Abstract
Background
The optimal treatment for early-stage esophageal cancer with positive surgical margins after an upfront esophagectomy is not well-defined. This study investigates the effect of post-operative radiotherapy (PORT) on overall survival (OS) in clinical stage I–II patients with positive margins.
Methods
We identified patients diagnosed between 2004 and 2012 with clinical stage I–II esophageal carcinoma from the National Cancer Data Base (NCDB) who underwent an upfront esophagectomy. For those patients with positive margins, administration of PORT was recorded, and OS was compared by the Kaplan-Meier estimator and log-rank test. Multivariable Cox regression analysis was performed to identify variables associated with improved survival.
Results
Among the 3,490 patients identified, 209 (5.8%) had positive margins. One hundred forty-two (67.9%) patients did not receive PORT while 67 (32.1%) did receive PORT. Compared to those receiving PORT, patients who did not receive PORT were significantly older (68.5 vs. 64.0 years, P=0.003), more likely to have pN0 disease (50.7% vs. 35.4%, P=0.026), and less likely to receive postoperative chemotherapy (21.1% vs. 86.6%, P<0.001). On multivariable logistic regression, only receipt of chemotherapy predicted for receipt of PORT (OR: 25.6, 95% CI: 9.9–65.8, P<0.001). OS was significantly higher for patients receiving PORT compared to those who did not (median OS: 32.2 vs. 16.9 months, log-rank P=0.008). Multivariable analysis confirmed an association with PORT and improved OS (HR: 0.39, 95% CI: 0.27–0.60, P<0.001). Subset analysis demonstrated that the OS benefit of PORT persisted in those patients who received adjuvant chemotherapy (HR: 0.33, 95% CI: 0.19–0.57, P<0.001).
Conclusions
PORT is associated with improved OS in clinical stage I–II esophageal cancer patients after an upfront esophagectomy with positive margins. In the absence of prospective randomized data, our findings suggest that PORT should be strongly considered in the setting of early-stage esophageal cancer resected with positive margins.
Keywords: Esophageal cancer, positive margins, adjuvant radiation
Introduction
The incidence of esophageal cancers is rising and is responsible for 10.3% of all gastrointestinal cancer related deaths in the United States (1-3). Currently, surgery is the mainstay of treatment (4). While advances have been made to improve surgical outcomes, the incidence of positive margins after upfront esophagectomy still ranges from 15.6–30.2% due to the natural biology of the disease and the complexity of the operation (5-7). Although most patients with locally advanced cancer receive pre-operative chemotherapy and radiation, upfront resection is often performed in patients with early-stage disease (8,9).
Several studies have confirmed that the presence of positive margins negatively impacts survival (1,5,10-12). However, there is a lack of data defining the optimal post-operative management for patients who receive incomplete resections. While the National Comprehensive Cancer Network (NCCN) guidelines recommend the use of post-operative radiotherapy (PORT) in addition to chemotherapy in both adenocarcinoma and squamous cell carcinoma resected with positive margins, this recommendation is based on low level evidence (6). To date, there have not been any randomized trials examining the use of PORT in margin-positive resections or retrospective studies directly comparing the outcomes of surgery with or without PORT. In light of the fact that several randomized trials not limited by margin status have failed to demonstrate that PORT confers a survival benefit in patients with esophageal cancer, the benefit of PORT in margin-positive patients is unclear and warrants further evaluation (13,14). The purpose of this study was to investigate the impact of PORT on overall survival (OS) in clinical stage I–II esophageal cancer patients with positive margins after upfront esophagectomy.
Methods
Data source
This retrospective analysis was performed using the National Cancer Data Base (NCDB), a joint project of the Commission on Cancer of the American College of Surgeons and the American Cancer Society. The NCDB records approximately 70% of newly diagnosed cancers in the United States and incorporates cancer registry records from over 1,500 accredited hospitals (15). Variables recorded in the database include patient demographics, tumor characteristics, Charlson-Deyo comorbidity score, socioeconomic status and details on the first course of therapy (defined as all modalities of treatment administered to the patient before disease progression or recurrence). Treatments delivered or withheld because of progressive disease, or other treatment modifications are not recorded. While details of anatomic treatment location, radiation dose, number of fractions throughout the course of radiation, and radiation technique are recorded, details regarding chemotherapy type, dose, treatment duration, and performance status are not available. The American College of Surgeons and the Commission on Cancer have not verified and are neither responsible for the analytic or statistical methodology employed, nor the conclusions drawn from these data by the investigator. This study was granted an exemption by the Yale Human Investigation Committee.
Study cohort
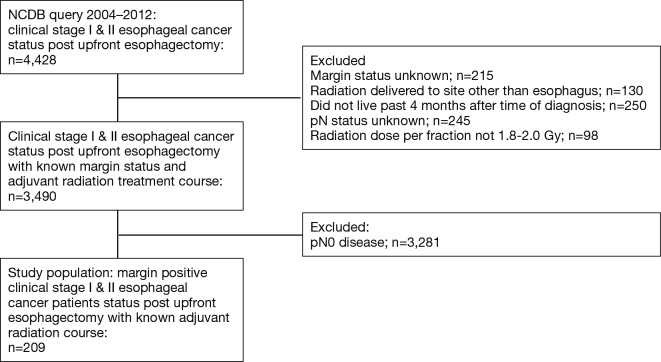
We identified patients diagnosed with clinical stage I and II esophageal cancer from 2004–2012 within the NCDB. Inclusion and exclusion criteria are summarized in Figure 1. Patients were excluded if they did not receive an esophagectomy (Facility Oncology Registry Data Standards codes 30–80), received neoadjuvant therapy, or if their treatment sequence related to surgery was unknown. Since many patients who receive upfront esophagectomy may wait 2–4 months after surgery before the receipt of adjuvant therapy, immortal time bias was controlled for by excluding patients who did not live past 4 months after surgery (16,17). Sequential landmark analysis was conducted at monthly intervals from 0 to 6 months to confirm that the results were robust (Figure S1).
Figure 1.
Study flow diagram.
Figure S1.
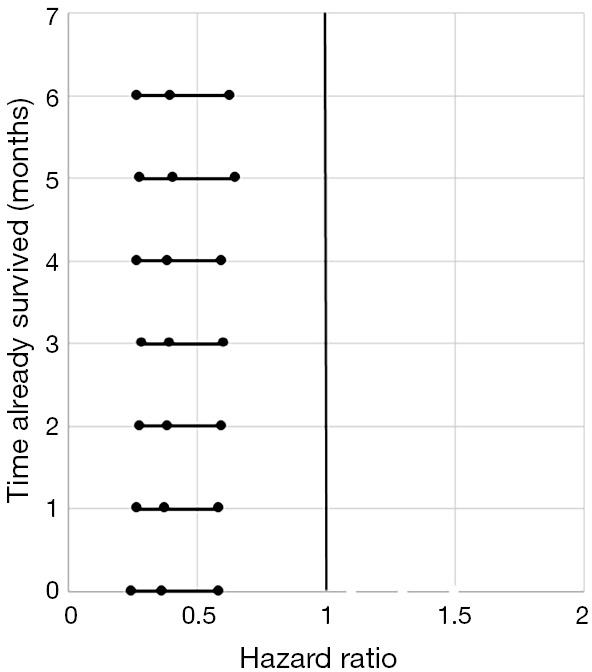
Sequential Landmark Analysis from 0 to 6 months demonstrating HR values for patients treated with PORT vs. no PORT. HR, hazard ratio; PORT, post-operative radiotherapy.
Variables
Socioeconomic characteristics were organized in the following manner: patients were dichotomized as living in an urban location (county population >250,000) or nonurban location (population <250,000), and having private or non-private insurance. Household income was recorded in quartiles of 2012 adjusted household annual income and dichotomized as ≥$63,000 or <$63,000.
Patient demographics were organized in the following manner: age was evaluated as a continuous variable. Race was categorized as white, black, or other. Sex was dichotomized as male or female. Comorbidity information derived from the Charlson-Deyo variation of the Charlson comorbidity index was categorized as 0, 1, or 2 (18).
Clinical characteristics were organized as follows: histology was dichotomized as squamous cell carcinoma or adenocarcinoma. Clinical stage group, clinical tumor stage (cT), clinical nodal stage (cN), and pathologic nodal stage (pN) were evaluated as categorical variables. Tumor size was evaluated as a continuous variable. Post-surgical margins were dichotomized as negative or positive. Positive margins were classified as R1 (microscopic residual tumor), R2 (macroscopic residual tumor), and residual tumor NOS (not otherwise specified).
Information on the course of treatment was organized in the following manner: facility types were assigned according to the Commission on Cancer accreditation category based on annual case volume and available oncology services and was dichotomized as academic or non-academic (including community cancer programs and comprehensive community cancer programs). Post-operative length of stay was included as a proxy for surgical complications, and measured as a continuous variable. PORT and adjuvant chemotherapy were dichotomized as delivered or not delivered. The total dose of PORT was not limited in order to include patients who could not complete their course of therapy. To exclude those who received salvage therapy, only patients who received radiation at fractions standard for curative treatment (1.8–2.0 Gy/fraction) were included.
Statistical analysis
Categorical variables were compared using χ2 tests, whereas continuous variables were compared using Wilcoxon rank sum tests. The primary endpoint was OS, which was defined as the time from diagnosis to death. Cohorts were dichotomized into two groups by receipt of PORT. Backwards stepwise Cox regression was used to conduct multivariable survival analyses. Multivariable backwards stepwise logistic regression was used to determine factors associated with positive margins. Variables were included in the initial multivariable model only if found to be associated with survival (P<0.10) on univariable analysis or if determined a priori to be clinically relevant. Time to locoregional or distant recurrence could not be analyzed, as the NCDB does not provide information on these factors. All tests of significance were two-sided. All analyses were performed using STATA SE 13.1 (Stata, College Station, TX, USA).
Results
Study cohort characteristics
Among 3,490 patients with clinical stage I and II disease who underwent upfront esophagectomy, positive margins were reported in 209 patients (5.8%) overall, including 2.3% of stage IA patients (R1: 1.5%; R2: 0.0%; residual tumor NOS: 0.8%), 4.3% of stage IB patients (R1: 3.2%; R2: 0.0%; residual tumor NOS: 1.1%), 10.4% of stage IIA patients (R1: 6.5%; R2: 1.1%; residual tumor NOS: 3.8%), and 12.5% of stage IIB patients (R1: 5.6%; R2: 0.3%; residual tumor NOS: 6.6%) (Figure 2). Multivariable logistic regression showed that cT2 (OR: 3.14, 95% CI: 2.20–4.49, P<0.001) and cT3 (OR: 6.54, 95% CI: 4.45–9.61, P<0.001) patients were more likely to have positive margins than cT1 patients (Table S1).
Figure 2.
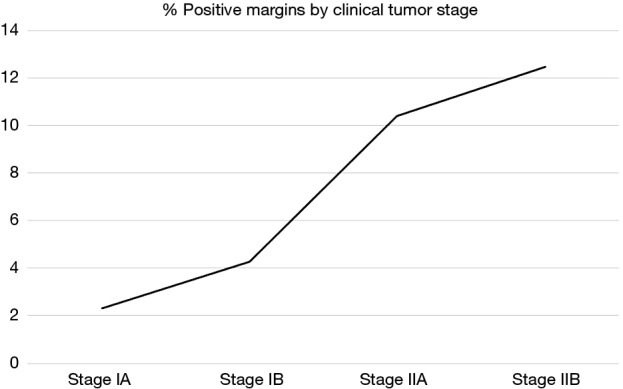
Incidence of positive margins based on clinical stage and pathologic nodal status (n=3,490).
Table S1. Multivariable logistic regression: predictors of margin positivity among all patients (n=3,490).
| Variable | Univariable analysis | Multivariable analysis | |||||
|---|---|---|---|---|---|---|---|
| OR | 95% CI | P | OR | 95% CI | P | ||
| Age | 1.01 | 0.99–1.02 | 0.105 | – | – | – | |
| Sex (female vs. male) | 0.96 | 0.67–1.37 | 0.829 | – | – | – | |
| Race | |||||||
| Black vs. white | 1.94 | 1.15–3.29 | 0.013 | – | – | – | |
| Other vs. white | 1.38 | 0.49–3.88 | 0.536 | – | – | – | |
| Charlson-Deyo score | |||||||
| 1 vs. 0 | 0.86 | 0.60–1.21 | 0.384 | – | – | – | |
| 2 vs. 0 | 1.63 | 1.03–2.58 | 0.037 | – | – | – | |
| Income (<$63,000 vs. ≥$63,000) | 0.98 | 0.72–1.32 | 0.892 | – | – | – | |
| Facility type (non-academic vs. academic) | 1.35 | 1.08–1.95 | 0.013 | 1.28 | 0.94–1.73 | 0.079 | |
| Insurance type (non-private vs. private) | 1.22 | 0.91–1.63 | 0.176 | – | – | – | |
| Urban population (metro vs. non-metro) | 0.75 | 0.51–1.10 | 0.144 | – | – | – | |
| cT status | |||||||
| cT2 vs. cT1 | 3.24 | 2.29–4.57 | <0.001 | 3.14 | 2.20–4.49 | <0.001 | |
| cT3 vs. cT1 | 7.01 | 4.83–10.19 | <0.001 | 6.54 | 4.45–9.61 | <0.001 | |
| Tumor size | 1.00 | 0.99–1.01 | 0.207 | – | – | – | |
| Length of stay at hospital | 1.01 | 1.00–1.01 | 0.003 | – | – | – | |
| Histology (squamous cell vs. adenocarcinoma) | 0.64 | 0.45–0.88 | 0.007 | – | – | – | |
| cN status (cN1 vs. cN0) | 1.53 | 0.98–2.37 | 0.061 | – | – | – | |
Pearson’s P=0.99; Homer-Lemeshow P=0.99.
Upon restriction of the cohort to only those with margin-positive disease, there were 209 patients remaining. Clinical and demographic characteristics of this population are shown in Table 1. 142 (67.9%) patients did not receive PORT after surgery and 67 (32.1%) patients received PORT. Of the patients who received PORT, 86.6% (n=58) also received adjuvant chemotherapy [39 (67.2%) concurrently and 19 (32.8%) sequentially]. The median age was 67 years old. PORT dose was 50.4 Gy in 43.3% of the patients and 45.0 Gy in 29.9% of patients, with dose <45.0 Gy in 5.9% of patients.
Table 1. Patient characteristics by adjuvant treatment type among margin-positive patients (n=209).
| Characteristic | No PORT, 67.9% (n=142) | PORT, 32.1% (n=67) | P |
|---|---|---|---|
| Age, y (median) | 68.5 | 64.0 | 0.003 |
| White race | 88.7% (n=125) | 92.4% (n=61) | 0.369 |
| Male sex (%) | 79.6% (n=113) | 83.6% (n=56) | 0.492 |
| Charlson-deyo score 0 (%) | 65.5% (n=93) | 74.6% (n=50) | 0.364 |
| Income ≥$63,000 (%) | 31.2% (n=43) | 36.9% (n=24) | 0.415 |
| Academic facility type (%) | 63.1% (n=82) | 50.0% (n=33) | 0.079 |
| Private insurance (%) | 35.2% (n=50) | 37.3% (n=25) | 0.767 |
| Urban residence (%) | 82.6% (n=109) | 86.2% (n=56) | 0.522 |
| Stage I (%) | 32.4% (n=46) | 26.9% (n=18) | 0.418 |
| Surgical margins | 0.678 | ||
| Microscopic margins (R1) | 59.2% (n=84) | 61.2% (n=41) | |
| Macroscopic margins (R2) | 1.4% (n=2) | 3.0% (n=2) | |
| Residual tumor NOS | 39.4% (n=56) | 35.8% (n=24) | |
| pN status (%) | 0.026 | ||
| pN0 | 50.7% (n=72) | 35.4% (n=23) | |
| pN+ | 49.3% (n=66) | 64.6% (n=42) | |
| Squamous cell carcinoma histology (%) | 24.6% (n=35) | 23.9% (n=16) | 0.904 |
| Chemotherapy | 21.1% (n=30) | 86.6% (n=58) | <0.001 |
| Length of stay (days) | 12 | 11 | 0.298 |
PORT, post-operative radiotherapy; NOS, not otherwise specified.
Compared to those receiving PORT, patients who did not receive PORT were significantly older (68.5 vs. 64.0 years, P=0.003), more likely to have pN0 disease (50.7% vs. 35.4%, P=0.026), and less likely to receive adjuvant chemotherapy (21.1% vs. 86.6%, P<0.001) (Table 1). On multivariable logistic regression, only receipt of chemotherapy predicted for receipt of PORT (OR: 25.6%, 95% CI: 9.9–65.8, P<0.001) (data not shown).
Survival analysis
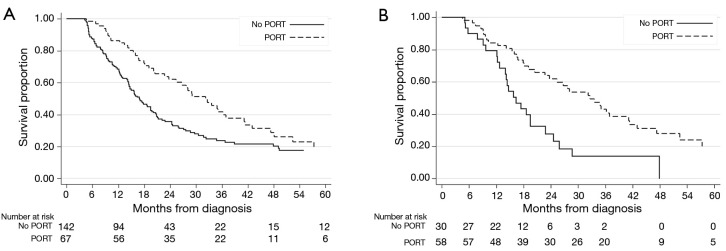
Median survival was nearly doubled at 32.2 months for patients treated with PORT compared to 16.9 months for patients not treated with PORT (P=0.009). On multivariable analysis, patients who received PORT had a significantly longer OS compared to those who did not receive PORT (HR: 0.39, 95% CI: 0.27–0.60, P<0.001) (Table 2, Figure 3A). Higher clinical stage, longer length of stay, and greater pN status were associated with decreased survival. When the patient population was divided based on histology, multivariable analysis showed that PORT was associated with significantly improved survival for those with squamous cell carcinoma (HR: 0.36, 95% CI: 0.15–0.89, P=0.028) and adenocarcinoma (HR: 0.43, 95% CI: 0.28–0.65, P<0.001).
Table 2. Univariable and multivariable analyses of predictors of overall survival among margin-positive patients (n=209).
| Variable | Univariable analysis | Multivariable analysis | |||||
|---|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | ||
| Treatment (PORT vs. no PORT) | 0.63 | 0.44–0.89 | 0.009 | 0.39 | 0.27–0.60 | <0.001 | |
| *Age | 1.03 | 1.01–1.04 | <0.001 | – | – | – | |
| Sex (female vs. male) | 1.03 | 0.69–1.53 | 0.894 | – | – | – | |
| Race | |||||||
| Black vs. white | 0.64 | 0.34–1.17 | 0.149 | – | – | – | |
| Other vs. white | 1.61 | 0.51–5.11 | 0.411 | – | – | – | |
| *Charlson-Deyo score | |||||||
| 1 vs. 0 | 0.85 | 0.56–1.27 | 0.423 | – | – | – | |
| 2 vs. 0 | 1.68 | 1.03–2.74 | 0.039 | – | – | – | |
| Income (<$63,000 vs. ≥$63,000) | 0.98 | 0.69–1.37 | 0.907 | – | – | – | |
| Facility type (non-academic vs. academic) | 1.10 | 0.79–1.53 | 0.563 | – | – | – | |
| *Insurance type (non-private vs. private) | 1.55 | 1.11–2.18 | 0.011 | – | – | – | |
| Urban population (metro vs. non-metro) | 1.21 | 0.79–1.85 | 0.378 | – | – | – | |
| *Clinical stage (II vs. I) | 2.07 | 1.42–3.04 | <0.001 | 1.83 | 1.18–2.83 | 0.007 | |
| *Tumor size | 0.99 | 0.98–0.99 | 0.037 | – | – | – | |
| *Length of stay at hospital | 1.01 | 1.00–1.01 | 0.003 | 1.01 | 1.01–1.02 | 0.001 | |
| Histology (squamous cell vs. adenocarcinoma) | 0.99 | 0.68–1.42 | 0.946 | – | – | – | |
| *pN status | |||||||
| pN1 vs. pN0 | 2.42 | 1.71–3.44 | <0.001 | 3.07 | 2.06–4.57 | <0.001 | |
| pN2 vs. pN0 | 1.28 | 0.65–2.51 | 0.469 | 1.85 | 0.87–3.91 | 0.109 | |
| pN3 vs. pN0 | 1.96 | 0.89–4.32 | 0.094 | 2.38 | 1.07–5.31 | 0.034 | |
| *Chemo (Yes vs. No) | 0.83 | 0.60–1.14 | 0.252 | – | – | – | |
| Margins | |||||||
| R2 vs. R1 | 2.31 | 0.72–7.35 | 0.158 | – | – | – | |
| Residual tumor NOS vs. R1 | 0.85 | 0.61–1.18 | 0.337 | – | – | – | |
*, indicates variables included in initial multivariable model. PORT, post-operative radiotherapy; NOS, not otherwise specified.
Figure 3.
Kaplan-Meier survival curve of no PORT vs. PORT in (A) the overall cohort and (B) patients who received chemotherapy. PORT, post-operative radiotherapy
Eighty patients (38%) had margins classified as residual tumor NOS. Multivariable analysis demonstrated that OS was not significantly different for patients in the residual tumor NOS category and patients with R1 margins (Table S2).
Table S2. Multivariable analysis comparing overall survival among patients with positive margins classified as R1, R2, and residual tumor NOS (n=209).
| Variable | Multivariable analysis | ||
|---|---|---|---|
| HR | 95% CI | P | |
| Margins | |||
| R2 vs. R1 | 3.25 | 0.77–13.61 | 0.107 |
| Residual tumor NOS vs. R1 | 0.90 | 0.63–1.29 | 0.572 |
| Insurance type (non-private vs. private) | 1.56 | 1.08–2.26 | 0.019 |
| Length of stay at hospital | 1.01 | 1.01–1.02 | 0.002 |
| Treatment (PORT vs. no PORT) | 0.63 | 0.43–0.91 | 0.016 |
NOS, not otherwise specified; PORT, post-operative radiotherapy.
Since patients who died within 4 months were excluded to account for immortal time bias, sequential landmark analysis was performed at 0, 1, 2, 3, 4, 5 and 6 months. This confirmed that our findings were robust regardless of the exclusion time criteria (Figure S1).
On multivariable analysis, receipt of chemotherapy was a strong predictor for receipt of PORT (data not shown). Therefore, a subset analysis was done in only patients who received chemotherapy. Multivariable analysis showed that PORT significantly improved OS in patients who received adjuvant chemotherapy (HR: 0.33, 95% CI: 0.19–0.57, P<0.001) (Table 3, Figure 3B).
Table 3. Univariable and multivariable analyses of predictors of overall survival among margin-positive patients who received chemotherapy (n=88).
| Variable | Univariable analysis | Multivariable analysis | |||||
|---|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | ||
| *Treatment (PORT vs. no PORT) | 0.41 | 0.24–0.69 | 0.001 | 0.33 | 0.19–0.57 | <0.001 | |
| *Age | 1.03 | 0.99–1.05 | 0.090 | – | – | – | |
| Sex (female vs. male) | 1.47 | 0.77–2.83 | 0.247 | – | – | – | |
| Race | |||||||
| Black vs. white | 0.51 | 0.12–2.10 | 0.351 | – | – | – | |
| Charlson-Deyo score | |||||||
| 1 vs. 0 | 0.91 | 0.47–1.77 | 0.788 | – | – | – | |
| 2 vs. 0 | 1.75 | 0.82–3.73 | 0.249 | – | – | – | |
| Income (<$63,000 vs. ≥$63,000) | 1.38 | 0.79–2.42 | 0.259 | – | – | – | |
| Facility type (non-academic vs. academic) | 1.11 | 0.67–1.86 | 0.671 | – | – | – | |
| *Insurance type (non-private vs. private) | 2.05 | 1.20–3.50 | 0.008 | 2.52 | 1.45–4.38 | 0.001 | |
| Urban population (metro vs. non-metro) | 1.59 | 0.78–3.24 | 0.206 | – | – | – | |
| Clinical stage (II vs. I) | 1.33 | 0.75–2.38 | 0.330 | – | – | – | |
| Tumor size | 0.99 | 0.99–1.00 | 0.272 | – | – | – | |
| Length of stay at hospital | 1.00 | 0.98–1.02 | 0.666 | – | – | – | |
| Histology (squamous cell vs. adenocarcinoma) | 1.28 | 0.63–2.61 | 0.493 | – | – | – | |
| *pN status | |||||||
| pN1 vs. pN0 | 1.95 | 1.06–3.62 | 0.033 | – | – | – | |
| pN2 vs. pN0 | 1.22 | 0.29–2.26 | 0.681 | – | – | – | |
| pN3 vs. pN0 | 1.34 | 0.38–4.71 | 0.650 | – | – | – | |
| Margins | |||||||
| R2 vs. R1 | 2.81 | 0.66–11.97 | 0.161 | – | – | – | |
| Residual tumor NOS vs. R1 | 0.76 | 0.46–1.31 | 0.346 | – | – | – | |
*, indicates variables included in initial multivariable model. PORT, post-operative radiotherapy; NOS, not otherwise specified.
Discussion
This study examines the impact of PORT on the OS of early-stage esophageal cancer patients with positive margins after upfront esophagectomy. While prior studies show that 15.6–30.2% of all esophageal cancer patients who receive upfront esophagectomy have positive post-resection margins, the incidence of positive margins in patients with early stage disease is less common and there is not prospective data in this subset of patients to guide postoperative therapy (5-7). This analysis of a large nationwide cohort shows that 6% of patients with clinical stage I and II esophageal cancer who underwent upfront esophagectomy had positive post-resection margins. We demonstrate that PORT confers a survival benefit in clinical stage I and II patients with margin positive resections.
While neoadjuvant chemoradiation followed by resection is the preferred method of treatment for locally advanced esophageal cancers based on level 1 data, it is not recommended for all patients with early-stage disease (6,19). There are no randomized trials demonstrating a survival benefit associated with neoadjuvant therapy in patients with stage I esophageal cancer, and the benefit of trimodality therapy in stage II disease is unclear. In 2008, the phase III French FFCD 9901 trial demonstrated that neoadjuvant chemoradiation did not prolong survival in stage I–II esophageal cancers, but was instead associated with a higher risk of peri-operative mortality (20). As such, upfront esophagectomy is commonly performed in patients with early-stage disease worldwide, including a substantial percentage of stage IIB patients (21).
However, the optimal management of patients who are left with residual tumor after an upfront esophagectomy is not well characterized. Previous studies have consistently demonstrated that patients with positive margins have significantly worse survival outcomes (7-9). PORT is commonly utilized along with chemotherapy in these patients in an effort to improve locoregional control and perhaps prolong survival. In addition, current NCCN guidelines support the use of PORT with chemotherapy in both adenocarcinoma and squamous cell carcinoma patients who have positive margins after upfront resection, regardless of stage or nodal status (6). Despite this, there is a lack of randomized data demonstrating that PORT is indeed associated with increased survival. Hence, a large retrospective study using a national clinical database comparing survival outcomes of patients who did and did not receive PORT after upfront esophagectomy can provide guidance in the management of these patients.
The results of our analysis support current NCCN guidelines and show that PORT is associated with significantly improved survival. Previous smaller retrospective studies reported consistent findings, including a comparison of patients with positive resection margins who received PORT and patients with negative resection margins who did not receive PORT. There were no significant differences in OS, disease-free survival, and locoregional control, suggesting that PORT improves outcomes for patients with positive surgical margins (22). However, there have not been any prior studies directly comparing the survival outcomes of margin-positive patients who do and do not receive PORT.
In this study, the majority of patients (86.5%) who received PORT also received adjuvant chemotherapy, whereas only 21.1% of patients who did not receive PORT received chemotherapy. Thus the combination of PORT and adjuvant chemotherapy likely contributes to the survival advantage over patients receiving chemotherapy alone or no adjuvant therapy. Nonetheless, in the subset of patients who received adjuvant chemotherapy, the addition of PORT was associated with improved survival. While further comparisons of survival after PORT alone and PORT with adjuvant chemotherapy were not feasible due to limited patient numbers, these results imply that in early-stage patients with positive margins, achieving local control with the addition of radiation to adjuvant chemotherapy contributes to prolonged survival. Our study also demonstrated that greater clinical T stage is significantly associated with increased risk of positive margins. Further highlighting this point, we found a linear correlation between increasing clinical stage (IA, IB, IIA, and IIB) and increased incidence of positive margins. Interestingly, clinical stage IIA and IIB patients had a pooled incidence of 10.8% whereas clinical stage IA and IB patients had a pooled incidence of 2.9%. Whether these patients would benefit from neoadjuvant chemoradiotherapy remains in question.
The primary strengths of our study lie in the relative large size of our cohort, which allowed for a direct comparison between margin-positive patients who did or did not receive PORT after upfront resection. However, there are limitations inherent to the retrospective nature of this study that must be considered. Selection bias in treatment allocation is unavoidable, even after accounting for age, comorbidities, length of hospital stay after surgery, and clinical stage. Certain patients in our analysis may not have received PORT because they were deemed medically unfit for treatment. While we controlled for performance status using the Charleson-Deyo score, there is no way to definitively exclude these patients. Additionally, certain patients in our study were understaged by pre-operative diagnostic procedures, as indicated by the presence of pN2 and pN3 disease in clinical stage I and II patients. Moreover, the NCDB does not give information on perioperative complications, local recurrence, distant recurrence, or disease-specific survival, all factors which influence OS.
In conclusion, the results of this study suggest that early stage esophageal cancer patients with positive margins who receive PORT and chemotherapy have better survival outcomes than patients who receive adjuvant chemotherapy alone or no adjuvant therapy, and support the consensus guidelines recommending the use of radiation therapy in this population (6). Future prospective studies are needed to confirm these results.
Acknowledgements
None.
Ethical Statement: This analysis was performed using the National Cancer Data Base, and thus was granted an exemption by the Yale Human Investigation Committee.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Markar SR, Gronnier C, Duhamel A, et al. Significance of microscopically incomplete resection margin after esophagectomy for esophageal cancer. Ann Surg 2016;263:712-8. 10.1097/SLA.0000000000001325 [DOI] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin 2016;66:7-30. 10.3322/caac.21332 [DOI] [PubMed] [Google Scholar]
- 3.Thrift AP, Whiteman DC. The incidence of esophageal adenocarcinoma continues to rise: analysis of period and birth cohort effects on recent trends. Ann Oncol 2012;23:3155-62. 10.1093/annonc/mds181 [DOI] [PubMed] [Google Scholar]
- 4.Boniface MM, Wani SB, Schefter TE, et al. Multidisciplinary management for esophageal and gastric cancer. Cancer Manag Res 2016;8:39-44. 10.2147/CMAR.S101169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jang R, Darling G, Wong RK. Multimodality approaches for the curative treatment of esophageal cancer. J Natl Compr Canc Netw 2015;13:229-38. 10.6004/jnccn.2015.0029 [DOI] [PubMed] [Google Scholar]
- 6.Ajani JA, D'Amico TA, Almhanna K, et al. Esophageal and esophagogastric junction cancers, version 1.2015. J Natl Compr Canc Netw 2015;13:194-227. 10.6004/jnccn.2015.0028 [DOI] [PubMed] [Google Scholar]
- 7.Gilbert S, Martel AB, Seely AJ, et al. Prognostic significance of a positive radial margin after esophageal cancer resection. J Thorac Cardiovasc Surg 2015;149:548-55; discussion 555. 10.1016/j.jtcvs.2014.10.040 [DOI] [PubMed] [Google Scholar]
- 8.Mulligan ED, Dunne B, Griffin M, et al. Margin involvement and outcome in oesophageal carcinoma: a 10-year experience in a specialist unit. Eur J Surg Oncol 2004;30:313-7. 10.1016/j.ejso.2003.11.009 [DOI] [PubMed] [Google Scholar]
- 9.Hughes BG, Yip D, Chao M, et al. Audit of postoperative chemoradiotherapy as adjuvant therapy for resected gastroesophageal adenocarcinoma: an Australian multicentre experience. ANZ J Surg 2004;74:951-6. 10.1111/j.1445-1433.2004.03218.x [DOI] [PubMed] [Google Scholar]
- 10.Kelsen DP, Winter KA, Gunderson LL, et al. Long-term results of RTOG trial 8911 (USA Intergroup 113): a random assignment trial comparison of chemotherapy followed by surgery compared with surgery alone for esophageal cancer. J Clin Oncol 2007;25:3719-25. 10.1200/JCO.2006.10.4760 [DOI] [PubMed] [Google Scholar]
- 11.Papachristou DN, Agnanti N, D'Agostino H, et al. Histologically positive esophageal margin in the surgical treatment of gastric cancer. Am J Surg 1980;139:711-3. 10.1016/0002-9610(80)90369-4 [DOI] [PubMed] [Google Scholar]
- 12.Deeter M, Dorer R, Kuppusamy MK, et al. Assessment of criteria and clinical significance of circumferential resection margins in esophageal cancer. Arch Surg 2009;144:618-24. 10.1001/archsurg.2009.115 [DOI] [PubMed] [Google Scholar]
- 13.Teniere P, Hay JM, Fingerhut A, et al. Postoperative radiation therapy does not increase survival after curative resection for squamous cell carcinoma of the middle and lower esophagus as shown by a multicenter controlled trial. French University Association for Surgical Research. Surg Gynecol Obstet 1991;173:123-30. [PubMed] [Google Scholar]
- 14.Fok M, Sham JS, Choy D, et al. Postoperative radiotherapy for carcinoma of the esophagus: a prospective, randomized controlled study. Surgery 1993;113:138-47. [PubMed] [Google Scholar]
- 15.Bilimoria KY, Stewart AK, Winchester DP, Ko CY. The National Cancer Data Base: a powerful initiative to improve cancer care in the United States. Ann Surg Oncol 2008;15:683-90. 10.1245/s10434-007-9747-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Macdonald JS, Smalley SR, Benedetti J, et al. Chemoradiotherapy after surgery compared with surgery alone for adenocarcinoma of the stomach or gastroesophageal junction. N Engl J Med 2001;345:725-30. 10.1056/NEJMoa010187 [DOI] [PubMed] [Google Scholar]
- 17.Park HS, Gross CP, Makarov DV, et al. Immortal time bias: a frequently unrecognized threat to validity in the evaluation of postoperative radiotherapy. Int J Radiat Oncol Biol Phys 2012;83:1365-73. 10.1016/j.ijrobp.2011.10.025 [DOI] [PubMed] [Google Scholar]
- 18.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol 1992;45:613-9. 10.1016/0895-4356(92)90133-8 [DOI] [PubMed] [Google Scholar]
- 19.Shapiro J, van Lanschot J, Hulshof MC, et al. Neoadjuvant chemoradiotherapy plus surgery versus surgery alone for oesophageal or junctional cancer (CROSS): long-term results of a randomised controlled trial. Lancet 2015;16:1090-8. 10.1016/S1470-2045(15)00040-6 [DOI] [PubMed] [Google Scholar]
- 20.Mariette C, Dahan L, Mornex F, et al. Surgery alone versus chemoradiotherapy followed by surgery for stage I and II esophageal cancer: final analysis of randomized controlled phase III trial FFCD 9901. J Clin Oncol 2014;32:2416-22. 10.1200/JCO.2013.53.6532 [DOI] [PubMed] [Google Scholar]
- 21.Boone J, Livestro DP, Elias SG, et al. International survey on esophageal cancer: part II staging and neoadjuvant therapy. Dis Esophagus 2009;22:203-10. 10.1111/j.1442-2050.2008.00930.x [DOI] [PubMed] [Google Scholar]
- 22.Song S, Chie EK, Kim HJ, et al. Role of postoperative radiotherapy for microscopic margin involvement in the squamous cell carcinoma of esophagus. Cancer Res Treat 2013;45:202-9. 10.4143/crt.2013.45.3.202 [DOI] [PMC free article] [PubMed] [Google Scholar]