Abstract
We investigated prescribing patterns for five opioid use disorder (OUD) medications: 1) injectable naltrexone, 2) oral naltrexone, 3) sublingual or oromucosal buprenorphine/naloxone, 4) sublingual buprenorphine, and 5) transdermal buprenorphine in a nationally representative claims-based database (Truven Health MarketScan®) of commercially insured individuals in the United States. We calculated the prevalence of OUD in the database for each year from 2010 to 2014 and the proportion of diagnosed patient months on OUD medication. We compared characteristics of individuals diagnosed with OUD who did and did not receive OUD medications with bivariate descriptive statistics. Finally, we fit a Cox proportional hazards model of time to discontinuation of therapy as a function of therapy type, controlling for relevant confounders.
From 2010 to 2014, the proportion of commercially insured individuals diagnosed with OUD grew by fourfold (0.12% to 0.48%), but the proportion of diagnosed patient-months on OUD medication decreased from 25% in 2010 (0.05% injectable naltrexone, 0.4% oral naltrexone, 23.1% sublingual or oromucosal buprenorphine/naloxone, 1.5% sublingual buprenorphine, and 0% transdermal buprenorphine) to 16% in 2014 (0.2% injectable naltrexone, 0.4% oral naltrexone, 13.8% sublingual or oromucosal buprenorphine/naloxone, 1.4% sublingual buprenorphine, and 0.3% transdermal buprenorphine). Individuals who received medication therapy were more likely to be male, younger, and have an additional substance use disorder compared with those diagnosed with OUD who did not receive medication therapy. Those prescribed injectable naltrexone were more often male, younger, and diagnosed with additional substance use disorders compared with those prescribed other medications for opioid use disorder (MOUDs). The proportion discontinuing MOUD 30 days or less after initiation was 52% for individuals treated with injectable naltrexone, 70% for individuals treated with oral naltrexone, 31% for individuals treated with sublingual or oromucosal buprenorphine/naloxone, 58% for individuals treated with sublingual buprenorphine, and 51% for individuals treated with transdermal buprenorphine. In the Cox proportional hazard model, use of injectable naltrexone, oral naltrexone, sublingual buprenorphine, and transdermal buprenorphine were all associated with significantly greater hazard of discontinuing therapy beginning more than 30 days after MOUD initiation (HR=2.17, 2.54, 1.15, and 2.21, respectively, 95% CIs 2.04–2.30, 2.45–2.64, 1.10–1.19, and 2.11–2.33), compared with the use of sublingual or oromucosal buprenorphine/naloxone.
This analysis demonstrates that the use of evidence-based medication therapies has not kept pace with increases in OUD diagnoses in commercially insured populations in the United States. Among those who have been treated, discontinuation rates more than 30 days after initiation are high. The proportion treated with injectable naltrexone, oral naltrexone, and transdermal buprenorphine grew over time but remains small, and the discontinuation rates are higher among those treated with these medications compared with those treated with sublingual or oromucosal buprenorphine/naloxone. In the face of the opioid overdose and addiction crisis, new efforts are needed at the provider, health system, and policy levels so that MOUD availability and uptake keep pace with new OUD diagnoses and OUD treatment discontinuation is minimized.
Keywords: injectable naltrexone, oral naltrexone, buprenorphine/naloxone, buprenorphine, opioid use disorder, treatment discontinuation
2. INTRODUCTION
Opioid use is a major public health challenge facing the United States, leading to what the Centers for Disease Control and Prevention has labeled an epidemic of drug overdose deaths (Rudd, Aleshire, Zibbell, & Gladden, 2016). Drug poisonings, most of which are opioid-related, are the leading cause of preventable injury and death, exceeding the deaths from firearms and motor vehicle crashes. Annual costs of opioid overdose in the United States are estimated to exceed $2.2 billion in direct medical costs and another $18.2 billion in costs from lost productivity due to morbidity and mortality (Inocencio, Carroll, Read, & Holdford, 2013). Public health, policy, and research leaders, including the United States Surgeon General, have called for the expansion of access to evidence-based medications for opioid use disorders (OUDs) as central to addressing this public health challenge (United States Department of Health and Human Services Office of the Surgeon General, 2016; Volkow, Frieden, Hyde, & Cha, 2014).
In 2010, extended-release injectable naltrexone (XR-NTX) joined oral naltrexone, buprenorphine, and methadone as an FDA-approved medication for treatment of OUDs (MOUDs) (U.S. Food and Drug Administration, 2010). The rationale for XR-NTX is that, in addition to reducing opioid craving and opioid use, it can improve adherence and decrease discontinuation compared with oral naltrexone, which requires daily dosing, because it is administered as an intramuscular injection approximately every 28 days (Krupitsky et al., 2011). Results from randomized controlled comparative effectiveness trials that include XR-NTX and other MOUDs are pending (Kunøe et al., 2016; Lee, Nunes, et al., 2016). These direct comparisons will provide evidence to clinicians and patients in determining how to choose among available medications. Simultaneously, understanding the characteristics of those currently on each type of therapy and the relative impact of each—including the frequency of discontinuation of MOUDs—can guide patients, health care providers, public health advocates, and policy makers seeking the best approach to mitigate the consequences of OUD among individuals and communities. National representative commercial claims data provide an opportunity to understand the prevalence of OUD in this population, explore the current uptake of different OUD medications among individuals insured by commercial payers, and examine the discontinuation rates of these medications.
Three of the MOUDs—oral naltrexone, XR-NTX, and buprenorphine—are currently available via prescription through outpatient, office-based treatment settings. In order to prescribe or dispense buprenorphine, physicians must qualify for a physician waiver, which includes completing an application and eight hours of required training (Substance Abuse and Mental Health Services Administration, 2016; Walley et al., 2008). In contrast, methadone is restricted to specially licensed and regulated clinics that are typically separate from the rest of the healthcare and addiction treatment systems and cannot be prescribed by office-based prescribers for MOUD. As these three medications have been available and prescribed through outpatient, office-based settings since 2010, we examined a large nationally representative database of commercially insured individuals from 2010–2014 for: a) the prevalence of OUD diagnoses and treatment with OUD medications; b) how the outpatient prescription of these medications has changed over time as the number of people diagnosed with OUDs has changed; c) the factors associated with being prescribed an OUD medication; and d) the rates of discontinuation for each of these medications once they have been initiated.
3. METHODS
3.1 Design, Population, and Data Collection
We identified a retrospective cohort of individuals with OUD in the Truven Health Analytics MarketScan® Commercial Claims Database (MarketScan®) for the years 2010–2014. MarketScan® is an insurance claims-based dataset that includes ambulatory and inpatient visits, laboratory and diagnostic testing, as well as outpatient pharmacy claims for more than 100 employer-sponsored plans covering more than 200 million unique individuals. The dataset is nationally representative of the U.S. commercially insured population (Truven Health Analytics, 2015). The data contain no identifying health information and the Boston University institutional review board reviewed this project and deemed it exempt.
Because some OUD medications can be used to treat alcohol use disorders as well as OUDs, we limited our population of interest to those with evidence of an OUD in their medical claims (Substance Abuse and Mental Health Services Administration, 2012). We classified individuals as having an OUD based on receiving an ICD-9 diagnosis for opioid abuse or dependence (ICD-9 302.0x, 304.7x, or 305.5x). The classification algorithm was based on expert review of ICD-9 codes as well as the previous literature (Cochran et al., 2014). All individuals with a diagnosis indicating OUD were included in the cohort.
3.2 Measures
Our exposure variable was OUD medication. We identified those treated with OUD medication based on their outpatient pharmacy claims. Using the Red Book (Micromedex 2.0, 2017), we extracted the national drug codes for XR-NTX, oral naltrexone, sublingual buprenorphine, transdermal buprenorphine, and sublingual or oromucosal buprenorphine/naloxone combinations (SO-B/N) (Supplemental Appendix Table 1) and used these to identify prescriptions filled at an outpatient pharmacy among those with an OUD diagnosis. Each of these medications are FDA-approved for the treatment of opioid use disorders, except transdermal buprenorphine, which is FDA-approved for the treatment of chronic pain. We chose to include transdermal buprenorphine in our analyses because we limited our population to people with OUD and thus it was likely that the prescribing of transdermal buprenorphine to people in this cohort was intended to treat OUD (Lanier, Umbricht, Harrison, Nuwayser, & Bigelow, 2007). Among patients receiving OUD medication, we recorded characteristics at the initiation visit, including the type of providers involved and the type of locations of care. Our main outcome variable was time to discontinuation of MOUD. Individuals began contributing follow-up time at their initial prescription, defined as the first prescription for an OUD therapy following at least three months without any OUD medication. Individuals ceased contributing time when they had a discontinuation event, defined as a gap of more than fourteen days between when a prescription for XR-NTX, oral naltrexone, SO-B/N, sublingual buprenorphine, or transdermal buprenorphine was scheduled to run out (based on the days supply of the prescription filled) and picking up a new prescription at the pharmacy (Stein et al., 2016; Wilder, Lewis, & Winhusen, 2015). Individuals were followed through the end of the database (December 31, 2014), or at the time of their exit from the commercial insurance plan.
Covariates measured included patients’ sex, age, and region of residence (Northeast, Midwest, South, West); type of commercial insurance coverage (PPO, HMO, POS, other); diagnosis of non-opioid substance use disorder at any time during the observation period (ICD-9 code definitions in Supplemental Appendix Table 2); the type of providers seen at the initiation visit (Internal Medicine, Family Practice, Psychiatry, Obstetrics and Gynecology, Surgery, or Pediatrics); type of setting of medication initiation visit (physician’s office not in a hospital, outpatient clinic located within a hospital, emergency department, or inpatient); and whether an individual had been seen at a detoxification facility during the observation period. Some individuals were seen at multiple settings by multiple providers on the day of the medication initiation visit, so we included a dichotomous indicator variable for each provider and setting covariate.
3.3 Analyses
We first analyzed how the prescription of medication to treat OUD changed over time. From January 1, 2010 to December 31, 2014 we calculated the total person-time of follow-up contributed by each OUD enrollee as well as the total person-months of treatment of XR-NTX, oral naltrexone, SO-B/N, sublingual buprenorphine, and transdermal buprenorphine. From this information, we calculated the proportion of time diagnosed with OUD spent on medication therapy and how prescribing trends among the therapies changed over time.
Next, we described clinical and demographic characteristics of patients prescribed each therapy type. We evaluated the differences in these characteristics using a chi-square test. We then compared patients with OUD who received any MOUD with those with OUD who received no treatment. We fit a multivariable logistic regression model in order to identify clinical and demographic characteristics associated with being prescribed an OUD medication. Finally, we developed a Cox proportional hazards model of the time to discontinuation of MOUD as a function of therapy type. Covariates in this model included sex, age, region of residence, insurance type, non-opioid SUD, initial provider specialty, initiation visit setting, and whether the patient went to a detoxification facility during follow-up. Buprenorphine formulations are prescribed and dispensed in several different quantities, such as weekly or monthly, and the rate of discontinuation is relatively continuous over time. XR-NTX, oral naltrexone, and transdermal buprenorphine, are typically prescribed on a monthly basis, leading to clustering of discontinuation events at discrete time intervals, especially after the first prescription (Cousins et al., 2016). Because this sudden drop occurs for XR-NTX, oral naltrexone, and transdermal buprenorphine, but not SO-B/N or sublingual buprenorphine, the hazards are not proportional over this period, as confirmed by examining the Schoenfeld residuals. We corrected for this non-proportionality by introducing time-dependent measures for type of therapy before and after 30 days (Allison, 2010).
4. RESULTS
Between 2010 and 2014, 340,017 unique individuals with OUD contributed 5.6 million person-months of follow-up. During this period, the proportion of individuals diagnosed with OUD as a proportion of the total MarketScan® population increased from 0.12% in 2010 to 0.48% in 2014. Over those four years, there were 7,330 prescription-months of XR-NTX, 19,175 months of oral naltrexone, 931,856 months of SO-B/N, 78,414 months of sublingual buprenorphine, and 14,812 months of transdermal buprenorphine prescriptions among individuals with OUD. The number of months on OUD medication grew 156%, from 102,895 prescription-months in 2010 to 278,771 prescription-months in 2014 (Table 1), but the proportion of months on OUD medication among people with OUD decreased from 25% in 2010 (0.05% injectable naltrexone, 0.4% oral naltrexone, 23.1% SO-B/N, 1.5% sublingual buprenorphine, and 0% transdermal buprenorphine) to 16% in 2014 (0.2% injectable naltrexone, 0.4% oral naltrexone, 13.8% SO-B/N, 1.4% sublingual buprenorphine, and 0.3% transdermal buprenorphine). Among those on MOUD, the proportion of SO-B/N prescribed decreased from 92.6% of all prescription-months in 2010 to 86.0% in 2014. XR-NTX, oral naltrexone, sublingual buprenorphine, and transdermal buprenorphine all increased as a proportion of total prescribed months between 2010 and 2014, from 0.2% to 1.0%, 1.4% to 2.2%, 5.8% to 8.8%, and 0% to 2.1%, respectively.
Table 1.
Medication treatment of OUD by year
| Year | Total OUD person- months | Injectable naltrexone | Oral naltrexone | Sublingual or oromucosal buprenorphine/naloxone | Sublingual buprenorphine | Transdermal buprenorphine* | Total Prescribed months /total OUD person months | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Prescribed mo. | % of total | Prescribed mo. | % of total | Prescribed mo. | % of total | Prescribed mo. | % of total | Prescribed mo. | % of total | |||
| 2010 | 411,936 | 195 | 0.2% | 1,435 | 1.4% | 95,299 | 92.6% | 5,966 | 5.8% | 0 | 0.0% | 25.0% (102,895/411,936) |
| 2011 | 875,105 | 637 | 0.3% | 2,929 | 1.6% | 169,075 | 90.7% | 12,021 | 6.4% | 1,805 | 1.0% | 21.3% (186,467/875,105) |
| 2012 | 1,286,954 | 1,425 | 0.6% | 4,041 | 1.6% | 219,655 | 89.3% | 17,392 | 7.1% | 3,399 | 1.4% | 19.1% 245,912/1,286,954) |
| 2013 | 1,315,446 | 2,243 | 0.9% | 4,626 | 1.9% | 208,195 | 87.6% | 18,612 | 7.8% | 3,867 | 1.6% | 18.1% (237,543/1,315,446) |
| 2014 | 1,739,560 | 2,830 | 1.0% | 6,144 | 2.2% | 239,632 | 86.0% | 24,423 | 8.8% | 5,742 | 2.1% | 16.0% (278,771/1,739,560) |
OUD = opioid use disorder
Transdermal mono buprenorphine is FDA-approved for the treatment of chronic pain, not opioid use disorder.
Demographic and clinical characteristics among those diagnosed with OUD that are associated with a particular medication appear in Table 2 (see Supplemental Appendix Table 3 for full logistic regression results). Individuals prescribed OUD medications were more often male (61%, versus 58%, p<0.001), younger (50% under 30 vs. 35%, p<0.001), and more commonly diagnosed with other substance use disorders—including alcohol, amphetamine, cannabis, cocaine, and sedative use (p<0.001). There were also significant differences between the groups based on region of residence and health plan type.
Table 2.
Descriptive characteristics of those treated and not treated for OUD
| Untreated OUD | Treated | p-value* | |||
|---|---|---|---|---|---|
| Parameter | n | % | n | % | |
| Total | 301,827 | 100% | 38,190 | 100% | |
| Sex | |||||
| Male | 173,820 | 58% | 23,196 | 61% | Ref. |
| Female | 128,007 | 42% | 14,994 | 39% | <0.001 |
| Age | |||||
| Less than 30 | 104,2897 | 35% | 18,973 | 50% | Ref. |
| 30 or older | 197,540 | 65% | 19,217 | 50% | <0.001 |
| Regions | |||||
| Northeast | 73,175 | 24% | 8,849 | 23% | Ref. |
| Midwest | 54,999 | 18% | 8,004 | 21% | <0.001 |
| South | 107,346 | 36% | 12,710 | 33% | <0.001 |
| West | 57,573 | 19% | 7,479 | 20% | 0.249 |
| Unknown | 8,734 | 3% | 1,148 | 3% | 0.108 |
| Health plan types | |||||
| PPO | 187,621 | 62% | 23,779 | 62% | Ref. |
| HMO | 29,992 | 10% | 4,175 | 11% | <0.001 |
| POS | 17,901 | 6% | 2,416 | 6% | <0.001 |
| Other | 66,313 | 22% | 7820 | 20% | <0.001 |
| Substance use disorder codes† | |||||
| Alcohol | 6,105 | 2% | 967 | 3% | <0.001 |
| Amphetamines | 11,155 | 4% | 2,353 | 6% | <0.001 |
| Cannabis | 33,657 | 11% | 7,039 | 18% | <0.001 |
| Cocaine | 17,514 | 6% | 3,810 | 10% | <0.001 |
| Hallucinogens | 2,003 | 1% | 403 | 1% | 0.069 |
| Sedative | 29,204 | 10% | 6,475 | 17% | <0.001 |
OUD = opioid use disorder; Ref. = reference group for logistic regression; HMO = health maintenance organization; PPO = preferred provider organization; POS = point of service Percentages may not sum to 100% due to rounding.
P-value is from a multivariable logistic regression and reflects the significance of the association between the covariate and treatment status.
Overlapping indicator variables: some individuals have multiple substance use disorder diagnoses during the observation period.
Descriptive statistics for OUD patients stratified by type of MOUD appear in Table 3. Patients prescribed XR-NTX were more often from the Northeast (32% vs. 29% for oral naltrexone, 23% for SO-B/N, 23% for sublingual buprenorphine, and 12% for transdermal buprenorphine, p<0.001), male (66% vs. 60%, 63%, 52%, and 41%, p<0.001), and under 30 years of age (68% vs. 61%, 50%, 51%, and 12%, p<0.001). The prevalence of an alcohol use disorder diagnosis was three times as high in those prescribed XR-NTX or oral naltrexone than those prescribed buprenorphine (6% vs. 2% for SO-B/N, sublingual buprenorphine, and transdermal buprenorphine, p<0.001). At the initiation visit, those on oral naltrexone were more likely to see a psychiatrist as part of their visit (23% vs. 11% for XR-NTX, 18% for SO-B/N, 16% for sublingual buprenorphine, and 5% for transdermal buprenorphine, p<0.001), while those receiving SO-B/N more often saw a family medicine practitioner (21%) compared with those on XR-NTX (14%) or oral naltrexone (11%) (p<0.001). At the time of initiation, those prescribed XR-NTX and oral naltrexone were less often seen in an office-based setting (both 52%) compared with those on buprenorphine (74% for SO-B/N, 74% for sublingual buprenorphine, and 85% for transdermal buprenorphine, p<0.001).
Table 3.
Descriptive characteristics of five treatments for OUD
| Parameter | Injectable naltrexone | Oral naltrexone | Sublingual or oromucosal buprenorphine/naloxone | Sublingual buprenorphine | Transdermal buprenorphine* | p-value† | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | n | % | n | % | ||
| Total‡ | 1,343 | 100% | 5,327 | 100% | 28,861 | 100% | 5,693 | 100% | 2,329 | 100% | |
| Sex | |||||||||||
| Male | 881 | 66% | 3,195 | 60% | 18,249 | 63% | 2,961 | 52% | 953 | 41% | <0.001 |
| Female | 462 | 34% | 2,132 | 40% | 10,612 | 37% | 2,732 | 48% | 1,376 | 59% | |
| Age | |||||||||||
| Younger than 30 | 918 | 68% | 3,270 | 61% | 14,523 | 50% | 2,919 | 51% | 271 | 12% | <0.001 |
| 30 and older | 425 | 32% | 2,057 | 39% | 14,338 | 50% | 2,774 | 49% | 2,058 | 88% | |
| Regions | |||||||||||
| Northeast | 436 | 32% | 1,526 | 29% | 6,733 | 23% | 1,304 | 23% | 284 | 12% | |
| Midwest | 249 | 19% | 1,143 | 21% | 6,412 | 22% | 898 | 16% | 414 | 18% | |
| South | 363 | 27% | 1,577 | 30% | 9,437 | 33% | 1,892 | 33% | 951 | 41% | <0.001 |
| West | 272 | 20% | 987 | 19% | 5,364 | 19% | 1,490 | 26% | 615 | 26% | |
| Unknown | 23 | 2% | 94 | 2% | 915 | 3% | 109 | 2% | 65 | 3% | |
| Health plan types | |||||||||||
| PPO | 846 | 63% | 3,151 | 59% | 17,896 | 62% | 3,680 | 65% | 1,595 | 68% | |
| HMO | 119 | 9% | 610 | 11% | 3,313 | 11% | 506 | 9% | 221 | 9% | <0.001 |
| POS | 110 | 8% | 429 | 8% | 1,853 | 6% | 421 | 7% | 152 | 7% | |
| Other | 268 | 20% | 1,137 | 21% | 5,799 | 20% | 1,086 | 19% | 361 | 16% | |
| Substance use disorder codes§ | |||||||||||
| Alcohol | 79 | 6% | 340 | 6% | 546 | 2% | 111 | 2% | 56 | 2% | <0.001 |
| Amphetamines | 159 | 12% | 594 | 11% | 1,642 | 6% | 418 | 7% | 61 | 3% | <0.001 |
| Cannabis | 441 | 33% | 1,694 | 32% | 5,227 | 18% | 969 | 17% | 147 | 6% | <0.001 |
| Cocaine | 255 | 19% | 972 | 18% | 2,751 | 10% | 603 | 11% | 55 | 2% | <0.001 |
| Hallucinogens | 30 | 2% | 108 | 2% | 285 | 1% | 50 | 1% | 5 | 0% | <0.001 |
| Sedative | 396 | 29% | 1,543 | 29% | 4,557 | 16% | 1,085 | 19% | 301 | 13% | <0.001 |
| Ever seen in detox facility | |||||||||||
| Yes | 1033 | 77% | 3,563 | 67% | 21,491 | 74% | 3,434 | 60% | 273 | 12% | <0.001 |
| No | 310 | 23% | 1,764 | 33% | 7,370 | 26% | 2,259 | 40% | 2,056 | 88% | |
| Days to discontinuation | |||||||||||
| 30 or less | 698 | 52% | 3,729 | 70% | 9,015 | 31% | 3,323 | 58% | 1,185 | 51% | <0.001 |
| More than 30 | 645 | 48% | 1,598 | 30% | 19,846 | 69% | 2,370 | 42% | 1,144 | 49% | |
| Provider at initiation§ | |||||||||||
| Internal Medicine | 166 | 12% | 668 | 13% | 5,280 | 18% | 1,081 | 19% | 612 | 26% | <0.001 |
| Family Practice | 183 | 14% | 592 | 11% | 6,006 | 21% | 1,130 | 20% | 422 | 18% | <0.001 |
| Psychiatry | 154 | 11% | 1,207 | 23% | 5,192 | 18% | 926 | 16% | 116 | 5% | <0.001 |
| Obstetrics and Gynecology | 11 | 1% | 32 | 1% | 282 | 1% | 203 | 4% | 15 | 1% | <0.001 |
| Surgery | 10 | 1% | 29 | 1% | 214 | 1% | 68 | 1% | 74 | 3% | <0.001 |
| Pediatrics | 8 | 1% | 33 | 1% | 221 | 1% | 49 | 1% | 8 | 0% | 0.097 |
| Place of initiation§ | |||||||||||
| Office | 694 | 52% | 2,786 | 52% | 21,419 | 74% | 4,208 | 74% | 1,987 | 85% | <0.001 |
| Outpatient clinic | 498 | 37% | 2,033 | 38% | 5,377 | 19% | 982 | 17% | 335 | 14% | <0.001 |
| ED | 30 | 2% | 174 | 3% | 524 | 2% | 159 | 3% | 61 | 3% | <0.001 |
| Inpatient | 29 | 2% | 258 | 5% | 655 | 2% | 126 | 2% | 14 | 1% | <0.001 |
OUD = opioid use disorder; HMO = health maintenance organization; PPO = preferred provider organization; POS = point of service; ED = emergency department Percentages may not sum to 100% due to rounding.
Transdermal mono buprenorphine is FDA-approved for the treatment of chronic pain, not opioid use disorder.
P-value from a Chi-square test for difference across the three groups.
Total reflects the total number of individuals prescribed each medication. It is possible that one individuals began therapy on one medication, discontinued, and then initiated a different medication. In that case, the individual would be included in both medication columns.
Overlapping indicator variables: some individuals have multiple substance use disorder diagnoses during the observation period and were seen by multiple providers or in multiple settings on the day of initiation.
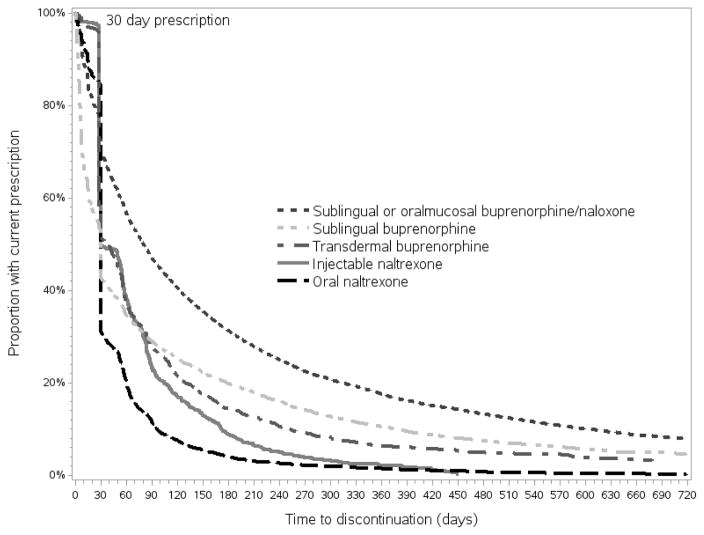
Figure 1 presents the results of time to discontinuation of therapy. As expected, the sharp increase in discontinuation after the first 30-day prescription for XR-NTX, oral naltrexone, and transdermal buprenorphine yields non-proportional hazards, which we correct by separately estimating the effect of therapy before and after 30 days. Discontinuation 30 days or less after initiation was high across therapies: 52% of those prescribed XR-NTX, 70% of those prescribed oral naltrexone, 31% of those prescribed SO-B/N, 58% of those prescribed sublingual buprenorphine, and 51% of those prescribed transdermal buprenorphine had discontinued (Table 2). The type of OUD therapy had the greatest impact on predicting discontinuation of MOUD. After the first 30 days of therapy, the hazard for discontinuation of therapy among those prescribed XR-NTX was 2.17 (95% CI 2.04–2.30) times that of SO-B/N (Table 4). Similarly, the hazard of discontinuing oral naltrexone was 2.54 (95% CI 2.25–2.64), the hazard of discontinuing sublingual buprenorphine was 1.15 (95% CI 1.10–1.19), and the hazard of discontinuing transdermal buprenorphine was 2.21 (95% CI 2.11–2.33) times that of that of SOB/N (Table 4). Additional factors significantly associated with a higher hazard of discontinuation include young age (HR=1.25, 95% CI 1.22–1.28); being initiated in an outpatient clinic, inpatient, or emergency department setting (HR=1.08, 1.23, 1.19, 95% CIs 1.05–1.12, 1.15–1.31, 1.11–1.28, respectively); and additional diagnoses of alcohol (HR=1.15, 95% CI 1.08–1.22), cannabis (HR=1.10, 95% CI 1.07–1.13), cocaine (HR=1.13 95% CI 1.09–1.17), amphetamines (HR=1.07, 95% CI 1.03–1.11) and sedative (HR=1.16, 95% CI 1.13–1.19) use disorders. Factors associated with a lower hazard of discontinuation include ever having been to a detoxification facility (HR=0.92, 95% CI 0.90–0.94); being seen by a family medicine practitioner (HR=0.94, 95% CI 0.91–0.97) or psychiatrist (HR=0.94, 95% CI 0.91–0.97); and initiating therapy in an office–based setting (HR=0.90, 95% CI 0.87–0.93). The model also controlled for region of residence and commercial insurance type (Supplemental Appendix Table 4).
Figure 1. Time to medication discontinuation among individuals treated for opioid use disorder in a United States commercially insured population.
Figure 1: Time to medication discontinuation among individuals treated for opioid use disorder in a United States commercially insured population This Kaplan–Meier survival curve displays the time to discontinuation for individuals prescribed sublingual or oromucosal buprenorphine/naloxone, sublingual buprenorphine, transdermal buprenorphine, injectable naltrexone, and oral naltrexone. The horizontal axis displays the time to discontinuation in days while the vertical axis displays the proportion of the population with a current prescription.
Table 4.
Hazard of discontinuing medication therapy
| Parameter | Hazard Ratio | 95% CI | p-value |
|---|---|---|---|
| Medication at initiation | |||
| Injectable naltrexone > 30 days | 2.17 | 2.04–2.30 | <0.001 |
| Oral naltrexone >30 days | 2.54 | 2.45–2.64 | <0.001 |
| Sublingual buprenorphine > 30 days | 1.15 | 1.10–1.19 | <0.001 |
| Transdermal buprenorphine* > 30 days | 2.21 | 2.11–2.33 | <0.001 |
| Sublingual or oromucosal buprenorphine/naloxone >30 days | Reference | ||
| Age at initiation | |||
| Younger than 30 | 1.25 | 1.22–1.28 | <0.001 |
| 30 or older | Reference | ||
| Sex | |||
| Male | Reference | ||
| Female | 1.01 | 0.99–1.03 | 0.563 |
| Substance use codes (indicators) | |||
| Alcohol | 1.15 | 1.08–1.22 | <0.001 |
| Amphetamines | 1.07 | 1.03–1.12 | 0.002 |
| Cannabis | 1.10 | 1.07–1.13 | <0.001 |
| Cocaine | 1.13 | 1.09–1.17 | <0.001 |
| Hallucinogens | 1.02 | 0.93–1.12 | 0.698 |
| Sedative | 1.16 | 1.13–1.19 | <0.001 |
| Ever seen in detox facility | |||
| Yes | 0.92 | 0.90–0.94 | <0.001 |
| No | Reference | ||
| Provider at initiation (indicators) | |||
| Internal medicine | 0.98 | 0.95–1.01 | 0.272 |
| Family practice | 0.94 | 0.91–0.97 | <0.001 |
| Pediatrics | 0.88 | 0.78–0.99 | 0.028 |
| Psychiatry | 0.94 | 0.91–0.97 | <0.001 |
| Obstetrics and Gynecology | 0.85 | 0.78–0.94 | 0.001 |
| Surgery | 1.17 | 1.05–1.30 | 0.005 |
| Place of initiation (indicators) | |||
| Office visit | 0.90 | 0.87–0.93 | <0.001 |
| Outpatient clinic | 1.08 | 1.05–1.12 | <0.001 |
| ED | 1.19 | 1.11–1.28 | <0.001 |
| Inpatient | 1.23 | 1.15–1.31 | <0.001 |
CI = confidence interval; ED = emergency department
All variables in this table are included in one model. Model controls for region of residence, commercial insurance type, and the effect of medication type in the first 30 days of treatment.
Transdermal buprenorphine is FDA-approved for the treatment of chronic pain, not opioid use disorder.
5. DISCUSSION
Although opioid misuse is a growing threat to public health in the United States, the use of medication therapy has not kept up with the increase in OUD diagnoses. In our analysis of a nationally representative commercial claims database, the proportion of OUD person-time covered by medication therapy decreased from 25% in 2010 to 16% in 2014. The low proportion of individuals diagnosed with OUD who received MOUD highlights the need for continued promotion of MOUD. Naltrexone-based therapies are growing as alternatives to SO-B/N but still make up a small share of the total, rising from 1.6% of medication therapies in 2010 to 3.2% in 2014.
The rate of discontinuation was high among all forms of therapy, although there were differences in the time until discontinuation. For XR-NTX, more than half of these discontinuations occur after the first month, a phenomenon that has been documented in other research (Comer et al., 2006; Mokri, Chawarski, Taherinakhost, & Schottenfeld, 2016). Randomized clinical trials conducted in Russia and the United States have demonstrated better retention, with approximately 60% receiving an XR-NTX injection in the sixth month of treatment (Krupitsky et al., 2011; Lee, Friedmann, et al., 2016). The patient selection, resources, structure, and incentives that accompany these clinical trials may promote retention. Behavioral interventions in clinical settings have improved retention for buprenorphine and naltrexone in other studies (Christensen et al., 2014; Nunes, Rothenberg, Sullivan, Carpenter, & Kleber, 2006; Sullivan et al., 2015). Wider adoption of these interventions may improve retention for patients receiving buprenorphine and injectable naltrexone. The rate of discontinuation we observe is also a function of how we define a gap in therapy–here, it is 14 days without prescription coverage. Many OUD patients face barriers to consistent care, so lapses may be more common. However, when we examined lengthening this gap period to 30 days before labelling it a discontinuation, our overall results were quite similar, with high discontinuation across therapies and the majority of individuals experiencing a gap in care during the observation period (Supplemental Appendix Table 5). Even beyond the initial drop-off, the type of medication is significantly associated with discontinuation. Part of this difference may be attributable to the lack of physical side effects associated with discontinuing an antagonist such as naltrexone compared with an opioid agonist such as buprenorphine, which can cause withdrawal symptoms. The discontinuation we observed for transdermal buprenorphine was more similar to treatment with injectable naltrexone. This may have been because transdermal buprenorphine was being used more frequently for short-term medically managed withdrawal than for long-term maintenance (Lanier et al., 2007). More direct comparisons of medication-assisted therapies are needed to understand the reasons for discontinuation and the subsequent risk of relapse to opioid use.
We were surprised to find that oral naltrexone was more common in this dataset than XR-NTX, with over 19,000 total prescription months compared with 7,330 for XR-NTX. Oral naltrexone was approved for use in OUD in 1984 (Sharafaddinzadeh, Moghtaderi, Kashipazha, Majdinasab, & Shalbafan, 2010), many years before XR-NTX, and some providers may start patients with an oral naltrexone prescription before transitioning to XR-NTX. However, this transitioning does not account for the use patterns observed in our data, where fewer than 8% of individuals initiating oral naltrexone subsequently initiate XR-NTX. We also find that almost a quarter of those on oral naltrexone saw a psychiatrist compared with 11% of those prescribed XR-NTX. Psychiatrists may not have the staff and office resources to provide injections. While oral naltrexone may be a good option for highly motivated patients (Lobmaier, Kornør, Kunøe, & Bjørndal, 2008), a systematic review failed to find enough evidence to recommend oral naltrexone, acknowledging that its effectiveness was limited by poor adherence (Minozzi et al., 2011). Further oral naltrexone may pose an elevated risk of overdose after discontinuation because oral naltrexone is cleared after only 24 hours, leaving patients with low opioid tolerance. More research is needed to understand why some providers prescribe oral naltrexone rather than the alternatives, and what clinical or other advantages they perceive may exist that may be absent from the empirical literature.
The number of individuals prescribed buprenorphine monotherapy (sublingual and transdermal buprenorphine) also surprised us. Monotherapy is recommended only for OUD patients who are pregnant, have a sensitivity to naloxone, have hepatic impairment, or those transferring from methadone. In some places monotherapy may be less costly than SO-B/N, which may promote its use by other OUD patients (Micromedex 2.0, 2017). Transdermal buprenorphine has not been approved as a treatment for OUD, but may be prescribed for treatment off-label. Our inclusion of transdermal buprenorphine may be subject to misclassification because some of the identified individuals may be receiving the medication to treat a chronic pain issue (the approved use) and not OUD (the off-label use). Indeed, we found that those prescribed transdermal buprenorphine to be both older (88% over 30) and more likely to be female (59%) than the other treated cohorts, suggesting that this may be a different population. However, like the other medications, we required individuals to have a previously recorded OUD diagnosis before we recorded transdermal buprenorphine as a treatment.
A major strength of this study is that our data are nationally representative and allow us to track the pattern of filled, rather than prescribed, OUD therapy medications over time. The limitation of administrative claims data is a lack of the clinical detail of a medical record. We use ICD-9 codes to identify individuals with OUD, and it is possible that misclassification or other coding errors affected the data. However, other research has demonstrated the reliability of diagnostic claims data (Maselli, Gonzales, & Colorado Medical Society Joint Data Project, 2001), and when comparing types of OUD therapy we do not expect the rate of misclassification to be different among the groups. Further, administrative claims data exclude many demographic variables that could allow us to explore subpopulations such as those who might have been exposed to the criminal justice system. We find young men are more likely to be treated, for example, and further research could explore whether this reflects the role of the criminal justice system in referring patients. More broadly, billing claims may underestimate the true prevalence of OUD. Because substance use is stigmatized, individuals may reluctant to seek care for OUD compared to a less stigmatized health conditions (e.g. hypertension). Our cohort represents a sample of individuals with an OUD diagnosis who were either comfortable enough with their provider to discuss substance use, or had an OUD-related adverse event such as an overdose. Another limitation of claims data is that it only records the care for which a claim was submitted. Thus, we are not able to report or analyze OUD medications or other treatments that patients received outside of their insurance plans, such as cash payments for treatment or publicly funded care not billed to insurance companies for reimbursement. We are also unable to determine the exact care context. Some patients have multiple providers or care locations associated with a single visit, and we are unable to deduce which provider wrote the prescription. By including all provider and practice variables as indicators, however, we measure the effect of the specific provider controlling for the fact that an individual can have multiple providers during a single visit, which acknowledges complex care contexts. Our MarketScan® data only include outpatient pharmacy prescriptions, so we are missing medications dispensed in the inpatient or detoxification setting. If a particular therapy is more often initiated in inpatient settings, for example, we may be underestimating the time to discontinuation for that patient. Finally, we focus only on three OUD therapies available from an outpatient pharmacy, so we exclude methadone maintenance therapy which is restricted to specially licensed and regulated clinics and largely separate from other healthcare services. For this reason, we were unable to reasonably identify methadone users and so, as in other published research, we excluded methadone maintenance therapy from our analysis (Thomas et al., 2013).
Opioid misuse is a major public health challenge, and the current use of FDA-approved, evidence-based medications for the treatment of OUD is lagging behind the increase in diagnoses. In this novel analysis of the use of these therapies in a commercially insured population, we found that discontinuation is common and that XR-NTX and oral naltrexone therapy had higher discontinuation rates than buprenorphine. Given the need for long-term maintenance of MOUD akin to other chronic disorders such as hypertension, our findings indicating short time on therapy and high discontinuation are alarming. In the face of the opioid overdose and addiction crisis, provider, health system, and policy-level efforts are needed so that MOUD availability and uptake keep pace with new OUD diagnoses and MOUD discontinuation. Unless new therapies are coupled with a reduction in the rates of discontinuation, their impact will be limited.
Supplementary Material
Highlights.
Prescription of opioid use disorder medication has not kept up with diagnosis.
Those prescribed were more often male, younger, and had additional substance use. Among those who have been treated, discontinuation rates after 30 days are high.
Naltrexone users more often discontinued therapy vs. buprenorphine/naloxone.
Acknowledgments
This study was sponsored by the National Institute on Drug Abuse (P30DA040500 and R01DA031059), and benefited from copy-editing from Casy Calver (R25DA013582). The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the funding agencies or the US government.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Allison P. Survival Analysis Using SAS: A Practical Guide. 2. Cary, NC, USA: SAS Institute Inc; 2010. [Google Scholar]
- Christensen DR, Landes RD, Jackson L, Marsch LA, Mancino MJ, Chopra MP, Bickel WK. Adding an Internet-delivered treatment to an efficacious treatment package for opioid dependence. J Consult Clin Psychol. 2014;82(6):964–972. doi: 10.1037/a0037496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cochran BN, Flentje A, Heck NC, Van Den Bos J, Perlman D, Torres J, … Carter J. Factors predicting development of opioid use disorders among individuals who receive an initial opioid prescription: Mathematical modeling using a database of commercially-insured individuals. Drug Alcohol Depend. 2014;138:202–208. doi: 10.1016/j.drugalcdep.2014.02.701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comer SD, Sullivan MA, Yu E, Rothenberg JL, Kleber HD, Kampman K, … O'Brien CP. Injectable, sustained-release naltrexone for the treatment of opioid dependence: a randomized, placebo-controlled trial. Arch Gen Psychiatry. 2006;63(2):210–218. doi: 10.1001/archpsyc.63.2.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cousins SJ, Radfar SR, Crevecoeur-MacPhail D, Ang A, Darfler K, Rawson RA. Predictors of Continued Use of Extended-Released Naltrexone (XR-NTX) for Opioid-Dependence: An Analysis of Heroin and Non-Heroin Opioid Users in Los Angeles County. J Subst Abuse Treat. 2016;63:66–71. doi: 10.1016/j.jsat.2015.12.004. [DOI] [PubMed] [Google Scholar]
- Inocencio TJ, Carroll NV, Read EJ, Holdford DA. The economic burden of opioid-related poisoning in the United States. Pain Med. 2013;14(10):1534–1547. doi: 10.1111/pme.12183. [DOI] [PubMed] [Google Scholar]
- Krupitsky E, Nunes EV, Ling W, Illeperuma A, Gastfriend DR, Silverman BL. Injectable extended-release naltrexone for opioid dependence: a double-blind, placebo-controlled, multicentre randomised trial. Lancet. 2011;377(9776):1506–1513. doi: 10.1016/S0140-6736(11)60358-9. [DOI] [PubMed] [Google Scholar]
- Kunøe N, Opheim A, Solli KK, Gaulen Z, Sharma-Haase K, Latif ZE, Tanum L. Design of a randomized controlled trial of extended-release naltrexone versus daily buprenorphine-naloxone for opioid dependence in Norway (NTX-SBX) BMC Pharmacol Toxicol. 2016;17(1):18. doi: 10.1186/s40360-016-0061-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanier RK, Umbricht A, Harrison JA, Nuwayser ES, Bigelow GE. Evaluation of a transdermal buprenorphine formulation in opioid detoxification. Addiction. 2007;102(10):1648–1656. doi: 10.1111/j.1360-0443.2007.01944.x. [DOI] [PubMed] [Google Scholar]
- Lee JD, Friedmann PD, Kinlock TW, Nunes EV, Boney TY, Hoskinson RA, Jr, O'Brien CP. Extended-Release Naltrexone to Prevent Opioid Relapse in Criminal Justice Offenders. N Engl J Med. 2016;374(13):1232–1242. doi: 10.1056/NEJMoa1505409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JD, Nunes EV, Mpa PN, Bailey GL, Brigham GS, Cohen AJ, … Rotrosen J. NIDA Clinical Trials Network CTN-0051, Extended-Release Naltrexone vs. Buprenorphine for Opioid Treatment (X:BOT): Study design and rationale. Contemp Clin Trials. 2016;50:253–264. doi: 10.1016/j.cct.2016.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobmaier P, Kornør H, Kunøe N, Bjørndal A. Sustained-release naltrexone for opioid dependence. Cochrane Database Syst Rev. 2008;16(2):CD006140. doi: 10.1002/14651858.CD006140.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maselli JH, Gonzales R Colorado Medical Society Joint Data Project I. Measuring antibiotic prescribing practices among ambulatory physicians: accuracy of administrative claims data. Journal of Clinical Epidemiology. 2001;54(2):196–201. doi: 10.1016/s0895-4356(00)00269-9. [DOI] [PubMed] [Google Scholar]
- Micromedex 2.0. Drug Topics Red Book Online. 2017 Retrieved from http://www.micromedexsolutions.com.
- Minozzi S, Amato L, Vecchi S, Davoli M, Kirchmayer U, Verster A. Oral naltrexone maintenance treatment for opioid dependence. Cochrane Database Syst Rev. 2011;(4):CD001333. doi: 10.1002/14651858.CD001333.pub4. [DOI] [PubMed] [Google Scholar]
- Mokri A, Chawarski MC, Taherinakhost H, Schottenfeld RS. Medical treatments for opioid use disorder in Iran: a randomized, double-blind placebo-controlled comparison of buprenorphine/naloxone and naltrexone maintenance treatment. Addiction. 2016;111(5):874–882. doi: 10.1111/add.13259. [DOI] [PubMed] [Google Scholar]
- Nunes EV, Rothenberg JL, Sullivan MA, Carpenter KM, Kleber HD. Behavioral therapy to augment oral naltrexone for opioid dependence: a ceiling on effectiveness? Am J Drug Alcohol Abuse. 2006;32(4):503–517. doi: 10.1080/00952990600918973. [DOI] [PubMed] [Google Scholar]
- Rudd RA, Aleshire N, Zibbell JE, Gladden RM. Increases in Drug and Opioid Overdose Deaths--United States, 2000–2014. MMWR Morb Mortal Wkly Rep. 2016;64(50–51):1378–1382. doi: 10.15585/mmwr.mm6450a3. [DOI] [PubMed] [Google Scholar]
- Sharafaddinzadeh N, Moghtaderi A, Kashipazha D, Majdinasab N, Shalbafan B. The effect of low-dose naltrexone on quality of life of patients with multiple sclerosis: a randomized placebo-controlled trial. Mult Scler. 2010;16(8):964–969. doi: 10.1177/1352458510366857. [DOI] [PubMed] [Google Scholar]
- Stein BD, Sorbero M, Dick AW, Pacula RL, Burns RM, Gordon AJ. Physician Capacity to Treat Opioid Use Disorder With Buprenorphine-Assisted Treatment. JAMA. 2016;316(11):1211–1212. doi: 10.1001/jama.2016.10542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration. An introduction to extended-release injectable naltrexone for the treatment of people with opioid dependence. SAMHSA Advisory. 2012;11(1):1–7. [Google Scholar]
- Substance Abuse and Mental Health Services Administration. Buprenorphine Waiver Management. 2016 Retrieved from http://www.samhsa.gov/medication-assisted-treatment/buprenorphine-waiver-management.
- Sullivan MA, Bisaga A, Glass A, Mishlen K, Pavlicova M, Carpenter KM, … Nunes EV. Opioid use and dropout in patients receiving oral naltrexone with or without single administration of injection naltrexone. Drug Alcohol Depend. 2015;147:122–129. doi: 10.1016/j.drugalcdep.2014.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas CP, Garnick DW, Horgan CM, Miller K, Harris AH, Rosen MM. Establishing the feasibility of measuring performance in use of addiction pharmacotherapy. J Subst Abuse Treat. 2013;45(1):11–18. doi: 10.1016/j.jsat.2013.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truven Health Analytics. Truven Health MarketScan® commercial claims and encounters user guide: data year 2014 edition. 2015. [Google Scholar]
- U.S. Food and Drug Administration. FDA approves injectable drug to treat opioid-dependent patients. 2010 Retrieved from http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm229109.htm.
- United States Department of Health and Human Services Office of the Surgeon General. Facing Addiction in America: The Surgeon General’s Report on Alcohol, Drugs, and Health. 2016 Retrieved from https://addiction.surgeongeneral.gov/surgeon-generals-report.pdf. [PubMed]
- Volkow ND, Frieden TR, Hyde PS, Cha SS. Medication-assisted therapies--tackling the opioid-overdose epidemic. N Engl J Med. 2014;370(22):2063–2066. doi: 10.1056/NEJMp1402780. [DOI] [PubMed] [Google Scholar]
- Walley AY, Alperen JK, Cheng DM, Botticelli M, Castro-Donlan C, Samet JH, Alford DP. Office-based management of opioid dependence with buprenorphine: clinical practices and barriers. J Gen Intern Med. 2008;23(9):1393–1398. doi: 10.1007/s11606-008-0686-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilder C, Lewis D, Winhusen T. Medication assisted treatment discontinuation in pregnant and postpartum women with opioid use disorder. Drug Alcohol Depend. 2015;149:225–231. doi: 10.1016/j.drugalcdep.2015.02.012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.