Abstract
Background
Hepatocellular carcinoma (HCC) is increasingly common, potentially fatal cancer type globally. Platelet-lymphocyte ratio (PLR) as a biomarker for systemic inflammation has recently been recognized as a valuable prognostic marker in multiple cancer types. The aim of the present study was to assess the prognostic value of PLR in HCC patients and determine the optimal cut-off value for risk stratification.
Methods
We retrospectively analyzed patients with diagnosis of HCC (screened by ICD-9 code, confirmed with radiographic examination and/or biopsy) at a large public hospital during 15 years (Jan 2000 through July 2015). PLR, among other serology laboratory values were collected at diagnosis of HCC. Its association with overall survival was evaluated with Cox proportional hazard model.
Results
Among 270 patients with HCC, 57 (21.1%) patients died within an average follow-up of 11.9 months. PLR at diagnosis was significantly different between survivors and deceased (128.9 vs. 186.7; P=0.003). In multivariate analysis, aspartate transaminase (AST) (HR 2.022, P<0.001) and PLR (HR 1.768, P=0.004) independently predicted mortality. The optimal cut-off value for PLR was determined to be 220 by receiver-operating characteristics curve, and high PLR group had significantly higher mortality (HR 3.42, P<0.001).
Conclusions
Our results indicated that elevated PLR at diagnosis above 220 predicted poor prognosis in HCC patients. PLR is a low-cost and convenient tool, which may serve as a useful prognostic marker for HCC.
Keywords: Hepatocellular carcinoma (HCC), platelet-lymphocyte ratio (PLR)
Introduction
Hepatocellular carcinoma (HCC) is currently the fifth most frequently diagnosed cancer in adult male and seventh in female globally, leading to 25,000 to one million deaths per annum (1-3). As the worldwide epidemics of obesity continues, the incidence of HCC increases with that of non-alcoholic fatty liver disease, and in contrast to the declining mortality rate seen among other cancer types, death rate of HCC is still on the rise (4-6). As tumor metastasis and recurrence remain the major cause of mortality, there is urgent need for identification of reliable and precise biomarkers to predict prognosis and guide individualized treatment in HCC patients (7,8).
Currently, an accumulating evidence suggests that systemic inflammation suppresses innate anti-tumor immune system, thus facilitates the initiation, progression and metastasis of cancer (7-12). Inflammatory biomarkers such as platelet-lymphocyte ratio (PLR), neutrophil-lymphocyte ratio (NLR), C-reactive protein have been proven as valuable prognostic factors in multiple gastrointestinal cancers including pancreatic ductal adenocarcinoma, colorectal cancer and gastric cancer (7,9-11,13-16). In HCC however, the results were still conflicting regarding the prognostic value of PLR to predict overall survival and the optimal cut-off of PLR remains unclear (11,17-20).
The present study aims to evaluate the clinical significance of PLR to predict mortality in patients of HCC.
Method
Study subjects
We retrospectively reviewed the electronic medical records of patients who presented to John H. Stroger Hospital of Cook County, Chicago IL from January 01, 2000 through July 31, 2015.
We identified potential patients using ICD-9 code (=155) and/or ICD-10 code (= C22) for malignant neoplasm of liver and intrahepatic biliary duct. We confirmed the diagnosis if they had histopathology-proven or radiographically proven HCC by triple-phase enhanced computed tomography and/or magnetic resonance imaging of the abdomen (intense enhancement during arterial phase followed by “washout patter” during delayed phase). We excluded patients <18 years of age, if they had incomplete data as outlined in Data Collection section, or had incomplete follow-up of less than 1 month in our institution.
The present study was approved by the Institutional Review Board of Cook County Health & Hospitals System, Chicago. The database was set up and maintained by the Department of Medicine, Cook County Health & Hospitals System (21).
Data collection
Eligible patients were censored if there was discontinuation of follow up. We abstracted demographic variables including age, gender, past medical history (hypertension, diabetes), body mass index (BMI) at diagnosis; tumor-related risk factors including etiology of liver disease, Child-Pugh score, number of intra-hepatic lesions, extra-hepatic metastasis, Barcelona staging of HCC20; treatment variables including chemotherapy with sorafenib, curative treatment (liver transplant, resection, radio-frequency ablation); laboratory values at diagnosis including neutrophil, lymphocyte, hemoglobin, platelet, INR, albumin, aspartate aminotransferase, total bilirubin.
VTE was diagnosed based on radiographic exams including compression ultrasonography, contrast enhanced computed tomography, and pulmonary angiogram. There was no systematic VTE screening. Portal vein thrombosis was not included given limitations on objective differentiation between tumor invasion and true thrombosis. Blinded event adjudication was conducted for all outcome data by three physicians; in case of discrepancy there was a majority vote to adjudicate.
Statistical analysis
Descriptive data was summarized to characterize the distribution of variables. We used the Kolmogorov-Smirnov and Shapiro-Wilk tests to evaluate if continuous variables were normally distributed. We used Student’s t-test to compare normally distributed continuous variables; we used Wilcoxon’s rank-sum test to compare non-parametric continuous variables; Chi-square test or Fisher’s exact test were used to compare categorical variables.
We plotted the bar graph to demonstrate difference of PLR between prognosis groups. Univariate analysis was performed for each laboratory value to predict mortality in Cox regression model. Variables with significance of <0.20 were included in multivariate analysis to identify independent risk factors for mortality. Multivariate analysis was then performed for PLR and epidemiologic factors significantly associated with mortality as demonstrated by prior literature (22,23). Receiver operating characteristics (ROC) curve was plotted for PLR to predict mortality and identify cut-off value by optimized Youden’s index. Kapan-Meier curve was then plotted for survival curves of different PLR groups.
Statistical analysis was performed with Stata version 13 (Stata Corp., College Station, Tex). P value of less than 0.05 indicated statistical significance in analysis.
Results
Patient characteristics
Among the 308 patients identified by initial screening, 270 patients were eventually included for analysis (34 patients were excluded as they had biopsy-proven cholangiocarcinoma or metastatic adenocarcinoma, 4 patients were excluded with a follow-up period less than 1 month) (Table 1). The mean age at diagnosis of HCC was 58.5 years for the cohort, 81.4% of patients were male. There were 155 (57.4%) patients who had advanced HCC with Barcelona C or D at diagnosis. A total of 33.7% patients underwent chemotherapy with sorafenib and 11.5% patients underwent curative treatment including surgical resection, radio-frequency ablation or liver transplant. We identified 16 cases of venous thromboembolism within a mean follow up period of 11.9 months, representing a 2-year cumulative incidence of 5.93%. Comparison between groups of survivor and deceased revealed that mortality was significantly higher in male patients, diabetics, patients with advanced HCC, patients who had VTE, and patients who underwent chemotherapy.
Table 1. Epidemiologic characteristics of patients with hepatocellular carcinoma, John H. Stroger Hospital of Cook County.
| Variables | Cohort (N=270) | Alive (N=213) | Deceased (N=57) | **P value | |||||
|---|---|---|---|---|---|---|---|---|---|
| Mean/N | SD/% | Mean/N | SD/% | Mean/N | SD/% | ||||
| †Age (years) | 58.5 | 8.9 | 58.6 | 9.4 | 58.2 | 7.4 | 0.760 | ||
| *≥65 (years) | 59 | 21.9 | 50 | 23.4 | 9 | 15.8 | 0.212 | ||
| *Gender | 0.028 | ||||||||
| Female | 50 | 18.5 | 46 | 21.6 | 5 | 8.8 | |||
| Male | 220 | 81.4 | 167 | 78.4 | 52 | 91.2 | |||
| *Past medical history | |||||||||
| Diabetes | 89 | 32.9 | 77 | 36.2 | 12 | 21.1 | 0.031 | ||
| Hypertension | 126 | 6.7 | 103 | 48.3 | 23 | 40.4 | 0.282 | ||
| Cirrhosis | 229 | 84.8 | 180 | 84.5 | 49 | 85.9 | 0.785 | ||
| *Barcelona stage | 0.002 | ||||||||
| 0, A, B | 115 | 42.6 | 101 | 47.4 | 14 | 24.6 | |||
| C, D | 155 | 57.4 | 112 | 52.6 | 43 | 75.4 | |||
| *VTE | 16 | 5.9 | 9 | 4.2 | 7 | 12.3 | 0.022 | ||
| *Chemotherapy | 91 | 33.7 | 64 | 30.0 | 27 | 47.3 | 0.014 | ||
| *Curative treatment | 31 | 11.5 | 26 | 12.2 | 5 | 8.80 | 0.470 | ||
†, normally distributed continuous variable, presented as mean value and standard deviation; *, categorical variables, presented as number and percentage of patients; **, P value obtained with Student’s t-test for continuous values, Chi-square test or Fisher’s exact test for categorical variables. N, number; SD, standard deviation.
Association between laboratory values and mortality
Direct comparison of laboratory values collected at diagnosis of HCC between groups revealed that survivors had significantly lower INR, aspartate transaminase (AST), bilirubin, PLR, and higher albumin (Table 2). This finding was anticipated as survivors were expected to have relatively preserved hepatic synthetic function and less hepatocellular inflammation and damage.
Table 2. Laboratory values at diagnosis of hepatocellular carcinoma.
| Variables | Cohort | Alive | Deceased | **P value |
|---|---|---|---|---|
| †Hgb (g/dL) | 12.3 | 12.4 | 11.9 | 0.273 |
| *Neutrophil count (109/L) | 4.46 | 4.23 | 5.30 | 0.152 |
| *Lymphocyte count (109/L) | 1.52 | 1.53 | 1.50 | 0.464 |
| *Platelet count (109/L) | 164.7 | 163.4 | 169.4 | 0.189 |
| *INR | 1.30 | 1.24 | 1.50 | 0.011 |
| †Albumin (g/dL) | 3.18 | 3.25 | 2.91 | 0.008 |
| *AST (unit/L) | 125.3 | 112.1 | 174.6 | 0.003 |
| *Bilirubin (mg/dL) | 2.36 | 2.14 | 3.18 | 0.047 |
| *PLR | 141.1 | 128.9 | 186.7 | 0.003 |
†, parametric continuous variable; *, non-parametric continuous variable; **, P value obtained with Student’s t-test for parametric continuous variables, Wilcoxon’s rank-sum test for non-parametric continuous variables. Hgb, hemoglobin; INR, international normalized ratio; AST, aspartate transaminase; PLR, platelet-lymphocyte ratio.
PLR, of note, was significantly lower in survivors (P=0.003) as demonstrated in Figure 1. In univariate analysis, hemoglobin, neutrophil count, INR, albumin, AST, bilirubin and PLR were significantly associated with mortality. However, the association remained significant in multivariate analysis only for AST (HR 2.022, P<0.001), and PLR (HR 1.768, P=0.004). Lower albumin did not reach statistically significant association with mortality (HR 0.655, P=0.053) (Table 3).
Figure 1.
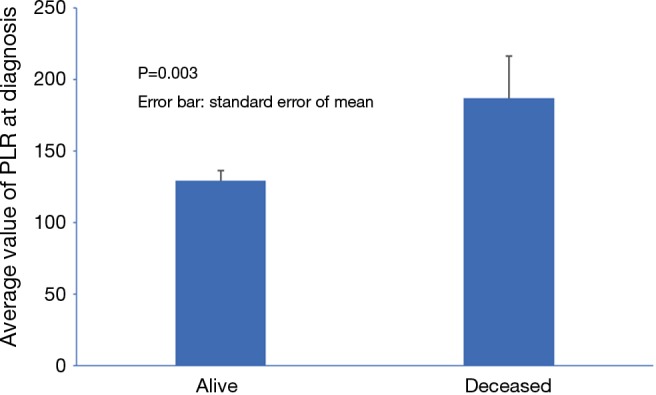
Bar graph of platelet-lymphocyte ratio (PLR) in alive and deceased group.
Table 3. Univariate and multivariate analysis of laboratory values for mortality prediction.
| Variables | Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|---|
| Hazard ratio | P value | Hazard ratio | P value | ||
| Hgb (g/dL) | 0.854 | 0.012 | 0.937 | 0.336 | |
| Neutrophil count (109/L) | 1.711 | 0.024 | 1.148 | 0.573 | |
| Lymphocyte count (109/L) | 0.795 | 0.329 | – | – | |
| Platelet count (109/L) | 1.305 | 0.245 | – | – | |
| INR | 4.717 | <0.001 | 1.378 | 0.517 | |
| Albumin (g/dL) | 0.487 | <0.001 | 0.655 | 0.053 | |
| AST (unit/L) | 2.257 | <0.001 | 2.022 | <0.001 | |
| Bilirubin (mg/dL) | 1.627 | 0.001 | 1.091 | 0.605 | |
| PLR | 1.860 | 0.002 | 1.768 | 0.004 | |
Hgb, hemoglobin; INR, international normalized ratio; AST, aspartate transaminase; PLR, platelet-lymphocyte ratio.
We then performed multivariate analysis for mortality on PLR, AST and epidemiologic factors predictive of mortality as demonstrated in previous literature, including male gender, advanced HCC, and occurrence of VTE (22,23). All variables remained significantly predictive of mortality (PLR: HR 1.56, P 0.028; AST: HR 1.92, P<0.001) (Table 4).
Table 4. Multivariate analysis of epidemiologic and laboratory values for prediction of mortality.
| Variables | Hazard ratio | P value | 95% confidence interval | |
|---|---|---|---|---|
| VTE | 6.49 | 0.001 | 2.21 | 19.1 |
| Gender | 3.06 | 0.022 | 1.17 | 7.98 |
| Advanced HCC stage | 2.61 | 0.007 | 1.29 | 5.22 |
| PLR | 1.56 | 0.028 | 1.05 | 2.33 |
| AST | 1.92 | <0.001 | 1.37 | 2.69 |
VTE, venous thromboembolism; HCC, hepatocellular carcinoma; PLR, platelet-lymphocyte ratio; AST, aspartate transaminase.
Utility of PLR in prediction of mortality
We plotted ROC curve of PLR for prediction of mortality (Figure 2) and identified the cut-off point of 218, with corresponding sensitivity and specificity of 24.6% and 89.7% respectively. We dichotomized the data based on this cut-off value of PLR into two groups and plotted Kaplan-Meier survival curve (Figure 3) which revealed high PLR group had significantly higher mortality rate (HR 3.42, P<0.001).
Figure 2.
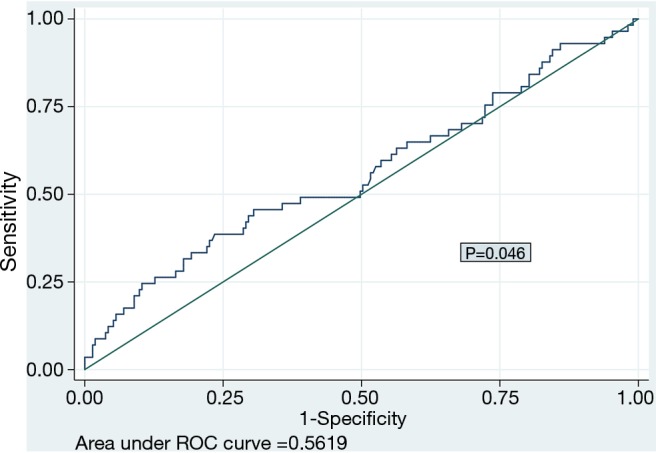
Receiver-operating characteristics curve of platelet-lymphocyte ratio (PLR) for prediction of mortality.
Figure 3.
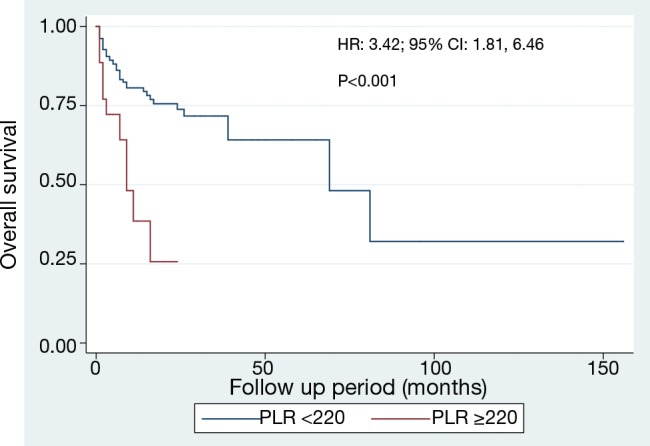
Kaplan-Meier curve demonstrating survival of different platelet-lymphocyte ratio (PLR) groups.
Discussion
In this cohort of 270 patients with HCC, 57 patients died within an average follow-up period of 11.9 months. Comparison of laboratory values at diagnosis between survivors and deceased patients revealed significant differences in the levels of INR, albumin, AST, bilirubin and PLR. Higher AST and PLR could independently predict mortality as demonstrated in multivariate analysis. The optimal cut-off point for PLR to predict mortality in HCC patients was determined to be 218 by ROC analysis, and after dichotomization, patients of high PLR group had significantly poor overall survival.
Our results were in line with a growing body of evidence supporting the utility of systemic inflammatory markers (e.g., PLR, NLR, C-reactive protein) to predict prognosis in patients of various cancer types including HCC (7-21). Yang et al. analyzed 778 HCC patients undergoing curative liver resection, concluding that PLR quintiles were significantly associated with poor survival in cirrhotic patients or patients with positive hepatitis B surface antigen (11). Similarly, Shirai et al. reviewed 131 patients of pancreatic ductal adenocarcinoma who underwent pancreatic resection and reported perioperative PLR independently predicted disease-free survival and overall survival (10). Fuentes et al. reported in their cohort of 112 patients with gastric cancer, that patients stratified as high risk by PLR had worse overall survival (22.6 vs. 42.8 months) (13). In the meta-analysis of patients with colorectal cancer, Huang et al. included 17 studies and concluded that elevated PLR was associated with poor overall survival, disease-free survival, as well as higher cancer stage and poor differentiation (7). Recently, the utility of PLR has also been extensively explored in various non-cancerous populations, including patients with acute coronary syndrome, immunoglobulin-resistant Kawasaki disease, premature ovarian insufficiency and acute pancreatitis (24-27). These associations were also attributed to the role of PLR as a systemic inflammation marker.
The exact underlying mechanism responsible for the role of PLR in HCC has yet to be elucidated (11). However, it is postulated to be closely related to the cancer-induced systemic inflammatory response, which suppresses recruitment of immunosuppressive cells such as regulatory T cells, leading to tumor progression and metastasis (7,9,28). Thrombocytosis results from megakaryocyte stimulation due to pro-inflammatory mediator secretion from tumor, and promotes tumor progression in several mechanisms (29). Platelet secretes various growth factors including vascular endothelial growth factor, platelet-derived growth factor, which promotes angiogenesis, cell proliferation and metastasis (14-17). In addition, tumor-induced platelet aggregation protects tumor cells from being cleared by innate immune surveillance of T cells (30,31). On the other hand, tumor-infiltrating lymphocytes induces apoptosis of tumor cells and play an important role in anti-tumor response (32,33). The lymphocyte count reflects the responsiveness of the host immune system and is inversely related with tumor proliferation and invasiveness (7).
Several limitations existed for the present study: (I) although the present data is from a relatively large cohort with heterogeneous HCC patients, our study was retrospective in design and limited by the inherent confounders as patients may have been systematically excluded; (II) all laboratory values were collected at initial diagnosis of HCC. A longitudinal follow up of series of PLR at intervals and important time points (e.g., pre-chemotherapy, perioperative) might provide more insights of its prognostic value.
In conclusion, elevated PLR of above 220 predicts poor prognosis in patients with HCC. As a readily available and low-cost test, PLR proves to be a valuable tool for prognosis stratification and individualized treatment. Further prospective studies are required to confirm the findings of the present study and the optimal cut-off value of PLR in HCC population.
Acknowledgements
None.
Ethical Statement: The study was approved by institutional ethics review board of Cook County Health and Hospitals System [IRB Organization (IORG) number: IORG0000119] and informed consent was taken from all the patients.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin 2011;61:69-90. 10.3322/caac.20107 [DOI] [PubMed] [Google Scholar]
- 2.Okuda K. Epidemiology of primary liver cancer. In: Tobe T, Kameda H, Okudaira M, et al. editors. Primary Liver Cancer in Japan. Springer, Tokyo, 1992:3-15. [Google Scholar]
- 3.Bosch FX, Munoz N. Hepatocellular carcinoma in the world: Epidemiologic questions. In: Tabor E, DiBisceglie AM, Purcell RH, editors. Etiology, Pathology and Treatment of Hepatocellular Carcinoma in America. Advances in Applied Technology Series, Gulf, Houston, 1991:35. [Google Scholar]
- 4.Bosetti C, Levi F, Boffetta P, et al. Trends in mortality from hepatocellular carcinoma in Europe, 1980-2004. Hepatology 2008;48:137-45. 10.1002/hep.22312 [DOI] [PubMed] [Google Scholar]
- 5.Ryerson AB, Eheman CR, Altekruse SF, et al. Annual Report to the Nation on the Status of Cancer, 1975-2012, featuring the increasing incidence of liver cancer. Cancer 2016;122:1312-37. 10.1002/cncr.29936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hashim D, Boffetta P, La Vecchia C, et al. The global decrease in cancer mortality: trends and disparities. Ann Oncol 2016;27:926-33. 10.1093/annonc/mdw027 [DOI] [PubMed] [Google Scholar]
- 7.Huang XZ, Chen WJ, Zhang X, et al. An Elevated Platelet-to-Lymphocyte Ratio Predicts Poor Prognosis and Clinicopathological Characteristics in Patients with Colorectal Cancer: A Meta-Analysis. Dis Markers 2017;2017:1053125. [DOI] [PMC free article] [PubMed]
- 8.Wang W, Bian C, Xia D, et al. Combining Carcinoembryonic Antigen and Platelet to Lymphocyte Ratio to Predict Brain Metastasis of Resected Lung Adenocarcinoma Patients. Biomed Res Int 2017;2017:8076384. [DOI] [PMC free article] [PubMed]
- 9.Song W, Tian C, Wang K, et al. Preoperative platelet lymphocyte ratio as independent predictors of prognosis in pancreatic cancer: A systematic review and meta-analysis. PloS One 2017;12:e0178762. 10.1371/journal.pone.0178762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shirai Y, Shiba H, Sakamoto T, et al. Preoperative platelet to lymphocyte ratio predicts outcome of patients with pancreatic ductal adenocarcinoma after pancreatic resection. Surgery 2015;158:360-5. 10.1016/j.surg.2015.03.043 [DOI] [PubMed] [Google Scholar]
- 11.Yang HJ, Jiang JH, Liu QA, et al. Preoperative platelet-to-lymphocyte ratio is a valuable prognostic biomarker in patients with hepatocellular carcinoma undergoing curative liver resection. Tumuor Biol 2017;39:1010428317707375. [DOI] [PubMed] [Google Scholar]
- 12.Koh CH, Bhoo-Pathy N, Ng KL, et al. Utility of pre-treatment neutrophil-lymphocyte ratio and platelet-lymphocyte ratio as prognostic factors in breast cancer. Br J Cancer 2015;113:150-8. 10.1038/bjc.2015.183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fuentes HE, Oramas DM, Paz LH, et al. Venous Thromboembolism Is an Independent Predictor of Mortality Among Patients with Gastric Cancer. J Gastrointest Cancer 2017. [Epub ahead of print]. 10.1007/s12029-017-9981-2 [DOI] [PubMed] [Google Scholar]
- 14.Kwon HC, Kim SH, Oh SY, et al. Clinical significance of preoperative neutrophil-lymphocyte versus platelet-lymphocyte ratio in patients with operable colorectal cancer. Biomarkers 2012;17:216-22. 10.3109/1354750X.2012.656705 [DOI] [PubMed] [Google Scholar]
- 15.Smith RA, Bosonnet L, Raraty M, et al. Preoperative platelet-lymphocyte ratio is an independent significant prognostic marker in resected pancreatic ductal adenocarcinoma. Am J Surg 2009;197:466-72. 10.1016/j.amjsurg.2007.12.057 [DOI] [PubMed] [Google Scholar]
- 16.Baranyai Z, Krzystanek M, Jósa V, et al. The comparison of thrombocytosis and platelet-lymphocyte ratio as potential prognostic markers in colorectal cancer. Thromb Haemost 2014;111:483-90. 10.1160/TH13-08-0632 [DOI] [PubMed] [Google Scholar]
- 17.Lai Q, Castro Santa E, Rico Juri JM, et al. Neutrophil and platelet-to-lymphocyte ratio as new predictors of dropout and recurrence after liver transplantation for hepatocellular cancer. Transpl Int 2014;27:32-41. 10.1111/tri.12191 [DOI] [PubMed] [Google Scholar]
- 18.Li X, Chen ZH, Xing YF, et al. Platelet-to-lymphocyte ratio acts as a prognostic factor for patients with advanced hepatocellular carcinoma. Tumuor Biol 2015;36:2263-9. 10.1007/s13277-014-2833-9 [DOI] [PubMed] [Google Scholar]
- 19.Kinoshita A, Onoda H, Imai N, et al. Comparison of the prognostic value of inflammation-based prognostic scores in patients with hepatocellular carcinoma. Br J Cancer 2012;107:988-93. 10.1038/bjc.2012.354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fan W, Zhang Y, Wang Y, et al. Neutrophil-to-lymphocyte and platelet-to-lymphocyte ratios as predictors of survival and metastasis for recurrent hepatocellular carcinoma after transarterial chemoembolization. PloS One 2015;10:e0119312. 10.1371/journal.pone.0119312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wisniewski MF, Kieszkowski P, Zagorski BM, et al. Development of a clinical data warehouse for hospital infection control. J Am Med Inform Assoc 2003;10:454-62. 10.1197/jamia.M1299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang Y, Attar BM, Fuentes HE, et al. Performance of Khorana Risk Score for Prediction of Venous Thromboembolism in Patients With Hepatocellular Carcinoma. Clin Appl Thromb Hemost 2017. [Epub ahead of print]. 10.1177/1076029617699088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang Y, Attar BM, Hinami K, et al. Characteristics and Impacts of Venous Thromboembolism in Patients with Hepatocellular Carcinoma. J Gastrointest Cancer 2017. [Epub ahead of print]. 10.1007/s12029-017-9945-6 [DOI] [PubMed] [Google Scholar]
- 24.İlhan M, İlhan G, Gök AF, et al. Evaluation of neutrophil–lymphocyte ratio, platelet–lymphocyte ratio and red blood cell distribution width–platelet ratio as early predictor of acute pancreatitis in pregnancy. J Matern Fetal Neonatal Med 2016;29:1476-80. 10.3109/14767058.2015.1051026 [DOI] [PubMed] [Google Scholar]
- 25.Kawamura Y, Takeshita S, Kanai T, et al. The Combined usefulness of the neutrophil-to-lymphocyte and platelet-to-lymphocyte ratios in predicting intravenous immunoglobulin resistance with Kawasaki disease. J Pediatr 2016;178:281-4.e1. 10.1016/j.jpeds.2016.07.035 [DOI] [PubMed] [Google Scholar]
- 26.Atalay K, Kaldirim Erdogan H, Kirgiz A, et al. Predictive role of neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio in normal-tension glaucoma. Med Hypotheses 2017;103:54-6. 10.1016/j.mehy.2017.04.001 [DOI] [PubMed] [Google Scholar]
- 27.Kurtul A, Murat SN, Yarlioglues M, et al. Association of platelet-to-lymphocyte ratio with severity and complexity of coronary artery disease in patients with acute coronary syndromes. Am J Cardiol 2014;114:972-8. 10.1016/j.amjcard.2014.07.005 [DOI] [PubMed] [Google Scholar]
- 28.Templeton AJ, Ace O, McNamara MG, et al. Prognostic role of platelet to lymphocyte ratio in solid tumors: a systematic review and meta-analysis. Cancer Epidemiol Biomarkers Prev 2014;23:1204-12. 10.1158/1055-9965.EPI-14-0146 [DOI] [PubMed] [Google Scholar]
- 29.McMillan DC. Systemic inflammation, nutritional status and survival in patients with cancer. Curr Opin Clin Nutr Metab Care 2009;12:223-6. 10.1097/MCO.0b013e32832a7902 [DOI] [PubMed] [Google Scholar]
- 30.Suzuki K, Aiura K, Ueda M, et al. The influence of platelets on the promotion of invasion by tumor cells and inhibition by antiplatelet agents. Pancreas 2004;29:132-40. 10.1097/00006676-200408000-00008 [DOI] [PubMed] [Google Scholar]
- 31.Palumbo JS, Talmage KE, Massari JV, et al. Platelets and fibrin(ogen) increase metastatic potential by impeding natural killer cell-mediated elimination of tumor cells. Blood 2005;105:178-85. 10.1182/blood-2004-06-2272 [DOI] [PubMed] [Google Scholar]
- 32.Clemente CG, Mihm MC, Jr, Bufalino R, et al. Prognostic value of tumor infiltrating lymphocytes in the vertical growth phase of primary cutaneous melanoma. Cancer 1996;77:1303-10. [DOI] [PubMed] [Google Scholar]
- 33.Ichihara F, Kono K, Takahashi A, et al. Increased populations of regulatory T cells in peripheral blood and tumor-infiltrating lymphocytes in patients with gastric and esophageal cancers. Clin Cancer Res 2003;9:4404-8. [PubMed] [Google Scholar]


