Abstract
Background
There are limited treatment options available for patients with advanced pancreatic ductal adenocarcinoma (PDAC). We conducted a phase II study evaluating the efficacy and safety of capecitabine/oxaliplatin (CAPOX) in patients with locally advanced and metastatic PDAC treated in the first and second lines.
Methods
Forty subjects with advanced PDAC and ECOG performance status ≥2 were enrolled. Treatment consisted of capecitabine 2,000 mg/m2 orally in two divided doses daily for 14 days and oxaliplatin 130 mg/m2 intravenously day 1 every 21 days. The primary endpoint was response rate (RR); secondary endpoints included safety analysis, progression free survival (PFS) and overall survival (OS).
Results
The overall RR was 12.5% (N=3); the disease control rate was 67% (N=16). Due to the protocol definition for eligibility of response evaluation, only 60% (N=24) were evaluable for the primary endpoint. Median progression free survival (mPFS) was 3.8 months (95% CI: 1.3, 6.2); median OS (mOS) was 7.4 months (95% CI: 4.8, 12.2). The most common grade 3/4 toxicities included: fatigue (19%), nausea (17%), and diarrhea (14%).
Conclusions
CAPOX is an active regimen in patients with advanced PDAC and is associated with acceptable toxicity. Careful consideration should be given to response endpoints and outcome measures when studying this characteristically ill population.
Keywords: Pancreatic cancer, capecitabine and oxaliplatin
Introduction
Despite recent advances in systemic therapy for advanced pancreatic ductal adenocarcinoma (PDAC), outcomes remain limited by poor survival and, for the most aggressive regimens, significant toxicities. Until 2011 when the FOLFIRINOX regimen [intravenous fluorouracil (5FU), leucovorin, oxaliplatin, and irinotecan] demonstrated superior overall survival (OS) compared to intravenous gemcitabine alone, single agent gemcitabine was the standard of care for first line treatment (1,2). Subsequently in 2013, the combination nab-paclitaxel/gemcitabine also demonstrated better OS than gemcitabine alone (3) in the first line setting. In addition, intravenous 5FU/leucovorin and nanoliposomal irinotecan in the second-line setting after gemcitabine-based chemotherapy has recently been shown to improve survival compared to intravenous 5FU/leucovorin alone or nanoliposomal irinotecan alone (4). Due to substantial toxicities, however, many PDAC patients are not able to tolerate these regimens. In particular, questions have been raised about how much toxicity (and benefit) irinotecan contributes to the 5FU-oxaliplatin backbone in FOLFIRINOX. In addition, the continuous infusion 5FU in FOLFIRINOX may add logistical and quality of life concerns for patients. In rectal cancer, oral 5FU (capecitabine) is non-inferior compared to continuous infusion 5FU (5) and may be more convenient for patients.
The activity of infusional 5FU combined with oxaliplatin without the addition of irinotecan for advanced PDAC has been reported in several small studies (Table 1). Intravenous 5FU and oxaliplatin appear active in first line (FOLFOX, OFF, OXFU) and second-line (weekly oxaliplatin/5FU/leucovorin, FOLFOX) regimens (6-8,10,12-14,16,17). Median OS and response rates (RRs) were superior in the first line compared to second line studies. The combination capecitabine and oxaliplatin showed similar efficacy and toxicity profile to FOLFOX4 in a study evaluating both XELOX and FOLFOX4 in the second line (XELOX PR 18%, SD 41%, mOS 21 weeks; FOLFOX PR 17%, SD 26%, mOS 25 weeks) (15).
Table 1. Clinical trials evaluating the combinations of capecitabine and/or fluorouracil with oxaliplatin in advanced pancreatic ductal adenocarcinoma.
| Author | Year | N | 1st or 2nd line treatment | 5FU or capecitabine | ORR (%) | DCR (%) | mPFS/TTP (months) | mOS (months) |
|---|---|---|---|---|---|---|---|---|
| Ducreux (6) | 2004 | 31 | 1st | 5FU | 10 | NA | 4.2 | 9.0 |
| Tsavaris (7) | 2005 | 30 | 1st | 5FU | 23.3 | 53.3 | 5.5 | 6.3 |
| Ghosn (8) | 2007 | 30 | 1st | 5FU | 27.6 | 62 | 4 | 7.5 |
| Boeck (9) | 2008 | 61 | 1st | Capecitabine | 13 | 49 | 4.2 | 8.1 |
| Mitry (10) | 2006 | 18 | 2nd | 5FU | 0 | 17 | 0.9 | 4.9 |
| Xiong (11) | 2008 | 41 | 2nd | Capecitabine | 3 | 28 | 2.5 | 5.8 |
| Novarino (12) | 2009 | 23 | 2nd | 5FU | 0 | 23.5 | 2.9 | 4.3 |
| Yoo (13) | 2009 | 30 | 2nd | 5FU | 7 | 17 | 1.5 | 3.7 |
| Pelzer (14) | 2011 | 46 | 2nd | 5FU | 0 | NA | NA | 4.8 |
| Berk (15) | 2012 | 85 | 2nd | 5FU and capecitabine | 18 | 59 | 4 | 5.3 |
| El-Hadaad (16) | 2013 | 30 | 2nd | 5FU | 6.7 | 26.7 | 3.3 | 5.5 |
| Oettle (17) | 2014 | 77 | 2nd | 5FU | NA | NA | 2.9 | 5.9 |
ORR, overall response rate (% partial plus complete response per RECIST); DCR, disease control rate (ORR plus % stable disease); mPFS, median progression free survival; mTTP, median time to progression; mOS, median overall survival. Results reported in weeks converted to months using 1 week, 7 days and 4 weeks, 1 month.
Two additional small studies have evaluated capecitabine combined with oxaliplatin in advanced PDAC (Table 1) (16,17). The first by Xiong and colleagues was a non-randomized phase II study evaluating capecitabine and oxaliplatin (CAPOX) in subjects who had progressed on gemcitabine (n=41) (16). Results were not as encouraging as those seen in studies of oxaliplatin combined with intravenous 5FU or gemcitabine (mOS =5.75 months; PR in 1 subject; SD in 10 of 39 (26%) evaluable subjects). The side effects were manageable, however, and the study population appeared more ill than in other studies: 95% had metastatic disease and almost 30% had an Eastern Cooperative Oncology Group (ECOG) Performance Score (PS) of 2. In the same year Boeck and colleagues published a randomized three-arm phase II trial evaluating CAPOX, combination capecitabine/gemcitabine, and combination gemcitabine/oxaliplatin (17). Results for the CAPOX arm showed median progression-free survival (mPFS) 4.2 months, mOS 8.1 months, and fewer grade 3 and 4 hematologic toxicities than in the gemcitabine containing arms. The authors concluded that each regimen demonstrated similar clinical efficacy and safety profiles in this population.
Given the scarcity of treatment options at the time of study initiation in the mid-2000s, we undertook a Phase II trial to assess CAPOX for first and second-line treatment in advanced PDAC. We present here the results of a multi-institutional Phase II single-arm trial that evaluated 40 patients treated with CAPOX for first or second-line treatment of locally advanced or metastatic PDAC.
Methods
Study population
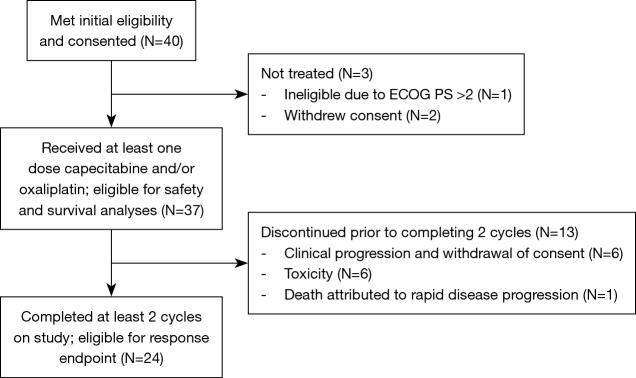
Forty subjects with locally advanced or metastatic PDAC were enrolled at three academic institutions for first or second line treatment (Figure 1). All subjects had histologically or cytologically confirmed PDAC and had received at most one prior chemotherapy regimen for advanced disease. Previous adjuvant chemotherapy was permitted if completed more than 12 months prior to initiation of study treatment. Other eligibility criteria included ECOG PS ≤2, adequate organ function and life expectancy ≥3 months (additional details in Supplementary 1). The Institutional Review Board approved this study, and all subjects provided written informed consent.
Figure 1.
Consort diagram.
Treatment
Participants self-administered capecitabine 1,000 mg/m2 orally twice daily (total daily dose 2,000 mg/m2), days 1–14 in 21-day cycles. Only 500 mg tablets were used, and doses were rounded to the nearest dose that could be administered with 500 mg tablets. Oxaliplatin 130 mg/m2 was administered intravenously on day 1 every 21±2 days. Treatment continued until tumor progression or toxicity requiring discontinuation of therapy. Oxaliplatin was provided by Sanofi-Synthelabo as investigational drug supply and capecitabine was prescribed commercially. Response was assessed by computed tomography (CT) or magnetic resonance imaging (MRI) every two cycles (42±2 days) using RECIST criteria (18). Complete responses (CR) and PR were confirmed by repeat scan one month later. Chemotherapy doses were modified for toxicities as outlined in the protocol (Supplementary 2). Capecitabine was held for any grade 2, 3, or 4 toxicity, and the dose was reduced when restarted after toxicity resolution. The oxaliplatin was dose-reduced for hematologic and neurologic toxicities, and the infusion was extended to six hours if a subject experienced acute laryngopharyngeal dysesthesia. Compliance with oral capecitabine was documented at each study visit by pill count.
Statistical analysis
The primary endpoint was RR, defined as objective response of measurable disease per RECIST criteria (18). Subjects were evaluable for response if two cycles of treatment on study were completed. An interim analysis was conducted after the first 12 subjects according to the Simon’s 2-stage design (19). Anticipating that 10% of subjects would not be evaluable for response, a total of 40 patients were enrolled to achieve 90% power to detect 20% difference in response in target lesions, with type 1 error of 10% and alpha <5%. A two-sided 80% CI was estimated considering the two-stage design based on Atkinson and Brown methods (20). Demographics, disease characteristics and adverse events are presented for the safety population. Progression free survival (PFS) reflects time from enrollment until progression or death. Subjects who did not reach progression or were lost to follow-up were censored at the date of last progression free disease evaluation. Disease control rate included data on subjects with CR, PR, and SD. PFS and OS were calculated by the Kaplan Meier method. Subjects were included in the PFS analysis if they completed two cycles on treatment (response evaluable population); subjects were included in the OS and safety analyses if they took any capecitabine or oxaliplatin dose on study.
We also performed a post-hoc literature review to search for all advanced pancreatic cancer studies evaluating capecitabine and oxaliplatin, and infusional 5FU and oxaliplatin. Search terms included: advanced or metastatic pancreatic cancer, 5FU, oxaliplatin, capecitabine, clinical trial. The following data was abstracted: First author, year of publication, number of subjects, line of treatment, and when available, ORR, DCR, mPFS and mOS.
Results
Patient characteristics
Forty subjects were enrolled between May 2004 and March 2010. Baseline demographics and disease characteristics are presented in Table 2. The majority of participants (95%) had an ECOG PS 0–1, had metastatic PDAC (95%), and had previously received gemcitabine for advanced PDAC (72.5%).
Table 2. Baseline characteristics.
| Characteristic | Number subjects, N [%] |
|---|---|
| Age in years, median [range] | 61 [40–78] |
| Gender | |
| Male | 19 [48] |
| Female | 21 [53] |
| Race | |
| Asian | 1 [3] |
| Black or African American | 2 [5] |
| White | 31 [78] |
| Other | 3 [8] |
| Unknown | 3 [8] |
| ECOG PS | |
| 0 | 10 [25] |
| 1 | 28 [70] |
| 2 | 2 [5] |
| Received prior chemotherapy | |
| Yes | 29 [72.5] |
| No | 11 [27.5] |
| Stage | |
| Locally advanced | 2 [5] |
| Metastatic | 38 [95] |
| CA 19-9 (U/mL); median (range) | 1,009 (3–678,332) |
| Number of metastatic sites, median (range) | 2 (0–4) |
Treatment completion and toxicity
Thirty-seven subjects received at least one dose of capecitabine and/or oxaliplatin on study (safety population). The reasons for not receiving any treatment on protocol were: ineligibility due to poor performance status and withdrawal of consent. The median number of cycles per subject was 2 (range, 1–12). Among subjects evaluable for response (n=24), the most common reasons for discontinuation were disease progression (n=17; 71%), followed by toxicity (n=4; 17%), and subject and/or physician preference (n=3; 13%). Subjects not evaluable for response withdrew prior to completing two cycles due to withdrawal of consent and/or clinical progression (n=6; 46.2%), toxicity (n=6; 46.2%), and death attributed to rapid disease progression (n=1; 7.7%). Dose reductions were performed for toxicity in nine (25%) subjects and treatment holds in 16 (44%) subjects. There were 36 subjected included in the toxicity analysis as one patient was missing adverse event data. The most prevalent toxicities are reported in Table 3. Two subjects died during treatment, one due to a cerebrovascular ischemic event and one due to colitis.
Table 3. Adverse events (maximum grade attributable to drug).
| Adverse event (n=36) | Grade 1, 2, n [%] | Grade 3, 4, n [%] | Overall, n [%] |
|---|---|---|---|
| Fatigue | 19 [53] | 7 [19] | 26 [72] |
| Nausea | 19 [53] | 6 [17] | 25 [69] |
| Neuropathy-sensory | 21 [58] | 1 [3] | 22 [61] |
| Vomiting | 15 [42] | 4 [1] | 19 [53] |
| Anorexia | 15 [42] | 1 [3] | 16 [44] |
| Anemia | 11 [31] | 3 [8] | 14 [39] |
| Diarrhea | 8 [22] | 5 [14] | 13 [36] |
| Hand-foot skin reaction | 8 [22] | 1 [3] | 9 [25] |
| Abdominal pain or cramping | 4 [11] | 3 [8] | 7 [19] |
| Thrombocytopenia | 7 [19] | 0 [0] | 7 [19] |
Note: one patient who received treatment was missing safety data.
Efficacy results
Due to the protocol definition of eligibility for evaluation of response (at least two cycles of treatment completed on study), only 24 subjects (60%) were evaluable for the primary endpoint. Four subjects were lost to follow up and were censored at the last visit.
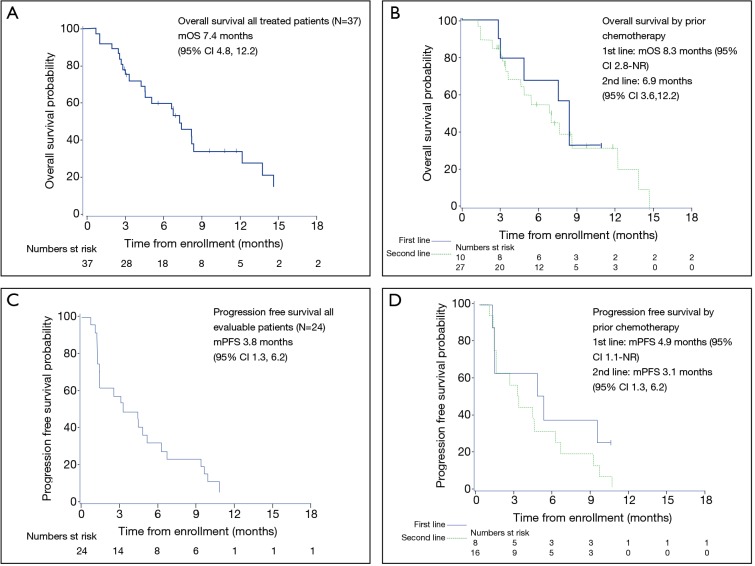
The most frequent response was SD (n=13; 54%), and three subjects experienced a PR (13%) (Table 4). At the first imaging after two cycles on treatment, 33% experienced disease progression (N=8). This result does not include those subjects who were taken off study due to clinical progression within the first two cycles as this choice was listed as ‘subject or clinician preference.’ The overall RR was 12.5% (80% CI: 3.5–20.1), and the disease control rate (DCR) was 67%. Twenty-four subjects were included in the PFS analysis, and 38 were included in the OS analysis. For evaluable subjects (n=24 patients with 22 PFS events), mPFS was 3.8 months (95% CI: 1.3, 6.2) (Figure 2). Among subjects treated in the first line mPFS was 4.9 months (95% CI: 1.1–NR) compared to 3.1 months (95% CI: 1.3, 6.2) among those treated in the second line. For all treated subjects (n=37 with 24 deaths), mOS was 7.4 months (95% CI: 4.8, 12.2). Median OS among subjects treated in the first line was 8.3 months (95% CI: 2.8–NR) compared to mOS 6.9 months (95% CI: 3.6, 12.2) among those treated in the second line.
Table 4. Best recorded response.
| Evaluable population (N=24) | Number subjects, N [%] |
|---|---|
| CR | 0 [0] |
| PR | 3 [13] |
| ORR | 3 [13] |
| SD | 13 [54] |
| DCR | 16 [67] |
| PD | 8 [33] |
| Not evaluable | 16 [67] |
CR, complete response per RECIST; PR, partial response per RECIST; ORR, overall response rate (CR + PR); SD, stable disease per RESIST; DCR, ORR + SD; PD, progressive disease per RECIST.
Figure 2.
Kaplan-Meier estimates for overall survival and progression-free survival for all treated patients and by line of therapy. (A) Median overall survival was 7.4 months (95% CI: 4.8, 12.2); (B) median overall survival among subjects treated in the first line was 8.3 months (95% CI: 2.8–NR) and in those treated in the second line 6.9 months (95% CI: 3.6, 12.2); (C) median progression free survival was 3.8 months (95% CI: 1.3, 6.2); (D) median progression free survival among subjects treated in the first line was 4.9 months (95% CI: 1.1–NR) and in those treated in the second line 3.1 months (95% CI: 1.3, 6.2).
Discussion
Despite the introduction of FOLFIRINOX and nab-paclitaxel/gemcitabine into clinical practice, advanced PDAC remains a highly lethal disease, and the burdens associated with treatment remain an important issue for patients and their caregivers. Our study contributes to the capecitabine and oxaliplatin (CAPOX) literature showing that this regimen can be administered to patients with locally advanced or metastatic PDAC with encouraging DCR (67%) and acceptable toxicity in either the first- or second-line setting. Thus, CAPOX may be a reasonable choice for those patients for whom the toxicities associated with the more aggressive combination regimens is not advised. This study, however, also highlights the challenges that exist in studying patients with an inherently aggressive disease like advanced PDAC.
In this study the majority of subjects (54%) achieved SD. Furthermore, eight subjects maintained SD for greater than six months, and disease remained controlled in three subjects for greater than 10 months, which is on par with results seen for more aggressive combination regimens (2-4,21).
The RR in our study, 13%, was similar to that reported by Boeck and colleagues in the first line (13%) (9) and similar to that reported by Berk and colleagues (18%) (15). Response was greater than that reported by Xiong and colleagues evaluating CAPOX (1%) (11) and by Yoo et al. evaluating FOLFOX in gemcitabine refractory disease (7%) (13). Response, however, was lower than that reported by Ghosn et al. in their study of FOLFOX in first line therapy (27.6%) (8) (Table 1). Results were also comparable to those reported by Demols et al., who assessed the benefit of oxaliplatin plus gemcitabine in subjects with gemcitabine-refractory advanced PDAC (PR 22.6%, SD 35.5%) (21) and that reported for second line 5-FU/nanoliposomal irinotecan (16%) (4) by Wang-Gillam et al.
Median PFS (3.8 months) and mOS (7.4 months) in the current study are similar to those reported by Xiong et al. (11) and Berk et al. (15), and similar to that reported for gemcitabine monotherapy in the first line setting (1). Results from studies of infusional 5FU have been variable with mOS ranging 3.7 to 9.0 months (6-8,10,12-14,16,17). Median OS was slightly lower than that reported by Boeck and colleagues for the same regimen (8.1 months) in the first-line (9), but our study also included second-line subjects. Median PFS and mOS were comparable to that reported for 5-FU/nanoliposomal irinotecan in the second line (mPFS 3.1 months, mOS 6.1 months) (4).
The RR, mPFS, and mOS in this trial are substantially lower than that reported for first line FOLFIRINOX. Thus, the addition of irinotecan to the intravenous 5FU/leucovorin/oxaliplatin backbone may provide additional benefit. However, our study does not directly compare these regimens, and the mechanism underlying this difference remains unclear. Irinotecan monotherapy has shown only modest benefit in advanced PDAC (22), and the results of other phase II studies of FOLFIRI (5FU/leucovorin, and irinotecan) and that of second line 5FU/nanoliposomal irinotecan are comparable to those reported here (4,23,24).
A substantial number of patients withdrew from this study prior to completing two chemotherapy cycles, and because of this only 24 were evaluable for response. The failure of so many patients to complete two cycles was a striking finding in and of itself. Recent trials evaluating FOLFIRINOX and nab-paclitaxel/gemcitabine included only patients with ECOG PS 0–1 (2,25), whereas this trial included patients with ECOG PS 0–2. In the current study, 5% had PS 2 while in the Xiong study 28% of subjects had PS 2 (11), and in the Boeck study 15% had KPS ≤70% (9). This suggests that either the combination oral capecitabine may be more toxic than previously thought, or that earlier studies of 5FU and oxaliplatin were conducted in a more select patient population. It is also plausible that the prognosis for patients with poor performance status is too limited for an objective response from treatment to be documented either because of short time period of benefit or inability of the patient to tolerate treatment long enough to achieve benefit. The large number of subjects who withdrew prematurely underscores the challenge of conducting trials in this inherently sick population. Future studies in advanced PDAC should include methods that account for early progression and for assessing study populations with poor performance status, multiple comorbidities, and rapidly progressive disease.
Also potentially affecting results is the fact that 72.5% (N=29) of subjects in our study had already been treated for advanced PDAC. Previous trials in advanced PDAC have suggested that patients whose disease is refractory to first line treatment are unlikely to benefit from second line therapy. In a study of weekly 5FU/leucovorin and oxaliplatin administered in the second line after gemcitabine, Tsavaris and colleagues noted that patients who had responded to first-line gemcitabine were more likely to show response or disease stability with second line treatment (7).
The findings of this study must be considered in the context of its limitations. The primary limitation is the lower than expected number of subjects evaluable for response. In addition to potentially affecting generalizability, the small evaluable population limits the power of the study for the primary endpoint. This limitation has implications for the design of future studies in advanced PDAC as the primary reason for non-evaluability of response was early study discontinuation. Early study discontinuation reflects both the toxicities caused by this regimen as well as the underlying disease state and poor functional status of the subjects. Alternate metrics for assessing therapeutic benefit may be important in this population such as symptom control, quality of life assessment, and earlier response endpoints. Although the study was conducted in a single geographic area, generalizability was enhanced by enrolling subjects at three different institutions.
This study shows that the combination capecitabine and oxaliplatin (CAPOX) is a reasonable treatment option for patients with advanced PDAC with acceptable toxicity and demonstrated benefit. It also adds to the body of literature supporting additional treatment strategies for patients who may not be candidates for more aggressive combination chemotherapy regimens. Finally, we emphasize the need to reassess outcome measures in advanced pancreatic cancer trials in a way that allows those patients whose disease progresses rapidly to contribute to primary endpoints.
Table S1. Capecitabine dose adjustments for non-hematologic AE’s.
| AE incidence | Grade 2 | Grade 3 | Grade 4 |
|---|---|---|---|
| 1st appearance | Interrupt treatment until resolved to grade 0–1, then continue at same dose with prophylaxis where possible | Interrupt treatment until resolved to grade 0–1, then continue at 75% of original dose with prophylaxis where possible | Discontinue treatment unless Investigator considers it to be in the best interests of the patient to continue at 50% of original dose, once toxicity has resolved to grade 0–1 |
| 2nd appearance of same toxicity | Interrupt treatment until resolved to grade 0–1, then continue at 75% of original dose |
Interrupt treatment until resolved to grade 0–1, then continue at 50% of original dose | – |
| 3rd appearance of same toxicity | Interrupt treatment until resolved to grade 0–1, then continue at 50% of original dose |
Discontinue treatment permanently (off study) unless it is considered to be by the investigator in the best interest of the patient to stay on treatment | – |
| 4th appearance of same toxicity | Discontinue treatment permanently (off study) unless it is considered to be by the investigator in the best interest of the patient to stay on treatment | – | – |
Acknowledgements
We thank the patients and their families, and all members of participating institutions.
This work was supported by Sanofi-Aventis.
Supplementary 1: Eligibility criteria
-
Inclusion criteria:
Hematologic function: neutrophils ≥1.5×109/L, platelet ≥100×109/L;
Renal function: creatinine <1.5× upper normal limit or estimated creatinine clearance >30 mL/min as calculated with Cockcroft-Gault equation;
Hepatic function: bilirubin <1.5× upper normal limit, ALT, AST <2.5× upper normal limit, alkaline phosphatase <2.5× upper normal limit.
-
Exclusion criteria:
Pregnant or lactating;
Serious or uncontrolled infection;
Prior oxaliplatin or fluoropyrimidine therapy (unless as part of adjuvant therapy completed more than 12 months prior);
Any active second malignancy or CNS metastases;
Clinically significant cardiac disease;
Major surgery within 4 weeks of study treatment start;
Uncontrolled coagulopathy;
Malabsorption syndrome;
Any other serious uncontrolled medical condition that the investigator felt might compromise study participation.
Supplementary 2: Chemotherapy dose adjustments for toxicity
Calculation of capecitabine dose reductions
(I) 75% of the original dose:
If the original dose is a total daily dose of 2,000 mg/m2 to be taken as two divided doses, then 75% of the original dose = a total daily dose of 1,500 mg/m2 to be taken as two divided doses.
If the original dose is a total daily dose of 1,500 mg/m2 (because of moderate renal impairment) to be taken as two divided doses, then 75% of the original dose = a total daily dose of 1,125 mg/m2 to be taken as two divided doses.
(II) 50% of the original dose:
If the original dose is a total daily dose of 2,000 mg/m2 to be taken as two divided doses, then 50% of the original dose = a total daily dose of 1,000 mg/m2 to be taken as two divided doses.
If the original dose is a total daily dose of 1,500 mg/m2 (because of moderate renal impairment), to be taken as two divided doses, then 50% of the original.
Ethical Statement: The Institutional Review Board approved this study, and all subjects provided written informed consent.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Burris HA, 3rd, Moore MJ, Andersen J, et al. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol 1997;15:2403-13. 10.1200/JCO.1997.15.6.2403 [DOI] [PubMed] [Google Scholar]
- 2.Conroy T, Desseigne F, Ychou M, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med 2011;364:1817-25. 10.1056/NEJMoa1011923 [DOI] [PubMed] [Google Scholar]
- 3.Von Hoff DD, Ervin T, Arena FP, et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med 2013;369:1691-703. 10.1056/NEJMoa1304369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang-Gillam A, Li CP, Bodoky G, et al. Nanoliposomal irinotecan with fluorouracil and folinic acid in metastatic pancreatic cancer after previous gemcitabine-based therapy (NAPOLI-1): A global, randomised, open-label, phase 3 trial. Lancet 2016;387:545-57. 10.1016/S0140-6736(15)00986-1 [DOI] [PubMed] [Google Scholar]
- 5.Hofheinz RD, Wenz F, Post S, et al. Chemoradiotherapy with capecitabine versus fluorouracil for locally advanced rectal cancer: A randomised, multicentre, non-inferiority, phase 3 trial. Lancet Oncol 2012;13:579-88. 10.1016/S1470-2045(12)70116-X [DOI] [PubMed] [Google Scholar]
- 6.Ducreux M, Mitry E, Ould-Kaci M, et al. Randomized phase II study evaluating oxaliplatin alone, oxaliplatin combined with infusional 5-FU, and infusional 5-FU alone in advanced pancreatic carcinoma patients. Ann Oncol 2004;15:467-73. 10.1093/annonc/mdh098 [DOI] [PubMed] [Google Scholar]
- 7.Tsavaris N, Kosmas C, Skopelitis H, et al. Second-line treatment with oxaliplatin, leucovorin and 5-fluorouracil in gemcitabine-pretreated advanced pancreatic cancer: A phase II study. Invest New Drugs 2005;23:369-75. 10.1007/s10637-005-1446-y [DOI] [PubMed] [Google Scholar]
- 8.Ghosn M, Farhat F, Kattan J, et al. FOLFOX-6 combination as the first-line treatment of locally advanced and/or metastatic pancreatic cancer. Am J Clin Oncol 2007;30:15-20. 10.1097/01.coc.0000235997.18657.a6 [DOI] [PubMed] [Google Scholar]
- 9.Boeck S, Hoehler T, Seipelt G, et al. Capecitabine plus oxaliplatin (CapOx) versus capecitabine plus gemcitabine (CapGem) versus gemcitabine plus oxaliplatin (mGemOx): Final results of a multicenter randomized phase II trial in advanced pancreatic cancer. Ann Oncol 2008;19:340-7. 10.1093/annonc/mdm467 [DOI] [PubMed] [Google Scholar]
- 10.Mitry E, Ducreux M, Ould-Kaci M, Boige V, Seitz JF, Bugat R, et al. Oxaliplatin combined with 5-FU in second line treatment of advanced pancreatic adenocarcinoma. Results of a phase II trial. Gastroenterol Clin Biol 2006;30:357-63. 10.1016/S0399-8320(06)73188-8 [DOI] [PubMed] [Google Scholar]
- 11.Xiong HQ, Varadhachary GR, Blais JC, et al. Phase 2 trial of oxaliplatin plus capecitabine (XELOX) as second-line therapy for patients with advanced pancreatic cancer. Cancer 2008;113:2046-52. 10.1002/cncr.23810 [DOI] [PubMed] [Google Scholar]
- 12.Novarino A, Satolli MA, Chiappino I, et al. Oxaliplatin, 5-fluorouracil, and leucovorin as second-line treatment for advanced pancreatic cancer. Am J Clin Oncol 2009;32:44-8. 10.1097/COC.0b013e31817be5a9 [DOI] [PubMed] [Google Scholar]
- 13.Yoo C, Hwang JY, Kim JE, et al. A randomised phase II study of modified FOLFIRI.3 vs modified FOLFOX as second-line therapy in patients with gemcitabine-refractory advanced pancreatic cancer. Br J Cancer 2009;101:1658-63. 10.1038/sj.bjc.6605374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pelzer U, Schwaner I, Stieler J, et al. Best supportive care (BSC) versus oxaliplatin, folinic acid and 5-fluorouracil (OFF) plus BSC in patients for second-line advanced pancreatic cancer: A phase III-study from the German CONKO-study group. Eur J Cancer 2011;47:1676-81. 10.1016/j.ejca.2011.04.011 [DOI] [PubMed] [Google Scholar]
- 15.Berk V, Ozdemir N, Ozkan M, et al. XELOX vs. FOLFOX4 as second line chemotherapy in advanced pancreatic cancer. Hepatogastroenterology 2012;59:2635-9. [DOI] [PubMed] [Google Scholar]
- 16.El-Hadaad HA, Wahba HA. Oxaliplatin plus 5-fluorouracil and folinic acid (off) in gemcitabine-pretreated advanced pancreatic cancer: A phase ii study. J Gastrointest Cancer 2013;44:313-7. 10.1007/s12029-013-9495-5 [DOI] [PubMed] [Google Scholar]
- 17.Oettle H, Riess H, Stieler JM, et al. Second-line oxaliplatin, folinic acid, and fluorouracil versus folinic acid and fluorouracil alone for gemcitabine-refractory pancreatic cancer: Outcomes from the CONKO-003 Trial. J Clin Oncol 2014;32:2423-9. 10.1200/JCO.2013.53.6995 [DOI] [PubMed] [Google Scholar]
- 18.Therasse P, Arbuck SG, Eisenhauer EA, et al. New Guidelines to Evaluate the Response to Treatment in Solid Tumors. J Natl Cancer Inst 2000;92:205-16. 10.1093/jnci/92.3.205 [DOI] [PubMed] [Google Scholar]
- 19.Simon R. Optimal two-stage designs for phase II clinical trials. Control Clin Trials 1989;10:1-10. 10.1016/0197-2456(89)90015-9 [DOI] [PubMed] [Google Scholar]
- 20.Atkinson EN, Brown BW. Confidence limits for probability of response in multistage phase II clinical trials. Biometrics 1985;41:741-4. 10.2307/2531294 [DOI] [PubMed] [Google Scholar]
- 21.Demols A, Peeters M, Polus M, et al. Gemcitabine and oxaliplatin (GEMOX) in gemcitabine refractory advanced pancreatic adenocarcinoma: a phase II study. Br J Cancer 2006;94:481-5. 10.1038/sj.bjc.6602966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yi SY, Park YS, Kim HS, et al. Irinotecan monotherapy as second-line treatment in advanced pancreatic cancer. Cancer Chemother Pharmacol 2009;63:1141-5. 10.1007/s00280-008-0839-y [DOI] [PubMed] [Google Scholar]
- 23.Zaniboni A, Aitini E, Barni S, et al. FOLFIRI as second-line chemotherapy for advanced pancreatic cancer: A GISCAD multicenter phase II study. Cancer Chemother Pharmacol 2012;69:1641-5. 10.1007/s00280-012-1875-1 [DOI] [PubMed] [Google Scholar]
- 24.Neuzillet C, Hentic O, Rousseau B, et al. FOLFIRI regimen in metastatic pancreatic adenocarcinoma resistant to gemcitabine and platinum-salts. World J Gastroenterol 2012;18:4533-41. 10.3748/wjg.v18.i33.4533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Von Hoff DD, Ramanathan RK, Borad MJ, et al. Gemcitabine plus nab-paclitaxel is an active regimen in patients with advanced pancreatic cancer: A phase I/II trial. J Clin Oncol 2011;29:4548-54. 10.1200/JCO.2011.36.5742 [DOI] [PMC free article] [PubMed] [Google Scholar]