | − | + | +++ | ++ | ++ | ||
| Provanna subglabra | − | − | + | − | + | + | + | |
| Mollusca: Bivalvia | Bathymodiolus aduloides | − | − | − | − | + | ++ | ++ |
| Annelida | Lamellibrachia sp. | − | − | − | − | − | ++++ | +++ |
| Alaysia sp. | − | − | − | − | − | +++ | +++ | |
| Amphisamytha sp. | − | + | +++ | − | + | ++ | ++ | |
| Paralvinella aff. hessleri | +++ | − | − | − | − | − | − | |
| Branchinotogluma sp. | +++ | ++ | + | ++++ | − | − | − | |
| Lepidonotopodium? sp. | − | − | − | − | − | + | − | |
| Arthropoda: Crustacea | Shinkaicaris leurokolos | ++++ | ++++ | +++ | ++ | − | − | − |
| Alvinocaris longirostris | ++ | + | +++ | +++ | ++++ | ++ | ++ | |
| Alvinocaris sp. | ++ | + | +++ | +++ | +++ | ++ | ++ | |
| Lebbeus shinkaiae | − | − | − | − | + | + | ++ | |
| Shinkaia crosnieri | − | + | +++ | − | + | ++ | ++ | |
| Munidopsis ryukyuensis | − | − | − | − | +++ | ++ | ++ | |
| Munidopsis longispinosa | − | − | − | − | + | + | + | |
| Neoverruca intermedia | − | − | − | − | − | + | + | |
On the two most active chimneys (figure 6a), Shinkaicaris leurokolos shrimps, Branchinotogluma sp. scale worms and Paralvinella aff. hessleri polychaetes lived in closest proximity to high-temperature effluents (figure 6b), with all three being present on Neuschwanstein but P. aff. hessleri being absent in Hohenschwangau. These three species found to dominate the area close to vent effluent are known to be the most heat-tolerant of vent animals found in OT, and are usually found close to a vent orifice [34,88]. Further away from the fluid source, a high abundance of two species of Alvinocaris shrimps (A. longirostris and A. sp. sensu [34]) was observed. Some individuals of the squat lobster Shinkaia crosnieri were present in the lower part of the chimney in Hohenschwangau, although this was not the case in Neuschwanstein. This distribution reflects different tolerance levels of the animals and that Alvinocaris and Shinkaia are less able tolerate strong vent effluents [34,36] of the two black-smoker chimneys. A commensal polychaete, Amphisamytha sp., was found on the body surface of S. crosnieri across the vent field.
At Heidelberg chimney where venting was less robust, zonation was not as clear with the two species of Alvinocaris being equally abundant but with S. crosnieri being present at much higher abundance (figure 6c). Paralvinella was not present, and Branchinotogluma was present only in low abundance. Three more peripheral molluscs including two snails in genus Provanna and one limpet in genus Bathyacmaea were present too, albeit in low abundances (category ‘occasional’, table 2). The Shisa chimney is rather strange in that although it was not vigorously venting at all, its entire surface was nonetheless covered by a high abundance of Branchinotogluma sp. scale worms (figure 6d). The top of the chimney appeared to be devoid of other megabenthos, although alvinocaridid shrimps became abundant in the lower parts. As Shisa chimney was not observed in detail and no samples were taken, it is currently not known why only Branchinotogluma sp. was abundant there.
The bases of all four chimneys were similar in faunal composition. Most notably, images of the bases are dominated by a high abundance of Alvinocaris shrimps and Munidopsis squat lobsters (mostly M. ryukyuensis with M. longispinosa co-occurring at low abundance; figure 6e). Shinkaia crosnieri is often present too but in low abundance, the same can be said for the shrimp Lebbeus shinkaiae. Upon closer examination, a high abundance of Provanna clathrata snails was revealed, occasionally together with low abundances of P. subglabra and Bathyacmaea sp. limpets. Very rarely, one or two Bathymodiolus aduloides mussels were seen. Further away from the chimney base, an unidentified demosponge begins to cover sulfide deposits.
Two types of peripheral aggregations were sighted in the Yokosuka site, including relatively focused diffuse flow areas dominated by tubeworm bushes (figure 6f) and large, weakly hydrothermally influenced areas dominated by a branching poecilosclerid sponge (figure 6g) that often engulfed the tubeworms. With the exception of Lamellibrachia being more abundant in tubeworm bush regions where poecilosclerid sponge was not at all present, megafaunal communities observed at these two peripheral regions were generally similar in composition and relative abundance. The mussel B. aduloides was common, as well as gastropods Provanna spp., Lepetodrilus nux and Bathyacmaea sp. No other Bathymodiolus species were found to inhabit the Yokosuka site, although more thorough exploration in the future may recover other species. The dominance of B. aduloides is nevertheless of interest because at all other OT vents it is never present in high abundance [89]), the dominant species being B. platifrons and B. japonicus. Perhaps, the fact that B. aduloides host sulfur-oxidizing endosymbiont (as opposed to methane-oxidizers in B. platifrons and B. japonicus [89]) contributes to its dominance in the Yokosuka site, but this is largely speculative at this point. Alaysia sp. tubeworm was equally abundant in both types of peripheral habitats. The two species of Alvinocaris shrimps were also common, although Shinkaicaris leurokolos was not seen. An unidentified demosponge was often seen covering hard substrates, more commonly in the sponge field. A single specimen of white-coloured Lepidonotopodium? sp. scale worm was seen in a tubeworm bush.
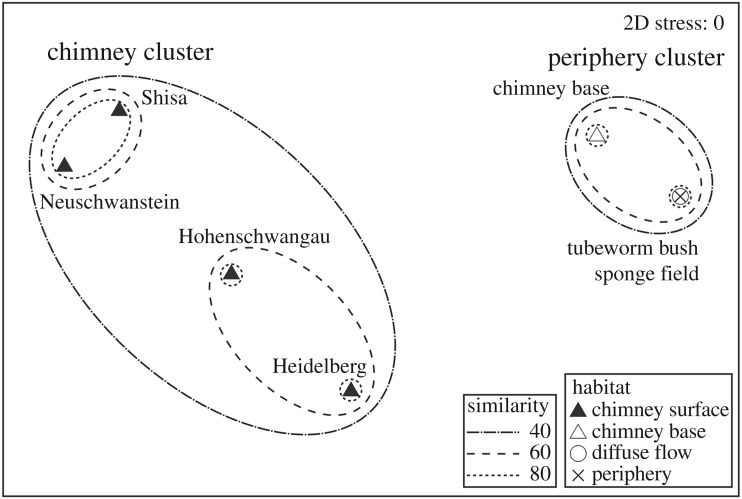
The results of similarity analysis of megabenthos composition among the different habitat types identified (figure 10) revealed two highly dissimilar clusters including a ‘chimney’ cluster composed of surfaces of the four active chimneys and a ‘periphery’ cluster composed of chimney bases, tubeworm-dominated diffuse flow and sponge-dominated periphery. All habitats within each cluster had a faunal similarity above 40%. Within the chimney cluster, Neuschwanstein and Shisa were most similar with a faunal similarity of 80%, Hohenschwangau and Heidelberg were rather similar with a similarity of 60%. The reason for detecting the Neuschwanstein–Shisa and Hohenschwangau–Heidelberg pairs may be attributed to the fact that the former lacks ‘peripheral’ taxa such as S. crosnieri completely, whereas the latter allows some intrusion of these taxa. Within the periphery cluster, the tubeworm-dominated diffuse flow and sponge-dominated periphery were almost identical in species composition but the chimney base habitat was slightly more dissimilar (60% similarity).
Figure 10.
Nonmetric multidimensional scaling (nMDS) plot visualizing similarity in megabenthos composition among the different habitat types identified, based on Jaccard's index of similarity calculated using species presence/absence data. The overlaid contours are based on results from group-average cluster analysis.
4. Conclusion
At a depth of 2190 m the Yokosuka hydrothermal vent site discovered in this study is the deepest OT vent known so far, extending the bathymetric range of hydrothermal activities within the OT by approximately 300 m. The highest fluid temperature recorded in the OT is also extended, as anticipated from the depth, up to 364°C. Neither the depth nor the temperature resulted in large changes in vent fluid compositions at this site compared with other OT sites. However, these fluids were distinct from other OT sites due to the notably high concentrations of H2 and CH4, probably resulting from inputs of thermogenic volatiles and the preferential venting of vapour-rich phase after subseafloor fluid boiling. Hydrogenotrophic sulfate-reducing bacteria were found to be abundant only on the Neuschwanstein chimney, which exhausts much more H2 than the other chimneys analysed. Although the potential of available energy yield from a unit of hydrothermal fluid for chemolithotrophic microbes is high, the total microbial cell density and the overall microbial composition of chimney habitats were not distinct from those known from other OT vent sites. The same is true for megafaunal composition, with species inhabiting the Yokosuka site being in common with those from other OT vents [36,66] despite water depth and aspects of fluid chemistry being distinct.
The preliminary results presented here suggest that there is probably no biogeographic barrier between the 2190 m deep Yokosuka site and other OT vents between 800 and 1650 m deep, and that the Yokosuka site is also part of the well-mixed gene pool of deeper OT vents [21,36]. However, it has been previously indicated that the two shallow sites between 550 and 800 m deep, Minami-Ensei Knoll and Yoron Hole, have different fauna composition compared with other sites [36]. Our early insights from the Yokosuka site implies that although the distribution of animal species may be linked to depth, the constraint is perhaps not water pressure and resulting chemical properties of the vent fluid but instead physical properties of the surrounding seawater, such as density and temperature. For example, a hydrographic barrier for the dispersal may exist at the bottom of thermocline around 700 m [90]. The colder water around the deeper vents may also pose developmental difficulties to larvae of species restricted to shallow vents (and vice versa). As knowledge in biological traits of vent larvae as well as circulation processes in the deep sea are still incomplete, expanding collaborative efforts between physical oceanography and biology (e.g. [20,21]) and detailed analyses of genetic connectivity of shared microbial and faunal taxa among the Yokosuka site and other OT vent sites are required in the future to elucidate whether or not the Yokosuka site is truly well connected with other sites genetically.
According to the bathymetric data available (figure 1) the deepest part of the OT is about 2400 m deep, meaning the Yokosuka site, being 2190 m deep, is in the deepest possible hydrogeographic zone for vents to exist in the OT. Therefore, the apparent lack of biogeographic barrier between the Yokosuka site and other known deeper (greater than 900 m) OT vents, as indicated by the present results, suggests that only two bathymetric biodiversity regions (and therefore gene pools) exist in the OT, with the barrier being around 700–900 m deep. This is an important result in understanding the biogeography and connectivity of vent microbes and fauna both within the OT as well as between OT and other regions such as the Izu–Ogasawara Arc, and also highly relevant to the future conservation and management of vent resources.
Supplementary Material
Supplementary Material
Acknowledgements
We thank the operation team of the ROV KAIKO and AUV URASHIMA, as well as the captains and crews of the R/Vs Yokosuka and Kairei for their skilful support during the relevant cruises (YK14-16, KR15-16, YK16-07 and KR16-16). Joseph Resing (PMEL/NOAA) and a second anonymous reviewer are thanked for providing comments which improved this paper. C.C. was supported by a JAMSTEC International Postdoctoral Fellowship at the time of writing. S.K. thanks Prof. Gretchen Bernasconi-Green for providing space and opportunity to draft the manuscript as an academic visitor of ETH Zurich.
Data accessibility
Raw sequence data: GenBank/EMBL/DDBJ accession no. DRA005734 [91]. Other datasets supporting this article have been uploaded as part of the electronic supplementary material.
Authors' contributions
J.M., K.N., K.O., H.Ku. and S.K. designed the study and led the relevant cruises. Seafloor and water column geophysics were conducted by A.T., K.K., K.N. and K.O. Fluid sampling and chemical analyses were conducted by A.M., Y.M., E.T., T.S., S.H., S.S. and J.-i.I. Volcanic rock geochemistry was analysed by J.T. and K.N. Microbial composition was analysed by J.M., H.Ka., S.N., M.H. and Y.T. Megabenthos were sampled and analysed by H.K.W. and C.C. The manuscript was drafted by J.-i.I., S.N., C.C. and S.K. All authors gave final approval for submission and publication.
Competing interests
We have no competing interests.
Funding
This study was supported by Council for Science, Technology, and Innovation (CSTI) as the Cross Ministerial Strategic Innovation Promotion Program (SIP), Next-generation Technology for Ocean Resource Exploration. Parts of this study were also supported by Ministry of Education, Culture, Sports, Science, and Technology (MEXT) grant-in-aids (KAKEN-HI) nos. 15K05263 (to K.O. and S.K.) and 15K12222 (S.K.). The analysis of YK14-16 cruise data was supported by JST CREST grant no. JPMJCR11A2, Japan.
References
- 1.Corliss JB, et al. 1979. Submarine thermal sprirngs on the Galapagos rift. Science 203, 1073–1083. (doi:10.1126/science.203.4385.1073) [DOI] [PubMed] [Google Scholar]
- 2.Spiess FN, et al. 1980. East pacific rise: hot springs and geophysical experiments. Science 207, 1421–1433. (doi:10.1126/science.207.4438.1421) [DOI] [PubMed] [Google Scholar]
- 3.Beaulieu SE, Baker ET, German CR, Maffei A. 2013. An authoritative global database for active submarine hydrothermal vent fields. Geochem. Geophys. Geosyst. 14, 4892–4905. (doi:10.1002/2013GC004998) [Google Scholar]
- 4.Baker ET, Resing JA, Haymon RM, Tunnicliffe V, Lavelle JW, Martinez F, Ferrini V, Walker SL, Nakamura K. 2016. How many vent fields? New estimates of vent field populations on ocean ridges from precise mapping of hydrothermal discharge locations. Earth Planet. Sci. Lett. 449, 186–196. (doi:10.1016/j.epsl.2016.05.031) [Google Scholar]
- 5.Elderfield H, Schultz A. 1996. Mid-ocean ridge hydrothermal fluxes and the chemical composition of the ocean. Annu. Rev. Earth Planet Sci. 24, 191–224. (doi:10.1146/annurev.earth.24.1.191) [Google Scholar]
- 6.German CR, Seyfried WE. 2013. Hydrothermal processes. In Treatise on geochemistry (ed. Elias SA.), pp. 191–233, 2nd edn Oxford, UK: Elsevier. [Google Scholar]
- 7.Tivey MK. 2007. Generation of seafloor hydrothermal vent fluids and associated mineral deposits. Oceanography 20, 50–65. (doi:10.5670/oceanog.2007.80) [Google Scholar]
- 8.Hannington M, Jamieson J, Monecke T, Petersen S. 2010. Modern sea-floor massive sulfides and base metal resources: toward an estimate of global sea-floor massive sulfide potential. Soc. Econ. Geol. Spec. Publ. 15, 317–338. [Google Scholar]
- 9.Huber JA, Mark Welch DB, Morrison HG, Huse SM, Neal PR, Butterfield DA, Sogin ML. 2007. Microbial population structures in the deep marine biosphere. Science 318, 97–100. (doi:10.1126/science.1146689) [DOI] [PubMed] [Google Scholar]
- 10.Takai K, et al. 2008. Cell proliferation at 122°C and isotopically heavy CH4 production by a hyperthermophilic methanogen under high-pressure cultivation. Proc. Natl Acad. Sci. USA 105, 10 949–10 954. (doi:10.1073/pnas.0712334105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Childress JJ, Girguis PR. 2011. The metabolic demands of endosymbiotic chemoautotrophic metabolism on host physiological capacities. J. Exp. Biol. 214, 312–325. (doi:10.1242/jeb.049023) [DOI] [PubMed] [Google Scholar]
- 12.Vrijenhoek RC. 2013. On the instability and evolutionary age of deep-sea chemosynthetic communities. Deep Sea Res II. 92, 189–200. (doi:10.1016/j.dsr2.2012.12.004) [Google Scholar]
- 13.Mullineaux LS. 2014. Deep sea hydrothermal vent communities. In Marine community ecology and conservation (eds Bertness M, Bruno M, Silliman B, Stachowicz J), pp. 383–400. Sunderland, MA: Sinauer. [Google Scholar]
- 14.McCollom TM, Shock EL. 1997. Geochemical constraints on chemolithoautotrophic metabolism by microorganisms in seafloor hydrothermal systems. Geochim. Cosmochim. Acta 61, 4375–4391. (doi:10.1016/S0016-7037(97)00241-X) [DOI] [PubMed] [Google Scholar]
- 15.Nakamura K, Takai K. 2014. Theoretical constraints of physical and chemical properties of hydrothermal fluids on variations in chemolithotrophic microbial communities in seafloor hydrothermal systems. Prog. Earth Planet. Sci. 1, 5 (doi:10.1186/2197-4284-1-5) [Google Scholar]
- 16.Shibuya T, et al. 2015. Hydrogen-rich hydrothermal environments in the Hadean ocean inferred from serpentinization of komatiites at 300°C and 500 bar. Prog. Earth Planet. Sci. 2, 46 (doi:10.1186/s40645-015-0076-z) [Google Scholar]
- 17.Shibuya T, Russell MJ, Takai K. 2016. Free energy distribution and hydrothermal mineral precipitation in Hadean submarine alkaline vent systems: importance of iron redox reactions under anoxic conditions. Geochim. Cosmochim. Acta 175, 1–19. (doi:10.1016/j.gca.2015.11.021) [Google Scholar]
- 18.Sekine Y, et al. 2015. High-temperature water-rock interactions and hydrothermal environments in the chondrite-like core of Enceladus. Nat. Commun. 6, 8604 (doi:10.1038/ncomms9604) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Waite JH, et al. 2017. Cassini finds molecular hydrogen in the Enceladus plume: evidence for hydrothermal processes. Science 356, 155–159. (doi:10.1126/science.aai8703) [DOI] [PubMed] [Google Scholar]
- 20.Breusing C, et al. 2016. Biophysical and population genetic models predict the presence of ‘phantom’ stepping stones connecting mid-Atlantic ridge vent ecosystems. Curr. Biol. 26, 2257–2267. (doi:10.1016/j.cub.2016.06.062) [DOI] [PubMed] [Google Scholar]
- 21.Mitarai S, Watanabe H, Nakajima Y, Shchepetkin AF, McWilliams JC. 2016. Quantifying dispersal from hydrothermal vent fields in the western Pacific Ocean. Proc. Natl Acad. Sci. USA 113, 2976–2981. (doi:10.1073/pnas.1518395113) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Smith CR, Baco AR. 2003. Ecology of whale falls at the deep-sea floor. Oceanogr. Mar. Biol. Annu. Rev. 41, 411–354. [Google Scholar]
- 23.Vrijenhoek RC. 2010. Genetic diversity and connectivity of deep-sea hydrothermal vent metapopulations. Mol. Ecol. 19, 4391–4411. (doi:10.1111/j.1365-294X.2010.04789.x) [DOI] [PubMed] [Google Scholar]
- 24.Beaulieu SE. 2015. Interridge global database of active submarine hydrothermal vent fields: prepared for InterRidge, version 3.4. Paris, France: World Wide Web electronic publication; See http://vents-data.interridge.org. [Google Scholar]
- 25.Rogers AD, et al. 2012. The discovery of new deep-sea hydrothermal vent communities in the Southern Ocean and implications for biogeography. PLoS Biol. 10, e1001234 (doi:10.1371/journal.pbio.1001234) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jang SJ, Park E, Lee WK, Johnson SB, Vrijenhoek RC, Won YJ. 2016. Population subdivision of hydrothermal vent polychaete Alvinella pompejana across equatorial and Easter Microplate boundaries. BMC Evol. Biol. 16, 235 (doi:10.1186/s12862-016-0807-9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ishibashi J-I, Ikegami F, Tsuji T, Urabe T. 2015. Hydrothermal activity in the Okinawa Trough back-arc basin: geological background and hydrothermal mineralization. In Subseafloor biosphere linked to hydrothermal systems: TAIGA concept (eds Ishibashi J-I, Okino K, Sunamura M), pp. 337–359. Tokyo, Japan: Springer. [Google Scholar]
- 28.Nakamura K, Kawagucci S, Kitada K, Kumagai H, Takai K, Okino K. 2015. Water column imaging with multibeam echo-sounding in the mid-Okinawa Trough: implications for distribution of deep-sea hydrothermal vent sites and the cause of acoustic water column anomaly. Geochem. J. 49, 579–596. (doi:10.2343/geochemj.2.0387) [Google Scholar]
- 29.Wen H-Y, et al. 2016. Helium and methane sources and fluxes of shallow submarine hydrothermal plumes near the Tokara Islands, Southern Japan. Sci. Rep. 6, 34126 (doi:10.1038/srep34126) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nishina A, Nakamura H, Park J-H, Hasegawa D, Tanaka Y, Seo S, Hibiya T. 2016. Deep ventilation in the Okinawa Trough induced by Kerama Gap overflow. J. Geophys. Res. Oceans. 121, 6092–6102. (doi:10.1002/2016JC011822) [Google Scholar]
- 31.Miyazaki J, et al. 2017. WHATS-3: an improved flow-through gas-tight fluid sampler for deep-sea geofluid research. Front. Earth. Sci. 5, 45 (doi:10.3389/feart.2017.00045) [Google Scholar]
- 32.Kawagucci S. 2015. Fluid geochemistry of high-temperature hydrothermal fields in the Okinawa Trough. In Subseafloor biosphere linked to hydrothermal systems: TAIGA concept (eds Ishibashi J-I, Okino K, Sunamura M), pp. 387–403. Tokyo, Japan: Springer. [Google Scholar]
- 33.Watanabe H, Tsuchida S, Fujikura K, Yamamoto H, Inagaki F, Kyo M, Kojima S. 2005. Population history associated with hydrothermal vent activity inferred from genetic structure of neoverrucid barnacles around Japan. Mar. Ecol. Prog. Ser. 288, 233–240. (doi:10.3354/meps288233) [Google Scholar]
- 34.Yahagi T, Watanabe H, Ishibashi J, Kojima S. 2015. Genetic population structure of four hydrothermal vent shrimp species (Alvinocarididae) in the Okinawa Trough, Northwest Pacific. Mar. Ecol. Prog. Ser. 529, 159–169. (doi:10.3354/meps11267) [Google Scholar]
- 35.Mino S, et al. 2017. Endemicity of the cosmopolitan mesophilic chemolithoautotroph Sulfurimonas at deep-sea hydrothermal vents. ISME J. 11, 909–919. (doi:10.1038/ismej.2016.178) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Watanabe H, Kojima S. 2015. Vent fauna in the Okinawa Trough. In Subseafloor biosphere linked to hydrothermal systems: TAIGA concept (eds Ishibashi J-I, Okino K, Sunamura M), pp. 449–459. Tokyo, Japan: Springer. [Google Scholar]
- 37.Ishiguro S, Yamauchi Y, Odaka H, Akiyama S. 2013. Development of mining element engineering test machine for operating in seafloor hydrothermal deposits. Mitsubishi Heavy Indust. Tech. Rev. 50, 21. [Google Scholar]
- 38.Nakajima R, Yamamoto H, Kawagucci S, Takaya Y, Nozaki T, Chen C, Fujikura K, Miwa T, Takai K. 2015. Post-drilling changes in seabed landscape and megabenthos in a deep-sea hydrothermal system, the Iheya North Field, Okinawa Trough. PLoS ONE 10, e0123095 (doi:10.1371/journal.pone.0123095) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fukuba T, Noguchi T, Fujii T. 2015. The Yoron Hole: the shallowest hydrothermal system in the Okinawa Trough. In Subseafloor biosphere linked to hydrothermal systems: TAIGA concept (eds Ishibashi J-I, Okino K, Sunamura M), pp. 489–492. Tokyo, Japan: Springer. [Google Scholar]
- 40.German CR. 2003. Hydrothermal activity on the eastern SWIR (50°–70° E): evidence from core-top geochemistry, 1887 and 1998. Geochem. Geophys. Geosyst. 4, 22 (doi:10.1029/2003GC000522) [Google Scholar]
- 41.Fryer P. 2012. Serpentinite mud volcanism: observations, processes, and implications. Ann. Rev. Mar. Sci. 4, 345–373. (doi:10.1146/annurev-marine-120710-100922) [DOI] [PubMed] [Google Scholar]
- 42.Nakamura K, et al. 2012. Discovery of new hydrothermal activity and chemosynthetic fauna on the Central Indian Ridge at 18°–20°S. PLoS ONE 7, e32965 (doi:10.1371/journal.pone.0032965) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ohara Y, et al. 2012. A serpentinite-hosted ecosystem in the Southern Mariana Forearc. Proc. Natl Acad. Sci. USA 109, 2831–2835. (doi:10.1073/pnas.1112005109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bischoff JL, Rosenbauer RJ. 1984. The critical point and two-phase boundary of seawater, 200–500°C. Earth Planet. Sci. Lett. 68, 172–180. (doi:10.1016/0012-821X(84)90149-3) [Google Scholar]
- 45.Suzuki R, Ishibashi J-I, Nakaseama M, Konno U, Tsunogai U, Gena K, Chiba H. 2008. Diverse range of mineralization induced by phase separation of hydrothermal fluid: case study of the Yonaguni Knoll IV hydrothermal field in the Okinawa Trough back-arc basin. Resour. Geol. 58, 267–288. (doi:10.1111/j.1751-3928.2008.00061.x) [Google Scholar]
- 46.Toki T, et al. 2016. Geochemical characteristics of hydrothermal fluids at Hatoma Knoll in the southern Okinawa Trough. Geochem. J. 50, 493–525. (doi:10.2343/geochemj.2.0449) [Google Scholar]
- 47.Ishibashi J-I, et al. 2014. Diversity of fluid geochemistry affected by processes during fluid upwelling in active hydrothermal fields in the Izena Hole, the middle Okinawa Trough back-arc basin. Geochem. J. 48, 357–369. (doi:10.2343/geochemj.2.0311) [Google Scholar]
- 48.Ishikawa M, Sato M, Furukawa M, Kimura M, Kato Y, Tsugaru R, Shimamura K. 1991. Report on DELP 1988 cruises in the Okinawa Trough Part 6: petrology of volcanic rocks. Bull. Earthquake Res. Inst. Univ. Tokyo. 66, 151. [Google Scholar]
- 49.Nakamura K, Toki T, Mochizuki N, Asada M, Ishibashi J-I, Nogi Y, Yoshikawa S, Miyazaki J-I, Okino K. 2013. Discovery of a new hydrothermal vent based on an underwater, high-resolution geophysical survey. Deep Sea Res I. 74, 1–10. (doi:10.1016/j.dsr.2012.12.003) [Google Scholar]
- 50.Clarke JH. 2006. Applications of multibeam water column imaging for hydrographic survey. Hydrogr. J. 120, 3–15. [Google Scholar]
- 51.Kumagai H, Tsukioka S, Yamamoto H, Tsuji T, Shitashima K, Asada M, Yamamoto F, Kinoshita M. 2010. Hydrothermal plumes imaged by high-resolution side-scan sonar on a cruising AUV, Urashima. Geochem. Geophys. Geosyst. 11, Q12013 (doi:10.1029/2010GC003337) [Google Scholar]
- 52.Kasaya T, Machiyama H, Kitada K, Nakamura K. 2015. Trial exploration for hydrothermal activity using acoustic measurements at the North Iheya Knoll. Geochem. J. 49, 597–602. (doi:10.2343/geochemj.2.0389) [Google Scholar]
- 53.Baker ET, German CR, Elderfield H. 1995. Hydrothermal plumes over spreading-center axes: global distributions and geological inferences. In Seafloor hydrothermal systems: physical, chemical, biological, and geological interactions (eds Humphris S, Zierenberg R, Mullineaux LS, Thomson R), pp. 47–71. Washington, DC: AGU. [Google Scholar]
- 54.Kawagucci S, et al. 2016. Fluid chemistry in the Solitaire and Dodo hydrothermal fields of the Central Indian Ridge. Geofluids 16, 988–1005. (doi:10.1111/gfl.12201) [Google Scholar]
- 55.Gieskes JM, Gamo T, Brumsack H. 1991. Chemical methods for interstitial water analysis aboard JOIDES Resolution. Technical Note 15. College Station, TX: Ocean Drilling Program Texas A&M University. See http://www-odp.tamu.edu/publications/tnotes/tn15/f_chem1.htm.
- 56.Okumura T, Kawagucci S, Saito Y, Matsui Y, Takai K, Imachi H. 2016. Hydrogen and carbon isotope systematics in hydrogenotrophic methanogenesis under H2-limited and H2-enriched conditions: implications for the origin of methane and its isotopic diagnosis. Prog. Earth. Planet. Sci. 3, 14 (doi:10.1186/s40645-016-0088-3) [Google Scholar]
- 57.Kato Y, Fujinaga K, Suzuki K. 2005. Major and trace element geochemistry and Os isotopic composition of metalliferous umbers from the Late Cretaceous Japanese accretionary complex. Geochem. Geophys. Geosyst. 6, Q07004 (doi:10.1029/2005GC000920) [Google Scholar]
- 58.Muto H, Takaki Y, Hirai M, Mino S, Sawayama S, Takai K, Nakagawa S. In press A simple and efficient RNA extraction method from deep-sea hydrothermal vent chimney structures. Microbes Environ. (doi:10.1264/jsme2.ME17048) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nunoura T, Takaki Y, Kazama H, Hirai M, Ashi J, Imachi H, Takai K. 2012. Microbial diversity in deep-sea methane seep sediments presented by SSU rRNA gene tag sequencing. Microbes Environ. 27, 382–390. (doi:10.1264/jsme2.ME12032) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Caporaso JG, et al. 2010. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 7, 335–336. (doi:10.1038/nmeth.f.303) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Edgar RC. 2010. Search and clustering orders of magnitude faster than BLAST. Bioinformatics 26, 2460–2461. (doi:10.1093/bioinformatics/btq461) [DOI] [PubMed] [Google Scholar]
- 62.Quast C, Pruesse E, Yilmaz P, Gerken J, Schweer T, Yarza P, Peplies J, Glöckner FO. 2013. The SILVA ribosomal RNA gene database project: improved data processing and web-based tools. Nucleic Acids Res. 41, D590–D596. (doi:10.1093/nar/gks1219) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Copley JT, Marsh L, Glover AG, Hühnerbach V, Nye VE, Reid WD. K., Sweeting CJ, Wigham BD, Wiklund H. 2016. Ecology and biogeography of megafauna and macrofauna at the first known deep-sea hydrothermal vents on the ultraslow-spreading Southwest Indian Ridge. Sci. Rep. 6, 39158 (doi:10.1038/srep39158) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Shinjo R, Chung S-L, Kato Y, Kimura M. 1999. Geochemical and Sr-Nd isotopic characteristics of volcanic rocks from the Okinawa trough and Ryukyu Arc: implications for the evolution of a young, intracontinental back arc basin. J. Geophys. Res. Solid Earth 104, 10 591–10 608. (doi:10.1029/1999JB900040) [Google Scholar]
- 65.Sakai H, et al. 1990. Unique chemistry of the hydrothermal solution in the mid-Okinawa Trough Backarc Basin. Geophys. Res. Lett. 17, 2133–2136. (doi:10.1029/GL017i012p02133) [Google Scholar]
- 66.Fujikura K, Okutani T, Maruyama T. 2012. Deep-sea life: biological observations using research submersibles, 2nd edn Tokyo, Japan: Tokai University Press. [Google Scholar]
- 67.Hashimoto J, Ohta S, Fujikura K, Miura T. 1995. Microdistribution pattern and biogeography of the hydrothermal vent communities of the Minami-Ensei Knoll in the mid-Okinawa Trough, Western Pacific. Deep Sea Res I. 42, 577–598. (doi:10.1016/0967-0637(94)00037-S) [Google Scholar]
- 68.Lilley MD, Butterfield DA, Lupton JE, Olson EJ. 2003. Magmatic events can produce rapid changes in hydrothermal vent chemistry. Nature 422, 878–881. (doi:10.1038/nature01569) [DOI] [PubMed] [Google Scholar]
- 69.Schmidt K, Garbe-Schönberg D, Hannington MD, Anderson MO, Bühring B, Haase K, Haruel C, Lupton J, Koschinsky A. 2017. Boiling vapour-type fluids from the Nifonea vent field (New Hebrides Back-Arc, Vanuatu, SW Pacific): geochemistry of an early-stage, post-eruptive hydrothermal system. Geochim. Cosmochim. Acta 207, 185–209. (doi:10.1016/j.gca.2017.03.016) [Google Scholar]
- 70.Reeves EP, et al. 2011. Geochemistry of hydrothermal fluids from the PACMANUS, Northeast Pual and Vienna Woods hydrothermal fields, Manus Basin, Papua New Guinea. Geochim. Cosmochim. Acta 75, 1088–1123. (doi:10.1016/j.gca.2010.11.008) [Google Scholar]
- 71.Seewald J, Cruse A, Saccocia P. 2003. Aqueous volatiles in hydrothermal fluids from the Main Endeavour Field, northern Juan de Fuca Ridge: temporal variability following earthquake activity. Earth Planet. Sci. Lett. 216, 575–590. (doi:10.1016/S0012-821X(03)00543-0) [Google Scholar]
- 72.Konn C, Charlou JL, Holm NG, Mousis O. 2015. The production of methane, hydrogen, and organic compounds in ultramafic-hosted hydrothermal vents of the Mid-Atlantic Ridge. Astrobiology 15, 381–399. (doi:10.1089/ast.2014.1198) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kawagucci S, et al. 2013. Geochemical origin of hydrothermal fluid methane in sediment-associated fields and its relevance to the geographical distribution of whole hydrothermal circulation. Chem. Geol. 339, 213–225. (doi:10.1016/j.chemgeo.2012.05.003) [Google Scholar]
- 74.Cruse AM, Seewald JS. 2006. Geochemistry of low-molecular weight hydrocarbons in hydrothermal fluids from Middle Valley, northern Juan de Fuca Ridge. Geochim. Cosmochim. Acta 70, 2073–2092. (doi:10.1016/j.gca.2006.01.015) [Google Scholar]
- 75.Horibe Y, Craig H. 1995. D/H fractionation in the system methane-hydrogen-water. Geochim. Cosmochim. Acta 59, 5209–5217. (doi:10.1016/0016-7037(95)00391-6) [Google Scholar]
- 76.Campbell BJ, Li C, Sessions AL, Valentine DL. 2009. Hydrogen isotopic fractionation in lipid biosynthesis by H2-consuming Desulfobacterium autotrophicum. Geochim. Cosmochim. Acta 73, 2744–2757. (doi:10.1016/j.gca.2009.02.034) [Google Scholar]
- 77.Kawagucci S, et al. 2011. Hydrothermal fluid geochemistry at the Iheya North field in the mid-Okinawa Trough: implication for origin of methane in subseafloor fluid circulation systems. Geochem. J. 45, 109–124. (doi:10.2343/geochemj.1.0105) [Google Scholar]
- 78.Mottl MJ, et al. 2011. Chemistry of hot springs along the Eastern Lau Spreading Center. Geochim. Cosmochim. Acta 75, 1013–1038. (doi:10.1016/j.gca.2010.12.008) [Google Scholar]
- 79.Sano Y, Marty B. 1995. Origin of carbon in fumarolic gas from island arcs. Chem. Geol. 119, 265–274. (doi:10.1016/0009-2541(94)00097-R) [Google Scholar]
- 80.Nakagawa S, Inagaki F, Takai K, Horikoshi K, Sako Y. 2005. Thioreductor micantisoli gen. nov., sp. nov., a novel mesophilic, sulfur-reducing chemolithoautotroph within the ϵ-Proteobacteria isolated from hydrothermal sediments in the Mid-Okinawa Trough. Int. J. Syst. Evol. Microbiol. 55, 599–605. (doi:10.1099/ijs.0.63351-0) [DOI] [PubMed] [Google Scholar]
- 81.Nunoura T, Takai K. 2009. Comparison of microbial communities associated with phase-separation-induced hydrothermal fluids at the Yonaguni Knoll IV hydrothermal field, the Southern Okinawa Trough. FEMS Microbiol. Ecol. 67, 351–370. (doi:10.1111/j.1574-6941.2008.00636.x) [DOI] [PubMed] [Google Scholar]
- 82.Schrenk MO, Kelley DS, Delaney JR, Baross JA. 2003. Incidence and diversity of microorganisms within the walls of an active deep-sea sulfide chimney. Appl. Environ. Microbiol. 69, 3580–3592. (doi:10.1128/aem.69.6.3580-3592.2003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Takai K, Komatsu T, Inagaki F, Horikoshi K. 2001. Distribution of archaea in a black smoker chimney structure. Appl. Environ. Microbiol. 67, 3618–3629. (doi:10.1128/aem.67.8.3618-3629.2001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Nakagawa S, et al. 2005. Variability in microbial community and venting chemistry in a sediment-hosted backarc hydrothermal system: Impacts of subseafloor phase-separation. FEMS Microbiol. Ecol. 54, 141–155. (doi:10.1016/j.femsec.2005.03.007) [DOI] [PubMed] [Google Scholar]
- 85.Takai K, Nakagawa S, Nunoura T. 2015. Comparative investigation of microbial communities associated with hydrothermal activities in the Okinawa Trough. In Subseafloor biosphere linked to hydrothermal systems: TAIGA concept (eds Ishibashi J-I, Okino K, Sunamura M), pp. 421–435. Tokyo, Japan: Springer. [Google Scholar]
- 86.Nakagawa S, Takaki Y. 2009. Nonpathogenic Epsilonproteobacteria. In eLS. Chichester, UK: John Wiley & Sons, Ltd; (doi:10.1002/9780470015902.a0021895) [Google Scholar]
- 87.Nakagawa S, Takai K. 2008. Deep-sea vent chemoautotrophs: diversity, biochemistry and ecological significance. FEMS Microbiol. Ecol. 65, 1–14. (doi:10.1111/j.1574-6941.2008.00502.x) [DOI] [PubMed] [Google Scholar]
- 88.Shigeno S, Tame A, Uematsu K, Miura T, Tsuchida S, Fujikura K. 2015. Dual cellular supporters: multi-layer glial wrapping and the penetrative matrix specialized in deep-sea hydrothermal vent endemic scale-worms. Biol. Bull. 228, 217–226. (doi:10.1086/BBLv228n3p217) [DOI] [PubMed] [Google Scholar]
- 89.Koito T, Hashimoto J, Nemoto S, Kitajima M, Kitada M, Inoue K. 2012. New distribution record of deep-sea mussel, Bathymodiolus aduloides (Mollusca: Bivalvia: Mytilidae) from a hydrothermal vent, Myojinsho. Mar. Biodivers. Rec. 5, 267 (doi:10.1017/S1755267212000267) [Google Scholar]
- 90.Oka E, Kawabe M. 1998. Characteristics of variations of water properties and density structure around the Kuroshio in the East China Sea. J. Oceanogr. 54, 605–617. (doi:10.1007/BF02823281) [Google Scholar]
- 91.Kanzaki H, Nakagawa S. 2017. Sequence data from ‘Deepest and hottest hydrothermal activity in the Okinawa Trough: Yokosuka site at Yaeyama Knoll’. GenBank/EMBL/DDBJ. DRA005734.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Kanzaki H, Nakagawa S. 2017. Sequence data from ‘Deepest and hottest hydrothermal activity in the Okinawa Trough: Yokosuka site at Yaeyama Knoll’. GenBank/EMBL/DDBJ. DRA005734.
Supplementary Materials
Data Availability Statement
Raw sequence data: GenBank/EMBL/DDBJ accession no. DRA005734 [91]. Other datasets supporting this article have been uploaded as part of the electronic supplementary material.