Abstract
Background
Programmed death-1 (PD-1) and programmed death ligand-1 (PD-L1) inhibitor therapy is showing marked efficacy in advanced non-small cell lung cancer (NSCLC). Meanwhile, it is concomitant with distinctive immune-related adverse effects. We aim to describe the incidence of pneumonitis and other rare but severe immune-related adverse effects (IRAEs), as well as treatment related deaths. In addition, we analyze the differences in incidence of pneumonitis between PD-1 and PD-L1 inhibitors and standard-of-care chemotherapy.
Methods
PubMed was searched up to 24 March 2017 for clinical trials of PD-1 inhibitors (nivolumab and pembrolizumab) and PD-L1 inhibitors (atezolizumab, avelumab and durvalumab) in treatment of NSCLC. Besides, references of relevant articles were screened.
Results
Finally, 22 trials were included in our study, 14 with data of pneumonitis, 19 with other severe IRAEs or treatment related deaths and 5 with control groups. Incidence of all-grade pneumonitis was 2.9% (95% CI, 2.0–4.8%) and grade 3 or higher pneumonitis 2.0% (95% CI, 1.0–2.0%). Incidence of all-grade pneumonitis in PD-1 and PD-L1 inhibitor therapy (n=1,313) was significantly higher than that in chemotherapy (n=918) (OR=2.35, 95% CI, 1.32–4.20, P=0.004), but had no significance in grade 3–5 pneumonitis. Incidence of cardiorespiratory arrest (n=537) was 1.0% (95% CI, 0–2.0%), cardiac failure (n=214) 2.0% (95% CI, 1.0–5.7%), myocardial infarction (n=402) 1.0% (95% CI, 0–3.8%), stroke (n=135) 2.0% (95% CI, 0–13.0%), disease progression (n=391) 1.0% (95% CI, 0–2.9%), pancreatitis (n=700) 1.0% (95% CI, 0–2.0%) and severe skin reactions (n=836) 2.0% (95% CI, 1.0–3.8%). Incidence of treatment related deaths was 0.7%.
Conclusions
Immune related adverse effects can on occasion be life-threatening even though usually rare. Incidence of pneumonitis in PD-1 and PD-L1 inhibitors was significantly higher than that in chemotherapy. More studies should be conducted to investigate the incidence of these rare but life-threatening IRAEs.
Keywords: Adverse drug events, immunotherapies, pneumonitis, programmed cell death 1 protein, programmed cell death 1 ligand 1
Introduction
Programmed death-1 (PD-1) and programmed death ligand-1 (PD-L1) inhibitors are thriving in anti-cancer treatment. PD-1 was expressed by activated T lymphocytes. It combines with its ligands PD-L1 to restrict the activation of T lymphocytes and prevent autoimmune disease. Monoclonal antibodies targeting PD-1 and PD-L1 can block the immune checkpoint and eliminate the inhibition of T lymphocytes activation. As a result, they largely enhance immune reactions to fight against malignant tumors (1,2). Several PD-1 inhibitors (nivolumab and pembrolizumab) and PD-L1 inhibitors (atezolizumab, avelumab and durvalumab) are showing distinct activities and efficacy in treatment of advanced melanoma, renal cell carcinoma and non-small cell lung cancer (NSCLC) or Hodgkin lymphoma.
However, for its character of blocking inhibitory regulation and leading to unregulated activation of immune reaction, when the potency of fighting against cancer increased significantly, it may also affect other organs and injure normal tissues, which consequently lead to immune-related adverse effects (IRAEs) (3). A wide range of organs and systems may be involved, and skin, gastrointestinal tract and liver are the most commonly affected. Preclinical researches, animal experiments and pathological specimens of clinical patients found abundant infiltration of activated T CD8 positive lymphocytes in involved organs. Usually, IRAEs of checkpoint inhibitors are mild and could be managed by clinicians. However, some serious adverse effects must be controlled by discontinuation of drugs, temporarily or even permanently. Pneumonitis is one of the IRAEs that reported by most clinical trials, and severe cases may lead to death. Besides, some rare toxic effects affecting cardiovascular and respiratory systems should raise our concern because they may be deadly once occur and possibly unrecognized as immune-related adverse effects. Incidence of pneumonitis in PD-1 and PD-L1 inhibitor therapy has been reported in several metanalysis, as well as differences in incidence between NSCLC and melanoma, and PD-1 and PD-L1 inhibitors (4,5). However, no metanalysis explored the incidence of these rare but life-threatening IRAEs, such as immune related myocarditis.
The objective of this study was to investigate the incidence of pneumonitis and other rare but life-threatening adverse effects, as well as treatment related deaths in PD-1 and PD-L1 inhibitor therapy for treatment of NSCLC. Meanwhile, we compare differences in incidence of pneumonitis between PD-1 and PD-L1 inhibitor therapy and standard-of-care chemotherapy.
Methods
Search methods and study selection
Clinical trials of PD-1 and PD-L1 inhibitors for treatment of lung neoplasms were searched through PubMed, using the key words of PD-1 and PD-L1 monoclonal antibody, nivolumab, pembrolizumab, atezolizumab, avelumab and durvalumab. Besides, references of relevant articles were searched for meeting abstracts and possible omission. Articles meeting the following criteria were included: (I) phases I–III clinical trials; (II) measure the safety profile of PD-1 and PD-L1 inhibitors; (III) single-arm trials of PD-1 and PD-L1 inhibitors or PD-1 and PD-L1 inhibitors versus chemotherapy. Trials of combined therapy of cytotoxic T lymphocyte associated antigen 4 (CTLA-4) inhibitors and PD-1 and PD-L1 inhibitors were excluded for that they would increase the incidence of pneumonitis and we may fail to distinguish the adverse effects of which drug. When results of the same clinical trial were reported at different time, only the most recent, or the most complete report was included. All the articles were screened independently by two authors (YB Hu and Q Zhang).
Data extraction
Data including clinical trial information of the study, first author, year of publication, trial phases, study drugs, total number of patients evaluated for safety, number of patients with pneumonitis, other rare but life-threatening adverse effects including cardiac failure, cardiorespiratory arrest, myocardial infarction, stroke, disease progression, pancreatitis, severe skin reactions, sepsis, pulmonary embolism, respiratory arrest, respiratory failure, constrictive pericarditis, cardiac tamponade, pericardial effusion, encephalitis, myocarditis, sarcoidosis, endophthalmitis, and myasthenia gravis, as well as treatment related deaths were extracted. Infectious related pneumonia was not included. Because phase I trials were commonly dose escalation, the number of an adverse event which developed from the study drug were counted together, regardless of doses. Any data not reported in the primary articles were represented as “not applicable”. We didn’t contact the author for detailed information. The main outcomes were incidence of pneumonitis and other rare but life-threatening adverse effects in PD-1 and PD-L1 inhibitor therapy, cases of treatment related deaths, and differences in incidence of pneumonitis between checkpoint inhibitor therapy and chemotherapy.
Statistical analysis
Review manager version 5.3 was used for statistical analysis. For trials with control groups, data type of dichotomous was selected to compare the incidence of pneumonitis between PD-1 and PD-L1 inhibitor therapy and standard-of-care chemotherapy. For single arm trials, data type of generic inverse variance was selected.
For the outcome of incidence, if P value was not close to 0 or 1 and both n*P and n*(1–P) were more than 5, P could be calculated by n/X [1], in which n and X referred to the number of patients with pneumonitis and total number of patients evaluated for safety, respectively. Standard error (SE) could be calculated using the formula of (P(1–P)/n)1/2 [2], in which P and n had the same meaning with above. On the other hand, if the above condition could not be met, P and SE must be calculated by the below formulas: P= ln(odds) = ln[X/(n–X)] [3], SE(P) = SE[ln(odds)] = [1/X+1/(n–X)]1/2[4]. Significantly, this is the method used for categorical data, thus the calculations OR should be transformed using the following formula: Pf = OR/(1+ OR) [5], lower limit (LL) of 95% confidence interval (CI) = LLOR/(1+ LLOR) [6], upper limit (UL) = ULOR/(1+ ULOR) [7]. Due to the low incidence of adverse effects in our study, the latter method was adopted.
Heterogeneity was evaluated by Cochran chi-square test and the I2 test. Heterogeneity was thought to be exist if P value was less than 0.05. If the homogeneity of ORs were fine (P>0.05), a fixed effect model was used; if not (P<0.05), a random effect model was used. I2<30% represented a slight level of heterogeneity, 30–60% was moderate while I2>60% meant the heterogeneity was high. Chi-square test was performed for rate differences between two or more categorical data by SPSS version 20.0.
Results
Eligible studies and characteristics
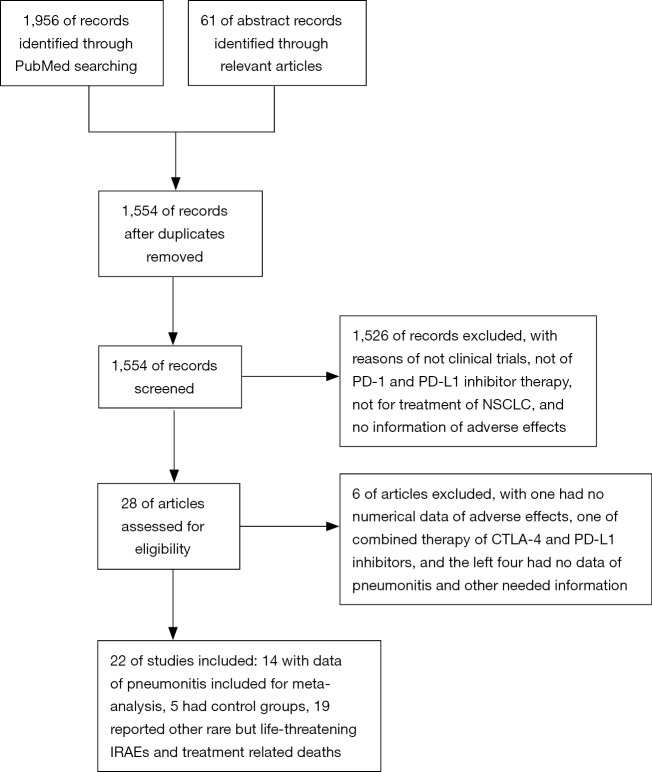
Totally, 1,956 publications were searched on PubMed up to 24 March 2017 and 61 abstracts were identified from reference lists of relevant articles. A total of 1,554 texts were screened after duplications removed, and 1,526 were excluded for reasons of not clinical trials, not of PD-1 and PD-L1 inhibitor therapy, not for treatment of NSCLC, and no information of adverse effects. Consequently, 28 articles were assessed for eligibility, and six were excluded, with one had no numerical data of adverse effects, one of combined therapy of CTLA-4 and PD-L1 inhibitor, and the left four had no data of pneumonitis and other needed information. Finally, 22 trials were included (6-27), 14 with data of pneumonitis were included for meta-analysis (6-10,12-18,20,21) (Figure 1). Five had control groups, with two of nivolumab vs. docetaxel (13,14), one of pembrolizumab vs. docetaxel (16), one of pembrolizumab vs. chemotherapy (pemetrexed plus carboplatin) (15) and one of pembrolizumab plus chemotherapy vs. chemotherapy (11). Nineteen reported other rare but life-threatening IRAEs and data of treatment related deaths (6-13,15-17,19,20,22-27).
Figure 1.
Flow diagram of study selection. NSCLC, non-small cell lung cancer.
Tables 1,2 are summary of the characteristics of all the included trials. Among them, 11 were phase I trials, 6 were phase II, 3 phase III, 1 phase II/III and the last one was phase IIIB/IV trial; 17 were PD-1 inhibitors (10 nivolumab and 7 pembrolizumab), and 5 were PD-L1 inhibitors (4 atezolizumab and 1 avelumab).
Table1. Characteristics of all included studies.
| Clinical trial information | First author | Year | Phase | Study drug | PD-1/PD-L1 | Study type | Control group | Dose of study drug | Population |
|---|---|---|---|---|---|---|---|---|---|
| NCT02066636 | Bauer | 2015 | IIIB/IV | Nivolumab | PD-1 | Single arm | – | 3 mg/kg q2w | Advanced NSCLC after failure |
| NCT02031458 | Besse | 2015 | II | Atezolizumab | PD-L1 | Single arm | – | 1,200 mg q3w | 1L or 2L/3L+ in PD-L1 selected advanced NSCLC |
| NCT01673867 | Borghaei | 2015 | III | Nivolumab | PD-1 | RCT | Docetaxel | 3 mg/kg q2w | Advanced NSQ NSCLC after failure |
| – | Brahmer | 2012 | I | Nivolumab | PD-1 | Single arm | – | 1, 3, 10 mg/kg q2w | Advanced NSCLC after failure |
| NCT00729664 | Brahmer | 2012 | I | Nivolumab | PD-1 | Single arm | – | 0.3, 1, 3, 10 mg/kg q2w | Advanced NSCLC after failure |
| NCT01642004 | Brahmer | 2015 | III | Nivolumab | PD-1 | RCT | Docetaxel | 3 mg/kg q2w | Advanced SQ NSCLC after failure |
| NCT01903993 | Ferenbacher | 2016 | II | Atezolizumab | PD-L1 | Single arm | – | 1,200 mg q3w | Advanced NSCLC after failure |
| NCT01295827 | Garon | 2014 | I | Pembrolizumab | PD-1 | Single arm | – | 10 mg/kg q2,3w | Advanced NSCLC after failure |
| NCT01295827 | Garon | 2015 | I | Pembrolizumab | PD-1 | Single arm | – | 2, 10 mg/kg q3w; 10 mg/kg q2w | Advanced NSCLC |
| NCT00730639 | Gettinger | 2015 | I | Nivolumab | PD-1 | Single arm | – | 1, 3, 10 mg/kg, q2w | Advanced NSCLC after failure |
| NCT01454102 | Gettinger | 2016 | I | Nivolumab | PD-1 | Single arm | – | 3 mg/kg q2w | Chemotherapy-naïve advanced NSCLC |
| NCT02085070 | Goldberg | 2016 | II | Pembrolizumab | PD-1 | Single arm | – | 10 mg/kg q2w | Advanced NSCLC with untreated brain metastasis |
| NCT01772004 | Gulley | 2015 | IB | Avelumab | PD-L1 | Single arm | – | 10 mg/kg q2w | Advanced NSCLC after failure |
| NCT01905657 | Herbst | 2016 | II/III | Pembrolizumab | PD-1 | RCT | Docetaxel | 2, 10 mg/kg q3w | NSCLC after failure |
| NCT01375824 | Horn | 2015 | IA | Atezolizumab | PD-L1 | Single arm | – | ≤20 mg/kg q3w | NSCLC |
| NCT02039674 | Langer | 2016 | II | Pembrolizumab | PD-1 | RCT | Pemetrexed + carboplatin | 200 mg | Chemotherapy-naïve NSQ NSCLC |
| NCT02142738 | Reck | 2016 | III | Pembrolizumab | PD-1 | RCT | Chemotherapy | 200 mg q3w, | Treatment-naïve |
| – | Rizvi | 2014 | I | Nivolumab | PD-1 | Single arm | – | 3 mg/kg q2w | Chemotherapy-naïve NSCLC |
| NCT01721759 | Rizvi | 2015 | II | Nivolumab | PD-1 | Single arm | – | 3 mg/kg q2w | Advanced SQ NSCLC after failure |
| NCT01295827 | Soria | 2015 | I | Pembrolizumab | PD-1 | Single arm | – | 2, 10 mg/kg q3w; 10 mg/kg q2w | Advanced NSCLC after failure |
| NCT01846416 | Spigel | 2015 | II | Atezolizumab | PD-L1 | Single arm | – | 1,200 mg q3w | Advanced NSCLC after failure |
| NCT00730639 | Topalian | 2012 | I | Nivolumab | PD-1 | Single arm | – | 1, 3, 10 mg/kg, q2w | Advanced NSCLC after failure |
PD-1, programmed death-1; PD-L1, programmed death ligand-1; NSCLC, non-small cell lung cancer; RCT, randomized control trial; q2w, every two weeks; q3w, every three weeks; NSQ, non-squamous cell; SQ NSCLC, squamous cell NSCLC.
Table2. Characteristics of all included studies.
| Clinical trial information | Total number of patients | Number of any grade pneumonitis | Number of grades 3–5 pneumonitis | Total in control group | Number of any grade pneumonitis in control | Number of grades 3–5 pneumonitis in control |
|---|---|---|---|---|---|---|
| NCT02066636 | 226 | 3 | 1 | – | – | – |
| NCT02031458 | 659 | 4 | 4 | – | – | – |
| NCT01673867 | 287 | 4 | 3 | 268 | 0 | 0 |
| – | 75 | 1 | 1 | – | – | – |
| NCT00729664 | 207 | – | – | – | – | – |
| NCT01642004 | 131 | 6 | 0 | 129 | 0 | 0 |
| NCT01903993 | 142 | – | – | – | – | – |
| NCT01295827 | 221 | 3 | 3 | – | – | – |
| NCT01295827 | 495 | 18 | 9 | – | – | – |
| NCT00730639 | 129 | 11 | 4 | – | – | – |
| NCT01454102 | 52 | 3 | 1 | – | – | – |
| NCT02085070 | 18 | 1 | 1 | – | – | – |
| NCT01772004 | 184 | – | – | – | – | – |
| NCT01905657 | 682 | 31 | 14 | 309 | 4 | 0 |
| NCT01375824 | 88 | – | – | – | – | – |
| NCT02039674 | 59 | – | – | 62 | – | – |
| NCT02142738 | 154 | 9 | 4 | 150 | 1 | 1 |
| – | 20 | – | – | – | – | – |
| NCT01721759 | 117 | 6 | 4 | – | – | – |
| NCT01295827 | 449 | – | – | – | – | – |
| NCT01846416 | 137 | – | – | – | – | – |
| NCT00730639 | 296 | 9 | 3 | – | – | – |
Incidence of pneumonitis
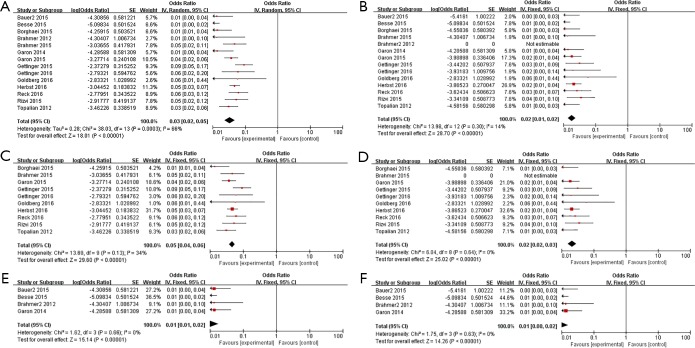
Incidence of all-grade pneumonitis (n=3,542) was 2.9% (95% CI, 2.0–4.8%, P=0.0003, I2=66%) (Figure 2A), and it was 2.0% (95% CI, 1.0–2.0%, P=0.30, I2=14%) of grade 3–5 pneumonitis (Figure 2B). Considering of the high heterogeneity, incidence of pneumonitis in full texts and abstracts were analyzed respectively. Results revealed that incidence of pneumonitis was 4.8% (95% CI, 3.8–5.7%, P=0.13, I2=34%) of all-grade (Figure 2C) and 2.0% (95% CI, 2.0–2.9%, P=0.64, I2=0) of grade 3–5 (Figure 2D) in published full texts (n=2,361). On the other hand, it was 1.0% (95% CI, 1.0–2.0%, P=0.66, I2=0) of all-grade (Figure 2E) and 1.0% (95% CI, 0–2.0%, P=0.63, I2=0) (Figure 2F) in reported abstracts (n=1,181).
Figure 2.
Forest plots of incidence of pneumonitis. (A) Incidence of all-grade pneumonitis; (B) incidence of grades 3–5 pneumonitis; (C) incidence of all-grade pneumonitis in published full-texts; (D) incidence of grades 3–5 pneumonitis in published full-texts; (E) incidence of all-grade pneumonitis in reported abstracts; (F) incidence of grades 3–5 pneumonitis in reported abstracts.
Incidence of pneumonitis in PD-1 and PD-L1 inhibitor therapy vs. standard-of-care chemotherapy
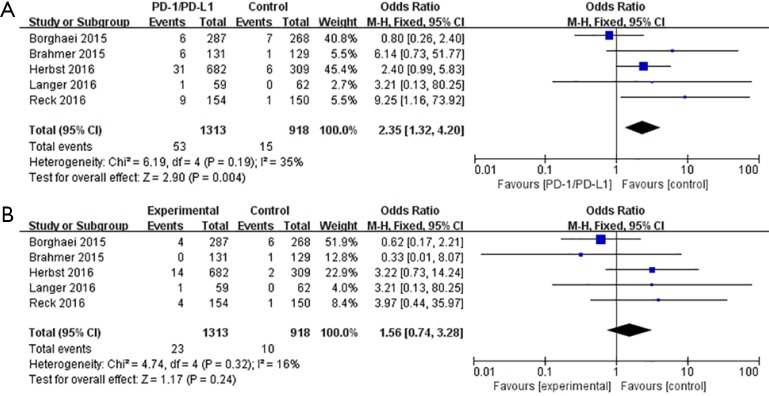
In the five trials with control groups, incidence of any grade pneumonitis in PD-1 and PD-L1 inhibitor therapy (n=1,313) was significantly higher than that in chemotherapy (n=918) (OR=2.35, 95%CI, 1.32–4.20, P=0.004) (Figure 3A). However, in the case of grade 3–5 pneumonitis, it was higher in PD-1 and PD-L1 inhibitor therapy, but was not statistically significant (P=0.24) (Figure 3B).
Figure 3.
Forest plots of differences in incidence of pneumonitis between PD-1 and PD-L1 inhibitors and chemotherapy. (A) Difference in incidence of all-grade pneumonitis between PD-1 and PD-L1 inhibitors and chemotherapy; (B) difference in incidence of grades 3–5 pneumonitis between PD-1 and PD-L1 inhibitors and chemotherapy. PD-1, programmed death-1; PD-L1, programmed death ligand-1
Incidence of other rare but life-threatening adverse effects
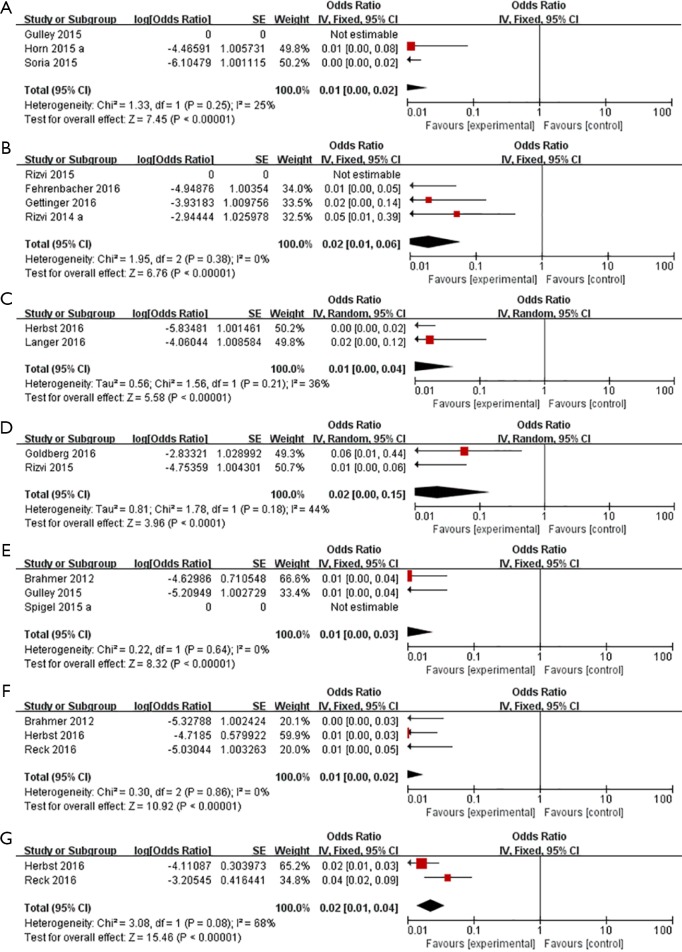
Incidence of some other rare adverse effects were 1.0% (95% CI, 0–2.0%) of cardiorespiratory arrest (n=537) (Figure 4A), 2.0% (95% CI, 1.0–5.7%) of cardiac failure (n=214) (Figure 4B), 1.0% (95% CI, 0–3.8%) of myocardial infarction (n=402) (Figure 4C), 2.0% (95% CI, 0–13.0%) of stroke (n=135) (Figure 4D), 1.0% (95% CI, 0–2.9%) of disease progression (n=391) (Figure 4E), 1.0% (95% CI, 0–2.0%) of pancreatitis (n=700) (Figure 4F) and 2.0% (95% CI, 1.0–3.8%) of severe skin reactions (n=836) (Figure 4G).
Figure 4.
Forest plots of incidence of some rare but severe adverse effects. (A) Cardiorespiratory arrest; (B) cardiac failure; (C) myocardial infarction; (D) stroke; (E) disease progression; (F) pancreatitis; (G) severe skin reactions.
Two cases of sepsis were reported and one of them led to death (n=59). Cases of pulmonary embolism, respiratory arrest, respiratory failure, constrictive pericarditis, cardiac tamponade, pericardial effusion, encephalitis, myocarditis, sarcoidosis, endophthalmitis and myasthenia gravis were reported each by one case. Three cases of respiratory arrest, respiratory failure and constrictive pericarditis had led to deaths of patients (treated with pembrolizumab, avelumab and atezolizumab respectively). All the other cases were treated with nivolumab. What’s more, except for myocarditis of grades 1–2, all the others were grades 3–4 in severity (Table 3).
Table 3. Incidence of other rare but life-threatening IRAEs.
| Adverse effects | First author | Year of publication | Study drugs | Total number of patients | Events |
|---|---|---|---|---|---|
| Cardiac failure | Gettinger | 2016 | Nivolumab | 52 | 1 |
| Rizvi | 2014 | Nivolumab | 20 | 1 | |
| Fehrenbacher | 2016 | Atezolizumab | 142 | 1 | |
| Cardiorespiratory arrest | Horn | 2015 | Atezolizumab | 88 | 1 |
| Soria | 2015 | Pembrolizumab | 449 | 1 | |
| Myocardial infarction | Langer | 2016 | Pembrolizumab | 59 | 1 |
| Herbst | 2016 | Pembrolizumab | 343 | 1 | |
| Stroke | Goldberg | 2016 | Pembrolizumab | 18 | 1 |
| Rizvi | 2015 | Nivolumab | 117 | 1 | |
| Disease progression | Gulley | 2015 | Avelumab | 184 | 1 |
| Brahmer | 2012 | Nivolumab | 207 | 2 | |
| Pancreatitis | Brahmer | 2012 | Nivolumab | 207 | 1 |
| Herbst | 2016 | Pembrolizumab | 339 | 3 | |
| Reck | 2016 | Pembrolizumab | 154 | 1 | |
| Severe skin reactions | Herbst | 2016 | Pembrolizumab | 682 | 11 |
| Reck | 2016 | Pembrolizumab | 154 | 6 | |
| Sepsis | Langer | 2016 | Pembrolizumab | 59 | 2 |
| Pulmonary embolism | Borghaei | 2015 | Nivolumab | 287 | 1 |
| Respiratory arrest | Soria | 2015 | Pembrolizumab | 449 | 1 |
| Respiratory failure | Gulley | 2015 | Avelumab | 184 | 1 |
| Constrictive pericarditis | Spigel | 2015 | Atezolizumab | 137 | 1 |
| Cardiac tamponade | Borghaei | 2015 | Nivolumab | 287 | 1 |
| Pericardial effusion | Borghaei | 2015 | Nivolumab | 287 | 1 |
| Encephalitis | Borghaei | 2015 | Nivolumab | 287 | 1 |
| Myocarditis | Brahmer | 2012 | Nivolumab | 207 | 1 |
| Sarcoidosis | Brahmer | 2012 | Nivolumab | 207 | 1 |
| Endophthalmitis | Brahmer | 2012 | Nivolumab | 207 | 1 |
| Myasthenia gravis | Brahmer | 2012 | Nivolumab | 207 | 1 |
IRAEs, immune-related adverse effects.
Treatment related deaths
Totally, 29 deaths related to treatment were reported (n=4,160). There was no significant difference between the rate of death in PD-1 and PD-L1 inhibitors (P=0.626). Four deaths were attribute to pneumonitis, four pneumonias and another one radiation pneumonitis. Other causes leading to deaths included cardiac failure, respiratory failure, respiratory arrest, constrictive pericarditis, encephalitis, ischemic stroke, myocardial infarction, sepsis (each n=1), and interstitial lung disease (ILD) and cardiorespiratory arrest (each n=2), as well as disease progression (n=3) and unknown causes (n=5) (Table 4).
Table 4. Trials reporting treatment-related deaths and causes of deaths.
| First author | Year | Study drug | PD-1/PD-L1 | Events | Total number of patients | Causes of deaths |
|---|---|---|---|---|---|---|
| Besse | 2015 | Atezolizumab | PD-L1 | 1 | 659 | Pneumonia |
| Fehrenbacher | 2016 | Atezolizumab | PD-L1 | 1 | 142 | Cardiac failure |
| Gulley | 2015 | Avelumab | PD-L1 | 3 | 184 | Radiation pneumonitis, acute respiratory failure, disease progression |
| Horn | 2015 | Atezolizumab | PD-L1 | 1 | 88 | Cardiorespiratory arrest |
| Spigel | 2015 | Atezolizumab | PD-L1 | 1 | 137 | Constrictive pericarditis |
| Borghaei | 2015 | Nivolumab | PD-1 | 1 | 287 | Encephalitis |
| Brahmer | 2012 | Nivolumab | PD-1 | 1 | 75 | Pulmonary toxicity |
| Brahmer | 2012 | Nivolumab | PD-1 | 2 | 207 | Disease progression |
| Gettinger | 2015 | Nivolumab | PD-1 | 1 | 129 | Pneumonitis |
| Rizvi | 2015 | Nivolumab | PD-1 | 2 | 117 | Pneumonia, ischemic stroke |
| Soria | 2015 | Pembrolizumab | PD-1 | 3 | 449 | Cardiorespiratory arrest, ILD, respiratory arrest |
| Topalian | 2012 | Nivolumab | PD-1 | 3 | 296 | Study drug toxicity |
| Garon | 2015 | Pembrolizumab | PD-1 | 1 | 495 | Interstitial lung disease |
| Herbst | 2016 | Pembrolizumab | PD-1 | 6 | 682 | 2 mg/kg (pneumonitis, pneumonia), 10 mg/kg (myocardial infarction, pneumonitis, pneumonia) |
| Langer | 2016 | Pembrolizumab | PD-1 | 1 | 59 | Sepsis |
| Reck | 2016 | Pembrolizumab | PD-1 | 1 | 154 | Unknown cause |
PD-1, programmed death-1; PD-L1, programmed death ligand-1; ILD, interstitial lung disease.
Discussion
This metanalysis revealed in patients with lung cancer treated with PD-1 and PD-L1 inhibitors the overall incidence of pneumonitis was 2.9% for all-grade and 2.0% for grade 3–5. Nishino et.al summarized in NSCLC treated with PD-1 inhibitor monotherapy the incidence of pneumonitis was 4.1% for all-grade and 1.8% for grade 3–5, both of which were significantly higher than that in melanoma (4). Besides, Khunger et al. found incidence of all-grade pneumonitis is 3.6% in PD-1 inhibitors and 1.3% in PD-L1 inhibitors, while it was 1.1% and 0.4% of grade 3–5 pneumonitis (5). Cardiovascular toxicities associated with immune checkpoint inhibitors have been serially reported mainly related to CTLA-4 inhibitors or combined therapy with PD-1 and PD-L1 antibodies, such as myocarditis, heart failure, cardiomyopathy, myocardial fibrosis, cardiac arrest, and complete heart block (28-31). Information of cardiac side effects of anti-PD-1 and anti-PD-L1 on patients with lung cancer were limited. Laubli et al. reported a case of heart failure due to autoimmune myocarditis after treating melanoma with pembrolizumab. Cardiac biopsy showed infiltration of CD8 positive T lymphocytes and a reduction of FOXP3 positive regulatory T cells (32). Semper et al. made known a patient with lung squamous cell carcinoma developed myocarditis after treatment of nivolumab (33); and Behling et al. gave an account of a case of myositis with treatment of nivolumab for metastatic melanoma, which then developed to third-degree atrioventricular block (34). Despite of the low incidence, they should be brought to the forefront. Any symptoms and signs should give rise to rapid and comprehensive cardiovascular evaluation, and prompt and effective therapeutic approaches are dictated, such as respiratory and hemodynamic support, even heart transplant if necessary.
Clinical trials demonstrated treatment-related adverse effects in PD-1 and PD-L1 inhibitors were fewer than that in docetaxel or chemotherapy (13-15). However, we proved patients treated with PD-1 and PD-L1 inhibitors had higher incidence of pneumonitis than chemotherapy. Nishijima et al. compared the safety and tolerability of PD-1 and PD-L1 inhibitors with chemotherapy and demonstrated incidence of any adverse effects of checkpoint inhibitors were significantly lower than chemotherapy, both all-grade and high grade, as well as the frequency of discontinued treatment for toxicity. However, PD-1 and PD-L1 inhibitors were associated with higher risk of all-grade rash, colitis, hypothyroidism, hyperthyroidism, and aminotransferase elevations, and all- and high-grade pneumonitis (35).
IRAEs were normal mild and could be managed by clinicians. However, some severe cases may be deadly and lead to deaths without effective measures could be taken. Thus, prevention, early recognition and prompt intervention are of great importance in management of these severe cases. Firstly, clinicians should evaluate the base-line status of patients and recognize possible risk factors that may contribute to the development of treatment-related adverse effects. Besides, patients and their family members should be educated to identify early symptoms so that prompt intervention could be taken. Meanwhile, during the course of treatment, regular examinations and closely monitor of patients were vital. Nevertheless, if IRAEs already developed, measures should be taken to control them without delay. For grades 1–2 AEs, discontinuation of therapeutic drugs may not be necessary. However, for grades 3–4 AEs, discontinuation of drugs is necessary, temporarily or even permanent. Besides, corticosteroid may be needed from time to time and it must be used rationally.
As well described by Naidoo, patients exposed to corticosteroids for IRAE become immunocompromised, as a consequence in cases of recurrence of respiratory event it become crucial to carefully ruled out some bacterial or fungal infections such as aspergillosis (36).
Finally, we found in the present metanalysis progression of disease as a side effect of treatment with an incidence of 1.0% (95% CI, 0–2.9%). Recently, Champiat et al. reported tumor hyperprogression related to anti-PD1 or anti-PDL1 treatments, defined as a doubling of the tumor growth rate. We believe that a homogenization of the term “hyperprogressive disease” is to be considered in future studies to compare this deleterious and rare side effect across studies (37).
There were some limitations in our study. Firstly, most included trials were open-label, which may lead to selection bias of patients. In addition, data in some abstracts were ambiguous and were not the final results. Moreover, patients included were those highly selective in clinical trials and may not reflect the condition in real world.
Conclusions
Immune related adverse effects can on occasion be life-threatening even though usually rare. This metanalysis demonstrated that incidence of pneumonitis in NSCLC treated with PD-1 and PD-L1 inhibitor therapy was significantly higher than that with chemotherapy. Further metanalysis should be conducted to deeply investigate the incidence of other rare and potentially unrecognized life-threatening IRAEs.
Acknowledgements
Funding: This work was supported by grants from the National Natural Science Foundation of China (grant number: 81401903, 81572937 and 81572273); the Natural Science Foundation of Jiangsu province (grant number: BL2013026, BK20140736 and BK20161386); Program of Nanjing Science and Technology of Nanjing Science and Technology Committee (grant number: 201605059); and Jiangsu Provincial Medical Youth Talent (grant number: QNRC2016125).
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Velcheti V, Schalper KA, Carvajal DE, et al. Programmed death ligand-1 expression in non-small cell lung cancer. Lab Invest 2014;94:107-16. 10.1038/labinvest.2013.130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Postow MA. Managing immune checkpoint-blocking antibody side effects. Am Soc Clin Oncol Educ Book 2015;35:76-83. 10.14694/EdBook_AM.2015.35.76 [DOI] [PubMed] [Google Scholar]
- 3.Weber JS, Yang JC, Atkins MB, et al. Toxicities of Immunotherapy for the Practitioner. J Clin Oncol 2015;33:2092-9. 10.1200/JCO.2014.60.0379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nishino M, Giobbie-Hurder A, Hatabu H, et al. Incidence of programmed cell death 1 inhibitor-related pneumonitis in patients with advanced cancer: a systematic review and meta-analysis. JAMA Oncol 2016;2:1607-16. 10.1001/jamaoncol.2016.2453 [DOI] [PubMed] [Google Scholar]
- 5.Khunger M, Rakshit S, Pasupuleti V, et al. Incidence of pneumonitis with use of PD-1 and PD-L1 inhibitors in non-small cell lung cancer: a systematic review and meta-analysis of trials. Chest 2017;152:271-81. 10.1016/j.chest.2017.04.177 [DOI] [PubMed] [Google Scholar]
- 6.Garon EB, Rizvi NA, Hui R, et al. Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med 2015;372:2018-28. 10.1056/NEJMoa1501824 [DOI] [PubMed] [Google Scholar]
- 7.Gettinger SN, Horn L, Gandhi L, et al. Overall Survival and Long-Term Safety of Nivolumab (Anti-Programmed Death 1 Antibody, BMS-936558, ONO-4538) in patients with previously treated advanced non-small-cell lung cancer. J Clin Oncol 2015;33:2004-12. 10.1200/JCO.2014.58.3708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gettinger S, Rizvi NA, Chow LQ, et al. Nivolumab monotherapy for first-line treatment of advanced non-small-cell lung cancer. J Clin Oncol 2016;34:2980-7. 10.1200/JCO.2016.66.9929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med 2012;366:2443-54. 10.1056/NEJMoa1200690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goldberg SB, Gettinger SN, Mahajan A, et al. Pembrolizumab for patients with melanoma or non-small-cell lung cancer and untreated brain metastases: early analysis of a non-randomised, open-label, phase 2 trial. Lancet Oncol 2016;17:976-83. 10.1016/S1470-2045(16)30053-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Langer CJ, Gadgeel SM, Borghaei H, et al. Carboplatin and pemetrexed with or without pembrolizumab for advanced, non-squamous non-small-cell lung cancer: a randomised, phase 2 cohort of the open-label KEYNOTE-021 study. Lancet Oncol 2016;17:1497-508. 10.1016/S1470-2045(16)30498-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rizvi NA, Mazières J, Planchard D, et al. Activity and safety of nivolumab, an anti-PD-1 immune checkpoint inhibitor, for patients with advanced, refractory squamous non-small-cell lung cancer (CheckMate 063): a phase 2, single-arm trial. Lancet Oncol 2015;16:257-65. 10.1016/S1470-2045(15)70054-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus Docetaxel in Advanced Nonsquamous Non-Small-Cell Lung Cancer. N Engl J Med 2015;373:1627-39. 10.1056/NEJMoa1507643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus Docetaxel in Advanced Squamous-Cell Non-Small-Cell Lung Cancer. N Engl J Med 2015;373:123-35. 10.1056/NEJMoa1504627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reck M, Rodriguez-Abreu D, Robinson AG, et al. Pembrolizumab versus Chemotherapy for PD-L1-Positive non-small-cell lung cancer. N Engl J Med 2016;375:1823-33. 10.1056/NEJMoa1606774 [DOI] [PubMed] [Google Scholar]
- 16.Herbst RS, Baas P, Kim DW, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet 2016;387:1540-50. 10.1016/S0140-6736(15)01281-7 [DOI] [PubMed] [Google Scholar]
- 17.Brahmer JR, Horn L, Antonia SJ, et al. Clinical activity and safety of anti-PD-1 (BMS-936558, MDX-1106) in Patients (Pts) with advanced non-small-cell lung cancer (NSCLC). J Clin Oncol 2012;30:S7509. [Google Scholar]
- 18.Garon EB, Leighl NB, Rizvi NA, et al. Safety and clinical activity of MK-3475 in previously treated patients (pts) with non-small cell lung cancer (NSCLC). J Clin Oncol 2014;32:8020. [Google Scholar]
- 19.Soria JC, Flatten O, Horn L, et al. Efficacy and Safety of Pembrolizumab (Pembro; MK-3475) for Patients (Pts) with previously treated advanced non-small cell lung cancer (NSCLC) Enrolled in KEYNOTE-001. Eur J Cancer 2015;51:S726-7. 10.1016/S0959-8049(15)30077-0 [DOI] [Google Scholar]
- 20.Besse B, Johnson M, Janne PA, et al. 16LBA Phase II, single-arm trial (BIRCH) of atezolizumab as first-line or subsequent therapy for locally advanced or metastatic PD-L1-selected non-small cell lung cancer (NSCLC). Eur J Cancer 2015;51:S717-8. 10.1016/S0959-8049(16)31938-4 [DOI] [Google Scholar]
- 21.Bauer TM, McCleod M, Chandler JC, et al. An ongoing phase IIIb/IV safety trial of nivolumab (NIVO) in patients (pts) with advanced or metastatic non-small-cell lung cancer (NSCLC) who progressed after receiving 1 or more prior systemic regimens. J Clin Oncol 2015;33:S3013. [Google Scholar]
- 22.Fehrenbacher L, Spira A, Ballinger M, et al. Atezolizumab versus docetaxel for patients with previously treated non-small-cell lung cancer (POPLAR): a multicentre, open-label, phase 2 randomised controlled trial. Lancet 2016;387:1837-46. 10.1016/S0140-6736(16)00587-0 [DOI] [PubMed] [Google Scholar]
- 23.Gulley JL, Spigel D, Kelly K, et al. Avelumab (MSB0010718C), an anti-PD-L1 antibody, in advanced NSCLC patients: A phase 1b, open-label expansion trial in patients progressing after platinum-based chemotherapy. J Clin Oncol 2015;33:S8034. [Google Scholar]
- 24.Horn L, Spigel DR, Gettinger SN, et al. Clinical activity, safety and predictive biomarkers of the engineered antibody MPDL3280A (anti-PDL1) in non-small cell lung cancer (NSCLC): update from a phase Ia study. J Clin Oncol 2015;33:S8029. [Google Scholar]
- 25.Brahmer JR, Tykodi SS, Chow LQ, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med 2012;366:2455-65. 10.1056/NEJMoa1200694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rizvi NA, Shepherd FA, Antonia SJ, et al. First-line monotherapy with nivolumab (Anti-PD-1; BMS-936558, ONO-4538) in advanced non-small cell lung cancer (NSCLC): safety, efficacy, and correlation of outcomes with PD-L1 Status. Int J Radiat Oncol Biol Phys 2014;90:S31 10.1016/j.ijrobp.2014.08.204 [DOI] [Google Scholar]
- 27.Spigel DR, Chaft JE, Gettinger SN, et al. Clinical activity and safety from a phase II study (FIR) of MPDL3280A (anti-PDL1) in PD-L1-selected patients with non-small cell lung cancer (NSCLC). J Clin Oncol 2015;33:S8028. [Google Scholar]
- 28.Heinzerling L, Ott PA, Hodi FS, et al. Cardiotoxicity associated with CTLA4 and PD1 blocking immunotherapy. J Immunother Cancer 2016;4:50. 10.1186/s40425-016-0152-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Johnson DB, Balko JM, Compton ML, et al. Fulminant Myocarditis with Combination Immune Checkpoint Blockade. N Engl J Med 2016;375:1749-55. 10.1056/NEJMoa1609214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Koelzer VH, Rothschild SI, Zihler D, et al. Systemic inflammation in a melanoma patient treated with immune checkpoint inhibitors-an autopsy study. J Immunother Cancer 2016;4:13. 10.1186/s40425-016-0117-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Geisler BP, Raad RA, Esaian D, et al. Apical ballooning and cardiomyopathy in a melanoma patient treated with ipilimumab: a case of takotsubo-like syndrome. J Immunother Cancer 2015;3:4. 10.1186/s40425-015-0048-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Laubli H, Balmelli C, Bossard M, et al. Acute heart failure due to autoimmune myocarditis under pembrolizumab treatment for metastatic melanoma. J Immunother Cancer 2015;3:11. 10.1186/s40425-015-0057-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Semper H, Muehlberg F, Schulz-Menger J, et al. Drug-induced myocarditis after nivolumab treatment in a patient with PDL1- negative squamous cell carcinoma of the lung. Lung Cancer 2016;99:117-9. 10.1016/j.lungcan.2016.06.025 [DOI] [PubMed] [Google Scholar]
- 34.Behling J, Kaes J, Munzel T, et al. New-onset third-degree atrioventricular block because of autoimmune-induced myositis under treatment with anti-programmed cell death-1 (nivolumab) for metastatic melanoma. Melanoma Res 2017;27:155-8. 10.1097/CMR.0000000000000314 [DOI] [PubMed] [Google Scholar]
- 35.Nishijima TF, Shachar SS, Nyrop KA, et al. Safety and Tolerability of PD-1/PD-L1 inhibitors compared with chemotherapy in patients with advanced cancer: a meta-analysis. Oncologist 2017;22:470-9. 10.1634/theoncologist.2016-0419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Naidoo J, Wang X, Woo KM, et al. Pneumonitis in Patients Treated With Anti-Programmed Death-1/Programmed Death Ligand 1 Therapy. J Clin Oncol 2017;35:709-17. 10.1200/JCO.2016.68.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Champiat S, Dercle L, Ammari S, et al. Hyperprogressive disease is a new pattern of progression in cancer patients treated by Anti-PD-1/PD-L1. Clin Cancer Res 2017;23:1920-8. 10.1158/1078-0432.CCR-16-1741 [DOI] [PubMed] [Google Scholar]