Abstract
123I-meta-iodobenzylguanidine (123I-MIBG) imaging is currently a mainstay in the evaluation of many neuroendocrine tumors, especially neuroblastoma. 123I-MIBG imaging has several limitations that can be overcome by the use of a PET agent. 18F-meta-fluorobenzylguanidine (18F-MFBG) is a PET analog of MIBG that may allow for single-day, high-resolution quantitative imaging. We conducted a first-in-human study of 18F-MFBG PET imaging to evaluate the safety, feasibility, pharmacokinetics, and dosimetry of 18F-MFBG in neuroendocrine tumors (NETs). Methods: Ten patients (5 with neuroblastoma and 5 with paraganglioma/pheochromocytoma) received 148–444 MBq (4–12mCi) of 18F-MFBG intravenously followed by serial whole-body imaging at 0.5–1, 1–2, and 3–4 after injection. Serial blood samples (a total of 6) were also obtained starting at 5 min after injection to as late as 4 h after injection; whole-body distribution and blood clearance data, lesion uptake, and normal-tissue uptake were determined, and radiation-absorbed doses to normal organs were calculated using OLINDA. Results: No side effects were seen in any patient after 18F-MFBG injection. Tracer distribution showed prominent activity in the blood pool, liver, and salivary glands that decreased with time. Mild uptake was seen in the kidneys and spleen, which also decreased with time. Urinary excretion was prominent, with an average of 45% of the administered activity in the bladder by 1 h after injection; whole-body clearance was monoexponential, with a mean biologic half-life of 1.95 h, whereas blood clearance was biexponential, with a mean biologic half-life of 0.3 h (58%) for the rapid α phase and 6.1 h (42%) for the slower β phase. The urinary bladder received the highest radiation dose with a mean absorbed dose of 0.186 ± 0.195 mGy/MBq. The mean total-body dose was 0.011 ± 0.011 mGy/MBq, and the effective dose was 0.023 ± 0.012 mSv/MBq. Both skeletal and soft-tissue lesions were visualized with high contrast. The SUVmax (mean ± SD ) of lesions at 1–2 h after injection was 8.6 ± 9.6. Conclusion: Preliminary data show that 18F-MFBG imaging is safe and has favorable biodistribution and kinetics with good targeting of lesions. PET imaging with 18F-MFBG allows for same-day imaging of NETs. 18F-MFBG appears highly promising for imaging of patients with NETs, especially children with neuroblastoma.
Keywords: 18F-MFBG, neuroendocrine, neuroblastoma, dosimetry, MIBG
Imaging plays a critical role in the diagnosis, staging, and follow-up of neuroendocrine tumors (NETs). Currently, 131I-meta-iodobenzylguanidine (131I-MIBG) and 123I-MIBG are widely used in NETs overexpressing the norepinephrine transporter, and 123I-MIBG is routinely used for staging and follow-up of patients with neuroblastoma (1–8). 123I-MIBG imaging is also used for the evaluation of disease extent and suitability for 131I-MIBG therapy.
However, 123I-MIBG imaging has significant limitations, including a 2-d imaging schedule, with injection on the first day and imaging 24 h later (3); and a need for thyroid protection with supersaturated potassium iodide before 123I-MIBG injection. Further, compared with PET, the images have poorer resolution and limited quantitative accuracy, which is typically associated with γ-camera imaging. These differences compromise sensitivity and the ability to evaluate tumor burden and tumor accumulation. Therefore, there is a clinical need for a PET imaging agent that would allow for same-day imaging with superior resolution and quantitation of the tracer uptake in lesions associated with PET imaging.
The PET imaging tracer 124I-MIBG has been studied previously (9), but the complex decay scheme, including the emission of high-energy cascade γ-rays, leads to poorer image quality, less reliable quantification, and unfavorable dosimetry (10). Furthermore, 124I is not widely available for clinical use. 18F-fluorodopamine, a dopamine analog, and 18F-fluorodopa PET have also been used to image noradrenaline and amino acid transporter expression, respectively, in neuroendocrine malignancies; however, the experience is limited, particularly in patients with neuroblastoma (11,12). 11C-hydroxyephedrine, a catecholamine analog (13), has also been shown to target tumors of the sympathetic nervous system. Current clinical data are again limited, and use of a short-lived isotope limits its use. These tracers are also currently limited in availability. There is a need for a NET imaging agent that allows for single-day imaging and that will enable faster evaluation of disease sites with high sensitivity within a more convenient imaging schedule. Given our extensive clinical experience with 123I-MIBG, a PET tracer such as an 18F-labeled analog of MIBG is appealing.
18F-labeled meta-fluorobenzylguanidine (18F-MFBG) was synthesized to overcome these limitations, and preclinical studies have shown a similarity to 123I-MIBG (14). In xenografts, 18F-MFBG showed a similar lesion uptake, with significantly faster blood clearance than 123I-MIBG, enabling high-contrast visualization of lesions as early as 1 h after injection (15).
On the basis of these preclinical data, we initiated a first-in-human study (NCT 02348749) to evaluate the safety, pharmacokinetics, and radiation dosimetry of 18F-MFBG in patients with neuroblastoma and pheochromocytoma/paragangliomas. We report the results of the safety, biodistribution, pharmacokinetics, and organ dosimetry of 18F-MFBG in these patients.
MATERIALS AND METHODS
A prospective study of PET imaging with 18F-MFBG was performed. 18F-MFBG was administered under an Investigational New Drug application (IND# 125108) approved by the Food and Drug Administration. The protocol was approved by the Institutional Review Board, and all patients or their legal guardians provided written informed consent.
Patients
Patients with confirmed neuroblastoma or paraganglioma/pheochromocytoma with evidence of evaluable disease or lesions on 123I-MIBG imaging were eligible. Additional eligibility criteria included a performance status of 60 or higher on the Karnofsky or Lansky scale and adequate hepatic and renal function defined as no toxicity greater than grade 2 (CTC 4.0).
18F-MFBG Preparation
18F-MFBG was manufactured at the Memorial Sloan Kettering Radiochemistry and Molecular Imaging Probes Core Facility in compliance with the requirements specified in the Chemistry, Manufacturing, and Controls section of a Food and Drug Administration–approved IND. Clinical 18F-MFBG batches for the first 4 patients were prepared using the original manual method described previously (16). Subsequently, the method was changed to a less complex synthesis, using the diaryliodonium salt (ALP)-MFBG precursor, supplied by Ground Fluor Pharmaceuticals, Inc. The revised synthesis, derived from the methods published by Hu et al. (17), involved nucleophilic incorporation of 18F-fluoride into the 18F-MFBG precursor, ALP-MFBG (7.5 mg dissolved in 1 mL of acetonitrile), at 120°C for 20 min, followed by removal of protective groups by acid hydrolysis, reversed-phase preparatory high-performance liquid chromatography (HPLC) purification, and terminal sterilization using a 0.22-μm sterilizing filter. The final 18F-MFBG drug product was formulated in 15 mL of ammonium acetate buffer and sterile water for injection (United States Pharmacopeia).
The final 18F-MFBG drug product batches underwent quality control testing, before batch release for patient administration. Radiochemical purity was more than 90%, as determined by reversed-phase HPLC; radiochemical identity was confirmed by comparison to a reference standard response on the HPLC; endotoxin content was less than 5 EU/mL, as measured by the portable test system supplied by Charles River Laboratories; sterilizing filter integrity pressure was more than 50 ψ, as measured by the bubble point method; pH was 3.5–8.0, as measured by pH strips; residual acetonitrile concentration was less than 270 μg/mL, as measured by gas chromatography; appearance was a clear and particle-free solution, as determined by a visual inspection check; and radionuclide identity was verified, as measured by radioactive half-life determination. Sterility testing, using the direct medium inoculation method, was performed after release. Specific activity (MBq/μg) determinations were performed on the initial 18F-MFBG validation batches and calibrated to the end of synthesis time; these were calculated by dividing the total measured radioactivity at the end of synthesis time by the total mass of 18F-MFBG present in the final product, as measured by HPLC.
18F-MFBG Administration
A dose of 148–444 MBq (4–12 mCi) of 18F-MFBG was administered as a slow intravenous bolus over 1 min, followed by a saline flush. Patients were not required to fast. No premedications were administered, and no patients were taking any medications known to interfere with MIBG uptake (3). Patients were monitored for at least 3 h after injection with vitals and for any reactions or adverse events and later at 24 h follow-up. Side effects and reactions were graded according to Common Terminology Criteria for Adverse Events, version 4.0.
18F-MFBG Scans
Scanning included dynamic imaging for the first 30 min with images acquired in list mode, 128 × 128 matrix (6 frames for 5 s each, followed by 3 frames for 10 s each, 4 frames for 1 min each, 2 frames for 2.5 min each, 2 frames for 5 min each, and 1 frame for 10 min) over the chest (including cardiac blood pool, lung, and liver) followed by whole-body (vertex to feet) imaging within the first hour after injection, a second whole-body scan at 1–2 h, and a third whole-body scan at 3–4 h after injection. A single low-dose CT scan, at 80 mA for adults and a weight-based scaled mA for children, was obtained. The 2 remaining PET/CT scans were acquired with an ultra-low-dose CT at 10 mA for attenuation. All scans were obtained on the same scanner (Discovery 710; 3-dimensional) in 3-dimensional mode with a 3-min acquisition time per field of view for the torso (vertex to pelvis) and 1- to 2-min field of view for the lower limbs. Images were reconstructed using a manufacturer-provided iterative reconstruction algorithm and attenuation and scatter corrections similar to 18F-FDG imaging.
Blood Clearance Measurements
Multiple venous blood samples were obtained, including a baseline sample before injection of 18F-MFBG, at 5 ± 2, 15 ± 5, 30 ± 5, 60 ± 5, 90 ± 10, 120 ± 10, and 180 ± 10 min after injection. Activity in blood (aliquots of about 500 μL) was measured in duplicate using a NaI (Tl) γ-counter (Wallac Wizard 1480 automatic γ-counter; Perkin Elmer) together with appropriate standards. The measured activity concentrations were converted to percentage injected activity per liter. Metabolite analysis of activity in plasma was performed by reversed-phase HPLC with in-line radiation detection on samples obtained up to at least 120 min after injection in all patients.
Whole-Body and Blood Parameters
Activity in the whole body was determined on the basis of whole-body scans; the first scan was obtained before voiding. A monoexponential function was fitted to the whole-body activity data and a biexponential function to the blood activity concentration data (18). Values of cumulated activity per unit administered activity (residence time) for whole body (in h) and blood (in h/L), τ, were calculated according to the formula where Ã, the cumulated activity, was estimated by integration of the time–activity curve and A0 was the administered activity. Effective and biologic clearance rates and corresponding half-times were derived from the fitted curves.
Normal-Organ Uptake and Dosimetry
Regions of interest were drawn on the PET images over normal organs, including lacrimal gland, salivary gland, thyroid gland, lung, right atrium, ventricular myocardium, liver, renal parenchyma, pancreas, spleen, adrenal gland, and bladder. Multiple regions of interest were used to generate volumes of interest of a representative site in organs that were copied to all scans (Hermes Medical Solutions). Activity concentration per unit mass (kBq/g) was generated for organs, and area under the activity concentration–time curves were integrated. Whole-organ areas under the curve were estimated by multiplying the activity concentration area under the curve by the respective organ masses, as given in the OLINDA/EXM dosimetry program, which was then used to derive the organ residence times (19).
Absorbed radiation doses to the whole body and various organs and the effective dose were calculated using the image-derived cumulated activities/residence time and the OLINDA/EXM program (19). The anatomic model in OLINDA for which whole-body mass most closely matched that of the patient was used, with the organ masses in OLINDA then scaled in proportion to the patient-to-anatomic model whole-body mass ratio.
RESULTS
Patients and 18F-MFBG Administration
Ten patients, including 5 with neuroblastoma (age, 5–23; 3 males and 2 females) and 5 with paraganglioma/pheochromocytoma (age 16–68; 2 males and 3 females), were imaged. All patients with neuroblastoma had recurrent disease. The patients with paraganglioma/pheochromocytoma had measurable and progressive disease as noted on standard imaging (CT/MRI/MIBG scanning), performed as standard of care. All patients had prior 123I-MIBG scans obtained within 4 wk before the 18F-MFBG PET scan that included whole-body planar imaging and SPECT/CT of the chest, abdomen, and pelvis. The injections were tolerated well, with no reactions or adverse events seen in any patients. The injected activity ranged from 162 to 436 MBq (4.37–11.8 mCi). The mean specific activity was 610.5 MBq/μg (16.5 mCi/μg) (range, 573.5 - > 1,061.9 MBq/μg or 15.5 - > 28.7 mCi/μg) for 18F-MFBG, produced via the initial radiosynthesis method, and 138.1 MBq/μg (3.73 mCi/μg) (range, 16.7–442.2 MBq/μg or 0.45–11.95 mCi/μg) for the ALP-MFBG–based radiosynthesis method. Radiochemical purity was greater than 95% in all batches.
Metabolite analysis showed that MFBG (mean ± SD), eluted with a retention time of 12.5 min, accounted for 90.0% ± 6.7% and 92.4 ± 4.6 of the plasma-borne activity in the blood samples obtained at 60 and 120 min after injection, respectively. The 18F-MFBG thus indicated excellent stability in vivo.
Whole-Body and Blood Kinetics
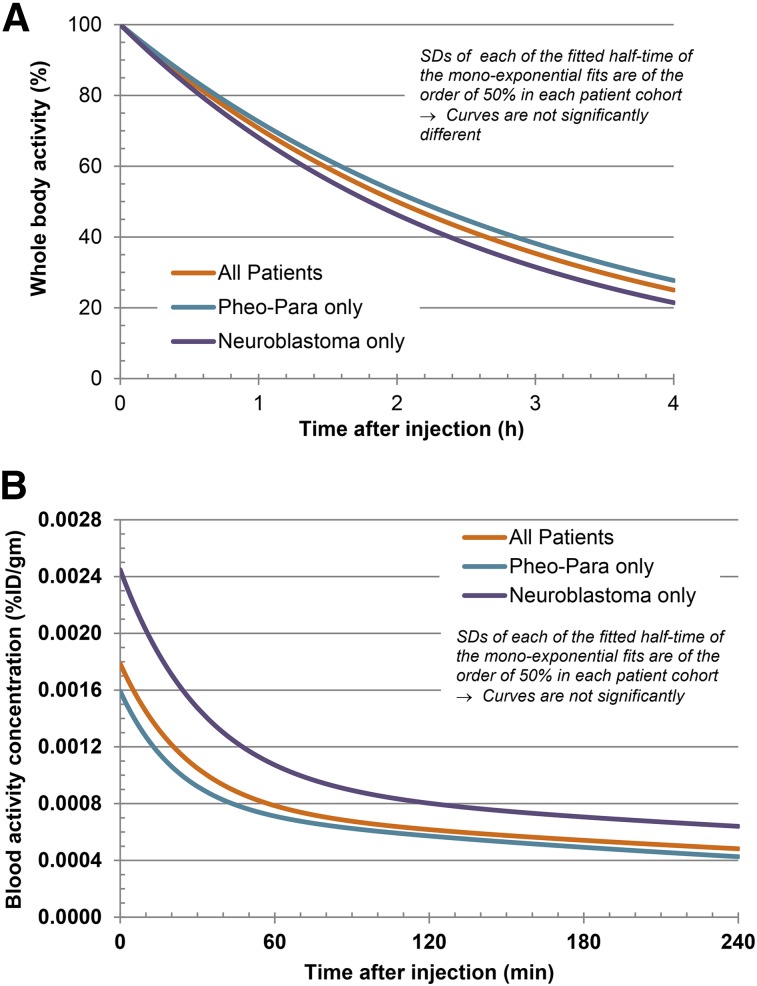
Blood clearance was biexponential, characterized by an initial rapid phase followed by a mean (±SD) half-time of biologic clearance of 0.31 ± 0.20 h (range, 0.12–0.35 h) for the fast component, α phase (57.6%), and 6.09 ± 3.8 h (range, 2.2–15.0 h) for the slow component, β phase (42.4%) (Fig. 1A). For patients with paraganglioma/pheochromocytoma, the mean (±SD) half-time of clearance was 0.26 ± 0.12 h for the α phase (57.3%) and 4.8 h ± 2.7 h (42.7%) for the β phase, whereas corresponding values for patients with neuroblastoma were 0.36 ± 0.26 h for the α phase (57.6%) and 7.3 ± 4.6 h for the β phase (42.4%). Whole-body biologic clearance was monoexponential, with a mean half-life (±SD) of 1.95 ± 1.22 h (range, 1.2–5.2 h) (Fig. 1B).
FIGURE 1.
Whole-body (A) and -blood (B) clearance time–activity curves. Whole-body activity showed monoexponential clearance, and blood activity showed biexponential clearance. Para = paraganglioma; Pheo = pheochromocytoma.
Biodistribution and Normal-Organ Uptake
18F-MFBG biodistribution (Fig. 2A) was characterized by a rapid drop in the cardiac blood-pool activity (Figs. 2A and 2C). Activity in the urinary bladder was seen early with significant excretion—an average of 45% within the first hour and 61%–95% excretion noted by 3–4 h after injection. Liver showed prominent activity that decreased with time; diffuse uptake in the left lobe of the liver (mean SUVmax, 6.0; range, 2.3–11.8) was greater than that in the right lobe (mean SUVmax, 3.9; range, 1.1–7.1) (Fig. 2F). The mean biologic liver clearance half-time (T1/2) was 80 min. Uptake in the ventricular myocardium (mean SUVmax, 3.9; range, 0.63–7.8 at 1 h after injection) showed an initial prominent decrease followed by minimal decrease at later times (Figs. 2A and 2C). Renal activity was mainly in the pelvicalyceal system, with minimal activity seen in the cortex. Diffuse mild splenic activity was seen (mean SUVmax, 1.5; range, 0.72–2.7 at 1 h after injection). Salivary gland uptake was prominently seen at all scanning time points, with a decrease in later scans (mean SUVmax, 8.6; range, 3.8–12.4 at 1 h after injection). Lacrimal gland activity was seen, also decreasing in later images (mean SUVmax, 3.1; range, 0.68–3.8 at 1 h after injection). Prostate gland uptake was seen in males (mean SUVmax, 8.6; range, 4.6–10.8 at 1 h after injection). Physiologic uptake was seen in adrenal glands and pancreas with an average SUVmax of 6.3 (range, 3.9–9.2) and 3.6 (range, 1.23–5.8), respectively. Diffuse physiologic thyroid uptake was also seen (mean SUVmax, 3.5; range, 0.7–6.8 at 1 h after injection). Organ uptake curves are shown in Figure 3.
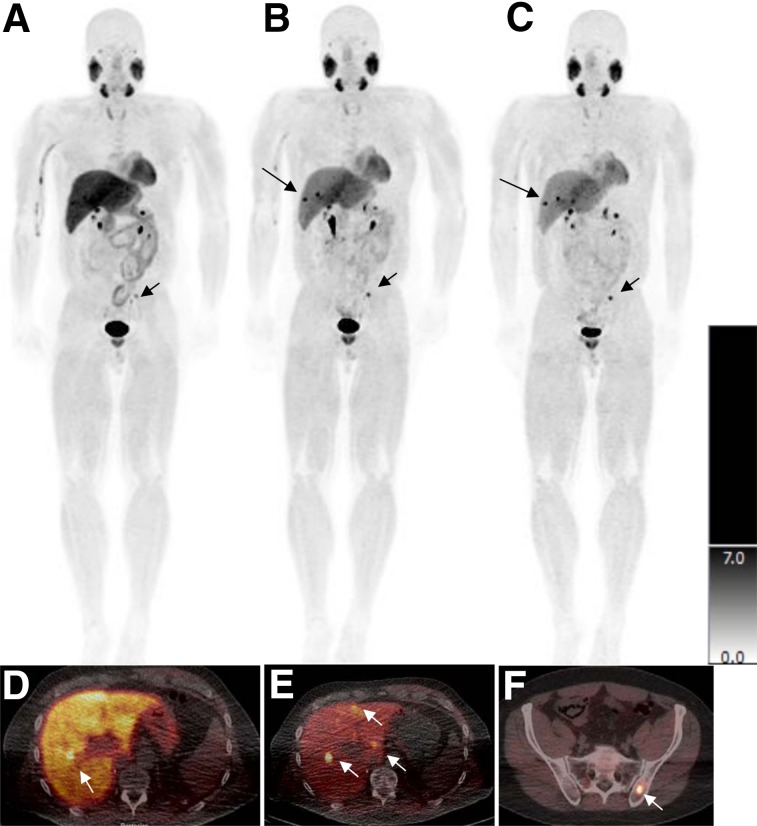
FIGURE 2.
Patient with metastatic pheochromocytoma. Whole-body maximum-intensity-projection scans of 18F-MFBG obtained 30–60 min after injection (A), 1–2 h after injection (B), and 3–4 h after injection (C) as against a uniform SUV scale (right bar). Lesions are distinctly seen in the liver at 1–2 h and 3–4 h after injection (B and C; arrows). Fused images show lesions more distinctly in liver (D and E; arrows). Lesion in maximum-intensity-projection image is localized to left iliac bone (F; short arrow).
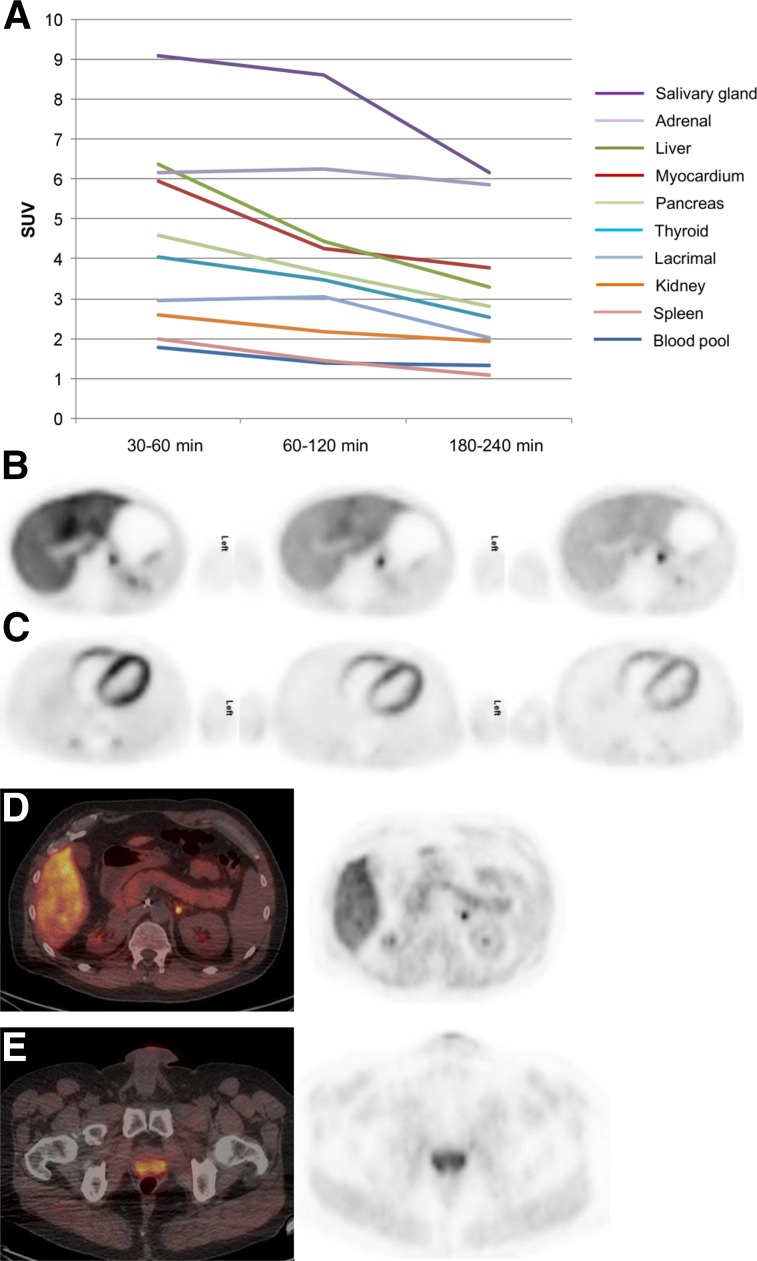
FIGURE 3.
Uptake in normal organs at various scan times after injection. (A) Uptake decreases from scan 1 (0.5–1 h after injection) to scan 2 (1–2 h after injection) and scan 3 (3–4 h after injection). (B) Prominent activity is seen in liver, which decreases over time. Focal uptake posteromedially is uptake along adrenal (SUV 5.6). (C) Cardiac activity is most prominent in early images, decreasing with time; distribution is seen along the ventricular myocardium. (D) Diffuse uptake is seen along pancreas (SUV 3.5); posteromedial uptake is physiologic uptake in adrenal gland. (E) Uptake is seen in prostate (SUV 5.6).
Radiation Doses to Normal Organs
The average absorbed dose estimates are summarized in Table 1. The urinary bladder wall received the highest radiation dose, with a mean absorbed dose (±SD) of 0.186 ± 0.195 mGy/MBq (0 0.689 ± 0.720 cGy/mCi). The mean absorbed dose to salivary glands was 0.058 ± 0.069 mGy/MBq (0.213 ± 0.253 cGy/mCi), liver was 0.046 ± 0.026 mGy/MBq (0.171 ± 0.097cGy/mCi), and kidney was 0.028 ± 0.025 mGy/MBq (0.105 ± 0.092 cGy/mCi). The myocardial/heart wall absorbed dose was 0.031 ± 0.016 mGy/MBq (0.115 ± 0.057 cGy/mCi). The mean total-body dose was 0.011 ± 0.011 mGy/MBq (0.042 ± 0.041 cGy/mCi), and the mean effective dose was 0.023 ± 0.012 mSv/MBq (0.085 ± 0.043 cSv [rem]/mCi).
TABLE 1.
18F-MFBG: Normal-Organ Absorbed Doses
| mGy/MBq |
cGy/mCi |
|||
| Organ | Mean | SD | Mean | SD |
| Salivary gland | 0.058 | 0.069 | 0.213 | 0.253 |
| Adrenals | 0.023 | 0.024 | 0.085 | 0.089 |
| Brain | 0.004 | 0.002 | 0.014 | 0.008 |
| Breasts | 0.005 | 0.002 | 0.017 | 0.008 |
| Gallbladder wall | 0.012 | 0.005 | 0.046 | 0.020 |
| Lower large intestine wall | 0.011 | 0.005 | 0.041 | 0.020 |
| Small intestine | 0.009 | 0.004 | 0.033 | 0.015 |
| Stomach wall | 0.007 | 0.003 | 0.027 | 0.012 |
| Upper large intestine wall | 0.009 | 0.004 | 0.032 | 0.014 |
| Heart wall | 0.031 | 0.016 | 0.115 | 0.057 |
| Kidneys | 0.028 | 0.025 | 0.105 | 0.092 |
| Liver | 0.046 | 0.026 | 0.171 | 0.097 |
| Lungs | 0.009 | 0.005 | 0.035 | 0.017 |
| Muscle | 0.006 | 0.003 | 0.024 | 0.010 |
| Ovaries | 0.011 | 0.005 | 0.041 | 0.019 |
| Pancreas | 0.032 | 0.021 | 0.119 | 0.078 |
| Red marrow | 0.006 | 0.002 | 0.022 | 0.008 |
| Osteogenic cells | 0.007 | 0.004 | 0.027 | 0.013 |
| Skin | 0.004 | 0.002 | 0.015 | 0.007 |
| Spleen | 0.015 | 0.008 | 0.057 | 0.028 |
| Testes | 0.008 | 0.006 | 0.030 | 0.023 |
| Thymus | 0.006 | 0.003 | 0.022 | 0.010 |
| Thyroid | 0.032 | 0.028 | 0.119 | 0.103 |
| Urinary bladder wall | 0.186 | 0.195 | 0.689 | 0.720 |
| Total body | 0.011 | 0.011 | 0.042 | 0.041 |
| Effective dose (mSv/MBq)/*(cSv or rem/mCi) | 0.023 | 0.012 | 0.085* | 0.043* |
Preliminary Assessment of Lesion Targeting and Uptake
Targeting of lesions was seen in all patients. Both skeletal and soft-tissue lesions were visualized with high contrast, even in the initial scans acquired 30–60 min after injection, with most lesions seen at 3–4 h after injection. The mean (±SD) lesion SUVmax for body weight was 8.6 ± 9.6 (range, 1.3–67.6) at 1–2 h after injection and 9.2 ± 11.4 (range, 1.1–80.7) at 3–4 h after injection. The tumor-to-background (T/BG) ratios obtained by comparison of bone with adjacent normal bone and muscle for soft tissue ranged from 1.35 to 36.2 at 1–2 h after injection and 1.22 to 28.5 at 3–4 h after injection for bone lesions and 1.2 to 35.2 at 1–2 h after injection and 1.4 to 31.4 at 3–4 h after injection for soft-tissue lesions. The lung lesions were visualized at lower uptake because of no uptake in the lungs, with an SUV range of 1.3–4.7 at 1–2 h after injection and 0.8–5.0 at 3–4 h after injection.
Assessment of lesions detected at various imaging times indicated that 103 lesions were seen at 30- to 60-min imaging, and 117 lesions were detected at 1- to 2-h imaging as compared with 122 lesions at 3- to 4-h imaging. The 5 additional lesions seen at the last time point of imaging were seen in the liver of 2 patients (Fig. 4). All lesions seen on 123I-MIBG imaging were seen on 18F-MFBG scans. There were no lesions seen by 123I-MIBG that were not targeted by 18F-MFBG. However, 18F-MFBG showed additional lesions in all patients, with an overall 122 lesions seen versus 63 by 123I-MIBG (Table 2). An additional 59 sites seen on 18F-MFBG included bones (n = 29) and 30 soft-tissue lesions, comprising lung (n = 6), liver (n = 8), nodes (n = 10), and other soft-tissue lesions (n = 6).
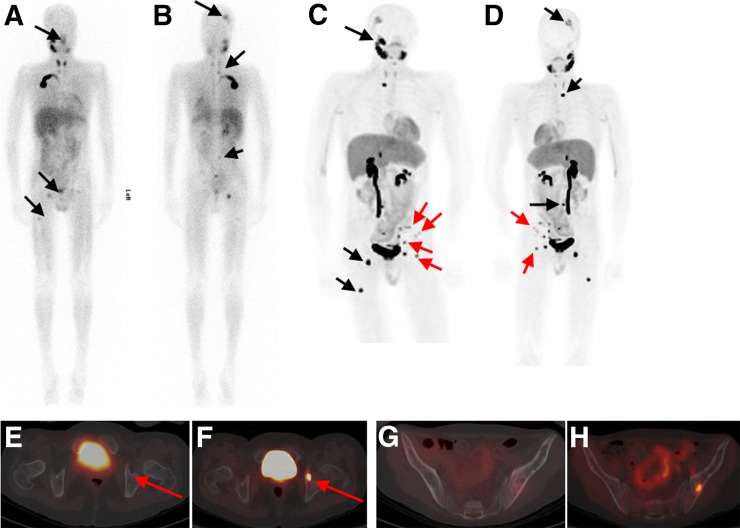
FIGURE 4.
Patient with neuroblastoma for follow-up evaluation and possible therapy with 131I-MIBG. 123I-MIBG images (A, anterior; B, posterior) show foci of suspicious activity in skull, lumbar vertebra, right and left acetabula, and right femur (black arrows). Patient underwent imaging with 162 MBq of 18F-MFBG a wk later. Whole-body maximum-intensity-projection scans with 18F-MFBG (C and D) show all lesions seen on 123I-MIBG scan but with greater contrast and clarity (black arrows). In addition, several lesions are seen on 18F-MFBG scan only (red arrows) that are not visible on 123I-MIBG images. For example, fused PET/CT transaxial 18F-MFBG image (F) shows intense uptake in left acetabulum (red arrow), suspicious for disease, that is not seen on 123I-MIBG SPECT/CT fused transaxial image (E). Also, left iliac bone lesions are clearly avid on 18F-MFBG (H) vs. 123I-MIBG imaging (G).
TABLE 2.
Lesion Detection Per Patient with 18F-MFBG and 123I-MIBG
| Patient no. | 123I-MIBG + lesion no. | 18F-MFBG + lesion no. |
| Neuroblastoma | ||
| 1 | 9 | 11 |
| 2 | 2 | 3 |
| 3 | 2 | 5 |
| 4 | 1 | 2 |
| 5 | 8 | 13 |
| Pheochromocytoma/paraganglioma | ||
| 1 | 2 | 6 |
| 2 | 14 | 29 |
| 3 | 2 | 4 |
| 4 | 9 | 20 |
| 5 | 14 | 29 |
DISCUSSION
Given the current limitations of imaging with 123I-MIBG, and in an effort to develop a better imaging biomarker for NETs, we performed first-in-human imaging with 18F-MFBG in 10 patients with metastatic neuroblastoma and paraganglioma/pheochromocytoma. The purpose of this study was to determine the safety, pharmacokinetics, normal-tissue distribution, and organ dosimetry of 18F-MFBG. In addition, an initial evaluation of tumor-targeting properties and detection of lesions at various time points of imaging after injection was performed.
18F-MFBG injections were well tolerated, with no toxicity or reactions seen in any patients. The overall distribution of 18F-MFBG appeared similar to that of 123I-MIBG. The 18F-MFBG distribution showed rapid clearance from the blood pool, with a mean biologic T1/2 of 18 min for early phase and 6 h for slower phase. Although prominent uptake was seen in normal liver, the activity decreased with time and lesions were detectable at 1 h after injection with high contrast, though later images showed more contrast and a higher number of lesions in some patients. The differential uptake in the right versus left lobe was noted, as with 123I-MIBG scans. Although the exact mechanism for this difference is unknown, it is postulated to be secondary to differential blood supply and not due to direct uptake mechanism (20). The kidneys were the primary route of excretion and the bladder was the critical organ, similar to 123I-MIBG. Because excreted activity accumulates in the bladder by 1 h, voiding before imaging will reduce bladder exposure. With respect to 123I-MIBG biodistribution, similar 18F-MFBG myocardial uptake was seen, and although prominent in the initial scans, the activity decreased with time. Gastrointestinal uptake was seen but was not prominent and did not affect the detection of small lesions in the abdomen and pelvis. Additionally, because of the superior resolution of PET compared with SPECT, it is possible to visualize uptake in lesions in the abdomen and pelvis that would otherwise be obscured by excreted activity in the bowel, ureters, and bladder.
The clearance of 18F-MFBG from the blood pool and organs is more rapid than 123I-MIBG when compared with published data (21,22). As with 123I-MIBG, the blood clearance of 18F-MFBG was biexponential with a shorter biologic T1/2 (second component) for 18F-MFBG (ranging between 2 and 15 h) compared with 123I-MIBG (ranging between 9 and 130 h) (22). In addition, there was faster and increased excretion into the urine of 18F-MFBG (61%–95%) compared with 123I-MIBG (11%–26%) (22) by 3 h after injection. The whole-body biologic T1/2 was monoexponential for 18F-MFBG up to 3- to 4-h imaging and was much shorter (1.2–5.2 h) in comparison to the biexponential 123I/131I-MIBG clearance, with a second-phase T1/2 of 19–45 h (21). The shorter half-time or faster clearance allows for earlier imaging and detection of lesions as a result of high contrast and T/BG ratios noted as early as 1 h after injection, compared with the recommended 24 h postinjection imaging for 123I-MIBG. The radiation exposure (overall effective dose) for 18F-MFBG (0.023 mSv/MBq) was comparable to that of 123I-MIBG (0.014 mSv/MBq for adults and 0.026 mSv/MBq for 10 y olds (3,21–23)). The relative organ doses from 18F-MFBG were also comparable to those of 123I-MIBG—lower for liver and spleen and slightly higher for heart with 18F-MFBG. The fact that the effective doses are similar despite faster blood and whole-body clearance is likely secondary to the higher energy associated with the 18F emissions.
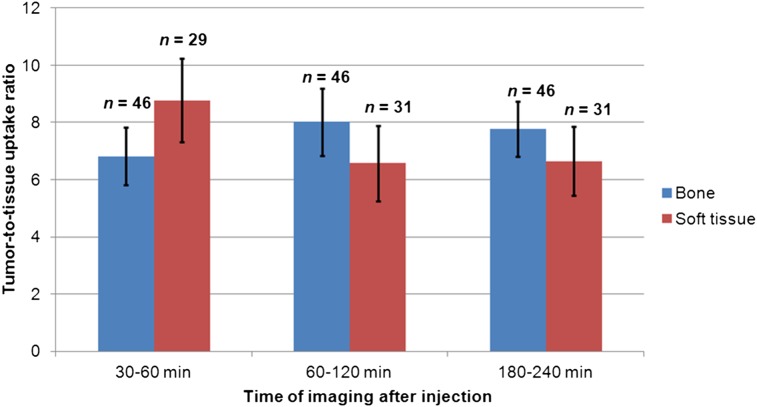
The PET images provided high-contrast visualization of lesions due to rapid blood and whole-body background clearance of 18F-MFBG, resulting in high T/BG ratios. 18F-MFBG showed good targeting of both bone and soft-tissue lesions, and high T/BG ratios were noted for both (Fig. 5). Despite modest uptake in the liver, lesions were detectable with relatively high contrast at the 3- to 4-h postinjection imaging times (Figs. 2 and 4). We found that both bone and soft-tissue lesions showed high uptake and were prominently visualized at both 1–2 h and 3–4 h postinjection imaging times, with no statistically significant difference between uptake in lesions at 1–2 h after injection versus 3–4 h after injection (P = 0.31 for bone and 0.23 for soft tissue). Given the high contrast of 18F-MFBG images, it is possible to image patients with lower activities, which can further reduce radiation exposure (images in Figs. 4C and 4D were obtained with a 162 MBq dose of 18F-MFBG).
FIGURE 5.
Tumor–to–normal bone and soft-tissue uptake ratios at different scan times after injection of 18F-MFBG (scan 1 at 30–60 min, scan 2 at 60–120 min, and scan 3 at 180–240 min). Uptake ratios were based on mean SUVs in respective tissues of 10 patients. Numbers = number of observations (i.e., lesions); error bars = SE of mean.
Although tracer uptake in lesions continued to increase up to the last imaging time point (3–4 h after injection), a decrease in the T/BG ratios was noted at 3–4 h after injection. Although the overall number of lesions detected was highest for the last time point of imaging, no statistically significant difference was evident in the number of lesions detected between 1 and 2 h after injection versus 3 and 4 h after injection (P = 0.24). Five additional lesions were detected at 3–4 h postinjection imaging versus 1- to 2-h imaging and were liver lesions seen in 2 patients. However, other liver lesions present in these 2 patients were detected at both 1- to 2-h and 3- to 4-h imaging. Given that imaging at 1–2 h after injection showed the highest T/BG ratios for both soft-tissue and bone lesions, this would be the optimal time point for imaging to provide adequate high-contrast images and good lesion detection (Fig. 3).
123I-MIBG is Food and Drug Administration–approved for imaging neuroendocrine malignancies and is extensively used in the clinical assessment of neuroblastoma (24). The biodistribution of 18F-MFBG is similar to that of 123I-MIBG, with the added advantage of clearing more rapidly from the body as well as higher resolution and improved assessment of lesion uptake (SUV) by PET imaging. Another advantage of 18F-MFBG, particularly in pediatric patients, is the ability to image with PET/MR, which would reduce radiation exposure by eliminating the CT component of imaging. 18F-MFBG PET/CT imaging also overcomes several limitations of 123I-MIBG SPECT/CT imaging, such as offering single-day imaging at 1–2 h after injection versus a 2-d imaging procedure as well as reducing the lag time for scanning after injection, thereby providing a more convenient option for patients.
CONCLUSION
Imaging with 18F-MFBG is feasible and safe. It is well tolerated by adults and pediatric patients. 18F-MFBG has favorable biodistribution and acceptable organ dosimetry and targets both bone and soft-tissue lesions with high contrast, enabling early imaging at 1–2 h after injection. 18F-MFBG is highly promising for imaging patients with NETs, especially children with neuroblastoma.
DISCLOSURE
This study was funded by the Department of Radiology seed grant of Memorial Sloan Kettering Cancer Center; NIH grant R01 CA204093 (Principal Investigator: Neeta Pandit-Taskar); and the MSK Radiochemistry & Molecular Imaging Probes Core, supported in part by NIH/NCI Cancer Center support grant P30 CA008748. The development of ALP-MFBG–based radiosynthesis was funded in part by a grant from The Hartwell Foundation (Principal Investigator: Scott E. Snyder) and by NIH Research Project grant R01 (NIBIB 5 R01EB015536) (Principal Investigator: Stephen DiMango). No other potential conflict of interest relevant to this article was reported.
REFERENCES
- 1.Monclair T, Brodeur GM, Ambros PF, et al. The International Neuroblastoma Risk Group (INRG) staging system: an INRG Task Force report. J Clin Oncol. 2009;27:298–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Decarolis B, Schneider C, Hero B, et al. Iodine-123 metaiodobenzylguanidine scintigraphy scoring allows prediction of outcome in patients with stage 4 neuroblastoma: results of the Cologne interscore comparison study. J Clin Oncol. 2013;31:944–951. [DOI] [PubMed] [Google Scholar]
- 3.Bombardieri E, Giammarile F, Aktolun C, et al. 131I/123I-metaiodobenzylguanidine (mIBG) scintigraphy: procedure guidelines for tumour imaging. Eur J Nucl Med Mol Imaging. 2010;37:2436–2446. [DOI] [PubMed] [Google Scholar]
- 4.Boubaker A, Bischof Delaloye A. MIBG scintigraphy for the diagnosis and follow-up of children with neuroblastoma. Q J Nucl Med Mol Imaging. 2008;52:388–402. [PubMed] [Google Scholar]
- 5.Katzenstein HM, Cohn SL, Shore RM, et al. Scintigraphic response by 123I-metaiodobenzylguanidine scan correlates with event-free survival in high-risk neuroblastoma. J Clin Oncol. 2004;22:3909–3915. [DOI] [PubMed] [Google Scholar]
- 6.Kushner BH, Yeh SD, Kramer K, Larson SM, Cheung NK. Impact of metaiodobenzylguanidine scintigraphy on assessing response of high-risk neuroblastoma to dose-intensive induction chemotherapy. J Clin Oncol. 2003;21:1082–1086. [DOI] [PubMed] [Google Scholar]
- 7.Boubaker A, Bischof Delaloye A. Nuclear medicine procedures and neuroblastoma in childhood: their value in the diagnosis, staging and assessment of response to therapy. Q J Nucl Med. 2003;47:31–40. [PubMed] [Google Scholar]
- 8.Sisson JC, Shulkin BL. Nuclear medicine imaging of pheochromocytoma and neuroblastoma. Q J Nucl Med. 1999;43:217–223. [PubMed] [Google Scholar]
- 9.Hartung-Knemeyer V, Rosenbaum-Krumme S, Buchbender C, et al. Malignant pheochromocytoma imaging with [124I]MIBG PET/MR. J Clin Endocrinol Metab. 2012;97:3833–3834. [DOI] [PubMed] [Google Scholar]
- 10.Pentlow KS, Graham MC, Lambrecht RM, et al. Quantitative imaging of iodine-124 with PET. J Nucl Med. 1996;37:1557–1562. [PubMed] [Google Scholar]
- 11.Timmers HJ, Chen CC, Carrasquillo JA, et al. Comparison of 18F-fluoro-L-DOPA, 18F-fluoro-deoxyglucose, and 18F-fluorodopamine PET and 123I-MIBG scintigraphy in the localization of pheochromocytoma and paraganglioma. J Clin Endocrinol Metab. 2009;94:4757–4767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Piccardo A, Lopci E, Foppiani L, Morana G, Conte M. 18F-DOPA PET/CT for assessment of response to induction chemotherapy in a child with high-risk neuroblastoma. Pediatr Radiol. 2014;44:355–361. [DOI] [PubMed] [Google Scholar]
- 13.Bonfiglioli R, Nanni C, Martignani C, et al. 11C-mHED for PET/CT: principles of synthesis, methodology and first clinical applications. Curr Radiopharm. 2014;7:79–83. [DOI] [PubMed] [Google Scholar]
- 14.Garg PK, Garg S, Zalutsky MR. Synthesis and preliminary evaluation of para- and meta-[18F]fluorobenzylguanidine. Nucl Med Biol. 1994;21:97–103. [DOI] [PubMed] [Google Scholar]
- 15.Zhang H, Huang R, Cheung NK, et al. Imaging the norepinephrine transporter in neuroblastoma: a comparison of [18F]-MFBG and 123I-MIBG. Clin Cancer Res. 2014;20:2182–2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang H, Huang R, Pillarsetty N, et al. Synthesis and evaluation of 18F-labeled benzylguanidine analogs for targeting the human norepinephrine transporter. Eur J Nucl Med Mol Imaging. 2014;41:322–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hu B, Vavere AL, Neumann KD, Shulkin BL, DiMagno SG, Snyder SE. A practical, automated synthesis of meta-[18F]fluorobenzylguanidine for clinical use. ACS Chem Neurosci. 2015;6:1870–1879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barrett PH, Bell BM, Cobelli C, et al. SAAM II: Simulation, Analysis, and Modeling Software for tracer and pharmacokinetic studies. Metabolism. 1998;47:484–492. [DOI] [PubMed] [Google Scholar]
- 19.Stabin MG, Sparks RB, Crowe E. OLINDA/EXM: the second-generation personal computer software for internal dose assessment in nuclear medicine. J Nucl Med. 2005;46:1023–1027. [PubMed] [Google Scholar]
- 20.Jacobsson H, Johansson L, Kimiaei S, Larsson SA. Concentration of 123I-metaiodobenzylguanidine in left and right liver lobes: findings indicate regional differences in function in the normal liver. Acta Radiol. 1999;40:224–228. [DOI] [PubMed] [Google Scholar]
- 21.Wafelman AR, Hoefnagel CA, Maes RA, Beijnen JH. Radioiodinated metaiodobenzylguanidine: a review of its biodistribution and pharmacokinetics, drug interactions, cytotoxicity and dosimetry. Eur J Nucl Med. 1994;21:545–559. [DOI] [PubMed] [Google Scholar]
- 22.Lashford LS, Moyes J, Ott R, et al. The biodistribution and pharmacokinetics of meta-iodobenzylguanidine in childhood neuroblastoma. Eur J Nucl Med. 1988;13:574–577. [DOI] [PubMed] [Google Scholar]
- 23.Adreview. Highlights of prescribing information. Food and Drug Administration website. http://www.accessdata.fda.gov/drugsatfda_docs/label/2008/22290lbl.pdf. Accessed October 26, 2017.
- 24.Brisse HJ, McCarville MB, Granata C, et al. Guidelines for imaging and staging of neuroblastic tumors: consensus report from the International Neuroblastoma Risk Group Project. Radiology. 2011;261:243–257. [DOI] [PubMed] [Google Scholar]