More than any other family, the IL-1 Family of 11 cytokines and 10 receptors provides the host with an endogenous innate immune system, whereas the family of Toll-like Receptors (TLR) provides the host with an exogenous innate system. Both endogenous and exogenous families evoke nearly the same biological effects because they share the same functional Toll IL-1 Receptor (TIR) domain of their respective receptors1. In addition to innate inflammation, the IL-1 and TLR families assist the acquired immune system. Ninety-five percent of living organisms use innate immune mechanisms for survival whereas only five percent depend on T and B cell functions. This volume of Immunological Reviews begins with an Overview of the IL-1 Family with emphasis on the role of the family in the treatment of disease1. Two members of the IL-1 Family used to treat inflammatory diseases, the IL-1 receptor antagonist (IL-1Ra) and the IL-18 Binding Protein (IL-18BP), are discussed. Clinical trials with antibodies to IL-1β, IL-1α, IL-1 Receptor type 1 (IL-1R1), IL-18 and IL-33 are also discussed in the Overview. Following the Overview, the volume examines the perennial issue of the role of inflammatory cytokines as mediators of disease contrasted to the role of cytokines in host defense. The review entitled “The Role of the Interleukin-1 Family in Trained Immunity”2 provides the reader with basic concepts of how the IL-1 Family contributes to trained immunity, a component of innate immunity and sometimes termed innate memory. Trained immunity affords protection to microbial challenges before the evolution of T and B cells. In this review, there are examples of large scale epidemiologic studies of BCG vaccination as well as to basic in vitro and animal studies.
Following the review on trained immunity, there is “Interleukin1 gene polymorphism and susceptibility to disease”3. This is a comprehensive study, as it provides the reader with a condensation of a large number of single nucleotide polymorphisms (SNP’s) in the family and their relationship to disease risk. The SNP’s in IL-1 family are timely data because of the emerging role of the IL-1 family for cancer risk. The review “IL-1 and IL-1 Regulatory Pathways in Cancer Progression and Therapy”4 is a cutting-edge review by the authors who initiated the field of inflammation and cancer progression. After many years characterizing tumor-associated macrophages5, those studies have now been validated with the therapeutic success of checkpoint inhibitors in cancer therapy. With the milestone study of neutralizing IL-1β for four years6, IL-1β blockade can now be classified as a safe, non-toxic, effective checkpoint inhibitor.
The volume is weighted towards IL-1β because of the central place IL-1β holds in autophagy, the NLRP3 and AIM2 inflammasomes, the clinical efficacy of IL-1β neutralization and the potential of oral NLRP3 inhibitors to specifically reduce IL-1β-mediated inflammation and cancer progression. In addition to NLRP3 and AIM2 inflammasomes, the NLRC4 inflammasome provides new information on its role in enteric infections and IL-18. This section begins with the pivotal role of IL-1β is presented in “Autophagy Limits Activation of the Inflammasomes”7, a topic that is fundamental to the reviews on inflammasomes. There are four reviews that address the unique properties of inflammasomes. Inflammasome activation by the filamentous oligomerization of the macromolecular complex containing ASC is visible in cells and called ‘specks’. In “The Intra- and Extracellular Functions of ASC Specks”8, we learn of how specks function to promote the autocleavage of the caspase1 precursor. The NLRP3 inflammasome is affected by and regulates several cellular metabolites, an area that has recently gained attention as it links the metabolic derangements of macrophage-mediated inflammation to the metabolic derangements of cancer. The metabolic upheaval that was described by Warburg in cancer cells is reviewed in “Metabolic Regulation of NLRP3”9. In “The AIM2 Inflammasome: Sensor of Pathogens and Cellular Perturbations”10, the importance of this inflammasome to bacterial and viral diseases provides new concepts on how the cell senses endogenous DNA. The NLRC4 inflammasome has a special place in this volume. In “The NLRC4 Inflammasome”11, we read that mutations in this inflammasome results is autoinflammatory diseases mediated by IL-18.
The next section in this volume provides the reader with reviews on specific cytokines in the IL-1 family. IL-1β is discussed in the Overview and reviews on autophagy and inflammasomes. A unique role for IL-1α in disease is presented in “Function and Regulation of IL-1α in Inflammatory Diseases and Cancer”12. As the field of cytokine-based therapies continues to impact on clinical medicine, blocking IL-1α is a rapidly evolving strategy for skin diseases and cancer13–16. Although in many ways IL-18 and IL-1β are closely related, IL-18 does not phenocopy IL-1β activity. The immunological and clinical significance of IL-18 is broadly addressed in “Interleukn-18: Biological Properties and Role in Disease”17. IL-33 has special place in the IL-1 family because this cytokine was unknown for many years. Nevertheless, using IL-1 Receptor Family ST2 (now IL-1R4), considerable advances were made on IL-33 as having a major role in the Th2 responses. Similar to IL-1α, IL-33 has a role in the nucleus. These two properties of IL-33 are reviewed in “Interleukin-33: a Nuclear Cytokine from the IL-1 family”18.
Five cytokines the IL-1 cytokine family were discovered by in silico research methods using DNA databases. The IL-36 subfamily is comprised of IL-36α, IL-36β, IL-36γ as well as the IL-36 Receptor antagonis (IL-36Ra). This subfamily is reviewed in ”Regulation and Function of Interleukin-36 Cytokines19, a timely assessment of these new members of the IL-1 family. IL-37 is a unique member of the IL-1 Family, as this cytokine is a fundamental inhibitor of innate inflammation as well as of acquired immunity. Since the first assessment of the function of IL-37 in 201020, reports on IL-37 during the past 7 years has risen remarkably. An update of this cytokines is presented in “Suppression of Inflammation and Acquired Immunity by IL-37”21. IL-38 is the most recent member of the IL-1 Family to be characterized. IL-38 and IL-37 exploit the ligand binding chains of the IL-36 Receptor and the IL-18 Receptor, respectively, and recruit two previous orphan co-receptors, IL-1R8 and IL-1R9, respectively. IL-38, like IL-37, suppresses innate inflammation and its role in immune responses and inflammation are presented in “Biology of IL-38 and its Role in Disease”22.
The volume closes with two reviews on IL-1 Family of Receptors. The IL-1 receptor family has a long history that began in 1988 with the identification of the IL-1R123, followed by the report of homology of IL-1R1 with the Drosophila Toll protein24 and completed in 1995 with discovery of the co-receptor, IL-1R325. Recently, IL-1R1 binding to IL-1β and the formation of signaling complex with IL-1R3 has been resolved26. The binding of IL-1β, IL-1827 and IL-3328 to their respective ligand binding chains is similar, and in each case, ligand binding results in a conformational change in structure of the receptor, which allows the co-receptor to bind and form the trimeric complex. The ligands never make contact with their co-receptors. The entire IL-1 family of receptors is presented in a comprehensive review “The family of interleukin-1 receptors”29. In “Tuning Inflammation and Immunity by the Negative Regulators IL-1R2 and IL-1R8”30, the function of the two negative receptors of the IL-1 family and their role in health and disease are reviewed.
Figure 1. IL-1 Family Has Evolved to Balance Itself.
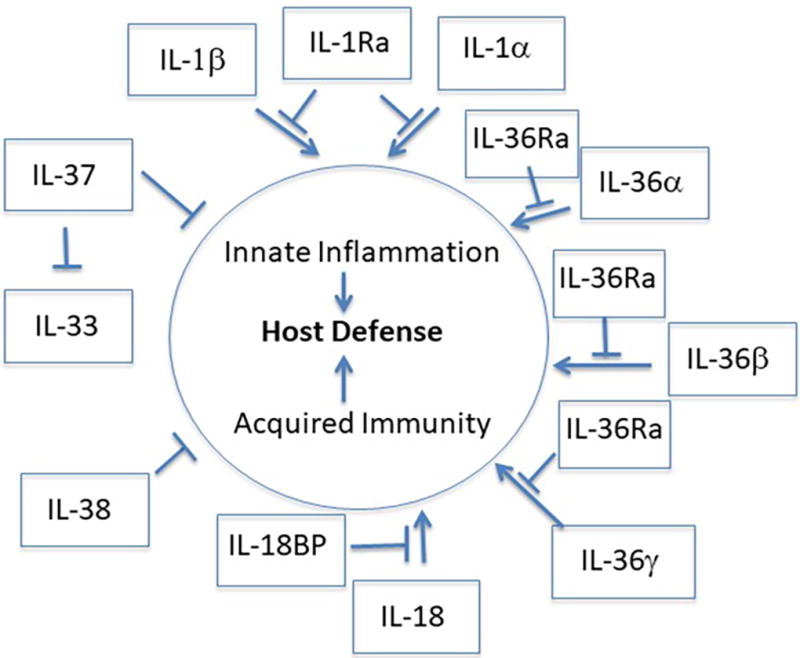
The IL-1 Family provides innate inflammation that is required for non-specific host defense as well as acquired immunity. Inflammation non-specifically contributes to host defense by increasing core temperature, white blood cell infiltration and microbial killing. Acquired immunity specifically contributes to host defense by acting as an adjuvant for Tcells and Bcells. Runaway inflammation and autoreactive Tcell activity is controlled by IL-1 Family members IL-1Ra, IL-36Ra, IL-37, IL-38 and IL-18BP.
Acknowledgments
These studies are supported by NIH Grant AI-15614
Footnotes
The Author declares no conflict of interest
References
- 1.Dinarello CA. Overview of the IL 1 Family in Innate Inflammation and Acquired Immunity. Immunol Rev. 2018;291 doi: 10.1111/imr.12621. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Netea MG. The role of the interleukin family in trained immunity. Immunolog Rev. 2018;281 doi: 10.1111/imr.12617. in press. [DOI] [PubMed] [Google Scholar]
- 3.Khaled K, Azulay E, Etti E, K B, Cohen I. Interleukin1 gene polymorphism and susceptibility to disease. Immunol Rev. 2018;281 doi: 10.1111/imr.12620. in press. [DOI] [PubMed] [Google Scholar]
- 4.Mantovani A, Barajon I, Garlanda C. IL-1 AND IL-1 Regulatory Pathways in cancer progression. Immunol Rev. 2018;281 doi: 10.1111/imr.12614. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mantovani A, Sica A, Allavena P, Garlanda C, Locati M. Tumor-associated macrophages and the related myeloid-derived suppressor cells as a paradigm of the diversity of macrophage activation. Hum Immunol. 2009;70:325–30. doi: 10.1016/j.humimm.2009.02.008. [DOI] [PubMed] [Google Scholar]
- 6.Ridker PM, MacFadyen JG, Thuren T, B E, Libby P, Glynn RJ. Anti-inflammatory therapy with canakinumab and lung cancer. New Engl J Med. 2017 in press. [Google Scholar]
- 7.Takahama M, Akira S, Saitoh T. Autophagy limits activation of 1 the inflammasomes. Immunol Rev. 2018;281 doi: 10.1111/imr.12613. in press. [DOI] [PubMed] [Google Scholar]
- 8.Franklin BS, Latz E, Schmidt FI. The intra- and extracellular functions of ASC specks. Immunol Rev. 2018;281 doi: 10.1111/imr.12611. in press. [DOI] [PubMed] [Google Scholar]
- 9.Hughes MM, O’Neill LA. Metabolic Regulation of NLRP3. Immunol Rev. 2018;281 doi: 10.1111/imr.12608. in press. [DOI] [PubMed] [Google Scholar]
- 10.Martinon F. The AIM2 Inflammasome: Sensor of Pathogens and Cellular Perturbations. Immunol Rev. 2018;281 doi: 10.1111/imr.12618. in press. [DOI] [PubMed] [Google Scholar]
- 11.Duncan JA, Canna SW. The NLRC4 Inflammasome. Immunol Rev. 2017;281 doi: 10.1111/imr.12607. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Malik A, Kanneganti T-D. Function and regulation of IL-1 in inflammatory diseases and cancer. Immunol Rev. 2018;281 doi: 10.1111/imr.12615. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kanni T, Argyropoulou M, Spyridopoulos T, et al. MABp1 Targeting Interleukin-1alpha for moderate to severe hidradenitis suppurativa not eligible for adalimumab: a randpomized study. J Invest Dermatol. 2017 doi: 10.1016/j.jid.2017.10.030. in press. [DOI] [PubMed] [Google Scholar]
- 14.Hickish T, Andre T, Wyrwicz L, et al. MABp1 as a novel antibody treatment for advanced colorectal cancer: a randomised, double-blind, placebo-controlled, phase 3 study. Lancet Oncol. 2017;18:192–201. doi: 10.1016/S1470-2045(17)30006-2. [DOI] [PubMed] [Google Scholar]
- 15.Hong DS, Janku F, Naing A, et al. Xilonix, a novel true human antibody targeting the inflammatory cytokine interleukin-1 alpha, in non-small cell lung cancer. Invest New Drugs. 2015;33:621–31. doi: 10.1007/s10637-015-0226-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hong DS, Hui D, Bruera E, et al. MABp1, a first-in-class true human antibody targeting interleukin-1alpha in refractory cancers: an open-label, phase 1 dose-escalation and expansion study. Lancet Oncol. 2014;15:656–66. doi: 10.1016/S1470-2045(14)70155-X. [DOI] [PubMed] [Google Scholar]
- 17.Kaplanski G. Interleukin-18: biological properties and role in disease pathogenesis. Immunol Rev. 2018;281 doi: 10.1111/imr.12616. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cayrol C, Girard J-P. Interleukin-33 (IL-33): a nuclear cytokine from the IL-1 family. Immunolog Rev. 2018;281 doi: 10.1111/imr.12619. in press. [DOI] [PubMed] [Google Scholar]
- 19.Bassoy EY, Towne JE, Gabay C. Regulation and function of interleukin-36 cytokines. Immunolog Rev. 2018;281 doi: 10.1111/imr.12610. in press. [DOI] [PubMed] [Google Scholar]
- 20.Nold MF, Nold-Petry CA, Zepp JA, Palmer BE, Bufler P, Dinarello CA. IL-37 is a fundamental inhibitor of innate immunity. Nat Immunol. 2010;11:1014–22. doi: 10.1038/ni.1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cavalli G, Dinarello CA. Suppression of inflammation and acquired immunity by IL-37. Immunol Rev. 2018;281 doi: 10.1111/imr.12605. in press. [DOI] [PubMed] [Google Scholar]
- 22.van der Veerdonk FL, De Graaf D, Dinarello CA. Biology of IL-38 and its role in disease. Immunol Rev. 2018;281 doi: 10.1111/imr.12612. in press. [DOI] [PubMed] [Google Scholar]
- 23.Sims JE, Smith DE. The IL-1 family: regulators of immunity. Nat Rev Immunol. 2010;10:89–102. doi: 10.1038/nri2691. [DOI] [PubMed] [Google Scholar]
- 24.Gay NJ, Keith FJ. Drosophila Toll and IL-1 receptor. Nature. 1991;351:355–6. doi: 10.1038/351355b0. [DOI] [PubMed] [Google Scholar]
- 25.Greenfeder SA, Nunes P, Kwee L, Labow M, Chizzonite RA, Ju G. Molecular cloning and characterization of a second subunit of the interleukin-1 receptor complex. J Biol Chem. 1995;270:13757–65. doi: 10.1074/jbc.270.23.13757. [DOI] [PubMed] [Google Scholar]
- 26.Wang D, Zhang S, Li L, Liu X, Mei K, Wang X. Structural insights into the assembly and activation of IL-1beta with its receptors. Nat Immunol. 2010;11:905–11. doi: 10.1038/ni.1925. [DOI] [PubMed] [Google Scholar]
- 27.Tsutsumi N, Kimura T, Arita K, et al. The structural basis for receptor recognition of human interleukin-18. Nat Commun. 2014;5:5340–12. doi: 10.1038/ncomms6340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu X, Hammel M, He Y, et al. Structural insights into the interaction of IL-33 with its receptors. Proc Natl Acad Sci U S A. 2013;110:14918–23. doi: 10.1073/pnas.1308651110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Boraschi D. The family of interleukin-1 receptors. Immunolog Rev. 2018;281 doi: 10.1111/imr.12606. in press. [DOI] [PubMed] [Google Scholar]
- 30.Garlanda C. Tuning inflammation and immunity by the negative regulators IL-1R2 and IL-1R8. Immunol Rev. 2018;281 doi: 10.1111/imr.12609. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]


