Abstract
Epithelial-mesenchymal transition (EMT) is a significant process in the invasion and metastasis of cancers including oral squamous cell carcinoma (OSCC), and the cadherin switch has been identified as one of the hallmarks of EMT. The aim of the present study was to evaluate the significance of the cadherin switch in the prognosis of OSCC and generate a model for prognostic predictions. Seventy-six biopsy and/or initial surgical specimens from OSCC patients were immunohistochemically analyzed for the expression of E-cadherin and N-cadherin in either overall OSCC cells in tumor nests or in OSCC cells at the invasive front. Among 76 OSCC cases, overall OSCC cells in tumor nests were negative for the expression of E-cadherin in 10 cases and positive for that of N-cadherin in 53 cases. Among 10 cases negative for the expression of E-cadherin, 4 cases were positive for that of N-cadherin. In OSCC cells at the invasive front, the expression of E-cadherin was negative in 62 cases, while that of N-cadherin was positive in 39 cases. Among 62 cases negative for the expression of E-cadherin, 33 cases were positive for that of N-cadherin. A logistic regression analysis showed that a model using the evaluation of N-cadherin expression in overall OSCC cells in tumor nests with a cut-off point of 70 years old was the best fit model. These results suggest that N-cadherin has significant value in prognostic predictions for OSCC patients.
Keywords: Oral squamous cell carcinoma, Cadherin, Epithelial-mesenchymal transition, Multivariate analysis, Prognosis
Introduction
Squamous cell carcinoma (SCC) is the most frequent malignant neoplasia in the oral cavity, with the lateral portion of the tongue being the most commonly affected site [1]. Smoking, drinking alcohol, and poor oral hygiene are regarded as key risk factors for oral squamous cell carcinoma (OSCC) [2–4]. OSCC has a well-known characteristic geographical distribution. The incidence of OSCC is higher in South Asian countries, in which the custom of chewing smokeless tobacco is popular, than in North America and European countries, and the epidemiological significance of smokeless tobacco and OSCC has been established [1, 4, 5]. Infections with the human papilloma virus have also been suggested as one of the risk factors for head and neck carcinomas; however, previous studies reported an association with oropharyngeal cancer rather than OSCC [6–8]. Although several risk factors for OSCC have been established, its prognosis remains unfavorable [7, 9, 10]. Periodical oral examinations are needed in order to improve the prognosis of OSCC.
In histopathological examinations, the development of markers and/or systems to predict prognoses may also contribute to improvements in the prognosis of OSCC. Epithelial-mesenchymal transition (EMT) is one of the imperative phenomena explaining the biological mechanisms underlying the infiltration of carcinoma cells into surrounding tissues [11, 12]. Carcinoma cells derived from epithelia are more likely to adhere to one another and form tumor nests, and the invasiveness of assemblages of carcinoma cells is typically milder than that of cellularized carcinoma cells, which are frequently observed in scirrhous carcinomas [13]. In EMT, the expression of the major adhesion molecule of epithelial cells, E-cadherin, is suppressed by the expression of Snail, the transcriptional repressor of E-cadherin, and carcinoma cells comprising tumor nests lose contact and are cellularized [14, 15]. Many in vitro studies have confirmed that transforming growth factor-beta (TGF-β) induces EMT, and treatments with TGF-β not only suppress the expression of E-cadherin, but also induce that of mesenchymal markers [16–18]. Furthermore, recent studies confirmed that cancer cells with the TGF-β signal induce matrix metalloproteinases and generate cancer stem cells and cancer-associated fibroblasts [11]. Therefore, the detection of EMT or EMT markers, including a decrease in or the loss of E-cadherin expression or the induction of N-cadherin, Snail, and vimentin expression in carcinoma cells, is expected to play a valuable role in prognostic predictions for patients with carcinomas.
EMT has been detected in OSCC specimens, similar to many other carcinoma specimens, and the expression of E-cadherin and N-cadherin has been analyzed [19–21]. A decrease in or the loss of E-cadherin has been analyzed in detail in previous studies on OSCC or head and neck SCC, and many studies including meta-analyses of published findings have suggested that the strong expression of E-cadherin is a positive prognostic factor, while its weak expression is a negative prognostic factor for OSCC or head and neck SCC [22–28]. The induction of N-cadherin expression has not been investigated as extensively as the suppression of E-cadherin expression and this may be because its induction is considered to be a more indirect marker of EMT than the suppression of E-cadherin expression. However, previous studies have suggested that the induction of N-cadherin expression is a negative prognostic factor for OSCC patients [29, 30].
In assessments of E-cadherin expression in histopathological specimens of OSCC cases, it is important that the evaluation is not attenuated by expression in well-differentiated OSCC cells in the core of tumor nests. In order to prevent this attenuation, the expression of E-cadherin has been analyzed in OSCC cells at the invasive front [31, 32]. In contrast, the expression of N-cadherin may be analyzed in overall OSCC cells in tumor nests because the mesenchymal phenotypes of carcinoma cells are one of the definitive markers of the infiltrative nature of carcinoma cells at any portion. We have been investigating the generation of statistical models for prognostic predictions in OSCC patients using several well-known biological markers. Vascular endothelial growth factors (VEGFs) have been identified as one of the essential factors for tumor invasion into surrounding tissues, and we generated a novel logic combination model for prognostic predictions in OSCC patients [33]. We also previously suggested that the expression of podoplanin, a reliable marker of lymphatic endothelial cells, in OSCC cells predicted the prognosis of OSCC patients [34]. In the present study, we analyzed the expression of E-cadherin and N-cadherin in overall OSCC cells in tumor nests and in those at the invasive front. The data obtained were extensively analyzed statistically in order to generate a novel model to predict the prognosis of OSCC patients and estimate an appropriate strategy for histopathological examinations.
Patients and Materials
This study was performed in accordance with the ethical standards of the Declaration of Helsinki, and was approved by the Ethics Committee of Nagasaki University Hospital (approved no. 17022746). This is a retrospective study using previously diagnosed samples and the clinical information. Written informed consent was not used and information regarding this study was disclosed on the website of Nagasaki University Clinical Research Center and assured the right for refusal to use samples and clinical information. Some of the subjects were already passed away and refusal by the proxy of the subject was also permitted.
Seventy-six patients, 51 males and 25 females ranging in age from 31 to 87 years with a median age of 64.9 years, who were admitted to Nagasaki University Dental Hospital between 1983 and 2002 with a histopathological diagnosis of OSCC were analyzed. All patients underwent either biopsy or surgery with or without preoperative treatment. Forty-four patients were treated with a standard program of preoperative irradiation using Linac (total, 30 Gy) and the preoperative continuous subcutaneous administration of peplomycin (5 mg/day; maximum dosage, 100 mg). Four patients were treated with preoperative irradiation only, 13 with the preoperative administration of peplomycin only, and 15 were not treated before surgery. Histopathological analyses diagnosed 63 patients with well-differentiated, 8 with moderately differentiated, and 5 with poorly differentiated OSCC. All patients were followed up at the hospital until 2005; 56 (73.6%) died during and 20 (26.4%) survived the follow-up period.
Histopathological Analyses
Specimens were routinely processed with a 10% buffered formalin fixative, three micrometer-thick sections were made from paraffin-embedded blocks of biopsy or surgical specimens, and sections were stained with hematoxylin and eosin (HE) for histopathological diagnoses. The expression of E-cadherin and N-cadherin was immunohistochemically analyzed with an anti E-cadherin antibody (ab40772, Abcam, Cambridge, UK) and anti N-cadherin antibody (ab98952, Abcam). Sections were incubated with each antibody described above at ×500 dilution with PBS at 4 °C overnight and an immunohistochemical analysis was performed on the EnVision + System (Dako, Carpinteria, CA).
The expression of E-cadherin and N-cadherin was evaluated either in overall OSCC cells in tumor nests, which invaded the submucosal region separate from the primary mucosa, or OSCC cells at the invasive front, the deep invasive margins of OSCC [35]. When evaluating overall OSCC cells in tumor nests, we analyzed the expression of E-cadherin and N-cadherin by intensity or the expression area (%). Intensities in OSCC cells were graded as –, ±, +, and ++ under a microscope, and indicated the absence of a positive signal, a weak signal, moderate signal, and strong signal, respectively. Expression at the invasive front was graded as – and +, which indicated the absence of a positive signal and a positive signal, respectively.
Statistical Analyses
The prognostic status was defined as poor for patients that died during the observation period and good for patients that were alive. In a univariate analysis, we used indicator variables for factors with more than two ordered categories in sex, age, and the expression profiles of E-cadherin and N-cadherin. The intensities of E-cadherin or N-cadherin, which were graded as –, ±, +, and ++, were analyzed for each grade. In statistical analyses, areas in OSCC cells were graded as the absence of a signal, <5%, ≥5% to <25%, ≥25% to <50%, ≥50% to <75%, and ≥75%, and were analyzed for each grade. Significant factors from the univariate analysis were further examined in a multivariate analysis using logistic regression models. However, variables for the expression of E-cadherin and N-cadherin were examined in the multivariate analysis irrespective of significance in the univariate analysis. In model selection, we used Akaike’s Information Criterion (AIC) [36]. Statistical procedures were performed with Statistical Language R. P-values <0.05 were considered to be significant.
Results
In 76 patients, the expression profiles for E-cadherin and N-cadherin were widely distributed. The detection of E-cadherin expression was slightly more common in relatively large tumor nests (Fig. 1a); however, it was also detected in relatively small tumor nests at variable intensities (Fig. 1b, c). A limited number of patients did not have a positive signal for E-cadherin (Fig. 1d). In contrast, N-cadherin expression was more likely to be negative in large tumor nests (Fig. 1e) and was more commonly detected in relatively small tumor nests at variable intensities (Fig. 1f-h). However, N-cadherin expression was also detected in large tumor nests, which revealed apparent squamous cell differentiation in some patients (Fig. 1g, arrows).
Fig. 1.
Representative views of immunohistochemical reactions for E-cadherin and N-cadherin in OSCC specimens. The expression of E-cadherin in each intensity grade from a strong (++), moderate (+), and weak (±) to no (−) signal (a-d), and the expression of N-cadherin in each intensity grade from no (−), a weak (±), and moderate (+) to a strong (++) signal (e-h). Arrows in (g) indicate a positive signal for N-cadherin in a tumor nest composed of OSCC cells with a squamous cell appearance. Original magnification ×200
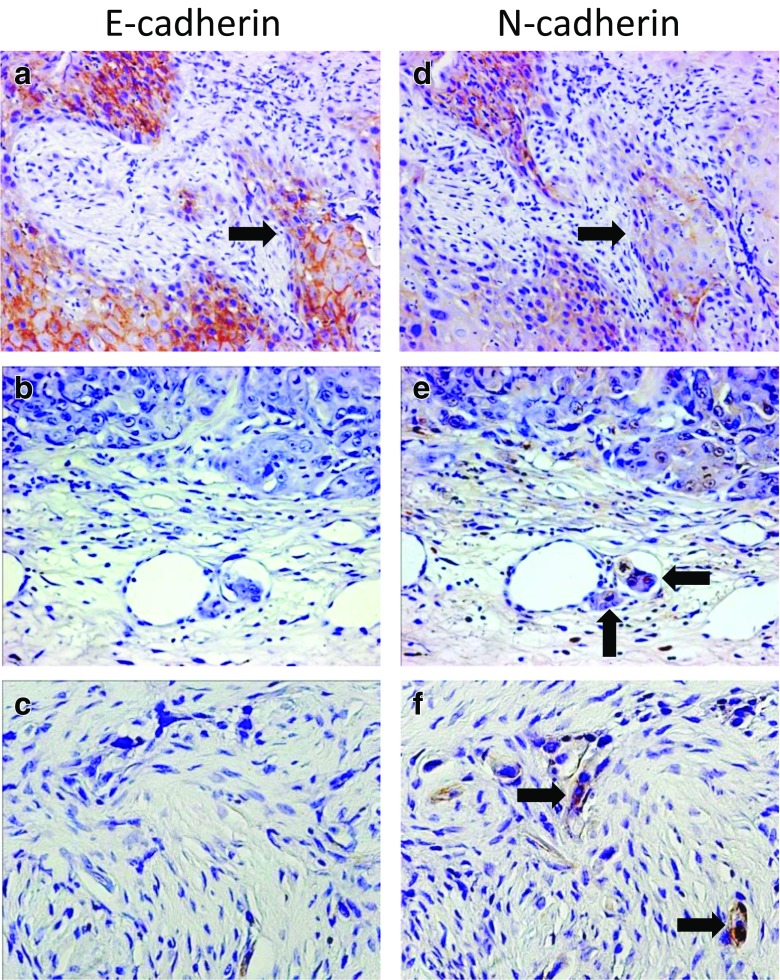
The results of the histopathological analysis also confirmed the presence of OSCC cells in tumor nests expressing E-cadherin and N-cadherin (Fig. 2a, d, arrows). A positive signal for N-cadherin was a useful marker for identifying small tumor satellites (Fig. 2e, arrows) and individual OSCC cells with EMT (Fig. 2f, arrows) in contrast to the negative reaction for the expression of E-cadherin (Fig. 2b, c).
Fig. 2.
Comparative immunohistochemical views of the expression of E-cadherin and N-cadherin in the same OSCC cases. (a, d) An OSCC case with the expression of both E-cadherin and N-cadherin. Arrows indicate a tumor nest at the invasive front, which reveals the expression of E-cadherin and N-cadherin. (b, e) An OSCC case with the expression of N-cadherin. Small satellites of OSCC invade a lymphatic vessel with a positive signal for N-cadherin (e, arrows). (c, f) An OSCC case with the expression of N-cadherin. OSCC cells with EMT show a strong signal for N-cadherin (f, arrows). Original magnification of (a, b, d, e) ×200; (c, f) ×400
The results of the univariate analysis revealed that the prognosis of OSCC patients was not associated with sex, whereas a poor prognosis correlated with age with a cut-off point of 70 years. Neither the intensities nor areas of E-cadherin expression evaluated in overall OSCC cells in tumor nests were associated with the prognosis of OSCC patients. In contrast, the expression of N-cadherin evaluated in overall OSCC cells in tumor nests correlated with the prognosis of OSCC patients. Similar results were obtained for OSCC cells at the invasive front. The expression of N-cadherin, but not that of E-cadherin at the invasive front was associated with the prognosis of OSCC patients (Table 1). Specification of E-cadherin and N-cadherin expression in overall OSCC cells in tumor nests and OSCC cells at the invasive front was shown in Table 2.
Table 1.
Study subjects by factors and their estimated Odds Ratio(OR)
| Factor | Category | Sample Size | Prognosis | Logistic Regression | ||
|---|---|---|---|---|---|---|
| Good | Poor | OR | p-Value | |||
| Sex | Female | 25 | 5 | 20 | 1.000 | – |
| Male | 51 | 15 | 36 | 0.600 | 0.384 | |
| Age | < 70 | 47 | 17 | 30 | 1.000 | – |
| ≥ 70 | 29 | 3 | 26 | 4.911 | 0.019 | |
| Overall OSCC cells in tumor nests | ||||||
| E-cad. Intensity | – | 10 | 3 | 7 | 1.000 | – |
| ± | 27 | 9 | 18 | 0.857 | 0.848 | |
| + | 32 | 6 | 26 | 1.857 | 0.453 | |
| ++ | 7 | 2 | 5 | 1.071 | 0.949 | |
| E-cad. Area (%) | 0 | 10 | 3 | 7 | 1.000 | – |
| >1 | 66 | 17 | 49 | 1.235 | 0.777 | |
| N-cad. Intensity | – | 23 | 11 | 12 | 1.000 | – |
| ± | 33 | 7 | 26 | 3.405 | 0.040 | |
| +, ++ | 20 | 2 | 18 | 8.250 | 0.013 | |
| N-cad. Area (%) | 0 | 23 | 11 | 12 | 1.000 | – |
| >1 | 53 | 9 | 44 | 4.481 | 0.007 | |
| OSCC cells at the invasive front | ||||||
| E-cad. Positive cells | – | 62 | 16 | 46 | 1.000 | – |
| + | 14 | 4 | 10 | 0.870 | 0.832 | |
| N-cad. Positive cells | – | 37 | 15 | 22 | 1.000 | – |
| + | 39 | 5 | 34 | 4.636 | 0.009 | |
Table 2.
Specification of E-cadherin and N-cadherin expression in overall OSCC cells in tumor nests and OSCC cells at the invasive front
| N-cad. Negative | N-cad. Positive | |
|---|---|---|
| Overall OSCC cells in tumor nests | ||
| E-cad. Positive | 17 | 49 |
| E-cad. Negative | 6 | 4 |
| OSCC cells at the invasive front | ||
| E-cad. Positive | 8 | 6 |
| E-cad. Negative | 29 | 33 |
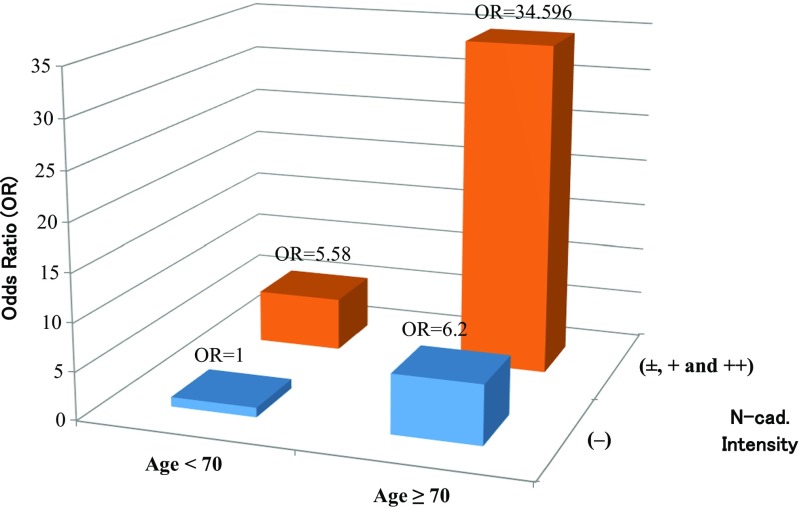
Variables including age and the expression of N-cadherin and E-cadherin were analyzed in more detail using a multiple logistic regression model. We found that the logistic model with the expression of N-cadherin (±, +, and ++) versus the other (−) evaluated in overall OSCC cells in tumor nests and age (≥70 versus <70) had a smaller AIC than the other multivariate models. (Table 3). Figure 3 shows the odds ratio against poor prognosis for OSCC patients evaluated by the model with the smallest AIC.
Table 3.
Result of multiple logisitic regression analyses
| Regression coefficient | Std. Error | p-value | Odds Ratio(OR) | 95% CI of OR | AIC | ||
|---|---|---|---|---|---|---|---|
| Low | Up | ||||||
| Overall OSCC cells in tumor nests | |||||||
| (Intercept) | −0.253 | 0.822 | 0.758 | 0.78 | 0.16 | 3.88 | 80.0 |
| E-cad. Intensity (±_ + _++) vs (−) | −0.458 | 0.894 | 0.609 | 0.63 | 0.11 | 3.65 | |
| N-cad. Intensity (±_ + _++) vs (−) | 1.818 | 0.645 | 0.005 | 6.16 | 1.74 | 21.83 | |
| Age ≥ 70 vs < 70 | 1.844 | 0.739 | 0.013 | 6.32 | 1.49 | 26.89 | |
| (Intercept) | −0.583 | 0.516 | 0.259 | 0.56 | 0.20 | 1.54 | 78.3 |
| N-cad. Intensity (±_ + _++) vs (−) | 1.719 | 0.610 | 0.005 | 5.58 | 1.69 | 18.44 | |
| Age ≥ 70 vs < 70 | 1.825 | 0.733 | 0.013 | 6.20 | 1.48 | 26.07 | |
| OSCC cells at the invasive front | |||||||
| (Intercept) | −0.082 | 0.416 | 0.845 | 0.92 | 0.41 | 2.08 | 81.2 |
| E-cad. Positive cells + vs - | −0.182 | 0.729 | 0.803 | 0.83 | 0.20 | 3.48 | |
| N-cad. Positive cells + vs - | 1.576 | 0.610 | 0.010 | 4.84 | 1.46 | 15.97 | |
| Age ≥ 70 vs < 70 | 1.661 | 0.713 | 0.020 | 5.27 | 1.30 | 21.31 | |
| (Intercept) | −0.114 | 0.395 | 0.773 | 0.89 | 0.41 | 1.94 | 79.2 |
| N-cad. Positive cells + vs - | 1.580 | 0.609 | 0.009 | 4.85 | 1.47 | 16.02 | |
| Age ≥ 70 vs < 70 | 1.643 | 0.708 | 0.020 | 5.17 | 1.29 | 20.71 | |
Fig. 3.
The odds ratio against poor prognosis for OSCC patients with (±, +, ++) or without (−) N-cadherin expression of the selected model
Discussion
The expression of E-cadherin has been suggested to contribute to prognostic predictions for OSCC patients. However, previous findings have been controversial [37–39]. EMT is regarded as one of the drivers of the infiltration of carcinoma cells into surrounding tissues by disassembled carcinoma cells at the invasive front. If most OSCC cells reveal scirrhous invasion, EMT may be evaluated in overall OSCC cells in a similar manner to OSCC cells at the invasive front. In contrast, most cases of OSCC exhibit squamous differentiation in the core of tumor nests, and the development of cancer pearls has occasionally been reported in cases of well-differentiated OSCC. In some tumor nests generated by EMT cells, mesenchymal-epithelial transition (MET) may occur and is considered to form a core composed of tumor cells with squamous differentiation [40, 41]. OSCC cells in tumor nests with the weak expression of E-cadherin may also completely lose expression at the invasive front [42]. Hence, positive signals in the core of tumor nests may affect the accuracy of evaluations of the EMT-related suppression of E-cadherin expression. This may be one of the reasons for the discrepancy in previous studies that analyzed the relationship between the expression of E-cadherin and prognosis of OSCC patients. The expression of N-cadherin has been reported to contribute to prognostic predictions for OSCC patients [29, 30]. However, previous findings have been controversial [37], with some studies indicating that the expression of N-cadherin in OSCC was limited more than that of E-cadherin, while the expression of N-cadherin was less applicable than that of E-cadherin to evaluations of the prognosis of OSCC [43, 44].
In the present study, we immunohistochemically analyzed the expression of E-cadherin and N-cadherin in OSCC specimens and statistically analyzed the data obtained in order to generate a model to predict the prognosis of OSCC patients. We analyzed the intensities and areas of expression of E-cadherin and N-cadherin in overall OSCC cells in tumor nests. In OSCC cells at the invasive front, we detected the presence or absence of OSCC cells with positive signals for E-cadherin or N-cadherin. The results of the univariate analysis showed that the expression of N-cadherin, but not E-cadherin correlated with the prognosis of OSCC patients in every analysis. Significant factors in a univariate analysis are typically only applied to a multivariate analysis in order to generate multiple logistic regression models. In the present study, data on the expression of E-cadherin was used in a multivariate analysis in addition to those for N-cadherin. The multivariate analysis confirmed that the expression of E-cadherin did not contribute to improving the fit of the model using the expression of N-cadherin for prognostic predictions in OSCC patients.
The frequency of the positive expression of E-cadherin was 87% in tumor nests and 18% at the invasive front. On the other hand, the positive frequency of N-cadherin was 70% in tumor nests and 51% at the invasive front. The expression of E-cadherin and N-cadherin were previously shown to be mutually exclusive [12, 45]. However, the results of the present study suggest that changes in these expression profiles were imperfectly reciprocal, and a number of OSCC cells expressed E-cadherin and N-cadherin. These results also indicate that the weak expression of E-cadherin was widely detected in a large number of OSCC cells irrespective of the nature of OSCC cells, including the grade of malignancy, and this may be one of the reasons why the expression of E-cadherin did not contribute to prognostic predictions for OSCC patients in this study. A quantitative evaluation of intensities in an immunohistochemical analysis is generally challenging because they are affected by fixatives and the fixing conditions of specimens. Therefore, the presence of EMT, namely, the weak expression of E-cadherin, was detected less accurately than that of N-cadherin, which was evaluated by a positive signal in the present study.
The frequency of the positive expression of N-cadherin was higher in overall OSCC cells in tumor nests than in OSCC cells at the invasive front. The reason for this may be related to the number of OSCC cells expressing N-cadherin. The number of OSCC cells expressing N-cadherin was limited and markedly lower than that expressing E-cadherin. Theoretically, an evaluation at the invasive front is the most accurate approach for detecting the cadherin switch if it is possible to evaluate all OSCC cells in whole specimens. However, we typically perform immunohistochemical examinations using a few representative histopathological sections in a pathological laboratory and this may affect the accuracy of analyzing a limited number of N-cadherin-positive OSCC cells in a small area such as the invasive front. This may also be the reason why we achieved the best fit model using N-cadherin expression in overall OSCC cells in tumor nests rather than in those at the invasive front. However, the AIC scores of a model evaluating the expression of N-cadherin in OSCC cells at the invasive front were not markedly different from those of the best fit model. These results not only confirmed that an evaluation of the expression of N-cadherin is useful for prognostic predictions in OSCC patients, but also suggest that it is possible to evaluate the expression of N-cadherin in overall OSCC cells in tumor nests in a histopathological examination.
In conclusion, we confirmed that the expression of N-cadherin was one of the reliable markers for prognostic predictions in OSCC patients. We suggest that it is possible to evaluate the expression of N-cadherin in overall OSCC cells in tumor nests. We also generated a model to evaluate the prognostic prediction of OSCC using the expression of N-cadherin and age of patients with a cut-off point of 70 years.
Sources of Funding
This study was supported by Japan Society for the Promotion of Science KAKENHI (25462865).
Compliance with Ethical Standards
Conflict of Interest
The authors declare that they have no potential conflicts of interest.
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional research committee, and with the 1964 Declaration of Helsinki and its latter amendments or comparable ethical standards. Nagasaki University Hospital Ethics Committee Approval No.: 17,022,746.
Informed Consent
Written informed consent was not used and information regarding this study was disclosed on the website of Nagasaki University Clinical Research Center and assured the right for refusal to use samples and clinical information. Some of the subjects were already passed away and refusal by the proxy of the subject was also permitted.
References
- 1.Neville BW, Damm DD, Allen CM, Bouquot JE, editors. Oral and maxillofacial pathology. 3. St. Louis: Saunders; 2009. pp. 409–421. [Google Scholar]
- 2.Chang JS, Lo HI, Wong TY, Huang CC, Lee WT, Tsai ST, et al. Investigating the association between oral hygiene and head and neck cancer. Oral Oncol. 2013;49:1010–1017. doi: 10.1016/j.oraloncology.2013.07.004. [DOI] [PubMed] [Google Scholar]
- 3.Tsai ST, Wong TY, CY O, Fang SY, Chen KC, Hsiao JR, et al. The interplay between alcohol consumption, oral hygiene, ALDH2 and ADH1B in the risk of head and neck cancer. Int J Cancer. 2014;135:2424–2436. doi: 10.1002/ijc.28885. [DOI] [PubMed] [Google Scholar]
- 4.Barness L, Eveson JW, Reichart P, Sidransky D, editors. Pathology and genetics of head and neck Tumours. World Health Organization classification of Tumours. Lyon: IARC Press; 2005. pp. 166–175. [Google Scholar]
- 5.Gupta B, Johnson NW. Systematic review and meta-analysis of association of smokeless tobacco and of betel quid without tobacco with incidence of oral cancer in South Asia and the Pacific. PLoS One. 2014;9:e113385. doi: 10.1371/journal.pone.0113385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marur S, Forastiere AA. Head and neck squamous cell carcinoma: update on epidemiology, diagnosis, and treatment. Mayo Clin Proc. 2016;91:386–396. doi: 10.1016/j.mayocp.2015.12.017. [DOI] [PubMed] [Google Scholar]
- 7.Malik UU, Zarina S, Pennington SR. Oral squamous cell carcinoma: key clinical questions, biomarker discovery, and the role of proteomics. Arch Oral Biol. 2016;63:53–65. doi: 10.1016/j.archoralbio.2015.11.017. [DOI] [PubMed] [Google Scholar]
- 8.Vigneswaran N, Williams MD. Epidemiologic trends in head and neck cancer and aids in diagnosis. Oral Maxillofac Surg Clin North Am. 2014;26:123–141. doi: 10.1016/j.coms.2014.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hunter KD, Parkinson EK, Harrison PR. Profiling early head and neck cancer. Nat Rev Cancer. 2005;5:127–135. doi: 10.1038/nrc1549. [DOI] [PubMed] [Google Scholar]
- 10.Scully C, Bagan J. Oral squamous cell carcinoma: overview of current understanding of aetiopathogenesis and clinical implications. Oral Dis. 2009;15:388–399. doi: 10.1111/j.1601-0825.2009.01563.x. [DOI] [PubMed] [Google Scholar]
- 11.Katsuno Y, Lamouille S, Derynck R. TGF-beta signaling and epithelial-mesenchymal transition in cancer progression. Curr Opin Oncol. 2013;25:76–84. doi: 10.1097/CCO.0b013e32835b6371. [DOI] [PubMed] [Google Scholar]
- 12.Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. J Clin Invest. 2009;119:1420–1428. doi: 10.1172/JCI39104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 14.Peinado H, Olmeda D, Cano A. Snail, Zeb and bHLH factors in tumour progression: an alliance against the epithelial phenotype? Nat Rev Cancer. 2007;7:415–428. doi: 10.1038/nrc2131. [DOI] [PubMed] [Google Scholar]
- 15.Vincent T, Neve EP, Johnson JR, Kukalev A, Rojo F, Albanell J, et al. A SNAIL1-SMAD3/4 transcriptional repressor complex promotes TGF-beta mediated epithelial-mesenchymal transition. Nat Cell Biol. 2009;11:943–950. doi: 10.1038/ncb1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moustakas A, Heldin CH. Induction of epithelial-mesenchymal transition by transforming growth factor beta. Semin Cancer Biol. 2012;22:446–454. doi: 10.1016/j.semcancer.2012.04.002. [DOI] [PubMed] [Google Scholar]
- 17.Xu J, Lamouille S, Derynck R. TGF-beta-induced epithelial to mesenchymal transition. Cell Res. 2009;19:156–172. doi: 10.1038/cr.2009.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ikushima H, Miyazono K. TGFbeta signalling: a complex web in cancer progression. Nat Rev Cancer. 2010;10:415–424. doi: 10.1038/nrc2853. [DOI] [PubMed] [Google Scholar]
- 19.Smith A, Teknos TN, Pan Q. Epithelial to mesenchymal transition in head and neck squamous cell carcinoma. Oral Oncol. 2013;49:287–292. doi: 10.1016/j.oraloncology.2012.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Scanlon CS, Van Tubergen EA, Inglehart RC, D'Silva NJ. Biomarkers of epithelial-mesenchymal transition in squamous cell carcinoma. J Dent Res. 2013;92:114–121. doi: 10.1177/0022034512467352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Krisanaprakornkit S, Iamaroon A (2012) Epithelial-mesenchymal transition in oral squamous cell carcinoma. ISRN Oncol 2012:681469 [DOI] [PMC free article] [PubMed]
- 22.Luo SL, Xie YG, Li Z, Ma JH, Xu X. E-cadherin expression and prognosis of oral cancer: a meta-analysis. Tumour Biol. 2014;35:5533–5537. doi: 10.1007/s13277-014-1728-0. [DOI] [PubMed] [Google Scholar]
- 23.Ren X, Wang J, Lin X, Wang X. E-cadherin expression and prognosis of head and neck squamous cell carcinoma: evidence from 19 published investigations. Onco Targets Ther. 2016;9:2447–2453. doi: 10.2147/OTT.S98577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.da Silva SD, Morand GB, Alobaid FA, Hier MP, Mlynarek AM, Alaoui-Jamali MA, et al. Epithelial-mesenchymal transition (EMT) markers have prognostic impact in multiple primary oral squamous cell carcinoma. Clin Exp Metastasis. 2015;32:55–63. doi: 10.1007/s10585-014-9690-1. [DOI] [PubMed] [Google Scholar]
- 25.Fan CC, Wang TY, Cheng YA, Jiang SS, Cheng CW, Lee AY, et al. Expression of E-cadherin, twist, and p53 and their prognostic value in patients with oral squamous cell carcinoma. J Cancer Res Clin Oncol. 2013;139:1735–1744. doi: 10.1007/s00432-013-1499-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tanaka N, Odajima T, Ogi K, Ikeda T, Satoh M. Expression of E-cadherin, alpha-catenin, and beta-catenin in the process of lymph node metastasis in oral squamous cell carcinoma. Br J Cancer. 2003;89:557–563. doi: 10.1038/sj.bjc.6601124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhao D, Tang XF, Yang K, Liu JY, Ma XR. Over-expression of integrin-linked kinase correlates with aberrant expression of snail, E-cadherin and N-cadherin in oral squamous cell carcinoma: implications in tumor progression and metastasis. Clin Exp Metastasis. 2012;29:957–969. doi: 10.1007/s10585-012-9485-1. [DOI] [PubMed] [Google Scholar]
- 28.Pectasides E, Rampias T, Sasaki C, Perisanidis C, Kouloulias V, Burtness B, et al. Markers of epithelial to mesenchymal transition in association with survival in head and neck squamous cell carcinoma (HNSCC) PLoS One. 2014;9:e94273. doi: 10.1371/journal.pone.0094273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.DID M, Pierantoni GM, Feola A, Esposito F, Laino L, DER A, et al. Prognostic significance of N-cadherin expression in oral squamous cell carcinoma. Anticancer Res. 2011;31:4211–4218. [PubMed] [Google Scholar]
- 30.Nguyen PT, Kudo Y, Yoshida M, Kamata N, Ogawa I, Takata T. N-cadherin expression is involved in malignant behavior of head and neck cancer in relation to epithelial-mesenchymal transition. Histol Histopathol. 2011;26:147–156. doi: 10.14670/HH-26.147. [DOI] [PubMed] [Google Scholar]
- 31.Yang TL, CT W, Ko JY, Wang CP, Lou PJ, Chang YL. Significance of tumor satellite variables in reflecting the epithelial-mesenchymal transition of tongue cancer. Oral Oncol. 2011;47:720–724. doi: 10.1016/j.oraloncology.2011.04.006. [DOI] [PubMed] [Google Scholar]
- 32.Soares MQ, Mendonca JA, Morais MO, Leles CR, Batista AC, Mendonca EF. E-cadherin, beta-catenin, and alpha2beta1 and alpha3beta1 integrin expression in primary oral squamous cell carcinoma and its regional metastasis. Histol Histopathol. 2015;30:1213–1222. doi: 10.14670/HH-11-616. [DOI] [PubMed] [Google Scholar]
- 33.Seki S, Fujiwara M, Matsuura M, Fujita S, Ikeda H, Asahina I, et al. Prediction of outcome of patients with oral squamous cell carcinoma using vascular invasion and the strongly positive expression of vascular endothelial growth factors. Oral Oncol. 2011;47:588–593. doi: 10.1016/j.oraloncology.2011.04.013. [DOI] [PubMed] [Google Scholar]
- 34.Seki S, Fujiwara M, Matsuura M, Fujita S, Ikeda H, Umeda M, et al. Prognostic value of podoplanin expression in oral squamous cell carcinoma--a regression model auxiliary to UICC classification. Pathol Oncol Res. 2014;20:521–528. doi: 10.1007/s12253-013-9723-0. [DOI] [PubMed] [Google Scholar]
- 35.Lindenblatt Rde C, Martinez GL, Silva LE, Faria PS, Camisasca DR, Lourenco Sde Q. Oral squamous cell carcinoma grading systems--analysis of the best survival predictor. J Oral Pathol Med. 2012;41:34–39. doi: 10.1111/j.1600-0714.2011.01068.x. [DOI] [PubMed] [Google Scholar]
- 36.Akaike H (1973) Information theory and an extension of the maximum likelihood principle. In: Petrov BN, Csaki F (eds) Proceeding of the second international symposium on information theory, Budapest, p 267–281
- 37.Ukpo OC, Thorstad WL, Zhang Q, Lewis JS., Jr Lack of association of cadherin expression and histopathologic type, metastasis, or patient outcome in oropharyngeal squamous cell carcinoma: a tissue microarray study. Head Neck Pathol. 2012;6:38–47. doi: 10.1007/s12105-011-0306-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hanemann JA, Oliveira DT, Nonogaki S, Nishimoto IN, de Carli ML, Landman G, et al. Expression of E-cadherin and beta-catenin in basaloid and conventional squamous cell carcinoma of the oral cavity: are potential prognostic markers? BMC Cancer. 2014;14:395. doi: 10.1186/1471-2407-14-395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Balasundaram P, Singh MK, Dinda AK, Thakar A, Yadav R. Study of beta-catenin, E-cadherin and vimentin in oral squamous cell carcinoma with and without lymph node metastases. Diagn Pathol. 2014;9:145. doi: 10.1186/1746-1596-9-145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Valastyan S, Weinberg RA. Tumor metastasis: molecular insights and evolving paradigms. Cell. 2011;147:275–292. doi: 10.1016/j.cell.2011.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Giampieri S, Manning C, Hooper S, Jones L, Hill CS, Sahai E. Localized and reversible TGFbeta signalling switches breast cancer cells from cohesive to single cell motility. Nat Cell Biol. 2009;11:1287–1296. doi: 10.1038/ncb1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Friedl P, Gilmour D. Collective cell migration in morphogenesis, regeneration and cancer. Nat Rev Mol Cell Biol. 2009;10:445–457. doi: 10.1038/nrm2720. [DOI] [PubMed] [Google Scholar]
- 43.Hashimoto T, Soeno Y, Maeda G, Taya Y, Aoba T, Nasu M, et al. Progression of oral squamous cell carcinoma accompanied with reduced E-cadherin expression but not cadherin switch. PLoS One. 2012;7:e47899. doi: 10.1371/journal.pone.0047899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Costa LC, Leite CF, Cardoso SV, Loyola AM, Faria PR, Souza PE, et al. Expression of epithelial-mesenchymal transition markers at the invasive front of oral squamous cell carcinoma. J Appl Oral Sci. 2015;23:169–178. doi: 10.1590/1678-775720140187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Angadi PV, Patil PV, Angadi V, Mane D, Shekar S, Hallikerimath S, et al. Immunoexpression of epithelial mesenchymal transition proteins E-cadherin, beta-catenin, and N-cadherin in oral squamous cell carcinoma. Int J Surg Pathol. 2016;24:696–703. doi: 10.1177/1066896916654763. [DOI] [PubMed] [Google Scholar]