Abstract
Objective
To characterize the effect of anti-tumor necrosis factor (TNF) therapy compared to conventional disease-modifying antirheumatic drugs (csDMARDs) in children with enthesitis-related arthritis (ERA) over the first year after diagnosis.
Methods
We conducted a multicenter retrospective comparative effectiveness study of children diagnosed with ERA. We estimated the effect of anti-TNF therapy on clinical parameters (active joint count, tender entheses count) and patient-reported pain and global assessment of disease activity over the first year after diagnosis using state-of-the-art comparative effectiveness analytic methods.
Results
During the study period, 217 newly diagnosed ERA patients had a total of 965 clinic visits the first year after disease diagnosis. Children (median age 11.6 years, IQR: 10–14) were treated with anti-TNF monotherapy (N=33; 15.2%), csDMARD monotherapy (N=73; 33.6%), or both (N=52; 23.9%) the first year after disease diagnosis. There was a statistically significant improvement in the primary outcome, active joint count over time, in children who received an anti-TNF drug versus those who did not (p=0.03). Additionally, use of anti-TNF therapy, versus no anti-TNF therapy, was associated with less patient-reported pain (p<0.01) and improved disease activity over time as assessed by the cJADAS10 (p<0.01). The magnitude of estimated effect on clinical outcomes was uniformly greater, with the exception of tender entheses count, in children treated with an anti-TNF drug versus a csDMARD.
Conclusion
During the first year after diagnosis, anti-TNF exposure was associated with benefits for several clinically meaningful outcomes in children with enthesitis-related arthritis.
INTRODUCTION
The comparative effectiveness of different treatment algorithms for children with enthesitis-related arthritis (ERA) remain unclear and without consensus. ERA is a category of arthritis characterized by arthritis, enthesitis, axial arthritis, symptomatic uveitis, and HLA-B27 positivity. Treatment regimens for ERA include monotherapy or combination therapy with any of the following: nonsteroidal anti-inflammatory drugs (NSAID), conventional synthetic disease-modifying anti-rheumatic drugs (csDMARD), such as methotrexate, sulfasalazine, and leflunomide, or biological DMARD (bDMARD) agents such as etanercept, adalimumab, and infliximab.
There are only 2 randomized clinical trials focused on children with ERA(1, 2); the majority of trials include ERA as one of several juvenile arthritis categories. Both of the published ERA trials included only children who had established disease and failed at least 1 NSAID and 1 csDMARD and who had at least 3 active joints(1, 2). In both trials, treatment with a bDMARD resulted in sustained clinical improvement. In another study which included a subset of children with prevalent ERA disease (average disease duration 2 years) and at least 2 active joints, etanercept resulted in improvement in the pediatric American College of Rheumatology (ACR) core set response criteria, tender entheses count, back pain, and back mobility(3). There are no published trials of therapy for children with a new diagnosis of ERA. The choice of induction treatment algorithms for children with ERA, according to the ACR treatment recommendations, is based solely on the number of active joints(4). Using these algorithms, the earliest a child with ERA might be treated with a bDMARD is after 3 months of therapy with a csDMARD. Sacroiliitis is considered separately in the ACR recommendations and earlier exposure to an anti-TNF drug is encouraged. Observational studies have shown that ERA is associated with a lower likelihood of good response to tumor necrosis factor inhibitors compared to other categories of JIA(5, 6). The comparative efficacy of csDMARD versus bDMARD therapy in children with a new diagnosis of ERA remains unclear.
In this retrospective study, we used a repeated measures design to evaluate the impact of bDMARD therapy compared to csDMARD therapy on relevant clinical and patient-reported outcomes in children from 5 centers with a new diagnosis of ERA. Treatments were based upon provider and family preferences, as per routine clinical practice. The use state-of-the-art comparative effectiveness analytic methods enabled assessment of bDMARD effect in this cohort with appropriate adjustment of time-invariant and time-variant confounders.
METHODS
This study was approved by the committees for the protection of human subjects at each of the participating institutions. Children’s Hospital of Philadelphia served as the coordinating center (IRB 12-009267).
Study sites and participants
The source population for this study was all children who fulfilled the International League of Associations for Rheumatology (ILAR) criteria for ERA(7) and had at least 6 months of documented follow-up at a rheumatology clinic at one of the following academic tertiary care referral centers: Children’s Hospital of Philadelphia (Philadelphia, PA), Children’s of Alabama (Birmingham, Alabama), Cincinnati Children’s Hospital Medical Center (Cincinnati, Ohio), Texas Scottish Rite Hospital for Children (Dallas, TX), and Meyer Children’s Hospital (Florence, Italy). Children who met ERA criteria but had a first-degree relative with psoriasis were not excluded (N=7). Children and adolescents who transferred care from another institution or who were already receiving systemic therapy (csDMARD or anti-TNF) at the time of initial evaluation were excluded.
Each institution queried their respective clinical databases for all children diagnosed with ERA in the outpatient health record at the initial or subsequent follow-up visit. The range of diagnosis dates included from each institution varied depending upon availability of searchable medical records and are as follows: Children’s Hospital of Philadelphia 2001–2012, Children’s of Alabama 2007–2012, Cincinnati Children’s Hospital Medical Center 2007–2012, Texas Scottish Rite Hospital for Children 1993–2011, and Meyer Children’s Hospital 1995–2012. All inclusion and exclusion criteria were verified by the coordinating center using the JIA Calculator(8). The JIA calculator is a web-based tool to help algorithmically classify children according to ILAR criteria(7); 39 children were excluded after this process. The disease characteristics and treatment approaches for an expanded selection of this cohort (including additional patients with an ERA diagnosis but limited follow-up) have been previously described(9).
Clinical characteristics
Baseline visit was defined as the first rheumatology visit at which the child presented with clinical signs of juvenile arthritis (enthesitis, arthritis, acute uveitis or inflammatory back pain). The following clinical data were abstracted from the medical record: demographics, family history of HLA-B27 associated disease, clinical features (including development of new sacroiliitis demonstrated on MRI), patient-reported outcomes (disease activity assessment and pain), and medication use. Medications evaluated included NSAIDs, intra-articular glucocorticoid injections, oral glucocorticoids, csDMARDs (methotrexate, leflunomide, sulfasalazine), and bDMARD therapy with tumor necrosis factor alpha blocking agents (adalimumab, etanercept, infliximab). Disease activity at each visit was measured using the juvenile spondyloarthritis disease activity (JSpADA) index(10), and the clinical juvenile arthritis disease activity score (cJADAS10) (11). The JSpADA is a validated composite measure (comprised of arthritis, enthesitis, patient pain assessment, inflammatory markers, morning stiffness, clinical sacroiliitis, uveitis, back mobility) developed specifically for children with juvenile spondyloarthritis that ranges from 0–8 with higher scores indicating more disease activity. The cJADAS10 (comprised of the active joint count, physician global and parent global disease activity evaluation) is also a validated composite disease measure but it was specifically developed for juvenile idiopathic arthritis and ranges from 0 (inactive disease) to 30 (highest disease activity).
Statistical analysis
In order to determine which clinical factors influence the decision to treat with anti-TNF therapy, we fit a multilevel mixed-effects logistic regression model with adjustment for clustering by patient and site. Covariates tested included: study day (baseline visit=day 1), age, sex, race, HLA-B27 status, csDMARD use, glucocorticoid use (yes/no) and presence of hip arthritis, wrist arthritis, sacroiliitis, or uveitis. The model included all visits up to and including the first visit where a bDMARD was prescribed. We used locally weighted scatterplot smoothing (Lowess) to also visually evaluate how disease activity scores influenced the probability of being prescribed anti-TNF medication. JSpADA scores were plotted against whether or not a bDMARD was prescribed at that visit or not.
Marginal structural models (MSMs) were used to estimate the causal effect of anti-TNF treatment(12). This approach appropriately estimates time-dependent treatment effects in the presence of time-dependent confounders that are themselves affected by previous treatment and also predict the subsequent treatment. The model is fitted in a two-stage process. First, at each time point each subject’s probability of receiving their own treatment and probability of being censored are derived as inverse-probability-of-treatment weights (IPTWs) and inverse-probability-of-censoring weights (IPCWs) respectively using pooled logistic regressions. Second, the association between the treatment and outcome measured repeatedly is evaluated in a generalized estimating equation (GEE) model that is weighted using the product of IPTWs and IPCWs. Weighting by the IPTWs in effect creates a pseudo-population in which no confounding exists. Therefore, the estimated treatment effect from the subsequent regression models based on this population can be interpreted as the true causal effect of the treatment on the outcome. Weighting by the IPCWs further accounts for the bias due to any loss of follow-up, which is common in longitudinal studies.
The pooled logistic regression was used to obtain the IPTWs at each visit as the conditional probability of receiving the anti-TNF therapy given the past treatment history, the baseline covariates, and time-varying clinical variables that might influence the receipt of treatment. The baseline variables included the demographics sex, race, and age, time-invariant clinical variables included HLA-B27 status and year of diagnosis, and the time-varying clinical variables included days since diagnosis, glucocorticoid use, prior anti-TNF prescription, hip arthritis, wrist arthritis, and acute anterior uveitis. Similarly, the IPCWs were obtained as the conditional probability of not receiving treatment (being censored) at each visit. Stability of the weights was assessed graphically at intervals of 60 study days.
For the second step of weighted MSM, the primary outcome was the active joint count measured over time. Secondary outcomes assessed included repeated measures of the tender entheses count, JSpADA index, cJADAS10, patient assessment of disease activity, and patient-reported pain. An exchangeable correlation structure was used in the GEE model which was equivalent to assuming random intercept among individuals to allow for potentially different baseline values. A negative binomial distribution with log link was assumed to account for the over-dispersion for active joint count and tender entheses count due to the number of zero counts. The trajectories of the outcome variables over time were visualized by making spaghetti plots, confirming the linear assumption of our model.
Normal distribution with identity link was used for the remainder of outcomes. The models for active joint count and tender entheses count included the following variables: anti-TNF use, csDMARD use, age, sex, study day, an interaction between study day and bDMARD use, HLA-B27 status and accounted for clustering within site and weighted inverse probability of bDMARD use (from step 1). The models for the JSpADA index, cJADAS10, patient assessment of disease activity, and patient-reported pain included the same variables with the exclusion of HLA-B27 status.
Data regarding development of new sacroiliitis was only available for 4 sites. The association of development of new sacroiliitis and anti-TNF use at these 4 sites was tested using chi-squared test.
Given the retrospective nature of the study, patient-reported outcomes and laboratory tests were collected at the discretion of the site. Missing data was not imputed. One site did not have reliable patient-reported outcome collection prior to March 2010, for which the MSM analysis was restricted to children diagnosed after 2010. Another site did not collect patient-reported disease activity, and this site was excluded from that particular MSM analysis.
All analyses were performed using Stata 14 (StataCorp. 2015, Stata Statistical Software. College Station, TX: StataCorp LP) and SAS software 9.4 (Copyright © 2011, SAS Institute Inc., Cary, NC, USA).
RESULTS
Subjects
During the study period, 217 newly diagnosed ERA patients had a total of 965 outpatient visits during the first year after disease diagnosis. One hundred forty-three (65.9%) children had follow-up for one year ± two months after baseline. Median follow-up for all patients was 335 days (IQR: 280–365 days). The median age for the cohort was 11.6 years and the median symptom duration at the time of baseline visit was 6 months (IQR: 3–12 months). Demographics and clinical characteristics of the cohort are shown in Table 1. Children presented with arthritis and enthesitis (N=176, 81.1%), arthritis plus 2 or more additional ILAR criteria (N=22; 10.1%), or enthesitis plus 2 or more additional ILAR criteria (N=19; 8.8%). One hundred ninety-eight (91.2%) children met ILAR criteria for ERA at the baseline visit; the remainder fulfilled ILAR criteria by 6 months. The population was predominantly male (71.9%) and 59.7% were HLA-B27 positive. Sixty-four (29.5%) had a polyarticular course. Thirty-two of 142 (23%) and 49 of 128 (38%) with at least one calculable score achieved a JSpADA of 0 or a cJADAS10 of ≤1 at some point during the first year after diagnosis indicating inactive disease. The median time to attain a JSpADA of 0 or a cJADAS10 of ≤1 was 232 days (IQR: 128–339) and 161 days (IQR: 72–287), respectively. One hundred twenty-seven (58.5%) children achieved a simultaneous active joint and tender entheses count of 0 a median of 145 days after the baseline visit.
Table 1. Patient Characteristics at Diagnosis.
Patient characteristics at visit where ILAR criteria was met. Children who met ERA criteria but had a first-degree relative with psoriasis were not excluded (N=7).
| N=217 | |
|---|---|
| Demographics | |
| Age, Median (IQR) | 11.6 (9.6, 13.8) |
| Sex (male), N (%) | 156 (71.9) |
| Race, Caucasian, N (%) | 181 (83.4) |
| ILAR ERA criteria, N (%) | |
| Arthritis | 187 (86.2) |
| Enthesitis | 144 (66.4) |
| Sacroiliac joint tenderness and/or inflammatory spinal pain | 50 (23.0) |
| Acute, symptomatic uveitis | 14 (6.5) |
| Onset of arthritis in a male >6 years | 132 (60.8) |
| Family history of HLA-B27+ associated disease in a first-degree relative | 37 (17.1) |
| Clinical Features and Patient Reported Outcomes at Diagnosis, Median (IQR) | |
| Active Joint Count | 2 (1, 4) |
| Tender Enthesis Count | 2 (0, 3) |
| Juvenile Spondyloarthritis Disease Activity Index (JSpADA) (0–8) | 3 (2, 4) |
| Clinical Juvenile Arthritis Disease Activity Score (cJADAS10) (0–30) | 9 (6, 14.5) |
| Patient/parent pain (VAS 0–10) | 4 (2, 7) |
| Patient/parent disease activity (VAS 0–10) | 4 (2, 6) |
The treating provider performed imaging for sacroiliitis based on clinical suspicion (MRI=65; Radiograph=25; MRI and radiograph within 90 days of each other=16 – 2 radiograph first, 1 MRI first, and 13 simultaneous). Twenty-one (32%) of the children who had an MRI performed had evidence of sacroiliitis on imaging at diagnosis. An additional 14 children developed sacroiliitis (defined by MRI) over the first year of follow-up. Of the 35 children with MRI-defined sacroiliitis, ten also had a radiograph performed within 90 days of the MRI, one of which was abnormal. Of the 35 with sacroiliitis at some point during the first year, 21 (60%) were HLA-B27+.
Medication use
Children were treated with anti-TNF monotherapy (N=33; 15.2%), csDMARD monotherapy (N=73; 33.6%), or simultaneous csDMARD and anti-TNF therapy (N=52; 23.9%) the first year after diagnosis. Two patients (1%) were switched from a csDMARD to an anti-TNF without any overlap in medication use. Children who received anti-TNF therapy received adalimumab (N=17; 19.5%), etanercept (N=63; 72.4%), or infliximab (N=7; 8.1%) as their primary medication. There were no bDMARD drugs other than anti-TNF drugs prescribed for children with newly diagnosed ERA during the study interval. Median time to first bDMARD prescription was 35 days (IQR: 0–99 days). Seventy-five (86%) children who started an anti-TNF remained on anti-TNF therapy for the duration of follow-up. Eight (9%) anti-TNF users switched anti-TNF drugs during the course of therapy. The median time to anti-TNF switch was 155 days (IQR: 62, 163). Of the 8 children who stopped the anti-TNF drug before the end of follow-up, 1 had previously tried a different anti-TNF drug.
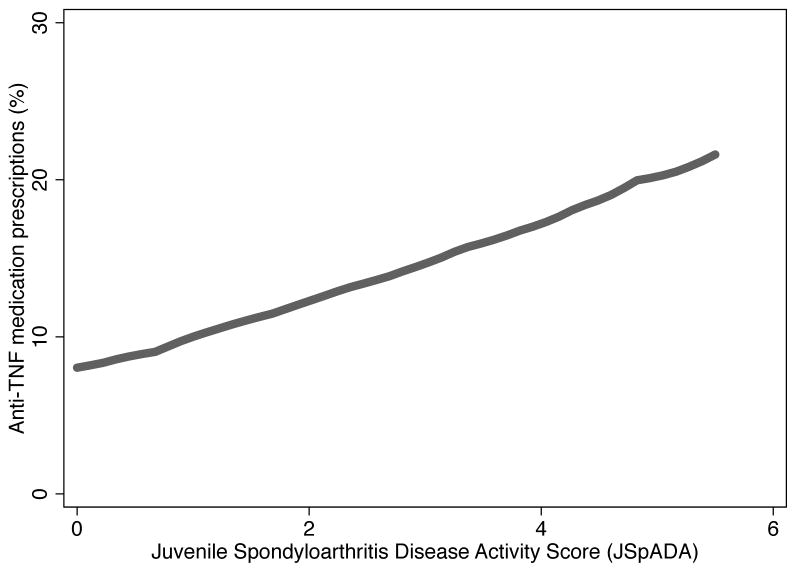
The severity of disease and certain disease manifestations were associated with a higher probability of physicians to prescribe an anti-TNF drug. As disease activity increased from low to high activity as measured by the JSpADA index, the probability of receiving an anti-TNF drug increased from less than 10% to greater than 20%, a finding consistent with confounding by indication (Figure 1)(13). In a multilevel mixed-effects logistic analysis, the presence of hip, wrist, or sacroiliac arthritis were all associated with increased odds of anti-TNF exposure (Table 2).
Figure 1. Confounding by indication between prescription of an anti-TNF drug and juvenile spondyloarthritis disease activity (JSpADA) index.
Unadjusted disease activity scores up to and including the first prescription of an anti-TNF drug were plotted against a binary variable defining if a patient was prescribed an anti-TNF drug at that time point or not.
Table 2. Factors associated with first anti-TNF drug prescription.
Results of multilevel mixed-effects logistic modeling to determine factors associated with prescription of first anti-TNF drug.
| Disease manifestation | Odds Ratio (95% CI) | p-value |
|---|---|---|
| Hip arthritis | 5.3 (2.0, 13.9) | <0.01 |
| Wrist arthritis | 3.1 (1.3, 7.6) | 0.01 |
| Sacroiliitis | 4.7 (1.8, 12.1) | <0.01 |
| Uveitis | 1.9 (0.6, 6.4) | 0.31 |
| HLA-B27 positivity | 1.2 (0.7, 2.3) | 0.49 |
The csDMARDs prescribed for children with ERA included methotrexate (N=100; 78.7%), sulfasalazine (N=26; 20.5%), and leflunomide (N=1; 0.8%). Median time to first csDMARD was 0 days (IQR: 0–63 days). Of the 127 children treated with any csDMARD, 27 (20%) discontinued csDMARD use before the end of follow-up. One hundred and eighty-eight (86.6%) and 58 (26.7%) were treated with non-steroidal anti-inflammatory drugs (NSAIDs) or systemic glucocorticoids, respectively. Sixty-two (28.6%) received at least 1 joint injection. The median number of joint injections in patients who underwent the procedure was 1 (IQR: 1, 2).
Outcomes
There were missing values on 38.3% and 34.2% of visits across all sites for patient disease activity assessment and patient-reported pain, respectively. No difference in age, sex, or median active joint count was observed between visits with and without patient-reported outcomes, or between visits with and without missing inflammatory markers.
Results of the MSM model, which adjusted for the confounding and censoring through weighting, are shown in Table 3. There was a statistically significant difference in the primary outcome, active joint count over time, in children who received an anti-TNF drug versus those who did not, when holding other covariates constant (p=0.03). Over the first year after disease, patients who received an anti-TNF drug also reported less pain (p<0.01) and had improved disease activity over time as assessed by the cJADAS10 (p<0.01). Use of an anti-TNF drug was associated with improvement, albeit statistically insignificant, in all remaining clinical, disease activity, and patient assessments.
Table 3. Association of treatment exposure on outcomes.
Results from repeated measures multivariate models. Higher scores for physician disease activity, JSpADA index, cJADAS10, patient-reported disease activity, and patient-reported pain indicate poorer outcomes.
| Outcome (over time) | Variable | Estimate (95% CI) | p-value |
|---|---|---|---|
| Active joint count | Anti-TNF | −0.78 (−1.49, −0.07) | 0.03 |
| csDMARD | −0.22 (−0.58, 0.15) | 0.25 | |
| HLA-B27 (−) | −0.07 (−0.66, 0.52) | 0.83 | |
| Age | 0.04 (−0.03, 0.12) | 0.26 | |
| Female Sex | −0.61 (−1.09, −0.14) | 0.01 | |
| Tender entheses count | Anti-TNF | −0.04 (−0.47, 0.40) | 0.87 |
| csDMARD | −0.26 (−0.47, −0.04) | 0.02 | |
| HLA-B27 (−) | 0.75 (0.48, 1.02) | <0.01 | |
| Age | 0.06 (0.02, 0.10) | <0.01 | |
| Female Sex | 0.26 (−0.03, 0.55) | 0.08 | |
| JSpADA (0, 8) | Anti-TNF | −0.51 (−1.06, 0.05) | 0.07 |
| csDMARD | −0.23 (−0.54, 0.07) | 0.14 | |
| Age | 0.07 (0.00, 0.13) | 0.04 | |
| Female Sex | 0.22 (−0.18, 0.63) | 0.28 | |
| cJADAS10 (0, 30) | Anti-TNF | −2.90 (−4.92, −0.88) | <0.01 |
| csDMARD | −0.28 (−1.47, 0.90) | 0.64 | |
| Age | 0.29 (0.05, 0.53) | 0.02 | |
| Female Sex | 1.10 (−0.54, 2.74) | 0.19 | |
| Patient-reported disease activity (0, 10) | Anti-TNF | −0.40 (−1.36, 0.56) | 0.42 |
| csDMARD | −0.06 (−0.66, 0.53) | 0.83 | |
| Age | 0.12 (−0.00, 0.24) | 0.04 | |
| Female Sex | 1.53 (0.65, 2.41) | <0.001 | |
| Patient-reported pain (0, 10) | Anti-TNF | −1.23 (−2.05, −0.41) | <0.01 |
| csDMARD | −0.42 (−0.97, 0.12) | 0.13 | |
| Age | 0.15 (0.03, 0.26) | 0.01 | |
| Female Sex | 1.64 (0.75, 2.54) | <0.001 |
Use of a csDMARD, holding all other covariates constant, was associated with significantly lower tender entheses count (p=0.02). csDMARD use, similar to anti-TNF drug use, was associated with improvement, albeit statistically insignificant in all other outcomes. The magnitude of the estimate, however, was dampened for all outcomes except tender enthesis count in comparison to the estimate for anti-TNF use.
Fourteen children were diagnosed with sacroiliitis by imaging over the course of follow-up. Twelve (86%) of these children were not being treated with an anti-TNF drug at the time of sacroiliitis diagnosis (p<0.01). Three of these children were subsequently started on bDMARD therapy and all children were treated with some form of medication. When stratified by HLA-B27 status, lack of anti-TNF exposure remained significantly associated with development of new sacroiliitis (both p <0.01).
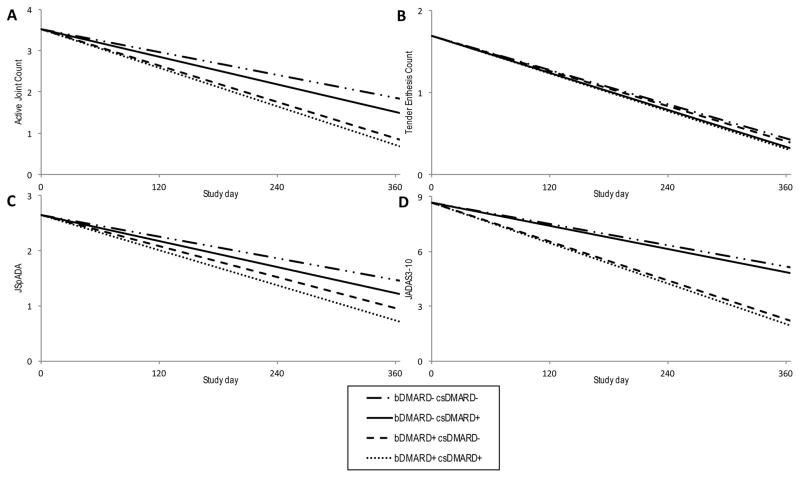
The change over time in all outcomes in children treated with anti-TNF drug plus csDMARD, anti-TNF monotherapy, csDMARD monotherapy, and supportive care only (NSAIDs, glucocorticoids, intraarticular joint injections) are shown in figures 2 and 3. For all outcomes the rate of improvement was greatest for those children treated with both an anti-TNF drug and a csDMARD, followed by anti-TNF monotherapy.
Figure 2. Patient disease manifestations and disease activity trajectories by treatment medication over the first year after diagnosis of ERA modeled using MSM.
Trajectories during the first year following diagnosis of (A) active joint count, (B) tender enthesis count, (C) JSpADA index, and (D) cJADAS10 for patients treated with bDMARD and csDMARD therapy, bDMARD monotherapy, csDMARD monotherapy, and supportive care only. Outcome trajectories are for an 11.6-year-old male.
Figure 3. Patient disease manifestation and disease activity trajectories by treatment medication over the first year following diagnosis of ERA modeled using MSM.
Trajectories during the first year following diagnosis of (A) patient pain scores and (B) patient reported disease activity scores activity scores for patients treated with bDMARD and csDMARD therapy, bDMARD monotherapy, csDMARD monotherapy, and supportive care only. Outcome trajectories are for an 11.6-year-old male.
DISCUSSION
This large multicenter comparative effectiveness study of clinical and patient-reported outcomes in children with newly diagnosed ERA revealed that anti-TNF exposure is associated with statistically significant improvements in active joint count, the cJADAS10, and patient-reported pain over the first year after disease diagnosis. Furthermore, the direction of our estimates was consistent across all outcomes measures. csDMARD therapy, as expected, also improved outcomes measures. The magnitude of estimated effect, however, was uniformly greater in children treated with an anti-TNF drug versus a csDMARD.
Several findings warrant additional discussion. First, as with any observational study of therapeutic intervention, the possibility of confounding by indication bias must be considered. This bias arises when children with more severe disease manifestations are more likely to receive the exposure of interest and experience poorer outcome(14). In this study, we did find evidence that children with higher disease activity and more severe disease manifestations (hip, wrist, axial arthritis) were more likely to receive bDMARD therapy within the first 3 months, as demonstrated in Table 2 and Figure 1. Our MSM model, which adjusted for confounding and censoring through weighting, likely minimized but did not completely remove this bias. Since we demonstrated that the children who received bDMARDs had a greater magnitude of beneficial effect than children who received csDMARDs and that rate of improvement over time was greatest in children who received an anti-TNF drug with or without a csDMARD, the possibility exists that anti-TNF agents have an even greater positive effect on clinical and patient-reported outcomes than we were able to demonstrate.
Second, in this multi-center cohort more than one-third of children attained a cJADAS10 indicating inactive disease activity a median of 161 days after diagnosis. This proportion of responders is in accordance with values previously reported in an observational study of children with ERA (38% after 15 months of therapy)(6). In the aforementioned study, the Wallace criteria for inactive disease(15) were used, which were developed for use in other categories of JIA. In another observational study, 43% of children with ERA attained inactive disease according to the Wallace criteria during a one-year follow-up period(5).
Third, this study was not designed to systematically evaluate for the presence of axial arthritis. Imaging for suspicion of axial disease was performed as per the treating physician. Interestingly, 11% of children in the cohort had axial involvement recognized on MRI evaluation at some point during the first year after disease diagnosis. Of these, 86% were not being treated with an anti-TNF drug. Whether early bDMARD use was “protective” against development of axial arthritis in those treated with an anti-TNF drug was unclear, as was whether early use of an anti-TNF drug suppressed axial disease symptoms and therefore the need for subsequent imaging. Prior studies have shown that in children with newly diagnosed juvenile spondyloarthritis and MRI evidence of sacroiliitis (both active and chronic lesions), up to two-thirds may not have back pain(16). Without the use of universal screening to detect subclinical sacroiliitis the true efficacy of anti-TNF drugs and other bDMARD agents for this disease manifestation will remain unknown. The role of early bDMARD use in juvenile spondyloarthritis, including ERA, remains unclear and has not been systematically evaluated.
In summary, this study supports the effectiveness of anti-TNF drugs within the first year after disease diagnosis in routine clinical practice. Children treated with bDMARD s had improvement in all clinical features and patient-reported outcomes, albeit some statistically insignificant. Efficacy trials of early bDMARD use versus traditional csDMARDs to assess impact on time to inactive disease, risk and treatment of sacroiliitis, patient-reported outcomes, and cost implications are critically needed.
Acknowledgments
Funding: Dr. Weiss’ work was supported by the NIH/National Institute of Arthritis and Musculoskeletal and Skin Diseases (grant 1-K23-AR-059749-01A1).
References
- 1.Burgos-Vargas R, Tse SM, Horneff G, Pangan AL, Kalabic J, Goss S, et al. A Randomized, Double-Blind, Placebo-Controlled Multicenter Study of Adalimumab in Pediatric Patients With Enthesitis-Related Arthritis. Arthritis Care Res (Hoboken) 2015;67:1503–12. doi: 10.1002/acr.22657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Horneff G, Foeldvari I, Minden K, Trauzeddel R, Kummerle-Deschner JB, Tenbrock K, et al. Efficacy and safety of etanercept in patients with the enthesitis-related arthritis category of juvenile idiopathic arthritis: results from a phase III randomized, double-blind study. Arthritis Rheumatol. 2015;67:2240–9. doi: 10.1002/art.39145. [DOI] [PubMed] [Google Scholar]
- 3.Horneff G, Burgos-Vargas R, Constantin T, Foeldvari I, Vojinovic J, Chasnyk VG, et al. Efficacy and safety of open-label etanercept on extended oligoarticular juvenile idiopathic arthritis, enthesitis-related arthritis and psoriatic arthritis: part 1 (week 12) of the CLIPPER study. Ann Rheum Dis. 2014;73:1114–22. doi: 10.1136/annrheumdis-2012-203046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beukelman T, Patkar NM, Saag KG, Tolleson-Rinehart S, Cron RQ, DeWitt EM, et al. 2011 American College of Rheumatology recommendations for the treatment of juvenile idiopathic arthritis: initiation and safety monitoring of therapeutic agents for the treatment of arthritis and systemic features. Arthritis Care Res (Hoboken) 2011;63:465–82. doi: 10.1002/acr.20460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Donnithorne KJ, Cron RQ, Beukelman T. Attainment of inactive disease status following initiation of TNF-alpha inhibitor therapy for juvenile idiopathic arthritis: enthesitis-related arthritis predicts persistent active disease. J Rheumatol. 2011;38:2675–81. doi: 10.3899/jrheum.110427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Otten MH, Prince FH, Twilt M, Ten Cate R, Armbrust W, Hoppenreijs EP, et al. Tumor necrosis factor-blocking agents for children with enthesitis-related arthritis--data from the dutch arthritis and biologicals in children register, 1999–2010. J Rheumatol. 2011;38:2258–63. doi: 10.3899/jrheum.110145. [DOI] [PubMed] [Google Scholar]
- 7.Petty RE, Southwood TR, Manners P, Baum J, Glass DN, Goldenberg J, et al. International League of Associations for Rheumatology classification of juvenile idiopathic arthritis: second revision, Edmonton, 2001. J Rheumatol. 2004;31:390–2. [PubMed] [Google Scholar]
- 8.Behrens EM, Beukelman T, Cron RQ. Juvenile idiopathic arthritis classification criteria: loopholes and diagnosis software. J Rheumatol. 2007;34:234. author reply -5. [PubMed] [Google Scholar]
- 9.Gmuca S, Xiao R, Brandon TG, Pagnini I, Wright TB, Beukelman T, et al. Multicenter inception cohort of enthesitis-related arthritis: variation in disease characteristics and treatment approaches. Arthritis Res Ther. 2017;19:84. doi: 10.1186/s13075-017-1297-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weiss PF, Colbert RA, Xiao R, Feudtner C, Beukelman T, DeWitt EM, et al. Development and retrospective validation of the juvenile spondyloarthritis disease activity index. Arthritis Care Res (Hoboken) 2014;66:1775–82. doi: 10.1002/acr.22411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McErlane F, Beresford MW, Baildam EM, Chieng SE, Davidson JE, Foster HE, et al. Validity of a three-variable Juvenile Arthritis Disease Activity Score in children with new-onset juvenile idiopathic arthritis. Ann Rheum Dis. 2013;72:6. doi: 10.1136/annrheumdis-2012-202031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Robins JM, Hernan MA, Brumback B. Marginal structural models and causal inference in epidemiology. Epidemiology. 2000;11:550–60. doi: 10.1097/00001648-200009000-00011. [DOI] [PubMed] [Google Scholar]
- 13.Walker AM. Confounding by indication. Epidemiology. 1996;7:335–6. [PubMed] [Google Scholar]
- 14.Csizmadi I, Collet J-P, Boivin J-F. Bias and Confounding in Pharmacoepidemiology. In: Strom BL, editor. Pharmacoepidemiology. 4. Chichester, UK: John Wiley & Sons, Ltd; 2007. pp. 791–809. [Google Scholar]
- 15.Wallace CA, Ruperto N, Giannini E. Preliminary criteria for clinical remission for select categories of juvenile idiopathic arthritis. J Rheumatol. 2004;31:2290–4. [PubMed] [Google Scholar]
- 16.Weiss PF, Xiao R, Biko DM, Chauvin NA. Assessment of Sacroiliitis at Diagnosis of Juvenile Spondyloarthritis by Radiography, Magnetic Resonance Imaging, and Clinical Examination. Arthritis Care Res (Hoboken) 2016;68:187–94. doi: 10.1002/acr.22665. [DOI] [PMC free article] [PubMed] [Google Scholar]