Abstract
HPV infected cervical cells secrete mediators that are gradually changed and have influence on infiltrating M2 phenotypic monocytes in cervical lesions. However, profiles of circulating immune cells in women with cervical lesions and M2 phenotypic monocyte activity in HPV infected cervical lesions are limited. This study aimed to investigate circulating monocyte populations correlated with M2 phenotype density and its activity in HPV infected cervical lesions. HPV DNA was investigated in cervical tissues using PCR. High risk HPV E6/E7 mRNA was detected using in situ hybridization. CD163 immunohistochemical staining was performed for M2 macrophage. CD163 and Arg1 mRNA expression were detected using real-time PCR. Circulating monocyte subpopulations were analyzed using flow cytometry. CD163 and Arg1 mRNA expression were increased according to cervical lesion severity and corresponding with density of M2 macrophage in HSIL and SCC in stroma and peri-tumoral areas. Additionally, the relationship between M2 macrophage infiltration and high risk HPV E6/E7 mRNA expression was found and corresponded with cervical lesion severity. Circulating CD14+CD16+ and CD14+CD163+ monocytes were elevated in No-SIL and cervical lesions. Interestingly, CD14+CD64+ monocyte was greatly elevated in HSIL and SCC, whereas intracellular IL-10+ monocytes were not significantly different between cervical lesions. The correlation between increasing ratio of circulating CD64+/CD163+ monocyte and density of infiltrating CD163+ monocytes was associated with severity of HPV infected cervical lesions. The elevated circulating CD64+/CD163+ monocyte ratio correlates to severity of HPV infected cervical lesions and might be a prognostic marker in cervical cancer progression.
Electronic supplementary material
The online version of this article (10.1007/s12307-017-0200-2) contains supplementary material, which is available to authorized users.
Keywords: Cervical lesions, Cervical cancer, Human papillomavirus (HPV), Monocyte, M2 macrophage, CD163
Introduction
Persistent infection with high-risk human papillomavirus (HR-HPV) is recognized as the cause of cervical cancer, the second most common cancer in women worldwide [1]. Oncoproteins E6 and E7 of HR-HPV induce cervical oncogenesis by disruption of host tumor-suppressor proteins, p53 and retinoblastoma protein (pRb), respectively [2]. During HPV infection, an abnormal cell is eradicated by a local cellular immune response that contains various tissue macrophages, which link innate to adaptive immune responses. Two main phenotypes of tumor-associated macrophages (TAMs) infiltrate into the tumor microenvironment: phenotype M1 promotes inflammatory responses and M2 suppresses inflammation to permit tissue resolution but also promotes tumor growth [3, 4]. However, HPV oncoproteins, notably E6 and E7, can manipulate host immune responses leading to evasion of immune recognition. Thus, despite the fact that HPV infection can induce high immune-cell infiltration by both lymphocytes and monocytes, the disease still progresses [5, 6]. It has been noted that both abnormal cervical cells and immune cells may produce and secrete mediators that support and promote tumor growth, as well as suppressing the immune response [7].
Tissue macrophages are recruited to tissues by chemo-attractant factors and are the professional antigen-presenting cells (APCs). In the conventional way, the cognate antigen, presented on MHC molecules, is recognized by CD4+ and CD8+ T cells and co-stimulatory molecules. After activation, T cells secrete cytokines with different functions depending on the cell type [8, 9]. On the other hand, this priming may attenuate the ability of T cells to increase co-inhibitory molecules and anti-inflammatory cytokines. M2 macrophages are characterized by specific cell-surface markers, such as CD163, and their anti-inflammatory role functions in homeostasis. Tissue M2 macrophages also promote tumor growth by releasing angiogenesis factors, anti-inflammatory cytokines or express co-inhibitory molecules on their cell surface [10].
Circulating monocytes are attracted to sites of chronic inflammation by chemokines produced by tumor cells, fibroblasts and resident macrophages. Many studies have shown that the phenotype of circulating monocytes can change, a situation found in many disease states [11, 12]. Monocytes express Fc gamma receptors (FCγRs), which recognize the Fc portion of a particular immunoglobulin gamma (IgG) isotype on their cell surface after monocyte activation. Subpopulations of monocytes are characterized by the particular subtype of FCγR that express: FcγRIII (CD16), FcγRII (CD32) and FcγRI (CD64). The activated monocytes express CD16 and CD64 molecules and also adhesion molecules before migration to the chemokine-releasing sites and differentiation to macrophages [13]. In addition, exposure of monocyte-derived dendritic cells and human monocyte (U937)-derived macrophages to tumor-produced prostaglandin E2 (PGE-2) and IL-6 results in M2 macrophage differentiation. This suggests that conventional M1 macrophages can change to the M2 macrophage phenotype, which expresses numerous scavenger surface receptors of CD163 (hemoglobin/haptoglobin complex). M2 macrophages, when activated, can produce anti-inflammatory mediators [IL-10, TGF-β, arginase 1 (Arg1), etc.] in tumor micro-environments [14, 15]. They can also produce factors which promote tumor growth (TGF-β), tumor metastasis (matrix metalloproteinase; MMPs), angiogenesis (vascular endothelial growth factor; VEGF) and immune-suppression (TGF-β, IL-10 and Arg1) [16].
M2 macrophages increase in numbers in the vicinity of various tumors (including breast cancer, colon cancer, head and neck cancer and cervical cancer) [17, 18] and usually express CD163 on their surfaces. This macrophage phenotype is recruited and developed from the circulating monocytes [19]. However, the correlations between circulating monocyte populations and the M2 macrophages in cervical tissues which represented monocyte activity are still unclear.
This study aimed to explore circulating monocyte subpopulations which relates to tumor M2 macrophage infiltration and monocyte activity in cervical lesions. This finding may suggest a prognostic marker for monitoring cervical lesion progression.
Materials and Methods
Patients, Clinical History and Sample Collection
The sample collection procedure was approved by the Khon Kaen University Ethics Committee for Human Research (HE531387) and the Khon Kaen Central Hospital Ethics Committee for Human Research (No. 20/04/2554). Patients were recruited from the Obstetrics and Gynecology outpatient department (OPD), Srinagarind Hospital, Faculty of Medicine, Khon Kaen University and Khon Kaen Central Hospital, Khon Kaen, Thailand. Blood samples (6 ml of heparinized blood) were collected from patients with normal cytology whereas blood, fresh cervical tissue and formalin-fixed, paraffin-embedded (FFPE) cervical tissue samples were collected from patients with abnormal cytology. FFPE cervical tissue samples were classified as no-squamous intraepithelial lesions (No-SIL), low-grade squamous intraepithelial lesions (LSIL), high-grade squamous intraepithelial lesions (HSIL) and squamous cell carcinoma (SCC).
DNA Extraction and HPV DNA Detection
FFPE cervical tissue samples were cut into 5 μm thickness for DNA extraction. Five tissue sections were de-paraffinized. DNA was extracted from de-paraffinized tissue samples using a DNeasy blood and tissue kit (QIAGEN®, Hilden, Germany). DNA quality was determined by amplify housekeeping gene (β-globin gene) before HPV DNA detection by PCR using GP5+/GP6+ primers as described previously [20].
Detection of HR-HPV E6/E7 mRNA by In Situ Hybridization
FFPE cervical tissue sections were stained for histopathology with hematoxylin and eosin (H&E; Bio-Optica, Milan, Italy). Dysplasia or tumor areas were selected for inclusion in a tissue microarray (TMA) platform. At least three areas of any tumor were punched with a biopsy needle gauge size 1.5 mm. (Miltex, inc., York, PA, USA) and re-embedded into an empty TMA paraffin block. TMA blocks were cut into 5 μm thickness and put onto adhesive SuperFrost® glass slide (Menzel-Gläser®, Braunschweig, Germany). The E6/E7 mRNAs of HR-HPV types were detected using a cocktail of 17HR-HPV probes, including HPV types 16, 18, 26, 31, 33, 35, 39, 45, 51, 52, 53, 56, 58, 59, 66, 68, 73, and 82 (ACDbio inc., Hayward, USA). Hybridization signal was developed using the RNAscope® 2.0 HD brown detection system (ACDbio inc., Hayward, USA) and sections counter-stained with hematoxylin dye (Bio-Optica, Milan, Italy). HPV E6/E7 mRNA was visualized in tumor cells as distinct or diffuse brown dots under the light microscope. Bacterial dihydrodipicolinate reductase (DapB) probe and human peptidylprolyl isomerase B (PPIB) probe served as internal negative and positive controls, respectively [21].
Peripheral Blood Mononuclear Cell (PBMC) Separation
PBMCs were separated from 6 ml of heparinized blood within 4 h after collection by using IsoPrep® density gradient solution (Robbins Scientific corp., Sunnyvale, CA, USA) [22]. The heparinized blood was diluted with an equal volume of sterile PBS pH 7.4, gently overlaid on the top of 5 ml of IsoPrep reagent and centrifuged at 2500 rpm. at 22 °C. PBMCs were collected, washed twice with sterile PBS pH 7.4 and re-suspended in 10% fetal bovine serum in RPMI 1640 media (10% FBS RPMI 1640; (Gibco®, Life Technologies™ Corp., Carlsbad, California, USA). The PBMCs were stored in 10% DMSO, 10% FBS RPMI 1640 and kept at −70 °C until used.
Flow Cytometry Analysis
The monocyte subpopulations were investigated by staining surface markers and flow cytometry analysis. Flow cytometry analysis was performed by thawing the frozen PBMCs immediately in a 37 °C waterbath until completely melted. The PBMCs were incubated in 10% FBS RPMI 1640 media at 37 °C with 5% CO2 atmosphere for 30 min to restore the cell morphology and surface markers. Before staining with specific antibodies, the Fc receptors on monocytes were blocked with 1 μg of purified human IgG (R&D systems, Minneapolis, MN, USA) at room temperature for 30 min. Then, specific antibodies were added; V500-conjugated mouse anti-human CD14 (Cat No. 561391: BD Horizon™, San Jose, CA, USA), eFluor® 450-conjugated mouse anti-human CD16 (Cat No. 9048-0168-025: eBioscience® DX, Vienna, Austria), FITC-conjugated mouse anti-human CD64 (Cat No. 555527: BD Pharmingen™, San Jose, CA, USA) and PE-conjugated mouse anti-human CD163 (Cat No. 556018: BD Pharmingen™, San Jose, CA, USA). For intracellular staining, V500-conjugated mouse anti-human CD14 antibody was first stained, fixed and permeabilized with BD Cytofix/Cytoperm™ solution (BD Biosciences, San Jose, CA, USA) and followed with Phycoerythrin-conjugated rat anti-human IL-10 (Cat No. 559330: BD Pharmingen™, San Jose, CA, USA) [23]. At least 1 × 104 of CD14+ monocytes were gated and analyzed using a FACScanthoII™ flow cytometer (Becton Dickinson, San Jose, CA, USA) and BD FACSDiva™ software. LPS-treated PBMCs were used for surface CD16, CD64 and IL-10 expression whereas dexamethasone-treated PBMCs served for surface CD163 expression control.
CD163 and Arginase 1 mRNA Expression
Fresh-frozen cervical biopsies were minced with a sterile surgical blade and extracted for total RNA with TRIZOL® reagent (Invitrogen, Life technologies™ Corp., Carlsbad, California, USA) according to instruction. Total mRNA was converted to cDNA by using Superscript® III first-strand synthesis system for RT-PCR and oligo-dT primer (Invitrogen, Life Technologies™ Corp., Carlsbad, California, USA). The expression of CD163 and Arg1 mRNA were determined using SsoAdvanced™ Universal SYBR® Green Supermix (Bio-Rad laboratories, Hercules, CA, USA) with specific primers (suplemental table 1). Relative quantitative real-time PCR was performed with a Bio-Rad C-1000 CFX96 Touch™ Real-Time PCR machine (Bio-Rad laboratories, Hercules, CA, USA). The target gene expression cycle thresholds were compared with expression of the house-keeping molecule glyceraldehyde 3-phosphate dehydrogenase (GAPDH) [24].
M2 Macrophage Immunohistochemistry (IHC) Staining
FFPE cervical tissues were cut into 5 μm sections and put onto SuperFrost® glass slides (Menzel-Gläser®, Braunschweig, Germany). The tissue sections were de-paraffinized with three washes in xylene and re-hydrated through a series of ethanol concentrations (100%, 95% and 70%), followed by a wash in distilled water for 10 min. The antigen retrieval step was performed with antigen retrieval buffer (10 mM sodium citrate, 0.05% Tween 20, pH 6.0) at high temperature (95-100 °C) for 30 min, after which the tissue was allowed to cool down at room temperature. The slides were soaked with IHC wash buffer (0.1% Tween 20 in PBS pH 7.4) for 10 min and endogenous peroxidase enzymes were blocked with 3% hydrogen peroxide for 10 min. After washing with wash buffer, the sections were incubated with mouse anti-human CD163 antibody (ab74604, Abcam®, Cambridge, United Kingdom) in a moisture chamber for 2 h, washed twice with wash buffer and followed with diluted 1:250 biotin conjugated goat anti-mouse IgG Fc part (ab97263, Abcam®, Cambridge, United Kingdom). The slides were washed twice again with wash buffer and 1:500 streptavidin-conjugated HRP (Invitrogen™, Life Technologies™ Corp., Carlsbad, California, USA) added. The color was developed with 3,3′-diaminobenzidine (DAB; Sigma Aldrich®, St. Louis, MO, USA) and counter-stained with hematoxylin dye (Bio-Optica, Milan, Italy) [25]. The CD163+ macrophages were observed and characterized as M2 macrophages by their brown staining under the light microscope. Tissue M2 macrophage densities were scored; score I (no CD163+ macrophage infiltration), score II (rare CD163+ macrophage infiltration) and score III (moderate or high CD163+ macrophage infiltration) [26].
Statistical Analysis
The monocyte subpopulations and mRNA expression in each sample group were analyzed using one-way ANOVA and Tukey’s multiple comparison test. All of values are indicated as mean ± standard deviation (SD). Percentage of HPV DNA and HR-HPV E6/E7 mRNA detection in FFPE cervical tissue samples were analyzed by using Chi-square test. Differences are considered statistically significant when P-value <0.05.
Results
M2 Macrophage Infiltration Increases with Cervical Lesion Severity
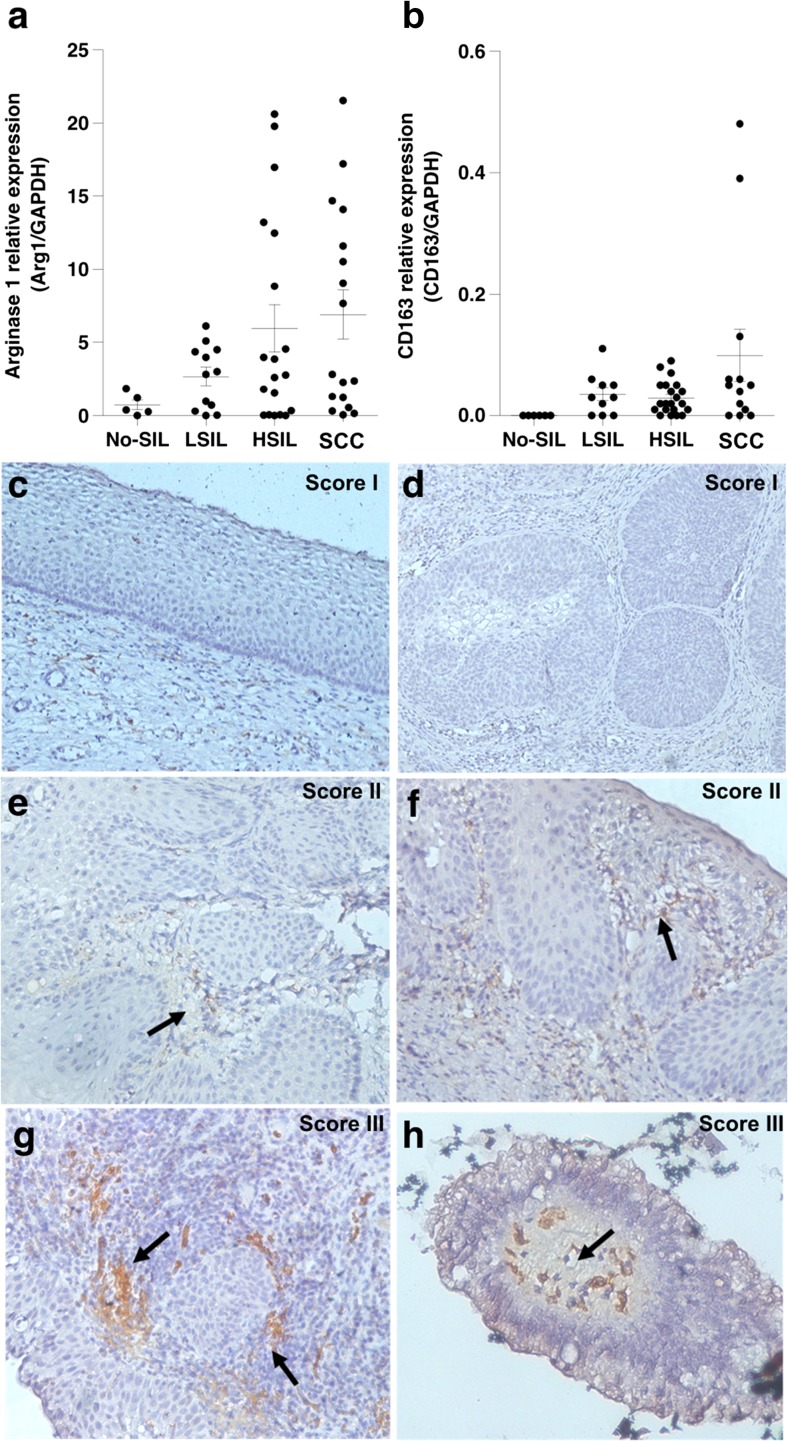
An immune suppressive mediator, Arg1, is mainly produced by M2 macrophages and considered a useful marker for monocyte activity of this cell class. Arg1 is an enzyme with immune-suppressive functions [14, 15]. M2 macrophages were detected by the presence of CD163 and Arg1 gene expression as markers and their activity, respectively. This was done in cervical tissues with the pathological diagnoses as No-SIL (10 cases), LSIL (16 cases), HSIL (25 cases) and SCC (18 cases). Levels of CD163 and Arg1 mRNA expression gradually increased depending on grade of cervical lesion severity (Fig. 1a–b).
Fig. 1.
Expression of CD163 and Arg1 in fresh-frozen cervical biopsy samples and CD163+ macrophages in cervical FFPE tissues. Quantification of (a) Arg1 and (b) CD163 mRNA expression was done using real time RT-PCR analysis and expression standardized relative to that of the internal control, GAPDH. (c–h) IHC using mouse anti-human CD163 antibody in cervical FFPE tissues. CD163+ macrophages are indicated with black arrows. Cell density was scored into three levels; (c–d) score I shows no CD163+ macrophage infiltration, (e–f) score II shows rare or slight infiltration and (g–h) score III show moderate or high infiltration
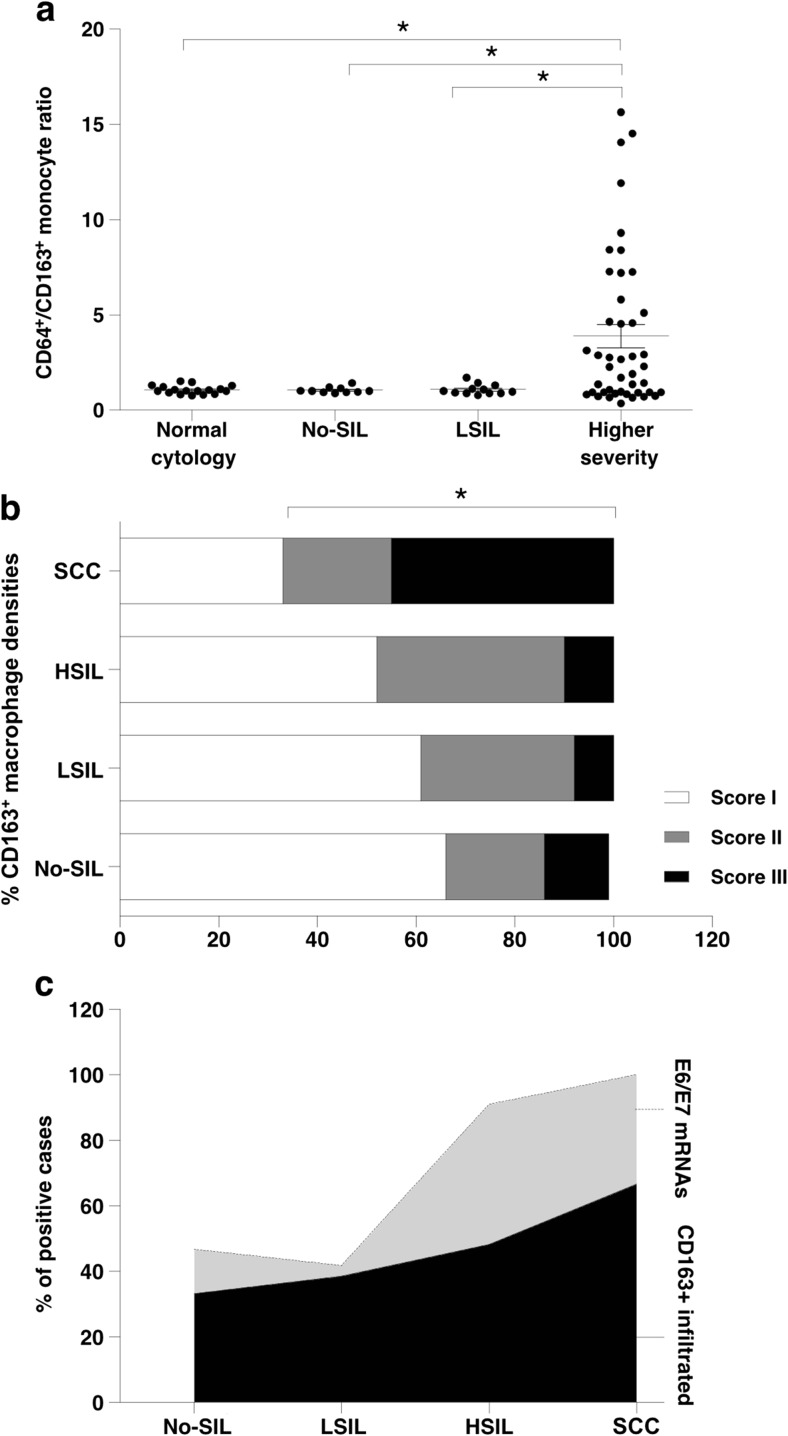
The density of M2 macrophages was further investigated in FFPE cervical tissue samples, including 17 No-SIL cases, 13 LSIL cases, 19 HSIL cases and 9 SCC cases by IHC staining, which identified CD163+ cells. Cell densities were graded into three categories (Fig. 1c–h). Scores II and III were considered positive for M2 macrophage infiltration, which increased in higher grade cervical lesions; HSIL and SCC than LSIL (P < 0.05) (Fig. 4b–c). These findings corresponded to increasing level of CD163 and Arg1 mRNA expression (Fig. 1a–b). Interestingly, the M2 macrophages were mainly detected in stromal and peri-tumoral areas.
Fig. 4.
CD64+/CD163+ ratio of circulating monocytes and density of stroma/peri-tumoral CD163+ monocyte in cervical cancer samples. a CD64+/CD163+ ratio of circulating monocytes and b Percentage of scoring CD163+ monocyte densities in cervical cancer samples, score I was adjusted as negative, score II and III were represented positive. (c) Correlation of HR-HPV E6/E7 mRNAs and infiltrated CD163+ monocyte densities in cervical cancer samples. Asterisks (*) represent statistically significant differences (P < 0.05)
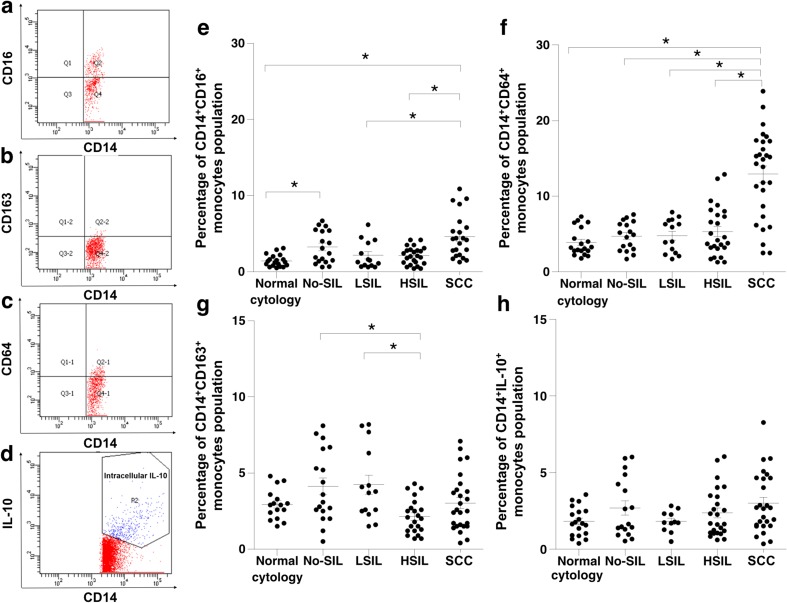
Increasing Proportions of CD14+CD64+ Circulating Monocytes with Increasing Cervical Lesion Severity
CD14+CD16+ monocytes are recognized as pro-inflammatory mediator cells and increase in density with increasing cervical lesion severity. Flow cytometry was used to detect markers [including intracellular IL-10 (CD14+IL10+)] on or in monocytes which were thus distinguished into three subpopulations: CD14+CD16+, CD14+CD64+ and CD14+CD163+ (Fig. 2a–d). The CD14+CD16+ population was significantly elevated in cervical lesions with No-SIL as well as in SCC (Fig. 2e). Interestingly, CD14+CD64+ cells were significantly more frequent in cervical lesions with HSIL and SCC than in LSIL, and were not found in No-SIL (Fig. 2f). The numbers of CD14+CD163+ monocytes (Fig. 2g) significantly elevated in No-SIL and LSIL and decreased in HSIL group. IL-10-expressing monocytes were rare in circulation (Fig. 2h). This result suggested that CD14+CD64+ monocytes in blood circulation might predict severity of cervical lesions.
Fig. 2.
Flow cytometry analysis was performed for monocyte subpopulation analysis in the PBMCs isolated from blood from women with different grades of cervical lesions. Monocyte population analysis of a CD14+CD16+, b CD14+CD163+, c CD14+CD64+ and d CD14+ with intracellular IL-10+ are shown. The percentages of each monocyte subpopulation were compared between different cervical lesion groups; e CD14+CD16+, f CD14+CD64+, g CD14+CD163+ and h CD14+ with intracellular IL-10+. Asterisks (*) represent statistically significant differences (P < 0.05)
Association of M2 Macrophage Polarization with HPV Infection
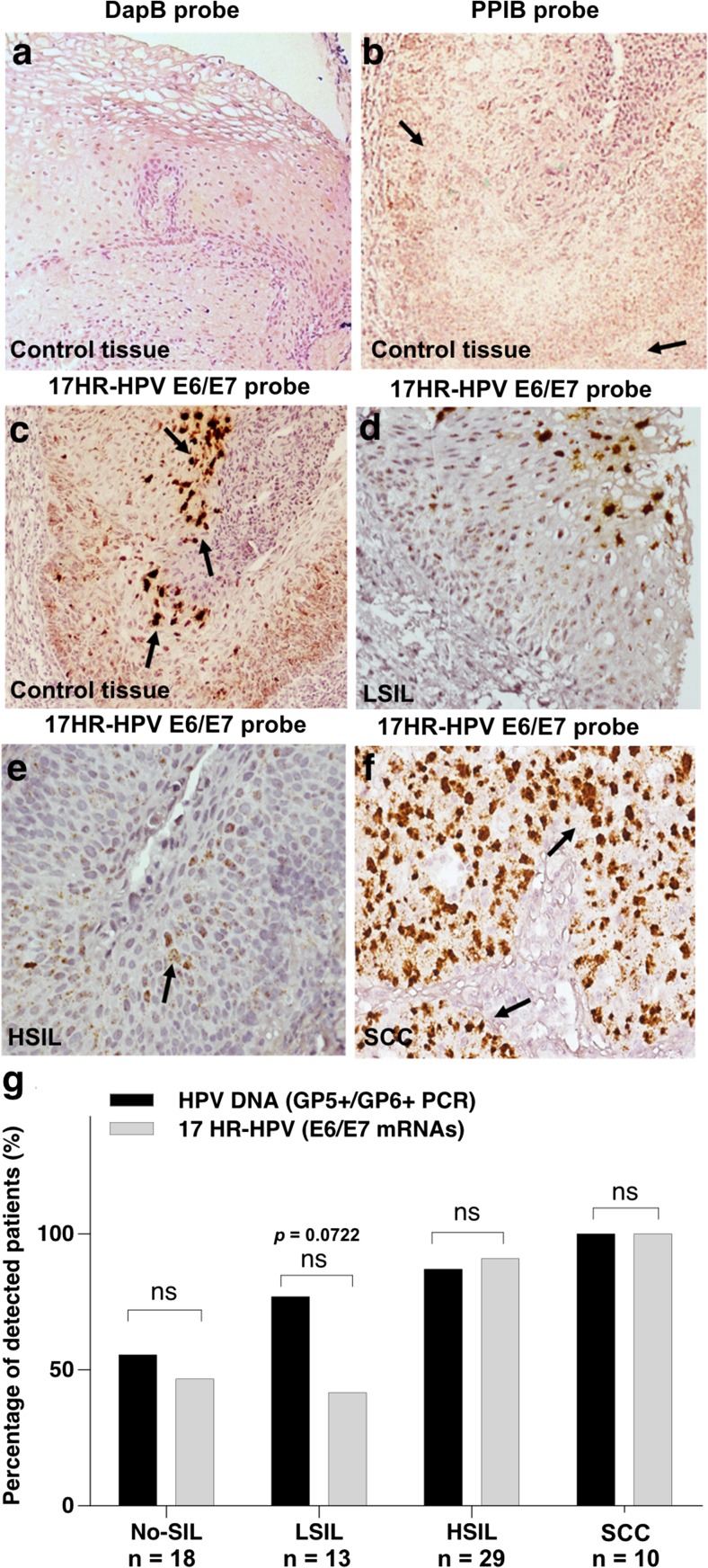
Monocytes are usually recruited to a site of infection. In HPV infection, monocytes may be phenotypically polarized under the influence of E6 and E7 oncoproteins that can promote an anti-inflammation milieu and increase densities of M2 macrophages. Therefore, we investigated the correlation of HPV infection, HPV DNA and mRNA expression with tissue M2 macrophage densities in cervical lesions of different grades including No-SIL and LSIL, HSIL and SCC. HPV infection was investigated using GP5+/GP6+ PCR for HPV DNA and expression of E6/E7 mRNA of 17HR-HPVs using ISH. The result showed that HPV DNA detection and mRNA expression of 17HR-HPVs were not significantly different in each cervical lesion group (Fig. 3a–f).
Fig. 3.
In situ hybridization of HR-HPV E6/E7 mRNA in cervical cancer samples. Brown dots indicated by black arrows represent the hybridization signals. Hybridization with DapB and PPIB probes served as internal (a) negative and (b) positive controls. (c) 17HR-HPV E6/E7 mRNA hybridization-positive controls was tested in HPV-positive cervical HSIL samples. ISH results of 17HR-HPV E6/E7 mRNA from cervical lesions are shown; (d) LSIL, (e) HSIL and (f) SCC. (g) Percentage of HPV DNA and HR-HPV E6/E7 mRNA detection in FFPE cervical tissue samples. Chi-square test for each cervical cancer stage was considered significant at P < 0.05
The expression of HR-HPV E6/E7 mRNA was used to analyze the correlation between M2 macrophage polarization and HPV infection. Fig. 4c shows that HR-HPV E6/E7 mRNA expression was correlated to tissue M2 macrophage density in the same tissue.
Correlation of CD64+/CD163+ Monocyte Ratio and stroma/peri-tumoral CD163+ Monocyte Density
The ratios between CD64+ and CD163+ monocytes (CD64+/CD163+ monocyte ratio) were calculated and showed a significant increase in the highly severity grade (HSIL and SCC) (Fig. 4a). This study also demonstrated the positive correlation of CD64+/CD163+ monocyte ratio and stroma/peri-tumoral CD163+ monocyte density with increasing cervical lesion severity (Fig. 4b).
Discussion
Monocytes are well known as precursors of tissue macrophages in various tissues. Circulating monocytes are recruited by chemokine ligands secreted from the site of inflammation. During this process, monocytes can express the adhesion molecules for endothelial attachment and the immunoglobulin receptors (CD16 or CD64), preparing for trans-endothelial activities are changed in monocyte subpopulations that are known to be associated with several diseases such as bacterial septicemia [27], coronary heart disease [28] and cholangiocarcinomas [11]. Increasing densities of CD14+CD16+ monocytes is considered normal in inflammatory responses to both acute and chronic conditions such as bacterial infection, arthrosclerosis or autoimmune diseases [27, 29]. In the present study, CD14+CD16+ monocytes were also found in the inflammatory condition (Fig. 2e).
Interestingly, circulating CD14+CD64+ monocytes was co-increased with tissue infiltrating M2 macrophages in cervical lesion grades (Fig. 1c–h). This might indicate that the CD14+CD64+ monocyte population was specifically responding to abnormal cervical lesions. Anti-inflammatory M2 phenotypes may be activated by the environment after entering tissues.
HR-HPV oncogenes in cervical tissue are known to play a role in immune evasion and cause phenotypic polarization of M1 to M2 macrophages, leading to an increase in the latter in malignancies including cervical cancer tissues [30, 31]. Infection with multiple HPV genotypes was rarely found in the HSIL and SCC groups. Previous report has shown that multiple infections were common in young women or women with low grade lesions because sexual activity is associated with sexual transmission of multiple HR-HPV types [32]. HPV 16, 58 and 18 were most common in our cervical lesion samples (data not shown). These are the predominant genotypes associated with cervical cancer in Asian populations which have high oncogenic and immune-evasion ability [33, 34].
The prevalence of HPV and expression of E6/E7 mRNA oncogenes increased with cervical lesion severity, as did the density of tissue M2 macrophages. The recruitment of monocytes, especially pro-inflammatory M1 macrophages, to tumor sites is essential to immune responses. HPV infection with an expression of E6/E7 mRNA was associated with the recruitment of anti-inflammatory tissue M2 macrophages, especially in the HSIL and SCC groups. This study agrees with previous studies that showed infiltration of M2 macrophages in HPV-induced vulva intraepithelial neoplasia (VIN) and in high grade cervical intraepithelial neoplasia (CIN), especially in HSIL and cervical cancer groups [5, 35].
SiHa and HeLa cells, which are cervical cancer cell lines containing HPV 16 and HPV 18 genomes, have been explored as sources of immunosuppressive factors and prostaglandin E2 and IL-6 can switch the M1 to the M2 phenotype [14, 15]. Thus, in the microenvironment of cervical lesions, the M1 phenotype might be switched to the M2 phenotype by mediators secreted from HPV-infected cells. This study demonstrated the increasing trend of HPV infection and tissue M2 macrophage density with increasing cervical lesion severity (Fig. 4c). This result suggested that an increasing in monocyte subpopulation and change them to the anti-inflammatory, tissue M2 macrophage may be under influence on HPV infection.
Several studies demonstrate alteration of immune cell populations as biomarkers for disease prediction. A recent study showed that infiltrating CD66b+ neutrophils are denser in the peri-tumoral compartment of cervical neoplasia tissues and are associated with patient recurrence-free survival [36]. High M1/M2 TAM ratios correlated with an increased survival rate and 5-year prognosis in ovarian cancer patients [37]. This study demonstrated that increasing of CD14+CD64+ monocytes and CD64+/CD163+ monocyte ratio in circulation were correlated with increasing of cervical lesion severity.
Conclusions
CD64+/CD163+ monocyte ratio of circulating monocytes was elevated corresponding with expression of HR-HPV oncogenes E6/E7 mRNA, cervical lesion severity and density of stroma/peri-tumoral CD163+ monocyte. These may be a candidate prognostic marker in cervical cancer progression.
Electronic supplementary material
(DOCX 8 kb)
Acknowledgements
We would like to acknowledge Prof. David Blair for editing the MS via Publication Clinic KKU.
Abbreviations
- APC
Antigen presenting cell
- Arg1
Arginase 1
- CD
Cluster of differentiation
- DapB
Bacterial dihydrodipicolinate reductase
- DNA
Deoxyribonucleic acid
- FFPE
Formalin-fixed paraffin-embedded tissue
- FITC
Fluorescein isothiocyanate
- GAPDH
Glyceraldehyde 3-phosphate dehydrogenase
- HR-HPV
High-risk human papillomavirus
- HSIL
High-grade squamous intraepithelial lesion
- IHC
Immunohistochemical staining
- IL-10
Interleukin 10
- ISH
In situ hybridization
- LSIL
Low-grade squamous intraepithelial lesion
- mRNA
Messenger ribonucleic acid
- No-SIL
No squamous intraepithelial lesion
- PBMC
Peripheral blood mononuclear cell
- PCR
Polymerase chain reaction
- PE
Phycoerythrin
- PPIB
Human peptidylpropyl isomerase B
- SA-HRP
Streptavidin horseradish peroxidase
- SCC
Squamous cell carcinoma
- TMA
Tissue microarray
Compliance with Ethical Standards
Conflict of Interest
The authors declare that they have no conflict of interest for this study.
Ethical Approval
Ethical approval of this study was obtained from the Khon Kaen University Ethics Committee for Human Research (KKUEC) and the Khon Kaen Central Hospital Ethics Committee for Human Research with reference numbers HE531387 and No. 20/04/2554, respectively.
Funding
Budgets for chemicals, reagents and sample collection processes were supported by Khon Kaen University (Grant numbers 551603, 564103 and 573003) and Faculty of Medicine, Khon Kaen University (Grant number I54141). PS was supported by the research fund for supporting lecturer to admit high potential student to study and research on his expert program from Graduate School (Grant number 511H1201). NS was supported by the Post-doctoral Program from Research Affairs and Graduate School, Khon Kaen University (Grant number 58222). The funders have no role in study design, data collection and analysis, preparation of the manuscript, and decision to publish.
Footnotes
Electronic supplementary material
The online version of this article (10.1007/s12307-017-0200-2) contains supplementary material, which is available to authorized users.
References
- 1.Castellsagué X. Natural history and epidemiology of HPV infection and cervical cancer. Gynecol Oncol. 2008;110:S4–S7. doi: 10.1016/j.ygyno.2008.07.045. [DOI] [PubMed] [Google Scholar]
- 2.Narisawa-Saito M, Kiyono T. Basic mechanisms of high-risk human papillomavirus-induced carcinogenesis: roles of E6 and E7 proteins. Cancer Sci. 2007;98:1505–1511. doi: 10.1111/j.1349-7006.2007.00546.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Verreck FA, de Boer T, Langenberg DM, van der Zanden L, Ottenhoff TH. Phenotypic and functional profiling of human proinflammatory type-1 and anti-inflammatory type-2 macrophages in response to microbial antigens and IFN-gamma- and CD40L-mediated costimulation. J Leukoc Biol. 2006;79:285–293. doi: 10.1189/jlb.0105015. [DOI] [PubMed] [Google Scholar]
- 4.Lawrence T, Natoli G. Transcriptional regulation of macrophage polarization: enabling diversity with identity. Nat Rev Immunol. 2011;11:750–761. doi: 10.1038/nri3088. [DOI] [PubMed] [Google Scholar]
- 5.van Esch EM, van Poelgeest MI, Trimbos JB, Fleuren GJ, Jordanova ES, van der Burg SH. Intraepithelial macrophage infiltration is related to a high number of regulatory T cells and promotes a progressive course of HPV-induced vulvar neoplasia. Int J Cancer. 2015;136:E85–E94. doi: 10.1002/ijc.29173. [DOI] [PubMed] [Google Scholar]
- 6.Heeren AM, Koster BD, Samuels S, Ferns DM, Chondronasiou D, Kenter GG, Jordanova ES, de Gruijl TD. High and interrelated rates of PD-L1+CD14+ antigen-presenting cells and regulatory T cells mark the microenvironment of metastatic lymph nodes from patients with cervical cancer. Cancer Immunol Res. 2015;3:48–58. doi: 10.1158/2326-6066.CIR-14-0149. [DOI] [PubMed] [Google Scholar]
- 7.Quail DF, Joyce JA. Microenvironmental regulation of tumor progression and metastasis. Nat Med. 2013;19:1423–1437. doi: 10.1038/nm.3394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shi C, Pamer EG. Monocyte recruitment during infection and inflammation. Nat Rev Immunol. 2011;11:762–774. doi: 10.1038/nri3070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Murray PJ, Wynn TA. Protective and pathogenic functions of macrophage subsets. Nat Rev Immunol. 2011;11:723–737. doi: 10.1038/nri3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen L. Co-inhibitory molecules of the B7-CD28 family in the control of T-cell immunity. Nat Rev Immunol. 2004;4:336–347. doi: 10.1038/nri1349. [DOI] [PubMed] [Google Scholar]
- 11.Subimerb C, Pinlaor S, Lulitanond V, Khuntikeo N, Okada S, McGrath MS, Wongkham S. Circulating CD14(+) CD16(+) monocyte levels predict tissue invasive character of cholangiocarcinoma. Clin Exp Immunol. 2010;161:471–479. doi: 10.1111/j.1365-2249.2010.04200.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang Y, Wang JS, Yin HJ, Chen KJ. The expression of CD14(+)CD16(+) monocyte subpopulation in coronary heart disease patients with blood stasis syndrome. Evid Based Complement Alternat Med. 2013;2013:416932. doi: 10.1155/2013/416932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fanger NA, Wardwell K, Shen L, Tedder TF, Guyre PM. Type I (CD64) and type II (CD32) Fc gamma receptor-mediated phagocytosis by human blood dendritic cells. J Immunol. 1996;157:541–548. [PubMed] [Google Scholar]
- 14.Sánchez-Reyes K, Bravo-Cuellar A, Hernández-Flores G, Lerma-Díaz JM, Jave-Suárez LF, Gómez-Lomelí P, de Celis R, Aguilar-Lemarroy A, Domínguez-Rodríguez JR, Ortiz-Lazareno PC. Cervical cancer cell supernatants induce a phenotypic switch from U937-derived macrophage-activated M1 state into M2-like suppressor phenotype with change in Toll-like receptor profile. Biomed Res Int. 2014;2014:683068. doi: 10.1155/2014/683068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Heusinkveld M, de Vos van Steenwijk PJ, Goedemans R, Ramwadhdoebe TH, Gorter A, Welters MJ, van Hall T, van der Burg SH. M2 macrophages induced by prostaglandin E2 and IL-6 from cervical carcinoma are switched to activated M1 macrophages by CD4+ Th1 cells. J Immunol. 2011;187:1157–1165. doi: 10.4049/jimmunol.1100889. [DOI] [PubMed] [Google Scholar]
- 16.Mantovani A, Sica A, Sozzani S, Allavena P, Vecchi A, Locati M. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 2004;25:677–686. doi: 10.1016/j.it.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 17.He KF, Zhang L, Huang CF, Ma SR, Wang YF, Wang WM, Zhao ZL, Liu B, Zhao YF, Zhang WF, Sun ZJ. CD163+ tumor-associated macrophages correlated with poor prognosis and cancer stem cells in oral squamous cell carcinoma. Biomed Res Int. 2014;2014:838632. doi: 10.1155/2014/838632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Edin S, Wikberg ML, Dahlin AM, Rutegård J, Öberg Å, Oldenborg PA, Palmqvist R. The distribution of macrophages with a M1 or M2 phenotype in relation to prognosis and the molecular characteristics of colorectal cancer. PLoS One. 2012;7:e47045. doi: 10.1371/journal.pone.0047045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Van Gorp H, Delputte PL, Nauwynck HJ. Scavenger receptor CD163, a Jack-of-all-trades and potential target for cell-directed therapy. Mol Immunol. 2010;47:1650–1660. doi: 10.1016/j.molimm.2010.02.008. [DOI] [PubMed] [Google Scholar]
- 20.Jacobs MV, de Roda Husman AM, van den Brule AJ, Snijders PJ, Meijer CJ, Walboomers JM. Group-specific differentiation between high- and low-risk human papillomavirus genotypes by general primer-mediated PCR and two cocktails of oligonucleotide probes. J Clin Microbiol. 1995;33:901–905. doi: 10.1128/jcm.33.4.901-905.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Evans MF, Peng Z, Clark KM, Adamson CS, Ma XJ, Wu X, Wang H, Luo Y, Cooper K. HPV E6/E7 RNA in situ hybridization signal patterns as biomarkers of three-tier cervical intraepithelial neoplasia grade. PLoS One. 2014;9:e91142. doi: 10.1371/journal.pone.0091142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ulmer AJ, Scholz W, Ernst M, Brandt E, Flad HD. Isolation and subfractionation of human peripheral blood mononuclear cells (PBMC) by density gradient centrifugation on Percoll. Immunobiology. 1984;166:238–250. doi: 10.1016/S0171-2985(84)80042-X. [DOI] [PubMed] [Google Scholar]
- 23.Bouhlel MA, Derudas B, Rigamonti E, Dièvart R, Brozek J, Haulon S, Zawadzki C, Jude B, Torpier G, Marx N, Staels B, Chinetti-Gbaguidi G. PPARgamma activation primes human monocytes into alternative M2 macrophages with anti-inflammatory properties. Cell Metab. 2007;6:137–143. doi: 10.1016/j.cmet.2007.06.010. [DOI] [PubMed] [Google Scholar]
- 24.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 25.Barros MH, Hauck F, Dreyer JH, Kempkes B, Niedobitek G. Macrophage polarisation: an immunohistochemical approach for identifying M1 and M2 macrophages. PLoS One. 2013;8:e80908. doi: 10.1371/journal.pone.0080908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maniecki MB, Etzerodt A, Ulhøi BP, Steiniche T, Borre M, Dyrskjøt L, Orntoft TF, Moestrup SK, Møller HJ. Tumor-promoting macrophages induce the expression of the macrophage-specific receptor CD163 in malignant cells. Int J Cancer. 2012;131:2320–2331. doi: 10.1002/ijc.27506. [DOI] [PubMed] [Google Scholar]
- 27.Belge KU, Dayyani F, Horelt A, Siedlar M, Frankenberger M, Frankenberger B, Espevik T, Ziegler-Heitbrock L. The proinflammatory CD14+CD16+DR++ monocytes are a major source of TNF. J Immunol. 2002;168:3536–3542. doi: 10.4049/jimmunol.168.7.3536. [DOI] [PubMed] [Google Scholar]
- 28.Li Y, Lee PY, Sobel ES, Narain S, Satoh M, Segal MS, Reeves WH, Richards HB. Increased expression of FcgammaRI/CD64 on circulating monocytes parallels ongoing inflammation and nephritis in lupus. Arthritis Res Ther. 2009;11:R6. doi: 10.1186/ar2590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Burbano C, Vasquez G, Rojas M. Modulatory effects of CD14+CD16++ monocytes on CD14++CD16- monocytes: a possible explanation of monocyte alterations in systemic lupus erythematosus. Arthritis Rheumatol. 2014;66:3371–3381. doi: 10.1002/art.38860. [DOI] [PubMed] [Google Scholar]
- 30.Riezebos-Brilman A, Regts J, Freyschmidt EJ, Dontje B, Wilschut J, Daemen T. Induction of human papilloma virus E6/E7-specific cytotoxic T-lymphocyte activity in immune-tolerant, E6/E7-transgenic mice. Gene Ther. 2005;12:1410–1414. doi: 10.1038/sj.gt.3302536. [DOI] [PubMed] [Google Scholar]
- 31.Boccardo E, Lepique AP, Villa LL. The role of inflammation in HPV carcinogenesis. Carcinogenesis. 2010;31:1905–1912. doi: 10.1093/carcin/bgq176. [DOI] [PubMed] [Google Scholar]
- 32.Cuschieri KS, Cubie HA, Whitley MW, Seagar AL, Arends MJ, Moore C, Gilkisson G, McGoogan E. Multiple high risk HPV infections are common in cervical neoplasia and young women in a cervical screening population. J Clin Pathol. 2004;57:68–72. doi: 10.1136/jcp.57.1.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chan PK. Human papillomavirus type 58: the unique role in cervical cancers in East Asia. Cell Biosci. 2012;2:17. doi: 10.1186/2045-3701-2-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bao YP, Li N, Smith JS, Qiao YL, ACCPAB M. Human papillomavirus type distribution in women from Asia: a meta-analysis. Int J Gynecol Cancer. 2008;18:71–79. doi: 10.1111/j.1525-1438.2007.00959.x. [DOI] [PubMed] [Google Scholar]
- 35.Hammes LS, Tekmal RR, Naud P, Edelweiss MI, Kirma N, Valente PT, Syrjänen KJ, Cunha-Filho JS. Macrophages, inflammation and risk of cervical intraepithelial neoplasia (CIN) progression--clinicopathological correlation. Gynecol Oncol. 2007;105:157–165. doi: 10.1016/j.ygyno.2006.11.023. [DOI] [PubMed] [Google Scholar]
- 36.Carus A, Ladekarl M, Hager H, Nedergaard BS, Donskov F. Tumour-associated CD66b+ neutrophil count is an independent prognostic factor for recurrence in localised cervical cancer. Br J Cancer. 2013;108:2116–2122. doi: 10.1038/bjc.2013.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang M, He Y, Sun X, Li Q, Wang W, Zhao A, Di W. A high M1/M2 ratio of tumor-associated macrophages is associated with extended survival in ovarian cancer patients. J Ovarian Res. 2014;7:19. doi: 10.1186/1757-2215-7-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 8 kb)