Abstract
The purpose of this study was to determine the relationship of 18F-FDG uptake in the primary tumor at diagnosis, during therapy, and after therapy with a histologic response and event-free survival in pediatric and young adult patients with osteosarcoma (OS). Methods: Serial (baseline and 5 and 10 wk after start of therapy) 18F-FDG PET/CT imaging was performed in patients with newly diagnosed OS treated uniformly in a therapeutic trial at a single institution. Whole-body images were obtained approximately 1 h after injection of 18F-FDG. Logistic regression was used to study the association of tumor uptake and changes in SUVmax between 0, 5, and 10 wk for both clinical endpoints. Results: Thirty-four patients (17 males; median age, 12.2 y; age range, 6.8–19.1 y) underwent PET imaging; 25 (74%) had localized disease. Primary tumor locations included the femur (n = 17; 50%), tibia (n = 9; 26%), and humerus (n = 5; 15%). Logistic regression showed that SUVmax at 5 wk (P = 0.034) and 10 wk (P = 0.022) and percentage change from baseline at 10 wk (P = 0.021) were highly predictive of a histologic response. Using SUVmax of 4.04 at week 5, SUVmax of 3.15 at week 10, and 60% decrease from baseline at week 10 as cutoff values, we determined that the respective sensitivities were 0.93, 0.93, and 0.79 and that the respective specificities were 0.53, 0.71, and 0.76. Conclusion: SUVmax on routine images at 5 or 10 wk and percentage change in SUVmax from baseline to week 10 were metabolic predictors of a histologic response in OS. These findings may be useful in the early identification of patients who are responding poorly to therapy and may benefit from a change in treatment.
Keywords: 18F-FDG, PET/CT, osteosarcoma, pediatrics
Although representing only 0.1% of all tumors, osteosarcoma (OS) is the most common primary bone malignancy in children (1). Long-term survival rates have been improved by the addition of multiagent chemotherapy to complete surgical resection of the primary tumor. Prognosis depends, at least partially, on postoperative histologic examination of the tumor; tumor necrosis of greater than 90% after preoperative therapy predicts a good outcome (1–4). PET may be able to measure the preoperative response to therapy in OS. A noninvasive method for assessing tumor viability during treatment and before surgery would be useful for distinguishing patients who are responding well to therapy from those who are not. This knowledge could allow a change in treatment regimen for nonresponsive tumors before definitive surgery, as changing treatment postoperatively does not improve patient outcomes (5).
The purpose of our prospective study was to determine the association of semiquantitative analysis of 18F-FDG uptake (SUVmax) at baseline, 5 wk (midtreatment), and 10 wk (before definitive surgery) with a histologic response and event-free survival (EFS) in pediatric patients with OS treated in a prospective therapeutic trial.
MATERIALS AND METHODS
Patients, Imaging, and Histologic Evaluation
Thirty-four consecutive patients with OS were prospectively enrolled in an institutional review board–approved phase 2 therapeutic trial (NCT00667342) from June 2008 to May 2012. Patient consent was obtained from patients 18 y and older, and parental/guardian assent was obtained for patients younger than 18 y. Patients with newly diagnosed, high-grade, biopsy-proven localized or metastatic OS were eligible. Patients underwent conventional initial radiographic evaluation (chest CT, MRI, and radiographs of the tumor bed) and whole-body 18F-FDG PET/CT at baseline and at 5 and 10 wk after the initiation of chemotherapy; the primary tumors were resected at week 10.
The histologic response was assessed by estimating the percentage of tumor necrosis in postchemotherapy resection specimens. One full sagittal and 1 transverse slice of resected tumors were mapped and blocked for histologic processing and microscopic analysis to estimate the percentage of therapy-induced tumor necrosis. A favorable response to therapy was defined as tumor necrosis of greater than 90%.
Neoadjuvant Chemotherapy
Chemotherapy for all patients consisted of cisplatin and doxorubicin at weeks 0 and 5 and high-dose methotrexate at weeks 3, 4, 8, and 9. Bevacizumab was administered 3 d before the first dose of chemotherapy and then on day 1 of weeks 3 and 5 of chemotherapy. Surgical resection of the primary tumors was performed at week 10. After surgery, chemotherapy was resumed. Patients with localized disease continued on a regimen of bevacizumab with cisplatin, doxorubicin, and methotrexate for a total of 29 wk of chemotherapy; patients with metastatic disease also received ifosfamide and etoposide for a total of 36 wk of chemotherapy.
PET/CT Technique
18F-FDG (5.3 MBq/kg [0.15 mCi/kg]; maximum, 440 MBq [12 mCi]) was injected intravenously after either an overnight fast or a minimum 4-h fast (for afternoon studies). Patients were kept in a quiet, dark room after injection and encouraged to remain recumbent and relaxed. Transmission CT and PET emission images were acquired approximately 1 h later using a GE Discovery Lightspeed PET/CT system (GE Healthcare). CT acquisition parameters were as follows: slice thickness, 0.5 cm; tube rotation, 0.8 s; table speed, 1.5 cm/rotation; pitch, 1.5:1; 120 kV; 90 mA; and dose modulation. PET images were obtained in 2-dimensional mode from the top of the skull through the feet for 5 min/bed position in separate acquisitions (upper body: top of head through pelvis; lower body: lower extremities). Images were reconstructed using standard vendor-supplied software. After August 2011, scans were obtained using a Discovery 690 PET/CT scanner (GE Healthcare). Images of the upper body were obtained in 3-dimensional mode at 5 min/bed position and images of the lower extremities were obtained in the 3-dimensional mode at 3 min/bed position in a single acquisition.
MRI Technique
Precontrast T1-weighted and short τ inversion recovery coronal images, T1-weighted and T2-weighted axial images, and postcontrast fat-suppressed T1-weighted coronal and axial images were obtained for comparison. The dose of the gadolinium contrast agent (Magnevist; Bayer) was 0.1 mL/kg.
Image Analysis
For determination of the SUVmax, regions of interest were drawn over the primary site of malignancy on PET images and expanded with reference to CT images to encompass the tumor broadly. MR images were transferred to an offline workstation for computation of tumor volume, calculated for a prolate ellipsoid shape as 4/3π (diameter × length).
Statistical Analysis
Logistic regression was used to explore potential associations between a histologic response and the SUVmax at each PET/CT time point. Patients with tumor necrosis of greater than 90% were considered to be responders. Cox proportional-hazards regression was used to explore associations between EFS and the SUVmax. EFS was defined as the time interval from study enrollment to either the date of the first event (i.e., relapsed or progressive disease, second malignancy, or death from any cause) or the date of last contact for patients without events. P values of less than 0.05 were considered to be statistically significant. No adjustments were made for multiple comparisons in this exploratory analysis.
Percentage change was calculated as 100 × [(pretreatment scan SUVmax – follow-up scan SUVmax)/pretreatment scan SUVmax]. The prognostic values for responses were determined by analysis of the areas under the receiver operating characteristic curves.
RESULTS
The characteristics of the 34 patients with PET/CT data are shown in Table 1. The most common locations for the primary tumors were the femurs, tibiae, and humeri (Figs. 1 and 2). Approximately 75% (25/34) of the patients had nonmetastatic OS. Nine patients had metastatic disease at diagnosis (Fig. 3).
TABLE 1.
Patient Characteristics
| Characteristic | No. of patients | Percentage of patients |
| Sex | ||
| Female | 17 | 50 |
| Male | 17 | 50 |
| Race | ||
| White | 18 | 53 |
| Black | 13 | 38 |
| Asian and white | 1 | 3 |
| Multiple races | 1 | 3 |
| Other | 1 | 3 |
| Histology of tumor | ||
| OS, not further specified | 29 | 85 |
| Chondroblastic OS | 2 | 6 |
| Telangiectatic OS | 2 | 6 |
| Fibroblastic OS | 1 | 3 |
| Primary site of tumor | ||
| Femur | 17 | 50 |
| Tibia | 9 | 26 |
| Humerus | 5 | 15 |
| Fibula | 1 | 3 |
| Mandible | 1 | 3 |
| Radius | 1 | 3 |
| Stage | ||
| Localized, resectable | 25 | 74 |
| Metastatic | 9 | 26 |
Median age of patients at study enrollment was 12.2 y (range, 6.8–19.1 y).
FIGURE 1.
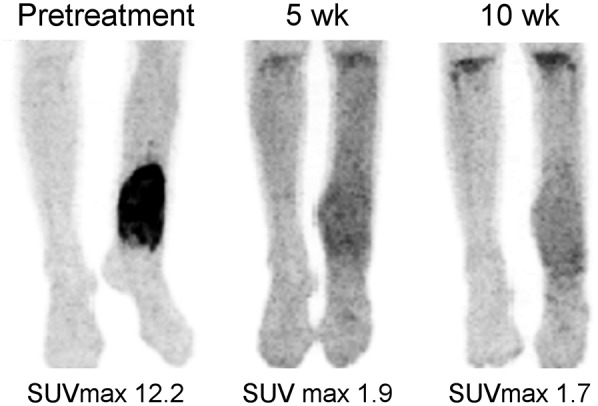
Maximum-intensity-projection images of 13-y-old girl with left tibial OS at presentation (left), after 5 wk of therapy (middle), and after 10 wk of therapy (right). SUVmax declined from 12.2 at baseline to 1.9 after 5 wk of therapy and then to 1.7 after 10 wk of therapy. Resected tumor specimen was greater than 95% necrotic. Patient remains free of disease 3 y after diagnosis.
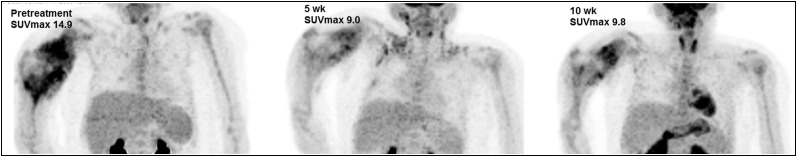
FIGURE 2.
Maximum-intensity-projection images of 8-y-old girl with right humeral OS. SUVmax declined from 14.9 at baseline to 9.0 after 5 wk of therapy and then rose slightly to 9.8 after 10 wk of therapy. Resected tumor specimen was 61% necrotic. After therapy, patient died of recurrent disease approximately 15 mo after diagnosis.
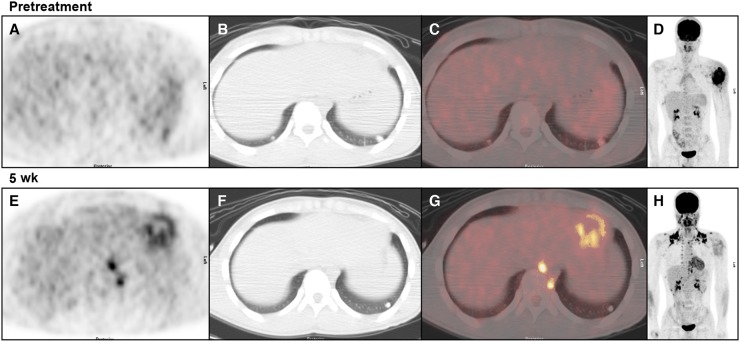
FIGURE 3.
Images of 14-y-old boy with metastatic OS of left humerus. (A–C) Transverse PET, CT, and fusion images, respectively, of lung bases before therapy. (D) Maximum-intensity-projection image depicting primary tumor. Slight uptake in 1-cm nodule at left base (SUVmax, 2.8) and no detectable uptake in 7-mm nodule at right base were noted. (E–G) Images from 5-wk evaluation. Left-base nodule has become smaller and has no detectable uptake. Right-base nodule has resolved. Intense uptake in brown fat (paravertebral on transverse images) and cardiac activity were present. (H) Uptake in left humeral OS declined from SUVmax of 12.4 to SUVmax of 4.2.
Because the SUVmax for patients with localized disease and the SUVmax for those with metastatic disease did not differ at any evaluation time point (data not shown), data from patients with localized disease and those with metastatic disease were combined. Table 2 shows summary statistics for the SUVmax at baseline, week 5, and week 10. The median SUVmax at baseline was 12.1, that at week 5 was 4.8, and that at week 10 was 3.9.
TABLE 2.
SUVmax and Timing of Imaging
| SUVmax |
|||
| Week | Mean ± SD | Median | Range |
| 0 | 11.7 ± 5.5 | 12.1 | 3.7–30.3 |
| 5 | 5.4 ± 2.7 | 4.8 | 1.9–14.7 |
| 10 | 4.7 ± 3.1 | 3.9 | 1.7–15.2 |
Differences and percentage changes in the SUVmax across time points were calculated for each patient. Changes from baseline to week 5 (P < 0.001), from baseline to week 10 (P < 0.001), and from week 5 to week 10 (P = 0.008) were statistically significant, as were percentage changes in the SUVmax across the same time periods.
Tumor volumes determined on MRI are shown in Table 3. The tumor volume did not change significantly from week 0 to week 5 (data not shown; P = 0.1315) but did decrease significantly from week 0 to week 10 (P < 0.0001). Neither the percentage change in tumor volume from week 0 to week 5 (P = 0.17) nor that from week 0 to 10 (P = 0.39) was significantly associated with EFS.
TABLE 3.
Tumor Volume and Percentage Change
| Variable | n | Mean | SD | Median | Range |
| Tumor volume (wk 0) | 30 | 310 | 288 | 243 | 193–1,558 |
| Tumor volume (wk 10) | 32 | 245 | 273 | 202 | 87–1,502 |
| % change in volume from wk 0 to wk 10 | 29 | −29.8 | 25.7 | −25.2 | 25–77 |
Volume measurements are in cubic centimeters.
Nine patients had metastatic disease: 7 in the lungs only, 1 in bone only, and 1 in both the lungs and bone (Fig. 3). Pulmonary involvement was confirmed by biopsy before therapy in 3 patients, after a clinical response in 3 patients, by biopsy after treatment in 1 patient, and by autopsy in 2 patients. In 2 patients, 18F-FDG uptake occurred in 3 nodules, with the largest diameters being 1 cm (SUVmax, 2.8), 7 mm (SUVmax, 2.1), and 5 mm (SUVmax, 1.4). Uptake in each resolved by 5 wk, and many smaller nodules without 18F-FDG uptake were present.
Patients were categorized as being responders or nonresponders on the basis of the histologic responses. The responses of 2 patients could not be evaluated in this analysis because their surgeries were delayed beyond 10 wk. On the basis of the traditional definition of a response in OS (tumor necrosis of >90%), 15 of the patients whose responses could be evaluated were considered to be responders. Table 4 shows the results of an exploratory analysis examining the SUVmax as a predictor of a histologic response and EFS. The baseline (week 0) SUVmax was not predictive of a response (P > 0.78). The SUVmax at week 5 (P = 0.034) and the SUVmax at week 10 (P = 0.022) were significant predictors of a response. Patients with a higher SUVmax at week 5 or 10 were less likely to be responders. The percentage change in the SUVmax from baseline to week 10 was also a significant predictor of a response (P = 0.021). Patients with a larger percentage decrease in the SUVmax were more likely to be responders.
TABLE 4.
Logistic Regression Analysis Examining SUVmax as Predictor of Response
| Variable | P | Odds ratio | 95% CI* |
| SUV at wk 0 | 0.7802 | ||
| SUV at wk 5 | 0.0335 | 0.62 | 0.40–0.96 |
| SUV at wk 10 | 0.0218 | 0.52 | 0.30–0.91 |
| % change from wk 0 to wk 5 | 0.0724 | 0.96 | 0.93–1.00 |
| % change from wk 0 to wk 10 | 0.0209 | 0.94 | 0.89–0.99 |
95% CI for odds ratio are included if P value was less than 0.10.
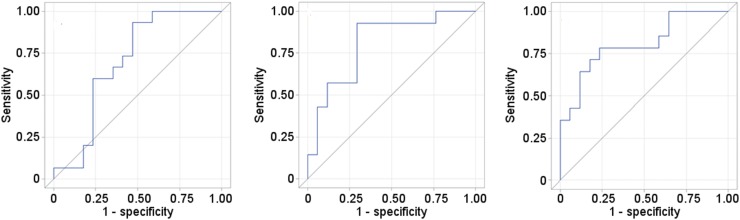
Figure 4 shows receiver operating characteristic curves for the SUVmax at week 5, the SUVmax at week 10, and the percentage change in the SUVmax from baseline to week 10. The estimated areas under the curve were 0.70, 0.81, and 0.81, respectively. The cutoff points that maximized the Youden Index were an SUVmax of 4.04 at week 5, an SUVmax of 3.15 at week 10, and a 60.24% decrease from baseline at week 10. At these cutoff points, the respective sensitivities were 0.93, 0.93, and 0.79, and the respective specificities was 0.53, 0.71, and 0.76. For the SUVmax at week 10, the positive predictive value was 72% and the negative predictive value was 92%. For the percentage change in the SUVmax from baseline to week 10, the positive predictive value was 73% and the negative predictive value was 81%.
FIGURE 4.
Receiver operating characteristic curves for SUVmax at 5 wk (left), SUVmax at 10 wk (middle), and percentage change from baseline to week 10 (right). Areas under curve for SUVmax were 0.698 at 5 wk, 0.807 at 10 wk, and 0.807 for percentage change.
Twenty-four of 34 patients were alive at the time of analysis, with a median follow-up time of 3.6 y (range, 1.4–5.3 y). All survivors had been seen or contacted within 6 mo of the time of analysis. Sixteen of 34 patients experienced events (disease relapse, progression, second malignancy, or death from any cause): 9 of 25 patients with localized disease at diagnosis and 7 of 9 patients with metastatic disease. We did not detect statistically significant associations between EFS and the SUVmax at baseline, week 5 (P = 0.11), or week 10 (P = 0.12); between EFS and changes in the SUVmax from baseline to week 5 or 10; or between EFS and percentage changes in the SUVmax from baseline to week 5 or 10. These results were true for all patients.
DISCUSSION
The present study demonstrated an association between the SUVmax during neoadjuvant therapy (at 5 wk), the SUVmax at the conclusion of neoadjuvant therapy (10 wk), and the percentage change from baseline to week 10 with tumor necrosis in patients who had newly diagnosed OS and who received standard chemotherapy in combination with bevacizumab. As in the present study, most uses of PET as a prognostic indicator of a histologic response in OS have been based on a criterion of tumor necrosis of at least 90% (6–11). In a study of 31 patients with OS, Bajpai et al. reported that both the posttherapy SUVmax (obtained after 3 cycles of chemotherapy) and the ratio of the posttherapy SUVmax to the pretherapy SUVmax were significantly associated with pathologic necrosis (10). Similarly, in a study of 70 patients who had high-grade OS and who underwent 18F-FDG PET/CT scanning before and after chemotherapy, the posttherapy SUVmax predicted a histologic response. A threshold of a posttherapy SUVmax of greater than 5 enabled the prediction of poor responders, with a positive predictive value of nearly 90% (7). A metaanalysis of 8 studies comprising 178 patients with OS found that a postchemotherapy SUVmax of less than or equal to 2.5 and a ratio of the posttherapy SUVmax to the pretherapy SUVmax of less than or equal to 0.5 were valuable for predicting a histologic response to chemotherapy (12). The studies in that metaanalysis used only PET scans obtained just before resection. A review of 10 prospectively and 12 retrospectively conducted studies concluded that despite variations in methods, 18F-FDG PET and 18F-FDG PET/CT appeared to be sensitive and reliable for assessing metabolic responses in patients with OS (13).
If poor outcomes could be predicted before the completion of neoadjuvant chemotherapy, then clinical outcomes might be changed because surgical intervention could be done sooner, preventing the development of resistance to chemotherapy agents (11). To our knowledge, no studies of OS have shown the SUVmax alone to be predictive of a response before the completion of neoadjuvant chemotherapy. However, metabolic tumor volume and total lesion glycolysis have predictive values after a single course of neoadjuvant chemotherapy in OS (14). These findings have potential clinical implications because changing treatment postoperatively does not improve patient outcomes (5). Thus, changing neoadjuvant treatment in nonresponders before surgery might increase survival in this population. In patients with breast cancer, nonresponders identified by PET had better outcomes if the neoadjuvant chemotherapy regimen was changed before surgery than did nonresponders who remained on the standard treatment protocol (15).
Tumor volume measurements are commonly used as response criteria in clinical oncology therapeutic trials. Although the present study showed small declines in tumor volumes from baseline measurements to presurgical measurements, the changes were not statistically related to EFS. Both Evilevitch et al. (16) and Benz et al. (17) showed that changes in CT-measured volumes before and after preoperative treatment in patients with high‐grade sarcomas did not lead to the prediction of a treatment response (16,17).
In addition to the SUVmax, metabolic tumor volume has prognostic significance, with the metabolic tumor volume for tumors with SUVs of greater than 2 predicting metastasis more accurately than the SUVmax (18). A preliminary radiomic analysis of our results suggested that baseline metabolic tumor volume may predict survival (19). The heterogeneity of tumors can affect imaging findings because of differences in size, vascularity, and location (20,21). Even when the strongest prognostic indicators (primary tumor location, histologic response, and metastasis present at diagnosis) are controlled for, young adults with OS have worse outcomes than do their pediatric counterparts (22).
Additional studies to monitor responses and better characterize tumors are still needed. Different radiotracers can be used to evaluate lesions. In a preclinical mouse model, different phenotypes of OS were demonstrated with 3 different radiotracers: 18F-FDG, 18F-fluoromisonidazole, and 18F-fluoride (23). 18F-FDG uptake was higher in osteolytic lesions, and 18F-fluoromisonidazole and 18F-fluoride uptake were higher in osteoblastic lesions (23). Such disease characterization provides the framework for the further evaluation of tumor biology to determine a treatment response.
The findings of the present study indicated that the maximal impact of therapy on tumor metabolism occurs early during treatment, that further effects on metabolism in subsequent treatments are slight, and that additional presurgical 18F-FDG PET/CT scans may not be useful for assessing a tumor metabolic response. Using PET/CT for the early determination of a tumor response (by week 5 of therapy) may be helpful in the tailoring of further treatment. However, subsequent PET/CT scans before surgery do not appear to offer additional benefit and will increase radiation exposure.
Few studies have described the results of interim 18F-FDG PET/CT during treatment for OS. Im et al. described interim 18F-FDG PET/CT findings in 14 patients with OS; in their study, a variety of chemotherapeutic regimens were used, and the number of courses of chemotherapy before the interim evaluation varied—with 11 patients having had 1 course of chemotherapy between the interim and presurgical evaluations and 3 patients having had 2 courses (14). In those 14 patients, the SUVmax declined from 7.73 ± 3.04 (mean ± SD) to 5.3 ± 3.44 at the interim evaluation (∼29%) and declined slightly further to 3.98 ± 2.18 before surgery (49%) (14). The results of the present study differ from those of Im et al. in that we found a much larger change in the SUVmax soon after the first course of chemotherapy (54%) but little change later. The mean change in the SUVmax from baseline to week 5 was −6.37 (P < 0.001), and that from week 5 to week 10 was less than 1.0 (P = 0.013).
If, indeed, the SUVmax indicates cellular viability, then most cell killing occurs early during treatment. In future studies, the use of 18F-FDG PET/CT for routine evaluation could be limited to either 5 or 10 wk because the results at these time points are similar. However, if an intervention is developed on the basis of a PET-determined response, then the 5-wk evaluation for a change in therapy seems preferable—to reduce the opportunity for chemotherapy-resistant clones to appear during subsequent courses of therapy.
The data from the present study did not demonstrate a statistically significant relationship between PET values at diagnosis, interim evaluation, or presurgical evaluation and EFS. The reason may be the small sample size; statistical significance may become apparent with larger numbers of subjects (for the week 5 PET study, P = 0.11; for the week 10 PET study, P = 0.12). One group did find a poor outcome for patients with a higher SUVmax at either baseline or the conclusion of chemotherapy (6). In that study, patients did not undergo interim 18F-FDG PET/CT (6). In agreement with our findings, separate multiinstitutional Children’s Oncology Group therapeutic trials in intermediate-risk rhabdomyosarcoma and high-risk rhabdomyosarcoma revealed no difference between the EFS of patients who experienced complete metabolic remission (either during chemotherapy or after chemotherapy) and the EFS of those who experienced a partial response, stable disease, or progressive disease (24). In Ewing sarcoma, PET correlates with survival in children and adults with recurrent disease (25,26). Although PET variables may correlate with responses in certain sarcomas, current evidence is insufficient to state definitively whether PET can be useful for prognosis in children and young adults with OS.
The present study differed from other reports in that it encompassed a group of patients who had a uniform diagnosis, were treated with the same protocol, and were evaluated at specific, predetermined time points. The limitations of the present study include the relatively small sample size as well as the addition of bevacizumab and its effect on the tumors and vascularity. Because the effect of antiangiogenic therapy on tumor glucose metabolism is unknown, whether our results are applicable to patients who receive only conventional, cytotoxic chemotherapy is uncertain.
We did not find 18F-FDG PET/CT to be useful for evaluating the response of metastatic disease within the time period of the present study. This finding reflects the pattern of metastatic disease that our patients experienced early in their disease process—that of predominantly pulmonary nodules, most very small and detected on CT but too small for an accurate characterization of metabolic activity. This observation does not preclude the potential utility of 18F-FDG PET/CT for monitoring a response or for surveillance for recurrent/metastatic disease after the completion of neoadjuvant therapy and definitive surgery. The imaging component of the present study was conducted at a single institution. Larger, cooperative group trials using a uniform treatment protocol with specific imaging intervals would be beneficial for validating our findings that the SUVmax is a clinically useful biomarker for assessing the tumor response during therapy in patients with OS.
CONCLUSION
The SUVmax on routine images at 5 and 10 wk and the percentage change in the SUVmax from baseline to week 10 were predictive of a histologic response in patients with OS. With further validation of our findings, perhaps treatment courses could be altered early—before surgical intervention—resulting in more favorable outcomes in patients with this highly malignant neoplasm.
DISCLOSURE
This work was supported, in part, by the American Lebanese Syrian Associated Charities (ALSAC) and by grant 5R25CA023944 from the National Cancer Institute (to James C. Davis). No other potential conflict of interest relevant to this article was reported.
Acknowledgments
We thank Sandra Gaither and Vella Laws-Bell for assistance in preparing this article and Cherise Guess, PhD, ELS, of the Department of Scientific Editing, St. Jude Children’s Research Hospital, for editorial expertise. This work was presented, in part, at the 2013 Annual Meeting of the Society of Nuclear Medicine and Molecular Imaging in Vancouver, British Columbia, Canada.
REFERENCES
- 1.Meyers PA, Schwartz CL, Krailo M, et al. Osteosarcoma: a randomized, prospective trial of the addition of ifosfamide and/or muramyl tripeptide to cisplatin, doxorubicin, and high-dose methotrexate. J Clin Oncol. 2005;23:2004–2011. [DOI] [PubMed] [Google Scholar]
- 2.Meyers PA, Heller G, Healey J, et al. Chemotherapy for nonmetastatic osteogenic sarcoma: the Memorial Sloan-Kettering experience. J Clin Oncol. 1992;10:5–15. [DOI] [PubMed] [Google Scholar]
- 3.Meyers PA, Gorlick R, Heller G, et al. Intensification of preoperative chemotherapy for osteogenic sarcoma: results of the Memorial Sloan-Kettering (T12) protocol. J Clin Oncol. 1998;16:2452–2458. [DOI] [PubMed] [Google Scholar]
- 4.Provisor AJ, Ettinger LJ, Nachman JB, et al. Treatment of nonmetastatic osteosarcoma of the extremity with preoperative and postoperative chemotherapy: a report from the Children’s Cancer Group. J Clin Oncol. 1997;15:76–84. [DOI] [PubMed] [Google Scholar]
- 5.Rosen G, Caparros B, Huvos AG, et al. Preoperative chemotherapy for osteogenic sarcoma: selection of postoperative adjuvant chemotherapy based on the response of the primary tumor to preoperative chemotherapy. Cancer. 1982;49:1221–1230. [DOI] [PubMed] [Google Scholar]
- 6.Costelloe CM, Macapinlac HA, Madewell JE, et al. 18F-FDG PET/CT as an indicator of progression-free and overall survival in osteosarcoma. J Nucl Med. 2009;50:340–347. [DOI] [PubMed] [Google Scholar]
- 7.Cheon GJ, Kim MS, Lee JA, et al. Prediction model of chemotherapy response in osteosarcoma by 18F-FDG PET and MRI. J Nucl Med. 2009;50:1435–1440. [DOI] [PubMed] [Google Scholar]
- 8.Kong CB, Byun BH, Lim I, et al. 18F-FDG PET SUVmax as an indicator of histopathologic response after neoadjuvant chemotherapy in extremity osteosarcoma. Eur J Nucl Med Mol Imaging. 2013;40:728–736. [DOI] [PubMed] [Google Scholar]
- 9.Huang TL, Liu RS, Chen TH, Chen WY, Hsu HC, Hsu YC. Comparison between F-18-FDG positron emission tomography and histology for the assessment of tumor necrosis rates in primary osteosarcoma. J Chin Med Assoc. 2006;69:372–376. [DOI] [PubMed] [Google Scholar]
- 10.Bajpai J, Kumar R, Sreenivas V, et al. Prediction of chemotherapy response by PET-CT in osteosarcoma: correlation with histologic necrosis. J Pediatr Hematol Oncol. 2011;33:e271–e278. [DOI] [PubMed] [Google Scholar]
- 11.Bajpai J, Gamnagatti S, Kumar R, et al. Role of MRI in osteosarcoma for evaluation and prediction of chemotherapy response: correlation with histological necrosis. Pediatr Radiol. 2011;41:441–450. [DOI] [PubMed] [Google Scholar]
- 12.Hongtao L, Hui Z, Bingshun W, et al. 18F-FDG positron emission tomography for the assessment of histological response to neoadjuvant chemotherapy in osteosarcomas: a meta-analysis. Surg Oncol. 2012;21:e165–e170. [DOI] [PubMed] [Google Scholar]
- 13.Caldarella C, Salsano M, Isgrò MA, Treglia G. The role of fluorine-18-fluorodeoxyglucose positron emission tomography in assessing the response to neoadjuvant treatment in patients with osteosarcoma. Int J Mol Imaging. 2012;2012:870301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Im HJ, Kim TS, Park SY, et al. Prediction of tumour necrosis fractions using metabolic and volumetric 18F-FDG PET/CT indices, after one course and at the completion of neoadjuvant chemotherapy, in children and young adults with osteosarcoma. Eur J Nucl Med Mol Imaging. 2012;39:39–49. [DOI] [PubMed] [Google Scholar]
- 15.Coudert B, Pierga JY, Mouret-Reynier MA, et al. Use of [18F]-FDG PET to predict response to neoadjuvant trastuzumab and docetaxel in patients with HER2-positive breast cancer, and addition of bevacizumab to neoadjuvant trastuzumab and docetaxel in [18F]-FDG PET-predicted non-responders (AVATAXHER): an open-label, randomised phase 2 trial. Lancet Oncol. 2014;15:1493–1502. [DOI] [PubMed] [Google Scholar]
- 16.Evilevitch V, Weber WA, Tap WD, et al. Reduction of glucose metabolic activity is more accurate than change in size at predicting histopathologic response to neoadjuvant therapy in high-grade soft-tissue sarcomas. Clin Cancer Res. 2008;14:715–720. [DOI] [PubMed] [Google Scholar]
- 17.Benz MR, Allen-Aeurbach MS, Eilber FC, et al. Combined assessment of metabolic and volumetric changes for assessment of tumor response in patients with soft-tissue sarcomas. J Nucl Med. 2008;49:1579–1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Byun BH, Kong CB, Park J, et al. Initial metabolic tumor volume measured by 18F-FDG PET/CT can predict the outcome of osteosarcoma of the extremities. J Nucl Med. 2013;54:1725–1732. [DOI] [PubMed] [Google Scholar]
- 19.Im H-J, Wu H, Yi Z, Wu J, Shulkin B, Cho S. Baseline metabolic tumor volume measured by FDG PET/CT before neoadjuvant chemotherapy predicts survival in pediatric osteosarcoma [abstract]. J Nucl Med. 2016;57(suppl 2):429. [Google Scholar]
- 20.Asselin MC, O’Connor JP, Boellaard R, Thacker NA, Jackson A. Quantifying heterogeneity in human tumours using MRI and PET. Eur J Cancer. 2012;48:447–455. [DOI] [PubMed] [Google Scholar]
- 21.Basu S, Kwee TC, Gatenby R, Saboury B, Torigian DA, Alavi A. Evolving role of molecular imaging with PET in detecting and characterizing heterogeneity of cancer tissue at the primary and metastatic sites, a plausible explanation for failed attempts to cure malignant disorders. Eur J Nucl Med Mol Imaging. 2011;38:987–991. [DOI] [PubMed] [Google Scholar]
- 22.Janeway KA, Barkauskas DA, Krailo MD, et al. Outcome for adolescent and young adult patients with osteosarcoma: a report from the Children’s Oncology Group. Cancer. 2012;118:4597–4605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Campanile C, Arlt MJ, Kramer SD, et al. Characterization of different osteosarcoma phenotypes by PET imaging in preclinical animal models. J Nucl Med. 2013;54:1362–1368. [DOI] [PubMed] [Google Scholar]
- 24.Harrison DJ, Parisi MT, Shulkin BL, et al. 18F 2fluoro-2deoxy-d-glucose positron emission tomography (FDG-PET) response to predict event-free survival (EFS) in intermediate risk (IR) or high risk (HR) rhabdomyosarcoma (RMS): a report from the Soft Tissue Sarcoma Committee of the Children's Oncology Group (COG) [abstract]. J Clin Oncol. 2016;34(suppl):10549. [Google Scholar]
- 25.Hawkins DS, Schuetze SM, Butrynski JE, et al. [18F]fluorodeoxyglucose positron emission tomography predicts outcome for Ewing sarcoma family of tumors. J Clin Oncol. 2005;23:8828–8834. [DOI] [PubMed] [Google Scholar]
- 26.Koshkin VS, Bolejack V, Schwartz LH, et al. Assessment of imaging modalities and response metrics in Ewing sarcoma: correlation with survival. J Clin Oncol. 2016;34:3680–3685. [DOI] [PMC free article] [PubMed] [Google Scholar]