Abstract
Dibutyl phthalate (DBP) is a widely used plasticizer, whose presence in the environment as a pollutant raises concern because of its endocrine-disrupting toxicity. Growth kinetics, glucose uptake, biodegradation constant of DBP (k), half-life of DBP biodegradation (t1/2) and percentage of removal efficiency (%E) were evaluated for Fusarium culmorum grown on media containing glucose and different concentrations of DBP (500 and 1000 mg/l). Intermediate compounds of biodegraded DBP were identified by GC–MS and a novel DBP biodegradation pathway was proposed on the basis of the intermolecular flow of electrons of the intermediates identified using quantum chemical modeling. F. culmorum degraded 99% of both 1000 and 500 mg of DBP/l after an incubation period of 168 and 228 h, respectively. %E was 99.5 and 99.3 for 1000 and 500 mg of DBP/l, respectively. The k was 0.0164 and 0.0231 h−1 for 500 and 1000 mg of DBP/l, respectively. DBP was fully metabolized to fumaric and malic acids, which are compounds that enter into the Krebs cycle. F. culmorum has a promising ability for bioremediation of environments polluted with DBP because it efficiently degrades DBP and uses high concentrations of this compound as carbon and energy source.
Electronic supplementary material
The online version of this article (10.1007/s13205-017-1065-2) contains supplementary material, which is available to authorized users.
Keywords: Constant of biodegradation, Dibutyl phthalate, Fusarium culmorum, Quantum chemical modeling, Removal efficiency
Introduction
Phthalates or esters of phthalic acid are xenobiotic organic compounds that are added to plastics to make them soft and flexible (plasticizers). Plasticizers are the largest group of additives in polymers. Low-molecular weight phthalates, such as dibutyl phthalate (DBP), are used to impart flexibility to thin films. It is one of the most abundantly produced and used plasticizers. It is used as a solvent in personal-care products (e.g., perfumes, lotions), elastomers, lacquers, explosives, printing inks, paper coatings, adhesives, resin solvents, and nail polish (Meeker et al. 2009). These compounds are not chemically bound to the polymer mesh, being released into the external environment during manufacture, use, disposal, leaking or evaporation from container or landfill sites. This has become a major current environmental concern. DBP is one of the predominant phthalate esters present in atmospheric particles as well as in fresh water and sediments (Gao and Wen 2016). At phthalates production sites, concentrations in surface water range from 1 to 220 μg/l and from 7.5 to 2045 mg/kg in sediment (Green Facts 2008). The highest levels in air occur around polyvinyl chloride (PVC) processing plants (ECB 2003). DBP can be taken up by plants and other living organisms, thereby entering the food supply in both marine and fresh environments (Muneer et al. 2001). Several studies have shown that DBP has anti-androgenic and estrogenic effects in male rats and fish and has also been reported to cause reproductive defects in humans (Chen et al. 2014; Xu et al. 2014). It has been reported that shorter ester chains such as DBP can be easier to biodegrade and mineralize than phthalates with long ester chains such as diethyl hexyl phthalate (DEHP) (Liang et al. 2008). Therefore, the occurrence of phthalate esters in the environment depends on their use, production and biodegradability. The hydrolysis and photolysis of phthalate esters are very slow. Microbial degradation plays a major role in mineralizing phthalate esters in the environment (Prasad and Suresh 2012; Pradeep et al. 2013; Ahuactzin-Pérez et al. 2014; Bouchiat et al. 2015; Meng et al. 2015). Bhardwaj et al. (2012) reported that microbial enzymes are one of the most powerful tools for biodegradation of plastics and that the activity of most enzymes is higher in fungi than in bacteria. Enzymes are able to transform pollutants and are potentially suitable to restore polluted environments (Rao et al. 2010). Esterases catalyze the hydrolysis of ester bonds of various compounds (i.e., lipids, oils, and phthalates) and break them down into the corresponding carboxylic acids (Benjamin et al. 2015). It is important to identify the pathways of biodegradation to insure that toxic compounds are not generated as a result of microbial degradation (Wackett 2014). Some species of the genus Fusarium have been shown to be highly efficient phthalate-degrading fungi due to their secretion of enzymes such as esterase (Kim et al. 2007; Obruca et al. 2012; Pradeep and Benjamin 2012; Chhaya and Gupte 2013; Aguilar-Alvarado et al. 2015; Bouchiat et al. 2015; Canavati-Alatorre et al. 2016). Quantum chemical calculations have been used to study biological systems from different approaches (Kaneco et al. 2006; Merz 2014; Ahuactzin-Pérez et al. 2016). Ahuactzin-Pérez et al. (2016) reported that calculation of the molecular quantum parameters was useful for proposing the DEHP biodegradation pathway by Fusarium culmorum. The calculation of quantum chemical parameters, such as the electrostatic potential of molecules, allows understanding of the molecular alignment and estimation of the electron transfer coefficient (ETC) (González-Pérez 2015). The quantum method, i.e., the semi-empirical parameterized model number 3 (SE-PM3), has been used to correlate molecular quantum chemical parameters and experimental data (Odunola and Semire 2007). Determination of chemical transformations helps to obtain a crucial understanding of how chemical reactions take place. In this work, the specific growth rate (µ), maximum biomass (Xmax), biodegradation constant of DBP (k), half-life of DBP biodegradation (t1/2) and percentage of removal efficiency (%E) were evaluated for F. culmorum grown on media containing glucose and different concentrations of DBP (500 and 1000 mg/l). Molecular quantum chemical parameters, such as the lowest unoccupied molecular orbital energy (ELUMO), the highest occupied molecular orbital energy (EHOMO), the electrostatic potential (Eδ), molecular partial positive charge (δ+), the molecular partial negative charge (δ−) and the energy of the band gap (EBg), were calculated to determine the ETC for each compound of DBP biodegradation, which were identified by gas chromatography coupled to mass spectroscopy (GC–MS). A biodegradation pathway of DBP based on quantum chemical modeling was proposed.
Materials and methods
Chemicals and media
DBP was purchased from Sigma (purity grade 99%). Three different kinds of liquid culture media were used: (1) glucose yeast extract (GYE), (2) GYE + 500 mg DBP/l, and (3) GYE + 1000 mg DBP/l. Each medium contained (in g/l): glucose (10), yeast extract (5), KH2PO4 (0.6), MgSO4·7H2O (0.5), K2HPO4 (0.4), CuSO4·5H2O (0.25), FeSO4·7H2O (0.05), MnSO4 (0.05), and ZnSO4·7H2O (0.001). The final pH was adjusted to 6.5 using either 0.1 M HCl or 0.1 M NaOH. All media also contained 400 µl of Tween 80 per liter. DBP (dibutyl phthalate) was added to the medium before autoclaving (boiling point 340 °C).
Strain and culture conditions
Fusarium culmorum from the culture collection of the Research Centre for Biological Sciences at Universidad Autónoma de Tlaxcala, Mexico (Fc1-CICBUAT), was used. This fungus was isolated from the pulping of the recycled paper industry in which phthalates can be present as emulsifiers in paper dyes and as adhesives for paper envelopes and inks (Aguilar-Alvarado et al. 2015). This organism was grown on malt extract agar (DIFCO) at 20 °C and kept on agar plates at 4 °C until it was needed. Erlenmeyer flasks (125 ml) containing 50 ml of culture medium were autoclaved at 120 °C for 15 min, cooled to room temperature, and then inoculated with three mycelial plugs (of 10 mm diameter) taken from the periphery of colonies of F. culmorum (7 days old) grown on potato dextrose agar (DIFCO).
Cultures were incubated for 10 days at 28 °C on a rotary shaker operated at 120 rpm. Analyses were performed on samples taken at 12-h intervals and performed in triplicate. The concentrations of DBP employed have been used previously in fungal studies (Suárez-Segundo et al. 2013).
Growth kinetics, glucose consumption and pH measurements
Biomass (X) was harvested from cultures by filtration using filter paper (pore size 20–25 µm), and the specific growth rate (μ) was calculated in terms of changes in dry weight (g/l) using the Velhurst–Pearl or logistic equation (Eq. 1), as previously reported (Ahuactzin-Pérez et al. 2016):
| 1 |
where µ is the maximal specific growth rate and Xmax is the maximal (or equilibrium) biomass level achieved when dX/dt = 0 for X > 0. The solution of Eq. (1) is as follows;
| 2 |
where, C = (Xmax − X0)/X0, and X = X0; the initial biomass value.
Estimation of kinetic parameters in the above equations was performed using a non-linear least square-fitting program “Solver” (Excel, Microsoft).
The specific biomass yield (YX/S) was calculated as the coefficient of the linear regression of biomass concentration versus substrate concentration in grams of biomass/grams of substrate consumed.
As the only reducing sugar present in the media, glucose was measured using the dinitrosalicylic acid reagent (DNS, SIGMA). Procedure details are described in our previous report (Ahuactzin-Pérez et al. 2016). The pH of the culture filtrate (CF) was measured every 12 h using a digital potentiometer (Hanna Instruments, México).
Identification of DBP biodegradation intermediates by GC–MS
Intermediate products of biodegradation of DBP were analyzed by GC–MS, using a GC/MSD Agilent 890A gas chromatograph coupled to a mass spectrometer (5975C VL MSD, USA), equipped with a triple-axis detector and a capillary Agilent column (HP-5MS, USA) with 5% phenyl methyl siloxane. 1 ml of each supernatant was previously placed at 95 °C for 2 min in a water bath to denature protein. A 2-µl aliquot of the supernatant from cultures was injected with an injection source (GC ALS) at a port temperature of 300 °C with the purge valve on (split mode), using a split ratio of 20:1 and split flow of 21.14 ml/min. Helium was the carrier gas and a flow rate of approximately 1.057 ml/min was used. The chromatographic conditions were; an initial oven temperature of 90 °C for 5 min, followed by a temperature ramp to 290 °C at 20 °C/min, and then held for 15 min using a syringe size of 10 µl in split mode to 300 °C at 20:1 in the injector. Data were analyzed with the MSD ChemStation software (Agilent Technologies), and intermediates were identified using the Nist MS 2.0 library (Ahuactzin-Pérez et al. 2016). Spectral match factors were greater than 90%.
Percentages of biodegradation and removal efficiency, and half-life of DBP
Percentage of biodegradation was determined by measuring the disappearance of DBP in the CF. The biodegradation percentage of DBP (%BDBP) in a sample was calculated as follows:
| 3 |
where C0 = initial concentration of DBP in the test solution, Ct = concentration of DBP in the test solution at time t (h).
Percentage of removal efficiency (%E) of DBP was calculated as follows:
| 4 |
where Cf = concentration of DBP at the end time (t = 240 h).
The half-life (t1/2) of DBP corresponded to the time interval of the concentration of DBP to decrease to half of its initial value (Benjamin et al. 2015). It was calculated according to the following equation:
| 5 |
where C = concentration of DBP in mg/l, t = time (h) and k = degradation constant:
| 6 |
Quantum chemical investigation
A DBP biodegradation pathway was proposed based on the compounds identified by GC–MS as intermediates from DBP and the quantum chemical parameters of each compound (according to the principles of molecular orbital theory). ELUMO, EHOMO, δ+ and δ− were calculated using the SE-PM3 as previously reported (Ahuactzin-Pérez et al. 2016). EBg, Eδ and ETC were calculated as follows;
| 7 |
Data analysis
All reported data are the mean and standard deviation of three replicates. Statistical analysis of data was performed using one-way ANOVA at the significance level of 0.05 and Tukey post-test using the Graph Pad Prism® program (San Diego, CA, USA).
Results
Growth kinetics, glucose consumption and pH of the cultures
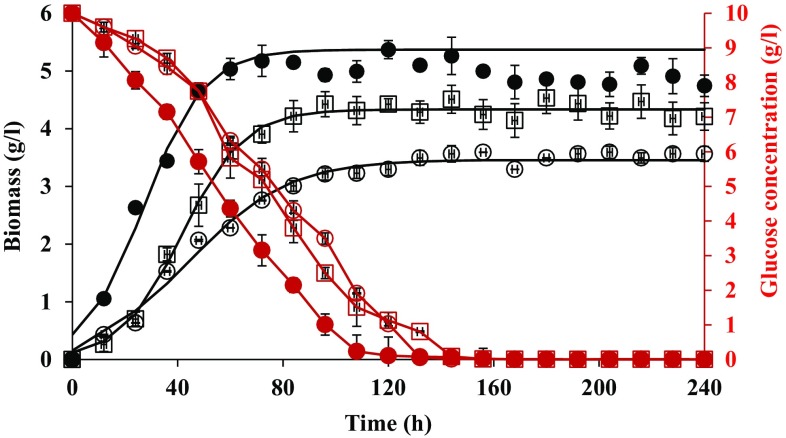
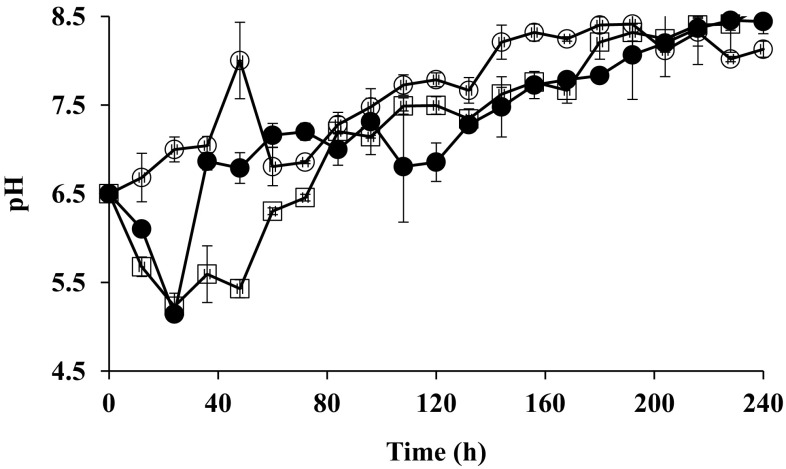
Biomass production and glucose consumption by F. culmorum on media supplemented with 500 and 1000 mg of DBP/l are shown in Fig. 1. F. culmorum produced a higher amount of biomass in medium supplemented with 1000 mg of DBP/l than in medium containing 500 mg of DBP/l. The organism reached the stationary phase after 72 and 84 h of growth in media supplemented with 1000 mg of DBP/l and 500 mg of DBP/l, respectively. Glucose was most rapidly consumed and the greatest amount of biomass was produced in the medium containing 1000 mg of DBP/l. Glucose was completely consumed by F. culmorum after 120 and 144 h in medium added with 1000 mg of DBP/l and in medium supplemented with 500 mg of DBP/l, respectively. Higher Xmax, µ and YX/S were shown in media supplemented with 1000 mg of DBP/l than in medium containing 500 mg of DBP/l (Table 1). Cultures of both DBP concentrations showed a similar pH pattern during the fungal growth. The pH of the cultures dropped from the starting pH 6.5–5.1 after 24 h. pH value of the cultures increased after 36 h of growth, reaching a pH value of 8.4 at the termination of the fermentation (Fig. 2).
Fig. 1.
Glucose consumption and biomass production by Fusarium culmorum on GYE medium (white circle), 500 (white square) and 1000 (black-filled circle) mg of DBP/l in submerged fermentation. Biomass curves were fitted (lines) using the logistic equation (Eqs. 1 and 2). Error bars are the SEM from triplicate experiments
Table 1.
Growth kinetic parameters of Fusarium culmorum grown in GYE medium and in DBP supplemented media under submerged fermentation conditions
| Growth kinetic parameters | Culture media | ||
|---|---|---|---|
| GYE | DBP (mg/l) | ||
| 500 | 1000 | ||
| Xmax (g/l) | 3.4c (0.10) | 4.3b (0.06) | 5.0a (0.21) |
| µ (h−1) | 0.05c (0.001) | 0.08b (0.006) | 0.10a (0.006) |
| YX/S (gX/gS) | 0.34c (0.01) | 0.43b (0.01) | 0.49a (0.02) |
Values are expressed as mean ± SD (n = 3); means within the same column not sharing common superscript letters (a–c) differ significantly at 5% level. Kinetic parameters (Xmax and µ) of the logistic equation were evaluated using a non-linear least squares fitting program
Fig. 2.
pH of Fusarium culmorum grown on GYE medium (white circle), 500 (white square) and 1000 (black-filled circle) mg of DBP/l in submerged fermentation. Error bars are the SEM from triplicate experiments
Percentages of biodegradation and removal efficiency of DBP
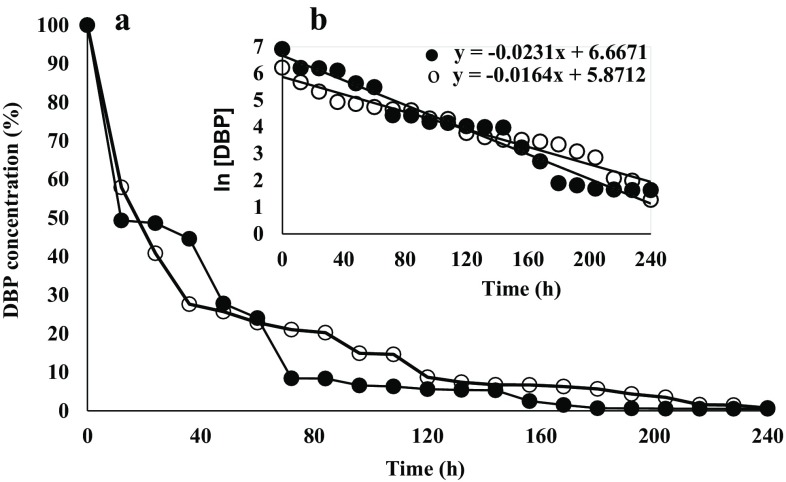
The disappearance of DBP (in percentage) during the fermentation is shown in Fig. 3a. F. culmorum degraded 99% of both 1000 mg of DBP/l and 500 mg of DBP/l after an incubation period of 168 and 228 h, respectively (Eq. 3) (Fig. 3a). The logarithm of DBP concentration versus time was plotted, and was used to calculate the first-order biodegradation constant of DBP (Fig. 3b). %E was 99.5 and 99.3 for 1000 and 500 mg of DBP/l, respectively, (Eq. 4). The k was around 50% higher for the medium supplemented with 1000 mg of DBP/l (0.0231 h−1) than that k containing 500 mg of DBP/l (0.0164 h−1) (Eq. 5). t1/2 was 42 and 30 h for 500 and 1000 mg of DBP/l, respectively, (Eq. 6) (Fig. 3b).
Fig. 3.
Biodegradation percentage of DBP by Fusarium culmorum (a) and DBP concentration (log-transformed) plotted across time used to determine k (b). Media with 500 (white circle) and 1000 (black-filled circle) mg of DBP/l added
Proposed DBP biodegradation pathway
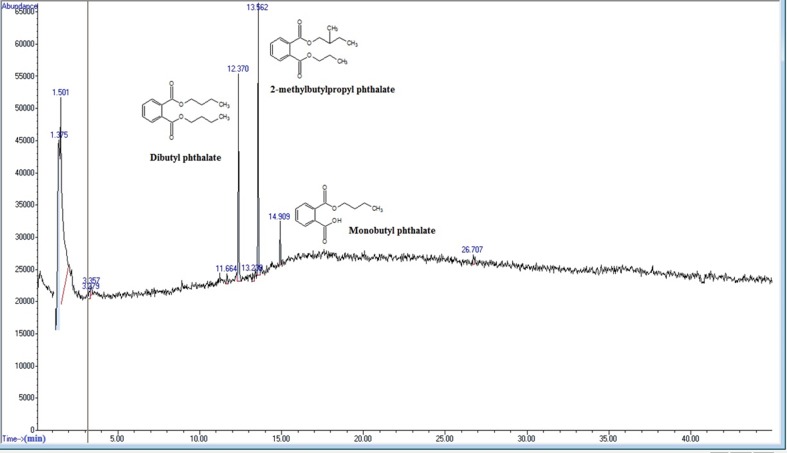
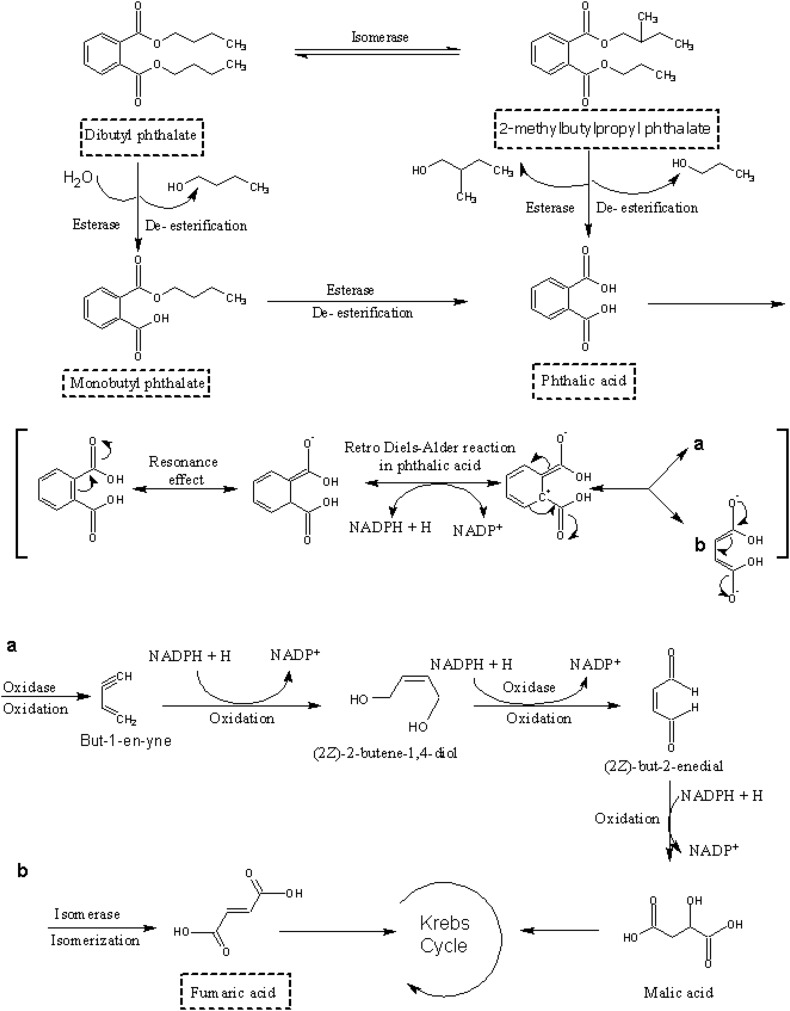
The DBP biodegradation pathway by F. culmorum was proposed on the basis of the intermolecular flow of electrons of the identified intermediate using quantum chemical modeling. Compounds identified by GC–MS (Figs. 4, 5) are shown by dashed lines and the rest of the compounds in the biodegradation pathway were proposed using quantum chemical modeling (Fig. 6). ETC values (Eq. 7) for the compounds of the DBP biodegradation pathway are shown in the table (Online Resource 1). The DBP biodegradation pathway was achieved by a DBP equilibrium isomerization reaction, with the formation of 2-methylbutylpropyl phthalate (MBPP). DBP is demethylated to produce MBPP, and the reaction moves from a less excited state to an excited state because the ETC value for DBP was lower than the ETC value for MBPP, which means that the electrons cannot easily move from DBP to MBPP. MBPP was likely metabolized by enzymatic ester hydrolysis to phthalic acid (PA). This reaction take place from an excited state to a less excited state because the ETC value for MBPP was higher than the ETC value for PA, which means that the electrons can easily move from MBPP to PA. Alternatively, DBP biodegradation seems to progress by enzymatic ester hydrolysis of DBP with the formation of monobutyl phthalate (MBP), and the reaction moves from a less excited state to an excited state because the ETC value for DBP was lower than the ETC value for MBP. This means that the electrons cannot easily move from DBP to MBP. MBP degradation would be continued by enzymatic ester hydrolysis with the production of PA. MBP had an ETC value higher than the ETC value of PA, which shows that the de-esterification occurs from an excited state to a less excited state, meaning that the electrons can easily move from MBP to PA. PA would be then metabolized to two different compounds: fumaric acid (FA) and but-1-e-yne (BE) by a retro Diels–Alder reaction and by the electron resonance effect. This reaction occurs for both compounds from a less excited state to an excited state because the ETC value for PA was lower than the ETC value for both FA and BE. This means that the electrons cannot easily move from PA to both compounds FA and BE. FA can enter into Krebs cycle to be metabolized to CO2 and H2O. On the other hand, BE would be oxidized to (2Z)-2-butene-1,4-diol (BD), and the reaction would occur from an excited state to a less excited state because the ETC value for BE was higher than the ETC value for BD, meaning that the electrons can easily move from BE to BD. BD would also be oxidized to (2Z)-but-2-enedial (BDL). This reaction moves from a less excited state to an excited state because the ETC value for BD was lower than the ETC value for BDL, meaning that the electrons cannot easily move from BD to BDL. Finally, BDL is oxidized to malic acid (MA), and the reaction occurs from an excited state to a less excited state because the ETC value for BDL was higher than the ETC value for MA. This means that the electrons can easily move from BDL to MA. MA can enter the Krebs cycle to be metabolized to CO2 and H2O (Fig. 4). In quantum chemical modeling, an enzyme should be used when a reaction occurs from a less excited state to an excited state. However, if a reaction occurs from an excited state to a less excited state, an enzyme may or may not be required.
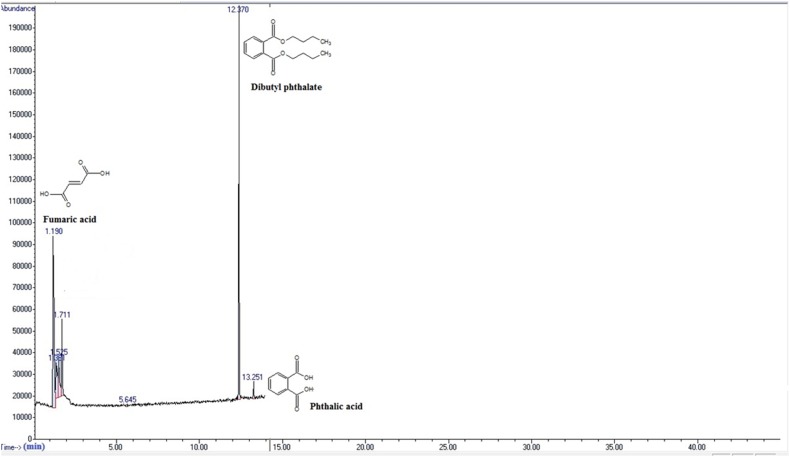
Fig. 4.
Chemical compounds produced during DBP degradation by Fusarium culmorum, as analyzed by GC/MS at a total run time of 60 min. Dibutyl phthalate (RT = 12.370 min), 2-methylbutylpropyl phthalate (RT = 13.562 min), monobutyl phthalate (R = 14.909 min)
Fig. 5.
Chemical compounds produced during DBP degradation by Fusarium culmorum, as analyzed by GC/MS at a total run time of 60 min. Fumaric acid (RT = 1.190 min), dibutyl phthalate (RT = 12.370 min), phthalic acid (RT = 13.251 min)
Fig. 6.
DBP biodegradation pathway by Fusarium culmorum proposed using quantum chemical modeling. The dashed line shows compounds identified by GC–MS
Discussion
Fusarium culmorum had higher biomass production and growth kinetic parameters in DBP-supplemented media, which shows that DBP was used by the fungus as a carbon and energy source. Biomass was much higher than the expected biomass in normal conditions. It could be due to the use of glucans (from mature hypha) by the fungus to growth (increasing biomass production). Sánchez et al. (2004) reported that hyphal ageing in the fungus involves glucan storage that is available, after cell wall lysis, as a carbon source for the growth of the fungus (at the end of the exponential phase). However, further studies on alkali-soluble cell-wall glucan (S-glucan) and alkali-insoluble cell-wall glucan (S-glucan) ratio need to be carried to corroborate this suggestion. For example, the S/R glucan ratio increased about 3- to 4-fold during fruit body formation in basidiomycetes (Schwalb, 1978). We previously reported glucose consumption and specific growth rate of F. culmorum in GYE medium (medium lacking dibutyl phthalate) (Ahuactzin-Pérez et al. 2016). In the present research, F. culmorum actively grew in media supplemented with DBP since the glucose uptake was higher in DBP-supplemented media compared to medium lacking DBP (Ahuactzin-Pérez et al. 2016). The use of phthalate as carbon source depends on the microorganism used and complexity of the phthalate structure. It has been reported than phthalates with shorter ester chains such as DBP can be easier to biodegrade and mineralize than phthalates with long ester chains such as DEHP (Liang et al. 2008). In the present study, F. culmorum reached the stationary phase after 72 h of growth in medium supplemented with 1000 of DBP/l. Ahuactzin-Pérez et al. (2016) reported that F. culmorum grown on DEHP (500 and 1000 mg/l) attained the stationary phase after 4 days of growth. Córdoba-Sosa et al. (2014) reported that the edible mushroom Pleurotus ostreatus grown on different concentrations of DEHP (750, 1200 and 1500 mg/l) reached to the stationary phase after 15 days of growth. In this study, F. culmorum degraded 99% of both 1000 mg of DBP/l and 500 mg of DBP/l after 168 and 228 h, respectively. These results show that DBP induced esterase production, which is in agreement with the increase in biomass in 1000 mg of DBP/l (Fig. 1). The significance of esterase in phthalate ester degradation and its induction by these compounds have already been reported. Córdoba-Sosa et al. (2014) reported that high concentrations of DEHP (1500 mg/l) induced esterase production, enhancing the growth of P. ostreatus. Moreover, Jin et al. (2016) reported that the DBP-degrading bacterium Gordonia sp. revealed the presence of putative hydrolase/esterase genes involved in phthalate esters. Gao and Chi (2015) reported that intracellular and extracellular esterases play key roles in the degradation of DBP. Ahuactzin-Pérez et al. (2016) found that F. culmorum degraded DEHP (initial concentration 1000 mg/l) within 224 h. In general, F. culmorum degraded higher concentrations of DBP and showed higher efficiency of removal than other organisms. Studies about DBP degradation (initial concentration 50 mg/l) by Mycobacterium sp. revealed that 80% was degraded after 5 days (Ren et al. 2016). Tang et al. (2016) found that Rhizobium sp. LMB-1 had a removal capacity of nearly 100 mg of DBP/l within 60 h. Kumar and Maitra (2016) found that Methylobacillus sp. degraded 70% of DBP (initial concentration 2000 mg/l) after 192 h. Lee et al. (2007) reported that the white rot fungus Polyporus brumalis nearly eliminated a concentration of 350 mg of DBP/l within 12 days and that the mycelial growth of the fungus was inhibited. Jin et al. (2016) found that Gordonia sp. degraded DBP (initial concentration 750 mg/l) after 32 h. It has been reported that Agrobacterium sp. completely degraded DBP within 48 h when the initial concentration was lower than 200 mg/l (Wu et al. 2011). In the present study, the k of DBP for F. culmorum was 0.0164 and 0.0231 h−1 for 500 and 1000 mg of DBP/l, respectively. Tang et al. (2016) studied DBP degradation (initial concentration 100 mg/l) by Rhizobium sp. LMB-1 and found that k, was 0.040 h−1. Ahuactzin-Pérez et al. (2016) reported that F. culmorum had a k of DEHP (initial concentration of 1000 mg/l) of 0.024 h−1. Hu et al. (2015) reported that k was 0.026 h−1 for DBP (initial concentration: 100 mg/l) in a bioaugmented reactor using Micrococcus sp. It has been reported that the k of DBP (initial concentration: 2000 mg/l) using microalgal species was 0.0169, 0.0035, 0.0034 h−1 for Cylindrotheca closterium, Dunaliella salina and Chaetoceros muelleri, respectively (Gao and Chi 2015). In the present study, %E was 99.5 and 99.3 for 500 and 1000 mg of DBP/l, respectively. Tang et al. (2016) studied DBP degradation (initial concentration 100 mg/l) by Rhizobium sp. LMB-1 and found that %E was 71.5. Ahuactzin-Pérez et al. (2016) found that the %E of DEHP (initial concentration 1000 mg/l) by F. culmorum was 99.8. Hu et al. (2015) reported that the %E was 85 for DBP (initial concentration 100 mg/l) in a bioaugmented reactor using Micrococcus sp. Microalgal species showed a %E of DBP by D. salina, C. muelleri and C. closterium of 40.0, 47.1 and 93.1, respectively (Gao and Chi 2015). In the present study, t1/2 was 42 and 30 h for 500 and 1000 mg of DBP/l, respectively. Ahuactzin-Pérez et al. (2016) reported that F. culmorum t1/2 was 28 h when the initial concentration was 1000 mg of DEHP/l. Tang et al. (2016) studied DBP degradation (initial concentration 100 mg/l) by Rhizobium sp. LMB-1 and found that t1/2 was 17 h. Fang et al. (2010) reported t1/2 by Enterobacter sp. to be about 21 h when DBP concentration was 500 mg/l. Xu et al. (2005) studied DBP biodegradation by Pseudomonas fluorescens at initial concentrations of 2.5–10 mg/l and found that t1/2 increased from approximately 14–24 h, respectively. Other methods of phthalate degradation are quite inefficient and slow. For example, it has been reported that the photodegradation and hydrolysis rates of phthalates are very slow under natural conditions. For example, butyl benzyl phthalate has an aqueous photolysis t1/2 of > 100 days, and dimethyl phthalate and DEHP have hydrolysis rates (at neutral pH) t1/2 of about 3 and 2000 years, respectively (Gao and Wen 2016). It is suggested that F. culmorum fully metabolized DBP with fumaric and malic acids as final products, which can enter the Krebs cycle. This fungus mineralized DBP, which is in accordance with alkaline pH in cultures at the end of the fermentation, since alkaline pH is observed when CO2 dissolves in H2O because it dissociates into bicarbonate ions. MBP and phthalic acid (PA) were reported as intermediate compounds found in degradation of DBP in a batch reactor bioaugmented with Micrococcus sp. (Hu et al. 2015). Kumar and Maitra (2016) also found MBP, PA and pyrocatechol as intermediate compounds during DBP degradation by Methylobacillus sp. Metabolites of degradation of DBP (initial concentration 1000 mg/l) by Rhizobium sp. were found to be diethyl phthalate (DEP), PA, dimethyl phthalate, monomethyl ester and tartaric acid, suggesting that the former metabolite can enter the Krebs cycle (Tang et al. 2016). On the other hand, the white rot fungus P. brumalis produced MBP, DEP and phthalic acid anhydride as DBP degradation products (Lee et al. 2007). Patil et al. (2006) studied DBP degradation by Delfia sp. and found MBP, phthalate and protocatechuate (PC) as intermediates, suggesting that this bacterium can mineralize PC by a meta-cleavage pathway. In this study, F. culmorum completely metabolized DBP (500 and 1000 mg/l), forming end products (fumaric and malic acids) that can enter the Krebs cycle. F. culmorum has a promising ability for bioremediation of environments polluted with DBP because it efficiently degrades DBP and uses high concentrations of this compound as carbon and energy source. For further understanding of DBP biodegradation processes, future studies need to be carried out on isolation, purification, characterization and eventual expression for large-scale production of the enzymes involved in the DBP-degradation process.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We are thankful to the Mexican Council for Science and Technology (CONACyT) for providing a doctoral scholarship (no. 351476) to Miriam Ahuactzin-Pérez.
Author contributions
MA-P; did the experimental work (Ph.D. thesis). ST-B and ES-J; drew the DBP biodegradation pathway. JG-D; did the GC–MS analysis. MG-P; did the quantum chemical studies. MCG-R; co-supervised MAP Ph.D. thesis and checked the MS. CS; co-supervised MAP Ph.D. thesis and wrote the MS.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest in the publication.
Footnotes
Electronic supplementary material
The online version of this article (10.1007/s13205-017-1065-2) contains supplementary material, which is available to authorized users.
References
- Aguilar-Alvarado Y, Báez-Sánchez MR, Martínez-Carrera D, Ahuactzin-Pérez M, Cuamatzi-Muñoz M, Sánchez C. Mycelial growth and enzymatic activities of fungi isolated from recycled paper wastes grown on di (2-ethylhexyl) phthalate. Pol J Environ Stud. 2015;24:1897–1902. doi: 10.15244/pjoes/58808. [DOI] [Google Scholar]
- Ahuactzin-Pérez M, Torres JL, Rodríguez-Pastrana BR, Soriano-Santos J, Díaz-Godínez G, Díaz R, Tlecuitl-Beristain S, Sánchez C. Fungal biodegradation of dibutyl phthalate and toxicity of its breakdown products on the basis of fungal and bacterial growth. World J Microbiol Biotechnol. 2014;30:2811–2819. doi: 10.1007/s11274-014-1705-1. [DOI] [PubMed] [Google Scholar]
- Ahuactzin-Pérez M, Tlecuitl-Beristain S, García-Dávila J, González-Pérez M, Gutiérrez-Ruíz MC, Sánchez C. Degradation of di (2-ethyl hexyl) phthalate by Fusarium culmorum: kinetics, enzymatic activities and biodegradation pathway based on quantum chemical modeling. Sci Total Environ. 2016;566–567:1186–1193. doi: 10.1016/j.scitotenv.2016.05.169. [DOI] [PubMed] [Google Scholar]
- Benjamin S, Pradeep S, Josh MKS, Kumar S, Masai E. A monograph on the remediation of hazardous phthalates. J Hazard Mater. 2015;298:58–72. doi: 10.1016/j.jhazmat.2015.05.004. [DOI] [PubMed] [Google Scholar]
- Bhardwaj H, Gupta R, Tiwari A. Microbial population associated with plastic degradation. Sci Rep. 2012;1(5):272–275. [Google Scholar]
- Bouchiat R, Veignie E, Grizard D, Soebert C, Vigier M, Rafin C. Ability of filamentous fungi to degrade four emergent water priority pollutants. Desalin Water Treat. 2015;57:1–7. [Google Scholar]
- Canavati-Alatorre MS, Águila I, Barraza-Soltero IK, Castillón E, Correa-Barrón AL, Sánchez-López E, Conde-Ávila V, González-Márquez A, Méndez-Iturbide D, Ruvalcaba D, Sánchez C (2016) Growth and cutinase activity of Fusarium culmorum grown in solid-state fermentation. Mex J Biotechnol 1(2):8–19
- Chen X, Xu S, Tan T, Lee ST, Cheng SH, Lee FWF, Xu SJL, Ho KC. Toxicity and estrogenic endocrine disrupting activity of phthalates and their mixtures. Int J Environ Res Public Health. 2014;11:3156–3168. doi: 10.3390/ijerph110303156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chhaya U, Gupte A. Possible role of laccase from Fusarium incarnatum UC-14 in bioremediation of bisphenol A using reverse micelles system. J Hazard Mater. 2013;254–255:149–156. doi: 10.1016/j.jhazmat.2013.03.054. [DOI] [PubMed] [Google Scholar]
- Córdoba-Sosa G, Torres JL, Ahuactzin-Pérez M, Díaz-Godínez G, Díaz R, Sánchez C. Growth of Pleurotus ostreatus ATCC 3526 in different concentrations of di (2-ethylhexyl) phthalate in submerged fermentation. JCBPSC. 2014;4:96–103. [Google Scholar]
- ECB (2003) European Chemicals Bureau, Summary risk assessment report (RAR 003) on dibutyl phthalate (DBP). http://www.greenfacts.org/en/dbp-dibutyl-phthalate/. Accessed 10 Jun 2016
- Fang CR, Yao J, Zheng YG, Jiang CJ, Hu LF, Wu Y, Dong-Sheng S. Dibutyl phthalate degradation by Enterobacter sp. T5 isolated from municipal solid waste in landfill bioreactor. Int Biodeterior Biodegrad. 2010;64:442–446. doi: 10.1016/j.ibiod.2010.04.010. [DOI] [Google Scholar]
- Gao J, Chi J. Biodegradation of phthalate acid esters by different marine microalgal species. Mar Pollut Bull. 2015;99(1–2):70–75. doi: 10.1016/j.marpolbul.2015.07.061. [DOI] [PubMed] [Google Scholar]
- Gao DW, Wen ZD. Phthalate esters in the environment: a critical review of their occurrence, biodegradation, and removal during wastewater treatment processes. Sci Total Environ. 2016;541:986–1001. doi: 10.1016/j.scitotenv.2015.09.148. [DOI] [PubMed] [Google Scholar]
- González-Pérez M. Methyl chloride vs ethyl chloride: a demonstration of quantum chemical theory in accordance with experimental chemical. IJAST. 2015;5:11–14. [Google Scholar]
- Green Facts (2008) Facts on health and the environment. http://www.greenfacts.org/en/dehp-dietylhexyl-phthalate/l-2/3-effects-environment.htm#3. Accessed 28 Jan 2007
- Hu J, Yang Q, Wang JL. Biodegradation of di-n-butyl phthalate in sequencing batch reactor bioaugmented with Micrococcus sp. and the bacterial community analysis. Int J Environ Sci Technol. 2015;12:2819–2828. doi: 10.1007/s13762-014-0683-z. [DOI] [Google Scholar]
- Jin D, Kong X, Liu H, Wang X, Deng Y, Jia M, Xiangyang Y. Characterization and genomic analysis of a highly efficient dibutyl phthalate-degrading bacterium Gordonia sp. strain QH-12. Int J Mol Sci. 2016;17(7):1012–1022. doi: 10.3390/ijms17071012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneco S, Katsumata H, Suzuki T, Ohta K. Titanium dioxide mediated photocatalytic degradation of dibutyl phthalate in aqueous solution—kinetics, mineralization and reaction mechanism. Chem Eng J. 2006;125:59–66. doi: 10.1016/j.cej.2006.08.004. [DOI] [Google Scholar]
- Kim YH, Seo HS, Min J, Kim YC, Ban YH, Han KY, Park JS, Bae KD, Gu MB, Lee J. Enhanced degradation and reduction of toxicity of di 2-ethylhexyl phthalate by Fusarium oxysporum f. sp. pisi cutinase. J Appl Microbiol. 2007;102:221–228. doi: 10.1111/j.1365-2672.2006.03095.x. [DOI] [PubMed] [Google Scholar]
- Kumar V, Maitra SS. Biodegradation of endocrine disruptor dibutyl phthalate (DBP) by a newly isolated Methylobacillus sp. V29b and the DBP degradation pathway. 3 Biotech. 2016;6:200–212. doi: 10.1007/s13205-016-0524-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SM, Lee JW, Koo BW, Kim MK, Choi DH, Choi IG. Dibutyl phthalate biodegradation by the white rot fungus, Polyporus brumalis. Biotechnol Bioeng. 2007;97:1516–1522. doi: 10.1002/bit.21333. [DOI] [PubMed] [Google Scholar]
- Liang DW, Zhang T, Fang HHP, He J. Phthalates biodegradation in the environment. Appl Microbiol Biotechnol. 2008;80:183–198. doi: 10.1007/s00253-008-1548-5. [DOI] [PubMed] [Google Scholar]
- Meeker JD, Sathyanarayana S, Swan SH. Phthalates and other additives in plastics: human exposure and associated health outcomes. Philos Trans R Soc B. 2009;364:2097–2113. doi: 10.1098/rstb.2008.0268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng X, Niu G, Yang W, Cao X. Di (2-ethylhexyl) phthalate biodegradation and denitrification by a Pseudoxanthomonas sp. strain. Bioresour Technol. 2015;180:356–359. doi: 10.1016/j.biortech.2014.12.071. [DOI] [PubMed] [Google Scholar]
- Merz KM. Using quantum mechanical approaches to study biological systems. Acc Chem Res. 2014;47:2804–2811. doi: 10.1021/ar5001023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muneer M, Theurich J, Bahnemann D. Titanium dioxide mediated photocatalytic degradation of 1,2-diethyl phthalate. J Photochem Photobiol A. 2001;143:213–219. doi: 10.1016/S1010-6030(01)00525-1. [DOI] [Google Scholar]
- Obruca S, Marova I, Matouskova P, Haronikova A, Lichnova A. Production of lignocellulose-degrading enzymes employing Fusarium solani F-552. Folia Microbiol. 2012;57:221–227. doi: 10.1007/s12223-012-0098-5. [DOI] [PubMed] [Google Scholar]
- Odunola OA, Semire B. Conformational analysis (semi-empirical PM3) and electronic properties of functionalized oligo (hexylpyrroles) Eur J Chem. 2007;4:363–371. [Google Scholar]
- Patil NK, Kundapur R, Shouche YS, Karegoudar TB. Degradation of a plasticizer, di n-butyl phthalate by Delfia sp. TBKNP-05. Curr Microbiol. 2006;52(5):369–374. doi: 10.1007/s00284-005-5258-2. [DOI] [PubMed] [Google Scholar]
- Pradeep S, Benjamin S. Mycelial fungi completely remediate di (2-ethylhexyl) phthalate, the hazardous plasticizer in PVC blood storage bag. J Hazard Mater. 2012;235–236:69–77. doi: 10.1016/j.jhazmat.2012.06.064. [DOI] [PubMed] [Google Scholar]
- Pradeep S, Faseela P, Sarath JMK, Balachandran S, Sudha-Devi R, Benjamin S. Fungal biodegradation of phthalate plasticizer in situ. Biodegradation. 2013;24:257–267. doi: 10.1007/s10532-012-9584-3. [DOI] [PubMed] [Google Scholar]
- Prasad B, Suresh S. Biodegradation of dimethyl phthalate, dibutyl phthalate and their mixture by Variovorax sp. Int J Environ Sci Dev. 2012;3:283–288. doi: 10.7763/IJESD.2012.V3.232. [DOI] [Google Scholar]
- Rao MA, Scelza R, Scotti R, Gianfreda L. Role of enzymes in the remediation of polluted environments. J Soil Sci Plant Nutr. 2010;10(3):333–353. doi: 10.4067/S0718-95162010000100008. [DOI] [Google Scholar]
- Ren L, Jia Y, Ruth N, Qiao C, Wang J, Zhao B, Yan Y. Biodegradation of phthalic acid esters by a newly isolated Mycobacterium sp. YC-RL4 and the bioprocess with environmental samples. Environ Sci Pollut Res. 2016 doi: 10.1007/s11356-016-6829-4. [DOI] [PubMed] [Google Scholar]
- Sánchez C, Téllez-Téllez M, Díaz G, Moore D. Simple staining detects ultrastructural and biochemical differentiation of vegetative hyphae and fruit body initials in colonies of Pleurotus pulmonarius. Lett Appl Microbiol. 2004;38(6):483–487. doi: 10.1111/j.1472-765X.2004.01517.x. [DOI] [PubMed] [Google Scholar]
- Schwalb MN. Regulation of fruiting. In: Schwalb MN, Miles PG, editors. Genetics and morphogenesis in the basidiomycetes. New York: Academic Press; 1978. [Google Scholar]
- Suárez-Segundo JL, Vázquez-López D, Torres-García JL, Ahuactzin-Pérez M, Montiel-Martínez N, Tlecuitl-Beristain S, Sánchez C. Growth of colonies and hyphal ultrastructure of filamentous fungi grown on dibutyl phthalate and di (2-ethylhexyl) phthalate. Rev Mex Ing Quim. 2013;2:499–504. [Google Scholar]
- Tang WJ, Zhang LS, Fang Y, Zhou Y, Ye BC. Biodegradation of phthalate esters by newly isolated Rhizobium sp. LMB-1 and its biochemical pathway of di-n-butyl phthalate. J Appl Microbiol. 2016;121(1):177–186. doi: 10.1111/jam.13123. [DOI] [PubMed] [Google Scholar]
- Wackett LP (2014) The metabolic pathways of biodegradation. In: Rosenberg E, DeLong EF, Stackebrandt E, Lory S, Thompson F (eds) The prokaryotes: applied bacteriology and biotechnology. Springer, Berlin, pp 383–393. 10.1007/978-3-642-31331-8_76
- Wu XL, Wang YY, Liang RX, Dai QY, Jin DC, Chao WL. Biodegradation of an endocrine-disrupting chemical di-n-butyl phthalate by newly isolated Agrobacterium sp. and the biochemical pathway. Process Biochem. 2011;46:1090–1094. doi: 10.1016/j.procbio.2011.01.031. [DOI] [Google Scholar]
- Xu XR, Li HB, Gu JD. Biodegradation of an endocrine-disrupting chemical di-n-butyl phthalate ester by Pseudomonas fluorescens B-1. Int Biodeterior Biodegrad. 2005;55:9–15. doi: 10.1016/j.ibiod.2004.05.005. [DOI] [Google Scholar]
- Xu N, Chen PY, Liu L, Zeng YQ, Zhou HX, Li S. Effects of combined exposure to 17a-ethynylestradiol and dibutyl phthalate on the growth and reproduction of adult male zebrafish (Danio rerio) Ecotoxicol Environ Saf. 2014;107:61–70. doi: 10.1016/j.ecoenv.2014.05.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.