Sir,
Dengue is a mosquito-borne disease affecting humans in tropical regions worlwide. A total of 2.5 billion people are at risk for contracting the infection, with an annual incidence of 50-100 million cases worldwide.
[1] Little has been reported on coinfections involving different dengue virus (DENV) serotypes. It has suggested that coinfection with DENV serotypes do not have a severe clinical course,[2] however, the pathology and clinical course associated with these simultaneous infections are not extensively described.
In 2009, a 21-year-old patient was admitted to the Manzanillo General Hospital in Colima State, Mexico with the suspicion of dengue infection. The patient presented with acute fever (>38.5°C) of 3-day progression. On physical examination, the patient did not have severe clinical symptoms, but he presented with myalgia, exanthema, arthralgia, abdominal pain, headache, fatigue, and nausea. Acute symptoms of dengue hemorrhagic fever were not observed. The tourniquet test was positive, but his platelet count was normal. The patient was only given acetaminophen 500 mg every 6 h for 3 days. He had no symptomatology of any other viral or bacterial infection. His symptoms were gone on the fifth day after their onset, and he experienced no chronic discomfort. The clinical diagnosis of dengue was confirmed through detection of the DENV NS1 antigen with the BIOLINE Dengue Duo rapid test. A molecular identification was performed as previously described,[3] revealing the presence of DENV-1 and DENV-2. The laboratory results during this viral coinfection are shown in Table 1.
Table 1.
Laboratory Results of the Dengue Virus 1/Dengue Virus 2 Coinfected Patient
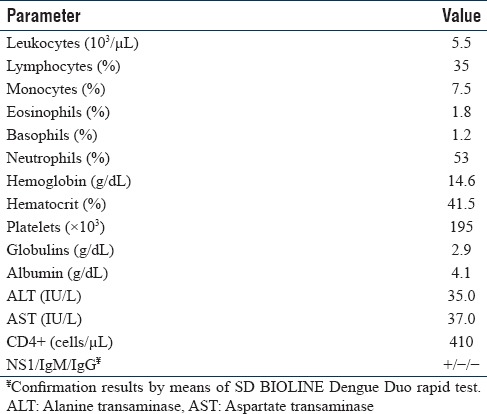
Our results suggest that the patient did not show with severe clinical complications during simultaneous infection among DENV-1 and DENV-2. These results are similar to those reported by do Santos (2003), in which no severe linical symptoms were observed in a DENV-1/DENV-2 coinfected patient in Brazil. Interestingly, both alanine transaminase (ALT) and aspartate transaminase (AST) remained at normal levels during this simultaneous infection, despite it has observed that dengue infection is associated with accelerated progression of liver damage during the acute infection phase.[4] At this point, we believe that it could be an effect of an innate immune response that could attenuate the expression of the proinflammatory cytokines involved in the increase of liver enzymes, suppressing the synthesis and release of the cytokines associated with severe dengue symptoms. Such is the case of interleukin-2, which is associated with an increase in ALT and AST levels, and therefore its inhibition could not only participate in the downregulation of the liver enzymes but also regulate the synthesis and release of the tumor necrosis factor-α, producing a less severe clinical course in those patients.[5]
In conclusion, our observations suggest that there are no severe clinical complications during this simultaneous infection. Further studies are required for understanding these viral coinfections, as well as the impact it has on the progression of dengue disease, especially in tropical and subtropical regions in which dengue is highly active.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
The study was supported by National Council for Science and Technology (CONACYT grant # 252229 and FORDECyT- CONACYT grant # 2009/1-000000117535).
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
The authors would like to thank the National Council for Science and Technology of Mexico for its financial assistance (CONACYT grant # 252229 and FORDECyT- CONACYT grant # 2009/1-000000117535).
REFERENCES
- 1.World Health Organization. Dengue and Severe Dengue. 2014. [Last updated on 2017 Apr]. Available from: http://www.who.int/mediacentre/factsheets/fs117/en/
- 2.dos Santos CL, Bastos MA, Sallum MA, Rocco IM. Molecular characterization of dengue viruses type 1 and 2 isolated from a concurrent human infection. Rev Inst Med Trop Sao Paulo. 2003;45:11–6. doi: 10.1590/s0036-46652003000100003. [DOI] [PubMed] [Google Scholar]
- 3.Lanciotti RS, Calisher CH, Gubler DJ, Chang GJ, Vorndam AV. Rapid detection and typing of dengue viruses from clinical samples by using reverse transcriptase-polymerase chain reaction. J Clin Microbiol. 1992;30:545–51. doi: 10.1128/jcm.30.3.545-551.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Samanta J, Sharma V. Dengue and its effects on liver. World J Clin Cases. 2015;3:125–31. doi: 10.12998/wjcc.v3.i2.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lorré K, Fransen L, Ceuppens JL. Interleukin-2 induces tumor necrosis factor-alpha production by activated human T cells via a cyclosporin-sensitive pathway. Eur Cytokine Netw. 1992;3:321–30. [PubMed] [Google Scholar]


