Abstract
We examined the prediction that the interaction between Glucocorticoid Receptor Gene (NR3C1) methylation, stress, and experienced maternal support predicts anxious and avoidant attachment development. This was tested in a general population sample of 487 children and adolescents (44% boys, Mage = 11.84, Sdage = 2.4). These children were followed over a period of 18 months. In line with the prediction, we found that NR3C1 methylation moderates the effect of maternal support during stress on anxious attachment development 18 months later. More stressed children who experienced less maternal support reported increased anxious attachment when their NR3C1 gene was highly methylated. This effect could not be explained by children’s level of psychopathology. No effects were found for attachment avoidance. These data provide the first prospective evidence that epigenetic processes are involved in attachment development.
Keywords: attachment, preadolescence, longitudinal, epigenetics, stress, maternal support
The ability for children to be securely attached to their mother and trust in her support during times of distress is a fundamental requisite of adaptive development (Bowlby, 1969). When trust is lacking, children either anxiously increase their attempts to elicit maternal care by displaying helplessness while fearing rejection (anxious attachment), or they physically and psychologically distance themselves from their mother and negative emotions (avoidant attachment; Brumariu, 2015). Both forms of insecure attachment put children at greater risk for a host of problems (Dujardin et al., 2016), including psychopathology (Madigan et al., in press), academic problems (Bosmans & De Smedt, 2015), and poor peer and romantic relationships (Benson, McWey, & Ross, 2006) with most maladjustment in more anxiously attached children.
Originally, Bowlby (1969) proposed that the quality of attachment development is mainly affected by maternal support or the lack thereof. But subsequent research found that a purely environmental explanation was inadequate and that additional factors are needed to explain insecure attachment development (Verhage et al., 2016). Since then, genetic factors have been investigated to understand individual differences in attachment development, but evidence supporting predominantly genetic influences (e.g., latent heritability via behavioral genetics and allelic variation in DNA) has been equivocal (Roisman & Fraley, 2008). Genetic research has, amongst other genes, pointed at the glucocorticoid receptor (GR) gene (NR3C1). This gene is considered important for individual differences in attachment development in humans (Mesquita et al., 2013). The lack of GRs enhances HPA-related stress reactivity through enhanced cortisol secretion (Dickerson & Kemeny, 2004). These higher levels of stress are harder to regulate by caregivers which reduces the effectiveness of parental care and the likelihood that children learn to trust in parents as a source for support (Borelli et al., 2016; Taylor, 2006). However, research has found little support for a direct association between genetic variability in NR3C1 and individual differences in attachment development. This leaves an unresolved gap in the explanation of individual differences in attachment development (for a review, see Bakermans-Kranenburg & van IJzendoorn, 2016).
The current study aimed to test whether epigenetic factors could help shed new light on this gap. According to epigenetic research, gene expression is affected by exposure to toxic environmental influences, including those related to diet, bacteria, and stress (e.g., Fish et al., 2004; Turecki & Meaney, 2016). While several mechanisms are involved in the epigenetic regulation of genetic expression, DNA methylation of CpG islands in gene promoters, associated with transcriptional repression, has been studied most extensively, both in animals and in humans. Genes that are highly methylated cannot be transcripted. This way, methylation can critically interfere with cell functioning (Turecki & Meaney, 2016). Applied to NR3C1, this means that high levels of methylation suppress the expression of GRs. This leads to elevated HPA-related stress responses like enhanced cortisol levels, and therefore to more difficulties with regulating negative affect and higher levels of experienced distress (Turecki & Meaney, 2016). We conjectured that taking into account epigenetic modulation of NR3C1 expression could help reveal thusfar unknown dynamics of attachment development.
Because there is a clear link between experienced level of distress and children’s need for maternal support and their tendency to seek this support (Bosmans, Braet, Heylen, & De Raedt, 2015), we hypothesized that lower levels of maternal support during distress would have a stronger impact on insecure attachment development in those children whose NR3C1 gene is more methylated. The reason for this hypothesis is that, attachment theory predicts no direct effect of stress experience on the development of attachment. Instead, the theory predicts that attachment development results from the experience of attachment figures providing support during times of distress (Bowlby, 1969). In the case of individuals with high levels of NR3C1 methylation, children experience elevated and prolonged levels of distress, and therefore an elevated need for support. Subsequently, the absence of support might be experienced more strongly in children with increased methylation of NR3C1 and could exacerbate the negative effect of reduced parental support on insecure attachment development.
Consistent with the importance of focusing on NR3C1 within a stressful environmental context for understanding attachment development, Weaver and colleagues’ pioneering rodent work showed that increased epigenetic methylation-related suppression of NR3C1 contributed to later negative developmental trajectories for offspring (Weaver, 2009). Research with humans further demonstrated a link between increased methylation of NR3C1 and ensuing negative outcomes (Turecki & Meaney, 2016). Taken together, these ideas and evidence suggest that methylation-related suppression of NR3C1 in children, especially in the context of low maternal care during high stress, can bridge nature and nurture explanations of insecure attachment development.
The current study
Therefore, the current study set out to test the hypothesis that attachment insecurity development can be predicted by the synergistic interaction of low maternal support and higher NR3C1 methylation, especially in the context of exposure to stress. This hypothesis was tested in a sample of early adolescents because research suggests that at this age significant changes in attachment (in)security across time can be found (e.g., Bosmans & Kerns, 2015; Pinquart, Feussner, & Ahnert, 2013), and these changes are relevant to understanding adolescents’ (mal)adaptive development (Bosmans & Kerns, 2015). Illustrating the importance of early adolescent attachment development, research shows that early adolescents’ attachment anxiety and avoidance is linked with the likelihood that they seek maternal support during distress, which in turn protects them against the maladaptive effects of new distress in adolescence (Dujardin et al., 2016). In this final section we introduce background and rationale for design and measurement considerations that would enable a rigorous examination of this hypothesis.
At the level of stress measurement, there is a vigorous debate about whether stressful experiences are best measured with contextual stress interviews or self-report questionnaires (Hammen, 2016; Harkness & Monroe, 2016). Both strategies have strengths and weaknesses. Therefore, we measured stress using both methods to evaluate robustness of the findings accounting for each strategy’s relative limitations and to provide a conceptual replication to ensure that any significant findings would be obtained across stress measure approaches. As suggested by Harkness and Monroe (2016), we measured chronic stress reflecting ongoing stressors in multiple domains of life with different stress assessments. First, at follow up children were interviewed using the gold standard Youth Life Stress Interview (YLSI; Rudolph & Flynn, 2007) to assess chronic stress that occurred between baseline and follow up. For the current study, we calculated a chronic stress severity score based on severity of chronic stress during the follow-up period in domains that occur outside of the parent-child relationship: school performance, exposure to violence, peer relationships, legal issues, and discrimination as experienced. As chronic stress may comprise family-related stress, this maximized the distinction between the chronic stress and the quality of the parent-child relationship variables. Second, youth completed the Adolescent Life Events Questionnaire (ALEQ; Hankin & Abramson, 2002), a survey of a broad range of stressful experiences relevant for youth, at baseline and every 3 months thereafter (7 time points) that longitudinally assessed ongoing stress exposure for 18 months after baseline. For the current study, we calculated a mean stress score across the 7 time points to estimate the level of longitudinal stress exposure experienced over the 18 months after baseline.
At the level of support, we focused on maternal support because the mother remains the primary attachment figure in middle childhood and continues to exert an important influence on child development (Bosmans & Kerns, 2015). When measuring maternal support, it is important to obtain different informants to avoid problems with mono-informant bias due to a single informant’s perceptions of maternal support. To account for potential effects of reporter bias, we measured maternal support as the average of ratings made at baseline by children and parents using the Network of Relationships Inventory (Furman & Buhrmester, 1985). This measures the extent to which the mother consistently provides and serves as a source for support, help, and affective companionship.
Finally, we measured individual differences in youths’ anxious and avoidant attachment patterns using the well-validated Experiences in Close Relationships-Relationship Structure Questionnaire (Fraley et al., 2011) at baseline and at 18 months after baseline. Because epigenetic research on attachment development is in its embryonic stage (Bakermans-Kranenburg & van IJzendoorn, 2016), little theory exists about whether different patterns of results should be expected for different attachment styles. Therefore, we tested the current study’s hypothesis for both insecure attachment anxiety and avoidance dimensions separately.
Because the abovementioned hypothesis implies that epigenetic processes should be mostly visible in how (in)secure attachment unfolds over time in interaction with environmental processes (stress exposure and supportive parenting), we used a longitudinal design to test for prospective changes in attachment anxiety and attachment avoidance. For each attachment dimension we tested the three-way interaction between NR3C1 methylation, stress, and maternal support in two separate analyses for each indicator of stress. To further provide a stringent, rigorous test of our hypotheses, we controlled for age and gender, because they are known to be related to attachment development in that age-group (e.g., Del Giudice, 2015). In supplementary analyses, we controlled for psychopathology covariates in analyses given well-known associations among maternal support, stress, insecure attachment and psychopathology levels. Psychopathology development is linked with NR3C1 methylation (Klengel et al., 2014), has a negative effect on the developing parent-child relationship (e.g., Stoolmiller, 2001), and is linked with insecure attachment (Madigan et al., 2016). To conduct these analyses, we included the measures of depressive symtoms and externalizing problems that were available in the larger dataset. This ensured that baseline symptoms were not a better explanation of insecure attachment development. Moreover, we could include a follow-up measure of depressive symptoms. This ensured that changes in insecure attachment did not just reflect changes in depressive symptoms. Controlling for this alternative interpretation is an additional, conservative test of the current study’s hypothesis that anxious and avoidant attachment development can be predicted from the interaction between NR3C1 methylation, stress, and maternal support. Finally, we controlled for attachment avoidance in the analyses predicting attachment anxiety change and vice versa, to test the extent to which the effects were unique for the predicted attachment style.
Method
Participants
The sample comes from the Gene Environment Mood (GEM) study, a longitudinal study focusing on child and adolescent social and emotional development. None of the currently presented data were reported in other publications. A full description of the participants and procedure can be found in Hankin et al. (2015). A total of 665 children and adolescents were recruited at two sites: University of Denver (DU) and Rutgers University (RU). Parent report established that both the parent and child were fluent in English, the child did not have an autism spectrum or psychotic disorder, and the child had an IQ above 70. Participating youth ranged in age from 7 to 16 years (M = 11.6, SD = 2.4). The sample was comparable to the community and school districts from which it was recruited. The sample was also generally comparable to the ethnic and racial characteristics of the overall population of the United States.
Of this sample, we had data at both measurement waves for 487 children. For these remaining children, 2% of the data was missing. Data was missing completely at random according to Little’s MCAR test χ2(167) = 173,298, p = .353. Missing data were listwise deleted. Drop-out analyses suggested that the remaining children did not differ from the children for which we had no data on all relevant study variables like gender, age, NR3C1 methylation, maternal support, anxious and avoidant attachment at base line, and stress (F-values < 1.83; p-values > .18).
NR3C1 Methylation
DNA methylation levels were determined using quantitative bisulfite pyrosequencing by EpigenDx. Pyrosequencing for allele quantification (PSQ H96A, Qiagen Pyrosequencing) is a real-time sequencing-based DNA analysis that quantifies multiple, and consecutive CpG sites individually as artificial T/C SNPs (Brakensiek et al., 2007; Liu et al., 2007). Briefly 500 ng of sample DNA was bisulfate treated using the Zymo DNA Methylation Kit (Zymo research, Orange, CA). Bisulfate treated DNA is eluted in 20 μl volume and 1 μl of it is used for each PCR.
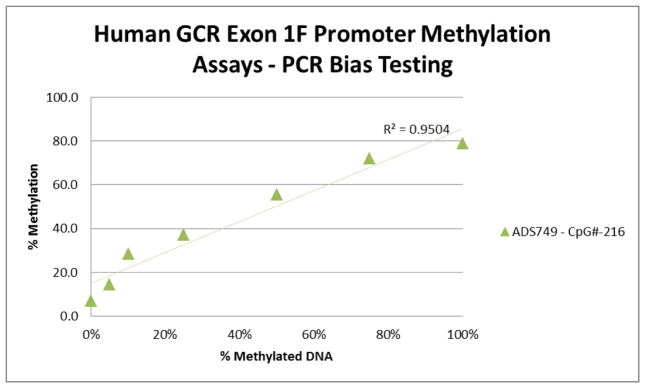
Human GCR Exon1F methylation assays covers thirty-nine CG dinucleotides in promoter region ranging from −630 ~ −354 from the transcriptional start site based on Ensembl ID ENST00000231509. ADS749FS covers seven CpG sites in the Exon1F region (hg19/chr5:142783664-142783608). The target sequences before and after bisulfite modification, and Pyrosequencing analysis dispensation order are listed in Table 1. The validation of results on PCR bias testing is showed as Figure 1. The PCR was performed using standard Pyrosequencing recommended PCR condition: 10X PCR buffer, 1.5 mM MgCl2, 200 μM of each dNTP, 0.2 μM each of forward and reverse primers, HotStar DNA polymerase (Qiagen Inc.) 1.25 U, and 1 μl of bisulfite converted DNA per 30 μl reaction. PCR cycling conditions were: 94ºC 15 min; 45 cycles of 94ºC 30 s; 46 ºC 30 s; 72ºC 30 s; 72ºC 5 min; and then products were held at 4°C. The PCR products (each 10 μl) were sequenced by Pyrosequencing PSQ96 HS System (PSQ H96A, Qiagen Pyrosequencing) following the manufacturer’s instructions (PSQ H96A, Qiagen Pyrosequencing). The methylation status of each locus was analyzed individually as a T/C SNP using QCpG software (PSQ H96A, Qiagen Pyrosequencing) (England R, Pettersson, 2005).
Table 1.
Bisulfite converted target sequence of Pyrosequencing assays
| Assay ID | Genomic Target Sequence | Bisulfite Converted Target Sequence | Pyrosequencing Dispensation order |
|---|---|---|---|
|
| |||
| ADS749 FS |
|
|
AGTCGATGTTCGAGT GTCGATGTCAGTCGA GTAGTCGGTCGAGAG TATGTCGA |
Figure 1.
Human GCR PCR Assay Preferential Amplication Test
Attachment
Anxious and avoidant attachment were measured with the Experiences of Close Relationships – Relationship Structures Questionnaire (ECR-RS; Fraley et al., 2011), a 10 item self-report measure to identify youth’s anxious (5 items; e.g., I often worry that my mother does not really care for me) and avoidant attachment (5 items; e.g., I don’t feel comfortable opening up to this person). Participants indicated for each item on a 7-point scale level of agreement (1 = strongly disagree; 7 = strongly agree). The questionnaire’s validity has been shown in different studies (Fraley et al., 2011), and youth’s answers to similar questions has been linked to observable support seeking behavior and the development of psychopathology (Dujardin et al., 2016). In the current study, alpha’s indicated adequate reliability (αanxiety pre =.82, αavoidance pre = .81, αanxiety post = .83, αavoidance post = .80).
Stress
Chronic Stress Severity was measured with the Youth Life Stress Interview (YLSI; Rudolph & Flynn, 2007), a revised version of the UCLA Child Episodic Life Stress and Chronic Stress Interview (Rudolph KD, Hammen, 1999). The YLSI is a reliable, valid, semistructured contextual stress interview used to assess youths’ ongoing stress level. The YLSI has demonstrated excellent reliability and validity (Conley & Rudolph, 2009;Rudolph & Flynn, 2007). To avoid conflation between the stress sources and maternal support, we concentrated on severity of chronic stress in stress domains located outside of the mother-child relationship: school performance, exposure to violence, peer relationships, legal issues, and discrimination. Interviewers ascertained from youth information relevant to chronic stress over the 18 months after baseline, including standardized questions. In line with the procedure that is most often used in previous research (for a thorough description, see Rudolph and Flynn, 2007), severity information based on responses to these questions were presented to a team of three or more blind raters, who came to an agreed upon severity score, from 1 (little/no stress), 2 (average/normal stress), 3 (moderate stress), 4 (serious stress), to 5 (severe stress).
Longitudinal Stress Exposure was measured with the Adolescent Life Events Questionnaire (Hankin & Abramson, 2002) which is designed to assess the occurrence of a broad range of negative events typically reported by adolescents, such as school problems (e.g., “You got in trouble with the teacher or principal”) or relationship difficulties (e.g., “You found out your boyfriend/girlfriend was cheating on you”). Each of the 37 events is rated for frequency in the past 3 months on a Likert-type scale ranging from A (never) to E (always). Reliability and validity for the ALEQ has been established in past studies (e.g., Calvete, 2011; Hankin, 2008; Hankin, Stone, & Wright, 2010). The ALEQ was administered at baseline and then every 3 months across 18 months of follow up (7 data points). For the current study, we calculated a mean score over the different measurement moments to obtain a single, reliable, robust stress exposure average over 18 months to parallel the 18 months of chronic stress exposure provided by the YLSI.
Maternal Support
Mothers and children filled out the Network of Relationships Inventory (NRI; Furman & Buhrmester, 1985), a questionnaire that consists of 13 items that assess perceived quality of relationships, both related to support and conflict. For the current study, we only focused on the child’s relationship with mother and the extent to which mother is perceived as providing support during stress (e.g., “How much does this person really care about you?”). Participants answered items on a 5-point scale ranging from ‘Little or none’ to ‘the most’. The NRI is a reliable and valid way to assess relationship perceptions in youth24. In the current study, child and mother-report yielded good reliability (αmother = .82, αchild = .85). Answers from the mother report and the child report were averaged to get a total maternal support score. Because mother- and child-report total scores correlated .36 (p < .001), these scores could be averaged to obtain a more parsimoneous, multi-informant maternal support score which is important to minimize social desirability-related response bias effects.
Psychopathology
Depressive Symptoms were measured with the Child Depression Inventory (Kovacs, 1985), a 27-item self-report questionnaire administered to the child. It measures the cognitive, affective, and behavioral symptoms of depression. Items are scored from 0 to 2, with higher scores indicating greater symptom severity. Youth are asked to circle a statement that describes him/her best. For instance, one item is ‘I am sad once in a while (0), I am sad many times (1), I am sad all the time (2)’. In the current study, reliability was good (αbaseline = .82, α18 months follow up = .91).
Externalizing Problems were measured with the externalizing factor from the Child Behavior Checklist (Achenbach, 2009). Using a 3-point scale ranging from 0 (not true ) to 2 (very true or often true ), mothers were asked how often their child showed each problem behavior. An externalizing score was computed by summing the scores on the Aggressive Behavior (19 items) and Destructive Behavior (11 items) scales. In the current study, reliability was good (α = .91).
Procedure
Data collection was conducted as part of the larger GEM study (Hankin et al., 2015). The sample for the methylation analyses was collected at baseline (Wave 1) together with baseline ECR-RS, NRI, CDI, and CBCL. The ALEQ was administered at baseline and every 3 months up to 18 months (Wave 2). At Wave 2, ECR-RS, and CDI were administered. The YLSI data that we used in the current study was collected at Wave 2 so that chronic stress in the various domains covers the time interval from Wave 1 to 18 months later at Wave 2. The caretaker and youth visited the laboratory for an in-person, in-depth assessment at baseline and then at 18 month follow-ups. Caretakers provided informed written consent for their child’s participation; youth provided written assent. The institutional review boards at both the University of Denver and Rutgers University approved all procedures. Both youth and the caretaker were compensated monetarily for their participation. The authors have no conflict of interest to declare.
Results
Table 2 provides the correlations and descriptives for all study variables. To test our hypothesis, we conducted four regression analyses to predict attachment insecurity at 18 months after baseline as the dependent variable, after controlling for baseline attachment to enable prediction of prospective change in individual differences in attachment insecurity over time. For two analyses, avoidant attachment served as the dependent measure, whereas anxious attachment was the outcome in the other two analyses. The different stress measures (YLSI and ALEQ) were included as separate measures of stress in different analyses. Each analysis tested the 3-way interaction of Stress X Maternal Support X NR3C1 methylation to predict longitudinal change in individual differences in attachment insecurity.
Table 2.
Correlations and Descriptives
| Measure | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
|---|---|---|---|---|---|---|---|---|---|---|
| 1. Gender | 1 | |||||||||
| 2. Age | −.017 | 1 | ||||||||
| 3. NR3C1 methylation | .039 | −.092* | 1 | |||||||
| 4. Maternal support | .132** | −.168* | .030 | 1 | ||||||
| 5. Avoidance Base line | −.095* | .234** | −.015 | −380** | 1 | |||||
| 6. Anxiety Base line | −.023 | .264** | −.015 | −.250** | .169** | 1 | ||||
| 7. Avoidance 18 months | −.035 | .136** | −0.15 | −.596* | .254** | .169** | 1 | |||
| 8. Anxiety 18 months | −.067 | −.065 | .054 | .411** | .169** | .254** | .367** | 1 | ||
| 9. Longitudinal Stress | −.106 | .418** | −.024 | −.175 | .181* | .231* | .213** | .119* | 1 | |
| 10. Chronic Stress Severity | −.044 | .224** | −102* | −116* | .136** | .178** | .162** | .155** | .402** | 1 |
| M | 1.57 | 11.78 | .16 | 29.96 | 16.38 | 6.62 | 17.09 | 6.21 | 56.62 | 1.57 |
| SD | 0.50 | 02.40 | .53 | 05.43 | 07.60 | 4.37 | 08.52 | 3.80 | 11.57 | 0.45 |
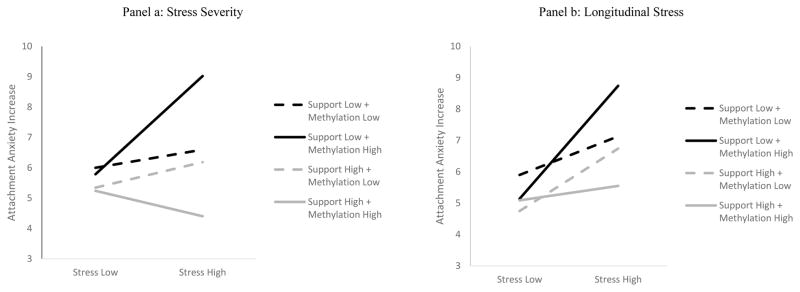
For avoidant attachment1, none of the three-way interaction effects reached significance (chronic stress severity via YLSI: t(471) = −.437, p = .662; longitudinal stress exposure via ALEQ: t(487) = −.781, p = .435). For anxious attachment, we found that the 3-way interactions were significant2. The pattern of results for this 3-way interaction predicting change in anxious attachment levels was similar for both stress measures (YLSI and ALEQ).
For the analysis that used YLSI chronic stress severity as moderator (Table 3, panel a, Figure 2, panel a) results show a significant three way interaction (f2 = .02). Slope analyses show that children with high levels of NR3C1 methylation and low levels of maternal support at baseline reported higher levels of anxious attachment after 18 months in the context of higher levels of stress severity t(470) = 4.32, p < .0001. In planned follow-up analyses, a Johnson-Neyman analysis determined the threshold level of stress above which the maternal support X NR3C1 methylation interaction was significant in this sample. Below that threshold, the interaction was not significant. Above that threshold, maternal support predicted an increase in anxious attachment in youth with high levels of NR3C1 methylation: t(305) = −3.886, p = .0001. Similarly, the analysis that used ALEQ longitudinal stress exposure over 18 months as moderator (Table 3, panel b, Figure 2, panel b) shows a significant three way interaction (f2 = .02). Slope analyses show that youth with high levels of NR3C1 methylation and low levels of maternal support at baseline exhibited higher levels of anxious attachment in the context of higher stress levels t(486) = −4.594, p < .0001. Follow-up analysis showed that low maternal support was only linked with anxious attachment increase in children with high levels of NR3C1 methylation among youth with stress levels above the threshold determined by the Johnson-Neyman analysis in this sample: t(164) = −2.50, p = .01. Among youth with lower stress levels, the interaction was not significant. The results were maintained after controlling for several potential covariates, including gender, age, baseline depressive symptoms, change in depressive symptoms from baseline to 18 months, baseline child externalizing behavior problems and baseline avoidant attachment (Supplementary Table 1).
Table 3.
Multiple Regression Analyses Testing the Stress X Maternal Support X NR3C1 Methylation Interaction to Predict Increased Anxious Attachment 18 Months after Baseline
| Panel a
|
Panel b | |||
|---|---|---|---|---|
|
| ||||
| Stress Severity | Longitudinal Stress | |||
|
|
|
|||
| β | ΔR2 | β | ΔR2 | |
| Step 1 | .06*** | .06*** | ||
| Baseline Anxious Attachment | .25*** | .25*** | ||
| Age | .04 | .04 | ||
| Gender | −.00 | −.00 | ||
|
| ||||
| Step 2 | .06*** | .10*** | ||
| Baseline Anxious Attachment | .16*** | .11* | ||
| Age | −.02 | −.13 | ||
| Gender | .03 | −.03 | ||
| Stress | .14** | .31*** | ||
| Maternal Support | −.21*** | −.18*** | ||
| NR3C1 Methylation | .03 | .02 | ||
|
| ||||
| Step 3 | .02** | .01 | ||
| Baseline Anxious Attachment | .16** | .11* | ||
| Age | −.02 | −.13** | ||
| Gender | .03 | −.03 | ||
| Stress | .14** | .30*** | ||
| Maternal Support | −.20*** | −.17*** | ||
| NR3C1 Methylation | .06 | .04 | ||
| Stress X Maternal Support | −.12** | −.07 | ||
| Stress X NR3C1 Methylation | .06 | .06 | ||
| NR3C1 Methylation X Maternal Support | −.08 | −.05 | ||
|
| ||||
| Step 4 | .01** | .01* | ||
| Baseline Anxious Attachment | .15** | .11* | ||
| Age | −.03 | −.13** | ||
| Gender | .04 | −.03 | ||
| Stress | .14** | .30*** | ||
| Maternal Support | −.22*** | −.17*** | ||
| NR3C1 Methylation | .01 | −.01 | ||
| Stress X Maternal Support | −.14** | −.10* | ||
| Stress X NR3C1 Methylation | .03 | .02 | ||
| NR3C1 Methylation X Maternal Support | −.16** | −.06 | ||
| Stress X NR3C1 Methylation X Maternal Support | −.15** | −.12* | ||
Note:
p < .05;
p < .01;
p < .001
Figure 2.
Stress X Maternal Support X NR3C1 Methylation on Increased Attachment Anxiety
Discussion
Overall, results showed that individual differences in insecure attachment levels at 18 months after baseline were predicted by high levels of NR3C1 methylation, low maternal support, and high chronic stress. This effect was robust over stress measures and could not be explained by youth’s age, gender, or youths’ psychopathology levels. Our findings corroborate with past animal work showing that epigenetic processes, specifically methylation-related suppression of NR3C1, are critically involved in the relationships among maternal care and rat pup negative outcomes (Weaver, 2009). These novel longitudinal findings with human children importantly replicate these animal effects. They also extend epigenetic research by showing effects on more interpersonal domains of human development, such as attachment which is essential for human development including psychosocial, psychopathological, and medical outcomes across the lifespan. Finally, our findings expand attachment theory by pointing to a more complex nature-nurture interplay involving epigenetic processes for attachment development.
These effects were unique for anxious attachment. This could be because anxious attachment is typically linked with an elevated need for parental support (Cassidy, 1994), and because high physiological stress-reactivity exacerbates the maladaptive effect of anxious attachment development (Bosmans et al., 2016). Consequently, children with high NR3C1 methylation could be more likely to experience the negative effects of insufficient maternal care, a learning experience that further contributes to anxious attachment development. The question could be raised whether attachment changes in this age-group are developmentally relevant or, rather, represent an innocuous phenomenon. To our knowledge, no research exists on whether attachment instability in this age-group is a risk factor. However, we used the current dataset to test the extent to which such changes are developmentally relevant. We found that increases in attachment anxiety uniquely predict increases in depressive symptoms (β = .37, p < .001) over and above baseline depressive symptoms (β = .45, p < .001) and baseline attachment anxiety (β = .21, p < .001; total model R2 = .30). This suggests that the current findings are important as the 3-way interaction of high NR3C1 methylation, low maternal support, and high chronic stress predicting change in anxious attachment has relevance as predicting this pattern of unstable anxious attachment is associated with later maladjustment and negative outcomes, such as elevated depressive symptoms.
Avoidant attachment development was not affected by NR3C1 methylation. One reason could be that attachment avoidance was under-reported in the current sample. The fact that we used self-report to assess avoidant attachment is a limitation of this study. Psychometric concerns have been raised about avoidantly attached individuals’ inclination to dismiss attachment needs (Borelli et al., 2016; Cassidy & Kobak, 1988; Main, Kaplan, & Cassidy, 1985). Consequently, it will be important in future research to use alternative measures of attachment anxiety and avoidance (e.g., the Child Attachment Interview, Borelli et al., 2016) to see whether the same pattern of results emerge. If the effects replicate, one possible explanation might be that avoidant attachment is affected by the methylation of other care-related genes, such as the Oxytocin Receptor Gene (Taylor, 2006).
These study findings need to be interpreted with various limitations in mind. First, the child was the primary informant for both the outcome measure and some predictor variables (attachment, stress, and maternal support). We sought to minimize mono-informant effects by using a mother-child composite score to evaluate maternal support. Moreover, the main variable of interest, NR3C1 methylation is not vulnerable to response bias. Also, the use of YLSI to assess chronic stress ensures a more objective indication of children’s stress levels because YLSI ratings are derived from independent, blind raters’ codes of the degree of children’s chronic stress levels. This chronic stress interview method is deemed to be optimal for measuring stress exposure (Harkness & Monroe, 2016). Finally, the fact that the findings replicated with both self-report of stress and the stress interview suggests that the stress effects in the three-way interactions cannot be merely explained as reflecting informant bias. However, for future research, it would be important to include other measurement strategies like observation to assess some of the variables. Second, it is a limitation that not all variables were measured at both measurement waves. Most importantly, NR3C1 methylation was only measured at baseline. Because too little is known about the plasticity and timing of epigenetic change (Talens et al., 2010), one cannot draw conclusions about baseline NR3C1 methylation scores as a causal factor in anxious attachment development. Notwithstanding this limitation, it is a strength of the current study that we did measure attachment at both waves. This allowed us to examine prospective change in attachment dimensions as a function of the main predictor variables that were assessed earlier in time.
Additionally, while NR3C1 methylation and maternal support were measured at a specific time point at baseline, stress was not measured at baseline, but either during the follow-up period (ALEQ) or at follow up (YSLI) to asses stress between the measurement moments. Although it is a limitation that the predictors were not all measured at the same time point or duration, we believe that the timing of these assessments may aid in the interpretation of these findings. Because attachment development results from learning experiences, it might have been an advantage that we measured the extent to which stress occurred during the follow-up period instead of before that period. This ensured that the degree of chronic stress that we assessed in the 18-month follow-up period was relevant for the learning experiences that affected attachment development in that period. Moreover, Cole and Maxwell (2003) made the point that longitudinal studies in which variables are measured at different time points are of high quality because this reduces the impact of reporter bias and overlap. Third, it is a limitation that we only investigated the role of methylation of a single, theory-driven, candidate gene. This is a typical strategy in contemporary attachment research (see also Borelli et al., 2016), but there is an increasing awareness that the development of complex human behavior like attachment is most associated with many genes, likely of small individual effects (Bakermans-Kranenburg & van IJzendoorn, 2016), so future research should look at methylation of a broader set of genes. Still, as this is the first of its kind prospective study with humans to investigate longitudinal prediction of attachment styles from a biologically plausible and theoretically defensible candidate gene for methylation, along with maternal support and chronic stress, the results provide initial preliminary evidence for future research to replicate the findings, ideally with a broader array of methylation patterns from multiple genes relevant for attachment development.
These findings are important because they provide the first prospective support for the growing idea that epigenetic mechanisms might be implicated in attachment development (Bakermans-Kranenburg & van IJzendoorn, 2016). This idea is important in light of different meta-analyses suggesting that parenting alone insufficiently explains attachment development. According to traditional attachment theory, attachment development refers to the internalization of caregiving experiences (Bowlby, 1969). Whether children become more or less securely attached has traditionally been considered to only result from differences in quality of caregiving and not from differences related to the biology of the child (Mangelsdorf & Frosch, 2000). Contradicting this theory, effect sizes of the association between parenting and attachment development appear to be rather modest (De Wolff & van IJzendoorn, 1997; Verhage et al., 2016). The current findings are more in line with recent attachment research suggesting that the association between parenting and children’s attachment development is conditional upon children’s susceptibility to environmental effects (e.g., Belsky, 1997; Cassidy et al., 2011). NR3C1 methylation might be a comparable susceptibility factor. In the current study, high NR3C1 methylation children with less supportive mothers showed the highest increase in attachment anxiety over time when they were exposed to distress. This might be because these children experience more distress due to the elevated cortisol response that is associated with NR3C1 methylation (Dickerson & Kemeny, 2004), and because increased levels of distress elicit more need for maternal support. As a consequence, these children might have experienced more negative effects of absent supportive care during distress which resulted in insecure attachment development. Further supporting this susceptibility interpretation, high levels of maternal support were linked with less attachment anxiety development when children with high levels of NR3C1 methylation were exposed to stress. This suggested a buffering effect of maternal support. In sum, this suggests that NR3C1 methylation might be a biological marker of which children might be most susceptible to the impact of parental responses to child stress on the development of the perceived quality of the attachment relationship.
As such, the current study’s finding might prove important because it suggests that studying epigenetic processes might improve our understanding of the mechanisms underlying attachment development. Apart from a need for replication, future research should focus on attachment and methylation development from younger ages in an attempt to distinguish levels of methylation that can be attributed to lack of maternal support during stress (e.g., Fish et al., 2014), attributed to diets high in methyl-donating nutrients (McGowan et al., 2008), and/or attributed to exposure to bacteria (Takahashi, 2014). Such research might be helpful to more accurately describe the processes through which parenting practices are linked with attachment development. This way, epigenetic research might prove valuable to help narrow the well-known gap in the transgenerational transmission of insecure attachment patterns (Verhage et al., 2016).
In sum, the current study showed that children’s anxious attachment levels can be prospectively predicted from an interaction between NR3C1 methylation, children’s stress exposure, and maternal support. More specifically, lower levels of maternal support were only linked to higher levels of attachment anxiety over time, when children were exposed to greater stress and had higher levels of NR3C1 methylation. The interaction did not significantly predict avoidant attachment. These results illustrate that investigating epigenetic processes can critically expand our understanding of intensively investigated, critical domains of human development, such as attachment, revealing explanations for inconsistencies that have puzzled clinicians for decades.
Supplementary Material
Acknowledgments
The content is solely the responsibility of the authors and does not necessarily represent the official views of the funders. We thank the many members of the GEM lab for assisting with data collection. We thank Andrew Smolen, Ph.D., for his invaluable assistance and discussions regarding genotyping and methylation assays. This research has been supported by NIMH grant R01MH077178 assigned to J.F. Youny, NIMH grants R01MH077195, R01MH105501, and R21MH102210 to B.L. Hankin, and by grants G.0934.12 and G.0774.15 of the Research Foundation Flanders (FWO), and grants C14/16/040, OT/12/043 and CREA/12/004 from the Research Fund KU Leuven, Belgium awarded to G. Bosmans,
Footnotes
All analyses were repeated after the imputation of missing data with expectation maximization. Because this did not change the effects, we decided to only report the most conservative test. Results of these analyses can be obtained from the authors.
Results were not dependent on who reported on maternal support (child versus mother). Detailed results can be obtained from the authors.
References
- Achenbach TM. The Achenbach System of Empirically Based Assessemnt (ASEBA): Development, Findings, Theory, and Applications. Burlington, VT: University of Vermont Research Center for Children, Youth, & Families; 2009. [Google Scholar]
- Bakermans-Kranenburg MJ, van IJzendoorn MH. Attachment, Parenting, and Genetics. In: Cassidy J, Shaver PR, editors. Handbook of attachment: Theory, research, and clinical applications. 3. New York: Guilford Press; 2016. pp. 155–180. [Google Scholar]
- Belsky J. Variation in susceptibility to rearing influences: An evolutionary argument. Psychological Inquiry. 1997a;8:182–186. [Google Scholar]
- Benson MJ, McWey LM, Ross JJ. Parental attachment and peer relations in adolescence: A Meta-Analysis. Research in Human Development. 2006;3:33–43. doi: 10.1207/s15427617rhd0301_4. [DOI] [Google Scholar]
- Borelli JL, Smiley PA, Rasmussen HF, *, Gómez A, *, Seaman LC, *, Nurmi EL. Interactive Effects of Attachment and FKBP5 Genotype on School-aged Children’s Emotion Regulation and Depressive Symptoms. Behavioural Brain Research. doi: 10.1016/j.bbr.2016.07.035. in press. [DOI] [PubMed] [Google Scholar]
- Borelli JL, Somers J, West JL, Coffey JK, De Los Reyes A, Shmueli-Goetz Y. Associations Between Attachment Narratives and Self-Report Measures of Attachment in Middle Childhood: Extending Evidence for the Validity of the Child Attachment Interview. Journal of Child and Family Studies. 2016 doi: 10.1007/s10826-015-0310-8. [DOI] [Google Scholar]
- Bosmans G. Cognitive behavior therapy for children and adolescents: Can attachment theory contribute to its efficacy? Clinical Child and Family Psychology Review. 2016;19:310–328. doi: 10.1007/s10567-016-0212-3. [DOI] [PubMed] [Google Scholar]
- Bosmans G, De Smedt B. Insecure attachment is associated with math anxiety in middle childhood. Frontiers in Psychology. 2015;6:1596. doi: 10.3389/fpsyg.2015.01596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosmans G, Kerns KA. Attachment in middle childhood: Progress and prospects. In: Bosmans G, Kerns KA, editors. Attachment in middle childhood: Theoretical advances and new directions in an emerging field. Vol. 148. New Directions for Child and Adolescent Development; 2015. pp. 1–14. [DOI] [PubMed] [Google Scholar]
- Bosmans G, Braet C, Heylen J, De Raedt R. Children’s Attentional Processing of Mother and Proximity Seeking. PLoS One. 2015;10(4) doi: 10.1371/journal.pone.0124038. art.nr. e0124038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosmans G, Poiana N, Van Leeuwen K, Dujardin A, De Winter S, Finet C, Heylen J, Van de Walle M. Attachment and depressive symptoms in middle childhood : The moderating role of skin conductance level variability. Journal of Social and Personal Relationships. doi: 10.1177/0265407515618278. in press. [DOI] [Google Scholar]
- Bowlby J. Attachment and loss. New York: Basic Books; 1969. [Google Scholar]
- Brakensiek K, Wingen LU, Langer F, Kreipe H, Lehmann U. Quantitative high-resolution CpG island mapping with PyrosequencingTM reveals disease-specific Methylation patterns of the CDKN2B gene in Myelodysplastic syndrome and myeloid leukemia. Clinical Chemistry. 2007;53:17–23. doi: 10.1373/clinchem.2007.072629. [DOI] [PubMed] [Google Scholar]
- Brumariu LE. Parent-child attachment and emotion regulation. New Directions in Child and Adolescent Development. 2015;148:31–45. doi: 10.1002/cad.20098. [DOI] [PubMed] [Google Scholar]
- Calvete E. Integrating sociotropy, negative inferences and social stressors as explanations for the development of depression in adolescence: interactive and mediational mechanisms. Cognitive Therapy and Research. 2011;3:477–490. [Google Scholar]
- Cassidy J. Emotion regulation: Influences of attachment relationships. Monographs for the Society for Research on Child Development. 1994;59:228–249. doi: 10.1111/j.15405834. [DOI] [PubMed] [Google Scholar]
- Cassidy J, Kobak R. Avoidance and its relation to other defensive processes. In: Belsky J, Nezworski T, editors. Clinical implications of attachment. Hillsdale, NJ: Erlbaum; 1988. pp. 300–323. [Google Scholar]
- Cassidy J, Woodhouse SS, Sherman LJ, Stupica B, Lejuez CW. Enhancing infant attachment security: an examination of treatment efficacy and differential susceptibility. Development and Psychopathology. 2011;23:131–148. doi: 10.1017/S0954579410000696. [DOI] [PubMed] [Google Scholar]
- Conley CS, Rudolph KD. The emerging sex difference in adolescent depression: Interacting contributions of puberty and peer stress. Developmental Psychopathology. 2009;21:593–620. doi: 10.1017/S0954579409000327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Wolff MS, van IJzendoorn MH. Sensitivity and attachment: A meta-analysis on parental antecedents of infant attachment. Child Development. 1997;68:571–591. [PubMed] [Google Scholar]
- Dickerson SS, Kemeny ME. Acute stressors and cortisol responses: A theoretical integration and synthesis of laboratory research. Psychological Bulletin. 2004;130:355–391. doi: 10.1037/0033-2909.130.3.355. [DOI] [PubMed] [Google Scholar]
- Dujardin A, Santens T, Braet C, De Raedt R, Vos P, Maes B, Bosmans G. Middle childhood support-seeking behavior during stress: links with self-reported attachment and future depressive symptoms. Child Development. 2016;87:326–340. doi: 10.1111/cdev.12491. [DOI] [PubMed] [Google Scholar]
- England R, Pettersson M. Pyro Q-CpG™: Quantitative analysis of methylation in multiple CpG sites by Pyrosequencing®. Nature Methods. 2005;2:1–2. doi: 10.1038/nmeth800. [DOI] [Google Scholar]
- Fish EW, Shahrokh D, Bagot R, Caldji C, Bredy T, Szyf M, Meaney MJ. Epigenetic programming of stress responses through variations in maternal care. Annals of the New York Academy of Science. 2004;1036:167– 180. doi: 10.1196/annals.1330.011. [DOI] [PubMed] [Google Scholar]
- Fraley RC, Heffernan ME, Vicary AM, Brumbaugh CC. The experiences in close relationships-Relationship structures questionnaire: A method for assessing attachment orientations across relationships. Psychological Assessessment. 2011;23:615–25. doi: 10.1037/a0022898. [DOI] [PubMed] [Google Scholar]
- Furman W, Buhrmester D. Children’s perceptions of the personal relationships in their social networks. Developmental Psychology. 1985;21:1016–24. doi: 10.1037/0012-1649.21.6.1016. [DOI] [Google Scholar]
- Hammen C. Depression and stressful environments: Identifying gaps in conceptualization and measurement. Anxiety, Stress, & Coping. 2016;29:335–351. doi: 10.1080/10615806.2015.1134788. [DOI] [PubMed] [Google Scholar]
- Harkness KL, Monroe SM. The assessment and measurement of adult life stress: Basic premises, operational principles, and design requirements. Journal of Abnormal Psychology. 2016;125:727–745. doi: 10.1037/abn0000178. [DOI] [PubMed] [Google Scholar]
- Hankin BL. Stability of cognitive vulnerabilities to depression: A short-term prospective multiwave study. Journal of Abnormal Psychology. 2008;117:324–33. doi: 10.1037/0021-843X.117.2.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hankin BL, Abramson LY. Measuring cognitive vulnerability to depression in adolescence: Reliability, validity, and gender differences. Journal of Clinical Child & Adolescent Psychology. 2002;31:491–504. doi: 10.1207/S15374424JCCP3104_8. [DOI] [PubMed] [Google Scholar]
- Hankin BL, Stone LB, Wright PA. Co-rumination, interpersonal stress generation, and internalizing symptoms: Accumulating effects and transactional influences in a multi-wave study of adolescents. Development and Psychopathology. 2010;22:217–235. doi: 10.1017/S0954579409990368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hankin BL, Young JF, Abela JRZ, Smolen A, Jenness JL, Gulley LD, Technow JR, Gottlieb AB, Cohen JR, Oppenheimer CW. Depression from childhood into late adolescence: Influence of gender, development, genetic susceptibility, and peer stress. Journal of Abnormal Psychology. 2015;124:803–16. doi: 10.1037/abn0000089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heard E, Martienssen RA. Transgenerational epigenetic inheritance: Myths and mechanisms. Cell. 2014;157:95–209. doi: 10.1016/j.cell.2014.02.045. Doi: http://dx.doi.org/10.1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klengel T, Pape J, Binder EB, Mehta D. The role of DNA methylation in stress-related psychiatric disorders. Neuropharmacology. 2014;80:115–132. doi: 10.1016/j.neuropharm.2014.01.013. [DOI] [PubMed] [Google Scholar]
- Kovacs M. The Children’s Depression Inventory (CDI) Psychopharmacology Bulletin. 1985;21:995–8. [PubMed] [Google Scholar]
- Liu T, Zhang X, So C, Wang S, Wang P, Yan L, et al. Regulation of Cdx2 expression by promoter methylation, and effects of Cdx2 transfection on morphology and gene expression of human esophageal epithelial cells. Carcinogenesis. 2007;28:488–96. doi: 10.1093/carcin/bgl176. [DOI] [PubMed] [Google Scholar]
- Madigan S, Brumariu LE, Villani V, Atkinson L, Lyons-Ruth K. Representational and questionnaire measures of attachment: A meta-analysis of relations to child internalizing and externalizing problems. Psychological Bulletin. 2016;142:367–99. doi: 10.1037/bul0000029. [DOI] [PubMed] [Google Scholar]
- Mangelsdorf SC, Frosch CA. Temperament and attachment: One construct or two? In: Reese HW, editor. Advances in child development and behavior. Vol. 57. San Diego, CA: Academic Press; 2000. pp. 181–220. [DOI] [PubMed] [Google Scholar]
- McGowan PO, Meaney MJ, Szyf M. Diet and the epigenetic (re)programming of phenotypic differences in behavior. Brain Research. 2008;1237:12–24. doi: 10.1016/j.brainres.2008.07.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesquita AR, Soares I, Roisman GI, van IJzendoorn M, Bakermans-Kranenburg M, Luijk M, et al. Predicting children’s attachment behaviors from the interaction between oxytocin and glucocorticoid receptors polymorphisms. Psychiatry Research. 2013;210:1322–3. doi: 10.1016/j.psychres.2013.08.005. [DOI] [PubMed] [Google Scholar]
- Mikulincer M, Shaver PR. Attachment in adulthood: Structure, dynamics, and change. 2. New York: Guilford Press; 2016. [Google Scholar]
- Roisman GI, Fraley RC. A behavior-genetic study of parenting quality, infant attachment security, and their covariation in a nationally representative sample. Developmental Psychology. 2008;44:831–9. doi: 10.1037/0012-1649.44.3.831. [DOI] [PubMed] [Google Scholar]
- Rudolph KD, Flynn M. Childhood adversity and youth depression: Influence of gender and pubertal status. Developmental Psychopathology. 2007;19:497–521. doi: 10.1017/S0954579407070241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudolph KD, Hammen C. Age and gender as determinants of stress exposure, generation, and reactions in youngsters: A Transactional perspective. Child Development. 1999;70:660–77. doi: 10.1111/1467-8624.00048. [DOI] [PubMed] [Google Scholar]
- Takahashi K. Influence of bacteria on epigenetic gene control. Cellular and Molecular Life Sciences. 2014;71:1045–2054. doi: 10.1007/s00018-013-1487-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talens RP, et al. Variation, patterns, and temporal stability of DNA methylation: considerations for epigenetic epidemiology. The FASEB Journal. 2010;24:3135–3144. doi: 10.1096/fj.09-150490. [DOI] [PubMed] [Google Scholar]
- Taylor SE. Tend and Befriend: Biobehavioral bases of affiliation under stress. Current Directions in Psychology. 2006;15:273–7. doi: 10.1111/j.1467-8721.2006.00451.x. [DOI] [Google Scholar]
- Turecki G, Meaney MJ. Effects of the social environment and stress on Glucocorticoid receptor Gene Methylation: A systematic review. Biological Psychiatry. 2016;79:87–96. doi: 10.1016/j.biopsych.2014.11.022. DOI: http://dx.doi.org/10.1016/j.biopsych.2014.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoolmiller M. Synergistic interaction of child manageability problems and parent-discipline tactics in predicting future growth in externalizing behavior for boys. Developmental Psychology. 2001;37:814–825. doi: 10.1037//0012-1649.37.6.814. [DOI] [PubMed] [Google Scholar]
- Verhage ML, Schuengel C, Madigan S, Fearon RMP, Oosterman M, Cassibba R, Bakermans-Kranenburg MJ, van IJzendoorn MH. Narrowing the transmission gap: A synthesis of three decades of research on intergenerational transmission of attachment. Psychological Bulletin. 2016;142:337–366. doi: 10.1037/bul0000038. Doi: http://dx.doi.org/10.1037/bul0000038. [DOI] [PubMed] [Google Scholar]
- Weaver ICG. Epigenetic effects of glucocorticoids. Seminars in Fetal and Neonatal Medicine. 2009;14:143–50. doi: 10.1016/j.siny.2008.12.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.