Abstract
Rationale
Nalfurafine is a G-protein-signaling-biased kappa opioid receptor (KOR) agonist approved in Japan for second-line treatment of uremic pruritus. Neither nalfurafine nor any other KOR agonist is currently approved anywhere for treatment of pain, but recent evidence suggests that G-protein-signaling-biased KOR agonists may have promise as candidate analgesics/antipruritics with reduced side effects compared to nonbiased or β-arrestin-signaling-biased KOR agonists.
Objectives
This study compared nalfurafine effects in rats using assays of pain-stimulated and pain-depressed behavior used previously to evaluate other candidate analgesics. Nalfurafine effects were also examined in complementary assays of itch-stimulated and itch-depressed behavior.
Methods
Intraperitoneal lactic acid (IP acid) and intradermal serotonin (ID 5HT) served as noxious and pruritic stimuli, respectively, in male Sprague Dawley rats to stimulate stretching (IP acid) or scratching (ID 5HT) or to depress positively reinforced operant responding in an assay of intracranial self-stimulation (ICSS; both stimuli).
Results
Nalfurafine was equipotent to decrease IP acid-stimulated stretching and ID 5HT-stimulated scratching; however, doses of nalfurafine that decreased these pain/itch-stimulated behaviors also decreased control ICSS performance. Moreover, nalfurafine failed to alleviate either IP acid- or ID 5HT-induced depression of ICSS.
Conclusions
These results suggest that nalfurafine-induced decreases in pain/itch-stimulated behaviors may reflect nonselective decreases in motivated behavior rather than analgesia or antipruritus against the noxious and pruritic stimuli used here. This conclusion agrees with the absence of clinical data for nalfurafine analgesia and the weak clinical data for nalfurafine antipruritus. Nalfurafine bias for G-protein signaling may not be sufficient for clinically safe and reliable analgesia or antipruritus.
Keywords: nalfurafine, kappa opioid receptor, pain, nociception, itch, pruritus, scratching, intracranial self-stimulation, pain-depressed behavior
INTRODUCTION
Nalfurafine is a high-efficacy kappa opioid receptor (KOR) agonist available clinically in Japan for treatment of uremic pruritus (Inui 2015). Relative to many other KOR agonists, nalfurafine is biased toward G-protein vs. β-arrestin intracellular signaling pathways coupled to KOR (Schattauer et al. 2017), and this signaling bias may contribute to an improved side-effect profile (Brust et al. 2016; Dogra and Yadav 2015; White et al. 2015). Consistent with this possibility, nalfurafine was initially evaluated as a candidate analgesic and found to be effective in a wide range of preclinical pain assays (Endoh et al. 1999; Endoh et al. 2001; Endoh et al. 2000; Nagase et al. 1998); however, it has not been approved for the treatment of pain in Japan or elsewhere, and there is no published evidence to suggest that it has clinical efficacy for treatment of pain in humans. Thus, nalfurafine may be similar to other centrally acting KOR agonists such as enadoline that have also shown efficacy to produce antinociception in conventional preclinical pain assays (Davis et al. 1992; Field et al. 1999; Hunter et al. 1990) but then failed to display adequate analgesic effectiveness and/or safety in clinical trials (Pande et al. 1996). The centrally acting kappa agonist salvinorin A, which is sometimes abused, has also been shown to produce antinociception in many conventional preclinical pain assays (Kivell and Prisinzano 2010; Listos et al., 2011), but analgesia has not been cited as an effect in humans under laboratory conditions (Johnson et al. 2011; MacLean et al. 2013) or in published anecdotal reports following its recreational use (Baggott et al. 2010). At present, no selective centrally acting kappa opioid agonists are clinically approved for pain treatment, and there is no evidence that these drugs are used to treat pain in humans.
One possible cause for the poor preclinical-to-clinical translation of results with centrally acting KOR agonists is the discordance in endpoints between animal and human studies of candidate analgesics. In humans, the primary endpoints for analgesic assessment are verbal reports by the patient using instruments such as numeric rating scales (Melzack and Katz 2013). Conversely, conventional procedures in laboratory animals measure behaviors that can be labeled as “pain-stimulated behaviors” (Negus 2013; Negus et al. 2006). These are behaviors that increase in rate, frequency, or intensity after delivery of a noxious stimulus, and examples include withdrawal responses from escapable stimuli (e.g. tail-withdrawal from a thermal stimulus) or other behaviors stimulated by inescapable stimuli (e.g. writhing/stretching responses elicited by intraperitoneal injection of chemical irritants). Analgesic drugs decrease expression of pain-stimulated behaviors, but false-positive effects can result from non-selective drug effects such as sedation or paralysis. Insofar as centrally acting KOR agonists are well known to inhibit expression of a wide range of behaviors, it is possible that apparent antinociception by KOR agonists in conventional preclinical assays of pain-stimulated behavior reflects non-selective motor inhibition rather than analgesia (Brust et al. 2016; Negus et al. 2010; Negus et al. 2015; Negus et al. 2012a).
A secondary category of pain behaviors in human studies focuses on pain-related impairment of function (e.g. decreased ability to walk, recreate, or work). We have referred to these as “pain-depressed behaviors” [i.e. behaviors that decrease in rate, frequency, or intensity after delivery of a noxious stimulus (Negus 2013; Negus et al. 2006)]. Although the degree of functional impairment and pain-depressed behavior in humans can be assessed by questionnaires answered by the patient, it can also be assessed by external observers in cases where the patient is not verbally capable (e.g. children) (Dworkin et al. 2005; McGrath and Unruh 2013; Melzack and Katz 2013). Measurement of functional impairment by external observers also plays a key role in veterinary pain assessment (Brown et al. 2008; National Research Council 2003), and relief of pain-related functional impairment is a goal in both human and veterinary medical practice. Thus, measures of pain-related functional impairment are clinically relevant, translationally viable, and suitable for assessment by external observers as part of a preclinical research program. Additionally, analgesia in assays of pain-depressed behavior manifests as an increase in the target behavior, and as a result, drugs that produce non-selective motor inhibition do not produce false-positive analgesic-like effects. Accordingly, we and others have developed preclinical assays of pain-related depression of unconditioned and operant conditioned behaviors in rodents (Cobos et al. 2012; Kandasamy et al. 2017; Negus et al. 2015; Pereira Do Carmo et al. 2009). The goal of the present study was to compare the effects of nalfurafine in complementary assays of pain-stimulated and pain-depressed behavior that have been used previously to examine other classes of candidate analgesics in rats (Altarifi et al. 2015; Freitas et al. 2015; Kwilasz and Negus 2012; Rosenberg et al. 2013), including KOR agonists (Brust et al. 2016; Negus et al. 2010; Negus et al. 2012a). In addition, because nalfurafine is approved clinically for the treatment of itch, we also evaluated nalfurafine effects in complementary assays of itch-stimulated and itch-depressed behavior.
METHODS
Subjects
A total of 47 adult male Sprague-Dawley rats (ENVIGO, Frederick, MD) had ad libitum access to food and water and were housed individually on a 12 hr light-dark cycle (6am –– 6pm, lights on) in a facility accredited by the Association for the Assessment and Accreditation of Laboratory Animal Care. Rats weighed between 300 and 350 g at the time of surgery for implantation of intracranial electrodes. All experiments were approved by the Institutional Animal Care and Use Committee at Virginia Commonwealth University in accordance with the National Institutes of Health guidelines (National Research Council 2011).
Assay of Intracranial Self-Stimulation (ICSS)
Surgery
ICSS studies were conducted using procedures similar to those described previously for studies with kappa, mu and delta opioid receptor agonists (Altarifi et al. 2015; Negus et al. 2010; Negus et al. 2012a; Negus et al. 2012b) and with other nonopioid drugs (Negus 2013; Negus and Miller 2014). Rats were anesthetized with isoflurane (3% in oxygen; Webster Veterinary, Phoenix, AZ) and secured in a stereotaxic apparatus for implantation of stainless steel electrodes (Plastics One, Roanoke, VA, USA). The cathode was implanted into the left medial forebrain bundle at the level of the lateral hypothalamus (2.8 mm posterior to bregma, 1.7 mm lateral to the midsagittal suture, and 8.8 mm ventral to the skull; skull flat method). The anode was wrapped around one of three skull screws to act as a ground, and dental acrylic secured the electrode to the screws and skull. Ketoprofen (5 mg/kg, IP) was administered as a postoperative analgesic immediately and 24 hr following surgery. Animals were allowed to recover for at least one week before ICSS training.
Apparatus
Operant conditioning chambers consisted of sound-attenuating boxes containing modular acrylic and metal test chambers (29.2 cm × 30.5 cm × 24.1 cm) (Med Associates, St. Albans, VT). Each chamber had a response lever (4.5 cm wide, 2.0 cm deep, 3.0 cm above the floor), three stimulus lights (red, yellow and green) centered 7.6 cm above the lever, a 2-watt house light, and an ICSS stimulator. Bipolar cables routed through a swivel-commutator (Model SL2C, Plastics One) connected the stimulator to the electrode. MED-PC IV computer software controlled all programming parameters and data collection (Med Associates).
Training
A house light was illuminated during behavioral sessions, and lever-press responding under a fixed-ratio 1 (FR1) schedule produced delivery of a 0.5-s train of square-wave cathodal pulses (0.1 ms per pulse) via the intracranial electrode. During brain stimulation, the stimulus lights over the lever were illuminated, and responding had no scheduled consequences. During initial 60-min training sessions, stimulation intensity was set at 150 μA, and stimulation frequency was set at 158 Hz. Stimulation intensity was then individually manipulated in each rat to identify an intensity that maintained reinforcement rates >30 stimulations/min (95–295 μA for this study). Once an appropriate intensity was identified, changes in frequency were introduced during sessions consisting of three consecutive 10-min components, each of which contained 10 consecutive 60-s trials. The stimulation frequency decreased from 158 to 56 Hz in ten 0.05 log unit steps across trials. Each trial began with a 10-s time-out period, during which responding had no scheduled consequences, and five non-contingent stimulations at the designated frequency were delivered. During the remaining 50 s of each trial, responding produced brain stimulation at the designated frequency and illumination of the lever lights under the FR1 schedule. ICSS performance was considered to be stable when frequency-rate curves were not statistically different over three consecutive days of training as indicated by lack of a significant effect of ‘day’ in a two-way analysis of variance (ANOVA) with day and frequency as the main effect variables (see Data Analysis below). All training was completed within six weeks of surgery. Rats that did not meet criteria for ICSS studies within six weeks were used for studies of acid-stimulated stretching or serotonin-stimulated scratching (see below).
Testing
Test sessions consisted of three consecutive baseline components followed first by a treatment period and then by a series of test components. Three general types of test sessions were conducted that varied details of the treatment period and test components. First, the potency, time-course, and KOR mediation of effects produced by nalfurafine in the absence of a noxious or pruritic stimulus were determined. For dose-effect studies, a 10-min treatment period was followed by two test components, and nalfurafine (0.001–0.1 mg/kg) or saline vehicle was administered IP at the beginning of the treatment period. For time-course studies, administration of 0.032 mg/kg nalfurafine was followed by pairs of test components beginning 10, 30, 100, 180, 300, and 1440 mins after drug administration. To assess KOR mediation of nalfurafine effects, new sets of drug-naïve rats were treated with saline vehicle or a dose of the KOR antagonist JDTic (10 mg/kg, SC) shown previously to block KOR agonist effects in rats for up to one week (Carroll et al. 2004; Runyon et al. 2010). Test sessions with nalfurafine and morphine were conducted in counterbalanced order 24 or 72 hr after saline/JDTic administration (i.e. some rats were tested with nalfurafine after 24 hr and morphine after 72 hr, and other rats were tested with morphine first then nalfurafine). During test sessions, rats received cumulative doses of nalfurafine (0.0032–0.1 mg/kg) or morphine (1.0–10 mg/kg) such that sequential doses were administered IP at 50-min intervals, each dose increased the total cumulative dose by 0.5 log units, and a pair of test components began 30 min after each dose.
Second, nalfurafine effects on acid-induced (i.e. pain-related) depression of ICSS were examined in a new set of drug-naïve rats. For these studies, a 30-min treatment period was followed by two test components. A dose of nalfurafine (0.001–0.01 mg/kg) or saline vehicle was administered IP at the beginning of each treatment period, and dilute lactic acid (1.8% in an injection volume of 1 ml/kg) was administered IP at the end of each treatment period. The acid treatment was identical to that used in previous studies of opioid agonist effects on acid-induced depression of ICSS (Altarifi et al. 2015; Negus et al. 2010; Negus et al. 2012a; Negus et al. 2012b).
Lastly, nalfurafine effects were examined in a final set of drug-naïve rats on depression of ICSS induced by serotonin (5HT) as an itch-related stimulus. 5HT was used as the pruritic agent because (a) previous studies found that 5HT produces more robust and reliable scratching than other pruritic agents in rats (Jinks and Carstens 2002; Klein et al. 2011; Thomsen et al. 2001), and (b) serotonin has been implicated as one mediator of uremic pruritus (Balaskas et al. 1998), the condition for which nalfurafine is clinically approved. For these studies, a 30-min treatment period was followed by four test components. A dose of nalfurafine (0.001–0.1 mg/kg), the mu opioid agonist morphine (1.0 mg/kg), the nonsteroidal anti-inflammatory drug ketoprofen (1.0 mg/kg), or saline vehicle was administered IP at the beginning of each treatment period, 5HT (0.5 mg in an injection volume of 50 μl) was administered intradermally (ID) 10-min later, and testing began 20-min after 5HT administration. The dose and temporal parameters of ID 5HT were based on previous studies (Thomsen et al. 2001) and on preliminary data showing that peak ICSS depression occurred after 20 min and lasted for at least 40 min. For ID 5HT administration, the midscapular region was shaved weekly (after test sessions on Fridays; at least 96 hr before the next test session), and 5HT was administered into the skin of the shaved region. As a control experiment, the same 5HT dose was administered IP without any drug pretreatment. The morphine and ketoprofen doses were selected because they have been shown previously to block acid-induced depression of ICSS (Altarifi et al. 2015; Negus et al. 2012a).
Test sessions were conducted on Tuesdays and Fridays, and three-component training sessions were conducted on other weekdays. All studies associated with any one set of treatments were completed before advancing to another treatment. Within each treatment condition, dose order was varied using a pseudo Latin-square design in groups of 5–8 rats.
Data Analysis
The first baseline component of each test session was considered a “warm up” component, and data were discarded. The primary dependent variable was reinforcement rate in stimulations per min during each frequency trial for the second and third baseline components and for all test components. To normalize these data, raw reinforcement rates from each trial in each rat were converted to percent maximum control rate (% MCR) for that rat on that day. MCR was defined as the mean of the maximal rates observed during the second and third baseline components of that test session. Subsequently, % MCR values for each trial were calculated as [(reinforcement rate during a frequency trial)/(MCR)] X 100. For each rat, data from baseline and test components were averaged to yield baseline and test frequency-rate curves. Baseline and test data were then averaged across rats to yield mean baseline and test frequency-rate curves for each manipulation. Results were compared by repeated measures two-way ANOVA with ICSS frequency as one factor and dose, time, or treatment type as the second factor. For these and all other ANOVA’s, the Greenhouse-Geisser correction was used to adjust for any violations of sphericity as determined by Mauchly’s test. A significant ANOVA was followed by the Holm-Sidak post-hoc test, and the criterion for significance for these and all other analyses was p < 0.05.
To provide an additional summary measure of ICSS performance, the total number of stimulations was determined across all 10 frequency trials of each component. Test data were expressed as a percentage of the average number of total stimulations per component during the second and third baseline components for that day. Thus, % Baseline Stimulations was calculated as (mean total stimulations per test component/mean total stimulations per baseline component) X 100. These data were then averaged across rats for each manipulation and analyzed by one- or two-way ANOVA as appropriate.
Assay of Acid-Stimulated Stretching
Behavioral Procedure
A subset of rats that failed to meet ICSS training criteria were used in studies of acid-stimulated stretching as described previously (Altarifi et al. 2015; Negus et al. 2010; Negus et al. 2012a; Negus et al. 2012b). During test sessions, IP nalfurafine (0.001–0.032 mg/kg) or saline vehicle was followed first by a pretreatment interval identical to that used for ICSS and then by IP injection of dilute lactic acid (1.8% in a volume of 1 ml/kg, identical to ICSS studies). Immediately after the acid injection, rats were placed into an amber acrylic test chamber (31.0 × 20.1 × 20.0 cm) for a 30-min observation period, and the number of stretches was counted. A stretch was operationally defined as a contraction of the abdomen followed by extension of the hindlimbs. Dose order across rats was counterbalanced using a pseudo Latin-square design, and at least 48 hr separated test sessions for each rat. Rats were drug naïve at the start of each study, and the experimenter who scored stretching was blind to the drug treatments. All rats were initially tested twice with acid alone, and only rats that exhibited greater than 10 mean stretches during these sessions were included in this study (7 of 12 rats evaluated).
Data Analysis
The primary dependent variable was the number of stretches counted during each observation period in each rat. The effect of nalfurafine dose was evaluated by one-way ANOVA.
Assay of Serotonin-Stimulated Scratching
Behavioral Procedure
A subset of rats that failed to meet ICSS training criteria and that were not used for acid-stimulated stretching were assigned to studies of 5HT-stimulated scratching. During test sessions, IP nalfurafine (0.001–0.1 mg/kg) or saline vehicle was followed first by a pretreatment interval identical to that used for ICSS studies and then by ID injection of either saline vehicle or 5HT (0.5 mg in a volume of 50 μl, identical to ICSS studies). Twenty min after 5HT, rats were placed into an amber acrylic test chamber (31.0 × 20.1 × 20.0 cm) for a 40-min observation period, and the number of scratching bouts was counted. A scratching bout was operationally defined as lifting of a hind limb followed by at least one scratching movement by a hindpaw to the midscapular region. Bouts were separated by return of the hind limb to the cage floor and a period in which the animal discontinued scratching of the midscapular region for at least 1 sec. Dose order across rats was counterbalanced using a pseudo Latin-square design, except for the highest dose of nalfurafine (0.1 mg/kg), which was the last dose tested in all rats. At least 48 hr separated test sessions for each rat. Rats were drug naïve for the start of each study, and the experimenter who scored scratching was blind to the drug treatments, except for the highest dose of nalfurafine (0.1 mg/kg). All rats were initially tested twice with 5HT alone, and only rats that exhibited greater than 10 mean scratches during these sessions were included in this study (8 of 12 rats evaluated).
Data Analysis
The primary dependent variable was the number of scratching bouts counted during each observation period in each rat. Treatment effects were evaluated by one-way ANOVA.
Drugs
Nalfurafine HCl and morphine sulfate were provided by the National Institute on Drug Abuse Drug Supply Program (Bethesda, MD). JDTic ((3R)-7-hydroxy- N-{(1S)-1-{[(3R,4R)-4-(3-hydroxyphenyl)-3,4-dimethyl-1-piperidinyl] methyl}-2-methylpropyl}-1,2,3,4-tetrahydro-3-isoquinoline-carboxamide) was synthesized by F. I. Carroll (Research Triangle Institute, Research Triangle Park, NC). Ketoprofen propionate (Spectrum Chemical Co., Gardena, CA), lactic acid (Sigma-Aldrich, St. Louis, MO) and serotonin HCl (Sigma-Aldrich, St. Louis, MO) were purchased from commercial suppliers. All chemicals were dissolved in sterile saline, and doses/concentrations refer to the forms described above.
RESULTS
Nalfurafine effects on acid-stimulated stretching and 5HT-stimulated scratching
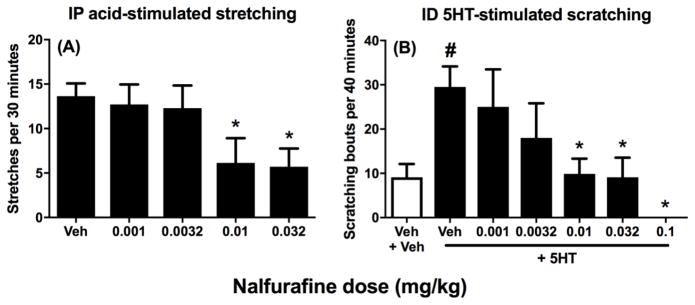
We have reported previously that IP saline does not elicit significant stretching, and peak stretching is elicited by a IP administration of 1.8% lactic acid (Altarifi et al. 2015). Figure 1A shows that nalfurafine produced a dose-dependent decrease in stretching stimulated by IP 1.8% lactic acid. Similarly, Figure 1B shows that ID 5HT stimulated significantly more scratching than ID vehicle, and nalfurafine produced a dose-dependent decrease in 5HT-stimulated scratching. Nalfurafine doses of ≥0.01 mg/kg were sufficient to significantly decrease both acid-stimulated stretching and 5HT-stimulated scratching. Statistical results for these and all other results are shown in the associated figure legends.
Figure 1. Effects of nalfurafine on IP lactic acid-stimulated stretching (A) and ID 5HT-stimulated stretching (B).
Left panel A shows effect of administration of IP lactic acid on stretching after pretreatment with vehicle (Veh) or increasing doses of nalfurafine. Abscissa: Nalfurafine dose in mg/kg. Ordinate: number of stretches per 30-min observation period. Asterisks show significant difference from Veh as determined by repeated-measures ANOVA followed by the Dunnett’s post hoc test, p<0.05. Right panel B shows baseline scratching (vehicle of both ID 5HT and nalfurafine, Veh+Veh) as the white bar and effect of ID 5HT on scratches after pretreatment with vehicle (Veh) or increasing doses of nalfurafine as the black bars. Abscissa: Nalfurafine dose in mg/kg. Ordinate: number of scratching bouts per 40-min observation period. Pound sign shows significant difference between Veh+Veh and Veh+5HT, and asterisks show differences of nalfurafine+5HT from Veh+5HT, as determined by repeated measures one-way ANOVA followed by a Holm-Sidak post hoc test, p < 0.05. Data presented are the mean ± SEM of seven rats (stretching) and eight rats (scratching), and all rats received all treatments within a given panel. ANOVA results are as follows: (A) Significant effect of treatment [F(3.03,18.15) = 7.60 p < 0.001]. (B) Significant effect of treatment [F(2.40, 16.81) = 4.39 p < 0.05].
Nalfurafine effects on ICSS in the absence of pain or itch stimuli
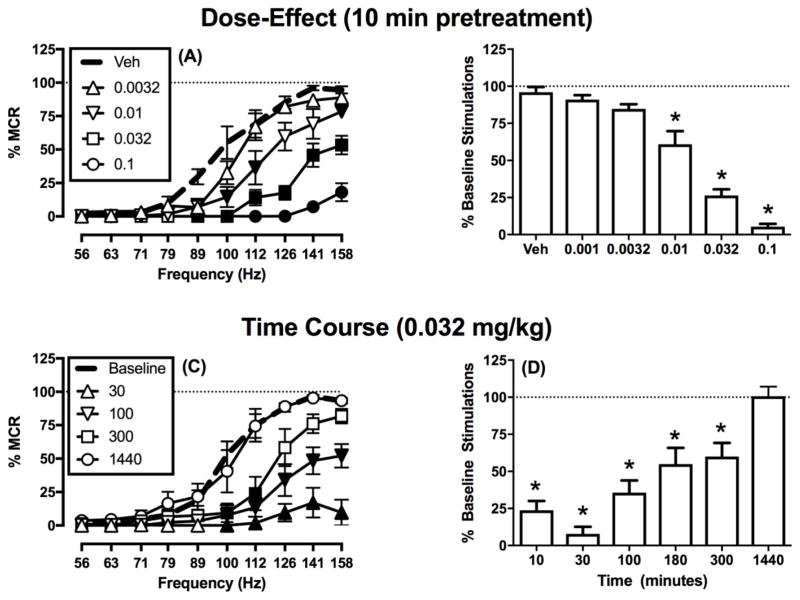
For all rats in the present study, the mean ± SEM maximum control rate was 57 ± 2 stimulations per trial, and the mean ± SEM number of total baseline stimulations per component summed across all brain-stimulation frequencies was 251 ± 12 stimulations per component. Figure 2 shows that electrical brain stimulation maintained a frequency-dependent increase in rates of ICSS responding, and nalfurafine produced a dose- and time-dependent decrease in responding for brain-stimulation reward. Dose-effect data are shown in Figures 2A,B, and doses ≥0.01 mg/kg significantly decreased rates of ICSS responding. Time-course data are shown in Figures 2C,D, and the ICSS rate-decreasing effects of nalfurafine peaked after 30 min and persisted for up to 300 min before recovering back to baseline after 1440 min (24 hr).
Figure 2. Effects of nalfurafine on intracranial self-stimulation in the absence of noxious or pruritic stimuli.
Left panels (A and C) show full ICSS frequency-rate curves. Data are shown only for selected doses and times to reduce clutter, but all data were included in statistical analysis. Abscissae: Frequency of electrical brain stimulation in Hz (log scale). Ordinates: Percent maximum control reinforcement rate (% MCR). Filled symbols show significant differences from (A) vehicle (Veh) and (C) baseline as determined by repeated-measures two-way ANOVA followed by the Holm-Sidak post hoc test, p < 0.05. Right panels (B and D) show the summary measure of the total number of stimulations per component for all doses and times tested. Abscissae: (B) Dose in mg/kg or (D) time post-injection in minutes. Ordinates: percent baseline stimulations per component (% Baseline Stimulations). Asterisks show significant difference from (B) Veh and (D) 1440 min as determined by repeated-measures one-way ANOVA followed by a Holm-Sidak post hoc test, p < 0.05. Data presented are the mean ± SEM of six rats, and all rats received all doses. Statistical results are as follows: (A) Significant main effects of frequency [F (1.36, 6.82) = 94.65 p < 0.001] and dose [F(2.89, 14.46) = 53.41 p < 0.001] and a significant interaction [F (3.26, 16.28) = 14.74 p < 0.001]. (B) Significant effect of treatment [F (1.82, 9.12) = 83.41 p < 0.001]. (C) Significant main effect of frequency [F(1.95, 9.76) = 62.50 p < 0.001] and time [F(2.01, 10.06) = 53.51 p < 0.001] and a significant interaction [F(3.4, 17.0) = 9.00 p < 0.05]. (D) Significant main effect of time [F(2.29, 11.44) = 80.38, p<0.001].
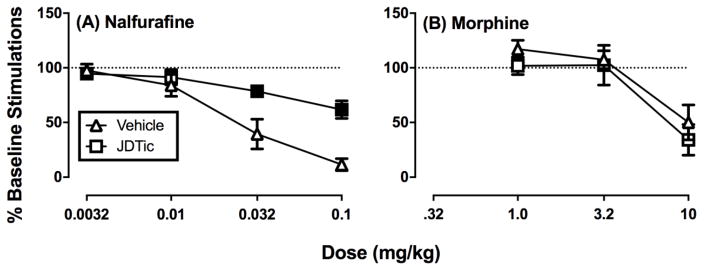
Figure 3 shows the effects of pretreatment with vehicle or the KOR antagonist JDTic on subsequent effects produced 24–72 hr later by cumulative doses of nalfurafine or morphine. After vehicle pretreatment, both nalfurafine and morphine produced dose-dependent decreases in ICSS. JDTic pretreatment antagonized the effects of nalfurafine but not of morphine.
Figure 3. Effects of nalfurafine and morphine on intracranial self-stimulation in the absence or presence of JDTic.
Graphs shows effect of vehicle or JDTic (10 mg/kg) on (A) nalfurafine-induced or (B) morphine-induced decreases of ICSS expressed as the total number of stimulations per component. Abscissae: Dose in mg/kg (log scale). Ordinates: percent baseline stimulations per component (% Baseline Stimulations). Filled symbols show significant differences between rats treated with vehicle vs. JDTic as determined by two-way ANOVA with JDTic dose as a between-subject variable and test-drug dose (nalfurafine or morphine) as a within-subject variable. A significant interaction was followed by the Holm-Sidak post hoc test, p < 0.05. Data presented are the mean ± SEM of five rats for all treatments, and all rats in the saline and JDTic treatment groups received all doses of both test drugs (nalfurafine and morphine). Statistical results are as follows: (A) Significant main effects of nalfurafine dose [F(3, 27) = 61.83, p < 0.001] and JDTic dose [F(1, 9) = 5.383 p < .05] and a significant interaction [F(3,27) = 13.28 p < 0.001]. (B) Significant main effect of morphine dose [F(1.19,9.53) = 19.14 p < .001], but neither the main effect of JDTic dose [F(1,8) = 1.134, p=0.32] nor the interaction [F(1.192,9.53) =0.13, p=0.77] were significant.
Nalfurafine effects on pain- or itch-related depression of ICSS
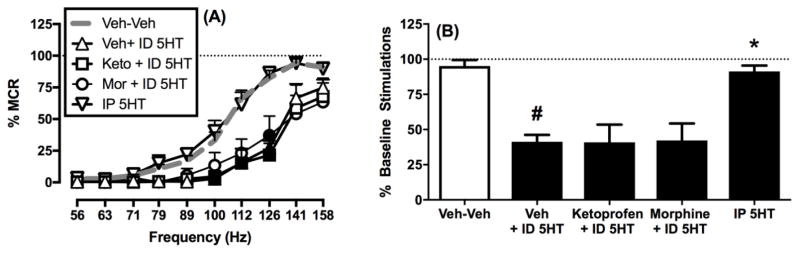
We have reported previously that IP acid produces a pain-related depression of ICSS (Altarifi et al. 2015; Negus 2013). Figure 4 shows that ICSS can also be depressed by ID 5HT as an itch-related stimulus. Specifically, ID 5HT produced a rightward shift in the ICSS frequency-rate curve (Figure 4A) and a significant decrease in the total number of stimulations per component (Figure 4B). This same dose of 5HT administered IP had no effect, and the analgesic drugs ketoprofen and morphine failed to block ID 5HT-induced depression of ICSS (Figure 4B).
Figure 4. Effects of ID 5HT on intracranial self-stimulation.
Left panel A shows full ICSS frequency-rate curves. Abscissa: Frequency of electrical brain stimulation in Hz (log scale). Ordinate: Percent maximum control reinforcement rate (% MCR). Filled symbols show significant differences from ID 5HT vehicle and drug vehicle (Veh + Veh) as determined by repeated measures two-way ANOVA followed by the Holm-Sidak post hoc test, p < 0.05. Right panel B shows the summary measure of the total number of stimulations per component. Abscissa: Treatment. Ordinate: percent baseline stimulations per component (% Baseline Stimulations). Asterisks show significant difference compared to Veh + Veh and pound sign shows significant difference from ID 5HT as determined by repeated measures one-way ANOVA followed by a Holm-Sidak post hoc test, p < 0.05. Data presented are the mean ± SEM of five rats, and all rats received all treatments. Statistical results are as follows: (A) Significant main effects of frequency [F(1.17, 4.67) = 71.05 p < 0.001] and treatment [F(2.12, 8.47) = 20.48 p < 0.01] and a significant interaction [F(3.34, 13.34) = 3.954 p < 0.05]. (B) Significant effect of treatment [F(1.83, 7.34) = 13.69 p < 0.01].
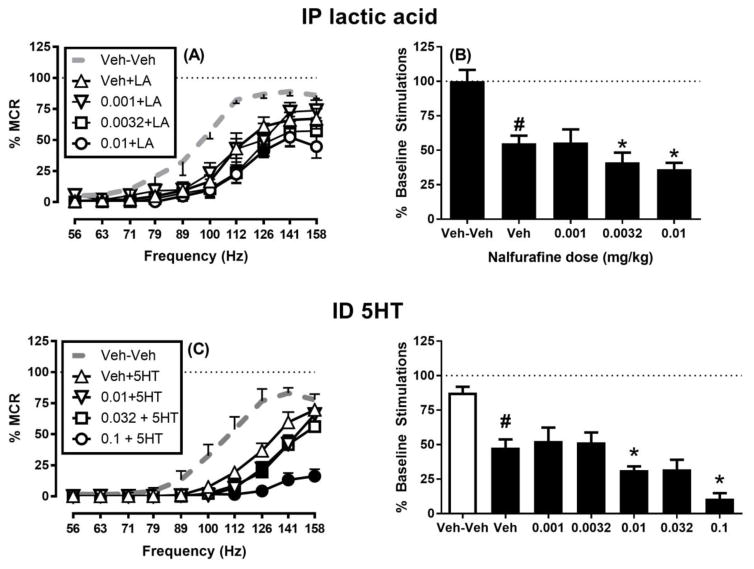
Figure 5 shows that nalfurafine doses sufficient to block acid- and 5HT-stimulated behaviors failed to block acid- or 5HT-induced depression of ICSS. Specifically, Figure 5A,B shows that nalfurafine (0.001–0.01 mg/kg) failed to block acid-induced depression of ICSS. Rather, doses of 0.0032 and 0.01 mg/kg only exacerbated acid-induced depression of ICSS as indicated by analysis of full frequency-rate curves (Figure 5A) and the summary measure of ICSS (Figure 5B). Similarly, Figure 5C,D shows that nalfurafine (0.001–0.1 mg/kg) failed to block 5HT-induced depression of ICSS; instead, nalfurafine doses ≥0.01 mg/kg generally produced only an exacerbation of 5HT-induced ICSS depression as indicated by both frequency-rate curve and summary analysis.
Figure 5. Effects of nalfurafine on IP acid-induced and ID 5HT-induced depression of intracranial self-stimulation.
Left panels (A and C) show full ICSS frequency-rate curves. Data are shown for only the highest three doses in panel C to reduce clutter, but all data were included in statistical analysis. Abscissae: Frequency of electrical brain stimulation in Hz (log scale). Ordinates: Percent maximum control reinforcement rate (% MCR). Filled symbols show significant differences from nalfurafine vehicle pretreatment to lactic acid (Veh+LA) or ID 5HT (Veh+5HT) as determined by repeated-measures two-way ANOVA followed by the Holm-Sidak post hoc test, p < 0.05. Right panels (B and D) show nalfurafine effects on the summary measure of total number of stimulations per component for all doses tested. Abscissae: Nalfurafine dose in mg/kg. Ordinates: percent baseline stimulations per component (% Baseline Stimulations). Pound sign shows significant difference between Veh+Veh and Veh+LA (B) or Veh+5HT (D), and asterisks show significant differences between Veh+LA and nalfurafine +LA (B) or Veh+5HT and nalfurafine+5HT (D) as determined by repeated-measures one-way ANOVA followed by a Holm-Sidak post hoc test, p < 0.05. All data show mean ± SEM of eight rats. Statistical results are as follows: (A) Significant main effects of frequency [F(3.08, 21.58) = 78.08 p < 0.001] and dose [F(2.37, 16.6) = 21.50 p < 0.001] and a significant interaction [F(4.92, 34.42) = 3.77 p <0.01]. (B) Significant effect of treatment [F(2.47,17.26) = 16.57, p < 0.001]. (C) Significant main effect of frequency [F(2.27, 15.85) = 91.64 p < 0.001] and dose [F(2.68, 18.76) = 15.62 p < 0.001] and a significant interaction [F(5.13, 35.94) = 8.80, p < 0.001]. (D) Significant effect of treatment [F(3.36, 23.50) = 20.82, p < 0.001].
DISCUSSION
Nalfurafine effects on pain- and itch-stimulated behaviors
Results of the present study agree with previous studies reporting that nalfurafine (Endoh et al. 1999; Endoh et al. 2000; Nagase et al. 1998; Togashi et al. 2002) and other centrally acting KOR agonists (Cowan et al. 2015; Kivell and Prisinzano 2010) decrease both pain-stimulated behaviors and scratching elicited in rodents by a range of noxious and pruritic stimuli, respectively. For example, in agreement with the present and previous studies in rats (Negus et al. 2010), both nalfurafine and the arylacetamide KOR agonist U69593 decreased IP acid-stimulated stretching in mice (Nagase et al. 1998; Seguin et al. 1995). Nalfurafine also displayed similar potency to decrease 5HT-stimulated scratching in rats (present study) and both substance P- and histamine-stimulated scratching in mice (Togashi et al. 2002). In the present study, nalfurafine effects on pain- and itch-stimulated behaviors were not evaluated for their sensitivity to a KOR antagonist; antagonism studies were conducted only for nalfurafine effects on ICSS (see below). However, previous studies found that nalfurafine effects on both acid-stimulated stretching and substance P-stimulated scratching in mice were blocked by the KOR antagonist norbinaltorphimine (Nagase et al. 1998; Togashi et al. 2002).
Nalfurafine effects on ICSS in the absence of pain and itch stimuli
As a prelude to evaluating nalfurafine effects on pain- and itch-depressed ICSS, nalfurafine was first evaluated alone in the absence of noxious or pruritic stimuli. Similar to many other centrally acting KOR agonists such as U50488, U69593 and salvinorin A (Carlezon et al. 2006; Negus et al. 2010; Negus et al. 2012a; Todtenkopf et al. 2004), nalfurafine produced a dose- and time-dependent decrease in ICSS. Furthermore, sensitivity of nalfurafine effects to antagonism by JDTic suggests that nalfurafine effects were mediated by KOR. Thus, the present results suggest that nalfurafine effects on ICSS are similar to those of other centrally acting kappa agonists.
Two other points warrant mention. First, the potency of nalfurafine to decrease ICSS was similar to its potency to decrease both acid-stimulated stretching and 5HT-stimulated scratching (i.e. 0.01 mg/kg was the lowest dose to significantly reduce all three behaviors). KOR agonist-induced decreases in ICSS may be related to sedative, dysphoric, and/or anhedonic effects that KOR agonists produce in humans (Todtenkopf et al. 2004; Carlezon and Chartoff 2007; Negus and Miller 2014; Wadenberg 2003), although nalfurafine may be less effective that other KOR agonists to produce dysphoric effects (Hasebe et al. 2004; Kumagai et al. 2012). This suggests that apparent antinociceptive and antipruritic effects of nalfurafine in our assays of pain- and itch-stimulated behaviors may have reflected non-selective effects on motor competence or motivated behavior rather than selective decreases in sensitivity to either the acid or 5HT stimuli. This conclusion contrasts with interpretations of previous studies in mice finding that nalfurafine and other KOR agonists are often more potent to decrease pain- or itch-stimulated behaviors than to decrease control behaviors such as rotorod performance or wheel-running (Endoh et al. 1999; Schattauer et al. 2017; Seguin et al. 1995; Togashi et al. 2002). Insofar as centrally acting KOR agonists have not succeeded as clinically effective analgesics, these findings suggest that such comparisons may not be sufficient either to predict clinical analgesic safety or to rule out nonselective effects as a contributing factor to apparent antinociception in conventional preclinical assays of pain-stimulated behavior.
Second, nalfurafine has been described as a biased agonist for G-protein signaling pathways coupled to KOR (Schattauer et al. 2017), but the present results with nalfurafine contrast with effects of other G-protein-signaling-biased KOR agonists that produced antinociception and antipruritus without decreasing ICSS (Brust et al. 2016; White et al. 2015). It is difficult to directly compare the degrees of G-protein signaling bias for nalfurafine and these other compounds given that bias was evaluated using different procedures (Brust et al. 2016; Schattauer et al. 2017; White et al. 2015; Zhou et al. 2013), and measures of bias can vary across procedures and with KOR from different species (DiMattio et al. 2015; Dogra and Yadav 2015; Schattauer et al. 2017). Although some G-protein signaling biased KOR agonists may have better safety profiles than non-biased or β-arrestin-signaling-biased agonists, nalfurafine apparently does not have sufficient bias in rats to produce antinociceptive and antipruritic effects without also decreasing ICSS.
Nalfurafine effects on pain-related depression of ICSS
The present results with nalfurafine agree with the failure of other centrally acting KOR agonists, such as U50488, U69593, and salvinorin A, to alleviate acid-induced decreases in ICSS (Brust et al. 2016; Negus et al. 2010; Negus et al. 2012a). Moreover, nalfurafine effects in this procedure contrast with the effects of clinically effective analgesics, including both mu opioid receptor agonists and cyclooxygenase inhibitors, which are effective to block acid-induced depression of ICSS in rats (Altarifi et al. 2015; Brust et al. 2016; Leitl et al. 2014; Negus et al. 2012a). Taken together with data discussed above to indicate that nalfurafine decreased acid-stimulated stretching only at doses that also decreased control ICSS performance, these data are consistent with the conclusion that nalfurafine produces non-selective inhibition of motivated behavior in rats either without producing analgesia or at doses below those that might produce analgesia. Thus, the present results do not support further consideration of nalfurafine as a stand-alone analgesic drug, a conclusion that appears consistent with the absence of published data on analgesic efficacy of nalfurafine in humans despite supportive preclinical data from assays of pain-stimulated behavior in mice, rats, and monkeys (Endoh et al. 1999; Endoh et al. 2001; Endoh et al. 2000; Nagase et al. 1998), and despite the approval of nalfurafine for treatment of a different indication (Inui 2015). Other centrally acting KOR agonists have also failed in to produce adequately safe and effective analgesic effects in humans (Pande et al. 1996), and the abused KOR agonist salvinorin A has also failed to yield evidence for analgesia among its many other effects in humans (Baggott et al. 2010; Johnson et al. 2011; MacLean et al. 2013). Although nalfurafine did not alleviate acid-induced depression of ICSS in the present study, another G-protein signaling biased KOR agonist (triazole 1.1) was effective (Brust et al. 2016). This discrepancy may reflect a lower degree of G-protein signaling bias for nalfurafine than for triazole 1.1, but further studies will be required to resolve this issue.
Nalfurafine effects on itch-related depression of ICSS
This study describes the first attempt to develop an assay of itch-depressed behavior that might complement assays of pain-depressed behavior and serve as a tool for evaluation of antipruritic drugs. ID 5HT was used as the pruritic stimulus because previous studies found that it is more effective than many other stimuli to elicit scratching in rats (Jinks and Carstens 2002; Klein et al. 2011; Thomsen et al. 2001) and because 5HT has been implicated as a mediator of itch in uremic pruritus (Balaskas et al. 1998). We found that an ID 5HT dose sufficient to stimulate scratching also depressed ICSS. The failure of 5HT administered by the IP route to depress ICSS suggests that, as with scratching (Thomsen et al. 2001), ID 5HT effects on ICSS are mediated locally by effects in skin. Moreover, the failure of analgesic drugs to block ID 5HT effects suggests that ID 5HT-induced decreases in ICSS are not pain related, and might therefore be itch related. However, nalfurafine doses that decreased scratching did not alleviate ID 5HT-induced decreases in ICSS. As a result, this procedure was not sensitive to any antipruritic effects of nalfurafine. The basis for this discrepancy warrants further study. Although serotonin has been implicated as one mediator of uremic pruritus in humans, other mechanisms may also contribute that are more sensitive to nalfurafine modulation (Balaskas et al. 1998). Moreover, it should be noted that, although nalfurafine is approved for treatment of uremic pruritus in Japan, it is not a primary treatment, its effect size has been modest in published studies (Inui 2015; Jaiswal et al. 2016), and it was not approved for use in Europe due to a lack of efficacy in clinical trials conducted there (European Medicines Agency 2013). Thus, the failure of nalfurafine to alleviate ID 5HT-induced decreases in ICSS depression in the present study may be consistent with the weak and unreliable efficacy of nalfurafine to treat itch in humans.
Summary
In summary, the present results do not support further consideration of nalfurafine as a stand-alone analgesic drug and suggest that nalfurafine efficacy and safety to treat itch may be limited. Nalfurafine has been described as a G-protein-signaling-biased KOR agonist (Schattauer et al. 2017), and recent studies suggest that G-protein biased signaling at KOR may produce analgesic and antipruritic effects with reduced side effects (Brust et al. 2016; White et al. 2015). However, nalfurafine effects reported here were not distinguishable from effects of relatively nonbiased or β-arrestin-signaling-biased KOR agonists, suggesting that nalfurafine may lack sufficient G-protein signaling bias to produce a desirable profile of analgesic and antipruritic efficacy and safety.
Acknowledgments
This grant was supported by NIH grants R01NS070715, R01DA039167, R01DA009045, and T32DA007027.
Footnotes
Conflict of Interest Statement
The authors have no conflicts of interest to report.
Author Contributions
Dr. Lazenka contributed as a postdoctoral fellow from the Negus Lab at VCU. He conducted most of the studies, analyzed data, prepared figures, and worked with the senior author to write manuscript drafts and incorporate author comments into the final version of the manuscript.
Dr. Moerke also contributed as a postdoctoral fellow from the Negus Lab at VCU. She conducted studies, performed initial data analysis and interpretation on the studies she conducted, contributed comments to manuscript drafts, and approved the final version of the manuscript.
Drs. Townsend and Freeman consulted on issues of experimental design regarding the behavioral pharmacology of nalfurafine, contributed comments to manuscript drafts, and approved the final version of the manuscript.
Dr. Carroll provided JDTic, consulted on issues of experimental design regarding the behavioral pharmacology of JDTic, contributed comments to manuscript drafts, and approved the final version of the manuscript.
Dr. Negus supervised all aspects of experimental design, conduct, analysis, and interpretation and worked the first author to write manuscript drafts and incorporate author comments into the final version of the manuscript.
References
- Altarifi AA, Rice KC, Negus SS. Effects of mu-opioid receptor agonists in assays of acute pain-stimulated and pain-depressed behavior in male rats: role of mu-agonist efficacy and noxious stimulus intensity. J Pharmacol Exp Ther. 2015;352:208–17. doi: 10.1124/jpet.114.219873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baggott MJ, Erowid E, Erowid F, Galloway GP, Mendelson J. Use patterns and self-reported effects of Salvia divinorum: an internet-based survey. Drug Alcohol Depend. 2010;111:250–6. doi: 10.1016/j.drugalcdep.2010.05.003. [DOI] [PubMed] [Google Scholar]
- Balaskas EV, Bamihas GI, Karamouzis M, Voyiatzis G, Tourkantonis A. Histamine and serotonin in uremic pruritus: effect of ondansetron in CAPD-pruritic patients. Nephron. 1998;78:395–402. doi: 10.1159/000044967. [DOI] [PubMed] [Google Scholar]
- Brown DC, Boston RC, Coyne JC, Farrar JT. Ability of the canine brief pain inventory to detect response to treatment in dogs with osteoarthritis. J Am Vet Med Assoc. 2008;233:1278–83. doi: 10.2460/javma.233.8.1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brust TF, Morgenweck J, Kim SA, Rose JH, Locke JL, Schmid CL, Zhou L, Stahl EL, Cameron MD, Scarry SM, Aube J, Jones SR, Martin TJ, Bohn LM. Biased agonists of the kappa opioid receptor suppress pain and itch without causing sedation or dysphoria. Sci Signal. 2016;9:ra117. doi: 10.1126/scisignal.aai8441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlezon WA, Jr, Beguin C, DiNieri JA, Baumann MH, Richards MR, Todtenkopf MS, Rothman RB, Ma Z, Lee DY, Cohen BM. Depressive-like effects of the kappa-opioid receptor agonist salvinorin A on behavior and neurochemistry in rats. J Pharmacol Exp Ther. 2006;316:440–7. doi: 10.1124/jpet.105.092304. [DOI] [PubMed] [Google Scholar]
- Carlezon WA, Jr, Chartoff EH. Intracranial self-stimulation (ICSS) in rodents to study the neurobiology of motivation. Nat Protoc. 2007;2:2987–95. doi: 10.1038/nprot.2007.441. [DOI] [PubMed] [Google Scholar]
- Carroll I, Thomas JB, Dykstra LA, Granger AL, Allen RM, Howard JL, Pollard GT, Aceto MD, Harris LS. Pharmacological properties of JDTic: a novel kappa-opioid receptor antagonist. Eur J Pharmacol. 2004;501:111–9. doi: 10.1016/j.ejphar.2004.08.028. [DOI] [PubMed] [Google Scholar]
- Cobos EJ, Ghasemlou N, Araldi D, Segal D, Duong K, Woolf CJ. Inflammation-induced decrease in voluntary wheel running in mice: a nonreflexive test for evaluating inflammatory pain and analgesia. Pain. 2012;153:876–84. doi: 10.1016/j.pain.2012.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowan A, Kehner GB, Inan S. Targeting Itch with Ligands Selective for kappa Opioid Receptors. Handb Exp Pharmacol. 2015;226:291–314. doi: 10.1007/978-3-662-44605-8_16. [DOI] [PubMed] [Google Scholar]
- Cunningham CW, Rothman RB, Prisinzano TE. Neuropharmacology of the naturally occurring kappa-opioid hallucinogen salvinorin A. Pharmacol Rev. 2011;63:316–47. doi: 10.1124/pr.110.003244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis RE, Callahan MJ, Dickerson M, Downs DA. Pharmacologic activity of CI-977, a selective kappa opioid agonist, in rhesus monkeys. J Pharmacol Exp Ther. 1992;261:1044–9. [PubMed] [Google Scholar]
- DiMattio KM, Ehlert FJ, Liu-Chen LY. Intrinsic relative activities of kappa opioid agonists in activating Galpha proteins and internalizing receptor: Differences between human and mouse receptors. Eur J Pharmacol. 2015;761:235–44. doi: 10.1016/j.ejphar.2015.05.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dogra S, Yadav PN. Biased agonism at kappa opioid receptors: Implication in pain and mood disorders. Eur J Pharmacol. 2015;763:184–90. doi: 10.1016/j.ejphar.2015.07.018. [DOI] [PubMed] [Google Scholar]
- Dworkin RH, Turk DC, Farrar JT, Haythornthwaite JA, Jensen MP, Katz NP, Kerns RD, Stucki G, Allen RR, Bellamy N, Carr DB, Chandler J, Cowan P, Dionne R, Galer BS, Hertz S, Jadad AR, Kramer LD, Manning DC, Martin S, McCormick CG, McDermott MP, McGrath P, Quessy S, Rappaport BA, Robbins W, Robinson JP, Rothman M, Royal MA, Simon L, Stauffer JW, Stein W, Tollett J, Wernicke J, Witter J Immpact. Core outcome measures for chronic pain clinical trials: IMMPACT recommendations. Pain. 2005;113:9–19. doi: 10.1016/j.pain.2004.09.012. [DOI] [PubMed] [Google Scholar]
- Endoh T, Matsuura H, Tajima A, Izumimoto N, Tajima C, Suzuki T, Saitoh A, Suzuki T, Narita M, Tseng L, Nagase H. Potent antinociceptive effects of TRK-820, a novel kappa-opioid receptor agonist. Life Sci. 1999;65:1685–94. doi: 10.1016/s0024-3205(99)00417-8. [DOI] [PubMed] [Google Scholar]
- Endoh T, Tajima A, Izumimoto N, Suzuki T, Saitoh A, Suzuki T, Narita M, Kamei J, Tseng LF, Mizoguchi H, Nagase H. TRK-820, a selective kappa-opioid agonist, produces potent antinociception in cynomolgus monkeys. Jpn J Pharmacol. 2001;85:282–90. doi: 10.1254/jjp.85.282. [DOI] [PubMed] [Google Scholar]
- Endoh T, Tajima A, Suzuki T, Kamei J, Narita M, Tseng L, Nagase H. Characterization of the antinociceptive effects of TRK-820 in the rat. Eur J Pharmacol. 2000;387:133–40. doi: 10.1016/s0014-2999(99)00815-8. [DOI] [PubMed] [Google Scholar]
- European Medicines Agency. Assessment report: Winfuran. European Medicines Agency. Committee for Medicinal Products for Human Use; 2013. [Google Scholar]
- Field MJ, Carnell AJ, Gonzalez MI, McCleary S, Oles RJ, Smith R, Hughes J, Singh L. Enadoline, a selective kappa-opioid receptor agonist shows potent antihyperalgesic and antiallodynic actions in a rat model of surgical pain. Pain. 1999;80:383–9. doi: 10.1016/s0304-3959(98)00237-1. [DOI] [PubMed] [Google Scholar]
- Freitas KC, Carroll FI, Negus SS. Effects of nicotinic acetylcholine receptor agonists in assays of acute pain-stimulated and pain-depressed behaviors in rats. J Pharmacol Exp Ther. 2015;355:341–50. doi: 10.1124/jpet.115.226803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasebe K, Kawai K, Suzuki T, Kawamura K, Tanaka T, Narita M, Nagase H, Suzuki T. Possible pharmacotherapy of the opioid kappa receptor agonist for drug dependence. Ann NY Acad Sci. 2004;1025:404–13. doi: 10.1196/annals.1316.050. [DOI] [PubMed] [Google Scholar]
- Hunter JC, Leighton GE, Meecham KG, Boyle SJ, Horwell DC, Rees DC, Hughes J. CI-977, a novel and selective agonist for the kappa-opioid receptor. Br J Pharmacol. 1990;101:183–9. doi: 10.1111/j.1476-5381.1990.tb12110.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inui S. Nalfurafine hydrochloride to treat pruritus: a review. Clin Cosmet Investig Dermatol. 2015;8:249–55. doi: 10.2147/CCID.S55942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaiswal D, Uzans D, Hayden J, Kiberd BA, Taennankore KK. Targeting the opioid pathway for uremic pruritis: A systematic review and meta-analysis. Can J Kidney Health Dis. 2016 doi: 10.1177/2054358116675345. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jinks SL, Carstens E. Responses of superficial dorsal horn neurons to intradermal serotonin and other irritants: comparison with scratching behavior. J Neurophysiol. 2002;87:1280–9. doi: 10.1152/jn.00431.2001. [DOI] [PubMed] [Google Scholar]
- Johnson MW, MacLean KA, Reissig CJ, Prisinzano TE, Griffiths RR. Human psychopharmacology and dose-effects of salvinorin A, a kappa opioid agonist hallucinogen present in the plant Salvia divinorum. Drug Alcohol Depend. 2011;115:150–5. doi: 10.1016/j.drugalcdep.2010.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandasamy R, Lee AT, Morgan MM. Depression of home cage wheel running: a reliable and clinically relevant method to assess migraine pain in rats. J Headache Pain. 2017;18:5. doi: 10.1186/s10194-017-0721-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kivell B, Prisinzano TE. Kappa opioids and the modulation of pain. Psychopharmacology (Berl) 2010;210:109–19. doi: 10.1007/s00213-010-1819-6. [DOI] [PubMed] [Google Scholar]
- Klein A, Carstens MI, Carstens E. Facial injections of pruritogens or algogens elicit distinct behavior responses in rats and excite overlapping populations of primary sensory and trigeminal subnucleus caudalis neurons. J Neurophysiol. 2011;106:1078–88. doi: 10.1152/jn.00302.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumagai H, Ebata T, Takamori K, Miyasato K, Muramatsu T, Nakamoto H, Kurihara M, Yanagita T, Suzuki H. Efficacy and safety of a novel k-agonist for managing intractable pruritus in dialysis patients. Am J Nephrol. 2012;36:175–83. doi: 10.1159/000341268. [DOI] [PubMed] [Google Scholar]
- Kwilasz AJ, Negus SS. Dissociable effects of the cannabinoid receptor agonists Delta9-tetrahydrocannabinol and CP55940 on pain-stimulated versus pain-depressed behavior in rats. J Pharmacol Exp Ther. 2012;343:389–400. doi: 10.1124/jpet.112.197780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leitl MD, Onvani S, Bowers MS, Cheng K, Rice KC, Carlezon WA, Jr, Banks ML, Negus SS. Pain-related depression of the mesolimbic dopamine system in rats: expression, blockade by analgesics, and role of endogenous kappa-opioids. Neuropsychopharmacology. 2014;39:614–24. doi: 10.1038/npp.2013.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Listos J, Merska A, Fidecka S. Pharmacological activity of salvinorin A, the major component of Salvia divinorum. Pharmacol Rep. 2011;63:1305–9. doi: 10.1016/s1734-1140(11)70694-6. [DOI] [PubMed] [Google Scholar]
- MacLean KA, Johnson MW, Reissig CJ, Prisinzano TE, Griffiths RR. Dose-related effects of salvinorin A in humans: dissociative, hallucinogenic, and memory effects. Psychopharmacology (Berl) 2013;226:381–92. doi: 10.1007/s00213-012-2912-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrath PJ, Unruh AM. Measurement and assessment of pediatric pain. In: McMahon SB, Koltzenburg M, Tracey I, Turk DC, editors. Wall and Melzack’s Textbook of Pain. 6. Elseivier; Philadelphia, PA: 2013. pp. 320–327. [Google Scholar]
- Melzack R, Katz J. Pain measurement in adult patients. In: McMahon SB, Koltzenburg M, Tracey I, Turk DC, editors. Wall and Melzack’s Textbook of Pain. 6. Elsevier; Philadelphia, PA: 2013. pp. 301–319. [Google Scholar]
- Nagase H, Hayakawa J, Kawamura K, Kawai K, Takezawa Y, Matsuura H, Tajima C, Endo T. Discovery of a structurally novel opioid kappa-agonist derived from 4,5-epoxymorphinan. Chem Pharm Bull (Tokyo) 1998;46:366–9. doi: 10.1248/cpb.46.366. [DOI] [PubMed] [Google Scholar]
- National_Research_Council. Guidelines for the Care and Use of Mammals in Neuroscience and Behavioral Research. The National Academies Press; Washington, D.C: 2003. [PubMed] [Google Scholar]
- National Research Council. Guide for the care and use of laboratory animals. National Academies Press; Washington DC: 2011. [Google Scholar]
- Negus SS. Expression and treatment of pain-related behavioral depression. Lab Anim (NY) 2013;42:292–300. doi: 10.1038/laban.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negus SS, Miller LL. Intracranial self-stimulation to evaluate abuse potential of drugs. Pharmacol Rev. 2014;66:869–917. doi: 10.1124/pr.112.007419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negus SS, Morrissey EM, Rosenberg M, Cheng K, Rice KC. Effects of kappa opioids in an assay of pain-depressed intracranial self-stimulation in rats. Psychopharmacology (Berl) 2010;210:149–59. doi: 10.1007/s00213-009-1770-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negus SS, Neddenriep B, Altarifi AA, Carroll FI, Leitl MD, Miller LL. Effects of ketoprofen, morphine, and kappa opioids on pain-related depression of nesting in mice. Pain. 2015;156:1153–60. doi: 10.1097/j.pain.0000000000000171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negus SS, O’Connell R, Morrissey E, Cheng K, Rice KC. Effects of peripherally restricted kappa opioid receptor agonists on pain-related stimulation and depression of behavior in rats. J Pharmacol Exp Ther. 2012a;340:501–9. doi: 10.1124/jpet.111.186783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negus SS, Rosenberg MB, Altarifi AA, O’Connell RH, Folk JE, Rice KC. Effects of the delta opioid receptor agonist SNC80 on pain-related depression of intracranial self-stimulation (ICSS) in rats. J Pain. 2012b;13:317–27. doi: 10.1016/j.jpain.2011.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negus SS, Vanderah TW, Brandt MR, Bilsky EJ, Becerra L, Borsook D. Preclinical assessment of candidate analgesic drugs: recent advances and future challenges. J Pharmacol Exp Ther. 2006;319:507–14. doi: 10.1124/jpet.106.106377. [DOI] [PubMed] [Google Scholar]
- Pande AC, Pyke RE, Greiner M, Cooper SA, Benjamin R, Pierce MW. Analgesic efficacy of the kappa-receptor agonist, enadoline, in dental surgery pain. Clin Neuropharmacol. 1996;19:92–7. doi: 10.1097/00002826-199619010-00009. [DOI] [PubMed] [Google Scholar]
- Pereira Do Carmo G, Stevenson GW, Carlezon WA, Negus SS. Effects of pain- and analgesia-related manipulations on intracranial self-stimulation in rats: further studies on pain-depressed behavior. Pain. 2009;144:170–7. doi: 10.1016/j.pain.2009.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prisinzano TE. Psychopharmacology of the hallucinogenic sage Salvia divinorum. Life Sci. 2005;78:527–31. doi: 10.1016/j.lfs.2005.09.008. [DOI] [PubMed] [Google Scholar]
- Rosenberg MB, Carroll FI, Negus SS. Effects of monoamine reuptake inhibitors in assays of acute pain-stimulated and pain-depressed behavior in rats. J Pain. 2013;14:246–59. doi: 10.1016/j.jpain.2012.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Runyon SP, Brieaddy LE, Mascarella SW, Thomas JB, Navarro HA, Howard JL, Pollard GT, Carroll FI. Analogues of (3R)-7-hydroxy-N-[(1S)-1-{[(3R,4R)-4-(3-hydroxyphenyl)-3,4-dimethyl-1-piperidinyl]methyl}-2-methylpropyl)-1,2,3,4-tetrahydro-3-isoquinolinecarboxamide (JDTic). Synthesis and in vitro and in vivo opioid receptor antagonist activity. J Med Chem. 2010;53:5290–301. doi: 10.1021/jm1004978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schattauer SS, Kuhar JR, Song A, Chavkin C. Nalfurafine is a G-protein biased agonist having significantly greater bias at the human than rodent form of the kappa opioid receptor. Cell Signal. 2017;32:59–65. doi: 10.1016/j.cellsig.2017.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seguin L, Le Marouille-Girardon S, Millan MJ. Antinociceptive profiles of non-peptidergic neurokinin1 and neurokinin2 receptor antagonists: a comparison to other classes of antinociceptive agent. Pain. 1995;61:325–43. doi: 10.1016/0304-3959(94)00194-J. [DOI] [PubMed] [Google Scholar]
- Thomsen JS, Petersen MB, Benfeldt E, Jensen SB, Serup J. Scratch induction in the rat by intradermal serotonin: a model for pruritus. Acta Derm Venereol. 2001;81:250–4. doi: 10.1080/00015550152572868. [DOI] [PubMed] [Google Scholar]
- Todtenkopf MS, Marcus JF, Portoghese PS, Carlezon WA., Jr Effects of kappa-opioid receptor ligands on intracranial self-stimulation in rats. Psychopharmacology (Berl) 2004;172:463–70. doi: 10.1007/s00213-003-1680-y. [DOI] [PubMed] [Google Scholar]
- Togashi Y, Umeuchi H, Okano K, Ando N, Yoshizawa Y, Honda T, Kawamura K, Endoh T, Utsumi J, Kamei J, Tanaka T, Nagase H. Antipruritic activity of the kappa-opioid receptor agonist, TRK-820. Eur J Pharmacol. 2002;435:259–64. doi: 10.1016/s0014-2999(01)01588-6. [DOI] [PubMed] [Google Scholar]
- Wadenberg ML. A review of the properties of spiradoline: a potent and selective kappa-opioid receptor agonist. CNS Drug Rev. 2003;9:187–98. doi: 10.1111/j.1527-3458.2003.tb00248.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White KL, Robinson JE, Zhu H, DiBerto JF, Polepally PR, Zjawiony JK, Nichols DE, Malanga CJ, Roth BL. The G protein-biased kappa-opioid receptor agonist RB-64 is analgesic with a unique spectrum of activities in vivo. J Pharmacol Exp Ther. 2015;352:98–109. doi: 10.1124/jpet.114.216820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L, Lovell KM, Frankowski KJ, Slauson SR, Phillips AM, Streicher JM, Stahl E, Schmid CL, Hodder P, Madoux F, Cameron MD, Prisinzano TE, Aube J, Bohn LM. Development of functionally selective, small molecule agonists at kappa opioid receptors. J Biol Chem. 2013;288:36703–16. doi: 10.1074/jbc.M113.504381. [DOI] [PMC free article] [PubMed] [Google Scholar]