To the Editor
The rising number of individuals affected by Alzheimer's disease (AD)[1, 2] is accelerating efforts to develop effective treatment and prevention approaches as policy makers, clinical researchers, drug developers, and other stakeholders fully recognize the scope of the problem. This increasing prioritization of AD-related research requires new recruitment efforts and clinical trial infrastructure to meet the changing scale of AD therapeutic development efforts. As recently identified by Fargo et al. in this journal, recruiting adequate numbers of qualified volunteer participants efficiently is among the biggest challenges facing AD investigators [3]. The pace of recruitment into trials directly impacts the cost of trials and their time to completion [4, 5].
To accelerate enrollment, the University of Kansas Alzheimer’s Disease Center (KU ADC) and the Global Alzheimer’s Platform Foundation [5] have created a novel recruitment model which 1) invests in extensive community-based efforts to promote research participation and 2) develops a centralized and integrated Recruitment Operations program. Here we focus on the latter effort. Rather than tasking study coordinators, already burdened with the demands of coordinating the trial, with identifying and pre-screening potential participants, we created a centralized, dedicated team led by our KU ADC Outreach and Recruitment Core leader and staffed by a lead recruitment coordinator, and two recruitment specialists (4 individuals total). The team supports recruitment and pre-screening operations, similar to recruitment efforts at many contract research organization. Importantly, these positions are replicable, allowing for additional scale as needed.
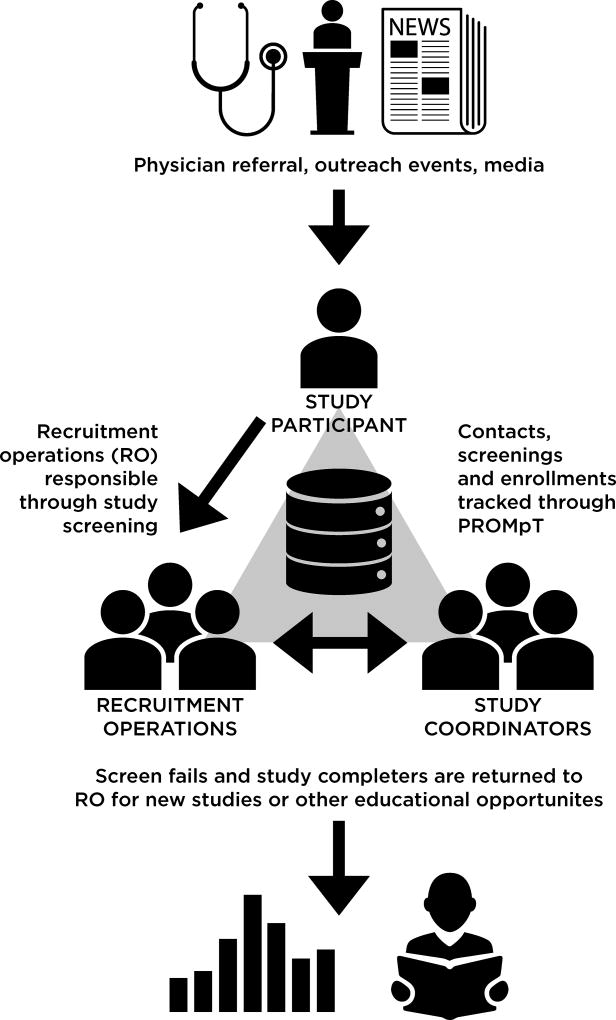
The Recruitment Operations team handles all inquiries into research with a strong focus on “customer service”. We have committed to returning calls in less than 48 business hours, warm transfer of misdirected phone calls, compassionate engagement with prospective participants that includes referral to resources beyond research participation, and consistent contact with Recruitment Operations staff throughout the screening process. We also emphasize continued engagement and offer regular educational opportunities, newsletters, support groups, an active social media presence, and appreciation events for the community regardless of study qualification. Importantly, the Recruitment Operations team is supported by a flexible system we developed in REDCap and Shiny [6, 7] we call our Participant Relations and Outreach Manager for Trials (PROMpT). PROMpT requires only basic database support experience and allows the team to manage interactions with potential participants, improve communication among staff and across units in our Center, and reliably manage handoffs of participants between staff. All contacts are recorded to create continuity and track timely interactions. The figure demonstrates flow through our recruitment process.
Figure.
Potential research participants are guided through the preliminary screening process by our Recruitment Operations (RO) team. All contact and eligibility information is stored in our Participant Relations and Outreach Manager for Trials (PROMpT) for analytics and future use by RO and study coordinators.
Our process begins with a centralized Intake Survey, which serves as the starting point for determining eligibility into all active studies. The survey can be self-administered and mailed in, completed electronically through our website via a PROMpT survey, or completed within PROMpT on the phone with a member of the research team. The survey provides sufficient information (demographics, medical history, current medications and the AD8 [8]) for the Recruitment Operations team to align potential participants with appropriate study options.
Completed Intake Surveys are reviewed collectively by Recruitment Operations staff who then contact the potential participant by phone to further discuss study options and solicit a signed release to obtain medical records. After discussing trial options with the participant, the Recruitment Operations team hands off the information to the appropriate study coordinator for final screening for a specific trial. This occurs at a weekly meeting led by the recruitment coordinator and attended by all study coordinators. These weekly meetings create contact deadlines to minimize loss-to-follow up and bidirectional flow of information regarding eligibility and interest of the potential participant, all of which is tracked in PROMpT.
The KU ADC has assessed preliminary efficiency and referral metrics to examine the impact of our Recruitment Operations team formed in September 2016. Three representative trials were selected, including foundation, pharmaceutical and NIH-sponsored trials; investigational medicine and lifestyle treatment and prevention trials: A4 (NCT02008357), APEx (NCT02000583), STEADFAST (NCT02080364). All studies were open for enrollment through at least the period of January 2016 through March 2017. We compared the first quarter of each calendar year (Table). The results suggest a meaningful improvement in the number of individuals considered for and enrolled into trials and the speed of enrollment, despite a small drop in Intake Surveys submitted over the previous year (303 in Q1 2016 vs. 254 in Q1 2017). Successful screenings by study coordinators (not lost-to-follow-up or screen failed) improved from 33% to 82% while median time from first contact to enrollment decreased by 54%. We attribute this improved efficiency to faster screening and triage to appropriate potential trials at first contact.
Table.
Recruitment performance metrics have improved following the reorganization of the recruitment operations
| Jan.–Mar. 2016 | Jan.–Mar. 2017 | |
|---|---|---|
| Screened for Trial (n) | 36 | 45 |
| Enrolled in Trial (n) | 12 | 37 |
| Median Time to Enrollment (days) | 115 | 53 |
Increased investment by governments, industry and philanthropic interests coupled with the numbers of individuals expected to develop dementia over the next 30 years will continue to drive need for faster recruitment into clinical trials. The KU ADC has benefitted from reorganization of our recruitment processes, improving efficiency and streamlining responsibilities for study coordinators. We do not suggest that our current model represents best practice for all clinical trial organizations as recruitment for trials by nature a highly local activity, with travel distance among the most cited reasons for declining participation[9]. However, the experience of the KU ADC suggests that a novel recruitment model can enhance recruitment into AD-related clinical trials. We believe that investing in recruitment infrastructure should be a priority. Centralizing recruitment for a portfolio of studies to a dedicated staff supported by specialized tracking systems (using free software platforms such as REDCap) is within the capacity of most clinical trial centers and can enhance sharing and flexibility between sites.
Fargo and colleagues have already proposed a National Core for recruitment. Such a Core could facilitate common data collection and identify best practices for AD research recruitment across the country. We believe centralized recruitment models with a strong focus on participant relations are a key component to addressing our great need for research volunteers.
Acknowledgments
The KU ADC recruitment effort is funded in part by grants from the National Institute on Aging P30 AG035982 and R01 AG043962, and by support from the Global Alzheimer’s Platform Foundation. John Dwyer is President of the Global Alzheimer’s Platform Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Scheltens P, Blennow K, Breteler MM, de Strooper B, Frisoni GB, Salloway S, et al. Alzheimer's disease. Lancet. 2016;388:505–17. doi: 10.1016/S0140-6736(15)01124-1. [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization. Dementia: a public health priority. Geneva: 2012. [Google Scholar]
- 3.Fargo KN, Carrillo MC, Weiner MW, Potter WZ, Khachaturian Z. The crisis in recruitment for clinical trials in Alzheimer's and dementia: An action plan for solutions. Alzheimers Dement. 2016;12:1113–5. doi: 10.1016/j.jalz.2016.10.001. [DOI] [PubMed] [Google Scholar]
- 4.Cummings J, Aisen PS, DuBois B, Frolich L, Jack CR, Jr, Jones RW, et al. Drug development in Alzheimer's disease: the path to 2025. Alzheimers Res Ther. 2016;8:39. doi: 10.1186/s13195-016-0207-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cummings J, Aisen P, Barton R, Bork J, Doody R, Dwyer J, et al. Re-Engineering Alzheimer Clinical Trials: Global Alzheimer's Platform Network. J Prev Alzheimers Dis. 2016;3:114–20. doi: 10.14283/jpad.2016.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–81. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.R Core Team. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2013. [Google Scholar]
- 8.Galvin JE, Roe CM, Powlishta KK, Coats MA, Muich SJ, Grant E, et al. The AD8: A brief informant interview to detect dementia. Neurology. 2005;65:559–64. doi: 10.1212/01.wnl.0000172958.95282.2a. [DOI] [PubMed] [Google Scholar]
- 9.Locke DE, Greenaway MC, Duncan N, Fields JA, Cuc AV, Snyder CH, et al. A patient-centered analysis of enrollment and retention in a randomized behavioral trial of two cognitive rehabilitation interventions for Mild Cognitive Impairment. J Prev Alzheimers Dis. 2014;1:143–50. doi: 10.14283/jpad.2014.27. [DOI] [PMC free article] [PubMed] [Google Scholar]