Abstract
Carbon-fiber electrodes (CFEs) are the gold standard for quantifying the release of oxidizable neurotransmitters from single vesicles and single cells. Over the last 15 years microfabricated devices have emerged as alternatives to CFEs that offer the possibility of higher throughput, subcellular spatial resolution of exocytosis, and integration with other techniques for probing exocytosis including microfluidic cell handling and solution exchange, optical imaging and stimulation, and electrophysiological recording and stimulation. Here we review progress in developing electrochemical electrode devices capable of resolving quantal exocytosis that are fabricated using photolithography.
1. Introduction
Quantal release of neurotransmitters is a fundamental step of synaptic transmission. Neurons and neuroendocrine cells communicate with their target cells by releasing “quanta” of neurotransmitters as individual intracellular vesicles fuse with the plasma membrane and release their contents into the extracellular space (exocytosis). Electrochemical detection of exocytosis has been widely investigated since the early 1990s, taking advantage of the oxidation reaction that occurs at the surface of polarized carbon fiber microelectrodes (CFEs) (19, 88, 89). In constant-potential amperometry, oxidation of electro-active molecules occurs rapidly following diffusion of the molecules to the electrode surface, therefore each release event (quantum) produces a pulse or spike of amperometric current if the electrode is nearby the release site on the cell surface. CFEs have been proven to be excellent tools for investigating the quantal nature of exocytosis, exhibiting excellent signal-to-noise ratio and fast response time and are therefore considered as the gold standard for measurements of quantal exocytosis of electroactive molecules (19).
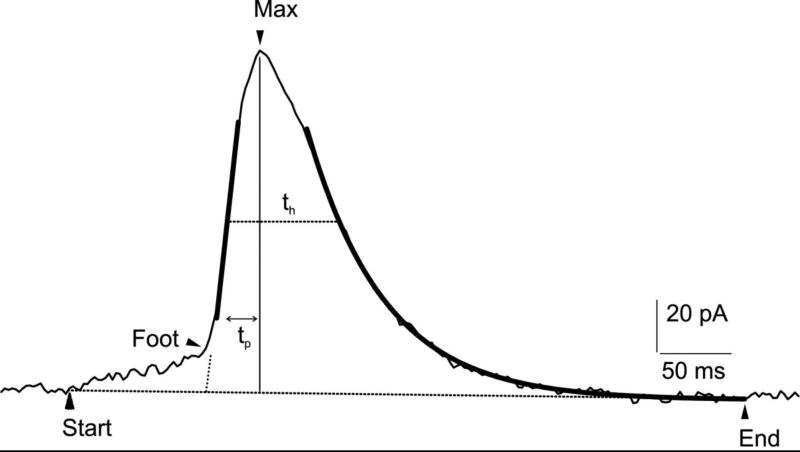
Quantal electrochemical measurements reveal at least three distinct stages within the exocytotic event: a small increase in current amplitude, corresponding to the catecholamine efflux through the fusion pore (foot) (1, 19); a rapid rise to a maximum spike amplitude value, associated to the increased catecholamine flux during the full pore expansion; a final exponentially descending phase, consistent with chemical dissociation of the intravesicular matrix or gel and the declining content of the vesicle (71). Abrupt declines in current, presumably due to rapid closing of the efflux pathway before the vesicle is emptied, have also been reported (54, 83, 100). As shown in Fig 1, the following spike parameters are often quantified: i) amplitude and duration of the foot, interpreted as the slow leak of secreted molecules through the nanometer-sized fusion pore preceding complete dilation, ii) height of the spike, corresponding to the maximum oxidation current. This parameter decreases with increasing distance between the electrode and cell due to diffusional delay (39); iii) spike area, evaluated as the amount of catecholamines detected per release event (charge, Q) (71). For example, in bovine chromaffin cells it has been estimated that approximately 2–3 million molecules can be detected for each unitary event (19); iv) radius of the vesicle, estimated from Q1/3, assuming spherical vesicles storing a uniform concentration of molecules (13, 28, 88). Also, kinetic parameters of the exocytotic event can be quantified, such as time to maximum current (tp) and the half-time width of the spike (th) (12, 57, 73).
Fig 1.
Amperometric spike recorded from a bovine chromaffin cell using a carbon fiber electrode (CFE). The distinct phases of the exocytotic event (foot, rising phase, decaying phase) can be quantified, as detailed in the text. Arrows indicate the event duration (start, end) and the presence of the foot (oblique dashed line). Thick lines represent the ascending slope on the rising phase and spike exponential decay. max: indicates the maximum oxidation current. tp is the time to reach the spike maximum. Analysis performed using the software “Quanta Analysis”, by Eugene Mosharov (57).
Whereas CFEs are excellent tools to resolve and quantify quantal exocytosis, probe electrodes suffer from some limitations. CFE amperometry is a time-consuming process because the probe must be positioned to the surface of the cell using a micromanipulator under observation with a microscope. Experiments are performed from only one cell at a time whereas a large number of cells must be tested to determine if an experimental condition changes quantal parameters because of substantial cell-to-cell variability (22). Thus this approach is not practical for drug or toxicity screening. In addition, the sensing area of the carbon fiber tip (approximately 5 µm radius) limits both the spatial resolution of exocytosis and the fraction of the surface area of the cell where release is detected (16).
As described in this review, these limitations can be overcome using microelectrode arrays fabricated using photolithography. Microfabrication not only allow almost unlimited flexibility in economically patterning large numbers of electrodes with any desired dimensions, but also can take advantage of a wider choice of materials, both for working electrodes and insulation layers (5). Recording amplifiers can be integrated with the electrode arrays using CMOS technology to enable truly high throughput. The cell culture chamber can be integrated with the electrode arrays (23) and stimulating electrodes, microfluidic solution exchange, and electrophysiological measurements (14) can be included on the chips. Finally, transparent devices enable experiments that simply cannot be carried out using CFEs, particularly the combination of fluorescent imaging of the release site simultaneous with electrochemical recording of the released quantum (47, 56).
Guiding design principles of the array geometry, either selectively designed for the analysis of cell populations or else for subcellular mapping of exocytosis (see (4) for a recent review), should fulfill some requisites, such as keeping cells close to the electrodes (within several µm), in order to reduce the diffusion distance and allow released catecholamine molecules to reach the electrode surface within a short time interval (18, 72). In addition, sensing electrodes must be small (hundreds of µm2 or less) in order to have low enough noise to resolve quantal exocytosis (see section 6, (94)). Even smaller electrodes (~10 µm2 or less (35, 85)) are needed for sub-cellular localization of release (see section 9). Another design principle is that ideally each electrode only records from a single cell so that cell-to-cell heterogeneity is revealed. This is accomplished by making electrodes small or constraining cells so only one cell is trapped adjacent to an electrode (26, 51).
Parallel or multiplexed detection simultaneously performed by different working electrodes is suitable for automated multisite and non-invasive detection of quantal exocytosis, thus lowering the cost per measurement, and enabling drug screening as well as toxicity analyses (e.g., (24, 41)).
The scope of this review is limited to electrochemical devices designed for quantal release detection and fabricated by means of photolithography. We will first provide an overview of the steps required for microchips realization, including microfabrication and electrical connection. Subsequent sections describe the materials commonly used for the working electrodes and insulating layers, and an analysis of the main noise sources is presented. Cell localization to the electrodes is discussed as well as approaches for stimulating exocytosis on microchip devices. Finally, we will address multisite subcellular detection of exocytosis and the combination of optical and electrochemical measurements.
2. Sample microfabrication process
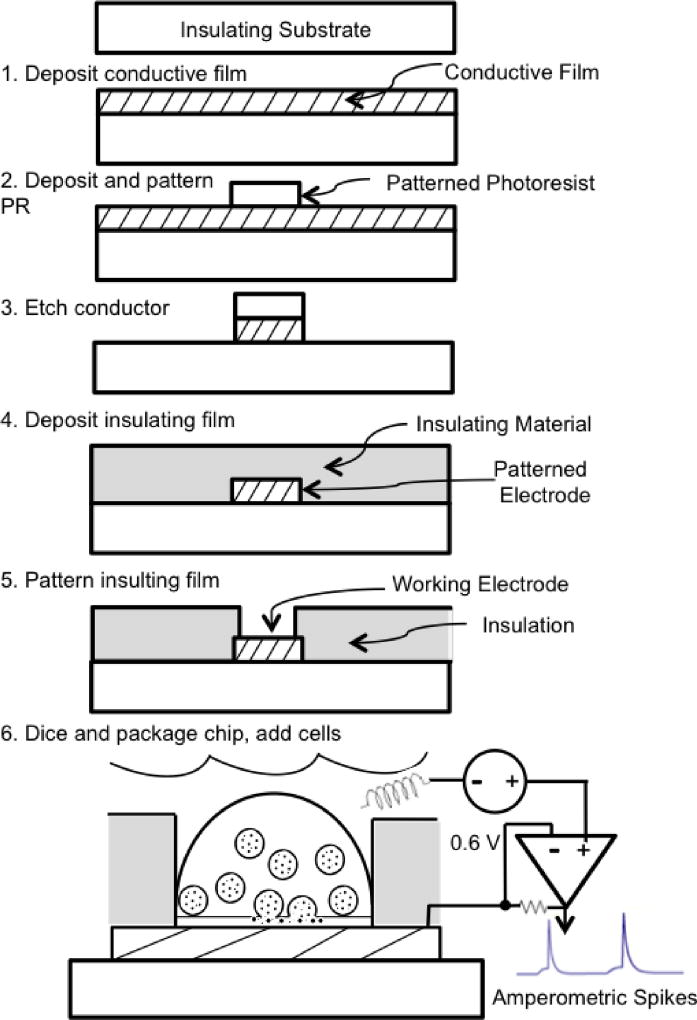
Fabrication of the electrode arrays is carried out using photolithography. Whereas the steps for fabricating microelectrode arrays vary considerably based on the type of array and the materials used, a sample process is illustrated in Fig 2 in order to orient those who are less familiar with microfabrication techniques.
A conductive film is deposited on an insulating substrate such as glass. In steps 2 and 3 the conductive film is patterned to serve as both the working electrodes and the conductive pathways (“wires”) to carry the current to pads around the perimeter of the chip that serve as the connection points to external amplifiers (also see Fig. 3).
A photoresist in liquid form is applied in a spin coating process followed by a baking step to result in a solid, light-sensitive film. The wafer is exposed to light through a mask to selectively illuminate portions of the photoresist. In the case of a negative-tone photoresist, the areas exposed to light are removed through a chemical development process.
This is followed by an etching step whereby the conductive film is removed in some areas, leaving behind only those regions protected by the overlying photoresist. The residual photoresist is then stripped using an organic solvent, completing the transfer of the pattern from the mask to the photoresist to the conductive film.
An insulating material is deposited on top of the patterned conductive film. Insulating the bulk of the conductive traces that contact the cell bath is necessary to decrease background noise.
Small openings in the insulating film are patterned using photolithographic processes similar to those of the preceding steps. These openings define the working electrodes for exocytosis measurements. The thick photoresist SU8 can be used as an insulating material that can be directly patterned using mask exposure and development. An opening in a thick insulating layer can also serve as a microwell to trap individual cells directly over the working electrode.
Wafers are diced into individual chips and packaged to make electrical connections to external amplifiers and to add a chamber to hold cells in a bath solution (Fig 3, described in further detail in the next section). Cells are seeded on the device and settle on the electrodes. A non-polarizable (e.g., Ag/AgCl) electrode makes contact with the bath solution to serve as the ground/reference. The voltage of the working electrode is held at a potential that is sufficient to oxidize catecholamines (typically 0.6 V relative to ground). An amperometric spike is recorded when the transmitter released from a vesicle is oxidized on the surface of the underlying electrode (Fig 2, bottom).
Fig 2.
Example photolithographic process for fabricating a microchip electrochemical microelectrode for measuring quantal exocytosis. Steps are described in the text.
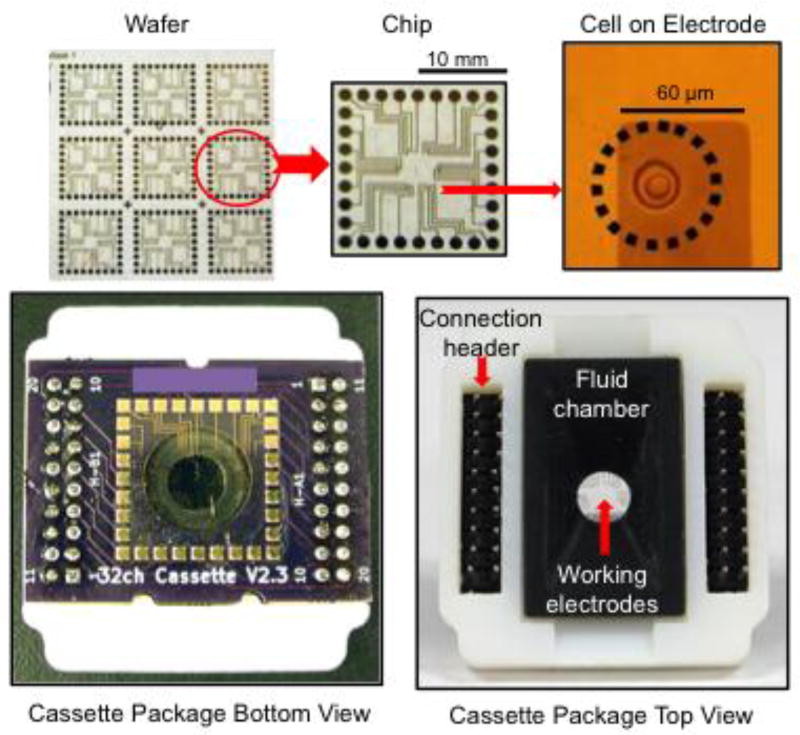
Fig 3.
Packaging of microchip devices is necessary to allow connection to amplifiers and addition of cells in solution. Top left: A wafer produced using photolighography is diced into individual chips (top middle) whereas the working electrodes that contact cells represent a small fraction of the chip typically in the middle (top right). Bottom left: The chip is electrically and mechanically connected to a PCB using conductive epoxy at each of the bonding pads located around the perimeter. A hole in the PCB allows the side of the chip with the electrodes to be exposed in a “flip chip” configuration. Bottom right: The PCB is attached to a plastic carrier that serves as the fluid chamber bonded to the chip surface through the hole in the PCB. Header pins are used to connect the PCB to the multi-channel amplifier socket.
3. Connecting/packaging microchip electrode arrays
Once electrode-array microchips are fabricated using photolithography, a significant task is to package the chips to enable cells in solution to be added and to support the acquisition of data using potentiostats and digitizers.
Electrical connection of microchips to external devices is generally carried out using conductive pads arranged around the perimeter of the chip. In the example depicted in Fig 3, the microchip pads are directly connected to complementary pads on a small PCB using a conductive epoxy. Wire bonding of microchip pads to a chip holder is also commonly used, e.g., (16). The PCB, in turn, has an array of terminal pins that conveniently plug into a socket array of a mult-channel potentiostat head stage to facilitate use and replacement of the disposable electrode array cassette. Since the microchip only has conductors on one side, direct bonding means the working electrodes face the PCB in a “flip-chip” configuration (35). Therefore the PCB has a cm-sized hole to allow access of cells and solution to the working electrodes. Since the bath solution must not contact the PCB nor the connection pads, some sort of waterproof material needs to be added to constitute a bath chamber. In the example depicted in Fig 3, a 3D-printed chamber sleeve is inserted through the hole in the PCB and bonded to the chip surface with a waterproof adhesive. This printed part also serves as the overall enclosing package for the cassette device.
It is relatively straightforward to pattern many thousands of electrodes on a cm2-sized microchip using photolithography, thus enabling massively parallel recordings of quantal exocytosis from many individual cells. However, a fundamental issue for multi-electrode arrays is that each electrode normally requires a dedicated amplifier to measure the signal from each cell independently. In the case when the potentiostats are external to the chip, there are practical limitations to the number of electrodes that can be recorded from due to the cost and complexity of developing instruments with many dozens of potentiostats as well as making all the connections through microchip bonding pads. Approaches to reduce the number of external connections and amplifiers include placing multiplexer electronics on the microchip (27), or addressing banks of electrodes isolated from each other in separate fluid compartments (95).
The ultimate solution to enable recording of quantal exocytosis from thousands of cells simultaneously is to integrate the potentiostats directly on the microchip and multiplex the data stream (6, 7, 45). For example, a CMOS device with a 10×10 array of electrodes with accompanying potentiostats was recently reported that is capable of recording quantal exocytosis (45). Each potentiostat takes up only ~ 500 µm2 of space on the chip (7), and the noise performance is comparable to a patch-clamp amplifier (6). CMOS devices are often fabricated using services such as the MOSIS Integrated Circuit Fabrication Service (Marina del Rey, CA) and then require post-processing steps to pattern electrochemical electrodes on the surface of the chip and to package the die into a recording chamber (6, 40, 45, 69).
It should be noted that the silicon substrate for CMOS devices is opaque, therefore these devices don’t support simultaneous fluorescence imaging of cells with an inverted microscope. Therefore lower-density electrode arrays on a transparent substrate are more convenient for single-cell experiments that combine fluorescent imaging with amperometry, whereas CMOS arrays will likely become the platform of choice for truly high-throughput measurement of changes in quantal parameters. In addition, high-density electrode/potentiostat arrays may be essential for reliable on-chip recording of quantal exocytosis of catecholamines from cultured neurons where the release sites may be dispersed at random locations across the chip surface. In this case a very large number of electrodes increases the odds that an electrode is in the immediate vicinity of a release site.
4. Electrode materials
A fundamental property of all electrochemical working electrodes are that they are “polarizable”, i.e., the voltage difference between the electrode and the solution drops within atomic distances from the surface of the electrode, creating an intense and highly localized electric field. A prerequisite for this is that background reactions at the electrode surface are minimal, as evidenced by a low background current, so that shot noise and iR voltage drops in the solution are low. So having a low background current over a wide window of applied potentials is a desirable property for electrode materials. In addition, for low-noise current measurements it is desirable that the admittance/capacitance of the electrode is low for the given area (94), which is achieved by making the electrode surface atomically smooth. A uniformly clean electrode surface is also important to ensure fast electron-transfer kinetics and good signal-to-noise ratio.
Other desirable properties of electrochemical working electrodes specific to cell measurements of quantal exocytosis are that the electrode material should not be toxic and ideally should promote cell attachment (described in section 7). Transparent electrode materials are advantageous for combining amperometry with fluorescence imaging. It is also desirable that the electrode material is not costly to fabricate and is robust and cleanable to support multiple uses.
To meet these requirements, a number of working electrode materials have been tested for detecting quantal exocytosis including noble metals (platinum, gold), indium tin oxide (ITO), and carbon-based materials (boron-doped diamond (BDD), diamond-like carbon (DLC), carbon nanotubes (CNTs) and conductive polymers). We will briefly describe how electrodes are tested and then summarize results relevant to measurements of quantal exocytosis.
Testing electrodes using cyclic voltammetry
Directly testing the oxidization of catecholamines on an electrode is complicated because catecholamines undergo cyclization reactions following oxidation and can passivate the electrode upon polymerization on the surface. Therefore alternative analytes are typically used for electrode tests. For example, the test analyte ferricyanide (Fe(CN)6) is easily reduced to ferrocyanide to form a readily reversible, well-behaved redox couple. Cyclic voltammetry is commonly used, whereby the applied potential is ramped between two limiting values while the current is recorded (Fig 4A). As the potential is ramped in the negative direction, a clean electrode with fast electron-transfer kinetics will show a steep transition at ~0.2 V (relative to a Ag/AgCl reference electrode) as reduction of ferricyanide occurs. The current peaks, then declines in magnitude as the ferricyanide near the surface of the electrode becomes depleted and the current reaches a quasi-steady-state value (Ilim) limited by the rate of diffusion of the analyte to the electrode surface. Ilim can be used to estimate the effective size of the working electrode. For a disk electrode on an insulated plane, the diffusion-limited current is given by:
where F is Faraday’s constant, D is the diffusion coefficient for the analyte, C is the concentration of the test analyte and r is the radius of the disk electrode. Thus measurement of the limiting current can be used to determine the “effective” electrode radius that is compared to the expected working electrode radius patterned using photolithography. Fig. 4A presents sample cyclic voltammograms for 20 µm-diameter microchip electrodes fabricated from ITO, Au and DLC:N. Electron-transfer kinetics are comparable among the films and generally faster than for carbon-fiber electrodes ((96) and our own unpublished measurements) perhaps due to oxygenated edge planes on CFEs (44). Values of Ilim are similar among the tested electrode materials and consistent with the value expected from the diffusion equation, verifying the active size of patterned electrodes.
Fig 4.
Comparison of microelectrode responses fabricated from DLC:N, Au, and ITO. Each electrode had a radius of 20 µm and was insulated with S1813 photoresist. Surfaces were cleaned with air plasma before testing. A: Cyclic voltammograms for a 1 mM K3Fe(CN)6 solution in 0.1 M KCl, pH 3.0, scan rate 1 V/s. B: Sample responses depicting quantal exocytosis from individual bovine chromaffin cells stimulated with a high K+ solution. Differences in the size or time course of these sample amperometric spikes do not necessarily represent consistent differences among electrode materials.
Measurement of the electrode double-layer capacitance is also an important method to validate the working electrode area and the integrity of the insulating film. Whereas the electrode capacitance varies with the test method/frequency, the test solution and the method of fabrication, a typical value is ~0.15 pF/µm2 in physiological cell bath solution measured at 1 kHz (58). Excessive measured capacitance can indicate cracks or pinholes in the insulation and is accompanied by larger background noise.
Background currents
Au has a background current that rises precipitously above ~ 0.65 V (Fig. 4A), presumably due to oxidation of chloride (96). Therefore a potential of +0.6 V (vs Ag/AgCl) is commonly used for amperometeric measurements of catecholamine exocytosis with Au electrodes. At this potential the background current in physiological bath solution is somewhat larger for noble metals (~0.1–0.3 pA/µm2) compared to ITO, BDD, and CFEs (~0.01–0.03 pA/µm2).
Optical transparency
Fluorescent measurements from cells are desired to measure cell [Ca2+]i, evaluate expression of GFP, monitor exocytosis of labeled vesicles using TIRF microscopy and other applications. These optical measurements are most conveniently carried out using inverted microscopes, so it is desirable to use optically transparent electrodes. ITO electrodes have been used as a classical transparent yet conductive material. It also has reasonable electrochemical properties and is suitable for recording quantal exocytosis (2, 17, 47, 55, 80). The transmittance of a 100-nm-thick ITO film is ~80% at 360 nm rising to >90% for wavelengths above 400 nm.
Diamond is also highly transparent over a wide range of wavelengths, and can be doped with boron to become conductive while maintaining reasonable transparency (see (15) for a review). Examples of 350 nm thin boron-doped electrode layer grown on undoped nanocrystalline diamond date back to 2010 (32, 33). BDD exhibit outstanding electrochemical properties, such as a broad working potential window (>3V), which enhance species detection to high anodic overpotentials, unreacheable by noble metals or glassy carbon electrodes, low background current, high signal to noise ratio, resistance to fouling (46), stability under anodic and cathodic polarization which is useful for detecting reducible species, inertness, and long life time (52, 60, 79). BDD has been grown on silicon substrates by chemical vapour deposition (43), and successively replaced by sapphire and glass to improve optical interfacing (11). More recently, high-temperature glass was used for diamond growth instead of sapphire or quartz. This strategy resolved issues of delamination and fractures and increased transparency by 50% in the visible to the near-UV range, thus allowing the excitation of fluorescent probes (15, 36). An additional method for BDD electrode fabrication consists in growing BDD electrode on a silicon substrate and bond the BDD electrode to a CMOS device with benzocyclobutene, followed by etching processes and deposition of connecting metals. An advantage of this method is that it allows integration of BDD electrodes with CMOS or other microfabrication processes which may be damaged during the conventional BDD fabrication process (40).
Diamond-like carbon (DLC) is a material with a mixture of carbon atoms in either the sp2 (graphite) and sp3 (diamond) hybridized states and therefore has properties intermediate between graphite and diamond. It is simpler to fabricate (e.g., using magnetron sputtering) compared to diamond and forms a hard, chemically inert film that is semi-transparent and modestly conductive. Nitrogen doping (DLC:N) during deposition increases the conductivity of the film and deposition on top of ITO results in a highly conductive material with the surface properties of DLC. The transmittance of a 40 nm DLC:N film on top of a 100 nm ITO film is ~70% at 400 nm. DLC:N has good electrochemical properties and has been used for measurements of quantal exocytosis (31, 47, 74).
Finally, metal films are partially transparent if they are very thin. A transmittance of 50–70% was measured at 400 nm for a 13 nm-thick Au film and quantal exocytosis has been measured on this Au films (47). It should be noted, however, that such a thin film is not mechanically robust and therefore not suitable for aggressive cleaning and reuse.
Ease of fabrication and cost
The material costs for Au and Pt are substantially higher than for ITO, BDD and DLC:N, although the cost of the materials is often not a substantial fraction of the total device fabrication cost. Noble metals, ITO and DLC:N are straightforward to deposit and pattern, although care must be taken when developing processes not to inadvertently remove DLC:N during plasma etching of insulating films. BDD requires somewhat more specialized equipment to deposit via hot filament or microwave plasma chemical vapor deposition (53).
Sensitivity for amperometric measurement of quantal exocytosis
There are reports of differing sensitivities among electrode materials for measurement of quantal exocytosis. An early report from the Amatore group indicated that an ITO electrode below the cell recorded charges per amperometric event that averaged over twofold larger than that measured with a CFE at the cell apex (3). This was interpreted as reflecting differences in exocytosis between the apex and the base of the cell. Surprisingly, though, amperometric spikes were reported to be ~50% smaller (peak amplitude and charge) for ITO electrodes compared to Au or DLC:N electrodes in paired recordings from the cell base (47). This was followed up with chronoamperometry experiments with known concentrations of analytes to reveal that the apparent number of electrons transferred per catecholamine molecule ranged from 2.0 – 2.7 for epinephrine on CFEs, DLC:N or Au electrodes, but was only ~1.5 for ITO (47). The reason for the lower oxidation yield of epinephrine on ITO is unclear. A yield of greater than two electrons per epinephrine molecule may be due to cyclization reactions that follow the initial oxidation (20) and may be captured on some electrode surfaces better than others. Thus care must be taken when comparing measurements of quantal exocytosis between different types of electrodes and differing recording configurations.
Alternative materials and surface modifications
Chemical modification of Au electrodes with mercaptopropionic acid has been used to improve the resistance of Au electrodes to fouling by dopamine electropolymerization (77, 78). Screen printed carbon electrodes have been used to detect quantal exocytosis and can be fabricated without the need for specialized photolithography equipment (92). Polypyrrole graphene electrodeposited onto Pt electrodes has been used to detect quantal exocytosis (87). Carbon nanotubes (CNTs) have been added as a surface modification to electrochemical sensors and are reported to be excellent substrates for cellular growth, exhibit high electrical conductivity and chemical stability (see (42) for a review). CNTs deposited on ITO electrodes have been used to detect quantal exocytosis (75) from individual PC12 cells as well as global dopamine release and action potentials from striatal slices (81). The conductive polymer PEDOT poly(3,4--ethylenedioxythiophene) doped with poly(styrene sulfonate) has high transparency, flexibility, and biocompatibility (59, 70) and has been shown to be suitable for amperometric detection of release (48), including quantal exocytosis (93). Microstructured graphitic multielectrode arrays embedded in a single-crystal diamond were realized as sub-superficial conductive micropaths by means of a deep ion beam lithography technique: by focusing ion beam writing through variable-thickness masks, the approach allows to regulate the depth at which the channels are formed and their emergence at specific locations of the sample surface (65–67). Micrographitic arrays can detect quantal exocytosis from isolated cells and adrenal slices, and are sensitive enough to distinguish full fusion from stand-alone foot signals (64, 82).
5. Insulating materials
Just as CFEs are insulated except for the working electrode at the tip, conductive films on the microchip must be insulated everywhere they contact the bath solution except for the small working electrodes in order to reduce the capacitance and the background noise level. Ideally the insulating material should be free of cracks and pinhole defects, bond tightly to the chip, be biocompatible, and robust enough to support long-term culture, cleaning and reuse. Conventional photoresist can be used as an insulator (17, 25), but is not long-term stable in solution. Bonding a slab of PDMS with an integrated channel placed at right angles to a conductive strip has been used to serve as both the insulation and a microfluidic channel (80). Conventional inorganic films that have been used to insulate electrodes include SiO2 (e.g., (10, 16)), Si3N4 (e.g., (87)), or alternating SiO2 and Si3N4 layers in order to prevent pinhole and stress defects (91). Teflon AF films have been used as an electrical insulator that also inhibits cell attachment, and thus promotes cell alignment to the working electrodes (9). The thick, hard photoresist SU8 is perhaps the most popular insulating material in recent studies. SU8 is appealing because openings can be directly patterned using photolithography and, once cured, it is a hard, stable, epoxy-like film that can be cleaned and reused. A drawback is that SU8 contains the toxic element antimony, although biocompatibility studies demonstrate that well-cured SU8 exhibits minimal cytotoxicity (61).
6. Determinants of noise
Amperometric spikes resulting from quantal exocytosis are very small in amplitude and charge, especially from small vesicles, therefore attempts to minimize background noise are important. The potentiostat can be the dominant noise source, particularly if it is not designed for pA-level measurements and, in any case, for electrodes smaller than several µm2 in area such that the electrode noise becomes very small. Instruments with GΩ-magnitude feedback resistors, such as found in patch-clamp amplifiers, are best for measurement of small signals because of their low thermal noise.
Under careful recording conditions, and for electrodes with areas ranging from several µm2 to many hundreds of µm2 and bandwidths up to several kHz, the dominant source is thermal noise at the electrode surface (94). Thermal noise is found in all processes that dissipate energy through a resistive pathway and is white in spectrum, i.e., uniform in intensity across a wide range of frequencies. Whereas an ideal electrochemical electrode behaves as a capacitor rather than a resistor, real-world electrodes are modeled by a “constant phase element” with electrical properties intermediate between those of a capacitor and a resistor (21, 94). The phase shift between an applied voltage sinusoid and the resulting current sinusoid (phase of the admittance) is ideally 90° for a purely capacitive electrode (i.e., the real component of admittance is zero), in which case it will not exhibit thermal noise. However, actual electrodes have admittance phases slightly smaller than 90°, denoting a resistive component that contributes noise. The thermal noise for a given electrode area depends on the material used, the method of fabrication and the cleanliness of the surface. In a recent study the rank order of thermal noise for different electrode materials was Au ~ carbon fiber > diamond-like carbon > indium-tin-oxide (94).
The standard deviation of thermal current noise scales with the square root of the working electrode area and also approximately with the square root of the bandwidth of the recording (94). This motivates making the working electrodes small and atomically smooth and low-pass filtering the signal with a cutoff frequency no larger than is necessary to preserve the dynamics of the spike.
Measuring very fast release events such as from dopaminergic neurons requires bandwidths exceeding several kHz (68), at which point non-thermal noise sources can become dominant. In particular, small fluctuations in the electrode introduced by the amplifier lead to fluctuations in the measured current that increase with frequency (76) because the admittance of the electrode, like a capacitor, increases ~linearly with frequency. This “input-voltage-capacitance” noise is also the dominant noise source even at modest bandwidths for electrodes with areas greater than several thousand µm2 (55, 94). Therefore high-bandwidth recordings are best carried out using potentiostats with low input voltage noise, such as patch-clamp amplifiers (76). In addition, input-voltage-capacitance noise has a standard deviation that increases approximately linearly with the area of the electrode (55, 80, 94), therefore minimizing the area of the electrode, as well as other sources of input capacitance, is particularly important for high-bandwidth measurements.
7. Localizing cells to electrodes
For measurement of quantal exocytosis using carbon-fiber electrodes the cells are normally adhered to the bottom of the recording chamber and a micromanipulator is used to position the electrode adjacent to the cell surface while under observation with a microscope. For microchip electrodes the situation is usually the opposite where the electrode is fixed and a cell needs to be positioned immediately adjacent to it (although see (90) for an interesting example of a microchip electrode array that is manipulated to a cell). Constraints include that at least a portion of the cell surface needs to be directly adjacent (within several µm) to an electrode in order to resolve amperometric spikes with out excessive temporal broadening due to diffusion (18, 72). It is also highly desirable that only one cell is adjacent to each electrode so that the statistical parameters derived from the recorded spikes are from a single cell (22).
A straightforward approach is to use a micropipette to pick up a cell and then precisely position the cell to the appropriate location with a micromanipulator (e.g., (38, 85)). However, this is a time-consuming process and cell damage may occur, for example, the cell will often undergo exocytosis in response to mechanical stimulation alone.
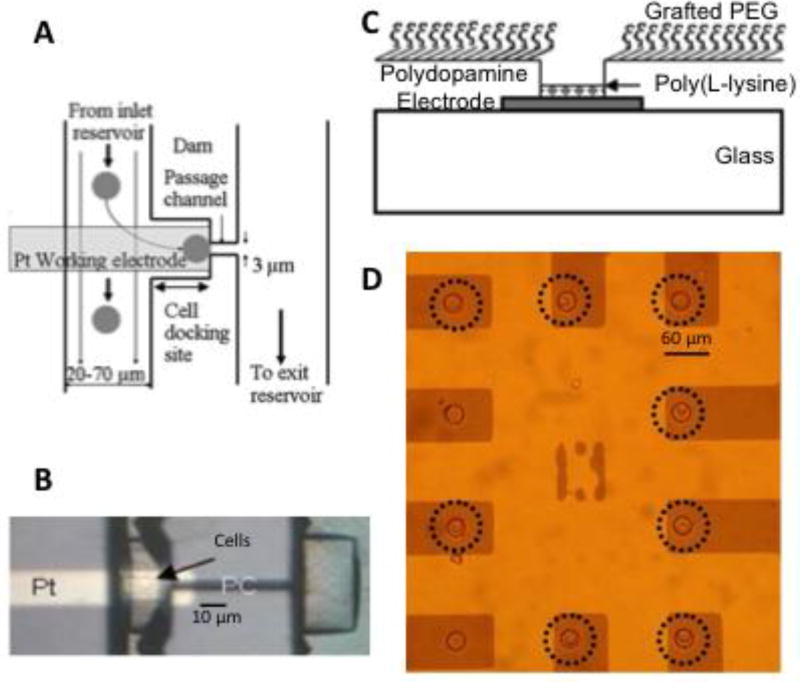
On-chip microfluidic dams or constrictions have been used to trap cells adjacent to electrodes for measurement of quantal exocytosis (Fig. 5A, (26, 29, 30, 78)). Limitations of these approaches are that it is challenging to trap only one cell near an electrode and cells are likely to become mechanically distressed when trapped by a microfluidic constriction.
Fig 5.
Approaches to trap cells in register with electrodes. A: Schematic illustration of how cells can be trapped in a constriction or dam in a microfluidic channel. B: Photomicrograph of a device trapping cells over a Pt electrode (from (30)). C: Schematic illustration of a microwell/electrode device whereby a PEG film grafted to the surface of the insulating layer prevents cell attachment. A cell-sized microwell traps individual cells over an electrode and a polylysine film at the bottom of the microwell helps promote cell attachment. D: Photomicrograph of a microwell-array device demonstrating that the microwells trap individual cells over 8 of 10 electrodes (circled), whereas the PEG film prevents attachment of cells elsewhere on the surface of the chip (from (51)).
Modifying the surface chemistry of microchips has also been used to target cells to electrodes. In this case “cytophilic” materials that promote cell attachment are used on electrodes whereas “cytophobic” materials that discourage cell attachment are used between electrodes so that cells are free to move (passively or actively) to electrode attachment sites. Fortunately, thin film that are typically use to promote cell attachment, such as collagen (55) and polylysine (51) do not passivate electrochemical electrodes. In addition, cells show variations in how well they stick to electrochemical electrode materials, e.g., chromaffin cells adhere in rank order to diamond-like carbon > indium-tin-oxide, Pt > Au (74). Materials that discourage cell attachment presumably do so by blocking adsorption of proteins that interact with cell receptors or extracellular matrix. Examples include polyethylene glycol (51) and teflon AF (9, 74). A study from the Gillis lab and collaborators demonstrates that teflon AF can be used as an effective insulating material that also facilitates concentration of cells to electrode areas on the chip (9).
Microwell traps are an effective method for targeting single cells to electrodes and also help prevent loosely adherent cells from washing away from the electrode during perfusion of the bath solution. For these devices the insulation on the chip is relatively thick, ~10 µm, so that a cell-sized opening over the conductor creates a microwell cell trap over the working electrode (Fig 2 and 5C). SU8 photoresist is a convenient material for this application because the thickness can be tailored, it cures into a hard, stable insulating material, and it is directly patterned with photolithography to open the holes. Addition of a very high density of cells leads to a high probability that a cell occupies each microwell whereas the microwell is too small to contain multiple cells, so single-cell recordings are assured.
The surface chemistry of SU8 can be modified in a straightforward manner to make the surface resistant to cell attachment so that cells are selectively targeted to the microwell electrodes. Stamping the surface of the devices with polydopamine results in an adherent film that is readily grafted with a high density of polyethylene glycol through a Schiff base reaction (Fig 5C, (51)). Subsequently a polylysine solution is added and selectively adheres to the electrode surface at the bottom of the microwell to promote cell adhesion. Fig 5D demonstrates effective loading of cells into microwell electrodes with few cells remaining outside the microwells following a washing step (51).
Loading of individual cells onto electrodes requires disruption of cell clumps, particularly for the case of microwell electrodes. This is straightforward if cells are loaded onto the devices directly after preparation, since clumps are disrupted during enzymatic treatment and trituration. However, cell clumping is a significant issue if cells are cultured in flasks for some days before seeding on the microchips for an acute experiment. In order the prevent chromaffin cells from clumping, the Gillis lab cultures chromaffin cells in a refrigerator (4° C) in Hibernate A media (BrainBits LLC, Springfield, IL, USA) in a refrigerator for use 1– 6 days after preparation (51).
8. Stimulating exocytosis on microchip devices
Once cells are localized to electrodes they need to be stimulated to undergo exocytosis. Cells placed on electrodes using a micropipette undergo exocytosis, likely due to a mechanically stimulated process (38). However, triggering exocytosis through pathways with clear physiological relevance is desirable. The most common method is to perfuse a solution containing a secretagogue, such as a high K+ solution, that induces depolarization of the cell, Ca2+ influx, and Ca2+-dependent exocytosis. A number of MEAs for detecting quantal exocytosis have been integrated with microfluidics to facilitate solution exchange (e.g., (17, 26, 49, 78, 80)). One complication with solution perfusion is that cells may be washed away, or at least move slightly relative to the electrode, which could potentially affect the recorded spike dynamics. Therefore, it is important that cells either be tightly adhered to the substrate or mechanically constrained, such as in a microwell. Another disadvantage of using common perfusion systems is that the rate that the stimulus can be turned on and off is limited, thus constraining dynamic information about stimulus-secretion coupling. In addition, the secretagogue concentration and time course may differ from cell to cell depending on their position in the perfusion stream. This has motivated the development of alternative on-chip methods to trigger exocytosis.
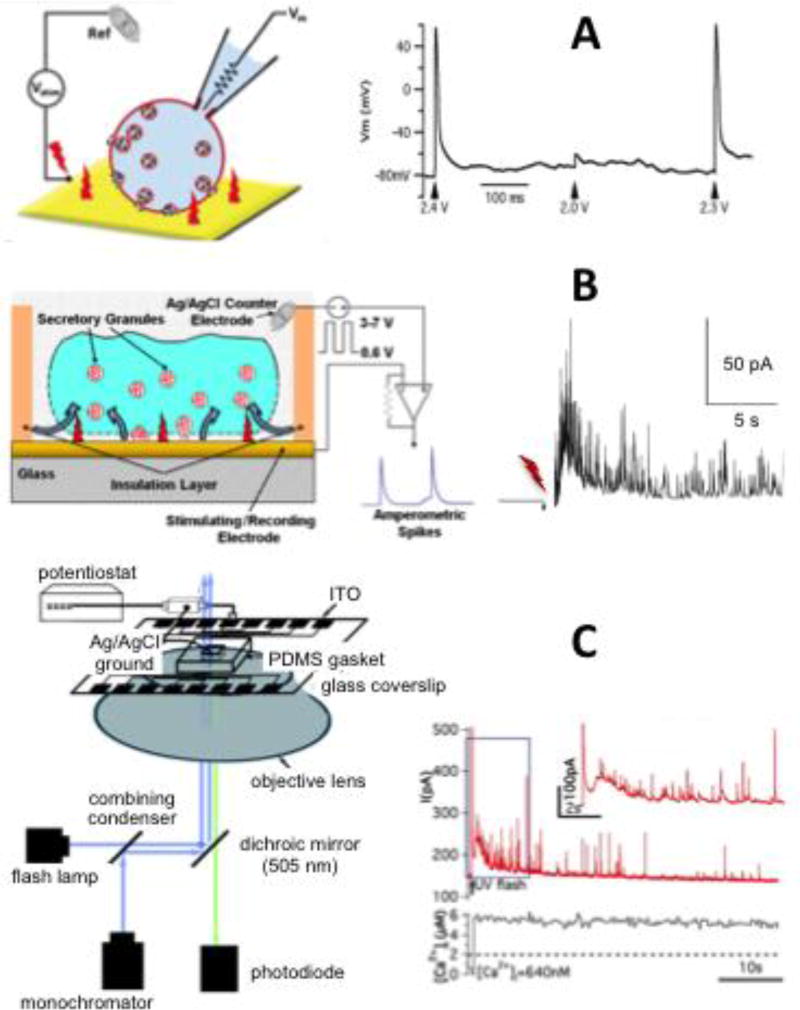
The Gillis lab has recently reported an approach to electrically stimulate cells using the same working electrode that is used to record quantal exocytosis (34). Brief voltage pulses are applied, which induce capacitive currents and voltage gradients as these currents pass through the solution adjacent to the electrode. Whole cell patch clamp recordings of membrane potential in current clamp mode demonstrate that electrode stimulation induces action potentials in an all-or-nothing manner in an overlying cell (Fig 6A, (34)). This raises the exciting possibility of measuring the secretion response to paced electric-field-induced action potentials. However, electrode voltage pulses, particularly applied in trains, often lead to a precipitous drop in membrane impedance presumably due to electropermeabilization, and a massive burst of amperometric spikes (Fig 6B, (34)). The spikes have charges and time courses similar to depolarization-evoked exocytosis, so they presumably reflect vesicle-release events, although it is possible that they result from vesicle lysis on the electrode surface (62) rather than normal exocytosis. Surprisingly, electropermeabilization-induced exocytosis is dependent on the presence of Cl− in the bath solution, but not Ca2+ (34). It should be noted that electronic modification of the potentiostat is necessary for it to pass sufficiently large currents through the electrode without saturating during stimulation (8, 34). Transient single-cell electroporation may offer the opportunity to introduce membrane-impermeant substances into a cell.
Fig 6.
On-chip approaches to stimulate cells to undergo exocytosis. A: Use of the working electrode beneath the cell to stimulate action potentials. Left: Schematic illustration of cell stimulation while recording membrane potential with whole-cell patch clamp pipette. Right: Recording made from a chromaffin cell illustrating how a transient voltage pulse applied to the working electrode of 2.3 or 2.4 V, but not 2.0 V, elicits an all-or-nothing action potential in the overlying cell (from (34)). B: Trains of voltage pulses to the working electrode results in massive quantal fusion events, presumably by electroporating the cell membrane. Left: schematic illustration of the process. Right: Sample recording whereby a train of 7, 0.1ms pulses to +6V (lightning bolt) leads to massive release. C: On-chip photolysis of caged Ca2+ leads to a step increase in intracellular Ca2+ concentration and a burst of exocytosis measured in the overlying electrode. Left: Schematic illustration of the recording setup. The light path of a flash lamp (to photolyze caged Ca2+) is combined with that of a monochromator (to excite fluorescent Ca2+ indicators). The light passes through a transparent ITO electrode and stimulates the cell. Right: Sample recording where a flash of UV light leads to a step increase in measured Ca2+ and a burst of exocytosis. An expanded view of the initial burst is depicted in the inset. (From (17)).
Electrically-evoked stimulation of exocytosis has also been obtained by means of BDD electrodes, resulting in amperometric signals comparable to those evoked by extracellular KCl-enriched solution (35).
On-chip optical stimulation of quantal exocytosis via photorelease of caged Ca2+ has also been demonstrated (17). Cells are loaded with the membrane-permeant acetoxymethyl ester derivatives of the Ca2+ cage NP-EGTA together with the Ca2+ indicator dye fura-4F and placed on transparent indium-tin-oxide electrodes fabricated on glass coverslips. Flash photolysis of the cage leads to Ca2+-stimulated exocytosis measured by the underlying electrode, whereas the concentration of Ca2+ in the cell is reported with fluorescent measurements of fura-4F using alternating-wavelength excitation with a monochromator (Fig 6C, (17)). The illumination and recording apparatus have apertures so that stimulation and measurements are restricted to an individual cell. This approach is particularly powerful because it allows the experimenter to directly probe the relationship between intracellular Ca2+ concentration and the vesicle priming and vesicle-fusion processes.
9. Multisite subcellular detection of exocytosis
It is desirable to resolve the site of exocytosis on the cell surface in order to identify hot spots of secretory activity and to understand how spatial patterns of exocytosis change with an experimental maneuver. It is also important to resolve the location of release using electrochemical electrodes so the information can be correlated with fluorescence imaging techniques that provide complementary information about the release process.
Two types of approaches have been used for subcellular electrochemical localization. The first approach uses small, µm-scale electrodes patterned close together so that a number of electrodes will contact the surface of a single cell (50, 90, 97, 98). The electrode that receives the signal identifies the site of release on the cell surface, thus the spatial resolution is essentially determined by the size of the electrode.
For example, the Ewing lab developed a movable microelectrode array probe, consisting of 16 platinum band electrodes on borosilicate glass, to detect quantal release from bovine chromaffin cells. In this case, 1.2 µm wide electrodes were tightly packed along two opposite rows within a 20 µm × 25 µm square area (90). Another example is an array of 9 rectangular electrodes patterned within a circular opening of 20 µm diameter fabricated from boron-doped nanocrystalline diamond grown on sapphire. This microarray was suitable for distinguishing variable secretory activity over the cell membrane with maximal resolution of 12 µm2, corresponding to the area of the smallest electrode (35). More recently, a different geometry has been proposed using BDD grown on high-temperature glass: the prototype consisted of 12 round-shaped sensing electrodes (2 µm diameter) within a total area of ~300 µm2, approximately corresponding to the area of a chromaffin cell (36). Further improved spatial resolution has been achieved using planar platinum ultramicroelectrodes (UMEAs). In his case, arrays with 16, 25, and 36 square microelectrodes (respectively of 4, 3, and 2 µm width), concentrated in a square area of 30 × 30 µm2, were suitable to perform multisite recordings from PC12 cells (85, 86). With this approach, subcellular heterogeneity of exocytosis could be monitored with 2 × 2 µm2 resolution by the 6 × 6 UMEA.
A limitation of these electrode array approaches is that the maximum packing density of electrodes, together with the conductive traces needed to carry the signal, is about a dozen per PC12 or chromaffin cell, therefore this approach is unlikely to ever match the resolution of optical imaging techniques.
A second approach pioneered by the Lindau lab is to arrange 3 or 4 electrodes in a cell-sized area with a gap in the middle (25, 38). If release occurs in the gap, several electrodes will capture a portion of the catecholamine with the nearest electrode reporting the largest signal. Comparing the relative charge detected by each electrode with diffusion simulations for the specific electrode geometry identifies the release site on the cell surface. The spatial resolution of this approach is not limited to the size of the electrode and sub-µm resolution is possible. Drawbacks of this approach are that the transmitter typically diffuses several µm before contacting one or more of the electrodes, so diffusional broadening occurs and signals can be small and slow. Only release from the portion of the cell in the electrode gap can be resolved because precise localization cannot occur if the signal is detected in only one or two electrodes. Also, the approach is complicated in that it requires correlating signals between several electrodes and performing diffusion simulations for each device.
Table 1 summarizes some of the features of multisite microchips reported in the literature.
TABLE 1.
Electrochemical microchips for multisite resolution of quantal exoytosis
| Electrode material |
Number of electrodes in the array |
Total sensing area | Experimental model |
Ref |
|---|---|---|---|---|
| carbon | 2-3-7 | 10–20 µm diameter | PC12 | (97) |
| carbon | 7 | 20 µm diameter | PC12 | (98) |
| carbon | 8-10-12-15 | 10–50 µm diameter | bovine adrenal chromaffin | (50) |
| Pt | 4 | 10 ×10 µm2 | bovine adrenal chromaffin | (25) |
| Pt | 4 | 10 ×10 µm2 | bovine adrenal chromaffin | (38) |
| Pt | 3 | 12 µm diameter | bovine adrenal chromaffin, mast cells | (10) |
| Pt | 16-25-36 | 30 ×30 µm2 | PC12 | (84, 86) |
| Pt | 16 | 20 µm × 25 µm2 | bovine adrenal chromaffin | (90) |
| BD-NCD | 4 | 16 µm diameter | bovine adrenal chromaffin | (32, 33) |
| BD-NCD | 4 | 18 µm diameter | bovine adrenal chromaffin | (63) |
| BD-NCD | 9 | 22 µm diameter | mouse/bovine adrenal chromaffin | (35) |
| ITO, gold, DLC | 4 | 12 µm × 12 µm2 | bovine adrenal chromaffin | (47) |
BD-NCD: boron-doped nanocrystalline diamond, DLC: diamond like carbon; ITO: indium tin oxide.
10. Combination of optical and electrochemical measurements of quantal exocytosis
Microchip devices to measure quantal exocytosis can enable simultaneously fluorescent imaging of the vesicle-release site, something that is not possible using carbon-fiber microelectrodes. The advantage of such a combination is that powerful fluorescent tools which track vesicle docking and release, or protein location or protein conformational changes (via FRET), can be combined with sub-millisecond resolution of fusion-poor opening and release via amperometry. This potentially enables the molecular steps of vesicle docking, priming, fusion, cargo release, and fission to be resolved at the single-vesicle level with a time resolution that exceeds that of typical video rates (99).
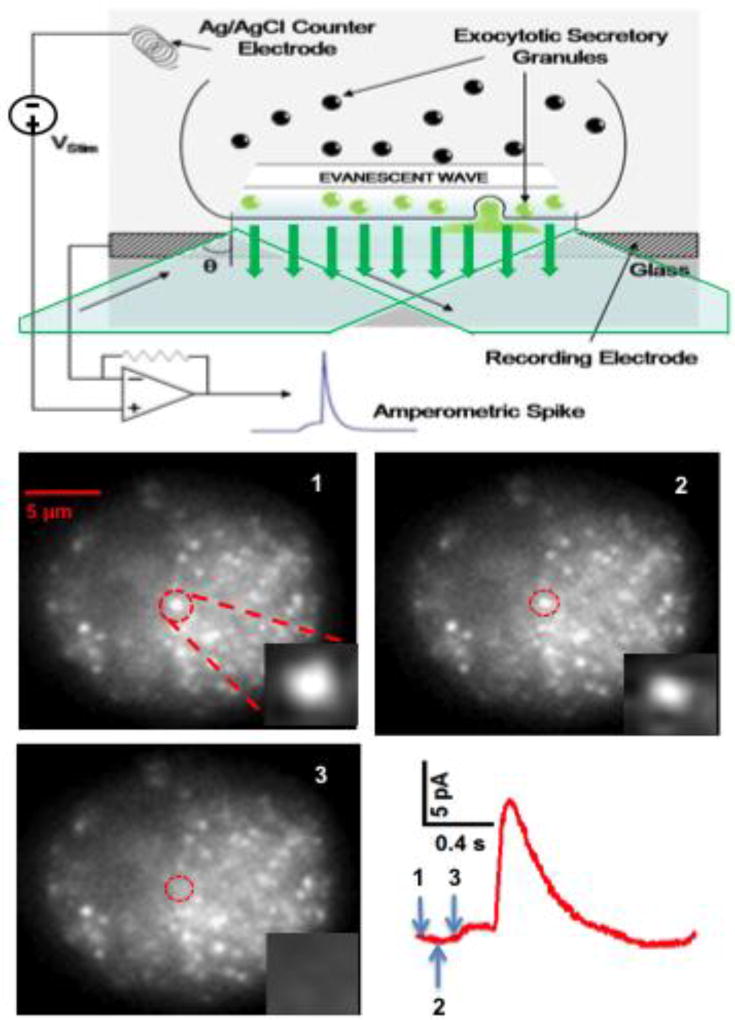
A straightforward way to combine imaging and electrochemical measurements is to use a thin cover glass substrate with transparent electrodes, and then image the cell through the electrode using an inverted microscope (Fig 7A). Total Internal Reflection Fluorescence Microscopy is a particularly effective imaging approach when combined with on-chip electrodes because it selectively illuminates the bottom surface of the cell with the evanescent field and the electrode electrochemically measures release from the same area of the membrane (47, 56). Fig 7 presents an example where a spot labeled with the false fluorescent neurotransmitter FFN511 (37) disappears in synchrony with the appearance of an amperometric foot signaling opening of the fusion pore. In these measurements the fluorescent and amperometric events need to be clearly coincident in time in order to assign them to the same vesicle-release event with high confidence. Therefore the frequency of release events must not be too high to minimize incorrect pairing of fluorescence and amperometric events and the fluorescent signal resulting from exocytosis, e.g., release of fluorescently labeled cargo from a vesicle, needs to be clearly resolved in the images.
Fig 7.
Combination of fluorescent imaging with amperometric recording of quantal exocytosis. Top: Schematic illustration of total internal reflectance fluorescence microscopy through a transparent electrode. Excitation light through the objective lens of an inverted microscope exceeds the critical angle and undergoes total internal reflection at the interface between the ITO transparent electrode and the cell. The evanescent field penetrates a short distance into the cell, selectively exciting fluorescent labels at the bottom surface of the cell. Bottom: TIRF images through an ITO electrode of a chromaffin cell with vesicles labeled with FFN511 (440 nm excitation). Punctae likely indicate individual granules docked to the bottom membrane. The sequence of 3 images taken at 10 frames per second depict the loss of a fluorescent spot near the center of the cell (circled) coincident with an amperometric spike measured by the underlying electrode. The inset of each image depicts the expanded region of interest. Arrows on the spike indicate the beginning of each frame. Note that the release of FFN511 in this example is coincident with the beginning of the foot signal (opening of the fusion pore).
“Electrochemical imaging” (38) is a clever approach to enable pairing of fluorescence and electrochemical signals originating from quantal exocytosis with high confidence (99). As explained previously, this approach allows approximate identification of the location of the release site based upon the relative charge from a single release event detected in at least three electrodes arranged around the cell. This facilitates combination of electrochemical detection with imaging in two ways. First, electrochemical imaging is carried out in regions between several electrodes, so the electrode is not in the way to attenuate the fluorescent signal. (Even mostly transparent electrode materials lead to some loss of light (47).) Second, fluorescent changes do not need to be obvious for them to be included in the analysis. For example, subtle fluorescent changes that would otherwise escape detection can be resolved by averaging many release events in the regions of interest identified by electrochemical imaging (99).
Event correlation microscopy, another innovation from the Lindau lab, enables measurement of changes in fluorescence in synchrony with amperometric release with a temporal resolution much higher than that of the video frame rate (99). The amperometric spike is used as a synchronization marker for a fusion event with millisecond precision. The fluorescence signal originating from a region of interest identified by electrochemical imaging is measured for a handful of frames before and after the event and aligned in time with the amperometric spike. Whereas the fluorescent time course for an individual event is only reported with a temporal resolution of the frame rate (e.g., 100 ms), averaging many sequences of fluorescent frames, with the start time of each frame occurring at random times relative to the amperometric spike, results in an averaged fluorescent signal that can achieve a temporal resolution of ~2 ms if the fluorescence signal-to-noise ratio is good (99). This has enabled the discovery that a conformational change in the SNAP-25 protein, reported as a change in FRET, precedes the opening of the exocytotic fusion pore by ~90 ms and is correlated with fusion pore opening, not dilation (99).
11. Conclusions and future directions
Microchips for quantal exocytosis have been shown to be a sensitive platform for subcellular identification of release sites, high-throughput detection of release from dozens of cells simultaneously, and combination of amperometric and TIRF imaging. The greatest promise of these microdevices is their ability to integrate two or more approaches to gain insight into exocytosis. This promise will be fully realized as these devices evolve from early-stage prototypes to fully-fledged systems that are disseminated to a multitude of research laboratories to serve as attractive tools for basic and applied research.
Acknowledgments
This work was supported by NIH grants R01NS048826, R01MH095046 and R44MH096650; by San Paolo Foundation, grant # CSTO 165284 and Italian Miur, grant # 2015 FNWP34 to VC. We thank our colleagues who have partnered with us throughout the years in carrying out some of the work described in this manuscript.
Footnotes
Disclosure
Kevin D. Gillis has an ownership interest in ExoCytronics, LLC, which is developing commercial microfabricated devices for assaying quantal exocytosis.
References
- 1.Alvarez de Toledo G, Fernandez-Chacon R, Fernandez JM. Release of secretory products during transient vesicle fusion. Nature. 1993;363:554–558. doi: 10.1038/363554a0. [DOI] [PubMed] [Google Scholar]
- 2.Amatore C, Arbault S, Chen Y, Crozatier C, Lemaitre F, Verchier Y. Coupling of electrochemistry and fluorescence microscopy at indium tin oxide microelectrodes for the analysis of single exocytotic events. Angew Chem Int Ed Engl. 2006;45:4000–4003. doi: 10.1002/anie.200600510. [DOI] [PubMed] [Google Scholar]
- 3.Amatore C, Arbault S, Lemaitre F, Verchier Y. Comparison of apex and bottom secretion efficiency at chromaffin cells as measured by amperometry. Biophys Chem. 2007;127:165–171. doi: 10.1016/j.bpc.2007.01.007. [DOI] [PubMed] [Google Scholar]
- 4.Amatore C, Delacotte J, Guille-Collignon M, Lemaitre F. Vesicular exocytosis and microdevices - microelectrode arrays. Analyst. 2015;140:3687–3695. doi: 10.1039/c4an01932f. [DOI] [PubMed] [Google Scholar]
- 5.Amatore C, Klymenko OV, Svir I. In situ and online monitoring of hydrodynamic flow profiles in microfluidic channels based upon microelectrochemistry: optimization of electrode locations. Chemphyschem. 2006;7:482–487. doi: 10.1002/cphc.200500400. [DOI] [PubMed] [Google Scholar]
- 6.Ayers S, Berberian K, Gillis KD, Lindau M, Minch BA. Post-CMOS fabrication of Working Electrodes for On-Chip Recordings of Transmitter Release. IEEE Trans Biomed Circuits Syst. 2010;4:86–92. doi: 10.1109/TBCAS.2009.2033706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ayers S, Gillis KD, Lindau M, Minch BA. Design of a CMOS Potentiostat Circuit for Electrochemical Detector Arrays. IEEE Trans Circuits Syst I Regul Pap. 2007;54:736–744. doi: 10.1109/TCSI.2006.888777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barbour B, Isope P. Combining loose cell-attached stimulation and recording. J Neurosci Methods. 2000;103:199–208. doi: 10.1016/s0165-0270(00)00318-6. [DOI] [PubMed] [Google Scholar]
- 9.Barizuddin S, Liu X, Mathai JC, Hossain M, Gillis KD, Gangopadhyay S. Automated targeting of cells to electrochemical electrodes using a surface chemistry approach for the measurement of quantal exocytosis. ACS chemical neuroscience. 2010;1:590–597. doi: 10.1021/cn1000183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Berberian K, Kisler K, Fang Q, Lindau M. Improved Surface-Patterned Platinum Microelectrodes for the Study of Exocytotic Events. Analytical Chemistry. 2009;81:8734–8740. doi: 10.1021/ac900674g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bonnauron M, Saada S, Mer C, Gesset C, Williams OA, Rousseau L, Scorsone E, Mailley P, Nesladek M, Arnault JC, Bergonzo P. Transparent diamond-on-glass micro-electrode arrays for ex-vivo neuronal study. physica status solidi (a) 2008;205:2126–2129. [Google Scholar]
- 12.Borges R, Camacho M, Gillis KD. Measuring secretion in chromaffin cells using electrophysiological and electrochemical methods. Acta Physiol (Oxf) 2008;192:173–184. doi: 10.1111/j.1748-1716.2007.01814.x. [DOI] [PubMed] [Google Scholar]
- 13.Bruns D, Riedel D, Klingauf J, Jahn R. Quantal release of serotonin. Neuron. 2000;28:205–220. doi: 10.1016/s0896-6273(00)00097-0. [DOI] [PubMed] [Google Scholar]
- 14.Carabelli V, Gosso S, Marcantoni A, Xu Y, Colombo E, Gao Z, Vittone E, Kohn E, Pasquarelli A, Carbone E. Nanocrystalline diamond microelectrode arrays fabricated on sapphire technology for high-time resolution of quantal catecholamine secretion from chromaffin cells. Biosensors & Bioelectronics. 2010;26:92–98. doi: 10.1016/j.bios.2010.05.017. [DOI] [PubMed] [Google Scholar]
- 15.Carabelli V, Marcantoni A, Picollo F, Battiato A, Bernardi E, Pasquarelli A, Olivero P, Carbone E. Planar Diamond-Based Multiarrays to Monitor Neurotransmitter Release and Action Potential Firing: New Perspectives in Cellular Neuroscience. ACS Chem Neurosci. 2017;8:252–264. doi: 10.1021/acschemneuro.6b00328. [DOI] [PubMed] [Google Scholar]
- 16.Chen P, Xu B, Tokranova N, Feng X, Castracane J, Gillis KD. Amperometric detection of quantal catecholamine secretion from individual cells on micromachined silicon chips. Anal Chem. 2003;75:518–524. doi: 10.1021/ac025802m. [DOI] [PubMed] [Google Scholar]
- 17.Chen X, Gao Y, Hossain M, Gangopadhyay S, Gillis KD. Controlled on-chip stimulation of quantal catecholamine release from chromaffin cells using photolysis of caged Ca2+ on transparent indium-tin-oxide microchip electrodes. Lab Chip. 2008;8:161–169. doi: 10.1039/b715308m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chow RH, Rüden Lv. Electrochemical detection of secretion from single cells. In: Sakmann B, Neher E, editors. Single Channel Recording. New York: Plenum Press; 1995. pp. 245–275. [Google Scholar]
- 19.Chow RH, von Ruden L, Neher E. Delay in vesicle fusion revealed by electrochemical monitoring of single secretory events in adrenal chromaffin cells. Nature. 1992;356:60–63. doi: 10.1038/356060a0. [DOI] [PubMed] [Google Scholar]
- 20.Ciolkowski EL, Maness KM, Cahill PS, Wightman RM, Evans DH, Fosset B, Amatore C. Disproportionation during Electrooxidation of Catecholamines at Carbon-Fiber Microelectrodes. Analytical Chemistry. 1994;66:3611–3617. [Google Scholar]
- 21.Cole KS, Cole RH. Dispersion and absorption in dielectrics: I. Alternating current characteristics. J Chem Phys. 1941;9:341–351. [Google Scholar]
- 22.Colliver TL, Hess EJ, Pothos EN, Sulzer D, Ewing AG. Quantitative and statistical analysis of the shape of amperometric spikes recorded from two populations of cells. J Neurochem. 2000;74:1086–1097. doi: 10.1046/j.1471-4159.2000.741086.x. [DOI] [PubMed] [Google Scholar]
- 23.Cooper JM. Towards electronic Petri dishes and picolitre-scale single-cell technologies. Trends Biotechnol. 1999;17:226–230. doi: 10.1016/s0167-7799(99)01325-6. [DOI] [PubMed] [Google Scholar]
- 24.Cui HF, Ye JS, Chen Y, Chong SC, Sheu FS. Microelectrode array biochip: tool for in vitro drug screening based on the detection of a drug effect on dopamine release from PC12 cells. Anal Chem. 2006;78:6347–6355. doi: 10.1021/ac060018d. [DOI] [PubMed] [Google Scholar]
- 25.Dias AF, Dernick G, Valero V, Yong MG, James CD, Craighead HG, Lindau M. An electrochemical detector array to study cell biology on the nanoscale. Nanotechnology. 2002;13:285. [Google Scholar]
- 26.Dittami GM, Rabbitt RD. Electrically evoking and electrochemically resolving quantal release on a microchip. Lab Chip. 2010;10:30–35. doi: 10.1039/b911763f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fiaccabrino GC, Koudelkahep M, Jeanneret S, Vandenberg A, Derooij NF. Array of Individually Addressable Microelectrodes. Sensor Actuat B-Chem. 1994;19:675–677. [Google Scholar]
- 28.Finnegan JM, Pihel K, Cahill PS, Huang L, Zerby SE, Ewing AG, Kennedy RT, Wightman RM. Vesicular quantal size measured by amperometry at chromaffin, mast, pheochromocytoma, and pancreatic beta-cells. J Neurochem. 1996;66:1914–1923. doi: 10.1046/j.1471-4159.1996.66051914.x. [DOI] [PubMed] [Google Scholar]
- 29.Gao C, Sun X, Gillis KD. Fabrication of two-layer poly(dimethyl siloxane) devices for hydrodynamic cell trapping and exocytosis measurement with integrated indium tin oxide microelectrodes arrays. Biomed Microdevices. 2013 doi: 10.1007/s10544-013-9744-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gao Y, Bhattacharya S, Chen X, Barizuddin S, Gangopadhyay S, Gillis KD. A microfluidic cell trap device for automated measurement of quantal catecholamine release from cells. Lab Chip. 2009;9:3442–3446. doi: 10.1039/b913216c. [DOI] [PubMed] [Google Scholar]
- 31.Gao Y, Chen X, Gupta S, Gillis KD, Gangopadhyay S. Magnetron sputtered diamond-like carbon microelectrodes for on-chip measurement of quantal catecholamine release from cells. Biomed Microdevices. 2008;10:623–629. doi: 10.1007/s10544-008-9173-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gao Z, Carabelli V, Carbone E, Colombo E, Demaria F, Dipalo M, Gosso S, Manfredotti C, Pasquarelli A, Rossi S, Xu Y, Vittone E, Kohn E. Transparent diamond microelectrodes for biochemical application. Diamond and Related Materials. 2010;19:1021–1026. [Google Scholar]
- 33.Gao Z, Carabelli V, Carbone E, Colombo E, Dipalo M, Manfredotti C, Pasquarelli A, Feneberg M, Thonke K, Vittone E, Kohn E. Transparent microelectrode array in diamond technology. Journal of Micro-Nano Mechatronics. 2011;6:33–37. [Google Scholar]
- 34.Ghosh J, Liu X, Gillis KD. Electroporation followed by electrochemical measurement of quantal transmitter release from single cells using a patterned microelectrode. Lab Chip. 2013;13:2083–2090. doi: 10.1039/c3lc41324a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gosso S, Turturici M, Franchino C, Colombo E, Pasquarelli A, Carbone E, Carabelli V. Heterogeneous distribution of exocytotic microdomains in adrenal chromaffin cells resolved by high-density diamond ultra-microelectrode arrays. J Physiol. 2014;592:3215–3230. doi: 10.1113/jphysiol.2014.274951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Granado TC, Neusser G, Kranz C, Filho JBD, Carabelli V, Carbone E, Pasquarelli A. Progress in transparent diamond microelectrode arrays. physica status solidi (a) 2015;212:2445–2453. [Google Scholar]
- 37.Gubernator NG, Zhang H, Stall RG, Mosharov EV, Pereira DB, Yue M, Balsanek V, Vadola PA, Mukherjee B, Edwards RH, Sulzer D, Sames D. Fluorescent false neurotransmitters visualize dopamine release from individual presynaptic terminals. Science. 2009;324:1441–1444. doi: 10.1126/science.1172278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hafez I, Kisler K, Berberian K, Dernick G, Valero V, Yong MG, Craighead HG, Lindau M. Electrochemical imaging of fusion pore openings by electrochemical detector arrays. Proc Natl Acad Sci U S A. 2005;102:13879–13884. doi: 10.1073/pnas.0504098102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Haller M, Heinemann C, Chow RH, Heidelberger R, Neher E. Comparison of secretory responses as measured by membrane capacitance and by amperometry. Biophys J. 1998;74:2100–2113. doi: 10.1016/S0006-3495(98)77917-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hayasaka T, Yoshida S, Inoue K, Nakano M, Matsue T, Esashi M, Tanaka S. Integration of Boron-Doped Diamond Microelectrode on CMOS-Based Amperometric Sensor Array by Film Transfer Technology. Journal of Microelectromechanical Systems. 2015;24:958–967. [Google Scholar]
- 41.Hondebrink L, Verboven AH, Drega WS, Schmeink S, de Groot MW, van Kleef RG, Wijnolts FM, de Groot A, Meulenbelt J, Westerink RH. Neurotoxicity screening of (illicit) drugs using novel methods for analysis of microelectrode array (MEA) recordings. Neurotoxicology. 2016;55:1–9. doi: 10.1016/j.neuro.2016.04.020. [DOI] [PubMed] [Google Scholar]
- 42.Jacobs CB, Peairs MJ, Venton BJ. Review: Carbon nanotube based electrochemical sensors for biomolecules. Anal Chim Acta. 2010;662:105–127. doi: 10.1016/j.aca.2010.01.009. [DOI] [PubMed] [Google Scholar]
- 43.Janischowsky K, Ebert W, Kohn E. Bias enhanced nucleation of diamond on silicon (100) in a HFCVD system. Diamond and Related Materials. 2003;12:336–339. [Google Scholar]
- 44.Ji X, Banks CE, Crossley A, Compton RG. Oxygenated edge plane sites slow the electron transfer of the ferro-/ferricyanide redox couple at graphite electrodes. Chemphyschem. 2006;7:1337–1344. doi: 10.1002/cphc.200600098. [DOI] [PubMed] [Google Scholar]
- 45.Kim BN, Herbst AD, Kim SJ, Minch BA, Lindau M. Parallel recording of neurotransmitters release from chromaffin cells using a 10×10 CMOS IC potentiostat array with on-chip working electrodes. Biosens Bioelectron. 2013;41:736–744. doi: 10.1016/j.bios.2012.09.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kiran R, Rousseau L, Lissorgues G, Scorsone E, Bongrain A, Yvert B, Picaud S, Mailley P, Bergonzo P. Multichannel Boron Doped Nanocrystalline Diamond Ultramicroelectrode Arrays: Design, Fabrication and Characterization. Sensors (Basel, Switzerland) 2012;12:7682–7700. doi: 10.3390/s120607669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kisler K, Kim BN, Liu X, Berberian K, Fang Q, Mathai CJ, Gangopadhyay S, Gillis KD, Lindau M. Transparent Electrode Materials for Simultaneous Amperometric Detection of Exocytosis and Fluorescence Microscopy. J Biomater Nanobiotechnol. 2012;3:243–253. doi: 10.4236/jbnb.2012.322030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Larsen ST, Taboryski R. All polymer chip for amperometric studies of transmitter release from large groups of neuronal cells. Analyst. 2012;137:5057–5061. doi: 10.1039/c2an35953g. [DOI] [PubMed] [Google Scholar]
- 49.Li LM, Wang W, Zhang SH, Chen SJ, Guo SS, Francais O, Cheng JK, Huang WH. Integrated microdevice for long-term automated perfusion culture without shear stress and real-time electrochemical monitoring of cells. Anal Chem. 2011;83:9524–9530. doi: 10.1021/ac202302t. [DOI] [PubMed] [Google Scholar]
- 50.Lin Y, Trouillon R, Svensson MI, Keighron JD, Cans AS, Ewing AG. Carbon-ring microelectrode arrays for electrochemical imaging of single cell exocytosis: fabrication and characterization. Anal Chem. 2012;84:2949–2954. doi: 10.1021/ac3000368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Liu X, Barizuddin S, Shin W, Mathai CJ, Gangopadhyay S, Gillis KD. Microwell device for targeting single cells to electrochemical microelectrodes for high-throughput amperometric detection of quantal exocytosis. Anal Chem. 2011;83:2445–2451. doi: 10.1021/ac1033616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Luong JH, Male KB, Glennon JD. Boron-doped diamond electrode: synthesis, characterization, functionalization and analytical applications. Analyst. 2009;134:1965–1979. doi: 10.1039/b910206j. [DOI] [PubMed] [Google Scholar]
- 53.Macpherson JV. A practical guide to using boron doped diamond in electrochemical research. Physical Chemistry Chemical Physics. 2015;17:2935–2949. doi: 10.1039/c4cp04022h. [DOI] [PubMed] [Google Scholar]
- 54.Mellander LJ, Trouillon R, Svensson MI, Ewing AG. Amperometric post spike feet reveal most exocytosis is via extended kiss-and-run fusion. Sci Rep. 2012;2:907. doi: 10.1038/srep00907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Meunier A, Fulcrand R, Darchen F, Guille Collignon M, Lemaitre F, Amatore C. Indium Tin Oxide devices for amperometric detection of vesicular release by single cells. Biophys Chem. 2012;162:14–21. doi: 10.1016/j.bpc.2011.12.002. [DOI] [PubMed] [Google Scholar]
- 56.Meunier A, Jouannot O, Fulcrand R, Fanget I, Bretou M, Karatekin E, Arbault S, Guille M, Darchen F, Lemaitre F, Amatore C. Coupling amperometry and total internal reflection fluorescence microscopy at ITO surfaces for monitoring exocytosis of single vesicles. Angew Chem Int Ed Engl. 2011;50:5081–5084. doi: 10.1002/anie.201101148. [DOI] [PubMed] [Google Scholar]
- 57.Mosharov EV, Sulzer D. Analysis of exocytotic events recorded by amperometry. Nat Methods. 2005;2:651–658. doi: 10.1038/nmeth782. [DOI] [PubMed] [Google Scholar]
- 58.Moulton SE, Barisci JN, Bath A, Stella R, Wallace GG. Studies of double layer capacitance and electron transfer at a gold electrode exposed to protein solutions. Electrochimica Acta. 2004;49:4223–4230. [Google Scholar]
- 59.Nambiar S, Yeow JT. Conductive polymer-based sensors for biomedical applications. Biosens Bioelectron. 2011;26:1825–1832. doi: 10.1016/j.bios.2010.09.046. [DOI] [PubMed] [Google Scholar]
- 60.Nebel CE, Shin D, Rezek B, Tokuda N, Uetsuka H, Watanabe H. Diamond and biology. Journal of the Royal Society, Interface. 2007;4:439–461. doi: 10.1098/rsif.2006.0196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nemani KV, Moodie KL, Brennick JB, Su A, Gimi B. In vitro and in vivo evaluation of SU-8 biocompatibility. Mater Sci Eng C Mater Biol Appl. 2013;33:4453–4459. doi: 10.1016/j.msec.2013.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Omiatek DM, Bressler AJ, Cans AS, Andrews AM, Heien ML, Ewing AG. The real catecholamine content of secretory vesicles in the CNS revealed by electrochemical cytometry. Sci Rep. 2013;3:1447. doi: 10.1038/srep01447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pasquarelli A, Carabelli V, Xu Y, Colombo E, Gao Z, Scharpf J, Carbone E, Kohn E. Diamond microelectrodes arrays for the detection of secretory cell activity. International Journal of Environmental Analytical Chemistry. 2011;91:150–160. [Google Scholar]
- 64.Picollo F, Battiato A, Bernardi E, Marcantoni A, Pasquarelli A, Carbone E, Olivero P, Carabelli V. Microelectrode Arrays of Diamond-Insulated Graphitic Channels for Real-Time Detection of Exocytotic Events from Cultured Chromaffin Cells and Slices of Adrenal Glands. Anal Chem. 2016;88:7493–7499. doi: 10.1021/acs.analchem.5b04449. [DOI] [PubMed] [Google Scholar]
- 65.Picollo F, Battiato A, Bernardi E, Plaitano M, Franchino C, Gosso S, Pasquarelli A, Carbone E, Olivero P, Carabelli V. All-carbon multi-electrode array for real-time in vitro measurements of oxidizable neurotransmitters. Sci Rep. 2016;6:20682. doi: 10.1038/srep20682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Picollo F, Battiato A, Carbone E, Croin L, Enrico E, Forneris J, Gosso S, Olivero P, Pasquarelli A, Carabelli V. Development and characterization of a diamond-insulated graphitic multi electrode array realized with ion beam lithography. Sensors. 2015;15:515–528. doi: 10.3390/s150100515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Picollo F, Gosso S, Vittone E, Pasquarelli A, Carbone E, Olivero P, Carabelli V. A New Diamond Biosensor with Integrated Graphitic Microchannels for Detecting Quantal Exocytic Events from Chromaffin Cells. Advanced Materials. 2013;25:4696–4700. doi: 10.1002/adma.201300710. [DOI] [PubMed] [Google Scholar]
- 68.Pothos EN, Davila V, Sulzer D. Presynaptic recording of quanta from midbrain dopamine neurons and modulation of the quantal size. J Neurosci. 1998;18:4106–4118. doi: 10.1523/JNEUROSCI.18-11-04106.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rothe J, Frey O, Stettler A, Chen Y, Hierlemann A. Fully integrated CMOS microsystem for electrochemical measurements on 32 × 32 working electrodes at 90 frames per second. Anal Chem. 2014;86:6425–6432. doi: 10.1021/ac500862v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rozlosnik N. New directions in medical biosensors employing poly(3,4-ethylenedioxy thiophene) derivative-based electrodes. Anal Bioanal Chem. 2009;395:637–645. doi: 10.1007/s00216-009-2981-8. [DOI] [PubMed] [Google Scholar]
- 71.Schroeder TJ, Borges R, Finnegan JM, Pihel K, Amatore C, Wightman RM. Temporally resolved, independent stages of individual exocytotic secretion events. Biophys J. 1996;70:1061–1068. doi: 10.1016/S0006-3495(96)79652-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Schroeder TJ, Jankowski JA, Kawagoe KT, Wightman RM, Lefrou C, Amatore C. Analysis of diffusional broadening of vesicular packets of catecholamines released from biological cells during exocytosis. Anal Chem. 1992;64:3077–3083. doi: 10.1021/ac00048a003. [DOI] [PubMed] [Google Scholar]
- 73.Segura F, Brioso MA, Gomez JF, Machado JD, Borges R. Automatic analysis for amperometrical recordings of exocytosis. J Neurosci Methods. 2000;103:151–156. doi: 10.1016/s0165-0270(00)00309-5. [DOI] [PubMed] [Google Scholar]
- 74.Sen A, Barizuddin S, Hossain M, Polo-Parada L, Gillis KD, Gangopadhyay S. Preferential cell attachment to nitrogen-doped diamond-like carbon (DLC:N) for the measurement of quantal exocytosis. Biomaterials. 2009;30:1604–1612. doi: 10.1016/j.biomaterials.2008.11.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Shi BX, Wang Y, Zhang K, Lam TL, Chan HL. Monitoring of dopamine release in single cell using ultrasensitive ITO microsensors modified with carbon nanotubes. Biosens Bioelectron. 2011;26:2917–2921. doi: 10.1016/j.bios.2010.11.037. [DOI] [PubMed] [Google Scholar]
- 76.Sigworth FJ. Electronic design of the patch clamp. In: Sakmann B, Neher E, editors. Single-Channel Recording. New York: Plenum Press; 1995. pp. 95–127. [Google Scholar]
- 77.Spégel C, Heiskanen A, Acklid J, Wolff A, Taboryski R, Emnéus J, Ruzgas T. On-Chip Determination of Dopamine Exocytosis Using Mercaptopropionic Acid Modified Microelectrodes. Electroanalysis. 2007;19:263–271. [Google Scholar]
- 78.Spegel C, Heiskanen A, Pedersen S, Emneus J, Ruzgas T, Taboryski R. Fully automated microchip system for the detection of quantal exocytosis from single and small ensembles of cells. Lab Chip. 2008;8:323–329. doi: 10.1039/b715107a. [DOI] [PubMed] [Google Scholar]
- 79.Stotter J, Zak J, Behler Z, Show Y, Swain GM. Optical and electrochemical properties of optically transparent, boron-doped diamond thin films deposited on quartz. Anal Chem. 2002;74:5924–5930. doi: 10.1021/ac0203544. [DOI] [PubMed] [Google Scholar]
- 80.Sun X, Gillis KD. On-chip amperometric measurement of quantal catecholamine release using transparent indium tin oxide electrodes. Anal Chem. 2006;78:2521–2525. doi: 10.1021/ac052037d. [DOI] [PubMed] [Google Scholar]
- 81.Suzuki I, Fukuda M, Shirakawa K, Jiko H, Gotoh M. Carbon nanotube multi-electrode array chips for noninvasive real-time measurement of dopamine, action potentials, and postsynaptic potentials. Biosens Bioelectron. 2013;49:270–275. doi: 10.1016/j.bios.2013.05.023. [DOI] [PubMed] [Google Scholar]
- 82.van Kempen GT, vanderLeest HT, van den Berg RJ, Eilers P, Westerink RH. Three distinct modes of exocytosis revealed by amperometry in neuroendocrine cells. Biophys J. 2011;100:968–977. doi: 10.1016/j.bpj.2011.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wang CT, Bai J, Chang PY, Chapman ER, Jackson MB. Synaptotagmin-Ca2+ triggers two sequential steps in regulated exocytosis in rat PC12 cells: fusion pore opening and fusion pore dilation. J Physiol. 2006;570:295–307. doi: 10.1113/jphysiol.2005.097378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wang J, Ewing AG. Simultaneous study of subcellular exocytosis with individually addressable multiple microelectrodes. The Analyst. 2014;139:3290–3295. doi: 10.1039/c4an00058g. [DOI] [PubMed] [Google Scholar]
- 85.Wang J, Trouillon R, Dunevall J, Ewing AG. Spatial resolution of single-cell exocytosis by microwell-based individually addressable thin film ultramicroelectrode arrays. Anal Chem. 2014;86:4515–4520. doi: 10.1021/ac500443q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wang J, Trouillon R, Lin Y, Svensson MI, Ewing AG. Individually addressable thin-film ultramicroelectrode array for spatial measurements of single vesicle release. Anal Chem. 2013;85:5600–5608. doi: 10.1021/ac4009385. [DOI] [PubMed] [Google Scholar]
- 87.Wang L, Xu H, Song Y, Luo J, Wei W, Xu S, Cai X. Highly sensitive detection of quantal dopamine secretion from pheochromocytoma cells using neural microelectrode array electrodeposited with polypyrrole graphene. ACS Appl Mater Interfaces. 2015;7:7619–7626. doi: 10.1021/acsami.5b00035. [DOI] [PubMed] [Google Scholar]
- 88.Wightman RM, Jankowski JA, Kennedy RT, Kawagoe KT, Schroeder TJ, Leszczyszyn DJ, Near JA, Diliberto EJ, Jr, Viveros OH. Temporally resolved catecholamine spikes correspond to single vesicle release from individual chromaffin cells. Proc Natl Acad Sci U S A. 1991;88:10754–10758. doi: 10.1073/pnas.88.23.10754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wightman RM, Schroeder TJ, Finnegan JM, Ciolkowski EL, Pihel K. Time course of release of catecholamines from individual vesicles during exocytosis at adrenal medullary cells. Biophys J. 1995;68:383–390. doi: 10.1016/S0006-3495(95)80199-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wigstrom J, Dunevall J, Najafinobar N, Lovric J, Wang J, Ewing AG, Cans AS. Lithographic Microfabrication of a 16-Electrode Array on a Probe Tip for High Spatial Resolution Electrochemical Localization of Exocytosis. Anal Chem. 2016;88:2080–2087. doi: 10.1021/acs.analchem.5b03316. [DOI] [PubMed] [Google Scholar]
- 91.Yakushenko A, Katelhon E, Wolfrum B. Parallel on-chip analysis of single vesicle neurotransmitter release. Anal Chem. 2013;85:5483–5490. doi: 10.1021/ac4006183. [DOI] [PubMed] [Google Scholar]
- 92.Yakushenko A, Schnitker J, Wolfrum B. Printed carbon microelectrodes for electrochemical detection of single vesicle release from PC12 cells. Anal Chem. 2012;84:4613–4617. doi: 10.1021/ac300460s. [DOI] [PubMed] [Google Scholar]
- 93.Yang SY, Kim BN, Zakhidov AA, Taylor PG, Lee JK, Ober CK, Lindau M, Malliaras GG. Detection of transmitter release from single living cells using conducting polymer microelectrodes. Advanced materials. 2011;23:H184–188. doi: 10.1002/adma.201100035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Yao J, Gillis KD. Quantification of noise sources for amperometric measurement of quantal exocytosis using microelectrodes. The Analyst. 2012;137:2674–2681. doi: 10.1039/c2an35157a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Yao J, Liu XA, Gillis KD. Two approaches for addressing electrochemical electrode arrays with reduced external connections. Anal Methods. 2015;7:5760–5766. doi: 10.1039/C5AY00229J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zachek MK, Hermans A, Wightman RM, McCarty GS. Electrochemical Dopamine Detection: Comparing Gold and Carbon Fiber Microelectrodes using Background Subtracted Fast Scan Cyclic Voltammetry. J Electroanal Chem (Lausanne Switz) 2008;614:113–120. doi: 10.1016/j.jelechem.2007.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zhang B, Adams KL, Luber SJ, Eves DJ, Heien ML, Ewing AG. Spatially and temporally resolved single-cell exocytosis utilizing individually addressable carbon microelectrode arrays. Anal Chem. 2008;80:1394–1400. doi: 10.1021/ac702409s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zhang B, Heien ML, Santillo MF, Mellander L, Ewing AG. Temporal resolution in electrochemical imaging on single PC12 cells using amperometry and voltammetry at microelectrode arrays. Anal Chem. 2011;83:571–577. doi: 10.1021/ac102502g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zhao Y, Fang Q, Herbst AD, Berberian KN, Almers W, Lindau M. Rapid structural change in synaptosomal-associated protein 25 (SNAP25) precedes the fusion of single vesicles with the plasma membrane in live chromaffin cells. Proc Natl Acad Sci U S A. 2013;110:14249–14254. doi: 10.1073/pnas.1306699110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Zhou Z, Misler S, Chow RH. Rapid fluctuations in transmitter release from single vesicles in bovine adrenal chromaffin cells. Biophys J. 1996;70:1543–1552. doi: 10.1016/S0006-3495(96)79718-7. [DOI] [PMC free article] [PubMed] [Google Scholar]