Abstract
Given the proportion of individuals with resistance to, and poor compliance or tolerance of, anti-hypertensive medication new drugs to treat this syndrome are required urgently. We show that peripheral chemoreceptors generate aberrant signalling contributing to high blood pressure in hypertension and thus reveal a novel target. We discovered that P2x3 receptor mRNA expression was up regulated substantially in chemoreceptive petrosal sensory neurones in hypertensive rats. These neurones generated both tonic drive and hyperreflexia in hypertensive (but not normotensive rats), and both phenomena were normalised by blockade of P2X3 receptors. Antagonism of P2X3 receptors also reduced arterial pressure and basal sympathetic activity and normalised carotid body hyperreflexia in conscious hypertensive rats; no effect was observed in normotensive rats. These preclinical data support the P2X3 receptor as a putative novel target for controlling human hypertension, a notion supported by our evidence of both P2X3 receptor expression in hypertensive human carotid body and the revelation of its hyperactivity.
Introduction
One third of the human population is hypertensive. In the USA, despite 75% of individuals using anti-hypertensive medication only 53% (~41 million) have blood pressure controlled1. This may, in part, reflect the significant drug adherence problem due to the asymptomatic nature of hypertension and the poorly tolerated side-effects of current medication. Significant savings in healthcare costs are predicted if blood pressure was reduced to <140/90 mm Hg2. The clear evidence that a 10 mmHg rise in diastolic BP >115/75 mm Hg doubles the risk of death from cardiovascular disease in adults over 40 years3. Further, at least 8% of an estimated 900 million hypertensive humans worldwide4 currently have developed drug-resistance5,6. Categorically, there remains an unmet clinical need for controlling blood pressure.
Aliskiren, a renin inhibitor, was the last anti-hypertensive drug introduced in 2007. This was the first new drug for thirteen years, yet its efficacy beyond the existing renin-angiotensin–aldosterone enzyme inhibitors and receptor blockers is questionable, and associated adverse effects question its long-term benefit-harm relationship7. The paucity of novel pharmacological anti-hypertensives has led to a series of device based interventional studies including renal denervation8, stimulation of carotid baroreceptors9, deep brain stimulation10 and arterial venous anastomosis11,12. Here, we introduce a novel mechanistic approach using a highly selective small molecule antagonist, and provide the pre-clinical and clinical data that justifies its use in a future clinical trial in hypertension.
Currently the carotid body is being considered as a novel therapeutic target for cardiovascular disease: (i) the carotid body chemoreflex-evoked sympathoexcitatory response is potentiated in both a pre-clinical model of hypertension - the spontaneously hypertensive (SH) rat - and humans with hypertension13–18; (ii) denervation of the carotid body is evidently an effective way to control both the development and maintenance of high blood pressure in the SH rat19,20. We concluded that in conditions of hypertension the carotid body chemoreceptors generate aberrant excitatory tone driving up sympathetic activity and causing high blood pressure14,21. We found that by switching off this carotid body tone using either hyperoxia or nerve section to disconnect the carotid body from the brain, arterial pressure and sympathetic activity were reduced in SH rats, but were unaffected in a normotensive strain14. In hypertensive human, hyperoxia lowered sympathetic activity18 and carotid body resection can reduce arterial pressure22. Thus, the animal and human data support the presence of aberrant carotid body discharge contributing to hypertension. Although the mechanistic basis for the carotid body aberrant tone is unknown, it may be pivotal for refining new pharmacological anti-hypertensive approaches as we propose here.
It is acknowledged that a plethora of mechanisms governs carotid body signalling23–27. We have considered in our current investigations the ATP gated ion channels (called purinergic P2X receptors), specifically the C-fiber localised P2X3 receptor subtypes, which are commonly associated with afferent sensitisation, and that apparently may contribute to hyperreflexic disease states in a variety of organs28,29. ATP is one of a number of transmitters involved in the transduction process of hypoxia in the carotid body30–35. P2X3 receptor expression is present in both carotid bodies and petrosal ganglia neurones of normotensive rats36. Moreover, combined P2X2 and P2X3 subunit deletion reduced the ventilatory response to hypoxia in mice37. We hypothesised that P2X3 receptors contribute to both the chemo-hyper reflexia and aberrant discharge of carotid body activity in hypertension and predict that their blockade would cause an anti-hypertensive effect. To address this, we have used highly selective P2X3 receptor antagonists. Herein, we demonstrate the translatability of our pre-clinical findings to hypertensive humans.
Results
Hyperactivity of carotid bodies in hypertensive rats
Carotid body afferent discharge was recorded from the carotid sinus nerve of the in situ preparation. Both basal discharge and chemoreflex evoked volleys (NaCN used to simulate hypoxia, 15–30 μg i.a.) were elevated in hypertensive versus normotensive rats (SH vs. Wistar: basal, 24.9 ± 2 vs 1.4 ± 0.3 spikes/s, respectively, P < 0.001; chemoreflex, 62.3 ± 3 vs 28.3 ± 2 spikes/s, respectively; P < 0.001, Fig. 1a – c). Next, low dose dopamine was used to inhibit carotid body discharge39. In SH rats in vivo, dopamine infusion (10 μg/kg/min i.v. as per39) depressed respiration, an effect not observed in Wistar rats (Fig. 1d), indicating the presence of carotid body tonicity in the hypertensive animals. Intracellular recordings revealed that physiologically characterised chemoreceptive primary afferent petrosal ganglion neurones from hypertensive rats in situ were more depolarised (−51.5 ± 0.9 vs −55.7 ± 1 mV; P < 0.001, Fig. 2a,c and Supplementary Fig. 1a), exhibited tonic firing (1.5 ± 0.8 vs 0 Hz; Fig. 2a) and displayed an enhanced chemoreflex evoked firing response to an equivalent dose of sodium cyanide (22.5 μg) compared to normotensive rats (123 ± 5 vs. 52 ± 3 spikes; P < 0.001, Fig. 2a,d). In sum, compared to the normotensive rat, the carotid body of SH rats is hyperactive generating both aberrant tone and hyperreflexia.
Figure 1.
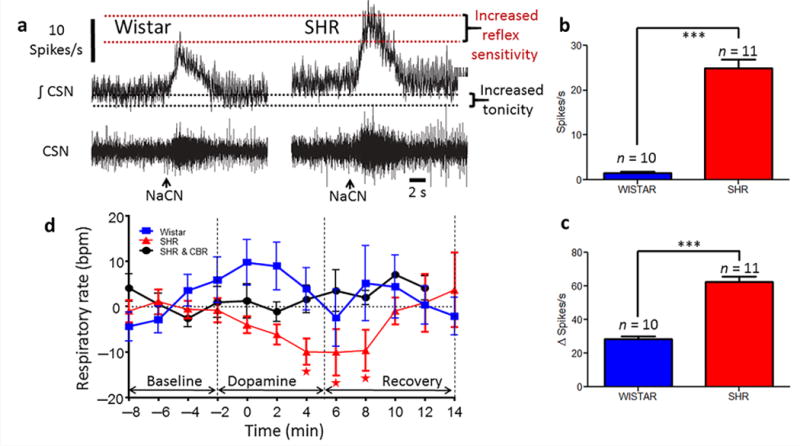
Overactive peripheral chemoreceptors in spontaneously hypertensive (SH) rats. Original recordings of carotid sinus nerve (CSN; (a)) activity showing both basal discharge (a,b) and that evoked reflexly by stimulating the carotid body with sodium cyanide (a,c; 22.5 μg NaCN i.a., arrowed in a) in SH and Wistar rats. CSN data from the in situ perfused preparation. (one-way ANOVA Bonferroni post-test; n = 10 or 11, ***P < 0.001). (d), dopamine infusion (10 μg/kg/min i.v.) while recording ventilation frequency in conscious radio-telemetered Wistar and SH rats (n = 5 each) before and after selective carotid body resection (SHR CBR). One-way ANOVA Dunnett’s post-test. * P < 0.05. ***P < 0.001. All data are mean ± s.e.m.
Figure 2.
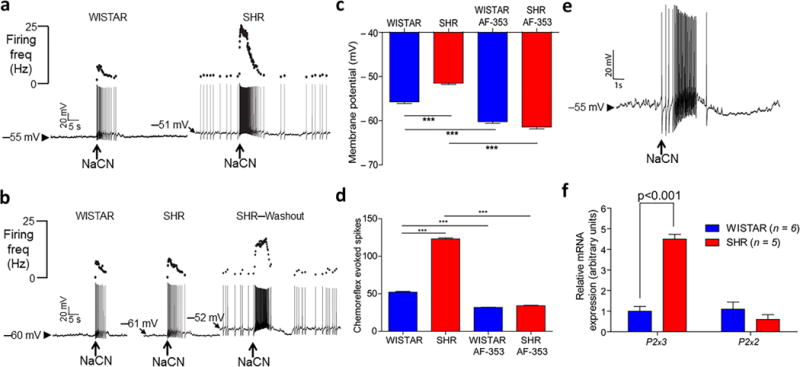
P2X3 receptor mediated hyperreflexia and tonicity of chemoreceptive petrosal neurones in spontaneously hypertensive (SH) rats are associated with upregulation of P2x3 receptor mRNA. (a), two representative whole cell patch clamp recordings from chemoreceptive petrosal neurones from a Wistar (left) and SH rat (right) recorded in the in situ preparation. Ongoing discharge, membrane potential and reflex evoked responses to carotid body stimulation (NaCN, sodium cyanide, 22.5 μg i.a. arrowed) were compared between rat strains. (b), responses of neurones in (a) following P2X3 receptor blockade with AF-353 (20 μM, 20 nl), which was delivered focally into the carotid body by picoinjection. Effects in SH rats were reversed upon washout. Grouped mean data summarising rat strain related differences and responses in membrane potential (c; see also Supplementary Fig. 1a,b) and chemoreflex evoked firing responses (d) before and after P2X3 receptor antagonism. Data in (c) are mean ± s.d. and those in (d) are mean ± s.e.m. One-way ANOVA Bonferroni post-test (n = 12 SH, n = 10 Wistar rats). The difference in expression of P2x3 and P2x2 receptor mRNA from petrosal chemoreceptive neurones (identified using sodium cyanide, NaCN, 22.5 μg, i.a.) during whole cell patch (e) was revealed using single cell PCR ((f); n = 5 or 6). Two-way ANOVA Bonferroni post-test. Data in (f) are mean ± s.e.m and were generated from the in situ arterially perfused preparation. *** P < 0.001.
Chemoreceptive petrosal neurone excitability in SH rats
We next compared the electrical excitability of chemoreceptive petrosal neurones and found that SH rats exhibited enhanced excitability to injected current pulses; for example, a 1nA current pulse evoked 25 ± 1.1 spikes vs 15 ± 1.7 spikes in Wistar rats (Supplementary Fig. 2a,b, n = 6; P < 0.05). Neither the membrane input resistance of these cells (119 ± 3 vs 117 ± 9 MΩ; Supplementary Fig. 1c) nor whole cell capacitance was different between rat strains (Supplementary Fig. 1d).
Normalizing petrosal neurone excitability in SH rats
As P2X3 receptors are found in the carotid body of normotensive rats36 and associated with pathological afferent sensitisation in numerous other organs28,29,33 we assessed whether they were involved in carotid body dysfunction in SH rats in situ. In SH rats, focal delivery via a glass micropipette inserted into the ipsilateral carotid body of a highly selective non-competitive P2X3 receptor antagonist (AF-353; 15 nl, 20 μM40) caused hyperpolarisation (−9.9 ± 2.1 mV; n = 12, P < 0.001, Fig. 2b,c), total abolition of ongoing activity (Fig. 2b) and reduced chemoreflex sensitivity of petrosal chemoreceptive neurones (from 123 ± 5 to 34 ± 2 spikes; n = 12, P < 0.001, Fig. 2b,d). In normotensive animals in situ, AF-353 also caused hyperpolarisation (−4.4 ± 1.8 mV; n = 10, P < 0.001, Fig. 2c) and reduced the chemoreflex evoked volley from 52 ± 3 to 32 ± 2 spikes; n = 10, P < 0.001, Fig. 2b,d). Both the magnitude of the hyperpolarisation and reduction in chemoreflex evoked spiking were greater in SH versus Wistar rats (P < 0.001, Fig. 2c,d). Notably, after P2X3 receptor antagonism, carotid body activation yielded comparable firing responses in petrosal neurones between rat strains (SH: 34 vs. Wistar 32 spikes; NS), which was reversible in SH rats (Fig. 2b). After AF-353 treatment the intrinsic firing response to injected depolarising current pulses was reduced in SH (e.g. at 1 nA: 25 ± 1 to 19 ± 1 spikes; P < 0.05, Supplementary Fig. 2a,b) but not Wistar rat neurones (15 ± 2 to 12 ± 2, NS). We next tested whether P2X3 receptors were upregulated in SH rat petrosal neurones.
Upregulated P2X3 receptors in petrosal neurones of SH rats
Single cell PCR analysis of characterised chemoreceptive petrosal ganglion neurones recorded from SH rats in situ revealed a >4 fold up regulation of P2x3 receptor mRNA relative to Wistar rats (Fig. 2e,f; n = 6, P < 0.001); this was confirmed at the protein level using western blot performed on carotid bodies (Fig. 3a). Interestingly, no change was found in P2x3 (or P2x2) receptor mRNA expression in non-chemoreceptive petrosal neurones (Supplementary Fig. 2e) indicative of specificity to chemoreflex neurones. There was no rat strain related change in: P2x2 receptor (Fig. 2f) and tyrosine hydroxylase (a known marker of glomus cells) mRNA expression in petrosal chemoreceptive neurones of SH rats (Supplementary Fig. 2c) or in chemoreceptive neurones located in the nucleus tractus solitarii (Supplementary Fig. 2f). Fig. 3b shows co-localisation of P2X3 receptors with tyrosine hydroxylase immunopositive glomus cells (and axons, Supplementary Fig. 3a,b). Based on the up regulation of P2x3 receptors in the petrosal neurones of SH rats, we tested whether there was a difference in sensitivity of these neurones to ATP between the rat strains.
Figure 3.
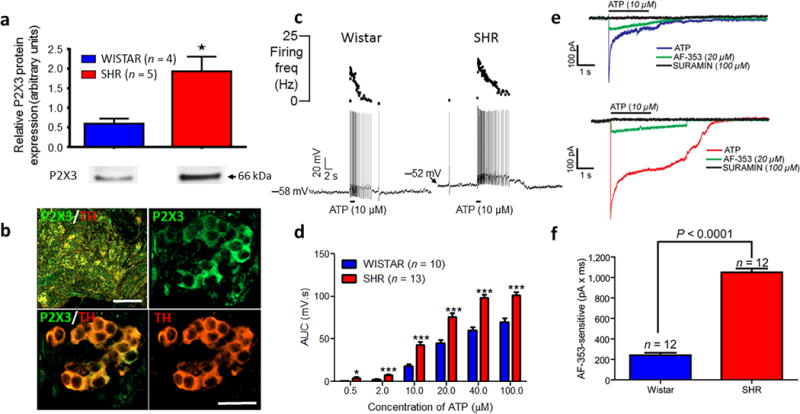
In spontaneously hypertensive (SH) rats P2X3 receptor protein was upregulated in carotid body and chemoreceptive petrosal neurones were sensitised to ATP relative to Wistar rats. (a), difference in P2X3 receptor protein in the carotid body of Wistar relative to SH rats (Mann–Whitney t-test; n = 4 or 5). (b) P2X3 receptor immunofluorescence labelling on glomus cells identified by the presence of tyrosine hydroxylase (TH; n = 3 Wistar, n = 3 SH rats). See, Supplementary Fig. 10 for control data on the specificity of the P2X3 receptor antibody. Scale bars 100 μm (top left) and 25 μm all others. (c), ATP microinfused into the carotid body while whole cell recording (current clamp) from petrosal chemoreceptive neurones in Wistar (n = 10) and SH rats (n = 13). (d), rat strain comparison of the ATP (applied to the carotid body) evoked membrane depolarisations from petrosal chemoreceptive neurones in both rat strains. AUC, area under the curve. (e), voltage clamp data indicating the ATP (applied to the carotid body) evoked inward current from identified petrosal chemoreceptive neurones before and after application to the carotid body of the P2X3 receptor antagonist (AF-253, 20 μM) and the non-selective P2X receptor antagonist, suramin (100 μM) in Wistar (top traces; n = 12) and SH rats (bottom traces; n = 12). (f), rat strain differences in the AF-353 sensi tive ATP evoked inward current. Neurophysiological data were from the in situ arterially perfused preparation and comparisons made using an unpaired t-test. All data are mean ± s.e.m. * P < 0.05; *** P < 0.001.
Sensitisation of SH rat chemoreceptive petrosal cells to ATP
Application (15 nl) of ATP or α–β–methylene-ATP focally to the carotid body produced dose-dependent increases in the firing and membrane depolarisation of petrosal neurones. Notably, the petrosal neurones in the SH rat were more sensitive than those from Wistar rats: for ATP the EC50% SH vs Wistar rat was 8.8 ± 2.2 vs 17.2 ± 1.3 (P < 0.001; Fig. 3c,d) and for α–β–methylene-ATP the EC50% SH vs Wistar rat was 9.4 ± 2.1 vs 16.2 ± 2.4 (P < 0.05; Supplementary Fig. 4a,b). These findings were consistent with greater P2X3 receptor-sensitive inward current evoked by ATP in chemoreceptive petrosal neurones (Fig. 3e,f). Next, we tested if antagonism of carotid body P2X3 receptors lowered arterial blood pressure in SH rats.
Blocking P2X3 receptors systemically is anti-hypertensive
One hour infusions of a highly selective non-competitive P2X3 receptor antagonist,AF-219, were made at 1, 4 and 8 mg/kg/h, i.v. This compound was preferred to the structurally related, more lipophilic antagonist AF-353, as AF-219 has been used clinically for other indications in humans29, and since it does not cross the blood brain barrier significantly its use in vivo was preferable. We performed a PK analysis to assess plasma concentrations in SH rats (Fig. 4a and Supplementary Fig. 5) and subsequently correlated these with the falls in systolic blood pressure (Fig. 4a). In SH rats, basal arterial pressure was 151±3/109±3 (SBP/DBP) mmHg. AF-219 produced dose182 dependent falls in arterial pressure (Fig. 4a); no response was observed in Wistar rats (Fig. 4b; Supplementary Fig. 6). At a dose of 8mg/kg/h SBP/DBP in SH rats fell by - 28±3/−26±4 mmHg (Fig. 4a; n = 7, P < 0.001). Heart rate and respiratory frequency also decreased (by −38±8 and −12±3/min, respectively; Supplementary Fig. 6, n = 7, P < 0.01). Interestingly, after carotid body resection a residual fall of −17±3 mmHg in SBP persisted with AF-219 infusion in SH rats (30 min, 8mg/kg/h; Fig. 4b, n = 7, P < 0.05; Supplementary Fig. 7). The chemoreflex evoked increase in arterial pressure in conscious SH rats was also reduced after systemic blockade of P2X3 receptors by AF-219 (bolus doses given i.v. >1 mg/kg; n = 4, P < 0.05; Fig. 4c). We next determined the mechanisms by which arterial pressure was lowered in SH rats by P2X3 receptor blockade.
Figure 4.
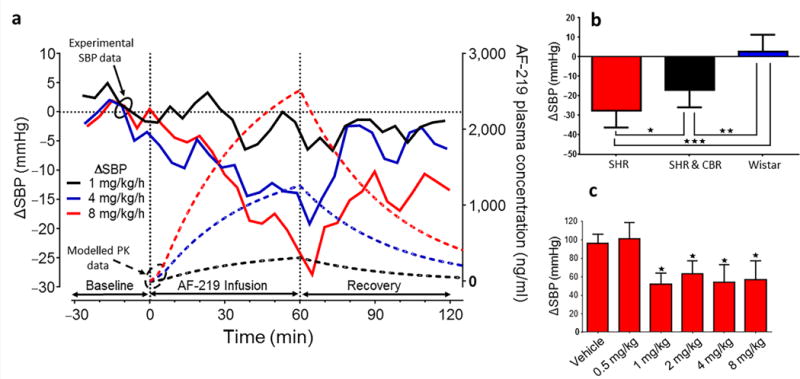
P2X3 receptor antagonism lowers arterial pressure in conscious spontaneously hypertensive (SH) rats. (a), Intra-venous infusions of a P2X3 receptor antagonist (AF-219, 1–8 mg/kg/h) showing dose-dependent responses in systolic blood pressure (SBP). Solid lines indicate the blood pressure responses and dotted lines the predicted PK value based on plasma sampling (see also Supplementary Fig. 5). (b), Comparison of the SBP responses induced with 8 mg/kg/h AF-219 i.v. in SH (n = 7) and Wistar rats (n = 7), and in SH rats before and after carotid body resection (CBR; n = 7). (c), the peripheral chemoreflex evoked pressor responses in SBP during infusion of vehicle and 0.5–8 mg/kg/h AF-219. Data from seven conscious radio-telemetered rats are mean ± s.e.m. One-way ANOVA Dunnett’s post-test. * P < 0.05, ** P < 0.01, *** P < 0.001.
P2X3 receptor blocker reduces sympathetic tone in SH rats
We evaluated the effect of P2X3 receptor antagonism on cardiovascular autonomic activity. Ongoing basal levels of thoracic SNA recorded in situ were reduced from 20.8±2.2 to 13.7±2.5 μV (Fig. 5a–c; n = 12, P < 0.001) following focal delivery of AF-353 (15 nl, 20 μM) to carotid bodies bilaterally in SH rats. This reduced value was similar to levels in normotensive rats either before (12.3±2.6 μV) or after AF-353 application (11.7±2.3 μV, Fig. 5c; n = 12, NS). Chemoreflex evoked increases in expiratory modulated SNA were 209±12% and 131±11% for SH and Wistar rats respectively, and after AF-353 were reduced significantly to 76±11% and 69±7.8% in SH and Wistar rats, respectively (Fig. 5a–e, n = 12, P < 0.001); these new basal values were not different to one another. Following drug washout, the depressant effects on SNA was reversed (basal and reflex evoked; Fig. 5b).
Figure 5.
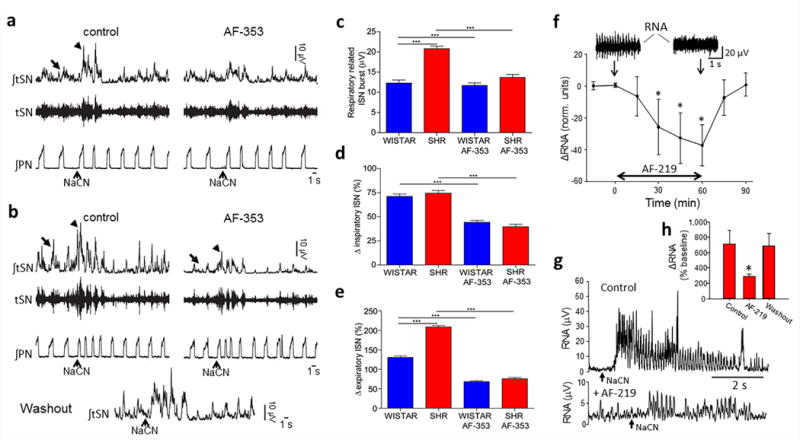
The anti-hypertensive action of P2X3 receptor antagonism is associated with a reduction in sympathetic activity in spontaneously hypertensive (SH) rats in situ and in vivo. A comparison of the effect of a P2X3 receptor antagonist (AF-353; 20 μM, 15 nl) applied locally to both carotid bodies on the ongoing thoracic chain sympathetic activity (arrow, raw: tSN; integrated: ∫tSN) and the sympathetic nerve reflex response (arrow head) to peripheral chemoreceptor stimulation (evoked by NaCN, 22.5 μg i.a.) in 12 Wistar (a) and 12 SH rats (b). The effect of P2X3 receptor antagonism within both carotid bodies on the basal (c), inspiratory- (d) and expiratory (e) modulated tSN for both rat strains is shown (n = 12 each). Inspiratory and expiratory modulation of tSN was determined using averaged triggering from the phrenic nerve (PN). One-way ANOVA Bonferroni post-test; data are mean ± s.e.m. from in situ rat preparations (n = 12 each strain). (f), renal sympathetic nerve activity (RNA) recorded from conscious radio697 telemetered in vivo SH rats (n = 5) during infusions of AF-219 (8 mg/kg/h i.v.). Note the similar time course of the response in RNA to the fall in systolic blood pressure depicted in Fig. 4(a). A representative example of the peripheral chemoreflex evoked sympathoexcitatory response (NaCN 22.5 μg i.a.) in a conscious radio-telemetered SH rat before and after AF-219 (g) with mean data shown in (h), which includes drug washout data. Repeated measures one-way ANOVA, with Holm-Sidek post-hoc comparison. n = 5, * P < 0.05, *** P < 0.001.
We replicated these data using TNP-ATP (Supplementary Fig. 8). a high affinity, non208 selective P2X receptor antagonist that inhibits P2X1, P2X3 and heteromeric P2X2/3 with a 1000-fold selectivity over P2X2, P2X4 and P2X7 receptors that is structurally distinct to AF-353. This evidence supports AF-353 blocking P2X3 homomeric and P2X2/3 heterotrimeric receptors rather than non-selective actions on ionic conductances and/or other receptors.
Recordings were made from renal sympathetic nerves using radio-telemetry in conscious SH rats in vivo during chronic systemic P2X3 receptor antagonism using AF-219. This reduced both the basal renal sympathetic activity (−37±13 normalised units relative to baseline, Fig. 5f; P < 0.05) and the chemoreflex evoked increase in renal sympathetic activity (Fig. 5g,h; P < 0.05). Spontaneous renal sympathetic baroreflex gain was enhanced after AF-219 (0.14±0.07 vs 0.01±0.02 %.mmHg−1; P < 0.05) but not vehicle infusion. Although no changes in autonomic activity to the heart were detected in conscious SH rats after AF-219 infusion (Supplementary Fig. 9a,b), spontaneous cardiac baroreflex was increased (0.13±0.04 to 0.27±0.05 ms/mmHg, Supplementary Fig. 9c; P < 0.001). We next tested the translatability of the SH rat data and potential use of P2X3 receptor antagonist in human hypertension.
Translating carotid body P2X3 receptors to human hypertension
If P2X3 receptors within the carotid body are to be considered a novel anti228 hypertensive target in humans, then evidence for their presence and pharmacokinetics of suitable antagonist are essential first steps. Carotid bodies were obtained from cadavers and processed for P2X3 receptors immunocytochemistry (n = 5) and western blot (n = 4). P2X3 receptor antibody specificity was determined (Supplementary Fig. 10). P2X3 receptor expression was prevalent in human carotid body (Fig. 6a and Supplementary Fig. 11a) showing co-expression with tyrosine hydroxylase, a marker for glomus cells. Axons within the carotid body also expressed P2X3 receptors (Supplementary Fig. 11b). This expression pattern was similar to that seen in the SH rat (Fig. 3b).
Figure 6.
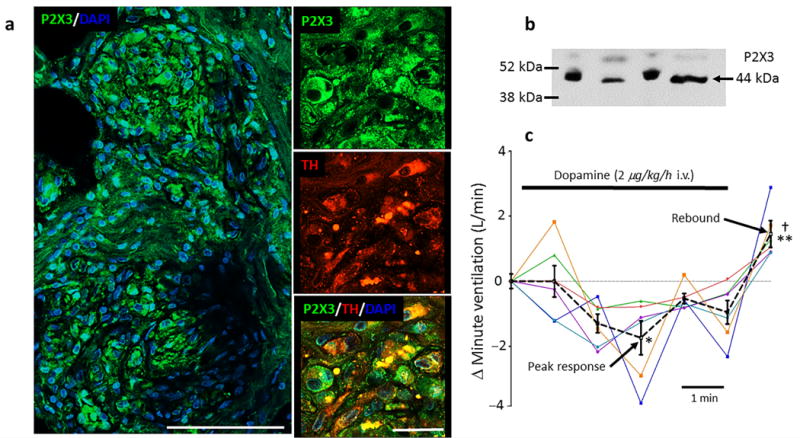
P2X3 receptor expression and aberrant tone generation in hypertensive human carotid bodies. (a), on the left is a section of a carotid body from a human cadaver showing P2X3 receptor immunofluorescence (green) with the nuclear stain - DAPI (blue; scale bar 100 μM). On the right, high power confocal images showing co-localisation of P2X3 receptors, tyrosine hydroxylase (TH) and DAPI (scale bar 25 μM). Data repeated in five human carotid bodies. See Supplementary Fig. 10 for P2X3 receptor antibody control and Supplementary Fig. 11 for axonal labelling. (b), western blot of the P2X3 receptor protein in human carotid body (n = 4). (c), low dose dopamine infusion (2 μg/kg/h i.v.) was used to inactivate the carotid bodies in six awake hypertensive humans (each colour coded) while recording minute ventilation in the supine position. A dextrose vehicle infusion was used as control and reference at time zero. The mean (± s.e.m.) response is indicated by the black dotted line. Note appearance of a rebound hyperventilatory response post-dopamine infusion. One-way ANOVA Bonferroni post-test. * P < 0.05 vehicle versus peak depression; ** P < 0.01, depression versus rebound (mean of 5 min); † P < 0.05, vehicle versus rebound.
With the presence of P2X3 receptors in hypertensive human carotid body, we next assessed whether there was tonicity in the carotid body of hypertensive humans by chemical and reversible inactivation of it. In six hypertensive humans, minute ventilation was measured during low dose dopamine infusion (5 min; 2 μg/kg/min i.v.) as described previously to inactivate the carotid body38. A bi-phasic response in minute ventilation to dopamine was observed (Fig. 6c) comprising a depression (−1.28± 0.52 L/min; P < 0.05) and over shoot following dopamine (1.28± 0.41 L/min; P < 0.05) relative to vehicle infusion. These data indicate that the carotid body of hypertensive humans can generate tonic drive and that dopamine infusion allows identification of it.
Discussion
Many transmitter mechanisms mediating reflex responses to hypoxia have been described within the carotid body and may contribute to its sensitisation in disease states; these include: TASK and ASIC channels14,25, hydrogen sulphide23,24, carbon monoxide24, nitric oxide24 and carotid body blood flow27. However, in vitro studies have also shown a major role for ATP release from glomus cells in response to hypoxia in both rat30, cat31 and human35. Whether there is a link between ATP release and any of the aforementioned mechanisms remains an open question. Moreover, P2X2 and P2X3 receptor subunits were both found to be present in the rat carotid body36 and using co257 cultures of glomus and petrosal cells in vitro Nurse and colleagues demonstrated that ATP acted via P2X receptors to excite petrosal neurones36. This evidence was based on using suramin, a weak and non-selective P2X antagonist. P2X2-containing channels (P2X2 homotrimers and/or P2X2/3 heterotrimers) likely dominate the normal physiological ventilatory response to hypoxaemia as the P2X2 single and P2X2 + P2X3 double KO mice, but not the P2X3 single KO, showed ventilatory hyporeflexia37. Thus, P2X3 subunit expression changes seem associated with pathological sensitisation of carotid body reflexes.
In the present study, using selective antagonists, we found that both exogenous and endogenous ATP released from the carotid body can act on P2X3 subunit containing receptors on petrosal neurons. A caveat is that we cannot fully establish the relative contribution of homotrimeric P2X3 versus heterotrimeric P2X2/3 receptors in such responses, although the antagonists we used have higher affinity for P2X3 homotrimeric receptors28, and a P2X3 heterotrimeric upregulation in carotid body could account for increased ATP sensitivity per se, as this channel form in recombinant studies displays significantly lower EC50 values to the nucleotide40. Our voltage clamp data indicate a non-desensitising current to α-β-methyl ATP and probable involvement of P2X3 homomeric and P2X2/3 heteromeric receptors. Our data provide the first evidence that P2X3 receptors in the carotid body play a vital role in controlling its reflex sensitivity in SH but not normotensive rats.
Up-regulation of P2X3 receptors in the carotid body appears, in part, causal for the aetiology of the tonicity, hyperreflexia of chemoreceptive petrosal neurones and downstream hyperactivity of the sympathetic nervous system in SH rats. We acknowledge that chemoreceptive petrosal neurones may have a larger receptive field in the carotid body of SH vs Wistar rats contributing to their hyperactive state. Notably, upregulated P2x3 receptor mRNA was exclusive to chemoreceptive petrosal neurons (i.e. not upregulated on non-chemoreceptive petrosal cells). The carotid body tonicity we report from in situ preparations (1.5 Hz per petrosal neuron) most likely underestimates that in conscious SH rats as recordings were made in hyperoxia, a condition known to temper carotid body discharge14,20; this may also explain the dearth of activity in neurones recorded from Wistar rats. Whether P2X3 receptor up290 regulation alone is sufficient for the pathological carotid body signalling in the SH rat (without the need for increased ATP release or reduced ATP clearance) is unclear. However, given that the microvasculature of the carotid body is hypertrophied in the SH rat41 and the possibility that arterioles feeding the carotid body receive high sympathetic drive41, the tissue is likely to be hypoxic/hypercapnic – a condition known to induce ATP release42. Additionally, inflammation, known to be present in the carotid body of SH rats41, will also drive ATP release43.
The association of P2X3 receptor up-regulation and aberrant afferent signalling is not new28 but consistent with sensitisation of visceral sensing mechanisms from, for example, the bladder44,45, urinary tract46, larynx29, lungs47, gastro-intestinal-tract48,49 and skeletal muscle50 in numerous diseased states including chronic pain51. Elucidation of the mechanisms controlling P2x3 receptor expression at both transcriptional and cytosolic trafficking levels within the carotid body and other organs now becomes an important issue to allow translation into the clinical arena.
The finding that P2X3 receptor activation evoked a rapidly inactivating current in vitro52 seems inconsistent with an ability of P2X3 receptors to maintain tonic drive from the carotid body (or any organ). Notably, low pH, a postulated condition in the carotid body of SH rats (see above;41), can potentiate P2X3 receptor inward currents and reduce de310 sensitisation53. Furthermore, the concept has been developed that P2X3 receptor activation may lead to local generator potentials and calcium transients in afferent terminals that lowers the threshold for activation by any other excitatory agent, as described in somatosensory nociceptor C-fibres as leading to a condition of hyperalgesic priming54,55. Such threshold attenuation and chemoceptive priming may be under the control of P2X3 receptor in the carotid body, although we do not rule out possible emergence of P2X2/3 heteromeric receptors, as these channels show less rapid de317 sensitisation52, which is consistent with our voltage clamp data (Fig. 3e,f).
We found a robust dose-dependent lowering of arterial pressure following systemic administration of AF-219 in SH rats. That a fall in blood pressure was seen in rats with Goldblatt hypertension (W. Pijacka, F.M. McBryde & J.F.R. Paton – unpublished) suggests this is not restricted to the SH rat. We propose that the effect of P2X3 receptor antagonism is, in most part, acting within the carotid body but not exclusively as the antagonist induced fall in arterial pressure was substantially reduced but not abolished after carotid body ablation (Fig. 4b and Supplementary Fig. 7). Further support for a direct action of the antagonist at the carotid body is that the fall in arterial pressure was accompanied with a reduction in sympathetic activity in vivo, a mechanism for the arterial pressure reduction, which was also found following direct injection of AF-353 into the carotid body in situ. The remaining residual depressor response seen with AF-219 after carotid body ablation suggests a contribution from other sensory afferents in which P2X3 receptors have become active. We propose this includes glomus tissue in the thorax and abdomen but this remains to be confirmed. Mechanistically, the decrease in sympathetic activity included a reduction in its respiratory modulation, particularly its expiratory component. It is this component that is upregulated in numerous models of hypertension56,57 supporting the importance of the central respiratory generator as a major driver of autonomic imbalance in hypertension57. We also found an improvement in the spontaneous cardiac baroreceptor reflex, possibly reflecting the removal of the inhibitory influence of the carotid body on the carotid sinus baroreceptor reflex as reported previously in both SH rats20 and human hypertensives58; this may contribute to the sympatho-inhibition and anti-hypertensive response of P2X3 receptor antagonism in the SH rat.
The clinical significance of the synergy of up-regulated P2X3 receptor expression in the carotid body and petrosal neurones with emerging pathology should not be underestimated. It follows that administration of a P2X3 receptor antagonist should attenuate aberrant signalling without affecting physiological function. Data herein supports this logic, as carotid body tonic drive and hyperreflexia were abated yet the system rem ained physiologically responsive to stimulation akin to control animals (Fig. 2b). This is entirely consistent with a recent human trial to treat chronic pathological cough where the latter was abated but physiological/protective cough reflexes did not appear to be altered29. Peripheral chemoreception also has both regulatory and protective reflex functions59. Simultaneously abolishing pathological signalling while preserving normal carotid body function with a P2X3 antagonist such as AF-219 is an ideal outcome clinically that would be paramount in drug safety and target specificity. An issue may be the known adverse effect of P2X3 receptor antagonism affecting taste perception29 but appropriate dosing may negate this.
Over the last 20 years there has been a dearth of novel anti-hypertensive therapeutics (e.g.7). We speculate that P2X3 receptor antagonism may be most efficacious in a sub360 population of uncontrolled hypertensive humans with demonstrable aberrant carotid body activity and an overactive sympathetic nervous system. The existence of P2X3 receptors in the carotid body of hypertensive human, the release of ATP from human glomus cells in response to hypoxia35 and the ability of dopamine to select those individuals with aberrant carotid body discharge (Fig. 6c) supports our contention of a translational trial to test the anti-hypertensive effect of P2X3 antagonism; this study now awaits commencement.
Methods
Methods and any associated references are available in the online version of the paper.
Online Methods
A. Animal studies
All procedures conformed to the UK Animals (Scientific Procedures) Act 1986, and were approved by the University of Bristol ethical review committee. We used male spontaneously hypertensive (SH) or Wistar rats, which were bred within the animal facility of the University of Bristol. Animals were selected from different litters and housed with controlled temperature (21 ± 2 °C) and humidity (55 ± 10%). Wistar and SH rats were exposed to 12 hour day-night shift with unlimited access to food and water. We used rats either 4-5 weeks old (70-100 g) for in situ preparations or 14-16 weeks for in vivo radio-telemetry. Power calculations were based on: determining response magnitude (arbitrarily or based on previous reports or pilot experiments), data variance (our previous experience, published papers, pilot experiments), taking into consideration number of drugs to be tested and drug doses, and expected technical success rate of each type of experiment. Given the highly technical nature and longevity of the studies it was not possible to blind them.
Arterially perfused in situ juvenile rat preparation
Male Wistar or SH rats, 4-5 weeks, weighing 70–100 g were prepared as originally described60. In brief, rats were anesthetized deeply using isoflurane (5%). Anesthetic depth was assessed by a failure to respond to a noxious pinch of either a paw or the tail. Anesthetized rats were transected below the diaphragm, their upper body submerged in ice-cooled Ringer solution and decerebrated pre-collicularly by gentle aspiration. The preparation was skinned, transferred to a recording chamber and a double-lumen catheter was inserted into the descending aorta. One lumen was used to deliver perfusate pumped using a roller pump (Watson Marlow 505S). The perfusate was an isosmotic Ringer solution (containing in mM: NaCl 120, NaHCO3 24, KCl 5, CaCl2 2.5, MgSO4 1.25, KH2PO4 1.25, glucose 10) containing an oncotic agent (polyethylene glycol, 1.5%; Sigma-UK), gassed with carbogen (95% O2 and 5% CO2), warmed to 32 °C, pH 7.3 after carbogenation, and filtered using a nylon screen (pore size: 25 μM diameter). A side connector to this catheter allowed administration of drugs directly into the arterial circulation. The second lumen of the catheter was used to monitor aortic perfusion pressure. The left phrenic nerve was isolated and its activity (PNA) recorded from the cut central end using a glass suction bipolar electrode held in a 3-D micromanipulator. Rhythmic ramping PNA gave a continuous physiological index of preparation viability. After respiratory-related movements commenced, a neuromuscular blocker (vecuronium bromide, 40 μg.ml−1, Norcuron Organon Teknika) was added to the perfusate to stabilize mechanically the preparation. Sympathetic nerve activity (SNA) was also recorded from the thoracic or lumbar sympathetic chain using a bipolar glass suction electrode. All nerve signals were AC-amplified, band-pass filtered (0.5 Hz−5 kHz), rectified and integrated and sampled at 2-5 kHz. At the end of each experiment the noise level was measured after application of lidocaine (2%) to the sympathetic chain; this level was subtracted from the integrated signal. The head of the preparation was fixed by ear bars.
Whole cell recordings from chemoreceptive neurones
The carotid body/carotid sinus nerve/petrosal ganglion complex was isolated on the animal’s right side. Using recently described techniques57,61 whole cell patch clamp recordings of chemoreceptive petrosal ganglionic or NTS neurones was performed with electrodes filled with a solution containing the following (in mM): 130 K-gluconate, 4.5 MgCl2; 14 trisphosphocreatine, 1 0 HEPES; 5 EGTA; 4 Na-ATP; 0.3 Na-GTP; pH 7.3, and ∼300 mOsmol and had resistances of 3–8 MΩ when tested in bath solution. Current777 and voltage- clamp experiments were performed using an Axopatch-200B integrating amplifier (Molecular Devices) and pClamp acquisition software (version 10.0, Molecular Devices). Gigaseals (>1 GΩ) were formed, and whole-cell configuration was obtained by suction. To allow stable whole cell recordings, the petrosal ganglion was opened along its lateral aspect. A mesh grid was lowered onto the ganglion for stabilization, while permitting visualisation of the petrosal ganglia. We used electrical stimulation of the carotid sinus nerve (the axons of petrosal neurones) to find the chemosensitive petrosal and NTS neurones as characterised by their excitatory response to sodium cyanide injected into the aorta (0.03%, 50 μl). ATP (15 nl of 0.5, 2, 10, 20, 40, 100 mM) or α-β-methylene ATP (15 nl of 0.5, 2, 10, 20, 40, 100 mM) or AF-353 (15 nl of 20 μM; a P2X3 receptor antagonist)40 were injected into the carotid body using a 2 μm tip diameter glass microelectrode attached to a picopump (Picospritzer II, Parker Instrumentation).
Single petrosal neurone PCR
The cytoplasm of the chemosensitive petrosal and NTS neurones were pulled into a patch pipette with a slight negative pressure as described recently61. The cytoplasm was then placed into a microtube containing High Capacity cDNA Reverse Transcription Kit reagents (Life Technologies) and nuclease-free water for subsequent transcription in a thermocycler (Mastercycler Gradient, Eppendorf). A pre-amplification of the cDNA was performed using the TaqMan PreAmp Master Mix Kit (Life Technologies) with the following probes: Rn04219592_g1 (P2x2), Rn00579301_m1 (P2x3), TH: Rn00562500_m1, Post-synaptic density-95: Rn00571479_m1 and β-Actin: NM_031144.2 (reference gene). The pre-amplification protocol consisted of a hold temperature at 95°C during 10 minutes and 14 cycles of 95°C and 60°C during 15 s and 14 minutes, respectively. The reactions for the single-cell qRT-PCR were performed in singleplex and triplicate (StepOnePlus System, Applied Biosystems) using the same probes described above and the TaqMan Universal PCR Master Mix kit (Life Technologies) according to the manufacture’s recommendations. Water was used instead of cDNA as a negative control. β-actin was used as a control gene to normalise the reactions. The relative quantitation was determined by the ΔΔCt method. For each sample, the threshold cycle (Ct) was determined and normalised to the average of the housekeeping genes . The fold change of mRNA content in the sample of petrosal neurones from SHR relative to the Wistar group was determined by , where . Data are presented as mRNA expression relative to the Wistar group. Dual probing of mRNA included: TH and PSD-95 in 11 petrosal chemoreceptive cells from Wistar rats; TH and PSD-95 in 9 petrosal chemoreceptive cells from SHR; P2x2 and P2x3 receptor expression in 6 petrosal chemoreceptive cells from Wistar and 5 petrosal chemoreceptive cells from SHR.
In vivo blood pressure and renal nerve monitoring
Most surgical techniques were described previously20. Rats were anaesthetised with ketamine (100mg/ml; Vetalar, Zoetis, London, UK)/medetomidine hydrochloride (1 mg/ml Domitor, Elanco Animal Health, Hampshire, UK) injected intra muscularly. For blood pressure recording only, an incision was made in the midline of the abdomen and the abdominal aorta was exposed and cannulated just above the iliac bifurcation. The cannula of the transmitter (PA-C40; DSI, USA) was inserted until the tip rested just below the left renal artery branch point, and held in place using a tissue adhesive (VetBond, 3M, USA) and cellulose matrix. The transmitter body was placed in the abdominal cavity. Animals were allowed a seven days recovery period. A non-steroidal anti-inflammatory analgesic, was administered for 3 to 5 days postoperatively (0.006 mg/100g of Metacam, Boehringer Ingelheim, Germany). Some rats were fitted with heparinised (TDMAC, Plolysciences Inc., Eppelheim, Germany) catheters placed into right jugular and left femoral vein for blood sampling and drug infusions, respectively. The catheter placed in the right jugular vein was perforated according to the protocol62. Animals were allowed five days recovery post vein catheterisation. Catheters were flushed with heparinised saline solution (0.9 % Saline/100U Heparin) every other day in order to maintain their patency.
For renal nerve recording, rats were implanted with telemetric devices (TRM56SP; Millar Inc, Houston, Texas, USA). Details for blood pressure procedures are given above. For renal nerve recording, the right renal artery was exposed via a retroperitoneal approach, the renal nerves gently freed from surrounding connective tissue and a small piece of parafilm placed underneath. The renal nerve(s) were then lifted over bipolar silver wire recording electrodes, isolated with a biocompatible silicone elastomer (Kiwk-Sil, WPI, Eu), and the nerve/electrode complex secured in place using tissue adhesive and cellulose mesh. The incision was closed, and rats recovered for at least seven days post-operatively, with analgesic treatment for 3 days (buprenorphine, 0.01 mg/kg per dose).
Experimental protocols for in vivo rat studies
These studies were repeated by two researchers working independently. Drug infusions (i.v.) were carried out in rats housed singularly. Animals were acclimatised to experimental environment for at least 2 hours. At this time blood pressure remained stable. AF-219 was dissolved in 0.9% saline containing 10 mM HCl (vehicle). In some animals in which arterial blood pressure was measured, a PK analysis of AF-219 was performed by withdrawing blood from the jugular catheter at two time points: one hour from the start of infusion and one hour post infusion. Rats received an infusion of vehicle and 1, 4 and 8 mg/kg/h AF-219 for 60 minutes at 5 ml/kg/h into their femoral vein followed by a 60 minute washout period. Administration order of the drug doses and vehicle was randomized. These doses were selected by modelling pharmacokinetic data to obtain a pharmacokinetic profile in rats similar to that achievable in humans. The modelling was validated by quantifying AF-219 plasma concentrations after the 60 minute infusion period by using an HPLC-MS/MS assay and stable-labelled AF-219 as internal standard. The peripheral chemoreflex was tested with sodium cyanide (0.1 ml bolus i.v. 0.04% NaCN; BDH, Poole, UK, flushed with 0.9% saline (0.1 ml)). A bolus injection of sodium cyanide (0.04% in 0.1 ml) was given 45 minutes before, and 60 and 90 minutes after the beginning of the infusion to check for chemoreflex sensitivity. Rats were allowed 24 hour recovery between vehicle/drug and each drug dose. Infusions were carried using a standard syringe pump (Harvard apparatus, MA, USA). Dopamine hydrochloride was infused (10 μg/kg/min; Sigma-Aldrich Company Ltd., Dorset, UK) to reversibly inactivate carotid bodies and assess their tonicity by measuring arterial pressure, heart rate and respiratory frequency.
Rats tested with NaCN and dopamine hydrochloride underwent selective bilateral carotid body resection. Via a midline incision through the skin on the ventral surface of the neck, and using a binocular dissecting microscope the carotid bifurcation was identified. From a medial perspective the carotid body was visualised and ablated using fine watchmakers forceps. Effective carotid body ablation was confirmed by an absence of a cardiovascular and respiratory response to NaCN (i.v.). Animals were allowed six days for recovery. Once confirmation of selective carotid body ablation rats were infused with vehicle, AF-219 (8 mg/kg/h i.v.) and dopamine hydrochloride (10 μg/kg/min i.v.) on separate days.
Western blotting and immunocytochemical studies
Rat left and right carotid bifurcations were surgically removed from deeply anaesthetised rats and immediately transferred into ice cold saline. Carotid bodies were dissected under a Motic K Series Stereo Microscope (Ted Pella, Inc.). Five SH and four Wistar rats were used for western blotting and six SH and six Wistar rats were used for immunohistochemistry. Specificity of the P2X3 receptor antibody was validated (Supplementary Fig. 10). Carotid artery bifurcations were removed and carotid bodies dissected from these and snap frozen in liquid nitrogen (left and right together) for western blotting or they were fixed in ice cold methanol/DMSO (4:1) for immunohistochemistry. The relative expression of protein corresponds to one pair of carotid bodies (left and right). Immunohistochemistry was carried out on the whole mount rat carotid body and anti-P2X3 receptor antibody (APR026AN0202, Alomone, Israel). Incubation with anti-P2X3 and TH antibody was carried out in 2% goat serum over night at 4°C, gently mixed. All other details are given below (Human Studies) and similar to those used in the human studies.
Statistical analysis
Digitally transmitted arterial pressure and renal SNA signals were collected continuously during the drug or vehicle infusion period. Arterial pressure was measured during resting conditions. The renal SNA signal was amplified, filtered (50-5000 Hz), full-wave rectified and integrated using a low pass filter with a 20 ms time constant. Arterial pressure and renal SNA were sampled at 1k Hz using a 1401 acquisition system and purpose-written scripts in Spike2 software (Cambridge Electronic Designs, Cambridge, UK). From the arterial pressure we derived pulse pressure, heart rate and respiratory frequency. Power spectral analysis was performed on heart rate using Spike 2 software (CED instruments, Cambridge, UK). The inter-burst interval in renal SNA was taken as the baseline noise level, and removed from the signal. The renal SNA signal was then scaled, with the 30 minutes immediately prior to infusion taken as the 100% normalised level. We report the change in renal SNA with AF-219 relative to vehicle. The mean differences between baseline and treatment were analysed by the GraphPad Prism 6 (GraphPad Software, Inc., USA). We analysed the spontaneous cardiac and renal sympathetic baroreflex gain (sBRG). The sBRG for the arterial pressure–renal SNA relationship was calculated for 10 minutes immediately before, and the final 10 minutes of the 60 minute infusion period of AF-219 or vehicle. Diastolic pressure was smoothed over 5 beats, and positive or negative pressure ramps of at least 5 consecutive beats were automatically identified using Spike2 scripts (CED instruments, Cambridge, UK). All ramps where both heart rate and renal SNA opposed the change in the corresponding DBP ramp were included for analysis. As described earlier, renal SNA was normalised with the baseline (pre-infusion) level set at 100%. A paired student t-test was used to compare the renal SNA sBRG between vehicle and AF-219 infusions.
The type of statistical test performed is indicated in the figure legends. Data were normally distributed and variance was comparable between groups statistically compared. Unless stated in the figure legends, data were expressed as mean ± SEM are presented and data were assumed significant when P < 0.05.
Drugs
Two highly potent and selective non-competitive P2X3 (homotrimeric) and P2X3/P2X2 (heterotrimeric) receptor antagonists were used in these studies: AF-353 and AF-219. Although the antagonists we used have higher affinity for P2X3 homotrimeric receptors28,40,44 they cannot fully establish the relative contribution of homotrimeric P2X3 versus heterotrimeric P2X2/3 receptors. AF-353 was used in all in situ studies and was only available to us when these were performed; this drug does cross the blood brain barrier. The in vivo experiments used AF-219 as this is known not to cross the blood brain barrier. The chemical formula for AF-219 is: (amino-(4-isopropyl-2-methoxyphenyl)sulfone)-5-yl-(2,4-diaminopyrimidine-5yl)ether. The structure of AF-219 is contained in a published patent (see: http://pdfaiw.uspto.gov/.aiw?PageNum=0&docid=20150057299&IDKey=DA73323D44 81&). Doses used were based on IC50 data63,64. For recombinant P2X3 homotrimers the IC50 for AF-35364 and AF-219 was ~8 nM and ∼30 nM, respectively. A higher IC50 was found for P2X2/3 heterotrimeric receptors – for example 100 to 250 nM for AF-219. Note that no inhibitory effect on any non-P2X3 subunit containing receptors was noted63,64.
B. Human studies
All participants gave informed consent to participate in the studies, which were approved by the Central Bristol Research Ethics Committee (REC numbers: 14/SW/0054 and 12/SW/0277).
Carotid body tone in hypertensive humans
Small increases in circulating dopamine can decrease peripheral chemoreceptor afferent input into the brainstem65. Thus, low dose intravenous dopamine infusion (2 μg/kg/min) was used to test for any tonicity from the carotid bodies. Six hypertensive humans (age 48.8±3 yr; BMI 28.6±4, three female) with an office blood pressure of >140/90 mmHg (ambulatory blood pressure: >139/91 mmHg) and on a mean of 2.83 anti-hypertensive medicines were selected. Participants were given an intravenous infusion of dextrose (5%) for 5 minutes via peripheral venous access while breathing room air as a vehicle control. After the vehicle infusion, the line was purged and the intravenous infusion of dopamine (infusion concentration: 100 μg/ml of dopamine in 5% dextrose) was started for 5 minutes while breathing room air. Equivalent volumes of dextrose and dopamine were administered. Participants were blinded to the order of the infusions. This was following by a recovery period of 5 minutes while breathing room air allowing time for the dopamine to be metabolised. Respiratory volumes were measured via a respiratory flow-head connected to the expiratory port of a non966 rebreathing valve attached to a face mask. Data were acquired using PowerLab and LabChart 7 software (AD Instruments) and analysed to calculate respiratory rate, tidal volume and minute ventilation using Spike2 (Cambridge Electronic Design) and MATLAB (MathWorks). The data was analysed by GraphPad Prism 5 (GraphPad Software, Inc., USA).
Western blotting and immunocytochemical studies
Human left and right carotid bodies were obtained from twelve cadavers. Briefly, carotid bifurcations were cut out, placed into cold x1 PBS and both carotid bodies were dissected and processed. The left carotid body was directly snap frozen and used for western blotting and right carotid body was immediately fixed in 4% paraformaldehyde and used for immunohistochemistry.
Western blotting
The left human carotid body was crushed in liquid nitrogen with a mortar and pestle. Samples were subsequently homogenised with RIPA Lysis Buffer (Santa Cruz Biotechnology, Inc). Homogenate was centrifuged at 10,000 g for 20 minutes at 4°C and the pellet was discarded. Protein concentration of the supernatant was determined by the Lowry method using the DC-protein assay kit (Bio-Rad Laboratories Ltd). Samples were mixed with loading dye (NuPage, LDS Sample Buffer 4x, Life Technologies) and 20 μg of protein was used for protein electrophoresis on 4-12% Bis-Tris gel (NuPage 4-12% Bis-Tris Gel, Life Technologies). The Amersham ECL Plex Western blotting system using a low-fluorescent PVDF membrane (GE Healthcare, Buckinghamshire, UK) and Alexa Fluor 488 secondary antibody (Life Technologies, UK) were used. This system enables detection and quantification with a broad dynamic range and high linearity. After protein transfer, the membrane was blocked for 1h at room temperature with 2% Advance blocking agent (GE Healthcare). Membranes were incubated with Anti-P2X3 (APR026AN0202; 1:200 dilution) over night at 4°C. The membranes were subsequently washed with 1xPBS/0.1%Tween three times for 10 minutes at room temperature. Incubation with secondary antibody Alexa Fluor 488 was carried out in the dark at room temperature. Before imaging, the membrane was thoroughly washed. Signal was detected by scanning the membrane on a fluorescent laser scanner, Typhoon (GE Healt hcare). Protein expressions were quantified using ImageQuant software (GE Healthcare). The specificity of anti-P2X3 antibody was confirmed by membrane incubation with blocking peptide (APR026AG0140, Alomone; Supplementary Fig. 10).
Immunofluoresce
Human right carotid bodies were cut 20 μM thick on a cryostat. Carotid body slices were incubated with 10% goat serum/0.1% Saponin (Sigma-Aldrich, UK) for 1h at room temperature and gently agitated overnight. Primary antibody incubation with anti-P2X3 receptor (APR026AN0202, Alomone, Israel; 1:25 dilution) and anti-tyrosine hydroxylase (TH, F-11, sc-25269, Santa Cruz Biotechnology, Inc; dilution 1:25) was carried out using manufacturers recommendations in 2% goat serum over night at 4°C, overnight. Samples were then washed in 1 × PBS four times for 10 minutes at room temperature and the secondary antibody was applied for 1 hour at room temperature. Anti-rabbit Alexa Fluor 488 was use to visualise the P2X3 receptor and anti-mouse Alexa Fluor 594 was used to visualise tyrosine hydroxylase. Secondary antibody incubation was followed by washing as described above and the samples were mounted on slides using mounting medium (Vectashield, H-1000, Vector Laboratories, Inc). Carotid bodies were examined under a Leica SP8 AOBS confocal laser scanning microscope attached to a Leica DM I6000 inverted epifluorescence microscope and imaging was performed using ‘hybrid’ GaAsP detectors for greater sensitivity. Images were processed in Adobe Illustrator CS3.
Supplementary Material
Acknowledgments
We wish to thank J.-C. Isner (School of Biological Sciences, University of Bristol) for his expertise on software used for analysing aspects of some of the in vivo cardiovascular data. Technical support of P. Chappell (mechanical workshop) and D. Carr (electronic workshop) is appreciated. The research support of the British Heart Foundation RG/12/6/29670 (J.F.R.P.) and Afferent Pharmaceuticals is acknowledged (A.P.F & J.F.R.P.). This research was supported by the National Institute for Health Research Biomedical Research Unit in Cardiovascular Disease at the University Hospitals Bristol National Health Service Foundation Trust and the University of Bristol (A.K.N & J.F.R.P.) In situ studies were supported by grants from ‘Fundação de Amparo à Pesquisa do Estado de São Paulo’ FAPESP Thematic Project 2013/06077-5 (B.H.M) and research grant 2013/10484-5 (D.J.A.M.). The University of Bristol’s Wolfson Bioimaging Facility BBSRC Alert 13 capital grant BB/L014181/1 is acknowledged.
Footnotes
Author contributions
W.P. conducted all the in vivo radio-telemetry blood pressure studies, the rat and human immunocytochemistry and western blotting; this also included data analysis, figure and manuscript preparation. D.J.A.M. performed all in situ rat nerve and petrosal neurone whole cell recording studies; this also included data analysis and figure preparation. M.P.d.S. performed the single neurone PCR study. L.E.K.R. with A.K.N. carried out the diagnosis and recruitment of humans with hypertension and, L.E.K.R. with E.C.H., performed and analysed data from the dopamine infusion study. B.H.M. supported all in situ studies, assisted in experimental design, data analysis and manuscript preparation. F.D.M. conducted the in vivo radio-telemetry study for recording renal sympathetic nerve activity; this also included data analysis and figure preparation. A.P.A. performed some of the first immunohistochemistry on human carotid bodies. A.P.F. provided the P2X3 receptor antagonists, carried out the PK analysis, assisted in drug trial design and manuscript preparation and revision. J.F.R.P. orchestrated the design of the project, provided supervision with data acquisition and analysis, wrote the manuscript and revised it.
Disclaimer
This article/paper/report presents independent research funded by the National Institute for Health Research (NIHR). The views expressed are those of the author(s) and not necessarily those of the National Health Service, the NIHR or the Department of Health.
Competing financial interests
A.P.F. is Chief Scientific Officer for Afferent Pharmaceuticals. The other authors declare no competing financial interests.
References
- 1.Go AS, et al. American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Executive summary: heart disease and stroke statistics–2014 update: a report from the American Heart Association. Circ. 2014;129:399–410. doi: 10.1161/01.cir.0000442015.53336.12. [DOI] [PubMed] [Google Scholar]
- 2.Lloyd A, Schmieder C, Marchant N. Financial and health costs of uncontrolled blood pressure in the United Kingdom. Pharmacoeconomics. 2003;21:33–41. doi: 10.2165/00019053-200321001-00004. [DOI] [PubMed] [Google Scholar]
- 3.Lewington S, Clarke R, Qizilbash N, Peto R, Collins R. Prospective Studies Collaboration Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet. 2002;360:1903–1913. doi: 10.1016/s0140-6736(02)11911-8. [DOI] [PubMed] [Google Scholar]
- 4.Kearney PM, et al. Global burden of hypertension: analysis of worldwide data. Lancet. 2005;365:217–223. doi: 10.1016/S0140-6736(05)17741-1. [DOI] [PubMed] [Google Scholar]
- 5.Carey RM. Resistant hypertension. Hypertension. 2013;61:746–750. doi: 10.1161/HYPERTENSIONAHA.111.00601. [DOI] [PubMed] [Google Scholar]
- 6.Plump A. Accelerating the pulse of cardiovascular R&D. Nat Rev Drug Discov. 2010;9:823–824. doi: 10.1038/nrd3315. [DOI] [PubMed] [Google Scholar]
- 7.Brown M. Aliskiren. Circ. 2008;118:773–784. doi: 10.1161/CIRCULATIONAHA.108.787630. [DOI] [PubMed] [Google Scholar]
- 8.Krum H, et al. Catheter-based renal sympathetic denervation for resistant hypertension: a multicentre safety and proof-of-principle cohort study. Lancet. 2009;373:1275–1281. doi: 10.1016/S0140-6736(09)60566-3. [DOI] [PubMed] [Google Scholar]
- 9.Heusser K, et al. Carotid baroreceptor stimulation, sympathetic activity, baroreflex function, and blood pressure in hypertensive patients. Hypertension. 2010;55:619–26. doi: 10.1161/HYPERTENSIONAHA.109.140665. [DOI] [PubMed] [Google Scholar]
- 10.Patel NK, et al. Deep brain stimulation relieves refractory hypertension. Neurology. 2011;76:405–407. doi: 10.1212/WNL.0b013e3182088108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Burchell AE, Lobo MD, Sulke N, Sobotka PA, Paton JF. Arteriovenous anastomosis: is this the way to control hypertension? Hypertension. 2014;64:6–12. doi: 10.1161/HYPERTENSIONAHA.114.02925. [DOI] [PubMed] [Google Scholar]
- 12.Lobo MD, et al. ROX CONTROL HTN Investigators. Central arteriovenous anastomosis for the treatment of patients with uncontrolled hypertension (the ROX CONTROL HTN study): a randomised controlled trial. Lancet. 2015;385:1634–1641. doi: 10.1016/S0140-6736(14)62053-5. [DOI] [PubMed] [Google Scholar]
- 13.Narkiewicz K, et al. Contribution of tonic chemoreflex activation to sympathetic activity and blood pressure in patients with obstructive sleep apnea. Circulation. 1998;97:943–945. doi: 10.1161/01.cir.97.10.943. [DOI] [PubMed] [Google Scholar]
- 14.Tan ZY, et al. Chemoreceptor hypersensitivity, sympathetic excitation, and overexpression of ASIC and TASK channels before the onset of hypertension in SHR. Circ Res. 2010;106:536–545. doi: 10.1161/CIRCRESAHA.109.206946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Przybylski J. Do arterial chemoreceptors play a role in the pathogenesis of hypertension? Med Hypotheses. 1981;7:127–131. doi: 10.1016/0306-9877(81)90109-2. [DOI] [PubMed] [Google Scholar]
- 16.Somers VK, Mark AL, Abboud FM. Potentiation of sympathetic nerve responses to hypoxia in borderline hypertensive subjects. Hypertension. 1988;11:608–612. doi: 10.1161/01.hyp.11.6.608. [DOI] [PubMed] [Google Scholar]
- 17.Trzebski A, Tafil M, Zoltowski M, Przybylski J. Increased sensitivity of the arterial chemoreceptor drive in young men with mild hypertension. Cardiovasc Res. 1982;16:163–172. doi: 10.1093/cvr/16.3.163. [DOI] [PubMed] [Google Scholar]
- 18.Sinski M, et al. Tonic activity of carotid body chemoreceptors contributes to the increased sympathetic drive in essential hypertension. Hypertens Res. 2012;35:487–491. doi: 10.1038/hr.2011.209. [DOI] [PubMed] [Google Scholar]
- 19.Abdala AP, et al. Hypertension is critically dependent on the carotid body input in the spontaneously hypertensive rat. J Physiol. 2012;590:4269–4277. doi: 10.1113/jphysiol.2012.237800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McBryde FD, et al. The carotid body as a putative therapeutic target for the treatment of neurogenic hypertension. Nat Communs. 2013;4:2395. doi: 10.1038/ncomms3395. [DOI] [PubMed] [Google Scholar]
- 21.Paton JF, et al. The carotid body as a therapeutic target for the treatment of sympathetically mediated diseases. Hypertension. 2013;61:5–13. doi: 10.1161/HYPERTENSIONAHA.111.00064. [DOI] [PubMed] [Google Scholar]
- 22.Nakayama K. Surgical removal of the carotid body for bronchial asthma. Dis Chest. 1961;40:595–604. doi: 10.1378/chest.40.6.595. [DOI] [PubMed] [Google Scholar]
- 23.Yuan G, et al. Protein kinase G-regulated production of H2S governs oxygen sensing. Sci Signal. 2015;8:ra37. doi: 10.1126/scisignal.2005846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Prabhakar NR, Peers C. Gasotransmitter regulation of ion channels: a key step in O2 sensing by the carotid body. Physiology. 2014;29:49–57. doi: 10.1152/physiol.00034.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Buckler KJ. TASK channels in arterial chemoreceptors and their role in oxygen and acid sensing. Pflugers Arch. 2015;467:1013–1025. doi: 10.1007/s00424-015-1689-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Evans AM, Peers C, Wyatt CN, Kumar P, Hardie DG. Ion channel regulation by the LKB1-AMPK signalling pathway: the key to carotid bodyactivation by hypoxia and metabolic homeostasis at the whole body level. Adv Exp Med Biol. 2012;758:81–90. doi: 10.1007/978-94-007-4584-1_11. [DOI] [PubMed] [Google Scholar]
- 27.Schultz HD, Marcus NJ, Del Rio R. Mechanisms of carotid body chemoreflex dysfunction during heart failure. Exp Physiol. 2015;100:124–129. doi: 10.1113/expphysiol.2014.079517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ford AP, et al. P2X3 receptors and sensitization of autonomic reflexes. Auton Neurosci. doi: 10.1016/j.autneu.2015.04.005. [DOI] [PubMed] [Google Scholar]
- 29.Abdulqawi R, et al. P2X3 receptor antagonist (AF-219) in refractory chronic cough: a randomised, double-blind, placebo-controlled phase 2 study. Lancet. 2015;385:1198–1205. doi: 10.1016/S0140-6736(14)61255-1. [DOI] [PubMed] [Google Scholar]
- 30.Zhang M, Zhong H, Vollmer C, Nurse CA. Co-release of ATP and ACh mediates hypoxic signalling at rat carotid bodychemoreceptors. J Physiol. 2000;525:143–158. doi: 10.1111/j.1469-7793.2000.t01-1-00143.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Varas R, Alcayaga J, Iturriaga R. ACh and ATP mediate excitatory transmission in cat carotid identified chemoreceptor units in vitro. Brain Res. 2003;988:154–63. doi: 10.1016/s0006-8993(03)03366-3. [DOI] [PubMed] [Google Scholar]
- 32.Zapata P. Is ATP a suitable co-transmitter in carotid body arterial chemoreceptors? Respir Physiol Neurobiol. 2007;157:106–115. doi: 10.1016/j.resp.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 33.Burnstock G. Purines and sensory nerves. Handb Exp Pharmacol. 2009;194:333–392. doi: 10.1007/978-3-540-79090-7_10. [DOI] [PubMed] [Google Scholar]
- 34.Icekson G, Dominguez CV, Dedios VP, Arroyo J, Alcayaga J. Petrosal ganglion responses to acetylcholine and ATP are enhanced by chronic normobaric hypoxia in the rabbit. Respir Physiol Neurobiol. 2013;189:624–631. doi: 10.1016/j.resp.2013.07.023. [DOI] [PubMed] [Google Scholar]
- 35.Kahlin J, et al. The human carotid body releases acetylcholine, ATP and cytokines during hypoxia. Exp Physiol. 2014;99:1089–1098. doi: 10.1113/expphysiol.2014.078873. [DOI] [PubMed] [Google Scholar]
- 36.Prasad M, et al. Expression of P2X2 and P2X3 receptor subunits in rat carotid body afferent neurones: role in chemosensory signalling. J Physiol. 2001;537:667–77. doi: 10.1111/j.1469-7793.2001.00667.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rong W, et al. Pivotal role of nucleotide P2X2 receptor subunit of the ATP-gated ion channel mediating ventilatory responses to hypoxia. J Neurosci. 2003;23:11315–11321. doi: 10.1523/JNEUROSCI.23-36-11315.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Welsh MJ, Heistad DD, Abboud FM. Depression of ventilation by dopamine in man. Evidence for an effect on the chemoreceptor reflex. J Clin Invest. 1978;61:708–713. doi: 10.1172/JCI108983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cardenas H, Zapata P. Dopamine-induced ventilatory depression in the rat, mediated by carotid nerve afferents. Neurosci Lett. 1981;24:29–33. doi: 10.1016/0304-3940(81)90354-2. [DOI] [PubMed] [Google Scholar]
- 40.Gever JR, et al. AF-353, a novel, potent and orally bioavailable P2X3/P2X2/3 receptor antagonist. Br J Pharmacol. 2010;160:1387–1398. doi: 10.1111/j.1476-5381.2010.00796.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Clarke JA, Daly MD, Ead HW. Vascular analysis of the carotid body in the spontaneously hypertensive rat. Adv Exp Med Biol. 1993;337:3–8. doi: 10.1007/978-1-4615-2966-8_1. [DOI] [PubMed] [Google Scholar]
- 42.Nurse CA. Synaptic and paracrine mechanisms at carotid body arterial chemoreceptors. J Physiol. 2014;592:3419–3426. doi: 10.1113/jphysiol.2013.269829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fan J, et al. Interleukin-6 increases intracellular Ca2+ concentration and induces catecholamine secretion in rat carotid body glomus cells. J Neurosci Res. 2009;87:2757–2762. doi: 10.1002/jnr.22107. [DOI] [PubMed] [Google Scholar]
- 44.Ford AP, Undem BJ. The therapeutic promise of ATP antagonism at P2X3 receptors in respiratory and urological disorders. Front Cell Neurosci. 2013;7:267. doi: 10.3389/fncel.2013.00267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Daly DM, et al. Age-related changes in afferent pathways and urothelial function in the male mouse bladder. J Physiol. 2014;592:537–549. doi: 10.1113/jphysiol.2013.262634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ford AP, Cockayne DA. ATP and P2X purinoceptors in urinary tract disorders. Handb Exp Pharmacol. 2011;202:485–526. doi: 10.1007/978-3-642-16499-6_22. [DOI] [PubMed] [Google Scholar]
- 47.Adriaensen D, Brouns I, Timmermans JP. Sensory input to the central nervous system from the lungs and airways: A prominent role for purinergic signalling via P2X2/3 receptors. Auton Neurosci. 2015 doi: 10.1016/j.autneu.2015.04.006. [DOI] [PubMed] [Google Scholar]
- 48.Burnstock G. Purinergic signalling in the gastrointestinal tract and related organs in health and disease. Purinergic Signal. 2014;10:3–50. doi: 10.1007/s11302-013-9397-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Deiteren A, et al. P2x3 receptors mediate visceral hypersensitivity during acute chemically-induced colitis and in the post-inflammatory phase via different mechanisms of sensitization. PLoS One. 2015;10:e0123810. doi: 10.1371/journal.pone.0123810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Li J, Xing J, Lu J. Nerve growth factor, muscle afferent receptors and autonomic responsiveness with femoral artery occlusion. J Mod Physiol Res. 2014;1:1–18. [PMC free article] [PubMed] [Google Scholar]
- 51.Hansen RR, et al. Chronic administration of the selective P2X3, P2X2/3 receptor antagonist, A-317491, transiently attenuates cancer-induced bone pain in mice. Eur J Pharmacol. 2012;688:27–34. doi: 10.1016/j.ejphar.2012.05.008. [DOI] [PubMed] [Google Scholar]
- 52.Liu M, et al. Coexpression of P2X(3) and P2X(2) receptor subunits in varying amounts generates heterogeneous populations of P2X receptors that evoke a spectrum of agonist responses comparable to that seen in sensory neurons. J Pharmacol Exp Ther. 2001;296:1043–1050. [PubMed] [Google Scholar]
- 53.Gerevich Z, et al. Dual effect of acid pH on purinergic P2X3 receptors depends on the histidine 206 residue. J Biol Chem. 2007;282:33949–33857. doi: 10.1074/jbc.M705840200. [DOI] [PubMed] [Google Scholar]
- 54.Reichling DB, Levine JD. Critical role of nociceptor plasticity in chronic pain. Trends Neurosci. 2009;32:611–618. doi: 10.1016/j.tins.2009.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schiavuzzo JG, et al. Neuronal P2X3 receptor activation is essential to the hyperalgesia induced by prostaglandins and sympathomimetic amines released during inflammation. Neuroscience. 2015;285:24–33. doi: 10.1016/j.neuropharm.2012.11.011. [DOI] [PubMed] [Google Scholar]
- 56.Zoccal DB, et al. Increased sympathetic outflow in juvenile rats submitted to chronic intermittent hypoxia correlates with enhanced expiratory activity. J Physiol. 2008;586:3253–3265. doi: 10.1113/jphysiol.2008.154187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Moraes DJ, Machado BH, Paton JF. Specific respiratory neuron types have increased excitability that drive presympathetic neurones in neurogenic hypertension. Hypertension. 2014;63:1309–1318. doi: 10.1161/HYPERTENSIONAHA.113.02283. [DOI] [PubMed] [Google Scholar]
- 58.Somers VK, Mark AL, Abboud FM. Interaction of baroreceptor and chemoreceptor reflex control of sympathetic nerve activity in normal humans. J Clin Invest. 1991;87:1953–1957. doi: 10.1172/JCI115221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Comroe JH., Jr The functions of the lung. Harvey Lect NY. 1954;48:110–144. [PubMed] [Google Scholar]
- 60.Paton JFR. A working heart-brainstem preparation. J Neurosci Meths. 1996;65:63–68. doi: 10.1016/0165-0270(95)00147-6. [DOI] [PubMed] [Google Scholar]
- 61.Moraes DJ, et al. Electrophysiological properties of rostral ventrolateral medulla presympathetic neurons modulated by the respiratory network in rats. J Neurosci. 2013;33:19223–19237. doi: 10.1523/JNEUROSCI.3041-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Waynforth HB, Flecknell P. Experimental and Surgical Techniques in the Rat. (Second) 1992 Jul 8; [Google Scholar]
- 63.Ford AP, Undem BJ. The therapeutic promise of ATP antagonism at P2X3 receptors in respiratory and urological disorders. Front Cell Neurosci. 2013;7:267. doi: 10.3389/fncel.2013.00267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gever JR, et al. AF-353, a novel, potent and orally bioavailable P2X3/P2X2/3 receptor antagonist. Br J Pharmacol. 2010;160:1387–1398. doi: 10.1111/j.1476-5381.2010.00796.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Stickland MK, et al. Carotid chemoreceptor modulation of blood flow during exercise in healthy humans. J Physiol. 2011;589:6219–6230. doi: 10.1113/jphysiol.2011.218099. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


