Abstract
Angiotensin II type 1 receptor (AT1R)-antibody has been linked to poor allograft outcomes in adult kidney transplantation. However, its clinical consequences in children are unknown. To study this, we examined the relationship of AT1R-antibody with clinical outcomes, biopsy findings, inflammatory cytokines, and HLA donor specific antibodies (DSA) in a cohort of pediatric renal transplant recipients. Sixty-five patients were longitudinally monitored for AT1R-antibody, HLA DSA, IL-8, TNF-α, IL-1β, IFN-γ, IL-17, and IL-6, renal dysfunction, hypertension, rejection, and allograft loss during the first two years post transplantation. AT1R-antibody was positive in 38 of the 65 of children but was not associated with HLA DSA. AT1R-antibody was associated with renal allograft loss (odds ratio of 13.1 (95% confidence interval (1.48 – 1728)), the presence of glomerulitis or arteritis, and significantly higher TNF-α, IL-1β, and IL-8 levels, but not rejection or hypertension. AT1R-antibody was associated with significantly greater declines in eGFR in patients both with and without rejection. Furthermore, in patients without rejection, AT1R-antibody was a significant risk factor for worsening eGFR over the two year follow-up period. Thus, AT1R-antibody is associated with vascular inflammation in the allograft, progressive decline in eGFR, and allograft loss. AT1R-antibody and inflammatory cytokines may identify those at risk for renal vascular inflammation and lead to early biopsy and intervention in pediatric kidney transplantation.
Keywords: Pediatric Nephrology, Inflammation, Endothelium, Cytokines, Angiotensin
Introduction
Antibody mediated rejection (AMR) remains a significant barrier to successful long-term outcomes in kidney transplantation.1–3 The role of alloantibody responses against Human Leukocyte Antigens (HLA) in mediating AMR has been a primary focus in transplantation.2, 4, 5 However, non-HLA autoantibodies have gained importance for their involvement in AMR.6–8 Moreover, the interplay between alloantibody and autoantibody responses is becoming important to our understanding and management of AMR. Antibodies to various non-HLA targets,6, 7 such as major-histocompatibility-complex class I-related chain A,9–14 endothelin type A receptor,15, 16 perlecan,17 collagen-IV,18 fibronectin,18 and angiotensin II type 1 receptor antibody (AT1R-Ab)19–24 have been associated with poor allograft outcomes in renal transplantation. Evidence for routine testing, however, remains insufficient.8
AT1R-Ab, in particular, is becoming recognized for its association with vascular injury and allograft failure in adult kidney transplant patients.19–24 Dragun and colleagues were the first to report the association of AT1R-Ab with acute AMR, endarteritis, and severe hypertension in kidney transplant recipients.19 Since that time, AT1R-Ab has also been linked to AMR in the absence of hypertension, allograft loss, acute cellular rejection (ACR), and decreased renal function in patients without this classical presentation,21–25 suggesting multiple clinical phenotypes exist for AT1R-Ab mediated allograft injury.
AT1R-Ab can directly injure endothelial and vascular smooth muscle cells, leading to elevated transcription factors associated with pro-inflammatory responses.26 Therefore, peripheral blood markers of inflammation such as cytokines might enhance our understanding of the effects of AT1R-Ab in clinical transplantation. Additionally, the relationship between allograft injury due solely to AT1R-Ab versus that due to the combination of AT1R-Ab and HLA DSA is unclear. Certain studies have found that the pathogenesis of AT1R-Ab-induced injury is independent of HLA DSA24 while others have found that AT1R-Ab and HLA DSA together portend inferior clinical outcomes.22
Recently, high AT1R-Ab levels have been shown to be more common in pediatric than adult renal transplant recipients.27, 28 However, the clinical significance and impact of elevated AT1R-Ab levels on allograft outcomes in pediatric patients remains unclear. Therefore, we examined the relationship of AT1R-Ab with clinical outcomes including allograft loss, biopsy findings, and renal function in a longitudinal cohort of pediatric renal transplant recipients. In addition, we investigated the association of AT1R-Ab with HLA DSA and a panel of inflammatory cytokines.
Results
Prevalence and Clinical Characteristics
The prevalence of AT1R-Ab >17 U/mL in our pediatric cohort was 58% (38/65) at any time pre-transplant to 2 years post-transplant. The cutoff of 17 U/mL was initially chosen based on the literature20, 29. Furthermore, we generated an area under the curve (AUC) for AT1R-Ab and clinical outcomes which confirmed a cut-off of 17 U/ml was within 6% of the optimal threshold in our pediatric cohort (Table S1). In AT1R-Ab positive patients, AT1R-Ab was present prior to transplantation (i.e., preformed) in 39% (15/38), de novo after transplant in 45% (17/38), and undetermined in 16% (6/38) due to the lack of pre-transplant sera. Of the patients who developed de novo AT1R-Ab, 59% (10/17) did so in the first 6 months, 35% (6/17) between 6–12 months, and 6% (1/17) between 12–24 months post-transplant. Figure 1 shows the longitudinal comparison of AT1R-Ab levels in AT1R-Ab positive (Figure 1a) versus negative (Figure 1b) patients during the first 2 years post-transplantation. Patients who were AT1R-Ab positive at any time during the monitoring period generally remained positive throughout. All patients who were positive pre-transplant continued to be AT1R-Ab positive at some time post-transplant.
Figure 1. Comparison of AT1R-Ab Levels over 2 year Follow-Up Period.

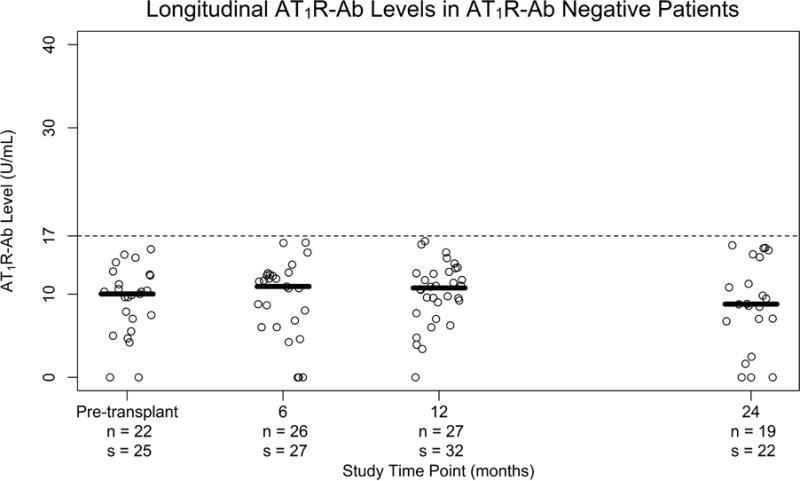
in a) patients positive for AT1R-Ab at any time point and b) patients who were negative for AT1R-Ab at all time points. Each plot point represents an individual patient blood sample taken pre-transplant and at 6, 12, and 24 months after transplantation. The median AT1R-Ab level at a given time point is represented by the bar in relation to the AT1R-Ab positive threshold of >17 units/mL (dashed line). n= number patients s= number of samples
There were no significant differences in demographic or baseline clinical characteristics between AT1R-Ab positive (defined as >17 units/mL at any time point) and negative patients (Table 1). Table 2 compares immunological characteristics and therapy between the two groups. There were no differences in pre-transplant sensitization risk factors including HLA mismatch, panel reactive antibody (PRA), and history of prior transplantation. There was an association between AT1R-Ab and anti-thymocyte globulin (ATG) induction (p=0.037). Four of six patients given ATG induction were sensitized with PRA Class I or II >30% and two were given ATG as part of a rapid steroid withdrawal protocol. The development of HLA DSA was notably not a risk factor for AT1R-Ab (p>0.99). Additional immunomodulatory treatments beyond our standard immunosuppression protocol (Table 2) were given for delayed graft function, acute rejection or disease recurrence. Overall, patients in the AT1R-Ab positive group received more total treatment days of augmented immunomodulation (p=0.010) and plasmapheresis (p=0.002). Using a mixed-effects longitudinal regression model, we found no temporal association between AT1R-Ab and the development of HLA DSA, acute rejection, or immunomodulatory treatments including plasmapheresis (data not shown).
Table 1. Demographics and Clinical Characteristics.
Comparison of demographic and clinical characteristics between AT1R-Ab positive and negative patients. IQR, interquartile range; ESRD, end stage renal disease; FSGS, focal segmental glomerulosclerosis; PKD, polycystic kidney disease; EBV, Epstein-Barr virus; CMV, cytomegalovirus; BKV, BK virus
| AT1R-Ab Positive (n=38) |
AT1R-Ab Negative (n=27) |
p-value | |
|---|---|---|---|
| Age, median (IQR) | 14.8 (12.8–17.6) | 16.4 (13.3–18.4) | 0.369 |
| Male Sex | 23 (60.5%) | 16 (59.3%) | >0.99 |
| Hispanic Ethnicity | 18 (47.4%) | 18 (66.7%) | 0.138 |
| Race | 0.243 | ||
| White | 28 (73.7%) | 19 (70.4%) | |
| Asian | 2 (5.3%) | 2 (7.4%) | |
| Black | 4 (10.5%) | 0 (0%) | |
| Other | 4 (10.5%) | 6 (22.2%) | |
| Dialysis Prior to Transplant | 32 (84.2%) | 19 (70.4%) | 0.227 |
| Years on Dialysis, median (IQR) | 2.4 (0.8–3.1) | 1.7 (1.1–2.5) | 0.355 |
| EBV Immune | 28 (73.7%) | 20 (74.1%) | >0.99 |
| CMV Immune | 24 (63.2%) | 14 (51.9%) | 0.446 |
| Deceased Donor | 26 (68.4%) | 14 (51.9%) | 0.204 |
| Etiology of ESRD | 0.931 | ||
| Dysplasia | 5 (13.2%) | 4 (14.8%) | |
| FSGS | 6 (15.8%) | 3 (11.1%) | |
| Glomerulonephritis | 5 (13.2%) | 3 (11.1%) | |
| IgA nephropathy | 0 (0%) | 1 (3.7%) | |
| Obstructive Uropathy | 9 (23.7%) | 7 (25.9%) | |
| PKD | 2 (5.3%) | 0 (0%) | |
| Other | 5 (13.2%) | 5 (18.5%) | |
| Unknown | 6 (15.8%) | 4 (14.8%) | |
| Cold Ischemia Time (hours), median (IQR) | 11.1 (1–13.8) | 6.3 (1–15) | 0.447 |
| Delayed Graft Function | 3 (7.9%) | 1 (3.7%) | 0.636 |
| EBV, CMV, or BK Viremia | 18 (52.9%) | 11 (44%) | 0.601 |
| Physician Assessed Non - Adherence | 11 (28.9%) | 5 (18.5%) | 0.393 |
Table 2. Immunological Characteristics and Therapy.
Comparison of sensitization status and immunomodulatory treatments between AT1R-Ab positive and negative patients. HLA, human leukocyte antigen; SD, standard deviation; PRA, panel reactive antibody; ATG, anti-thymocyte globulin; IL-2, interleukin-2; DSA, donor specific antibody; ACE, angiotensin converting enzyme; ARB, angiotensin receptor blocker; IQR, interquartile range; IVIG, intravenous immunoglobulin
| AT1R-Ab Positive (n=38) |
AT1R-Ab Negative (n=27) |
p-value | |
|---|---|---|---|
| HLA Mismatch, mean (SD) | 1.2 (0.6) | 1.2 (0.5) | 0.730 |
| Primary Transplant | 33 (86.8%) | 26 (96.3%) | 0.388 |
| Baseline PRA Class I > 20% | 3 (7.9%) | 1 (3.7%) | 0.636 |
| Baseline PRA Class II > 20% | 6 (15.8%) | 1 (3.7%) | 0.224 |
| ATG Induction (vs. IL - 2 Inhibitor) | 6 (15.8%) | 0 (0%) | 0.037 |
| Steroids Based Immunosuppression | 17 (44.7%) | 14 (51.9%) | 0.621 |
| Presence of HLA DSA in the First 2 Years Post-Transplant | >0.99 | ||
| Negative | 27 (71.1%) | 19 (70.4%) | |
| Class I Only | 3 (7.9%) | 2 (7.4%) | |
| Class II Only | 7 (18.4%) | 5 (18.5%) | |
| Class I and Class II | 1 (2.6%) | 1 (3.7%) | |
| ACE Inhibitor Treatment | 10 (26.3%) | 3 (11.1%) | 0.209 |
| ARB Treatment | 1 (2.6%) | 0 (0%) | >0.99 |
| Immunomodulatory Treatment Days, median (IQR) | 6 (0–21.5) | 0 (0–3) | 0.010 |
| Any Immunomodulatory Treatment | 23 (60.5%) | 12 (44.4%) | 0.219 |
|
Immunomodulatory Treatment by Medication
|
|||
| High Dose Steroids | 15 (39.5%) | 8 (29.6%) | 0.444 |
| IVIG | 11 (28.9%) | 4 (14.8%) | 0.239 |
| ATG | 15 (39.5%) | 5 (18.5%) | 0.102 |
| Rituximab | 6 (15.8%) | 1 (3.7%) | 0.224 |
| Plasmapheresis | 11 (28.9%) | 0 (0%) | 0.002 |
| Bortezomib | 2 (5.3%) | 0 (0%) | 0.507 |
AT1R-Ab and Allograft Loss
AT1R-Ab positive status within the first 2 years post-transplant was associated with renal allograft loss (p=0.036) (Figure 2). Seven patients experienced allograft loss: 1 between 0–6 months, 3 between 6–12 months, and 3 between 12–24 months. Five of 7 patients had a positive AT1R-Ab at the time of allograft failure. The remaining 2 patients were AT1R-Ab positive at the time of treatment resistant ACR episodes with vascular involvement and were treated with rituximab and/or bortezomib plus plasmapheresis. The AT1R-Ab became negative after treatment; however, these two patients subsequently developed progressive fibrosis and allograft failure.
Figure 2. Clinical Outcomes by AT1R-Ab Status.
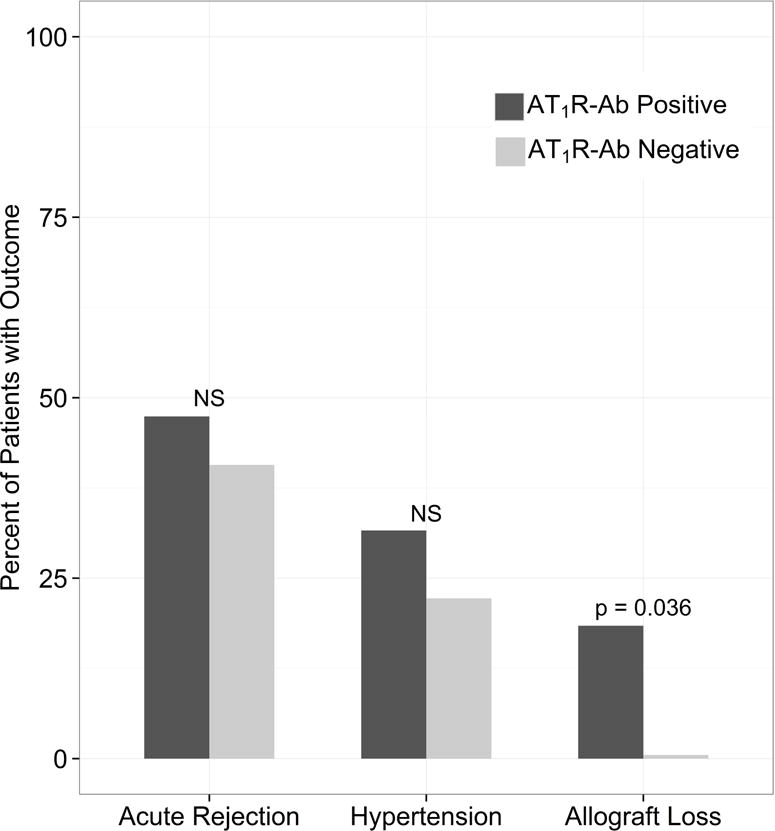
AT1R-Ab Positive status was defined as having AT1R-Ab >17 units/mL at any time point from pre-transplant through the first 2 years post-transplant. AT1R-Ab positive status was associated with allograft loss but not acute rejection or hypertension. Of the 65 patients, 29 had acute rejection, 18 had hypertension, and 7 had allograft loss.
We conducted univariate and multivariable analysis to further assess risk factors for allograft loss (Table S2). We limited the number of variables in our model given the small number of events and excluded potential intermediate outcomes. On univariate analysis, AT1R-Ab positive status (OR 95% CI of 13.1 (1.48 – 1728.44), p=0.036), deceased donor transplant (p=0.038), mean HLA mismatch (p=0.005), and physician assessed non-adherence (p=0.008) were associated with renal allograft loss (Table S2). As significant collinearity existed between donor type and HLA mismatch, only HLA mismatch was included in the final model (see Statistical Methods). AT1R-Ab positive status remained associated with allograft loss (OR 95% CI of 9.24 (0.51–168.38), p=0.061) after accounting for mean HLA mismatch and physician assessed non-adherence in the multivariable model (Table S2).
AT1R-Ab and Biopsy Findings
AT1R-Ab was not associated with ACR, C4d negative AMR, or C4d positive AMR (Table S3). AT1R-Ab was not associated with elevated acute interstitial or tubular inflammation scores. A combination score reflecting the presence of vascular inflammation represented by either glomerulitis or arteritis was statistically significant (p=0.037). AT1R-Ab was not associated with acute peritubular capillaritis or other combination acute vascular inflammation scores. AT1R-Ab was not associated with chronic change scores or degree of interstitial fibrosis and tubular atrophy (Table S3).
AT1R-Ab and Renal Function
Patients with AT1R-Ab demonstrated larger declines in eGFR over the first 2 years post-transplant compared to those without AT1R-Ab (p=0.013) (Figure 3a). Because AT1R-Ab may exert direct effects on the allograft endothelium outside the context of biopsy proven acute rejection, we investigated AT1R-Ab as a risk factor for worsening renal function in patients both with and without rejection. In both groups, patients with AT1R-Ab had a greater median decline in eGFR than those without AT1R-Ab (p=0.003) (Figure 3b). Furthermore, we longitudinally analyzed eGFR in patients without rejection and found that patients with AT1R-Ab had significantly greater decline in eGFR over the first 2 years post-transplant (p=0.032, Figure 3c).
Figure 3. Relationship between Renal Function and AT1R-Ab.

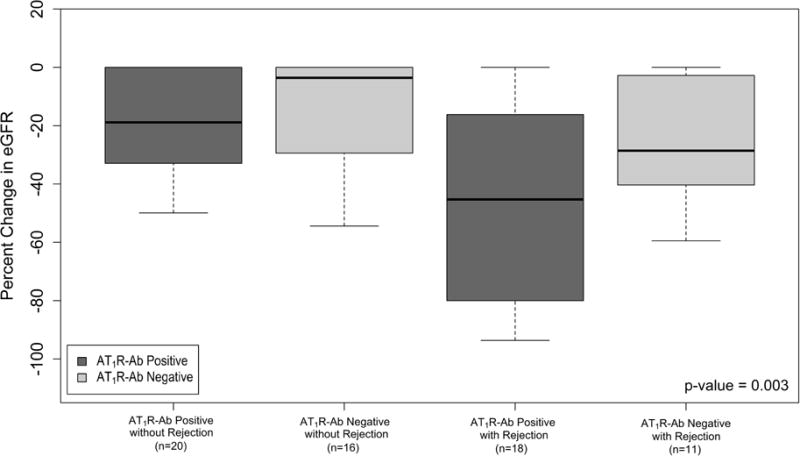
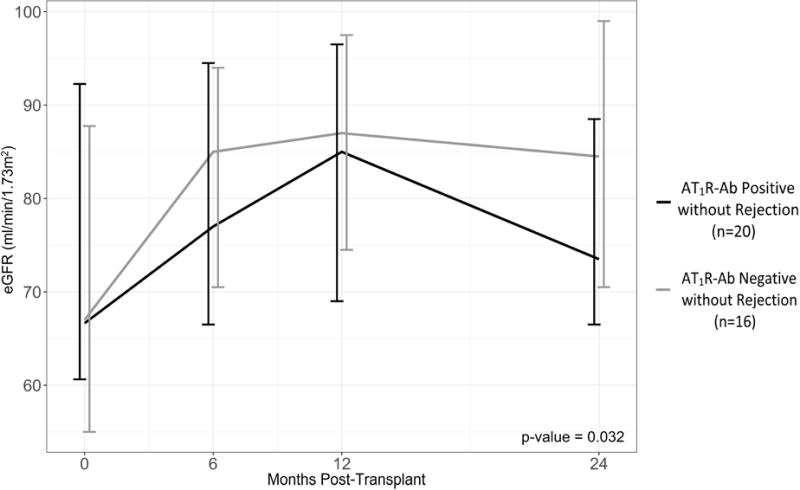
Median percent change in eGFR by a) AT1R-Ab status and b) AT1R-Ab and Rejection Status shown, p-value for 4 group comparison. Percent change in eGFR was taken from hospital discharge to lowest eGFR value during the 2 year follow up period. c) The impact of AT1R-Ab status on eGFR over time in patients without rejection assessed by a mixed-effects regression model. Median eGFR and interquartile range at each time point post-transplant is shown. All patients in this analysis had developed AT1R-Ab by the 12 month time-point. Notably, patients with AT1R-Ab, in the absence of rejection, had progressive decline in renal function in contrast to patients who remained free from AT1R-Ab development over the 2 year period. β = 0.85 (0.00 – 1.69).
AT1R-Ab and Cytokine Analysis
AT1R-Ab was associated with vascular inflammation and a decline in allograft function that was not mediated by acute rejection. Therefore, a panel of serum cytokines associated with activation of the AT1R in hypertension, scleroderma, and preeclampsia30–35 was measured post-transplantation to assess the activity of AT1R mediated inflammatory pathways in kidney transplant patients. Although these cytokines have been associated with AT1R activation in other disease states, it remained unclear which of the 6 cytokines were important in kidney transplantation, therefore, they were analyzed individually. Patients positive for AT1R-Ab had higher TNF-α (p=0.043), IL-1β (p=0.045), and IL-8 (p=0.016) levels (Figure 4). There was no association between AT1R-Ab status and IFN-γ, IL-17, or IL-6 levels. We further investigated the temporal relationship between these cytokines and AT1R-Ab positivity in blood samples over the 2 year period. The blood sample analysis was based on the AT1R-Ab status (>17 units/mL considered positive) and cytokine levels in individual blood samples controlled for patient-level random effects. In this longitudinal analysis, TNF-α, IL-1β, and IL-8 were all significantly elevated in AT1R-Ab positive versus negative blood samples (Figure S1 a–c, p-values 0.037, 0.005, and 0.004 respectively). A similar analysis did not reveal any differences in IFN-γ, IL-17, or IL-6 levels.
Figure 4. Cytokine Levels in Patients by AT1R-Ab Status.

Comparison of cytokine levels in patients with (n=38) and without AT1R-Ab (n=27) in the first 2 years post-transplant. For each patient, the median of all the post-transplant values for each cytokine over the 2 year period was used as representative.
Discussion
In the largest, comprehensive pediatric cohort to date, we longitudinally examined the association of AT1R-Ab with clinical outcomes, development of HLA DSA, biopsy findings, and inflammatory cytokines. Consistent with previous studies, AT1R-Ab was highly prevalent in pediatric renal transplant recipients.27, 28 Notably, we are the first to show the detrimental association of AT1R-Ab on allograft survival and function in children, separate from the effects of HLA DSA. Furthermore, we found a significant correlation between AT1R-Ab, arteritis or glomerulitis on renal biopsy, and elevated serum TNF-α, IL-1β, and IL-8 levels, which may represent vascular inflammation. Taken together, our data suggests that renal transplant recipients with AT1R-Ab may manifest a pattern of vascular injury as evidenced by renal biopsy findings, decrement in renal function, increased incidence of allograft failure, and elevated serum cytokines.
The lack of a standardized cutoff for determining AT1R-Ab positivity presents a challenge for comparing and generalizing studies; many adult studies have found lower levels of AT1 R-Ab (>9,21, 36, 3710,24, 38 and 1522 units/mL) to be associated with poor renal transplant outcomes. A prior pediatric study27 showed that pediatric patients with stable allograft function have higher levels of AT1R-Ab compared to adults. To address this question, we constructed an AT1R-Ab AUC that confirms 17 U/mL to be the optimal threshold to predict poor transplant outcomes including allograft loss, eGFR decline, and glomerulitis/arteritis in a pediatric population. Our study supports the use of the 17 U/mL AT1R-Ab cutoff in children to avoid excessive false positive values and may guide future studies.
Pre-transplant AT1R-Ab was present in 23% (15/65) of the total population, which is within the range of 17–33% that has been described in adults using similar cutoffs,20, 22 but higher than the 0% reported in a pediatric study of 20 patients.28 Importantly, 26% (17/65) of our cohort developed de novo AT1R-Ab. By contrast, the largest analogous study in adult kidney transplant recipients found only 3% of patients with de novo AT1R-Ab.22 The higher prevalence of AT1R-Ab found in children compared to adults is most likely multifactorial. Inflammatory events at the time of transplant, such as ischemia reperfusion injury, may trigger non-HLA antibody formation.6 Younger patients may be at higher risk of ischemic injury39, 40 secondary to surgical and hemodynamic challenges related to their size. Additionally, children are more susceptible to post-transplant infections.41 Activation of inflammatory pathways by infectious agents, particularly Parvovirus,42 have been implicated in the development of AT1R-Ab formation. Unfortunately, we did not routinely measure Parvovirus. Although we did not find an association between CMV, EBV, and BK viremia and AT1R-Ab, we may have been underpowered to detect a difference and this requires further investigation in a larger cohort.
Consistent with other studies, the presence of HLA DSA was not associated with AT1R-Ab in our cohort. Giral et al reported no association between pre-transplant AT1R-Ab and HLA DSA in adult recipients.24 Furthermore, Hesemann et al found no association between the development of HLA DSA with de novo AT1R-Ab in 8 children.28 In contrast, Cuevas and colleagues reported an association between high levels of pre-transplant AT1R-Ab and the formation of de novo HLA DSA,23 and Taniguchi and colleagues found that patients with both HLA DSA and AT1R-Ab had inferior allograft survival.22 These conflicting results support the need for continued investigation to understand the relationship of auto- and allo-immune responses in kidney transplantation.
Surprisingly, ATG induction was the only significant risk factor we found for the presence of AT1R-Ab in the first 2 years post-transplant. Patients receiving ATG induction were more likely to be sensitized against HLA antibodies which could lead to subsequent autoimmune responses and development of AT1R-Ab.6 ATG may also deplete T regulatory cells43, 44 thereby promoting autoimmunity. However, the association of AT1R-Ab with ATG induction was unexpected since cell depletion diminishes antibody production by reducing CD4 helper T cells. Furthermore, we, like others,21, 22, 24 have not found an association between pre-transplant PRA and AT1R-Ab. ATG induction has been associated with lower risk of de novo HLA DSA in sensitized patients45 and a recent study reported a potential benefit of using an ATG induction protocol in AT1R-Ab positive patients.46 Therefore, our results must be interpreted with caution.
Perhaps the most important findings of our study were the associations of AT1R-Ab with poor clinical outcomes. We are the first to show an association between AT1R-Ab and allograft loss in children. Heseman et al found no association between AT1R-Ab and rejection in 29 pediatric patients.28 Bjerre et all showed that AT1R-Ab levels are higher in stable pediatric kidney transplant patients when compared to adults, however, history of rejection events in the pediatric group were too rare to be analyzed.27 Furthermore, AT1R-Ab was correlated with greater declines in renal function in our cohort, even in the absence of rejection, which has also been reported in adults.21, 23 Our longitudinal analysis, demonstrating significant progressive decline in eGFR in patients without rejection, suggests a sustained, long-term impact of AT1R-Ab. This finding is particularly compelling as percent change in eGFR has been shown to predict long term allograft outcomes.47 Given the high prevalence of AT1R-Ab in pediatric kidney transplant recipients shown in our study and by others,27, 28 a better understanding of this relationship is essential to their clinical management.
We performed a detailed examination of sequential biopsies and analyzed serum cytokines associated with AT1R activation found in other disease states such as scleroderma and preeclampsia.30–35 We showed that patients with AT1R-Ab were more likely to have glomerulitis or arteritis on biopsy which is consistent with other studies reporting associations between AT1R-Ab and vascular inflammation.21, 48 Furthermore, we are the first to report that kidney transplant patients with AT1R-Ab have higher TNF-α, IL-1β, and IL-8 levels. These elevations occur simultaneously with AT1R-Ab positivity. Angiotensin II has been shown to stimulate TNF-α production in glomerular endothelial cells in rats.49 Endothelial cells can also produce IL-1β and IL-8, which are known to promote leukocyte chemotaxis and artherogenesis.35, 50–52 Taken together, our data support an association between AT1R-Ab and renovascular inflammation, which is consistent with previous work.19, 48 It appears that this process can worsen functional outcomes in the absence of rejection. Thus, we hypothesize that AT1R-Ab can activate the endothelium19 and mediate vascular inflammation leading to progressive decline in renal function and eventual allograft loss. This hypothesis requires testing in prospective mechanistic studies. Additionally, validating the use of AT1R-Ab and cytokine testing to identify patients at highest risk for poor allograft outcomes may be warranted.
There are important limitations of our study. Although some studies have shown an association between AT1R-Ab and hypertension,19, 21, 24 our study and others23, 28 have not. Furthermore, this retrospective examination shows association and not causation. Nevertheless, our findings are hypothesis generating for prospective mechanistic studies. We also cannot rule out the potential of unmeasured confounders or confounders with limited events, such as other non-HLA antibodies, recurrent disease, medication toxicity, or infection. Our study only examined patients for the first 2 years post-transplantation, which may not allow sufficient time for chronic change to develop on biopsy. Our small sample size makes negative findings more difficult to interpret and restricted our ability to do extensive multivariable and subgroup analyses. A larger cohort is required to validate the clinical impact of AT1R-Ab, importance of pre-transplant versus post-transplant development of AT1R-Ab, interaction of AT1R-Ab with both HLA and other non-HLA antibodies, and the role of cytokines as a predictor of AT1R-Ab mediated allograft injury. No treatment effects were observed in our study, however, this may be secondary to the timing of sample collection. Success with the use of angiotensin receptor blockade (ARB), plasmapheresis, IVIG, and ATG in the treatment19, 53 and peri-operative prevention46 of AT1R-Ab mediated AMR have been reported. Randomized controlled trials are warranted to examine the efficacy of these treatments for AT1R-Ab mediated AMR and the potential role of ARBs in preventing subacute decline in renal function.
In conclusion, our comprehensive observational study demonstrates an association between AT1R-Ab and allograft loss, decline in renal function, and vascular inflammation in a cohort of pediatric renal transplant recipients. Our data suggests cytokines may be useful to identify renal vascular inflammation in conjunction with renal biopsy and AT1R-Ab testing, leading to early intervention with ARB therapy and potential attenuation of AT1R-Ab mediated allograft injury.46 Our study highlights the clinical consequences of AT1R-Ab in pediatric kidney transplant recipients and supports the use of an AT1R-Ab cut-off of 17 U/mL in children.
Methods
Patients and Study Design
In this retrospective study, 65 pediatric kidney transplant patients were monitored for 2 years post-transplant. From August 2005 to November 2014, 83 patients were enrolled in the UCLA Pediatric Kidney Transplant Immune Monitoring Study, and 18 patients were excluded from analysis secondary to missing > 1 study sample at the below time points. This study was approved by the UCLA Institutional Review Board (#11-002375) and conforms with the 1964 Helsinki declaration and its later amendments or comparable ethical standards and the Principles of the Declaration of Istanbul. Informed consent and when appropriate patient assent was obtained for all patients. Blood samples were obtained pre-transplant and at 6, 12, and 24 months post-transplant and during episodes of kidney transplant rejection. In longitudinal analyses, blood samples were grouped by periods within 3 months of the time point. Demographic and clinical data including age, race, ethnicity, etiology of ESRD, transplant type (deceased/living donor), sensitization history, time on dialysis, delayed graft function (defined as dialysis in the first week post-transplant), immunosuppression regimen, cytomegalovirus, Epstein Barr virus, and BK Virus levels, medication nonadherence, and blood pressure were collected. Non-adherence by staff report was defined as physician and/or clinic staff documentation of patient report, undetectable drug levels, or missed appointments.4 Patients were monitored for rejection, allograft loss, and decrease in eGFR as determined by the updated Schwartz equation54 in patients <18 years old at the time of transplant and by the abbreviated Modification of Diet in Renal Disease (MDRD) equation55 in patients ≥18 at the time of transplant. Hypertension was determined by age, sex, and height percentile56 for children <18 years old and based on American Society of Hypertension and the International Society of Hypertension guidelines for patients ≥18.57 Additional approaches for eGFR are shown in Table S4. Study data were collected and managed using a secure REDCap (Research Electronic Data Capture) electronic data capture tools hosted at UCLA.58 Of the 65 patients, 54 patients had complete 2 year follow up, 7 patients suffered graft loss, and 4 patients transferred care to a different institution. No patients died during the study period. A total of 9.1 patient-years of follow up were analyzed.
Clinical Protocols and Biopsy Evaluation
UCLA immunosuppressive regimen for pediatric transplant recipients included induction with either ATG for PRA ≥ 30%, delayed graft function, or rapid-steroid withdrawal protocol or anti-CD25 monoclonal antibody for those with PRA<30%. Maintenance immunosuppression consisted of steroid free or steroid based immunosuppression, a calcineurin inhibitor, and an anti-metabolite. Acute and chronic rejection were treated with previously described protocols.59
Patients underwent protocol biopsies at 6, 12, and 24 months post-transplantation or for clinical indication. Biopsies were evaluated based on the 2013 Banff Criteria.60 Two patients in the AT1R-Ab positive group and one patient in the AT1R-Ab negative group were not included in the biopsy score data as they had missing values.
Antibody Testing
HLA typing of recipient and donor was performed using molecular methods as previously described.59 HLA antibodies were detected using a Luminex single antigen bead (SAB) assay (Immucor, Stanford, CT) and quantified by mean fluorescence intensity (MFI). Antibodies were considered positive when MFI was ≥1000 for HLA-A, -B, -DR, -DQ, and ≥2000 for HLA-C and –DP.61 AT1R-Ab was measured by enzyme-linked immunosorbent based assay (One Lambda, Canoga Park, CA). Sera were diluted 1:100, tested in duplicate and AT1R-Ab concentrations were determined by a standard curve. AT1R-Ab IgG >17 units/ml was considered positive.29, 62 AT1R-Abs exceeding the limit of the standard curve were further diluted and re-tested to determine concentration.
Cytokine Testing
Cytokines were selected based on a literature review of cytokines that have been associated with activation of the AT1R30–35 and measured in serial post-transplant samples to avoid effects of dialysis and end-stage renal disease. A custom magnetic bead kit including IL-8, TNF-α, IL-1β, IFN-γ, IL-17, and IL-6 (EMD Millipore, Darmstadt, Germany) was used per manufacturer’s instructions. Fluorescence was quantified using a Luminex 200TM instrument. For the patient level analysis of cytokines, the median of the post-transplant values of each cytokine across samples was used as representative. Prior to statistical analysis, cytokines were transformed using the log(x+1) transformation.
Statistical Methods
A receiver operating characteristic analysis was performed to validate the AT1R-Ab >17 U/mL cutoff suggested by the literature. This was done by generating thresholds to predict various clinical outcomes and comparing these thresholds to an AT1R-Ab >17 U/mL cutoff. The best threshold for each clinical outcome was determined using the Youden Index.63 Categorical variables were compared between groups using Fisher’s exact test. Continuous variables were compared between groups using either the Wilcoxon Rank Sum test or a t-test based on the distribution of the data. To assess the relationship of clinical factors with the outcome of allograft loss, we constructed univariate logistic regression models. Variables that had a p-value on univariate analysis below 0.10 were included in a multivariable model. Due to small number of events of allograft loss, we used Firth’s penalized likelihood approach to fit the logistic regression models. To address concerns about collinearity between donor type and HLA mismatch, separate models were fit to using these variables and the model with the highest c-statistic is presented. An additional longitudinal mixed-effect regression model was constructed to assess the effect of AT1R-Ab positivity over time on various clinical characteristics (i.e., viremia, rejection, HLA antibodies). Separate models were used for each characteristic with each model including a random slope and random intercept for time from transplant. Mixed effects regression models were used to evaluate the effect of AT1R-Ab positivity on eGFR and cytokine levels over time. The model for longitudinal cytokine analysis was controlled for patient level random effects. The regression coefficients, represented by “β” are reported. A p- value below 0.05 was considered statistically significant and all tests were two-sided. The R Statistical Computing Environment was used for analysis (R Core Team; Vienna, Austria).
Supplementary Material
Acknowledgments
This study was supported by the Ruth L. Kirschstein National Research Service Award T32 DK104687 UCLA Translational Research Grant in Pediatric Nephrology Program (M.H.P); the Casey Lee Ball Foundation (E.W.T and M.H.P); the Today’s and Tomorrow’s Children Fund (E.W.T); the National Institute of Allergy and Infectious Diseases Grant R01AI042819 (E.F.R.); and by the NIH National Center for Advancing Translational Sciences UCLA CTSI Grant Number UL1TR001881. We would also like to acknowledge Shanti Seaman for her work in organizing data for this project.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: Elaine F. Reed has an industry grant from Immucor that was not used to fund this project. All the authors declared no competing interests
References
- 1.Meier-Kriesche HU, Schold JD, Srinivas TR, et al. Lack of improvement in renal allograft survival despite a marked decrease in acute rejection rates over the most recent era. Am J Transplant. 2004;4:378–383. doi: 10.1111/j.1600-6143.2004.00332.x. [DOI] [PubMed] [Google Scholar]
- 2.Sellares J, de Freitas DG, Mengel M, et al. Understanding the causes of kidney transplant failure: the dominant role of antibody-mediated rejection and nonadherence. Am J Transplant. 2012;12:388–399. doi: 10.1111/j.1600-6143.2011.03840.x. [DOI] [PubMed] [Google Scholar]
- 3.Wiebe C, Gibson IW, Blydt-Hansen TD, et al. Rates and Determinants of Progression to Graft Failure in Kidney Allograft Recipients With De Novo Donor-Specific Antibody. Am J Transplant. 2015;15:2921–2930. doi: 10.1111/ajt.13347. [DOI] [PubMed] [Google Scholar]
- 4.Wiebe C, Gibson IW, Blydt-Hansen TD, et al. Evolution and clinical pathologic correlations of de novo donor-specific HLA antibody post kidney transplant. Am J Transplant. 2012;12:1157–1167. doi: 10.1111/j.1600-6143.2012.04013.x. [DOI] [PubMed] [Google Scholar]
- 5.Terasaki PI, Ozawa M. Predicting kidney graft failure by HLA antibodies: a prospective trial. Am J Transplant. 2004;4:438–443. doi: 10.1111/j.1600-6143.2004.00360.x. [DOI] [PubMed] [Google Scholar]
- 6.Zhang Q, Reed EF. The importance of non-HLA antibodies in transplantation. Nat Rev Nephrol. 2016;12:484–495. doi: 10.1038/nrneph.2016.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dragun D, Catar R, Philippe A. Non-HLA antibodies against endothelial targets bridging allo- and autoimmunity. Kidney international. 2016;90:280–288. doi: 10.1016/j.kint.2016.03.019. [DOI] [PubMed] [Google Scholar]
- 8.Tait BD, Susal C, Gebel HM, et al. Consensus guidelines on the testing and clinical management issues associated with HLA and non-HLA antibodies in transplantation. Transplantation. 2013;95:19–47. doi: 10.1097/TP.0b013e31827a19cc. [DOI] [PubMed] [Google Scholar]
- 9.Zou Y, Stastny P, Susal C, et al. Antibodies against MICA antigens and kidney-transplant rejection. The New England journal of medicine. 2007;357:1293–1300. doi: 10.1056/NEJMoa067160. [DOI] [PubMed] [Google Scholar]
- 10.Sanchez-Zapardiel E, Castro-Panete MJ, Castillo-Rama M, et al. Harmful effect of preformed anti-MICA antibodies on renal allograft evolution in early posttransplantation period. Transplantation. 2013;96:70–78. doi: 10.1097/TP.0b013e3182943506. [DOI] [PubMed] [Google Scholar]
- 11.Panigrahi A, Gupta N, Siddiqui JA, et al. Post transplant development of MICA and anti-HLA antibodies is associated with acute rejection episodes and renal allograft loss. Human immunology. 2007;68:362–367. doi: 10.1016/j.humimm.2007.01.006. [DOI] [PubMed] [Google Scholar]
- 12.Cox ST, Stephens HA, Fernando R, et al. Major histocompatibility complex class I-related chain A allele mismatching, antibodies, and rejection in renal transplantation. Human immunology. 2011;72:827–834. doi: 10.1016/j.humimm.2011.05.004. [DOI] [PubMed] [Google Scholar]
- 13.Terasaki PI, Ozawa M, Castro R. Four-year follow-up of a prospective trial of HLA and MICA antibodies on kidney graft survival. Am J Transplant. 2007;7:408–415. doi: 10.1111/j.1600-6143.2006.01644.x. [DOI] [PubMed] [Google Scholar]
- 14.Suárez-Alvarez B, Alonso-Arias R, Bravo-Mendoza C, et al. Identification of epitopes and immunodominant regions on the MICA protein defined by alloantibodies from kidney transplant patients. Transplantation. 2009;88:S68–77. doi: 10.1097/TP.0b013e3181afeb7a. [DOI] [PubMed] [Google Scholar]
- 15.Banasik M, Boratyńska M, Kościelska-Kasprzak K, et al. The impact of non-HLA antibodies directed against endothelin-1 type A receptors (ETAR) on early renal transplant outcomes. Transplant immunology. 2014;30:24–29. doi: 10.1016/j.trim.2013.10.007. [DOI] [PubMed] [Google Scholar]
- 16.Banasik M, Boratynska M, Koscielska-Kasprzak K, et al. Long-term follow-up of non-HLA and anti-HLA antibodies: incidence and importance in renal transplantation. Transplantation proceedings. 2013;45:1462–1465. doi: 10.1016/j.transproceed.2012.11.025. [DOI] [PubMed] [Google Scholar]
- 17.Cardinal H, Dieude M, Brassard N, et al. Antiperlecan antibodies are novel accelerators of immune-mediated vascular injury. Am J Transplant. 2013;13:861–874. doi: 10.1111/ajt.12168. [DOI] [PubMed] [Google Scholar]
- 18.Angaswamy N, Klein C, Tiriveedhi V, et al. Immune responses to collagen-IV and fibronectin in renal transplant recipients with transplant glomerulopathy. Am J Transplant. 2014;14:685–693. doi: 10.1111/ajt.12592. [DOI] [PubMed] [Google Scholar]
- 19.Dragun D, Muller DN, Brasen JH, et al. Angiotensin II type 1-receptor activating antibodies in renal-allograft rejection. The New England journal of medicine. 2005;352:558–569. doi: 10.1056/NEJMoa035717. [DOI] [PubMed] [Google Scholar]
- 20.Reinsmoen NL, Lai CH, Heidecke H, et al. Anti-angiotensin type 1 receptor antibodies associated with antibody mediated rejection in donor HLA antibody negative patients. Transplantation. 2010;90:1473–1477. doi: 10.1097/TP.0b013e3181fd97f1. [DOI] [PubMed] [Google Scholar]
- 21.Banasik M, Boratynska M, Koscielska-Kasprzak K, et al. The influence of non-HLA antibodies directed against angiotensin II type 1 receptor (AT1R) on early renal transplant outcomes. Transplant international : official journal of the European Society for Organ Transplantation. 2014;27:1029–1038. doi: 10.1111/tri.12371. [DOI] [PubMed] [Google Scholar]
- 22.Taniguchi M, Rebellato LM, Cai J, et al. Higher risk of kidney graft failure in the presence of anti-angiotensin II type-1 receptor antibodies. Am J Transplant. 2013;13:2577–2589. doi: 10.1111/ajt.12395. [DOI] [PubMed] [Google Scholar]
- 23.Cuevas E, Arreola-Guerra JM, Hernandez-Mendez EA, et al. Pretransplant angiotensin II type 1-receptor antibodies are a risk factor for earlier detection of de novo HLA donor-specific antibodies. Nephrol Dial Transplant. 2016 doi: 10.1093/ndt/gfw204. [DOI] [PubMed] [Google Scholar]
- 24.Giral M, Foucher Y, Dufay A, et al. Pretransplant sensitization against angiotensin II type 1 receptor is a risk factor for acute rejection and graft loss. Am J Transplant. 2013;13:2567–2576. doi: 10.1111/ajt.12397. [DOI] [PubMed] [Google Scholar]
- 25.Hernandez-Mendez EA, Arreola-Guerra JM, Morales-Buenrostro LE, et al. Pre-transplant angiotensin II type 1 receptor antibodies: A risk factor for decreased kidney graft function in the early post-transplant period? Clinical transplants. 2013:343–350. [PubMed] [Google Scholar]
- 26.Dragun D, Catar R, Kusch A, et al. Non-HLA-antibodies targeting Angiotensin type 1 receptor and antibody mediated rejection. Human immunology. 2012;73:1282–1286. doi: 10.1016/j.humimm.2012.07.010. [DOI] [PubMed] [Google Scholar]
- 27.Bjerre A, Tangeraas T, Heidecke H, et al. Angiotensin II type 1 receptor antibodies in childhood kidney transplantation. Pediatr Transplant. 2016 doi: 10.1111/petr.12728. [DOI] [PubMed] [Google Scholar]
- 28.Hesemann LE, Subramanian V, Mohanakumar T, et al. De novo development of antibodies to kidney-associated self-antigens angiotensin II receptor type I, collagen IV, and fibronectin occurs at early time points after kidney transplantation in children. Pediatr Transplant. 2015;19:499–503. doi: 10.1111/petr.12531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.In JW, Park H, Rho EY, et al. Anti-angiotensin type 1 receptor antibodies associated with antibody-mediated rejection in patients without preformed HLA-donor-specific antibody. Transplant Proc. 2014;46:3371–3374. doi: 10.1016/j.transproceed.2014.09.096. [DOI] [PubMed] [Google Scholar]
- 30.Gunther J, Kill A, Becker MO, et al. Angiotensin receptor type 1 and endothelin receptor type A on immune cells mediate migration and the expression of IL-8 and CCL18 when stimulated by autoantibodies from systemic sclerosis patients. Arthritis Res Ther. 2014;16:R65. doi: 10.1186/ar4503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kamat NV, Thabet SR, Xiao L, et al. Renal transporter activation during angiotensin-II hypertension is blunted in interferon-γ−/− and interleukin-17A−/− mice. Hypertension. 2015;65:569–576. doi: 10.1161/HYPERTENSIONAHA.114.04975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xie P, Joladarashi D, Dudeja P, et al. Modulation of angiotensin II-induced inflammatory cytokines by the Epac1-Rap1A-NHE3 pathway: implications in renal tubular pathobiology. Am J Physiol Renal Physiol. 2014;306:F1260–1274. doi: 10.1152/ajprenal.00069.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kauma S, Takacs P, Scordalakes C, et al. Increased endothelial monocyte chemoattractant protein-1 and interleukin-8 in preeclampsia. Obstet Gynecol. 2002;100:706–714. doi: 10.1016/s0029-7844(02)02169-5. [DOI] [PubMed] [Google Scholar]
- 34.Jonsson Y, Ruber M, Matthiesen L, et al. Cytokine mapping of sera from women with preeclampsia and normal pregnancies. J Reprod Immunol. 2006;70:83–91. doi: 10.1016/j.jri.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 35.Sprague AH, Khalil RA. Inflammatory cytokines in vascular dysfunction and vascular disease. Biochem Pharmacol. 2009;78:539–552. doi: 10.1016/j.bcp.2009.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee H, Kim JI, Moon IS, et al. Investigation of Serum Angiotensin II Type 1 Receptor Antibodies at the Time of Renal Allograft Rejection. Ann Lab Med. 2015;35:314–320. doi: 10.3343/alm.2015.35.3.314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee J, Huh KH, Park Y, et al. The clinicopathological relevance of pretransplant anti-angiotensin II type 1 receptor antibodies in renal transplantation. Nephrol Dial Transplant. 2015 doi: 10.1093/ndt/gfv375. [DOI] [PubMed] [Google Scholar]
- 38.Fuss A, Hope CM, Deayton S, et al. C4d-negative antibody-mediated rejection with high anti-angiotensin II type I receptor antibodies in absence of donor-specific antibodies. Nephrology (Carlton, Vic) 2015;20:467–473. doi: 10.1111/nep.12441. [DOI] [PubMed] [Google Scholar]
- 39.North American Pediatric Renal Trials and Collaborative Studies 2014 Annual Transplant Report. https://web.emmes.com/study/ped/annlrept/annlrept.html.
- 40.Grenda R. Delayed graft function and its management in children. Pediatr Nephrol. 2016 doi: 10.1007/s00467-016-3528-9. [DOI] [PubMed] [Google Scholar]
- 41.Dharnidharka VR, Agodoa LY, Abbott KC. Risk factors for hospitalization for bacterial or viral infection in renal transplant recipients–an analysis of USRDS data. Am J Transplant. 2007;7:653–661. doi: 10.1111/j.1600-6143.2006.01674.x. [DOI] [PubMed] [Google Scholar]
- 42.Herse F, Verlohren S, Wenzel K, et al. Prevalence of agonistic autoantibodies against the angiotensin II type 1 receptor and soluble fms-like tyrosine kinase 1 in a gestational age-matched case study. Hypertension. 2009;53:393–398. doi: 10.1161/HYPERTENSIONAHA.108.124115. [DOI] [PubMed] [Google Scholar]
- 43.Pearl JP, Parris J, Hale DA, et al. Immunocompetent T-cells with a memory-like phenotype are the dominant cell type following antibody-mediated T-cell depletion. Am J Transplant. 2005;5:465–474. doi: 10.1111/j.1600-6143.2005.00759.x. [DOI] [PubMed] [Google Scholar]
- 44.Sewgobind VD, Kho MM, van der Laan LJ, et al. The effect of rabbit anti-thymocyte globulin induction therapy on regulatory T cells in kidney transplant patients. Nephrol Dial Transplant. 2009;24:1635–1644. doi: 10.1093/ndt/gfn778. [DOI] [PubMed] [Google Scholar]
- 45.Brokhof MM, Sollinger HW, Hager DR, et al. Antithymocyte globulin is associated with a lower incidence of de novo donor-specific antibodies in moderately sensitized renal transplant recipients. Transplantation. 2014;97:612–617. doi: 10.1097/TP.0000000000000031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Carroll RP, Riceman M, Hope CM, et al. Angiotensin II type-1 receptor antibody (AT1Rab) associated humoral rejection and the effect of peri operative plasma exchange and candesartan. Human immunology. 2016 doi: 10.1016/j.humimm.2016.08.009. [DOI] [PubMed] [Google Scholar]
- 47.Clayton PA, Lim WH, Wong G, et al. Relationship between eGFR Decline and Hard Outcomes after Kidney Transplants. Journal of the American Society of Nephrology : JASN. 2016;27:3440–3446. doi: 10.1681/ASN.2015050524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Philogene MC, Bagnasco S, Kraus ES, et al. Anti-Angiotensin II Type 1 Receptor and Anti-Endothelial Cell Antibodies: A Cross-Sectional Analysis of Pathological Findings in Allograft Biopsies. Transplantation. 2016 doi: 10.1097/TP.0000000000001231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ruiz-Ortega M, Ruperez M, Lorenzo O, et al. Angiotensin II regulates the synthesis of proinflammatory cytokines and chemokines in the kidney. Kidney international Supplement. 2002:S12–22. doi: 10.1046/j.1523-1755.62.s82.4.x. [DOI] [PubMed] [Google Scholar]
- 50.Strieter RM, Kunkel SL, Showell HJ, et al. Endothelial cell gene expression of a neutrophil chemotactic factor by TNF-alpha, LPS, and IL-1 beta. Science. 1989;243:1467–1469. doi: 10.1126/science.2648570. [DOI] [PubMed] [Google Scholar]
- 51.Russo RC, Garcia CC, Teixeira MM, et al. The CXCL8/IL-8 chemokine family and its receptors in inflammatory diseases. Expert Rev Clin Immunol. 2014;10:593–619. doi: 10.1586/1744666X.2014.894886. [DOI] [PubMed] [Google Scholar]
- 52.Koch AE, Polverini PJ, Kunkel SL, et al. Interleukin-8 as a macrophage-derived mediator of angiogenesis. Science. 1992;258:1798–1801. doi: 10.1126/science.1281554. [DOI] [PubMed] [Google Scholar]
- 53.Guzzo I, Morolli F, Camassei FD, et al. Acute kidney transplant rejection mediated by angiotensin II type 1 receptor antibodies in a pediatric hyperimmune patient. Pediatr Nephrol. 2016 doi: 10.1007/s00467-016-3500-8. [DOI] [PubMed] [Google Scholar]
- 54.Schwartz GJ, Munoz A, Schneider MF, et al. New equations to estimate GFR in children with CKD. Journal of the American Society of Nephrology : JASN. 2009;20:629–637. doi: 10.1681/ASN.2008030287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Levey AS, Coresh J, Greene T, et al. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med. 2006;145:247–254. doi: 10.7326/0003-4819-145-4-200608150-00004. [DOI] [PubMed] [Google Scholar]
- 56.National High Blood Pressure Education Program Working Group on High Blood Pressure in C, Adolescents. The fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents. Pediatrics. 2004;114:555–576. [PubMed] [Google Scholar]
- 57.Weber MA, Schiffrin EL, White WB, et al. Clinical practice guidelines for the management of hypertension in the community a statement by the American Society of Hypertension and the International Society of Hypertension. J Hypertens. 2014;32:3–15. doi: 10.1097/HJH.0000000000000065. [DOI] [PubMed] [Google Scholar]
- 58.Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (REDCap)–a metadata-driven methodology and workflow process for providing translational research informatics support. Journal of biomedical informatics. 2009;42:377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pearl MH, Nayak AB, Ettenger RB, et al. Bortezomib may stabilize pediatric renal transplant recipients with antibody-mediated rejection. Pediatr Nephrol. 2016;31:1341–1348. doi: 10.1007/s00467-016-3319-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Haas M, Sis B, Racusen LC, et al. Banff 2013 meeting report: inclusion of c4d-negative antibody-mediated rejection and antibody-associated arterial lesions. Am J Transplant. 2014;14:272–283. doi: 10.1111/ajt.12590. [DOI] [PubMed] [Google Scholar]
- 61.Blumberg JM, Gritsch HA, Reed EF, et al. Kidney paired donation in the presence of donor-specific antibodies. Kidney international. 2013;84:1009–1016. doi: 10.1038/ki.2013.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Vo AA, Choi J, Kim I, et al. A Phase I/II Trial of the Interleukin-6 Receptor Specific Humanized Monoclonal (Tocilizumab) + Intravenous Immunoglobulin in Difficult to Desensitize Patients. Transplantation. 2015 doi: 10.1097/TP.0000000000000741. [DOI] [PubMed] [Google Scholar]
- 63.YOUDEN WJ. Index for rating diagnostic tests. Cancer. 1950;3:32–35. doi: 10.1002/1097-0142(1950)3:1<32::aid-cncr2820030106>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


