Abstract
Objectives
To define the incidence of obstructive sleep apnea (OSA) in patients with rheumatoid arthritis (RA) and determine if OSA diagnosis predicts future cardiovascular disease (CVD) and non-cardiac vascular events.
Methods
Medical information pertaining to RA, OSA, CVD and vascular diagnoses was extracted from a comprehensive medical record system for a geographically defined population of 813 patients previously diagnosed with RA and 813 age and sex matched comparator subjects.
Results
The risk for OSA in persons with RA vs comparators was elevated although not reaching statistical significance (HR: 1.32; 95% CI: 0.98, 1.77, p=0.07). Patients with RA were more likely to be diagnosed with OSA if they had traditional risk factors for OSA, including male sex, current smoking status, hypertension, diabetes, dyslipidemia, and increased body mass index. Features of RA disease associated with OSA included large joint swelling and joint surgery. Patients with RA with decreased renal function were also at higher risk of OSA. The increased risk of overall CVD among patients with RA who have OSA was similar to the increased CVD risk associated with OSA in the comparator cohort (interaction p-value = 0.86). OSA diagnosis was associated with an increased risk of both CVD (HR 1.9, 95% CI 1.08-3.27), and cerebrovascular disease (HR 2.4, 95% CI 1.14-5.26) in patients with RA.
Conclusions
Patients with RA may be at increased risk of OSA secondary to both traditional and RA-related risk factors. Diagnosis with OSA predicts future CVD in RA and may provide an opportunity for CVD intervention.
Key Indexing Terms: arthritis, rheumatoid; cardiovascular disease; apnea, obstructive sleep; assessment, risk
Introduction
Many extra articular manifestations of rheumatoid arthritis (RA) are linked to underlying systemic inflammation. RA-associated cardiovascular disease (CVD), likely accentuated by the pro-inflammatory state, is responsible for significant mortality (1, 2). The excess CVD risk seen in RA is independent of standard cardiovascular (CV) risk factors and correlates with RA disease activity (3-5). Prediction of CVD events and associated risk is crucial for informing preventative measures to help alleviate the increased CVD mortality seen in patients with RA.
Within the general population, obstructive sleep apnea (OSA) is an independent risk factor for numerous CVD events (6, 7) and is associated with many CV risk factors including obesity, inactivity, hypertension, hypercholesterolemia and diabetes mellitus (5). Similar to RA, OSA appears to increase the CVD risk through inflammatory mechanisms and is a predictor of CVD events (6, 7). Treatment of OSA through the use of positive airway pressure, which maintains open airways and prevents injurious hypoxic events, may decrease CV risk in affected populations (6).
The incidence of OSA in patients with RA is understudied. Knowledge of the effect of concomitant diagnosis of OSA and RA on CV risk could help to predict and manage CVD events in this particularly high risk population. The overlap of these two conditions may identify a population of patients who should be especially targeted for CVD evaluation and intervention or may represent an unchanged CVD risk from baseline RA secondary to an already increased risk.
This study defines the risk of OSA development in a cohort of patients newly diagnosed with RA in comparison to an age and sex matched comparator cohort, and examines the association of OSA diagnosis on the risk of subsequent CVD and non-cardiac vascular events among patients with RA.
Materials and Methods
Study population
An inception cohort of all cases of RA first diagnosed between January 1, 1980 and December 31, 2007 (n=813) among Olmsted County residents ≥18 years of age was assembled as previously described (8). The incidence date was defined as the earliest date at which the patient fulfilled at least 4 of the 7 American College of Rheumatology 1987 classification criteria for RA (9). A comparison cohort of subjects without RA with similar age sex and calendar year was also previously assembled. All patients in both cohorts were followed up longitudinally through their complete medical records until death, migration from Olmsted County, or July 1, 2016. Two subjects in the non-RA cohort denied use of their medical records for research purposes per Minnesota law and were excluded from these analyses. The study protocol was approved by Review Boards from the Mayo Clinic (675-99) and Olmsted Medical Center (018-OMC-06).
Data relevant to OSA diagnosis were extracted from the medical record. OSA diagnoses were defined by formal diagnosis by a physician paired with an abnormal sleep study (either plethysmography or overnight oximetry) indicating OSA. Additional information collected at the time of OSA diagnosis included relevant data from sleep studies (apnea / hypoapnea index, respiratory disturbance index, Epworth sleepiness score), measures of inflammation (C-reactive protein, erythrocyte sedimentation rate, blood neutrophils, blood lymphocytes), height, weight, medication information (concerning statin, prednisone and TNF inhibitor usage) and whether the patient was provided with a prescription for devices to treat OSA. At follow-up, compliance data, updated apnea / hypoapnea index and Epworth sleepiness scale values were collected where available. Information on long-term oxygen therapy for OSA treatment was also collected.
Previous data collection included CVD and non-cardiac vascular events occurring at any time during the patient's history. CVD events included myocardial infarction (MI; hospitalized or silent), revascularization procedures, angina and heart failure (HF). MI and HF were both defined using objective criteria commonly used in epidemiologic studies, as previously described (10). Non-cardiac vascular diseases, including venous thromboembolism (deep venous thrombosis or pulmonary embolism), cerebrovascular events (hemorrhagic stroke, nonhemorrhagic stroke, transient ischemic attack, or amaurosis fugax), and peripheral arterial events (abdominal aortic aneurysm, renal artery stenosis, peripheral artery disease, or arterial thromboembolism), were previously collected based on fulfillment of previously defined criteria (11).
Smoking status and body mass index (BMI) were obtained at RA incidence/index date. Hypertension, dyslipidemia and diabetes mellitus were abstracted from the medical records at RA incidence/index date and throughout follow-up and were defined using standardized diagnostic criteria (10). Reduced kidney function was defined as 2 consecutive estimated GFRs (eGFRs) < 60 mL/min/1.73 m2 at least 90 days apart using the CKD Epidemiology Collaboration creatinine equation (12, 13). The date the patient was considered to have reduced kidney function is the second date of eGFR < 60 mL/min/1.73 m2. Chronic alcoholism was included as previously identified from physician diagnosis in the medical record (10).
The information on RA characteristics included rheumatoid factor positivity, erythrocyte sedimentation rate (ESR) at RA incidence, date of first large-joint swelling, joint erosions/destructive changes on radiographs, joint surgeries (i.e., arthroplasty and synovectomy) and severe extraarticular manifestations of RA (10). Severe extraarticular manifestations were defined according to Malmö criteria and included pericarditis, pleuritis, Felty's syndrome, glomerulonephritis, vasculitis, peripheral neuropathy, scleritis, and episcleritis (14). Data regarding start and stop dates for use of systemic glucocorticoids, disease-modifying antirheumatic drugs (DMARD; methotrexate, hydroxychloroquine, other conventional DMARD and biologic response modifiers) were collected for all patients. Data on the use of nonsteroidal anti-inflammatory drugs (NSAID) including coxibs were also recorded.
Statistical methods
Descriptive statistics (means, percentages, etc.) were used to summarize the data. Characteristics were compared between cohorts using Chi-square and rank sum tests. The cumulative incidence of OSA adjusted for the competing risk of death was estimated (15). These methods are similar to Kaplan-Meier method with censoring of patients who are still alive at last follow-up. Patients who die before experiencing OSA are appropriately accounted for to avoid overestimation of the rate of occurrence of OSA, which can occur if such subjects are simply censored. Patients who were diagnosed with OSA prior to the diagnosis of RA, or prior to the index date for subjects in the non-RA comparison cohort, were excluded from the analysis of cumulative incidence. Poisson regression models with smoothing splines were used to examine trends in OSA according to time calendar year with direct adjustment for age and sex.
Cox proportional hazards models were used to compare the rate of development of OSA between patients with RA and the non-RA comparison cohort. In addition, Cox models were used to assess the association of risk factors on the development of OSA among patients with RA.
Cox models were also used to assess the effect of OSA on the development of CVD or mortality among patients with RA and non-RA subjects. Traditional cardiovascular risk factors were included in these models as adjustors. Time-dependent covariates were used to model risk factors that developed over time. These time-dependent covariates allowed patients to be modeled as unexposed to the risk factor during the follow-up time prior to development of the risk factor, then change to exposed following development of the risk factor. For the modeling of OSA, patients with OSA prior to index date were considered as exposed from the index date forward and those who developed OSA during follow-up were considered as unexposed prior to diagnosis of OSA and exposed after diagnosis of OSA. Interactions between cohort and OSA were examined. Analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC, USA) and R 3.2.3 (R Foundation for Statistical Computing, Vienna, Austria).
Results
The study population included 813 patients with RA and 811 subjects without RA. The average age at RA incidence (index date for the non-RA cohort) was 55.9 years (standard deviation [SD] 15.7), and 556 (68%) were female in each cohort (Table 1). There was no difference in the presence of OSA at RA incidence/ index date between cohorts (p=0.99).
Table 1.
Characteristics of 813 patients with rheumatoid arthritis (RA) and 811 subjects without RA at RA incidence/index date.
| Characteristic | RA (n=813) | Non-RA (n=811) | p-value |
|---|---|---|---|
| Age, years, mean ± SD | 55.9 ± 15.7 | 55.9 ± 15.7 | 0.95 |
| Sex, female, n (%) | 556 (68%) | 554 (68%) | 0.97 |
| Length of follow-up, years, mean ± SD | 14.8 ± 7.8 | 15.7 ± 8.2 | -- |
| Smoking status, n (%) | 0.002 | ||
| Never | 364 (45%) | 435 (54%) | |
| Current | 177 (22%) | 142 (18%) | |
| Former | 272 (33%) | 234 (29%) | |
| Body mass index, kg/m2, mean ± SD | 27.7 ± 5.9 | 27.7 ± 5.8 | 0.99 |
| Diabetes mellitus, n (%) | 81 (10%) | 68 (8%) | 0.27 |
| Hypertension, n (%) | 314 (39%) | 278 (34%) | 0.07 |
| Any prior CVD or non-cardiac vascular events, n (%) | 121 (15%) | 112 (14%) | 0.54 |
| Presence of OSA, n (%) | 21 (3%) | 21 (3%) | 0.99 |
Abbreviations: CVD=cardiovascular disease; n = number; OSA=obstructive sleep apnea; RA= Rheumatoid Arthritis; SD = standard deviation
The cumulative incidence of OSA was somewhat higher in the patients with RA (97 patients with OSA out of 792 at risk) compared to the subjects without RA (80 patients with OSA out of 790 at risk). This equated to a 10 year cumulative incidence (CI) of OSA of 6.6% (95% CI 4.8%, 8.4%) in the RA cohort, compared to 5.6% (95% CI 4.0%, 7.2%) in the non-RA cohort (Figure 1). The 20 year cumulative incidence was similarly increased in the RA cohort (12.6%, 95% CI 10.0%, 15.1%) compared to the non-RA cohort (11.4%, 95% CI 8.8, 14.0%). In other words, patients with RA were somewhat more likely to develop OSA during follow-up (hazard ratio [HR]: 1.32; 95% CI 0.98, 1.77 adjusted for age, sex and calendar year of RA incidence/index date), but this difference did not reach statistical significance (p=0.07). The rate of OSA development increased over time in both cohorts.
Figure 1.
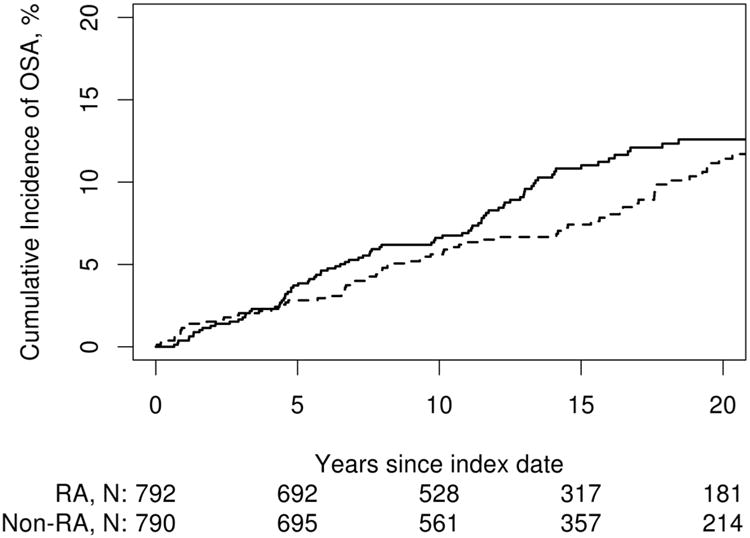
Cumulative incidence of obstructive sleep apnea (OSA) in patients with rheumatoid arthritis (RA; solid line) vs. non-RA comparators (dashed line). The cumulative incidence of OSA was 6.6% (95% confidence interval [CI}: 4.8-8.4%) in RA compared with 5.6% (95% CI: 4.0-7.2) in non-RA at 10 years and 12.6% (95% CI: 10.0-15.1) in RA compared with 11.4% (95% CI: 8.8-14.0) in non-RA at 20 years after RA incidence/index date. This difference correspond to a 32 % increase in RA compared with non-RA (hazard ratio: 1.32; 95% CI: 0.98, 1.77 adjusted for age, sex and calendar year), but this association did not reach statistical significance (p=0.07).
Among those who were diagnosed with OSA, there were few differences between RA and non-RA regarding characteristics at the time of OSA diagnosis (Table 2). In particular, several measures of OSA activity (Epworth sleepiness scale, apnea hypoapnea index, respiratory disturbance index) were similar between the groups. Additionally, inflammatory markers measured at OSA diagnosis, including erythrocyte sedimentation rate and C-reactive protein were similar, although raw blood neutrophil levels were increased in the RA cohort compared to the non-RA cohort. Follow-up values, including prescriptions for OSA machines and use compliance, as well as Epworth sleepiness scale and apnea hypoapnea index were also similar.
Table 2. Characteristics of patients with rheumatoid arthritis (RA) and non-RA comparator subjects diagnosed with obstructive sleep apnea (OSA) after RA incidence/index date.
| Characteristic | RA (n=97) | Non-RA (n=80) | p-value |
|---|---|---|---|
| Age at OSA diagnosis, years, mean ± SD | 64.4 ± 11.4 | 64.9 ± 10.8 | 0.79 |
| Sex, female, n (%) | 59 (61%) | 45 (56%) | 0.54 |
| AHI, mean ± SD (n) | 27.0±23.0 (92) | 26.4 ± 25.9 (77) | 0.56 |
| RDI, mean ± SD (n) | 39.7±29.7 (86) | 40.4 ± 25.4 (77) | 0.52 |
| ESS, mean ± SD (n) | 9.3±5.2 (81) | 8.0 ± 4.8 (66) | 0.14 |
| CRP, mg/L, mean ± SD (n) | 7.3±17.3 (51) | 2.4±2.4 (11) | 0.47 |
| ESR, mm/1 hr, mean ± SD (n) | 20.4±18.3 (69) | 15.3±15.7 (26) | 0.12 |
| Blood neutrophils, mean ± SD (n) | 5.3±2.6 (82) | 4.5±1.9 (62) | 0.035 |
| Blood lymphocytes, mean ± SD (n) | 1.6±0.6 (84) | 1.7±0.7 (63) | 0.14 |
| Neutrophil/lymphocyte ratio, mean ± SD (n) | 4.3±3.4 (82) | 3.3±3.3 (62) | 0.011 |
| BMI at OSA diagnosis, kg/m2, mean ± SD (n) | 34.2±8.4 (97) | 33.6±7.0 (80) | 0.74 |
| BMI≥30 kg/m2 at OSA diagnosis, n (%) | 69 (71%) | 53 (66%) | 0.48 |
| Statin use, n (%) | 23 (24%) | 27 (34%) | 0.14 |
| Glucocorticoid use, n (%) | 35 (36%) | 3 (4%) | |
| Biologic use, n (%) | 12 (12%) | 0 (0%) | |
| OSA machine prescription, n (%) | 78 (80%) | 67 (84%) | 0.50 |
| CPAP | 57 | 56 | |
| BiPAP | 8 | 2 | |
| APAP | 0 | 2 | |
| ASV | 4 | 0 | |
| Oral appliance | 0 | 1 | |
| Follow-up % machine compliance, mean ± SD (n) | 80%±30 (62) | 80%±20 (53) | 0.51 |
| Follow-up AHI, mean ± SD (n) | 4.2±5.2 (25) | 7.9±9.8 (25) | 0.34 |
| Follow-up ESS, mean ± SD (n) | 6.2±3.6 (51) | 6.7±4.6 (47) | 0.84 |
| Oxygen use, n (%) | 9 (9%) | 4 (5%) | 0.28 |
Abbreviations: AHI=apnea hypoapnea index; APAP = automatic titrating positive airway pressure; ASV= adaptive servo-ventilation; BiPAP = bilevel positive airway pressure; BMI = body mass index; CPAP = continuous positive airway pressure; CRP= C-reactive protein; ESR = erythrocyte sedimentation rate; ESS=Epworth Sleepiness Scale; n=number; OSA= obstructive sleep apnea; RA=rheumatoid arthritis; RDI= respiratory disturbance index; SD= standard deviation
Due to the known association between CV risk factors and OSA in the general population, examination was undertaken of the potential association of RA disease characteristics and CV risk factors with the development of OSA in patients with RA (Table 3). These included measures of RA disease activity and severity. In particular, various CV risk factors including smoking, increased BMI, hypertension, hyperlipidemia and diabetes mellitus were found to be associated with the development of OSA among the patients with RA (Table 3). In addition, some features of RA disease, including decreased kidney function (p=0.001), presence of large joint swelling (p=0.007), joint surgery (p<0.001) and the use of DMARDs (including methotrexate, other DMARDs, and biologics) and glucocorticoids were associated with increased risks for developing OSA in patients with RA.
Table 3.
Association between rheumatoid arthritis (RA) disease characteristics and the development of obstructive sleep apnea in 813 patients with RA. Hazard ratios are adjusted for age, sex and calendar year.
| RA characteristic | Hazard ratio (95% CI) | p-value |
|---|---|---|
| Age (per 10 year increase) | 1.12 (0.97, 1.29) | 0.12 |
| Male sex | 1.64 (1.09, 2.47) | 0.017 |
| Calendar year of incidence/index date | 1.10 (1.06, 1.14) | <0.001 |
| ESR at index (per 10 mm/hr) | 1.05 (0.95, 1.16) | 0.297 |
| Rheumatoid factor positive | 1.28 (0.84, 1.95) | 0.243 |
| Current smoker | 0.51 (0.28, 0.94) | 0.031 |
| Former smoker | 1.13 (0.74, 1.71) | 0.565 |
| Time dependent characteristics | ||
| Hypertension | 2.32 (1.40, 3.85) | 0.001 |
| Diabetes mellitus | 1.85 (1.17, 2.92) | 0.008 |
| Dyslipidemia | 2.40 (1.31, 4.37) | 0.004 |
| Prior cardiovascular disease | 1.73 (1.05, 2.87) | 0.032 |
| BMI ≥30 kg/m2 | 4.99 (2.98, 8.36) | <0.001 |
| BMI <20 kg/m2 | 0.28 (0.12, 0.65) | 0.003 |
| Alcoholism | 0.53 (0.21, 1.31) | 0.170 |
| Reduced kidney function* | 2.06 (1.32, 3.20) | 0.001 |
| Rheumatoid nodules | 1.21 (0.80, 1.85) | 0.369 |
| Erosions/destructive changes | 1.46 (0.97, 2.19) | 0.070 |
| Severe extra-articular manifestations* | 1.41 (0.75, 2.65) | 0.286 |
| Large joint swelling | 2.25 (1.25, 4.05) | 0.007 |
| Joint surgery | 2.30 (1.47, 3.61) | <0.001 |
| Methotrexate | 1.86 (1.18, 2.92) | 0.007 |
| Hydroxychloroquine | 1.14 (0.75, 1.73) | 0.545 |
| Other DMARD | 1.69 (1.10, 2.60) | 0.016 |
| Biologic | 2.44 (1.55, 3.84) | <0.001 |
| Glucocorticoids | 2.18 (1.22, 3.88) | 0.008 |
| COX2 | 2.35 (1.53, 3.64) | <0.001 |
| NSAID | 1.24 (0.60, 2.58) | 0.561 |
Abbreviations: BMI=body mass index; COX2=Cox2 inhibitors; CVD=cardiovascular disease; DMARD= disease-modifying anti-rheumatic drug; ESR=erythrocyte sedimentation rate; HCQ=hydroxychloroquine; MTX=methotrexate; NSAID=nonsteroidal antiinflammatory drug; RA=rheumatoid arthritis
For study definition, see Methods - Study Population section
The diagnosis of OSA in both the RA and the non-RA cohorts was significantly associated with an increased risk of CVD events (Table 4). The associations between OSA and the outcomes of interest were similar in patients without RA and those with RA (Table 4). However, OSA appeared to be more strongly associated with peripheral arterial events among the non-RA (HR: 3.09; 95% CI: 1.15, 8.28) than among the patients with RA (HR: 1.17; 95% CI: 0.34, 3.98), though this difference did not reach statistical significance (interaction p=0.057). Results were similar but slightly attenuated after additional adjustment for CV risk factors, including smoking, hypertension, obesity (BMI ≥ 30 kg/m2), diabetes mellitus and dyslipidemia (Table 4). For example, the HR for any CVD or non-cardiac vascular event reduced from 1.76 to 1.68 among the RA and from 1.62 to 1.57 among the non-RA. Within the RA cohort, there was some evidence that OSA was more strongly associated with development of CVD among patients who were rheumatoid factor negative (HR: 2.90) than those who were RF positive (HR: 1.17; interaction p=0.046).
Table 4.
Associations between obstructive sleep apnea and cardiovascular disease/ non-cardiac vascular disease / any cardio- or cerebrovascular disease related mortality in 813 patients with rheumatoid arthritis (RA) and 811 subjects without RA.
| Rheumatoid arthritis | Non-rheumatoid arthritis | ||||||
|---|---|---|---|---|---|---|---|
| Outcome | N events | HR† (95% CI) | HR* (95% CI) | N events | HR† (95% CI) | HR* (95% CI) | p-value** |
| Cardiovascular disease | 168 | 1.88 (1.08, 3.27) | 1.77 (1.01, 3.10) | 124 | 1.87 (1.02, 3.42) | 1.64 (0.89, 3.03) | 0.84 |
| Myocardial infarction | 50 | 0.94 (0.32, 2.74) | 0.92 (0.31, 2.72) | 45 | 2.09 (0.84, 5.21) | 2.02 (0.78, 5.21) | 0.31 |
| Heart failure | 10 | 1.07 (0.47, 2.41) | 0.98 (0.43, 2.23) | 83 | 1.37 (0.61, 3.10) | 1.29 (0.56, 2.96) | 0.71 |
| Non-cardiac vascular event | 91 | 1.93 (0.95, 3.92) | 1.71 (0.84, 3.50) | 51 | 2.10 (0.97, 4.51) | 2.04 (0.92, 4.51) | 0.24 |
| Cerebrovascular disease | 65 | 2.44 (1.14, 5.26) | 2.01 (0.92, 4.39) | 31 | 2.18 (0.86, 5.55) | 2.46 (0.91, 6.66) | 0.49 |
| Peripheral vascular disease | 43 | 1.17 (0.34, 3.98) | 1.28 (0.37, 4.46) | 25 | 3.09 (1.1, 8.28) | 2.58 (0.92, 7.26) | 0.057 |
| Any CVD or non-cardiac vascular event | 211 | 1.76 (1.04, 2.98) | 1.68 (0.98, 2.85) | 157 | 1.62 (0.93, 2.83) | 1.57 (0.89, 2.76) | 0.86 |
| Overall mortality | 323 | 1.20 (0.81, 1.78) | 1.24 (0.82, 1.86) | 248 | 0.69 (0.38, 1.26) | 0.76 (0.42, 1.40) | 0.12 |
Abbreviations: CI= confidence interval; CVD=cardiovascular disease; HF=heart failure; HR= hazard ratio; MI=myocardial infarction; N=number; PAD= peripheral arterial disease; RA=rheumatoid arthritis.
Adjusted for age, sex and calendar year of RA incidence/index date.
Adjusted for ages, sex, calendar year of RA incidence/index date, smoking, hypertension, obesity (BMI sity on, obesity x, calendar year of RA incidenc.
p-value for interaction between RA/non-RA status and obstructive sleep apnea status
Discussion
In this population-based study of patients with RA, there was a trend toward an increased risk of OSA. Diagnosis of OSA in RA was associated with several features of RA, including decreased kidney function, large joint swelling and joint surgery, as well as DMARD and glucocorticoid medication use. An OSA diagnosis in patients with RA was associated with an increased risk of future CVD events, similar to that seen in the general population.
A few previous studies have reported a borderline increase in the incidence of OSA in patients with RA compared to the general population. One study within the same geographic area as this study showed an increased risk, as determined by the Berlin Sleep score, of OSA in RA, but only a borderline increase (p=0.13) in diagnosis of OSA in the setting of RA, suggesting under-diagnosis of OSA in this population (16). This mirrors the finding of the current study: subjects with RA were slightly more likely to be diagnosed with OSA, although this finding did not reach statistical significance (p=0.07).
Additional studies of OSA have focused on subsets of populations diagnosed with RA. In patients with RA involvement in anatomical areas relevant for OSA, specifically the occipitocervical area (17) or temporomandibular joint (18), about 80% had evidence of OSA. In addition, hospitalized patients with RA also had an increased risk of severe apnea syndrome (19). These findings may relate to severe cases of RA or to patients with RA with specific anatomic involvement. Unlike the current study, which accounted for RA beginning at diagnosis, previous studies on this topic focused on prevalent RA cases without accounting for time since diagnosis, thus representing more established, and potentially more severe RA.
The link between OSA and CV risk is well-established within the general population. Although OSA predisposes individuals to CVD events, the diligent use of corrective sleep apparati, including continuous positive airway pressure (CPAP) and bi-level positive airway pressure (BiPAP) attenuates this risk (6, 7). In this study, many of the same risk factors that predispose to OSA in the general population, including male sex, obesity, smoking, hypertension and diabetes (7), also predisposed to OSA in RA, reflecting common risk factors in both populations. In addition, some RA related disease features, including joint swelling, joint surgery and medications used to treat RA, as well as decreased renal function in the RA cohort, also correlated with OSA diagnosis in this population.
It is unclear whether these two general areas of OSA disease risk seen in RA, namely lifestyle / metabolic syndrome (as in the general population) and the RA disease burden, are at all related. The vast majority of patients with both RA and OSA are reportedly overweight, with many of the traditional cardiovascular risk factors (16, 19). However, a small minority of patients with RA, generally with high disease activity, were significantly underweight (19). A relationship between RA disease severity and OSA has been previously documented in retrospective studies, case reports and case series (17, 18, 20-25), which describe individuals with RA in whom critical structures around the upper airway, including the temporomandibular joint and occipitocervical area, are eroded. In these patients, who presumably have a high RA disease burden, mechanical change in the bone structure could compromise the airway independent of visceral adiposity. It was not possible to adequately assess this issue in the current retrospective study.
In the setting of RA, OSA diagnosis predicts future CVD. CVD risk is increased by both the burden of RA disease and OSA diagnosis, suggesting that patients with both conditions are at an increased risk of CVD events and should be closely monitored. In this cohort there was a clear additional effect of co-occurrence of OSA and RA on CVD risk. This observation underscores OSA as an important risk factor for CVD in this population, and suggests that the contribution of each of these conditions to CVD risk might be via independent mechanisms. In RA, the increased CVD risk is linked to RA-related disease activity and burden (26) including lymphocyte-driven inflammation, which may be secondary to the increased quantity of circulating activated and more differentiated T cells (27). In contrast, OSA results in intermittent hypoxia and hypercapnia, causing chronic microarousals and sleep fragmentation. This leads to sympathetic activation, oxidative stress and systemic inflammation (7) which contributes to endothelial cell damage (28).
Interestingly, in this study, peripheral arterial event risk was unaffected by the diagnosis of OSA in RA. As with many other CV-related conditions, RA diagnosis is associated with an increased risk of peripheral arterial events (29-31). The observation that OSA diagnosis in patients with RA correlates with increased risk of central but not peripheral atherosclerotic disease may relate to the anatomy of the artery (i.e., the femoral artery is muscular; the carotid artery is elastic), location of the artery, or on the pathological process leading to formation of atherosclerotic plaque. Similarly, the effect of OSA on the development of peripheral arterial events might not be directly related to conventional pathologic-anatomic mechanisms. RA-related immunopathogenesis may contribute to initiation of peripheral artery plaques, but not to the advancing state of the lesions (29). If OSA also interacted at the same early step in this process, the combined impact might be redundant resulting in no increased risk over that seen from RA alone.
The association between OSA and CVD in patients with RA is most pronounced in RF negative subjects. One previous study showed greatly increased risk of CVD events in patients with RF positive RA, but CVD event frequency comparable to that seen in the general population for patients with RF negative RA (32). The increased CVD risk seen in RF positive RA may be secondary to more inflammation, while the CVD risk in RF negative RA may be primarily based on traditional CV risk factors, as seen in the general population. Thus, OSA confers increased risk similar that seen in the general population in those patients with RF negative RA, but has less of an impact on increasing the CVD risk in RF positive RA, likely because, in this population, the majority of the risk is driven by inflammation.
This study is the first to examine the whether OSA diagnosis in patients with RA predicts future CVD, and one of few to document the incidence of OSA within the setting of RA. This study benefitted from a geographical medical record linkage system allowing for the selection of sex and age matched comparators from the same underlying population. The use of complete inpatient and outpatient medical records provided comprehensive ascertainment of all study outcomes that came to medical attention for these residents of Olmsted County. Potential limitations are those inherent in a retrospective study, and that both the RA and the comparator cohort populations are predominantly Caucasian and generally overweight. These specific demographics may limit applicability to other patient populations, particularly in patients from other races and those with different risk factor profiles. However, results from studies in Olmsted County have generally been consistent with national data, where available (33).
This study relied on the physician diagnosis of OSA paired with an abnormal sleep study, predominantly polysomography, although some patients with abnormal overnight oximetry highly predictive of OSA were included. This strict definition leads to the exclusion of many cases of clinically unrecognized OSA or patients who were not interested in a complete OSA evaluation and treatment. As a result, the full extent of undiagnosed OSA could not be ascertained as completely as might be possible in a prospective study; confounding cannot be excluded. This could have affected some results and lead to underestimation of the total incidence of OSA, however this under-ascertainment would likely affect both the RA and non-RA cohorts. This is an obstacle in most research concerning OSA, as it is likely that a significant proportion of patients who satisfy diagnostic criteria are untested due to a lack of symptom recognition.
In this study, the possible under-diagnosis of OSA, secondary to general fatigue being mistakenly attributed to RA, may result in an underestimate of the incidence of OSA in the cohort of patients with RA, leading to a false negative result concerning the incidence of OSA in patients with RA. This is especially relevant considering the statistically non-significant increase in OSA diagnosis in this RA cohort (p=0.07). In terms of the ascertainment of cardiac risk, the inclusion of some patients with RA and OSA in the RA-only group would likely result in an increased CV risk in this group, thereby biasing the study against showing an increased risk of CVD given a patient with OSA and RA. Given these limitations, which bias against positive findings, the incidence of OSA in RA and the association of OSA with increased cardiovascular risk in patients with RA may be underestimated.
Given these limitations, it is important to note that many of the symptoms of OSA could be mistaken for a RA disease activity, particularly fatigue, trouble sleeping and inability to focus. In patients with RA presenting with new instances of these symptoms or with what appears to be increased RA disease activity, evaluation of sleeping habits may be warranted, and in some cases, testing for OSA may prove beneficial both in terms of quality of life and CVD risk. Especially considering the effectiveness of CPAP intervention in alleviating symptoms and reducing CVD risk in the general population (6, 7), diagnostic testing for OSA in patients with RA could be warranted.
In conclusion, this study indicates that an increased risk of OSA is likely in RA, and that diagnosis of both RA and OSA in an individual patient signals additional CVD risk that should be accounted for in clinical practice. Diagnosis of OSA in RA is associated with many of the same risk factors as in the general population and should be considered in the assessment of CVD risk. It is possible that symptoms of OSA such as fatigue may be attributed to RA disease activity and affect disease management decisions.
Acknowledgments
This publication was funded by a grant from the National Institutes of Health, NIAMS (R01 AR046849). This research was made possible by the Rochester Epidemiology Project (R01 AG 034676 from the National Institutes of Health) and the CTSA Grant Number UL1 TR000135 from the National Center for Advancing Translational Sciences (NCATS), a component of the National Institutes of Health (NIH). KMW was supported by the National Institutes of General Medical Sciences (grant T32-GM-65841) and the Mayo Clinic College of Medicine's Medical Scientist Training Program. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
- 1.Kremers HM, Crowson CS, Therneau TM, Roger VL, Gabriel SE. High ten-year risk of cardiovascular disease in newly diagnosed rheumatoid arthritis patients: a population-based cohort study. Arthritis Rheum. 2008;58:2268–74. doi: 10.1002/art.23650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Maradit-Kremers H, Nicola PJ, Crowson CS, Ballman KV, Gabriel SE. Cardiovascular death in rheumatoid arthritis: a population-based study. Arthritis Rheum. 2005;52:722–32. doi: 10.1002/art.20878. [DOI] [PubMed] [Google Scholar]
- 3.Gonzalez A, Maradit Kremers H, Crowson CS, Ballman KV, Roger VL, Jacobsen SJ, et al. Do cardiovascular risk factors confer the same risk for cardiovascular outcomes in rheumatoid arthritis patients as in non-rheumatoid arthritis patients? Ann Rheum Dis. 2008;67:64–9. doi: 10.1136/ard.2006.059980. [DOI] [PubMed] [Google Scholar]
- 4.Solomon DH, Reed G, Kremer JM, Curtis JR, Farkouh ME, Harrold LR, et al. Disease Activity in Rheumatoid Arthritis and the Risk of Cardiovascular Events. Arthritis rheumatol. 2015;67:1449–55. doi: 10.1002/art.39098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.del Rincon ID, Williams K, Stern MP, Freeman GL, Escalante A. High incidence of cardiovascular events in a rheumatoid arthritis cohort not explained by traditional cardiac risk factors. Arthritis Rheum. 2001;44:2737–45. doi: 10.1002/1529-0131(200112)44:12<2737::AID-ART460>3.0.CO;2-%23. [DOI] [PubMed] [Google Scholar]
- 6.Maeder MT, Mueller C, Schoch OD, Ammann P, Rickli H. Biomarkers of cardiovascular stress in obstructive sleep apnea. Clinica Chimica Acta. 2016;460:152–63. doi: 10.1016/j.cca.2016.06.046. [DOI] [PubMed] [Google Scholar]
- 7.Maeder MT, Schoch OD, Rickli H. A clinical approach to obstructive sleep apnea as a risk factor for cardiovascular disease. Vasc Health Risk Manag. 2016;12:85–103. doi: 10.2147/VHRM.S74703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Myasoedova E, Crowson CS, Kremers HM, Therneau TM, Gabriel SE. Is the incidence of rheumatoid arthritis rising?: results from Olmsted County, Minnesota, 1955-2007. Arthritis Rheum. 2010;62:1576–82. doi: 10.1002/art.27425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, Cooper NS, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31:315–24. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- 10.Myasoedova E, Crowson CS, Nicola PJ, Maradit-Kremers H, Davis JM, 3rd, Roger VL, et al. The influence of rheumatoid arthritis disease characteristics on heart failure. J Rheumatol. 2011;38:1601–6. doi: 10.3899/jrheum.100979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bacani AK, Gabriel SE, Crowson CS, Heit JA, Matteson EL. Noncardiac vascular disease in rheumatoid arthritis: increase in venous thromboembolic events? Arthritis Rheum. 2012;64:53–61. doi: 10.1002/art.33322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hickson LJ, Crowson CS, Gabriel SE, McCarthy JT, Matteson EL. Development of reduced kidney function in rheumatoid arthritis. Am J Kidney Dis. 2014;63:206–13. doi: 10.1053/j.ajkd.2013.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, 3rd, Feldman HI, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–12. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Turesson C, O'Fallon WM, Crowson CS, Gabriel SE, Matteson EL. Extra-articular disease manifestations in rheumatoid arthritis: incidence trends and risk factors over 46 years. Ann Rheum Dis. 2003;62:722–7. doi: 10.1136/ard.62.8.722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gooley TA, Leisenring W, Crowley J, Storer BE. Estimation of failure probabilities in the presence of competing risks: new representations of old estimators. Stat Med. 1999;18:695–706. doi: 10.1002/(sici)1097-0258(19990330)18:6<695::aid-sim60>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 16.Reading SR, Crowson CS, Rodeheffer RJ, Fitz-Gibbon PD, Maradit-Kremers H, Gabriel SE. Do rheumatoid arthritis patients have a higher risk for sleep apnea? J Rheumatol. 2009;36:1869–72. doi: 10.3899/jrheum.081335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shoda N, Seichi A, Takeshita K, Chikuda H, Ono T, Oka H, et al. Sleep apnea in rheumatoid arthritis patients with occipitocervical lesions: the prevalence and associated radiographic features. Eur Spine J. 2009;18:905–10. doi: 10.1007/s00586-009-0975-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Redlund-Johnell I. Upper airway obstruction in patients with rheumatoid arthritis and temporomandibular joint destruction. Scand J Rheumatol. 1988;17:273–9. doi: 10.3109/03009748809098796. [DOI] [PubMed] [Google Scholar]
- 19.Mutoh T, Okuda Y, Mokuda S, Sawada N, Matoba K, Yamada A, et al. Study on the frequency and risk factors of moderate-to-severe sleep apnea syndrome in rheumatoid arthritis. Mod Rheumatol. 2016:1–4. doi: 10.3109/14397595.2015.1131352. [DOI] [PubMed] [Google Scholar]
- 20.Ataka H, Tanno T, Miyashita T, Isono S, Yamazaki M. Occipitocervical fusion has potential to improve sleep apnea in patients with rheumatoid arthritis and upper cervical lesions. Spine (Phila Pa 1976) 2010;35:E971–5. doi: 10.1097/BRS.0b013e3181c691df. [DOI] [PubMed] [Google Scholar]
- 21.Alamoudi OS. Sleep-disordered breathing in patients with acquired retrognathia secondary to rheumatoid arthritis. Med Sci Monit. 2006;12:CR530–4. [PubMed] [Google Scholar]
- 22.Davies SF, Iber C. Obstructive sleep apnea associated with adult-acquired micrognathia from rheumatoid arthritis. Am Rev Respir Dis. 1983;127:245–7. doi: 10.1164/arrd.1983.127.2.245. [DOI] [PubMed] [Google Scholar]
- 23.Pepin JL, Della Negra E, Grosclaude S, Billon C, Levy P. Sleep apnoea syndrome secondary to rheumatoid arthritis. Thorax. 1995;50:692–4. doi: 10.1136/thx.50.6.692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Drossaers-Bakker KW, Hamburger HL, Bongartz EB, Dijkmans BA, Van Soesbergen RM. Sleep apnoea caused by rheumatoid arthritis. Br J Rheumatol. 1998;37:889–94. doi: 10.1093/rheumatology/37.8.889. [DOI] [PubMed] [Google Scholar]
- 25.Hamilton J, Dagg K, Sturrock R, Anderson J, Banham S. Sleep apnoea caused by rheumatoid arthritis. Rheumatology (Oxford) 1999;38:679–80. doi: 10.1093/rheumatology/38.7.679. [DOI] [PubMed] [Google Scholar]
- 26.Holman AJ. Considering cardiovascular mortality in patients with rheumatoid arthritis from a different perspective: a role for autonomic dysregulation and obstructive sleep apnea. J Rheumatol. 2007;34:671–3. [PubMed] [Google Scholar]
- 27.Winchester R, Giles JT, Nativ S, Downer K, Zhang HZ, Bag-Ozbek A, et al. Association of Elevations of Specific T Cell and Monocyte Subpopulations in Rheumatoid Arthritis With Subclinical Coronary Artery Atherosclerosis. Arthritis Rheumatol. 2016;68:92–102. doi: 10.1002/art.39419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hollan I, Meroni PL, Ahearn JM, Cohen Tervaert JW, Curran S, Goodyear CS, et al. Cardiovascular disease in autoimmune rheumatic diseases. Autoimmun Rev. 2013;12:1004–15. doi: 10.1016/j.autrev.2013.03.013. [DOI] [PubMed] [Google Scholar]
- 29.Stamatelopoulos KS, Kitas GD, Papamichael CM, Kyrkou K, Zampeli E, Fragiadaki K, et al. Subclinical peripheral arterial disease in rheumatoid arthritis. Atherosclerosis. 2010;212:305–9. doi: 10.1016/j.atherosclerosis.2010.05.007. [DOI] [PubMed] [Google Scholar]
- 30.Chuang YW, Yu MC, Lin CL, Yu TM, Shu KH, Huang ST, et al. Risk of peripheral arterial occlusive disease in patients with rheumatoid arthritis. A nationwide population-based cohort study. Thromb Haemost. 2016;115:439–45. doi: 10.1160/TH15-07-0600. [DOI] [PubMed] [Google Scholar]
- 31.Pujades-Rodriguez M, Duyx B, Thomas SL, Stogiannis D, Rahman A, Smeeth L, et al. Rheumatoid Arthritis and Incidence of Twelve Initial Presentations of Cardiovascular Disease: A Population Record-Linkage Cohort Study in England. PLoS One. 2016;11:e0151245. doi: 10.1371/journal.pone.0151245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Crowson CS, Matteson EL, Roger VL, Therneau TM, Gabriel SE. Usefulness of risk scores to estimate the risk of cardiovascular disease in patients with rheumatoid arthritis. Am J Cardiol. 2012;110:420–4. doi: 10.1016/j.amjcard.2012.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.St Sauver JL, Grossardt BR, Leibson CL, Yawn BP, Melton LJ, 3rd, Rocca WA. Generalizability of epidemiological findings and public health decisions: an illustration from the Rochester Epidemiology Project. Mayo Clin Proc. 2012;87:151–60. doi: 10.1016/j.mayocp.2011.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]


