Abstract
Background
CRLX101 is an investigational nanoparticle-drug conjugate with a camptothecin payload. Preclinical evidence indicated preferential uptake in tumors, and tumor xenograft models demonstrate superiority of CRLX101 over irinotecan. A pilot trial was conducted at recommended phase 2 dosing (RP2D) using the bimonthly schedule to assess preferential uptake of CRLX101 in tumor vs. adjacent normal tissue in endoscopically accessible tumors in chemotherapy-refractory gastroesophageal cancer. Results from the biopsies were previously reported and herein we present the clinical outcomes.
Methods
Patients initiated CRLX101 dosed at RP2D (15 mg/m2) on days 1 and 15 of a 28-day cycle. Detection of preferential CRLX101 tumor uptake was the primary endpoint and objective response rate (ORR) was a secondary endpoint. With a sample size of ten patients, the study had 90% power to detect ≥1 responder if the true response rate is ≥21%.
Results
Between Dec. 2012 and Dec. 2014, ten patients with chemotherapy-refractory (median 2 prior lines of therapy, range 1–4) gastric adenocarcinoma were enrolled. The median time-to-progression was 1.7 months. Best response was seen in one patient with stable disease (SD) for 8 cycles. Only ≥ grade 3 drug-related toxicity occurred in one patient with grade 3 cardiac chest pain who was able to resume therapy after CRLX101 was reduced to 12 mg/m2.
Conclusions
Bimonthly CRLX101 demonstrated minimal activity with SD as best response in this heavily pretreated population. Future efforts with CRLX101 in gastric cancer should focus on combination and more dose-intensive strategies given its favorable toxicity profile and evidence of preferential tumor uptake.
Keywords: CRLX101, gastric cancer, esophageal cancer, clinical trial
Introduction
Advanced gastroesophageal cancer remains a deadly disease with most historic studies showing a median overall survival (OS) of approximately 10 months (1-3). Current first line treatment for the majority of patients with gastric and gastroesophageal junction adenocarcinoma is predominated by platinum and fluoropyrimidine based doublet therapy (4). New options for second-line therapy have been limited until the recent FDA-approval of the VEGFR2 monoclonal antibody inhibitor ramucirumab that was based on positive survival results from two phase III studies (5,6). Other agents for refractory disease include the camptothecin derivative irinotecan, which has demonstrated modest activity as second-line therapy in advanced gastroesophageal cancer with reported response rates of 0–15%, and median progression-free survival limited to approximately 2 months (7,8). As such, when refractory disease develops, new therapeutics are still needed to overcome drug resistance.
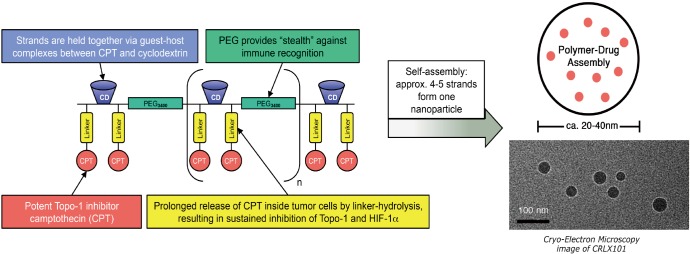
CRLX101 is an investigational nanoparticle drug conjugate (NDC) with a camptothecin payload (Figure 1) (10). The phase I first-in-human trial demonstrated a favorable safety profile and established 15 mg/m2 delivered intravenously every 2 weeks as the recommended phase 2 dose (RP2D) (10). Preclinical evidence indicates preferential uptake in tumors vs. normal tissue, and several mouse cancer xenograft models have demonstrated superior antitumor activity of CRLX101 over irinotecan, including in gastroesophageal cancer (11-13). We subsequently conducted a pilot trial at the RP2D using the bimonthly schedule to assess preferential uptake of CRLX101 in tumor vs. adjacent normal tissue in endoscopically accessible tumors in patients with chemotherapy-refractory gastroesophageal cancer (Clinicaltrials.gov registration NCT01612546). Data demonstrating preferential tumor vs. normal tissue uptake of CRLX101 for patients enrolled in this trial were previously published (9). Here we report on the clinical outcomes of the patients enrolled as pre-planned secondary analyses of the study.
Figure 1.
CRLX101 is a novel nanoparticle drug conjugate (NDC) that contains a cyclodextrin-containing polymer (CDP) conjugated to camptothecin (CPT) that self assembles into 20 to 40 nm diameter nanoparticles. Adapted from Clark et al. (9).
Methods
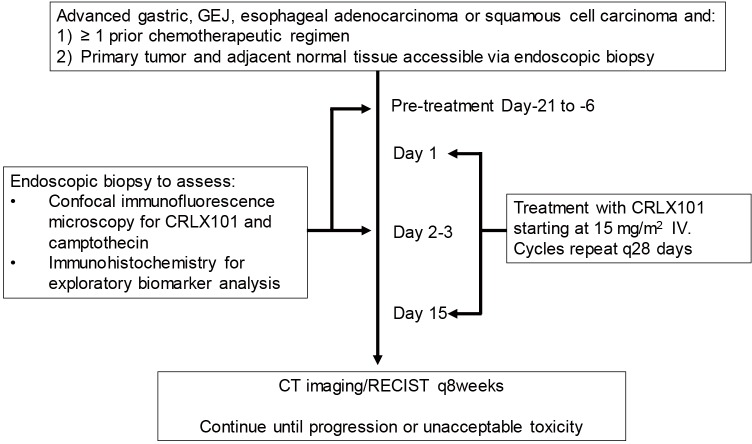
This study was a single center pilot trial for patients refractory to at least one line of systemic therapy for advanced, unresectable or metastatic gastric, gastroesophageal junction, or esophageal squamous cell or adenocarcinoma. Patients with human epidermal growth factor receptor 2 (HER2)-overexpressing adenocarcinoma were included in the trial provided they progressed on trastuzumab-containing therapy. Other eligibility criteria included age ≥18 years, ECOG performance status ≤2, and acceptable organ function defined on standard laboratory testing. Given the primary endpoint of the pilot study was to assess for preferential CRLX101 tumor uptake, patients were also required to have a primary tumor in place accessible for endoscopic biopsy of the tumor and adjacent normal tissue. All patients initiated CRLX101 at the RP2D of 15 mg/m2 delivered intravenously on days 1 and 15 of a 28-day cycle with timepoints of tissue collection as per the Study Schema (Figure 2). Patients continued on therapy until disease progression or intolerant toxicity. Tumor responses were assessed using RECIST1.1 and toxicities were graded per NCI CTCAE version 4.03. Time to progression (TTP) was defined as length of time from start of CRLX101 therapy until evidence of radiographic progression by RECIST1.1 and/or disease-related symptomatic progression per investigator assessment. OS was defined as the length of time from start of CRLX101 therapy until patient death due to any cause. While detection of preferential CRLX101 tumor uptake was the primary endpoint, with ten patients enrolled a secondary analysis could be performed with the study having 90% power to detect ≥1 responder if the true response rate is ≥21%. The study received local IRB approval and was conducted in accordance with Good Clinical Practice Guidelines and the Declaration of Helsinki. All patients provided written informed consent prior to undergoing study-related procedures.
Figure 2.
Study schema indicating timepoints of pre- and post-treatment biopsies with initiation of CRLX101 therapy. GEJ, gastroesophageal junction; RECIST, response evaluation criteria in solid tumors.
Results
Between Dec. 2012 and Dec. 2014, ten patients were enrolled with a median age of 64 years (range, 48–73 years) (Table 1). Patients all had adenocarcinoma histology with primary tumors located beyond the gastric cardia when assessed by endoscopy. Patients all had at least one line of platinum and fluoropyrimidine therapy with median number of prior cycles being 2. Only 1 patient harbored a HER2-overexpressing tumor and 3 patients had previous treatment with irinotecan-containing therapy. One patient received prior therapy with the VEGFR2 inhibitor ramucirumab. Best response by RECIST1.1 was stable disease in one patient who was on treatment for 8.7 months prior to stopping therapy for disease progression (Table 2). That patient had received only one previous line of therapy with docetaxel, cisplatin, and fluorouracil combination chemotherapy with a modified infusional schedule. The median TTP for the entire study population was 1.7 months (range, 0.6–8.7 months) and median OS was 5.5 months (range, 1.5–28.1 months) (Figure 3). At the time of final survival analysis all patients had expired.
Table 1. Patient characteristics.
| Patient sequence | Gender | Age (years) | ECOG PS | Histology (Lauren classification) | Prior therapy [no. lines] |
|---|---|---|---|---|---|
| 01 | M | 48 | 0 | Adenocarcinoma (intestinal) | mDCF [1] |
| 02 | F | 63 | 1 | Adenocarcinoma (diffuse) | EOX, mDCF [2] |
| 03 | F | 68 | 1 | Adenocarcinoma (diffuse) | mDCF, FOLFOX [2] |
| 04 | M | 63 | 1 | Adenocarcinoma (intestinal) | EOX, ELF [2] |
| 05 | M | 55 | 0 | Adenocarcinoma (intestinal) | mDCF [1] |
| 06 | F | 69 | 1 | Adenocarcinoma (intestinal) | mDCF, FOLFOX [2] |
| 07 | M | 66 | 0 | Adenocarcinoma (intestinal) | Paclitaxel/carboplatin/capecitabine [1] |
| 08 | F | 65 | 1 | Adenocarcinoma (intestinal) | EOX, FOLFIRI, docetaxel/cisplatin [3] |
| 09* | F | 73 | 0 | Adenocarcinoma (intestinal) | FOLFOX/trastuzumab, docetaxel/trastuzumab, irinotecan/trastuzumab [3] |
| 10 | F | 50 | 1 | Adenocarcinoma (diffuse) | mDCF, FOLFOX, FOLFIRI, paclitaxel/ramucirumab [4] |
*, only patient with tumor HER2 overexpression. mDCF, modified docetaxel/cisplatin/fluorouracil; EOX, epirubicin/oxaliplatin/capecitabine; FOLFOX, folinic acid/fluorouracil/oxaliplatin; ELF, etoposide/leucovorin/fluorouracil; FOLFIRI, folinic acid/fluorouracil/irinotecan; HER2, human epidermal growth factor receptor 2; ECOG PS, ECOG performance status; M, man; F, female.
Table 2. Best response, time to progression (TTP), and survival.
| Patient sequence | Best response | TTP (months) | Survival (months) |
|---|---|---|---|
| 01 | Stable disease | 8.7 | 28.1 |
| 02 | Progression | 2.0 | 5.2 |
| 03 | Progression | 3.1 | 5.7 |
| 04 | Progression | 1.9 | 19.3 |
| 05 | Progression | 1.8 | 13.0 |
| 06 | Progression | 1.3 | 2.7 |
| 07 | Progression | 1.6 | 3.5 |
| 08 | Progression | 1.4 | 3.4 |
| 09 | Progression | 1.6 | 5.9 |
| 10 | Progression | 0.6 | 1.5 |
Figure 3.

Kaplan-Meier plots for (A) time-to-progression and (B) overall survival.
Toxicities attributed to CRLX101 were predominately grade 1–2 as summarized in Table 3. Most common grade 1–2 toxicities observed were fatigue, nausea, proteinuria, and anemia. Incidences of proteinuria were asymptomatic and detected on protocol-mandated urinalysis collected prior to every dose of CRLX101. No adverse elevations in creatinine were detected to indicate nephrotoxicity and no patients developed any nephrotic range proteinuria. The only grade 3 event related to CRLX101 occurred in one patient with grade 3 cardiac chest pain. The patient’s electrocardiogram (EKG) at the time of the event demonstrated atrial fibrillation and the patient also had a history of paroxysmal atrial fibrillation. No ischemic changes were noted on EKG and cardiac enzymes monitoring did not indicate any evidence for myocardial infarction with the event. After resolution of the chest pain and control of atrial fibrillation with beta-blockers, discussion was had within the study team to allow the patient to resume therapy with CRLX101 reduced to a dose of 12 mg/m2. With the dose reduction the patient did not experience any recurrence of drug-related toxicity. Dose reductions were required for one other patient with persisting grade 2 cystitis through 2 cycles manifesting as complaints of urinary urgency and frequency interfering with instrumental activities of daily living (e.g., driving and shopping). With the onset of cycle 7, the patient’s dose was reduced to 12 mg/m2. Symptoms of grade 2 cystitis recurred such that the patient required a dose reduction to 9 mg/m2 with the onset of cycle 8 after which cystitis improved to grade 1. Throughout the episodes of grade 2 cystitis the only hematuria ever recorded was microscopic on urinalysis, and symptoms were manageable with phenazopyridine and solifenacin pharmacologic interventions.
Table 3. Incidence of treatment-related adverse events.
| No. adverse events | Grade 1–2 | Grade 3* |
|---|---|---|
| Non-hematologic | ||
| Fatigue | 5 | 0 |
| Nausea | 3 | 0 |
| Proteinuria | 3 | 0 |
| Hematuria | 2 | 0 |
| Vomiting | 1 | 0 |
| Diarrhea | 1 | 0 |
| Infusion reaction | 1 | 0 |
| Pruritus | 1 | 0 |
| Myalgia | 1 | 0 |
| Cystitis | 1 | 0 |
| Cough | 1 | 0 |
| Hypertension | 1 | 0 |
| Alk Phos elevation | 1 | 0 |
| AST elevation | 1 | 0 |
| Cardiac chest pain | 0 | 1 |
| Hematologic | ||
| Anemia | 3 | 0 |
| Leukopenia | 2 | 0 |
| Neutropenia | 2 | 0 |
| Lymphopenia | 1 | 0 |
| Thrombocytopenia | 1 | 0 |
*, no grade 4 or 5 toxicities were observed. Alk Phos, alkaline phosphatase; AST, aspartate aminotransferase.
Discussion
Bimonthly CRLX101 demonstrated minimal activity in this heavily pre-treated advanced gastric cancer patient population in which median OS was 5.5 months. The single patient who had stable disease as best response for 8.7 months prior to developing radiographic metastatic disease progression did not have viable tumor tissue on endoscopic pre- and post-treatment biopsies despite satisfying the eligibility criterion of having a known primary tumor in place. As such, exploratory analyses to examine molecular factors which may have accounted for this patient’s benefit from CRLX101 therapy compared to the remaining patients enrolled were not possible. The median time-to-progression of 1.7 months for the overall study population is in line with a median progression-free survival of 2.3 and 2.5 months observed in two phase III studies with second-line irinotecan (7,8). Both studies also exhibited very low objective response rates (ORRs) of 0% and 13.6%. The more recent French Intergroup FFCD 0307 trial also demonstrated a low 13.7% ORR with combination irinotecan, fluorouracil, leucovorin therapy (FOLFIRI) as second-line cross-over treatment after patients received upfront epirubicin, cisplatin, and capecitabine (ECX) (14). Interestingly, when FOLFIRI was moved up into the frontline setting, the ORR improved to 37.8% while subsequent cross-over to second-line ECX only yielded an ORR of 10.1%. This trial further highlights the challenge of drug resistance that develops in gastric cancer which appears to be universal regardless of choice of first-line cytotoxic chemotherapy utilized. We were able to demonstrate that CRLX101 exhibits evidence of preferential uptake of drug into patient gastric tumor tissue as well as evidence of downregulation of putative drug targets such as carbonic anhydrase IX and HIF-1α (9). Consistent with the lack of non-tumor tissue camptothecin uptake, CRLX101 demonstrated a favorable toxicity profile. Persisting grade 2 cystitis did occur in the one patient that achieved stable disease as best response. With employment of appropriate dose reductions currently utilized across all ongoing CRLX101 trials, the patient was able to continue on with protocol therapy until disease progression. The cystitis profile still compares favorably to historic studies with native camptothecin in which severe hemorrhagic cystitis halted further development of this agent despite evidence of anti-tumor activity (15,16).
Bimonthly CRLX101 monotherapy, however, demonstrated minimal activity in this trial. Several possibilities could explain the lack of efficacy. CRLX101 accumulation could be less in human tumors than what had been observed previously in preclinical mouse xenograft models, consistent with what was observed in the gastric pharmacodynamic study, though the assay was not quantitative (9). Second, mechanisms of resistance to camptothecin and its derivatives likely could be present contributing to the lack of anti-tumor activity. Potential possibilities reported in the literature predominantly derived from cell line models include increased drug efflux, Topo I resistance mutations, improved repair of irinotecan-induced DNA damage, and activation of anti-apoptotic NF-κB signaling (17). Finally, biweekly CRLX101 monotherapy may not be sufficient in this setting. More frequent dosing and/or combination therapy may be necessary to obtain better antitumor activity to overcome primary and secondary drug resistance mechanisms.
Future efforts with CRLX101 in advanced gastric cancer should thus focus on alternative dosing and combinatorial drug strategies. Recently, CRLX101 monotherapy was tested on a more dose-intensive schedule (18). In this study, the RP2D was the same as the bimonthly schedule of 15 mg/m2 though given weekly. Tolerability of CRLX101 remained acceptable, and moreover evidence of objective responses in renal cell carcinoma and gastrointestinal malignancies were seen including a patient with rectal cancer refractory to irinotecan therapy. Based on these results, a phase I study is ongoing combining weekly CRLX101 in combination with fluorouracil/leucovorin/oxaliplatin combination chemotherapy (modified FOLFOX6) in advanced solid tumors including gastrointestinal cancers (Clinicaltrials.gov NCT02648711). Phase II studies of the triplet combination of irinotecan/fluorouracil/oxaliplatin (FOLFOXIRI or FOLFIRINOX) have demonstrated promising ORRs of >60% as first-line therapy for advanced gastric cancer (19-21). As such, the combination of CRLX101 with mFOLFOX6 could be promising in gastroesophageal cancer patients. Other combinatorial opportunities include poly(ADP-ribose) polymerase (PARP) inhibitors potentiating the activity of Topo I inhibitors which have led to phase I studies combining irinotecan with PARP inhibitors in solid tumors (22,23). However, these studies to date have reported significant gastrointestinal and hematologic toxicities limiting PARP inhibitor and chemotherapy combinations to RP2D lower than their established monotherapy prescribing. Such was the approach utilized in the recently reported phase III GOLD trial in which olaparib was dosed at 100 mg twice daily, as opposed to its approved monotherapy dosing of 400 mg, in combination with weekly paclitaxel for the second-line treatment of patients with advanced or metastatic gastric cancer (24). Low tumor levels of the DNA damage response protein ataxia-telangiectasia mutated (ATM) as measured by immunohistochemistry (IHC) appeared promising as a biomarker of response to olaparib in the initial randomized phase II trial, and validated preclinical gastric cancer cell line findings (25). The phase III GOLD trial demonstrated numerical improvement in OS for both the entire study population and the preplanned subset analysis of patients with ATM low expression by IHC. However, neither group met the study’s predefined boundary for statistical significance to conclude that the addition of olaparib to paclitaxel can meaningfully improve survival in chemotherapy-refractory gastric cancer. The favorable toxicity profile of CRLX101 may thus serve as a more attractive partner to PARP inhibitors and permit higher drug dosing to improve the therapeutic index in ATM low expressing gastric cancers.
Lastly, preferential tumor uptake of CLX101 may promote selective release of tumor antigens into the tumor microenvironment that may augment immuno-oncology drug approaches. Chemotherapy is commonly associated with immunosuppressive toxicities, though it is also increasingly recognized that chemotherapy can promote tumor immunity through induction of immunogenic cell death (26). The lack of any observed grade 3 or higher leukopenia or lymphopenia in our patient cohort supports that CRLX101 may leverage chemotherapeutic drug responses in favor of anti-tumor immunogenicity. Immune checkpoint inhibitors are currently undergoing large scale phase III investigation in all lines of therapy in advanced gastric cancer, after small patient cohorts in earlier phase studies demonstrated promising evidence of clinical activity with programmed death-1 (PD-1) inhibitors (27-29). However, durable objective responses to immune checkpoint inhibitors as monotherapy are still confined to a limited proportion of patients. This is exemplified by the recent reporting of the phase III ONO-4538-12 study, which in the context of a randomized, placebo-controlled trial, assigned 330 advanced gastric cancer patients refractory to at least two chemotherapy regimens to nivolumab monotherapy (30). The study met its primary endpoint of improving OS in comparison to placebo, though ORR was 11.2% and median OS was still limited to 5.32 months for the nivolumab-treated patient population. Biomarker analyses were ongoing at the study’s initial reporting, but exemplified observations that the majority of an unselected patient population do not achieve durable responses. As such, combinatorial immuno-oncology strategies are predominating the next wave of clinical trials. Efforts focusing on preclinical investigation into patient-derived xenograft mouse models with humanized immune systems may help ascertain if CRLX101 can increase tumor immune cell infiltration. Such evidence would support future trials combining CRLX101 and immuno-oncology agents.
Acknowledgements
We thank all the participating patients and families and the research support staff involved in carrying out this study.
Funding: This study was supported by Cerulean Pharma Inc., as well as National Cancer Institute Grant L30 CA179788-01 and National Institutes of Health Grant 5K12CA001727-22. Research reported in this publication included work performed in the Biostatistics Core supported by the National Cancer Institute of the National Institutes of Health under award number P30CA033572.
Disclaimer: The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Ethical Statement: The study was approved by the institutional ethics board of City of Hope (IRB no. 11276) and informed consent was taken from all the patients.
Footnotes
Conflicts of Interest: E Garmey and S Eliasof are current or former employees of Cerulean Pharma Inc. ME Davis is a consultant for Cerulean Pharma Inc. and owns stock in the company. The remaining authors have no conflicts of interest to declare.
References
- 1.Van Cutsem E, Moiseyenko VM, Tjulandin S, et al. Phase III study of docetaxel and cisplatin plus fluorouracil compared with cisplatin and fluorouracil as first-line therapy for advanced gastric cancer: a report of the V325 Study Group. J Clin Oncol 2006;24:4991-7. 10.1200/JCO.2006.06.8429 [DOI] [PubMed] [Google Scholar]
- 2.Cunningham D, Starling N, Rao S, et al. Capecitabine and oxaliplatin for advanced esophagogastric cancer. N Engl J Med 2008;358:36-46. 10.1056/NEJMoa073149 [DOI] [PubMed] [Google Scholar]
- 3.Al-Batran SE, Hartmann JT, Probst S, et al. Phase III trial in metastatic gastroesophageal adenocarcinoma with fluorouracil, leucovorin plus either oxaliplatin or cisplatin: a study of the Arbeitsgemeinschaft Internistische Onkologie. J Clin Oncol 2008;26:1435-42. 10.1200/JCO.2007.13.9378 [DOI] [PubMed] [Google Scholar]
- 4.Ajani JA, D'Amico TA, Almhanna K, et al. Gastric Cancer, Version 3.2016, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 2016;14:1286-312. 10.6004/jnccn.2016.0137 [DOI] [PubMed] [Google Scholar]
- 5.Fuchs CS, Tomasek J, Yong CJ, et al. Ramucirumab monotherapy for previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (REGARD): an international, randomised, multicentre, placebo-controlled, phase 3 trial. Lancet 2014;383:31-9. 10.1016/S0140-6736(13)61719-5 [DOI] [PubMed] [Google Scholar]
- 6.Wilke H, Muro K, Van Cutsem E, et al. Ramucirumab plus paclitaxel versus placebo plus paclitaxel in patients with previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (RAINBOW): a double-blind, randomised phase 3 trial. Lancet Oncol 2014;15:1224-35. 10.1016/S1470-2045(14)70420-6 [DOI] [PubMed] [Google Scholar]
- 7.Thuss-Patience PC, Kretzschmar A, Bichev D, et al. Survival advantage for irinotecan versus best supportive care as second-line chemotherapy in gastric cancer--a randomised phase III study of the Arbeitsgemeinschaft Internistische Onkologie (AIO). Eur J Cancer 2011;47:2306-14. 10.1016/j.ejca.2011.06.002 [DOI] [PubMed] [Google Scholar]
- 8.Hironaka S, Ueda S, Yasui H, et al. Randomized, open-label, phase III study comparing irinotecan with paclitaxel in patients with advanced gastric cancer without severe peritoneal metastasis after failure of prior combination chemotherapy using fluoropyrimidine plus platinum: WJOG 4007 trial. J Clin Oncol 2013;31:4438-44. 10.1200/JCO.2012.48.5805 [DOI] [PubMed] [Google Scholar]
- 9.Clark AJ, Wiley DT, Zuckerman JE, et al. CRLX101 nanoparticles localize in human tumors and not in adjacent, nonneoplastic tissue after intravenous dosing. Proc Natl Acad Sci U S A 2016;113:3850-4. 10.1073/pnas.1603018113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weiss GJ, Chao J, Neidhart JD, et al. First-in-human phase 1/2a trial of CRLX101, a cyclodextrin-containing polymer-camptothecin nanopharmaceutical in patients with advanced solid tumor malignancies. Invest New Drugs 2013;31:986-1000. 10.1007/s10637-012-9921-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schluep T, Hwang J, Cheng J, et al. Preclinical efficacy of the camptothecin-polymer conjugate IT-101 in multiple cancer models. Clin Cancer Res 2006;12:1606-14. 10.1158/1078-0432.CCR-05-1566 [DOI] [PubMed] [Google Scholar]
- 12.Schluep T, Hwang J, Hildebrandt IJ, et al. Pharmacokinetics and tumor dynamics of the nanoparticle IT-101 from PET imaging and tumor histological measurements. Proc Natl Acad Sci U S A 2009;106:11394-9. 10.1073/pnas.0905487106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gaur S, Chen L, Yen T, et al. Preclinical study of the cyclodextrin-polymer conjugate of camptothecin CRLX101 for the treatment of gastric cancer. Nanomedicine 2012;8:721-30. 10.1016/j.nano.2011.09.007 [DOI] [PubMed] [Google Scholar]
- 14.Guimbaud R, Louvet C, Ries P, et al. Prospective, randomized, multicenter, phase III study of fluorouracil, leucovorin, and irinotecan versus epirubicin, cisplatin, and capecitabine in advanced gastric adenocarcinoma: a French intergroup (Fédération Francophone de Cancérologie Digestive, Fédération Nationale des Centres de Lutte Contre le Cancer, and Groupe Coopérateur Multidisciplinaire en Oncologie) study. J Clin Oncol 2014;32:3520-6. 10.1200/JCO.2013.54.1011 [DOI] [PubMed] [Google Scholar]
- 15.Creaven PJ, Allen LM, Muggia FM. Plasma camptothecin (NSC-100880) levels during a 5-day course of treatment: relation to dose and toxicity. Cancer Chemother Rep 1972;56:573-8. [PubMed] [Google Scholar]
- 16.Muggia FM, Creaven PJ, Hansen HH, et al. Phase I clinical trial of weekly and daily treatment with camptothecin (NSC-100880): correlation with preclinical studies. Cancer Chemother Rep 1972;56:515-21. [PubMed] [Google Scholar]
- 17.Xu Y, Villalona-Calero MA. Irinotecan: mechanisms of tumor resistance and novel strategies for modulating its activity. Ann Oncol 2002;13:1841-51. 10.1093/annonc/mdf337 [DOI] [PubMed] [Google Scholar]
- 18.Lakhani N, Piha-Paul SA, Senderowicz A, et al. Evaluation of weekly dosing of CRLX101 alone and in combination with bevacizumab (bev) in patients (pts) with advanced solid tumors. Ann Oncol 2016;27:114-35.http:// 10.1093/annonc/mdw368.3626487588 [DOI] [Google Scholar]
- 19.Lee J, Kang WK, Kwon JM, et al. Phase II trial of irinotecan plus oxaliplatin and 5-fluorouracil/leucovorin in patients with untreated metastatic gastric adenocarcinoma. Ann Oncol 2007;18:88-92. 10.1093/annonc/mdl317 [DOI] [PubMed] [Google Scholar]
- 20.Cao W, Yang W, Lou G, et al. Phase II trial of infusional fluorouracil, leucovorin, oxaliplatin, and irinotecan (FOLFOXIRI) as first-line treatment for advanced gastric cancer. Anticancer Drugs 2009;20:287-93. 10.1097/CAD.0b013e3283273509 [DOI] [PubMed] [Google Scholar]
- 21.Lockhart AC, Krajewski KA, Wang-Gillam A, et al. FOLFIRINOX as first-line therapy in patients with metastatic gastroesophageal cancers (GEC). J Clin Oncol 2015;33:177 10.1200/jco.2015.33.3_suppl.177 [DOI] [Google Scholar]
- 22.Chen EX, Jonker DJ, Siu LL, et al. A Phase I study of olaparib and irinotecan in patients with colorectal cancer: Canadian Cancer Trials Group IND 187. Invest New Drugs 2016;34:450-7. 10.1007/s10637-016-0351-x [DOI] [PubMed] [Google Scholar]
- 23.LoRusso PM, Li J, Burger A, et al. Phase I Safety, Pharmacokinetic, and Pharmacodynamic Study of the Poly(ADP-ribose) Polymerase (PARP) Inhibitor Veliparib (ABT-888) in Combination with Irinotecan in Patients with Advanced Solid Tumors. Clin Cancer Res 2016;22:3227-37. 10.1158/1078-0432.CCR-15-0652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bang YJ, Boku N, Chin K, et al. Olaparib in combination with paclitaxel in patients with advanced gastric cancer who have progressed following first-line therapy: Phase III GOLD study. Ann Oncol 2016;27:1-36. 10.1093/annonc/mdw435.16 [DOI] [PubMed] [Google Scholar]
- 25.Bang YJ, Im SA, Lee KW, et al. Randomized, Double-Blind Phase II Trial With Prospective Classification by ATM Protein Level to Evaluate the Efficacy and Tolerability of Olaparib Plus Paclitaxel in Patients With Recurrent or Metastatic Gastric Cancer. J Clin Oncol 2015;33:3858-65. 10.1200/JCO.2014.60.0320 [DOI] [PubMed] [Google Scholar]
- 26.Emens LA, Middleton G. The interplay of immunotherapy and chemotherapy: harnessing potential synergies. Cancer Immunol Res 2015;3:436-43. 10.1158/2326-6066.CIR-15-0064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Muro K, Chung HC, Shankaran V, et al. Pembrolizumab for patients with PD-L1-positive advanced gastric cancer (KEYNOTE-012): a multicentre, open-label, phase 1b trial. Lancet Oncol 2016;17:717-26. 10.1016/S1470-2045(16)00175-3 [DOI] [PubMed] [Google Scholar]
- 28.Chung HC, Arkenau HT, Wyrwicz L, et al. Avelumab (MSB0010718C; anti-PD-L1) in patients with advanced gastric or gastroesophageal junction cancer from JAVELIN solid tumor phase Ib trial: Analysis of safety and clinical activity. J Clin Oncol 2016;34:abstr 4009.
- 29.Janjigian YY, Bendell JC, Calvo E, et al. CheckMate-032: Phase I/II, open-label study of safety and activity of nivolumab (nivo) alone or with ipilimumab (ipi) in advanced and metastatic (A/M) gastric cancer (GC). J Clin Oncol 2016;34:abstr 4010.
- 30.Kang YK, Satoh T, Ryu MR, et al. Nivolumab (ONO-4538/BMS-936558) as salvage treatment after second or later-line chemotherapy for advanced gastric or gastro-esophageal junction cancer (AGC): A double-blinded, randomized, phase III trial. J Clin Oncol 2017;35:2 10.1200/JCO.2017.35.4_suppl.2 [DOI] [Google Scholar]