Abstract
Background
Parenchymal changes after stereotactic body radiation therapy (SBRT) make differential diagnosis between treatment outcomes and disease recurrence often difficult. The purpose of our study was to identify the radiographic features detectable at computed tomography (CT) scan [high-risk features (HRFs)] that allow enough specificity and sensitivity for early detection of recurrence.
Methods
We retrospectively evaluated patients who underwent SBRT for inoperable early stage non-small cell lung cancer (NSCLC). The median delivered dose performed was 50 Gy in 5 fractions prescribed to 80% isodose. All patients underwent chest CT scan before SBRT and at 3, 6, 12, 18, 24 months after, and then annually. Each CT scan was evaluated and benign and HRFs were recorded. 18F-fluorodeoxyglucose-CT was not used routinely.
Results
Forty-five patients were included (34 males, 11 females; median age: 77 years; stage IA: 77.8%, stage IB: 22.2%; median follow-up: 21.7 months). Two year and actuarial local control was 77%. HRFs were identified in 20 patients. The most significant predictor of relapse was an enlarging opacity at 12 months (P<0.001) with 84.6% sensitivity and 71.8% specificity. The presence of ≥2 HRFs demonstrated a high sensibility (92.3%) and specificity (71.9%) (P<0.0001).
Conclusions
Detection of HRFs is predictive of relapse with a sensibility that increases with the number of HRFs observed. This observation may allow to better define the diagnostic follow algorithm up suggesting to performing further exams only in patients with >2 HRFs.
Keywords: Stereotactic body radiation therapy (SBRT), non-small cell lung cancer (NSCLC), radiological predictor, local recurrence
Introduction
Stereotactic body radiation therapy (SBRT) treatments are delivered with few high-dose fractions with a dose distribution conformed to the target and a steep dose gradient at the target boundary.
SBRT is now the gold standard treatment in patients with early stage non-small cell lung cancer (NSCLC) unfit for surgery (1-4). Furthermore, the outcome after SBRT is at least not inferior to surgery in operable patients with early stage NSCLC (5). Although local recurrences after SBRT for early stage NSCLC are uncommon (3,6), an early detection may allow curative surgical treatment in selected patients (6).
Benign parenchymal changes after SBRT are different from those observed after 3-dimensional conformal radiotherapy (7) and can mimic the appearance of recurrent disease because of similar size and morphology (8,9). Huang and colleagues after a systematic review of the literature identified radiological patterns of benign (early and late) parenchymal changes and high-risk features (HRFs) for recurrence. Acute benign changes (within 6 months post SBRT) included diffuse ground glass opacities (GGO), patchy GGO, diffuse consolidation, and patchy consolidation. Late changes (after 6 months post SBRT) were subdivided in a modified conventional pattern, mass-like fibrosis, and scar-like fibrosis. On the contrary, HRFs were represented by an enlarging opacity at the primary site, enlarging opacity after 12 months, sequential enlarging opacity, bulging margin, loss of linear margin and loss of air bronchogram (including partial loss) (10). One year later, the same authors identified a new HRF: growth in the cranio-caudal direction (11).
Therefore, the aim of this study is to validate the HRFs described in the literature as predictive of recurrence, and to determine the sensitivity and specificity of each of them.
Methods
We reviewed all patients with early stage NSCLC treated with curative SBRT in the Radiation Department of Bellaria Hospital, Bologna from January 2006 to March 2012 with follow-up performed in the same. The study was approved by the local Ethical Committee and all the patients have given written informed consent for the study. In 29 patients the diagnosis of NSCLC was based on transthoracic fine needle aspiration; in 16 patients with poor respiratory status a biopsy was not possible. The criteria followed to make diagnosis of primary lung tumor were a lung nodule increasing in size at a subsequent computed tomography (CT) scan performed after 3 months and positive at 18F-fluorodeoxyglucose-positron emission tomography/CT (18F-FDG-PET/CT).
Patients immobilization and treatment features were previously reported (12). Briefly, a vacuum pillow in the stereotactic body frame (SBF©; Elekta, Stockholm, Sweden) was used for immobilization and abdominal compression was employed to reduce the movements of the diaphragm. The dose delivered was adapted to the location of the tumor and to the constraints for normal tissues. Median dose was 50 Gy in 5 fractions (range, 40–60 Gy), prescribed to 80% isodose. Treatment was delivered with a linear accelerator using 7–12 fixed coplanar or non-coplanar fields with 10 MV photon energy. A cone beam CT was performed daily before each treatment fraction to check the position of the target volume, and online set-up adjustments were obtained by mean of a robotic couch (HEXAPOD-EVO©; Elekta, Stockholm, Sweden).
In the study were included only patients who underwent a contrast or non-contrast enhanced CT scans before and after SBRT (at 3, 6, 12, 18, 24 months and then annually). More frequent CT imaging was obtained in cases of suspected recurrence. 18F-FDG-PET/CT scanning was not routinely used in staging and follow-up. Only when disease recurrence was suspected, a 18F-FDG-PET/CT was performed. PET/CT was interpreted as indicative of recurrence when standardized uptake value (SUV) was >5 or SUV was higher than that observed at initial 18F-FDG-PET/CT scan. The HRFs were defined radiologically as previously described (10,11).
One radiologist and one radiation oncologist with experience in interpreting SBRT changes independently reviewed all CT scan to identify benign and HRFs and any discrepancies were resolved by consensus. Chi-square test was used to compare the radiographic changes between recurrences and non-recurrence group. Sensitivity and specificity for each individual HRF were calculated, along with the sensitivity and specificity for each additional cumulative HRF.
Results
Forty-five patients with stage I NSCLC unsuitable for surgery and treated with SBRT were included in this retrospective study. Median age was 77 years (range, 60–96 years), 11 patients were female and 34 were male. All CT scan images from May 2006 to March 2015 were reviewed. Thirty-five patients had stage IA and ten patients stage IB NSCLC with a median follow-up of 21.7 months (range, 4–69 months), 13 patients had local failure (4 local failure within 12 months after SBRT, 6 local failure between 12–24 months and 3 after 24 months). Two-year local control was 77%. Eleven patients had lymph nodes metastases or distant failure. Table 1 shows a summary of patients and tumors features.
Table 1. Summary of patient and tumor features.
| Characteristics | Number of patients (%) | Local recurrence (%) | No recurrence (%) | P value |
|---|---|---|---|---|
| Sex | 0.370 | |||
| Male | 34 (75.6) | 11 (32.4) | 23 (67.6) | |
| Female | 11 (24.4) | 2 (18.2) | 9 (81.8) | |
| Stages | 0.370 | |||
| IA | 35 (77.8) | 12 (34.3) | 23 (65.7) | |
| IB | 10 (22.2) | 1 (10.0) | 9 (90.0) | |
| Tumor size | 0.630 | |||
| T1a | 20 (44.4) | 5 (25.0) | 15 (75.0) | |
| T1b | 16 (35.6) | 6 (37.5) | 10 (62.5) | |
| T2a | 9 (20.0) | 2 (22.2) | 7 (77.8) | |
| BED | 0.720 | |||
| <100 Gy | 29 (64.4) | 11 (37.9) | 18 (62.1) | |
| >100 Gy | 16 (35.6) | 2 (12.5) | 14 (87.5) | |
| Margins | 0.048 | |||
| Spiculated | 28 (62.2) | 5 (17.9) | 23 (82.1) | |
| Polylobate | 7 (15.6) | 5 (71.4) | 2 (28.6) | |
| Smooth | 4 (8.9) | 1 (25.0) | 3 (75.0) | |
| Irregular | 6 (13.3) | 2 (33.3) | 4 (66.7) |
BED, biologically effective dose.
The radiological finding of tumor margins in the pre-SBRT CT scan was assessed. The most common was spiculated margin in 28 (62.2%) patients. Other tumor margins aspects were polylobate in 7 (15.6%) patients, irregular in 6 (13.3%) patients, and smooth in 4 (8.9%) patients. Acute benign CT scan changes were identified in 74.4% of patients. In order of decreasing frequency, the acute benign patterns were patchy consolidation (50%), patchy GGO (18.7%), diffuse consolidation (15.6%) and diffuse GGO (15.6%). Late benign features were identified in 88.3% of CT scans and the most frequent was mass-like fibrosis (41.5%). Other patterns were conventional changes (32%), and scar-like fibrosis (26.4%).
High-risk CT features were identified in 20 (44.4%) patients. No HRFs was observed at 3 months after SBRT while at 6, 12 and >12 months the number of identified HRFs was identified 13, 37 and 21, respectively. The most frequent were enlarging opacity after 12 months (44.4%) and loss of air bronchograms (40.0%). Other identified HRFs were growth in the cranio-caudal direction (33.3%), enlarging opacity at the primary site (20.0%), bulging margin (15.6%) and loss of linear margin (4.4%). Except the last one (loss of linear margin), all the other HRFs were individually significantly associated to local recurrence. Table 2 shows a summary of the results. Figure 1 shows CT images of patients after SBRT with local recurrence and Figure 2 CT images of benign and high-risk CT features.
Table 2. Summary of frequency of high-risk features.
| HRFs | Patients (%) | Recurrence (%) | Non-recurrence (%) | P value | Sensibility | Specificity | Odd ratio |
|---|---|---|---|---|---|---|---|
| Bulging margin | 7 (15.6) | 6 (85.7) | 1 (14.3) | <0.001 | 46.1 | 96.8 | 26.5 |
| Cranio-caudal growth | 15 (33.3) | 10 (66.7) | 5 (33.3) | <0.001 | 76.9 | 84.4 | 18.0 |
| Enlargement after 12 months | 20 (44.4) | 11 (55.0) | 9 (45.0) | 0.001 | 84.6 | 71.8 | 14.0 |
| Loss of air bronchogram | 18 (40.0) | 10 (55.6) | 8 (44.4) | 0.001 | 76.9 | 75.0 | 10.0 |
| Linear margin disappearance | 2 (4.4) | 1 (50.0) | 1 (50.0) | 0.500 | 7.7 | 96.9 | 2.5 |
| Enlarging opacity | 9 (20.0) | 3 (33.3) | 6 (66.7) | 0.740 | 23.0 | 81.0 | 1.3 |
HRF, high-risk feature.
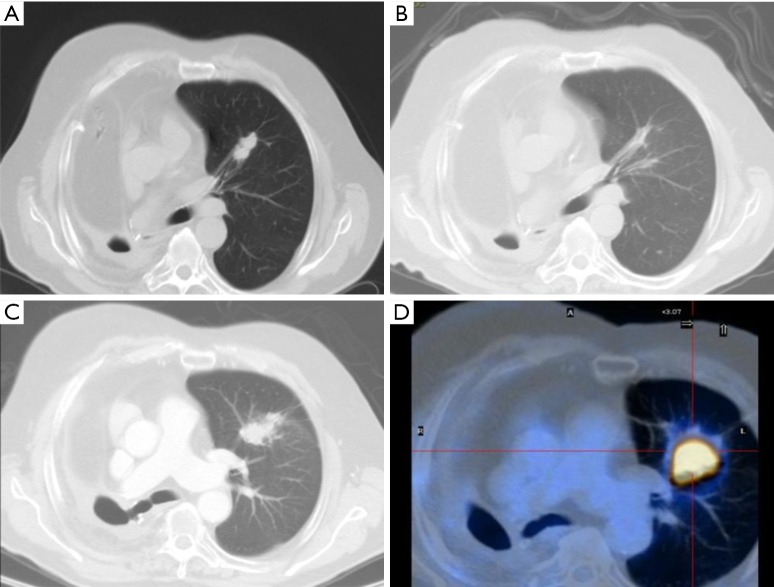
Figure 1.
CT images of patients after SBRT with local recurrence. (A) Seventy-four years old male, CT scan pre-SBRT NSCLC stage IB. Previously right pneumonectomy for lung cancer; (B) 6 months after SBRT: scar-like fibrosis; (C) 12 months after SBRT: enlargement after 12 months, loss of air bronchogram, bulging margin; (D) 18 months after SBRT relapse SUVmax: 25.8. CT, computed tomography; SBRT, stereotactic body radiation therapy; NSCLC, non-small cell lung cancer; SUV, standardized uptake value.
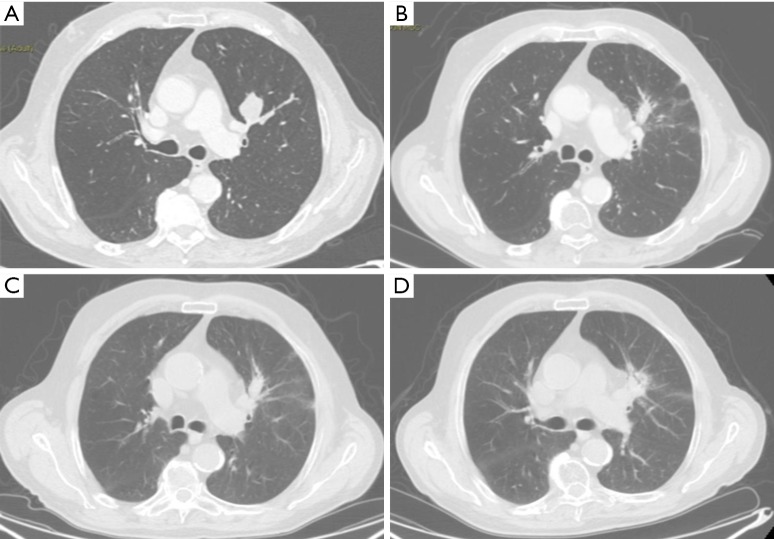
Figure 2.
CT images of benign and high-risk CT features. (A) Eighty-three years old male, CT scan pre-SBRT, NSCLC stage IB; (B) 6 months after SBRT: scar-like fibrosis, patchy GGO; (C) 12 months after SBRT: no differences respect the first one; (D) 24 months after SBRT: mass-like fibrosis. CT, computed tomography; SBRT, stereotactic body radiation therapy; NSCLC, non-small cell lung cancer; GGO, ground glass opacities.
All patients with recurrence had one or more HRFs. Both caudal cranial growth and enlargement of the opacity after 12 months showed high values of sensitivity (76.9% and 84.6%, respectively) and specificity (84.4% and 71.8%, respectively). The appearance of a consolidation with bulging edges was a feature with low sensitivity (46.1%) but with high specificity (96.8%). Patients with a bulging margin was observed a 26.5 times higher risk of tumor recurrence compared to patients without this feature.
Table 3 shows a summary of the sensitivity and specificity of detecting recurrence based on cumulative number of HRFs. The presence of only one HRFs showed high sensitivity (92.3%) but a low specificity (59.4%), reflecting a high false-positive rate with a low cut-off. With an increasing number of HRFs, specificity increased while sensitivity declined.
Table 3. Specificity and sensibility of CT based on number of HRFs.
| No. of HRF | Patients (n) | Recurrence (%) | Non-recurrence (%) | P value | Sensibility | Specificity |
|---|---|---|---|---|---|---|
| ≥1 | 25 | 12 (48.0) | 13 (52.0) | 0.002 | 92.3 | 59.4 |
| ≥2 | 21 | 12 (57.1) | 9 (42.9) | <0.001 | 92.3 | 71.9 |
| ≥3 | 13 | 9 (69.2) | 4 (30.8) | <0.001 | 69.2 | 87.5 |
| ≥4 | 9 | 7 (77.8) | 2 (22.2) | <0.001 | 53.8 | 93.7 |
CT, computed tomography; HRFs, high-risk features.
No other factor [gender, tumor size, stage, biological effective dose (BED)] was significantly associated with local recurrences, although a lower BED (<100 Gy) was associated to a lower local control as previously reported (13).
In our study with 45 patients, 13 patients had local failure. All patients with local failure had one or more HRFs. Except loss of linear margin, all the other HRFs were individually associated to local failure. With an increasing number of HRFs, specificity increased while sensitivity declined.
Our study has some limitations: diagnosis of NSCLC without pathological confirmation, retrospective nature, modest sample size, recurrence defined only by CT imaging without biopsy.
However, most series of SBRT had patients without pathological confirmation due to comorbidity (14,15).
SBRT is a standard approach for inoperable patients with early stage NSCLC (1-4). The efficacy of SBRT even in patients with early and operable NSCLC has been suggested by a recent pooled analysis of two randomized trials [ROSEL (NCT00687986) and STARS (NCT00840749)] comparing SBRT versus surgery in operable stage I NSCLC. Three-year overall survival after SBRT and surgery was 95% and 79%, respectively. Three-year recurrence-free survival was 86% in the SBRT group and 80% in the surgery group, respectively. Therefore, the authors concluded that SBRT could be an option even in operable stage I NSCLC (5).
The possibility to extend the prescription of SBRT also in the operable setting makes an early detection of recurrences crucial for salvage treatment (6). Local recurrence after SBRT are not common, and most of them appear in the first 2 years after treatment (3,6). Benign lung changes after SBRT are common. Late changes can mimic recurrence making early diagnosis difficult (5,6). Hence, the identification of HRFs to predict recurrences is very important.
Unfortunately, the classics Response Evaluation Criteria In Solid Tumors (RECIST) are poorly predictive.
In fact, Hayashi et al. reported the overall accuracy of RECIST for predicting tumor recurrence at 2–5 months and at 5–8 months after SBRT: 52.2% [false negative rate (FNR): 25.0% and false positive rate (FPR): 72.7%) and 65.2% (FNR: 45.5% and FPR: 27.3%), respectively, confirming that criteria based on the size of the lesions showing an increased density are not reliable (16).
Also, 18F-FDG-PET/CT has limitations, FDG avidity can appear transiently following SBRT and can persist at a low value for over 12 months due to inflammatory reaction lung parenchyma and in tumor (17). The optimal time recommendation of PET/CT examination after SBRT has not yet been established, also if it is advisable to perform a PET scan not before than 6 months due to the possible interference of the inflammatory changes in the lungs which follow high-dose irradiation. However, in presence of high suspicion of recurrence at CT, SUVmax ≥5.0 after SBRT or greater than the original pretreatment SUVmax appear suggestive of recurrent disease and a biopsy is recommended if salvage surgery is possible (10,18).
On the contrary, in a study similar to ours, Hayashi and coworkers evaluated CT patterns of 81 patients with early stage NSCLC who underwent SBRT (main delivered dose was 48 Gy in 4 fractions, 8–11 conformal static field). The authors classified CT patterns into two categories: mass-like fibrosis and others. Then was made a subdivision of mass-like fibrosis category into: mass-like consolidation and mass-like opacity. Six patients had local recurrences with pathological confirmation. Five patients with local recurrences presented with the mass-like opacity pattern, compared with 33% of patients from the non-recurrent group (P=0.01) and showed an increase in maximum diameter at ≥12 months after SBRT. Also, a significantly higher SUV was reported in the recurrences group (P<0.001), with all values >5 (range, 5.7–25.4). The authors concluded that a mass-like opacity pattern, an increasing maximum diameter ≥12 months after SBRT, and SUVmax >5 of the mass-like fibrosis should be considered indicators of local recurrence (18).
Furthermore, Huang and colleagues analysed 36 patients with early stage NSCLC treated with SBRT (delivered dose was 54–60 Gy, 3–8 risk-adapted fractions, based on tumor size and location) who developed pathology-proven local recurrence (n=12). These patients were matched 1:2 to patients without recurrences (n=24). Serial CT images were assessed by blinded radiation oncologists. The combination of HRFs permitted a highly sensitive and specific diagnosis of local recurrence without functional imaging (11).
Our study confirmed the resulted reported in Huang’s series that the most accurate predictor of local recurrence is an enlarging opacity after 12 months, which does not allow a confinable detection of recurrence within the first year after diagnosis.
The results of our study showed that the identification of HRFs can detect recurrence. These results seem better than the classical criteria of RECIST (17).
The identification of local recurrence after SBRT is a challenging problem but is very important, seems the indication of this method are increasing. An improvement of performance of imaging tests in the future may result from the use of method of radiomics which involves the high-throughput extraction of quantitative imaging features with the intent of creating mineable databases from radiological images (19).
Conclusions
Detection of HRFs is predictive of relapse with a sensibility that increases with the number of HRF’s observed. This observation allows us to better define the diagnostic algorithm in the follow-up suggesting performing further exams only in patients that have two or more HRFs. However, prospective studies are needed since our data are retrospective, the sample size is modest, and recurrence was defined on CT or PET/CT imaging without biopsy.
Acknowledgements
The authors thank all the physicians, physicist and radiographers who have been involved in the development of the stereotactic ablative radiotherapy program.
Ethical Statement: The study was approved by the Bellaria Hospital Ethical Committee and all the patients have given written informed consent for the study.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Baumann P, Nyman J, Hoyer M, et al. Outcome in a prospective phase II trial of medically inoperable stage I non-small-cell lung cancer patients treated with stereotactic body radiotherapy. J Clin Oncol 2009;27:3290-6. 10.1200/JCO.2008.21.5681 [DOI] [PubMed] [Google Scholar]
- 2.Onishi H, Shirato H, Nagata Y, et al. Hypofractionated stereotactic radiotherapy (HypoFXSRT) for stage I non-small cell lung cancer: updated results of 257 patients in a Japanese multi-institutional study. J Thorac Oncol 2007;2:S94-100. 10.1097/JTO.0b013e318074de34 [DOI] [PubMed] [Google Scholar]
- 3.Timmerman R, Paulus R, Galvin J, et al. Stereotactic body radiation therapy for inoperable early stage lung cancer. JAMA 2010;303:1070-6. 10.1001/jama.2010.261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ricardi U, Frezza G, Filippi AR, et al. Stereotactic Ablative Radiotherapy for stage I histologically proven non-small cell lung cancer: an Italian multicenter observational study. Lung Cancer 2014;84:248-53. 10.1016/j.lungcan.2014.02.015 [DOI] [PubMed] [Google Scholar]
- 5.Chang JY, Senan S, Paul MA, et al. Stereotactic ablative radiotherapy versus lobectomy for operable stage I non-small-cell lung cancer: a pooled analysis of two randomised trials. Lancet Oncol 2015;16:630-7. 10.1016/S1470-2045(15)70168-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Verstegen NE, Lagerwaard FJ, Hashemi SM, et al. Patterns of Disease Recurrence after SABR for Early Stage Non-Small-Cell Lung Cancer: Optimizing Follow-Up Schedules for Salvage Therapy. J Thorac Oncol 2015;10:1195-200. 10.1097/JTO.0000000000000576 [DOI] [PubMed] [Google Scholar]
- 7.Larici AR, del Ciello A, Maggi F, et al. Lung abnormalities at multimodality imaging after radiation therapy for non-small cell lung cancer. Radiographics 2011;31:771-89. 10.1148/rg.313105096 [DOI] [PubMed] [Google Scholar]
- 8.Takeda A, Kunieda E, Takeda T, et al. Possible misinterpretation of demarcated solid patterns of radiation fibrosis on CT scans as tumor recurrence in patients receiving hypofractionated stereotactic radiotherapy for lung cancer. Int J Radiat Oncol Biol Phys 2008;70:1057-65. 10.1016/j.ijrobp.2007.07.2383 [DOI] [PubMed] [Google Scholar]
- 9.Linda A, Trovo M, Bradley JD. Radiation injury of the lung after stereotactic body radiation therapy (SBRT) for lung cancer: a timeline and pattern of CT changes. Eur J Radiol 2011;79:147-54. 10.1016/j.ejrad.2009.10.029 [DOI] [PubMed] [Google Scholar]
- 10.Huang K, Dahele M, Senan S, et al. Radiographic changes after lung stereotactic ablative radiotherapy (SABR)--can we distinguish recurrence from fibrosis? A systematic review of the literature. Radiother Oncol 2012;102:335-42. 10.1016/j.radonc.2011.12.018 [DOI] [PubMed] [Google Scholar]
- 11.Huang K, Senthi S, Palma DA, et al. High-risk CT features for detection of local recurrence after stereotactic ablative radiotherapy for lung cancer. Radiother Oncol 2013;109:51-7. 10.1016/j.radonc.2013.06.047 [DOI] [PubMed] [Google Scholar]
- 12.Frakulli R, Salvi F, Balestrini D, et al. Stereotactic Radiotherapy in the Treatment of Lung Metastases from Bone and Soft-tissue Sarcomas. Anticancer Res 2015;35:5581-6. [PubMed] [Google Scholar]
- 13.Boily G, Filion É, Rakovich G, et al. Stereotactic Ablative Radiation Therapy for the Treatment of Early-stage Non-Small-Cell Lung Cancer: CEPO Review and Recommendations. J Thorac Oncol 2015;10:872-82. 10.1097/JTO.0000000000000524 [DOI] [PubMed] [Google Scholar]
- 14.Haidar YM, Rahn DA, 3rd, Nath S, et al. Comparison of outcomes following stereotactic body radiotherapy for non-small cell lung cancer in patients with and without pathological confirmation. Ther Adv Respir Dis 2014;8:3-12. 10.1177/1753465813512545 [DOI] [PubMed] [Google Scholar]
- 15.Takeda A, Kunieda E, Sanuki N, et al. Stereotactic body radiotherapy (SBRT) for solitary pulmonary nodules clinically diagnosed as lung cancer with no pathological confirmation: comparison with non-small-cell lung cancer. Lung Cancer 2012;77:77-82. 10.1016/j.lungcan.2012.01.006 [DOI] [PubMed] [Google Scholar]
- 16.Hayashi S, Tanaka H, Hoshi H. Imaging characteristics of local recurrences after stereotactic body radiation therapy for stage I non-small cell lung cancer: Evaluation of mass-like fibrosis. Thorac Cancer 2015;6:186-93. 10.1111/1759-7714.12162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mattonen SA, Palma DA, Haasbeek CJ, et al. Early prediction of tumor recurrence based on CT texture changes after stereotactic ablative radiotherapy (SABR) for lung cancer. Med Phys 2014;41:033502. 10.1118/1.4866219 [DOI] [PubMed] [Google Scholar]
- 18.Huang K, Palma DA, IASLC Advanced Radiation Technology Committee Follow-up of patients after stereotactic radiation for lung cancer: a primer for the nonradiation oncologist. J Thorac Oncol 2015;10:412-9. 10.1097/JTO.0000000000000435 [DOI] [PubMed] [Google Scholar]
- 19.Lambin P, Rios-Velazquez E, Leijenaar R, et al. Radiomics: extracting more information from medical images using advanced feature analysis. Eur J Cancer 2012;48:441-6. 10.1016/j.ejca.2011.11.036 [DOI] [PMC free article] [PubMed] [Google Scholar]