Abstract
The rise of multidrug-resistant pathogens and the dearth of new antibiotic development place an existential strain on successful infectious disease therapy. Breakthrough strategies that go beyond classical antibiotic mechanisms are needed to combat this looming public health catastrophe. Reconceptualizing antibiotic therapy in the richer context of the host-pathogen interaction is required for innovative solutions. By defining specific virulence factors, the essence of a pathogen, and pharmacologically neutralizing their activities, one can block disease progression and sensitize microbes to immune clearance. Likewise, host-directed strategies to boost phagocyte bactericidal activity, enhance leukocyte recruitment, or reverse pathogen-induced immunosuppression seek to replicate the success of cancer immunotherapy in the field of infectious diseases. The answer to the threat of multidrug-resistant pathogens lies “outside-the-box” of current antibiotic paradigms.
Keywords: Antibiotic resistance, Bacterial infections, Virulence factors, Neutrophils, Macrophages, Immunotherapy
Introduction
An alarming and persistent rise in antibiotic resistance among many important pathogenic bacterial species poses one of the greatest contemporary challenges to the public health. A 2014 U.K. Government “Review on Antimicrobial Resistance” performed in collaboration with the Wellcome Trust concluded that, without a dramatic change in our response to the crisis, the true cost of antimicrobial resistance will be 300 million premature deaths and up to $100 trillion lost to the global economy by 2050, at which point it will exceed cancer as a cause of human mortality [1]. As World Health Organization Director-General Margaret Chan recently addressed the United Nations General Assembly: “Antimicrobial resistance is a global crisis – a slow motion tsunami. The situation is bad, and getting worse. With few replacement products in the pipeline, the world is heading towards a post-antibiotic era in which common infections, especially those caused by Gram-negative bacteria, will once again kill.” [2]
The effects of the antibiotic resistance epidemic are particularly distressing in hospitals and chronic care facilities, where such infections tend to strike the most vulnerable patient groups with chronic diseases and weakened immune systems. Highest-risk populations include the elderly, cancer patients, diabetics, premature newborns, surgical patients, and those fighting for their lives in the intensive care unit. Antibiotic resistance also disproportionately impacts developing countries with poor public health infrastructure that cannot deploy costly last-line antibiotic treatments.
The roots of the current dilemma are multifactorial. Ill-considered over-prescription of antibiotics for self-resolving conditions, physician reliance on unnecessarily broad-spectrum treatment regimens, widespread use of antibiotics in agricultural feed for growth promotion, an innovation gap from the exodus of most major pharmaceutical companies from antibiotic research and development they judge unprofitable, and pure Darwinian evolution of bacteria subjected to life-or-death selective pressures each contribute deeply. While conventional antibiotics have cured more disease than all other drug classes combined, this “golden era” is coming to a close, and increasingly complex patients and multi-resistant pathogens are exacting high morbidity and mortality in the face of an monolithic and uncreative approach to therapy. Broadening the definition of “antibiotic” is essential for innovation [3], and a prerequisite to escape from the difficult situation that has emerged due to our unfortunate collective complacency and neglect.
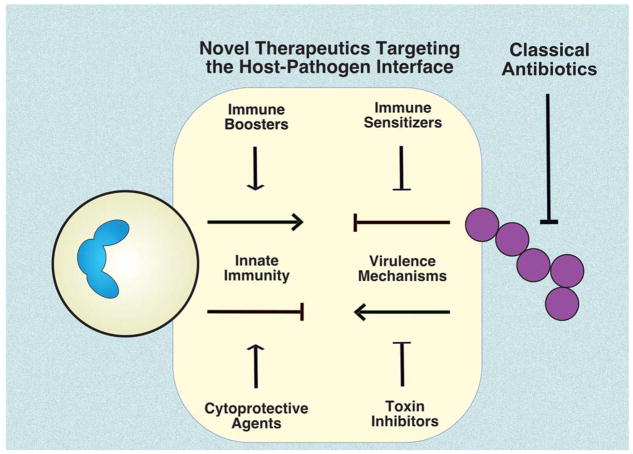
A barrier to innovation exists because the conceptual and scientific basis for current antibiotics centers solely on the bacterium, yet serious infection is more properly understood as a disease of the host-pathogen interaction. Most leading agents of human bacterial infection frequently colonize the skin or mucosal surfaces of healthy individuals without producing symptoms. However, if a bacterium has invaded into the bloodstream or deep tissues to sicken the patient, one can say that the sentinel defense functions of our innate immune system have failed. A more opportune definition of antibiotic therapy that centers on understanding correcting the dysfunctional host-pathogen interaction can unlock opportunities for therapeutic discovery (Fig. 1). Such novel pharmacological concepts come at the question from both sides: (a) pathogen-directed therapeutics that target virulence factors to reduce bacterial toxicity and/or sensitize the pathogen to normal immune clearance; or (b) host-directed therapeutics that boost the endogenous antimicrobial activity of host innate immune cells.
Figure 1. Potential for novel infectious disease therapeutics targeting the host-pathogen interface.
Classical antibiotics, drugs that kill or suppress the growth of pathogens, have been the cornerstones of infectious disease therapy for decades. However, continual evolution of antibiotic resistance has eroded their once reliable efficacy. Considering serious bacterial infection as a perturbation of the host-pathogen interaction, novel therapeutic drug classes are under evaluation. These drugs seek to inhibit bacterial toxins and immune resistance factors, or stimulate immune cell resilience and expression of antimicrobial effectors.
Neutralization of Virulence Factors: Disarming the Pathogen
Virulence factors are those characteristics that differentiate disease-causing bacteria from the hundreds of species of beneficial bacteria that comprise the normal flora of our intestine, mucosal surfaces and skin. Virulence factors include bacterial surface structures or secreted molecules that promote mucosal/epithelial adherence, biofilm formation, and/or intracellular invasion to breech host cell barriers. Additional virulence determinants promote resistance to immunological clearance by host antimicrobial peptides, complement or phagocytes, thereby allowing the pathogen to continue replication in normally sterile sites and produce a deep-seated or systemic infection. Finally, bacterial factors that directly injure host cells or membranes, impair critical host cell functions, or elicit exaggerated and deleterious pro-inflammatory responses, collectively referred to as “toxins”, are important contributors to virulence.
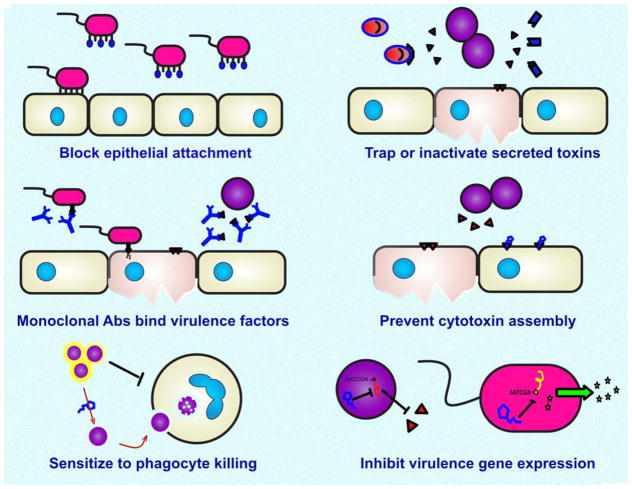
The basic pharmacological concept at play here is rather than identifying drugs than directly kill or suppress the growth (classical bactericidal or bacteriostatic antibiotic activities), one could screen or design drugs to target a virulence factor of the pathogen, rendering the pathogen “harmless” or readily susceptible to immune clearance (Fig. 2). The ideal virulence factor target should be universally or widely expressed among disease-associated strains of the pathogenic bacterial species, and play an essential role in disease pathophysiology. An express advantage to the virulence factor neutralization concept is that the therapy is envisioned to be very specific for the pathogen in question, avoiding the extensive “collateral damage” to the normal flora that accompanies courses of broad-spectrum antibiotic therapy and increases the risk of opportunistic infection or metabolic derangements. Virulence factor inhibitor therapy can also be contemplated as a companion or adjunct to effective classical antibiotic therapy (when available) in order to improve clinical outcome in severe or recalcitrant infections, or as a prophylactic therapy for patients entering a high-risk window for nosocomial infections (e.g. chemotherapy, major surgery).
Figure 2. Potential targets for pathogen-directed anti-virulence therapies against multidrug-resistant bacterial pathogens.
Small molecules, nanoparticles, engineered proteins or monoclonal antibodies are under investigation to block expression or function of bacterial toxins and other virulence factors. Anti-virulence therapies hold promise of being more specific to the infectious bacterium while sparing the healthy microbiome, and can be used as adjuncts to classical antibiotics for difficult infections.
Block epithelial adherence or biofilm formation
An essential first step in disease pathogenesis for many mucosal pathogens is to gain a secure foothold on the epithelium of the target organ, either by specific adhesin-mediated interactions with a host epithelial cell receptor, indirect binding to mucus or extracellular matrix components, or formation of a polymeric self-produced matrix of extracellular substances known as a biofilm. The opportunistic and frequently multidrug-resistant Gram-negative bacterial pathogen Pseudomonas aeruginosa binds to glycostructures on mucosal surfaces via two carbohydrate-binding proteins (lectins), PA-IL and PA-IIL. D-galactose or peptides mimicking human natural killer-1 antigen (HNK-1), polysialic acid or fucose blocked PA-IL-mediated airway cell binding, while L-fucose and pHNK-1 blocked PA-IIL-mediated binding, restoring normal ciliary beat frequency [4]. P. aeruginosa also produces a fucose-specific lectin, LecB, which participates in tissue attachment and the formation of biofilms. High-affinity LecB ligands obtained by screening combinatorial libraries of multivalent fucosyl-peptide dendrimers induced total dispersion of established biofilms in several clinical P. aeruginosa isolates [5]. Uropathogenic Escherichia coli (UPEC) is the most common cause of urinary tract infections, and its type I fimbrial (FimH) lectin is a key factor in bladder cell adherence and colonization. A small molecular weight orally bioavailable “mannoside” inhibitor that targets FimH in its mannose-binding pocket showed good therapeutic efficacy against UPEC infections, including antibiotic-resistant strains [6]. In Gram-positive pathogens such as Staphylococcus aureus, many surface proteins, including the fibronectin-binding protein A (FbpA) critical for epithelial adherence, are anchored to the cell envelope by the action of transpeptidase enzymes called sortases, which recognize a motif (LPXTG) in the target protein and catalyze a covalent linkage to cell wall peptidoglycan. Small molecules with potent inhibitory activity against S. aureus sortase A and B in vitro blocked adherence of the living pathogen to fibronectin [7].
Monoclonal antibodies
The antibiotic resistance crisis has spurred renewed interest in monoclonal antibody (mAb) therapeutics against specific antigens expressed by key pathogens. Many such passive immunotherapies bind conserved surface targets of the pathogen, which themselves may or may not be virulence factors, to promote opsonization and phagocytic clearance of the organism, as recently reviewed [8, 9]. Here we highlight some novel mAb therapeutic concepts that target toxins or other virulence factors of pathogens to improving disease outcome without directly promoting opsonophagocytic clearance/killing of the pathogen.
Alpha-hemolysin (“alpha toxin”) of S. aureus is a small beta-barrel pore-forming toxin with a broad range of cellular specificities that binds to its receptor (ADAM10) on target cells to trigger membrane disruption [10]. Alpha-toxin is an important virulence factor, as demonstrated through targeted mutagenesis of the encoding hla gene in strains of the globally disseminated epidemic USA 300 clone of methicillin-resistant S. aureus (MRSA), which markedly reduces host cell injury and virulence potential in murine models of infection, particularly pneumonia [11]. mAbs targeting alpha-toxin prevent assembly of its stable oligomer on the target cell and protect against lethal S. aureus pneumonia in mice [12]. Treatment with such a mAb provided dose-dependent increases in murine survival across a variety of different S. aureus clinical isolates, reduced proinflammatory cytokine and chemokine release, improved lung function independent of Fc activities, and showed synergistic or additive effects with concurrent antibiotics [13]. Two anti-alpha toxin mAbs (MEDI4982/Medimmune and AR301/Aridis) have now entered Phase I/II human clinical trials for prevention of staphylococcal pneumonia [9]. S. aureus also produces a family of related bi-component leukocidins that contribute to disease severity: Panton-Valentine leukocidin (PVL), gamma hemolysin (HlgABC) and leukocidin ED (LukED). A recent study described a single human mAb capable of recognizing a conformational epitope shared by the bi-component staphylococcal toxins plus alpha-toxin that prevented lysis of human phagocytes and provided high level protection in murine pneumonia and sepsis models [14].
Another key aspect of S. aureus pathogenesis is subversion or dysregulation of host coagulation through specific virulence determinants that bind or activate key clotting factors and platelets to promote pathogen clumping, endovascular clot formation and/or development of tissue abscesses. A mAb against S. aureus surface protein clumping factor A (ClfA) has been evaluated in a Phase II trial of hospitalized patients with documented S. aureus bacteremia (www.clinicaltrials.gov #NCT00198302). In a recent study, mice systemically challenged with L. lactis expressing ClfA succumbed to death within 24 h; passive immunization of such mice with an anti-ClfA mAb antibody to block fibrinogen binding dramatically increased survival compared to a control anti-ClfA mAb antibody that allowed fibrinogen binding [15]. This result suggests that inhibition of virulence functions (fibrinogen-binding) and not simply antibody-mediated opsonization contributed to the efficacy of the passive immunization. Coagulase A (CoA) and von Willebrand factor binding protein (vWbp) are two additional S. aureus proteins that synergize with ClfA to allow the pathogen to create fibrin cables in vivo. In a murine endocarditis model, passive immunization against all three virulence factors (CoA, vWbp, ClfA) reduced the development of heart lesions and increased survival [16]. A combination of prothrombin inhibitors plus anti-ClfA mAb also prolonged time to death in S. aureus sepsis [16].
Newer mAbs target bacterial toxins with mechanisms of action other than membrane pore formation and also improve specific bacterial disease outcomes. The virulence potential of P. aeruginosa is associated with a type III secretion system (TTSS), a “molecular syringe” that directly injects cytotoxins into host cells, inducing cell death. Protein PcrV makes an indispensable contribution to P. aeruginosa TTSS toxin translocation, and an engineered humanized anti-PcrV IgG antigen-binding fragment, KB001, has been developed for clinical use in ventilator-associated pneumonia and the chronic pneumonitis of cystic fibrosis [17]. Other mAbs targeting PcrV likewise show efficacy in pre- and post-challenge animal models of P. aeruginosa infection [18]. Obiltoxaximab, a mAb targeting protective antigen (PA), a component of anthrax lethal toxin (LT) and edema toxin (ET) required for toxin entry, prevents dissemination of Bacillus anthracis and reduces mortality in pre- and post-exposure rabbit or cynomolgus macaque models of inhalational anthrax, a foremost biodefense concern [19]. A cocktail of one fully human MAb specific to the receptor binding domain of Clostridium difficile toxin A and two fully human MAbs specific to nonoverlapping regions of the glucosyltransferase domain of C. difficile toxin B reduced severity and duration of diarrhea and mortality upon challenge with highly virulent C. difficile strains [20]. Lastly, an interesting derivation of mAb therapeutics was reported in the treatment of difficult S. aureus infections, where a slow-growing intracellular reservoir of the pathogen is often resistant to antibiotic clearance. An anti-S. aureus mAb was conjugated to an antibiotic (rifalogue) activated only after it is released in the proteolytic environment of the phagolysosome [21]. This unique mAb-drug conjugate showed superior efficacy vs. vancomycin in a mouse model of MRSA bacteremia.
An overarching advantage of mAb therapeutics for the treatment of bacterial infections is the opportunity for specific targeting of an individual pathogen or molecular virulence factor, allowing narrow spectrum precision and sparing the healthy microbiome. Their long half-life may allow prophylactic administration to high-risk patients, and newer antibody engineering technologies to enhance Fc effector functions or create bi-specific platforms can provide additional benefit. Limitations of mAbs include constraints on tissue penetration due to their large size, inability to enter cells to access intracellular pathogens and pathways, and requirement for parenteral, and not oral, administration.
Strategies for toxin neutralization
Beyond specific mAbs, other approaches have been devised to prevent the action of bacterial toxins by their physical sequestration or interfering with their key initial interactions with host cell receptors or cellular trafficking pathways.
The essential mechanism of action of bacterial pore-forming toxins reflects their natural affinity for the lipid bilayer of host cell membranes, wherein they assemble to disrupt the integrity of the membrane and promote cell death through hypo-osmotic lysis. By designing pharmacological agents that mimic structural features or biochemical properties of the host cell membrane, a “decoy” strategy can be used to sequester toxin away from the target cells and protect the host from pathogenic insult. For example, cholesterol, packaged within methyl-beta-cyclodextrin (CD), was used as a topical therapy to sequester alpha-toxin and prevent corneal erosions in a rabbit model of ocular keratitis with live S. aureus or purified toxin [22]. Another modified CD compound, IB201, blocked alpha-toxin-induced lysis of human alveolar epithelial cells, preventing formation of the lytic pore, and prevented or treated pulmonary infections with highly virulent S. aureus strains in mice [23]. Another group engineered artificial liposomes composed exclusively of naturally occurring lipids tailored to compete effectively with host cells for toxin binding. Liposome-bound toxins such as alpha-toxin or pneumolysin from Streptococcus pneumoniae did not lyse mammalian cells in vitro, and administration of such liposomes up to 10 hours after infectious challenge rescued mice from fatal septicemia caused by S. aureus and S. pneumoniae [24]. Another innovative approach derives biomimetic “nanosponges”, consisting of a polymeric nanoparticle core surrounded by freshly isolated red blood cell membranes. These functioned as toxin decoys in vivo to scavenge S. aureus alpha-toxin and improve survival in mice challenged with the toxin [25], or to reduce lesion size in a skin infection model [26]. Platelet-membrane derived nanosponge mimics also been bind pathogens and enhance the therapeutic efficacy of vancomycin in systemic S. aureus infection [27].
Blocking host receptors and entry pathways used by toxins is a promising therapeutic concept, because extensive adaptations by the microbe would be required to enable it to switch to a new receptor that can still support pathogenesis. For example, phage display was used to select a peptide that binds both natural host cell receptors for anthrax toxins (ANTXR1 and ANTXR2), and polyvalent presentation of this peptide on a synthetic scaffold neutralized anthrax toxin action upon intravenous administration in rats [28]. The cell surface metalloprotease ADAM 10 is a receptor for S. aureus alpha-toxin, and a small molecule ADAM 10 inhibitor protects against alpha-toxin-mediated disease pathogenesis in murine staphylococcal pneumonia [11] and abscess formation [29]. Upon receptor binding, a common mechanism for cellular entry of bacterial toxins requires trafficking to an acidified endosome, promoting translocation across the host membrane. The most active compound identified in a 30,000 compound screen against anthrax lethal toxin activity, 4-bromobenzaldehyde N-(2,6-dimethylphenyl) semicarbazone (EGA), effectively blocked entry of lethal toxin and other acid-dependent bacterial toxins into mammalian cells [30].
Direct inhibition of bacterial protease toxins has also been explored therapeutically. For example, a small molecule hydroxamate compound that inhibits the metalloprotease activity of anthrax lethal toxin, provides a significant survival advantage to mice given a lethal challenge of vegetative bacilli or rabbits given a lethal inhalation challenge of spores [31]. Likewise, ebselen inhibits the cysteine protease activity of C. difficile toxins TcdA and TcdB in nanomolar concentrations. Ebselen administration increased survival of mice and reduced pathological injury to the gastrointestinal mucosa in toxin or C. difficile gastrointestinal infection [32]. Several bacterial pathogens with pore-forming toxins induce macrophage necroptosis, a pro-inflammatory mode of cell death regulated by receptor interacting protein kinases RIP1 and RIP3 and mediated by the effector mixed-lineage kinase domain-like protein MLKL; macrophages deficient in MLKL are consequently resistant to pore-forming toxin-induced cell death. Treatment of mice with necrostatin-5, an inhibitor of RIP1 and/or GW806742X, an inhibitor of MLKL, reduced severity of Serratia marcescens pneumonia in mice, protecting alveolar macrophages from cell death and reducing bacterial burden [33]; necrostatin-5 also reduced pneumolysin-mediated macrophage necroptosis and cardiac damage following pneumococcal bacteremia and myocardial invasion [34].
Reducing bacterial virulence factor gene expression
In most medically important human pathogens, transcription of genes encoding key virulence determinants or their biosynthetic pathways is regulated by complex, intersecting pathways to allow fine tuning of expression in response to environmental cues encountered at each stage of colonization and disease progression. Virstatin is a virulence inhibitor drug against Vibrio cholerae disease that inhibits dimerization of transcriptional activator, ToxT. When ToxT is blocked, transcription of genes encoding cholera toxin, which triggers the hallmark watery diarrhea, and the toxin-coregulated pilus (Tcp), which promotes intestinal colonization by the pathogen, are both shut off [35]. Virstatin also inhibits pilus biogenesis, motility and biofilm formation in the multidrug-resistant nosocomial pathogen Acinetobacter baumannii [36]. Regacin is a drug that inhibits the ability of an AraC-like virulence regulator, RegA, to bind DNA and activate target promoters in the genome of the model gastrointestinal pathogen Citrobacter rodentium. Regacin reduced C. rodentium toxicity to intestinal epithelial cells, and treatment of infected mice with the drug reduced colonization and bacterial virulence [37]. Similar results have been observed with small molecule inhibitors of VirF, an AraC-like transcriptional regulator in the foodborne pathogen Shigella flexneri, blocking expression of numerous virulence genes and inhibiting intestinal epithelial cell invasion [38].
S. aureus virulence has been targeted using enol-acyl carrier protein inhibitor AFN-1252. AFN-1252 treatment rapidly increased S. aureus expression of fatty acid synthetic genes, perturbing membrane dynamics to influence signaling through the SaeRS two-component regulator and repress expression of virulence genes including alpha-toxin, β- and γ-hemolysins, and two fibrinogen-binding adhesins. Oral treatment of mice with AFN-1252 in an S. aureus air pouch infection model reduced viable bacteria recovered [39]. Two small molecule compounds (CCG-2979 and its analog CCG-102487) were identified in a high content screen for reduced expression of streptokinase (SK), a virulence factor of Streptococcus pyogenes that co-opts and activates host plasminogen to facilitate systemic spread of the pathogen during necrotizing fasciitis (“flesh-eating disease”). Microarray analysis of GAS grown in the presence of these inhibitors showed down-regulation of of other important S. pyogenes virulence factors in addition to SK, including the antiphagocytic surface anchored M protein and the cytolytic toxins streptolysin O and S, consistent with disruption of a general virulence gene regulatory network. Drug treatment increased neutrophil phagocytosis and killing of S. pyogenes, protecting mice from mortality in a systemic infection model [40].
Interference with bacterial quorum sensing
Quorum sensing is an important regulatory principle in numerous bacterial species in which the organisms produce and release chemical signal molecules called autoinducers that increase in concentration as a function of cell density. Many bacterial pathogens use quorum sensing to coordinate gene expression according to the density of their local population within host tissues. The natural product 6-gingerol, a pungent oil derived from fresh ginger, competitively inhibited the binding of two related homoserine lactone autoinducers of P. aeruginosa to their cognate receptors, reducing the expression of known virulence factors including elastase and pyocyanin, and blocking biofilm formation. Pre-treatment with 6-gingerol reduced mouse mortality from P. aeruginosa infection in a dose-dependent manner [41]. Another strategy for competitive inhibition and disruption of P. aeruginosa quorum sensing utilized meta-bromo-thiolactone (mBTL), which resulted in reduced biofilm formation, pyocyanin production, and increased survival of Caenorhabditis elegans and human lung epithelial cells at low μM levels after P. aeruginosa infection [42]. Likewise the antimetabolite compound S-phenyl-l-cysteine sulfoxide inhibited the kynurenine pathway implicated in P. aeruginosa alkyl quinolone autoinducer production and quorum sensing, reducing production of pyocyanin and other toxins [43].
In S. aureus, the accessory gene regulator (agr) quorum sensing system decreases expression of several cell surface proteins and increases expression of many secreted toxins in the transition from late-exponential growth to stationary phase. As the Agr system, and in particular its response regulator AgrA, are required for full S. aureus virulence, they have been explored as a therapeutic target. Treatment with naringenin, a flavonoid present in grapefruits and tomatoes, reduced transcription of S. aureus genes encoding AgrA and alpha-toxin, attenuating alpha-toxin mediated injury to alveolar epithelial cells, and reduced pulmonary inflammation and injury in a mouse pneumonia model [44]. Very recently, the nonsteroidal anti-inflammatory drug diflunisal was shown to block S. aureus AgrA transcriptional regulation of small peptide toxin virulence factors known as phenol soluble modulins (PSMs); diflunisal treatment reduced PSM production and diminished bone destruction in a murine osteomyelitis model of infection [45].
Sensitization of the pathogen to host innate immune clearance
When a patient is suffering a serious infection with a multidrug-resistant pathogen, it is important to consider not only last-line antibiotic options, but also that the pathogen is being combatted by multiple endogenous molecular and cellular effectors of microbial clearance, such as phagocytic cells, reactive oxygen species, and cationic host defense peptides. One emerging concept in alternative infectious disease therapeutics is to identify genes that deprive the pathogen of virulence factors it uses to defend itself against host defense mechanisms, thus re-sensitizing it to innate immune destruction. For example, the polysialic acid capsule of K1 serotype E. coli defends against complement- and phagocyte-mediated immune clearance and plays a role in invasive disease potential (e.g. urosepsis). Small molecule inhibitors identified in a screen for inhibition of E. coli capsule biogenesis render the pathogen highly sensitive to active human serum and provided near total protection to mice in lethal systemic infection with a virulent K1 serotype isolate [46]. Recent research has also shown that certain antibiotics that have no demonstrable activity against a particular pathogen in standard laboratory minimum inhibitory concentration (MIC) testing do nevertheless sensitize the pathogen to killing by endogenous host defense peptides, such as human cathelicidin LL-37. Examples include (a) the use of the β-lactam antibiotic nafcillin against MRSA, which promotes LL-37 killing and bacterial clearance in human whole blood, in a murine necrotizing skin infection model and in human patients with recalcitrant bacteremia [47]; and (b) the use of azithromycin to sensitize highly multidrug-resistant Gram-negative bacterial pathogens such as P. aeruginosa, A. baumannii, Klebsiella pneumoniae and Stenotrophomonas maltophilia to LL-37 killing, human serum and clearance in murine models of pneumonia with each pathogen [48, 49].
Phagocytic cells, including neutrophils and macrophages, generate reactive oxygen species (ROS) including hydrogen peroxide, hypochlorite and singlet oxygen through the NADPH oxidase-mediated “oxidative burst”, a key component of phagolysosomal killing of pathogens after their engulfment. S. aureus expresses a hallmark golden carotenoid pigment, staphyloxanthin, with antioxidant properties, which promoted resistance to ROS and phagocyte killing, increasing virulence in mouse infection models [50]. Interestingly, a high degree of similarity exists between a key enzyme required for production of the staphylococcal pigment (CrtM, dehydrosqualene synthase) and a human enzyme (squalene synthase) in the cholesterol biosynthesis pathway. Phosphonosulfate drug BPH-652, a squalene synthase inhibitor in clinical development for its cholesterol lowering properties, blocked S. aureus pigment production, sensitizing the pathogen to ROS, neutrophil killing, and reducing the bacterial burden in kidneys of mice following systemic infection [51]. Interestingly, the next enzyme in the S. aureus carotenoid pigment synthesis pathway is the diapophytoene desaturase, CrtN, which is inhibited by nanomolar concentrations by the FDA-approved antifungal drug, naftifine. Pigment inhibition by naftidine treatment decreased bacterial burden and increased mouse survival in systemic S. aureus challenge model [52]. Blocking carotenoid synthesis also increased cell membrane fluidity to sensitize S. aureus to the antimicrobial action of cationic host defense peptides [53].
Pharmacologically Boosting The Bactericidal Activity of Phagocytic Cells
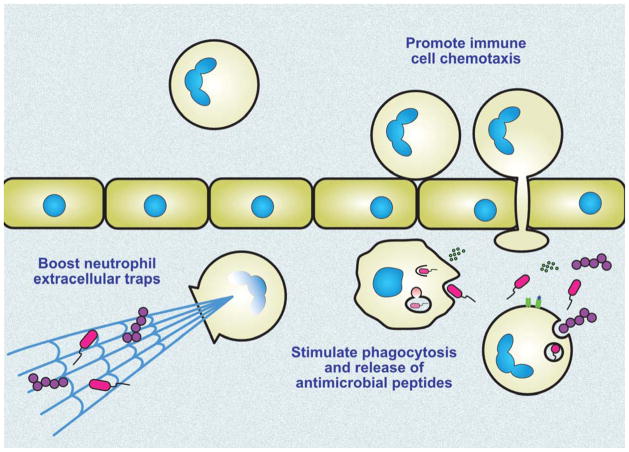
Development of a serious bacterial infection, by definition, declares a functional failure of innate immune cells to execute their frontline antimicrobial defense function. And while there are legions of medicines used in clinical medicine today whose purpose is to dial down immune cell activity in the treatment of inflammatory disorders such as rheumatoid arthritis or multiple sclerosis (e.g. corticosteroids, anti-cytokine therapies), the management of serious acute bacterial infections is performed agnostic to immune cell function. However, since the activation states of all immune cells are controlled through intricate pathways that rapidly deploy but promptly counter-regulate inflammatory processes (“accelerators” and “brakes”), opportunity to pharmacologically target more powerful immune cell killing function is gaining attention (Fig. 3).
Figure 3. Potential targets for host-directed immune boosting therapies against multidrug-resistant bacterial pathogens.
Taking advantage of evolving knowledge regarding chemokines and cytokines, pattern recognition receptors, and the regulatory programs they control, pharmacological approaches are being explored to boost the intrinsic antibacterial activity of macrophages and neutrophils.
Manipulating Cytokines and Chemokines
One group of strategies pursued in this vein envisions administration of endogenous pro-immune cytokines or chemokine to augment clearance of the potentially antibiotic-resistant pathogen. For example, treating mice with macrophage activating lipopeptide-2 (MIP-2), a C-X-C family cytokine akin to human interleukin-8, increased neutrophil and macrophage recruitment to the lung in S. pneumoniae lung infection, decreasing bacterial counts and promoting survival [54]. S. aureus resistance to lysozyme results suppresses the induction IFN-β production in from resident dendritic cells in skin, but addition of exogenous IFN-β can reverse this defect and promote bacterial clearance in vivo [55]. Intranasal administration of the mitogen keratinocyte growth factor (KGF) increased GM-CSF-dependent killing of Mycobacterium tuberculosis through enhanced macrophage phagolysosome fusion and nitric oxide production, improving clearance of the pathogen [56]. Similar results were found in E. coli pulmonary challenge, where KGF treatment protected animals by enhancing alveolar macrophage function and release of antimicrobial peptides into the broncheoalveolar fluid [57]. Finally, certain pathogens, such as K. pneumoniae, can immunosuppress the host by stimulating its production of anti-inflammatory cytokines such as interleukin-10 (IL-10). Pretreatment of K. pneumoniae-infected mice with anti-IL-10 serum boosted levels of TNFα, MIP-2 and neutrophil-derived myeloperoxidase, reducing bacterial load and increasing survival [58].
Lipid mediators of innate immunity to bacterial infection
Leukotrienes are a family of eicosanoid lipid inflammatory mediators arising from the oxidative metabolism of arachadonic acid. Treatment of macrophages with leukotriene B4 (LTB4) enhanced NADPH oxidase dependent production of reactive oxygen species and killing of S. pyogenes. [59]. Aerosolized administration of LTB4 to mice improved bacterial clearance and protected against mortality in pneumonia challenge models with S. pneumoniae or Klebsiella pneumoniae [60, 61]. Other specialized endogenous mediators (SPMs) explored for proimmune properties are derived from the omega-3 fatty acid docosahexaenoic acid (DHA), including molecules termed protectins, resolvins and maresins. Protectins, naturally produced by human M2 macrophages, enhance resolution of inflammation cause by infection. Administration of a synthetic protectin enhanced macrophage recruitment, phagocytosis of E. coli, while decreasing neutrophil infiltration, promoting efferocytosis of dead cells, and blunting production of pro-inflammatory lipid mediators [62]. Likewise, maresins and resolvins promote resolution of experimental murine E. coli or S. aureus infections, respectively, by increasing macrophage phagocytosis and efferocytosis while limiting neutrophil and eicosanoid production [63, 64]. Additional compounds, termed 13-series resolvins, were recently identified from neutrophil-endothelial co-cultures as a product of cyclooxygenase-2 (COX-2) activity, further induced by atorvastatin via S-notrosylation of COX-2, and present in human tissues after sterile inflammation or infection. Atorvastatin and 13-series resolvins had additive therapeutic effects to accelerate resolution of inflammation and protect against mortality in E. coli peritonitis [65]. Moreover, dietary modulations may skew lipid profiles in a beneficial manner, as mice fed a high fat diet containing the omega-3 unsaturated fatty acid (HFD-ω3) survived 5-fold better upon intravenous S. aureus challenge than mice fed with a control high fat diet with saturated fatty acids (HFD-S). Mice fed with HFD-ω3 had reduced bacterial loads in the kidney, better neutrophil stores in their bone marrow, and enhanced phagocytic activity of their neutrophils vs. S. aureus [66].
Identifying and Targeting Immune Regulatory Pathways in Phagocytes
Studies of altered infectious disease susceptibility in knockout mice have also inspired pharmacological approaches to host-directed innate immune boosting. While studying the role of leptin receptor (LepR)/STAT3 signaling, it was found that mutant mice deficient in leptin-induced STAT3 phosphorylation had improved bacterial clearance and survival when challenged with S. pneumoniae. Treatment of normal mice with a pharmacologic cysteinyl-leukotriene receptor antagonist increased resistance to pneumococcal infection [67]. Similarly acrophages and neutrophils deficient in the transcriptional regulator hypoxia-inducible factor-1 (HIF-1) have defects in glycolysis and ATP generation, leading to impaired microbicidal function and increased susceptibility of myeloid-specific HIF-1 knockout mice to infection [68]. Pharmacological stabilization of HIF-1 with a prolyl hydroxylase inhibitor drug (AKB-4924) to block its degradation pathway increased macrophage and neutrophil killing of MRSA and several Gram-negative bacterial pathogens, providing protection against skin and urinary tract infection in mouse challenge models [69, 70]. Mice in which deletion of C/EBPε mimics neutrophil-specific granule deficiency are hypersusceptible to S. aureus infection. Vitamin B3 (nicotinamide) pretreatment of human whole blood increased C/EBPε levels and clearance of S. aureus (as well as P. aeruginosa and K. pneumoniae) attributable to enhance phagocytic cell function. Treating mice with vitamin B3 also decreased S. aureus bacterial loads in kidneys and spleens [71]. Finally, in cystic fibrosis, mutations in the CFTR ion transporter lead to abnormal mucus dynamics, persistent bacterial pneumonia, and chronic debilitating loss of pulmonary function. CFTR-deficient macrophages have impaired autophagy-mediated killing of Burkholderia cenocepacia in association with exaggerated pro-inflammatory cytokine IL-1β production; stimulation of autophagy with rapamycin in CFTR-deficient macrophages and mice reduced bacterial burden and IL-1β secretion [72].
Immunomodulatory peptides
Therapeutic strategies are emerging in which particular immune-stimulatory peptides are derived or modified from endogenous molecules to exploit key host signaling pathways. For example, a natural fragment of lactoferrin, the peptide HLR1r, alters cytokine production and antimicrobial responses in macrophages, facilitating their recognition and effector killing functions against S. aureus and Candida albicans, and providing protection in ex vivo and in vivo skin infection models [73, 74]. Erythropoietin (Epo) participates in the stress responses to dampen pro-inflammatory signaling. An Epo analogue (ARA290) that selectively binds to a tissue protective receptor comprised of one subunit of the Epo receptor disulfide linked to CD131 caused an altered response to uropathogenic E. coli (UPEC) infection, modulating IL-8 secretion and reducing UPEC cellular invasion by dampening β-1 integrin signaling [75]. A novel peptide derived from the endogenous bovine neutrophil peptide bactenecin, named IDR-1002, induced potent chemokine production by human and murine macrophages, and when injected locally at the site of infection, enhanced neutrophil recruitment to clear both Gram-positive and Gram-negative bacterial infections [76].
Enhancing Toll-like receptor (TLR) signaling pathways
Toll-like receptors (TLRs) recognize “pathogen-associated microbial patterns” (PAMPs, e.g. lipopolysaccharide, peptidoglycan, flagellin) and host-derived “danger-associated molecular patterns” (DAMPs, e.g. HMGB1, serum amyloid A) to initiate signaling cascades that increase expression of innate immune response genes. Stimulating TLR4 with a synthetic lipid A mimetic induced macrophage and dendritic cell proinflammatory cytokine production, leading to an IFN-γ dependent reduction in organ bacterial burdens and increased survival in Francisella tularensis infection [77]. Stimulating TLR5 with flagellin protected mice from lethal pneumococcal pulmonary challenge, increasing pro-inflammatory cytokine expression and neutrophil recruitment to the lung [78]. Stimulation of TLR9 with CpG motif-containing oligodeoxynucleotides reduced bacterial burden in mice infected with Listeria monocytogenes, with immunological correlates of an IFN-γ dependent sustaining IL-12 production [79]. Injecting mice with CpG motif-rich F. tularensis DNA also showed protective efficacy in this model, promoting long-term pathogen-specific B cell-mediated responses against the Listeria [80]. Finally, targeting TLR co-receptor CD14 with leucine rich repeat peptides enhanced TLR2- and TLR4-dependent pro-inflammatory responses to bacteria, increasing phagocyte recruitment and accelerating bacterial clearance in mouse models of Gram-negative Gram-positive bacterial peritonitis. This CD14-directed strategy also rescued the pro-inflammatory response of peripheral blood mononuclear cells from immunosuppressed sepsis patients ex vivo [81].
Current drugs with unanticipated immune boosting effects
Imatinib (Gleevec) is a breakthrough drug targeting the ATP-binding pocket of ABL tyrosine kinase with remarkable efficacy as a “magic bullet” in treatment of chronic myelogenous leukemia and certain other malignancies. A number of intracellular pathogens including M. tuberculosis manipulate imatinib-sensitive kinases during cellular entry and phagolysosomal trafficking. Imatinib treatment of mice infected with Mycobacterium marinum reduced bacterial burden and liver pathology, and synergy with the antibiotic rifampicin further decreased bacterial load [82]. The antimicrobial efficacy of imatinib may in part be attributed to increased expression of vATPase leading to better phagolysosomal acidification [83]. Nonsteroidal anti-inflammatory drug (NSAID) celecoxib is used to treat pain or inflammation. A celecoxib derivative (AR-12) has off-target autophagy-inducing properties that promote macrophage clearance of intracellular pathogens Francisella and Salmonella, prolonging survival in murine challenge models [84, 85]. Finally, estrogen receptor antagonist tamoxifen is a mainstay of treatment for many forms of breast cancer. Treating human neutrophils with tamoxifen increased chemotaxis and formation of neutrophil “extracellular traps” (NETs), a special type of cell death wherein nuclear DNA is released in a sticky meshwork to ensnare bacteria, exposing them to high concentrations of antimicrobial peptides and histones [86]. Tamoxifen neutrophil boosting was independent of estrogen receptor antagonism, but rather depended upon PKCζ-dependent increases in intracellular ceramide levels. Tamoxifen NET induction boosted neutrophil bacterial killing, and treatment of mice with tamoxifen decreased bacterial burden in multiple organs and protected against mortality in systemic MRSA infection [86].
Statins: Repurposing a leading human medication at the host-pathogen interface
Statins bind to the active site of 3-hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA) reductase, the rate-limiting enzyme involved in cholesterol biosynthesis. Due to their oral availability, favorable pharmacokinetic properties, and relatively low level of associated side effects, these drugs have become a mainstay of LDL cholesterol-lowering therapy, now used to treat tens of millions of individuals with hyperlipidemia worldwide. Interestingly, recent meta-analyses have compiled a heterogeneous collection of retrospective and prospective cohort studies and randomized trials to conclude that statin use may provide a beneficial effect in reducing morbidity and mortality associated with different infectious disease conditions, including pneumonia, bacteremia, and sepsis [87, 88]. Thus it has been hypothesized that statins may have pharmacological effects, either through cholesterol reduction itself or via an independent “off-target” mechanism, to aid in pathogen resistance. Since these drugs have limited direct in vitro antimicrobial activity against the leading pathogens, recent studies have focused on the effects of statins on both sides of the host-pathogen interaction.
Statin treatment modulates certain key bacterial virulence phenotypes. At subinhibitory concentrations, statins reduce biofilm formation in P. aeruginosa, S. aureus and Staphylococcus epidermidis [89–91], and suppress production of S. aureus cytolysins alpha-toxin and Panton-Valentine leukocidin [90]. Statins may also block host epithelial or endothelial cell invasion by P. aeruginosa, S. aureus, or the neonatal pathogen group B Streptococcus [92–94]; the later study suggesting that mevastatin antagonism of Rho-family GTPases involved in endocytotic uptake might inhibit pathogen host cell entry.
Likewise, statins may augment defense against bacterial pathogens by mechanisms that work through the host cell. Reduction of cholesterol may reduce host cell susceptibility to lysis by bacterial pore-forming toxins such as pneumolysin, which utilize cholesterol as a receptor for engagement and assembly in the host cell membrane [95]. Killing of macrophages by anthrax lethal toxin is also reduced significantly by statins because they antagonize Rho-family GTPases [96]. Statin depletion of intracellular cholesterol also impacts the formation of lipid rafts, plasma membrane subdomains important in cell signaling, which can also be exploited by certain intracellular pathogens for intracellular trafficking and survival, e.g. Chlamydia pneumoniae and L. monocytogenes. Cellular transmission of C. pneumoniae in macrophage co-culture experiments with vascular smooth muscle cells is reduced by statin treatment [97], which reduced disease severity in a murine C. pneumoniae pulmonary infection model [98]. Statins protect against L. monocytogenes infection by reducing membrane cholesterol in macrophages and blocking the ability of the cholesterol-dependent cytolysin listeriolysin O to mediate escape of the bacterium from the phagosome to the cytoplasm [99]. Lastly, statins increased neutrophil and macrophage extracellular trap-based killing of S. aureus, protecting against pulmonary challenge with the pathogen and enhancing extracellular trap formation in vivo [100].
Concluding Remarks
Decreasing treatment options for antibiotic-resistant pathogens places an imperative both on the medical community and upon academic and industry drug discovery initiatives to discover innovative therapeutic approaches to reduce morbidity and mortality in the increasingly complex patient populations at greatest risk thinking beyond classical antibiotics to envision strategies that carefully analyze the host-pathogen interaction for opportunities to “tip the balance” back in favor of host immune clearance and preservation of cell and tissue integrity would be a welcome and overdue paradigm shift with the potential for considerable upside. Virulence factor inhibitors can offer increased specificity and personalization of therapy to the infection at hand, with reduction of undesired side effects placed by broad-spectrum antibiotics on the integrity of the normal host microflora. Definition of virulence determinants for treatment, however, becomes more challenging in immunocompromised patients, where organisms with little de novo virulence potential in normal hosts can sometimes produce severe infectious consequences, but much less may be understood about their pathogenic mechanisms.
We should draw inspiration from host-directed immunotherapeutics that have revolutionized the field of cancer therapeutics, e.g. PDL-1 checkpoint inhibitors that boost antitumor T cells in melanoma to dramatically improved outcomes. These successes can inspire future precision medicines that modulate host targets to enhance antimicrobial function of innate immune cells such as macrophages and neutrophils for infectious disease therapeutics. In this manner, we may also theoretically help cure or prevent deep tissue foci of antibiotic-resistant pathogens with minimal collateral damage our microbiota. In our experience, promotion of host-directed immune boosting therapeutics has raised theoretical concerns among some drug developers regarding pro-inflammatory consequences of the therapy. We believe antibiotic-resistant pathogens are themselves causing even more deleterious inflammatory effects on the host until effective medical cure is achieved, and that wise host immune pathway targeting can overcome such hurdles.
The many proofs-of-concept provided in this review are provided to illustrate that both screening based and targeted approaches to discovery of virulence factor inhibitors and immune boosters are feasible. Analysis of the biological action of current FDA-approved drugs, used for different indications but with known effects on cellular biochemistry and metabolism, offer the opportunity for repurposing as adjunctive antimicrobial agents to modulate virulence factor expression or fortify host immune function and resiliency against bacterial infection.
Trends Box.
To address the ever-increasing problem of multidrug-resistant pathogens, the narrow conceptualization of antibiotic pharmacology must be expanded to a holistic analysis of the host-pathogen interaction.
Pharmacologically targeting bacterial virulence factors is an alternative strategy to halt disease progression and enhance immune clearance. By “disarming” specific pathogens rather than killing them with broad-spectrum antibiotics the normal microbiome and its critical functions are preserved.
Host-directed therapies to boost the resilience and microbicidal capacities of immune cells may enhance clearance of antibiotic-resistant pathogens, mirroring successful cancer immune-therapeutics.
When pharmacological activities are studied in the richer context of bacterial immune systems interactions, antibiotics deemed inactive in standard laboratory testing show hidden activities and drugs approved for other clinical indications may be repurposed to expand our antimicrobial armamentarium.
Outstanding Questions Box.
Will a pharmaceutical agent that does not directly kill a bacterial pathogen be readily accepted into modern clinical dogma for treating multidrug-resistant infections?
How receptive will the FDA and other government agencies be to adopting novel clinical trial designs for pathogen-specific approaches in smaller targeted patient groups in order to get innovative therapies to patients?
Can rapid, sensitive and specific (culture-independent) diagnostic assays for bacterial identification be developed to allow prompt deployment of anti-virulence therapies in a cost-effective manner?
Will bacterial pathogens have the same potential for stepwise evolution of resistance to anti-virulence therapies as they do for classical antibiotics?
Is there a potential for undesired inflammatory or autoimmune side effects when innate immune cells are stimulated to fight off bacterial infections?
Will virulence factor inhibiting and immune cell boosting antibacterial therapies better preserve the patient’s normal microbial flora and their critical roles in immune and metabolic homeostasis?
References
- 1.O’Neill J. Trust, U.G.a.t.W, editor. Antimicrobial Resistance: Tackling a Crisis for the Health and Wealth of Nations. 2014. Review on Antimicrobial Resistance. [Google Scholar]
- 2.Chan M., Director-General of the World Health Organization . Speech, to the United Nations General Assembly. 2016. [Google Scholar]
- 3.Nizet V. Stopping superbugs, maintaining the microbiota. Sci Transl Med. 2015;7(295):295ed8. doi: 10.1126/scitranslmed.aab2373. [DOI] [PubMed] [Google Scholar]
- 4.Gustke H, et al. Inhibition of the bacterial lectins of Pseudomonas aeruginosa with monosaccharides and peptides. Eur J Clin Microbiol Infect Dis. 2012;31(2):207–15. doi: 10.1007/s10096-011-1295-x. [DOI] [PubMed] [Google Scholar]
- 5.Johansson EM, et al. Inhibition and dispersion of Pseudomonas aeruginosa biofilms by glycopeptide dendrimers targeting the fucose-specific lectin LecB. Chem Biol. 2008;15(12):1249–57. doi: 10.1016/j.chembiol.2008.10.009. [DOI] [PubMed] [Google Scholar]
- 6.Cusumano CK, et al. Treatment and prevention of urinary tract infection with orally active FimH inhibitors. Sci Transl Med. 2011;3(109):109ra115. doi: 10.1126/scitranslmed.3003021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Oh KB, et al. Inhibition of sortase-mediated Staphylococcus aureus adhesion to fibronectin via fibronectin-binding protein by sortase inhibitors. Appl Microbiol Biotechnol. 2006;70(1):102–6. doi: 10.1007/s00253-005-0040-8. [DOI] [PubMed] [Google Scholar]
- 8.Oleksiewicz MB, et al. Anti-bacterial monoclonal antibodies: back to the future? Arch Biochem Biophys. 2012;526(2):124–31. doi: 10.1016/j.abb.2012.06.001. [DOI] [PubMed] [Google Scholar]
- 9.Sause WE, et al. Antibody-based biologics and their promise to combat Staphylococcus aureus infections. Trends Pharmacol Sci. 2016;37(3):231–41. doi: 10.1016/j.tips.2015.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Berube BJ, Bubeck Wardenburg J. Staphylococcus aureus alpha-toxin: nearly a century of intrigue. Toxins (Basel) 2013;5(6):1140–66. doi: 10.3390/toxins5061140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Inoshima I, et al. A Staphylococcus aureus pore-forming toxin subverts the activity of ADAM10 to cause lethal infection in mice. Nat Med. 2011;17(10):1310–4. doi: 10.1038/nm.2451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ragle BE, Bubeck Wardenburg J. Anti-alpha-hemolysin monoclonal antibodies mediate protection against Staphylococcus aureus pneumonia. Infect Immun. 2009;77(7):2712–8. doi: 10.1128/IAI.00115-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hua L, et al. Assessment of an anti-alpha-toxin monoclonal antibody for prevention and treatment of Staphylococcus aureus-induced pneumonia. Antimicrob Agents Chemother. 2014;58(2):1108–17. doi: 10.1128/AAC.02190-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rouha H, et al. Five birds, one stone: neutralization of alpha-hemolysin and 4 bi-component leukocidins of Staphylococcus aureus with a single human monoclonal antibody. MAbs. 2015;7(1):243–54. doi: 10.4161/19420862.2014.985132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Scully IL, et al. Demonstration of the preclinical correlate of protection for Staphylococcus aureus clumping factor A in a murine model of infection. Vaccine. 2015;33(41):5452–7. doi: 10.1016/j.vaccine.2015.08.029. [DOI] [PubMed] [Google Scholar]
- 16.McAdow M, et al. Preventing Staphylococcus aureus sepsis through the inhibition of its agglutination in blood. PLoS Pathog. 2011;7(10):e1002307. doi: 10.1371/journal.ppat.1002307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sawa T, et al. Anti-PcrV antibody strategies against virulent Pseudomonas aeruginosa. Hum Vaccin Immunother. 2014;10(10):2843–52. doi: 10.4161/21645515.2014.971641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Warrener P, et al. A novel anti-PcrV antibody providing enhanced protection against Pseudomonas aeruginosa in multiple animal infection models. Antimicrob Agents Chemother. 2014;58(8):4384–91. doi: 10.1128/AAC.02643-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yamamoto BJ, et al. Obiltoxaximab prevents disseminated Bacillus anthracis infection and improves survival during pre- and post-exposure prophylaxis in animal models of inhalational anthrax. Antimicrob Agents Chemother. 2016;60(10):5796–805. doi: 10.1128/AAC.01102-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Anosova NG, et al. A combination of three fully human toxin A- and toxin B-specific monoclonal antibodies protects against challenge with highly virulent epidemic strains of Clostridium difficile in the hamster model. Clin Vaccine Immunol. 2015;22(7):711–25. doi: 10.1128/CVI.00763-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lehar SM, et al. Novel antibody-antibiotic conjugate eliminates intracellular S. aureus. Nature. 2015;527(7578):323–8. doi: 10.1038/nature16057. [DOI] [PubMed] [Google Scholar]
- 22.McCormick CC, et al. Chemical inhibition of alpha-toxin, a key corneal virulence factor of Staphylococcus aureus. Invest Ophthalmol Vis Sci. 2009;50(6):2848–54. doi: 10.1167/iovs.08-3157. [DOI] [PubMed] [Google Scholar]
- 23.Ragle BE, et al. Prevention and treatment of Staphylococcus aureus pneumonia with a beta-cyclodextrin derivative. Antimicrob Agents Chemother. 2010;54(1):298–304. doi: 10.1128/AAC.00973-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Henry BD, et al. Engineered liposomes sequester bacterial exotoxins and protect from severe invasive infections in mice. Nat Biotechnol. 2015;33(1):81–8. doi: 10.1038/nbt.3037. [DOI] [PubMed] [Google Scholar]
- 25.Hu CM, et al. A biomimetic nanosponge that absorbs pore-forming toxins. Nat Nanotechnol. 2013;8(5):336–40. doi: 10.1038/nnano.2013.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang F, et al. Hydrogel retaining toxin-absorbing nanosponges for local treatment of methicillin-resistant Staphylococcus aureus infection. Adv Mater. 2015;27(22):3437–43. doi: 10.1002/adma.201501071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hu CM, et al. Nanoparticle biointerfacing by platelet membrane cloaking. Nature. 2015;526(7571):118–21. doi: 10.1038/nature15373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Basha S, et al. Polyvalent inhibitors of anthrax toxin that target host receptors. Proc Natl Acad Sci U S A. 2006;103(36):13509–13. doi: 10.1073/pnas.0509870103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sampedro GR, et al. Targeting Staphylococcus aureus alpha-toxin as a novel approach to reduce severity of recurrent skin and soft-tissue infections. J Infect Dis. 2014;210(7):1012–8. doi: 10.1093/infdis/jiu223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gillespie EJ, et al. Selective inhibitor of endosomal trafficking pathways exploited by multiple toxins and viruses. Proc Natl Acad Sci USA. 2013;110(50):E4904–12. doi: 10.1073/pnas.1302334110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shoop WL, et al. Anthrax lethal factor inhibition. Proc Natl Acad Sci USA. 2005;102(22):7958–63. doi: 10.1073/pnas.0502159102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bender KO, et al. A small-molecule antivirulence agent for treating Clostridium difficile infection. Sci Transl Med. 2015;7(306):306ra148. doi: 10.1126/scitranslmed.aac9103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gonzalez-Juarbe N, et al. Pore-forming toxins induce macrophage necroptosis during acute bacterial pneumonia. PLoS Pathog. 2015;11(12):e1005337. doi: 10.1371/journal.ppat.1005337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gilley RP, et al. Infiltrated macrophages die of pneumolysin-mediated necroptosis following pneumococcal myocardial invasion. Infect Immun. 2016;84(5):1457–69. doi: 10.1128/IAI.00007-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hung DT, et al. Small-molecule inhibitor of Vibrio cholerae virulence and intestinal colonization. Science. 2005;310(5748):670–4. doi: 10.1126/science.1116739. [DOI] [PubMed] [Google Scholar]
- 36.Nait Chabane Y, et al. Virstatin inhibits biofilm formation and motility of Acinetobacter baumannii. BMC Microbiol. 2014;14:62. doi: 10.1186/1471-2180-14-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yang J, et al. Disarming bacterial virulence through chemical inhibition of the DNA binding domain of an AraC-like transcriptional activator protein. J Biol Chem. 2013;288(43):31115–26. doi: 10.1074/jbc.M113.503912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Koppolu V, et al. Small-molecule inhibitor of the Shigella flexneri master virulence regulator VirF. Infect Immun. 2013;81(11):4220–31. doi: 10.1128/IAI.00919-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Parsons JB, et al. Perturbation of Staphylococcus aureus gene expression by the enoyl-acyl carrier protein reductase inhibitor AFN-1252. Antimicrob Agents Chemother. 2013;57(5):2182–90. doi: 10.1128/AAC.02307-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sun H, et al. Inhibitor of streptokinase gene expression improves survival after group A streptococcus infection in mice. Proc Natl Acad Sci USA. 2012;109(9):3469–74. doi: 10.1073/pnas.1201031109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kim HS, et al. 6-Gingerol reduces Pseudomonas aeruginosa biofilm formation and virulence via quorum sensing inhibition. Sci Rep. 2015;5:8656. doi: 10.1038/srep08656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.O’Loughlin CT, et al. A quorum-sensing inhibitor blocks Pseudomonas aeruginosa virulence and biofilm formation. Proc Natl Acad Sci USA. 2013;110(44):17981–6. doi: 10.1073/pnas.1316981110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kasper SH, et al. Chemical inhibition of kynureninase reduces Pseudomonas aeruginosa quorum sensing and virulence factor expression. ACS Chem Biol. 2016;11(4):1106–17. doi: 10.1021/acschembio.5b01082. [DOI] [PubMed] [Google Scholar]
- 44.Zhang Y, et al. Inhibition of alpha-toxin production by subinhibitory concentrations of naringenin controls Staphylococcus aureus pneumonia. Fitoterapia. 2013;86:92–9. doi: 10.1016/j.fitote.2013.02.001. [DOI] [PubMed] [Google Scholar]
- 45.Hendrix AS, et al. Repurposing the nonsteroidal anti-inflammatory drug diflunisal as an osteoprotective, anti-virulence therapy for Staphylococcus aureus osteomyelitis. Antimicrob Agents Chemother. 2016;60(9):5322–30. doi: 10.1128/AAC.00834-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Goller CC, et al. Lifting the mask: identification of new small molecule inhibitors of uropathogenic Escherichia coli group 2 capsule biogenesis. PLoS One. 2014;9(7):e96054. doi: 10.1371/journal.pone.0096054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sakoulas G, et al. Nafcillin enhances innate immune-mediated killing of methicillin-resistant Staphylococcus aureus. J Mol Med (Berl) 2014;92(2):139–49. doi: 10.1007/s00109-013-1100-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kumaraswamy M, et al. Standard susceptibility testing overlooks potent azithromycin activity and cationic peptide synergy against MDR Stenotrophomonas maltophilia. J Antimicrob Chemother. 2016;71(5):1264–9. doi: 10.1093/jac/dkv487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lin L, et al. Azithromycin synergizes with cationic antimicrobial peptides to exert bactericidal and therapeutic activity against highly multidrug-resistant Gram-negative bacterial pathogens. EBioMedicine. 2015;2(7):690–8. doi: 10.1016/j.ebiom.2015.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Liu GY, et al. Staphylococcus aureus golden pigment impairs neutrophil killing and promotes virulence through its antioxidant activity. J Exp Med. 2005;202(2):209–15. doi: 10.1084/jem.20050846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Liu CI, et al. A cholesterol biosynthesis inhibitor blocks Staphylococcus aureus virulence. Science. 2008;319(5868):1391–4. doi: 10.1126/science.1153018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chen F, et al. Small-molecule targeting of a diapophytoene desaturase inhibits S. aureus virulence. Nat Chem Biol. 2016;12(3):174–9. doi: 10.1038/nchembio.2003. [DOI] [PubMed] [Google Scholar]
- 53.Mishra NN, et al. Carotenoid-related alteration of cell membrane fluidity impacts Staphylococcus aureus susceptibility to host defense peptides. Antimicrob Agents Chemother. 2011;55(2):526–31. doi: 10.1128/AAC.00680-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Reppe K, et al. Immunostimulation with macrophage-activating lipopeptide-2 increased survival in murine pneumonia. Am J Respir Cell Mol Biol. 2009;40(4):474–81. doi: 10.1165/rcmb.2008-0071OC. [DOI] [PubMed] [Google Scholar]
- 55.Kaplan A, et al. Failure to induce IFN-beta production during Staphylococcus aureus infection contributes to pathogenicity. J Immunol. 2012;189(9):4537–45. doi: 10.4049/jimmunol.1201111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pasula R, et al. Keratinocyte growth factor administration attenuates murine pulmonary mycobacterium tuberculosis infection through granulocyte-macrophage colony-stimulating factor (GM-CSF)-dependent macrophage activation and phagolysosome fusion. J Biol Chem. 2015;290(11):7151–9. doi: 10.1074/jbc.M114.591891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wu H, et al. Keratinocyte growth factor augments pulmonary innate immunity through epithelium-driven, GM-CSF-dependent paracrine activation of alveolar macrophages. J Biol Chem. 2011;286(17):14932–40. doi: 10.1074/jbc.M110.182170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Greenberger MJ, et al. Neutralization of IL-10 increases survival in a murine model of Klebsiella pneumoniae. J Immunol. 1995;155(2):722–9. [PubMed] [Google Scholar]
- 59.Soares EM, et al. Leukotriene B4 enhances innate immune defense against the puerperal sepsis agent Streptococcus pyogenes. J Immunol. 2013;190(4):1614–22. doi: 10.4049/jimmunol.1202932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mancuso P, et al. Intrapulmonary administration of leukotriene B4 enhances pulmonary host defense against pneumococcal pneumonia. Infect Immun. 2010;78(5):2264–71. doi: 10.1128/IAI.01323-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Batra S, et al. Intrapulmonary administration of leukotriene B(4) augments neutrophil accumulation and responses in the lung to Klebsiella infection in CXCL1 knockout mice. J Immunol. 2012;188(7):3458–68. doi: 10.4049/jimmunol.1101985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ramon S, et al. The Protectin PCTR1 Is produced by human M2 macrophages and enhances resolution of infectious inflammation. Am J Pathol. 2016;186(4):962–73. doi: 10.1016/j.ajpath.2015.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dalli J, et al. Identification and actions of a novel third maresin conjugate in tissue regeneration: MCTR3. PLoS One. 2016;11(2):e0149319. doi: 10.1371/journal.pone.0149319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Winkler JW, et al. Resolvin D4 stereoassignment and its novel actions in host protection and bacterial clearance. Sci Rep. 2016;6:18972. doi: 10.1038/srep18972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dalli J, et al. Elucidation of novel 13-series resolvins that increase with atorvastatin and clear infections. Nat Med. 2015;21(9):1071–5. doi: 10.1038/nm.3911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Svahn SL, et al. Dietary omega-3 fatty acids increase survival and decrease bacterial load in mice subjected to Staphylococcus aureus-induced sepsis. Infect Immun. 2016;84(4):1205–13. doi: 10.1128/IAI.01391-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mancuso P, et al. Disruption of leptin receptor-STAT3 signaling enhances leukotriene production and pulmonary host defense against pneumococcal pneumonia. J Immunol. 2011;186(2):1081–90. doi: 10.4049/jimmunol.1001470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Peyssonnaux C, et al. HIF-1alpha expression regulates the bactericidal capacity of phagocytes. J Clin Invest. 2005;115(7):1806–15. doi: 10.1172/JCI23865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Okumura CY, et al. A new pharmacological agent (AKB-4924) stabilizes hypoxia inducible factor-1 (HIF-1) and increases skin innate defenses against bacterial infection. J Mol Med (Berl) 2012;90(9):1079–89. doi: 10.1007/s00109-012-0882-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lin AE, et al. Role of hypoxia inducible factor-1alpha (HIF-1alpha) in innate defense against uropathogenic Escherichia coli infection. PLoS Pathog. 2015;11(4):e1004818. doi: 10.1371/journal.ppat.1004818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kyme P, et al. C/EBPepsilon mediates nicotinamide-enhanced clearance of Staphylococcus aureus in mice. J Clin Invest. 2012;122(9):3316–29. doi: 10.1172/JCI62070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Abdulrahman BA, et al. Autophagy stimulation by rapamycin suppresses lung inflammation and infection by Burkholderia cenocepacia in a model of cystic fibrosis. Autophagy. 2011;7(11):1359–70. doi: 10.4161/auto.7.11.17660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.van der Does AM, et al. Antimicrobial peptide hLF1-11 directs granulocyte-macrophage colony-stimulating factor-driven monocyte differentiation toward macrophages with enhanced recognition and clearance of pathogens. Antimicrob Agents Chemother. 2010;54(2):811–6. doi: 10.1128/AAC.00652-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bjorn C, et al. Anti-infective efficacy of the lactoferrin-derived antimicrobial peptide HLR1r. Peptides. 2016;81:21–8. doi: 10.1016/j.peptides.2016.04.005. [DOI] [PubMed] [Google Scholar]
- 75.Polgarova K, et al. The erythropoietin analogue ARA290 modulates the innate immune response and reduces Escherichia coli invasion into urothelial cells. FEMS Immunol Med Microbiol. 2011;62(2):190–6. doi: 10.1111/j.1574-695X.2011.00801.x. [DOI] [PubMed] [Google Scholar]
- 76.Nijnik A, et al. Synthetic cationic peptide IDR-1002 provides protection against bacterial infections through chemokine induction and enhanced leukocyte recruitment. J Immunol. 2010;184(5):2539–50. doi: 10.4049/jimmunol.0901813. [DOI] [PubMed] [Google Scholar]
- 77.Lembo A, et al. Administration of a synthetic TLR4 agonist protects mice from pneumonic tularemia. J Immunol. 2008;180(11):7574–81. doi: 10.4049/jimmunol.180.11.7574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Munoz N, et al. Mucosal administration of flagellin protects mice from Streptococcus pneumoniae lung infection. Infect Immun. 2010;78(10):4226–33. doi: 10.1128/IAI.00224-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Krieg AM, et al. CpG DNA induces sustained IL-12 expression in vivo and resistance to Listeria monocytogenes challenge. J Immunol. 1998;161(5):2428–34. [PubMed] [Google Scholar]
- 80.Elkins KL, et al. Bacterial DNA containing CpG motifs stimulates lymphocyte-dependent protection of mice against lethal infection with intracellular bacteria. J Immunol. 1999;162(4):2291–8. [PubMed] [Google Scholar]
- 81.Raby AC, et al. Targeting the TLR co-receptor CD14 with TLR2-derived peptides modulates immune responses to pathogens. Sci Transl Med. 2013;5(185):185ra64. doi: 10.1126/scitranslmed.3005544. [DOI] [PubMed] [Google Scholar]
- 82.Napier RJ, et al. Imatinib-sensitive tyrosine kinases regulate mycobacterial pathogenesis and represent therapeutic targets against tuberculosis. Cell Host Microbe. 2011;10(5):475–85. doi: 10.1016/j.chom.2011.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bruns H, et al. Abelson tyrosine kinase controls phagosomal acidification required for killing of Mycobacterium tuberculosis in human macrophages. J Immunol. 2012;189(8):4069–78. doi: 10.4049/jimmunol.1201538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Chiu HC, et al. Eradication of intracellular Salmonella enterica serovar Typhimurium with a small-molecule, host cell-directed agent. Antimicrob Agents Chemother. 2009;53(12):5236–44. doi: 10.1128/AAC.00555-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Chiu HC, et al. Eradication of intracellular Francisella tularensis in THP-1 human macrophages with a novel autophagy inducing agent. J Biomed Sci. 2009;16:110. doi: 10.1186/1423-0127-16-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Corriden R, et al. Tamoxifen augments the innate immune function of neutrophils through modulation of intracellular ceramide. Nat Commun. 2015;6:8369. doi: 10.1038/ncomms9369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Janda S, et al. The effect of statins on mortality from severe infections and sepsis: a systematic review and meta-analysis. J Crit Care. 2010;25(4):656e7–22. doi: 10.1016/j.jcrc.2010.02.013. [DOI] [PubMed] [Google Scholar]
- 88.Chopra V, et al. Is statin use associated with reduced mortality after pneumonia? A systematic review and meta-analysis. Am J Med. 2012;125(11):1111–23. doi: 10.1016/j.amjmed.2012.04.011. [DOI] [PubMed] [Google Scholar]
- 89.Graziano TS, et al. Statins and antimicrobial effects: simvastatin as a potential drug against Staphylococcus aureus biofilm. PLoS One. 2015;10(5):e0128098. doi: 10.1371/journal.pone.0128098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Thangamani S, et al. Exploring simvastatin, an antihyperlipidemic drug, as a potential topical antibacterial agent. Sci Rep. 2015;5:16407. doi: 10.1038/srep16407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hennessy E, et al. Statins inhibit in vitro virulence phenotypes of Pseudomonas aeruginosa. J Antibiot (Tokyo) 2013;66(2):99–101. doi: 10.1038/ja.2012.95. [DOI] [PubMed] [Google Scholar]
- 92.Horn MP, et al. Simvastatin inhibits Staphylococcus aureus host cell invasion through modulation of isoprenoid intermediates. J Pharmacol Exp Ther. 2008;326(1):135–43. doi: 10.1124/jpet.108.137927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Shibata H, et al. Simvastatin represses translocation of Pseudomonas aeruginosa across Madin-Darby canine kidney cell monolayers. J Med Invest. 2012;59(1–2):186–91. doi: 10.2152/jmi.59.186. [DOI] [PubMed] [Google Scholar]
- 94.Burnham CA, et al. Rac1, RhoA, and Cdc42 participate in HeLa cell invasion by group B streptococcus. FEMS Microbiol Lett. 2007;272(1):8–14. doi: 10.1111/j.1574-6968.2007.00768.x. [DOI] [PubMed] [Google Scholar]
- 95.Rosch JW, et al. Statins protect against fulminant pneumococcal infection and cytolysin toxicity in a mouse model of sickle cell disease. J Clin Invest. 2010;120(2):627–35. doi: 10.1172/JCI39843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.deCathelineau AM, Bokoch GM. Inactivation of rho GTPases by statins attenuates anthrax lethal toxin activity. Infect Immun. 2009;77(1):348–59. doi: 10.1128/IAI.01005-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Dechend R, et al. Hydroxymethylglutaryl coenzyme A reductase inhibition reduces Chlamydia pneumoniae-induced cell interaction and activation. Circulation. 2003;108(3):261–5. doi: 10.1161/01.CIR.0000083367.93022.78. [DOI] [PubMed] [Google Scholar]
- 98.Erkkila L, et al. Effect of simvastatin, an established lipid-lowering drug, on pulmonary Chlamydia pneumoniae infection in mice. Antimicrob Agents Chemother. 2005;49(9):3959–62. doi: 10.1128/AAC.49.9.3959-3962.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Parihar SP, et al. Simvastatin enhances protection against Listeria monocytogenes infection in mice by counteracting Listeria-induced phagosomal escape. PLoS One. 2013;8(9):e75490. doi: 10.1371/journal.pone.0075490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Chow OA, et al. Statins enhance formation of phagocyte extracellular traps. Cell Host Microbe. 2010;8(5):445–54. doi: 10.1016/j.chom.2010.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]