Abstract
Bacteria are social creatures that are able to interact and coordinate behaviors with each other in a multitude of ways. The study of such group behaviors in microbes was coined “sociomicrobiology” in 2005. Two such group behaviors in bacteria are quorum sensing (QS) and biofilm formation. At a very basic level, QS is the ability to sense bacterial density via cell-to-cell signaling using self-produced signals called autoinducers, and biofilms are aggregates of cells that are attached to one another via a self-produced, extracellular matrix. Since cells in biofilm aggregates are in close proximity, biofilms represent an ecologically relevant environment for QS. While QS is known to affect biofilm formation in both Gram-negative and Gram-positive species, in this review, we will focus exclusively on Gram-negative bacteria, with an emphasis on Pseudomonas aeruginosa. We will begin by describing QS systems in P. aeruginosa and how they affect P. aeruginosa biofilm formation. We then expand our review to other Gram-negative bacteria and conclude with interesting questions with regard to the effect of biofilms on QS.
Keywords: sociomicrobiology, quorum sensing, biofilm, signaling
1. Quorum Sensing in Pseudomonas aeruginosa
Bacteria are social creatures that interact and coordinate behaviors with each other in a multitude of ways. One mechanism involves cell-to-cell signaling, also known as quorum sensing (QS). QS is commonly found in both Gram-negative and Gram-positive species, and utilizes self-produced signals. These signals come in a variety of forms, e.g. small peptides, acyl-homoserine lactones (AHL), and quinolones [1,2,3,4]. Currently, there are a wide range of QS systems that have been identified, but AHL-based QS represents one of the most thoroughly studied systems [5,6].
The basic AHL-based QS system usually consists of three components: a cytoplasmic AHL synthase protein of the LuxI family, an AHL-responsive DNA-binding transcriptional regulator belonging to the LuxR family, and an acyl-homoserine lactone signal, which has a conserved homoserine lactone ring linked by an amide bond to side chains that vary depending upon the species and the system [1,4]. In canonical QS systems, the signal synthase and the transcriptional regulator are located on the chromosome in tandem or are separated by one or two genes [7]. In some species, there are luxR homologs that are not in the vicinity of a luxI-like gene, which are referred to as solos or orphans.
Cells produce and release AHL signals at a low basal rate. AHL signals are capable of freely diffusing across the cell membrane, eliminating the need for a specific AHL-signal receptor on the cell surface. When the signals reach a critical threshold concentration in the local environment, they interact with the LuxR-family transcriptional regulator, which in turn changes gene expression by binding to a conserved DNA sequence in the promoter region (called a lux box). In several systems, the gene encoding the LuxI signal synthase is positively regulated by QS, leading to positive feedback of the system [8,9].
In Pseudomonas aeruginosa, there are two QS systems: las and rhl. The las system is composed of LasI, which synthesizes N-3-oxo-dodecanoyl-l-homoserine lactone, and LasR, the cognate transcriptional regulator that senses the signal. In the rhl system, RhlI is responsible for the production of N-butanoyl-l-homoserine lactone, which is recognized by RhlR. In addition, there is an “orphan” LuxR regulator called QscR that has been shown to bind the signal produced by LasI and to affect both the las and rhl systems. As there are las-boxes upstream of both lasI and rhlI, LasR not only positively auto-regulates the las system, but also promotes early activation of the rhl system. Strains lacking a functional las system where rhl is still functional have been reported, but rhl activation is delayed [10,11].
One relevant non-AHL QS system in P. aeruginosa is the pqs system. This system uses 2-alkyl-4-quinolones (AQs) as signals, and expression studies have shown that it has a relationship with the las and rhl regulons. The system is composed of the pqsABCDE operon, and three other transcriptional units, pqsR (also known as mvfR), pqsH, and pqsL. P. aeruginosa produces dozens of AQs and the most commonly associated with QS are 2-heptyl-3-hydroxy-4-quinolone (PQS) and its precursor 2-heptyl-4-hydroxyquinoline (HHQ). The synthesis of these molecules is dependent on the pqsABCDE operon, but production of PQS also requires pqsH under aerobic conditions via the oxidation of HHQ. Like AHL QS systems, pqs is subject to positive autoregulation via its LysR-family transcriptional regulator PqsR, which is known to regulate pqsABCDE-phnAB expression.
QS in P. aeruginosa is very complex and can be modulated by transcriptional regulators (RsaL and MvaT), sigma factors (RpoS and RpoN), post-transcriptional regulators (RsmA), and even other QS systems (pqs system) [3,8,9,12,13,14,15,16]. Detailing all these levels of regulation is not within the scope of this review. The genes regulated by QS in P. aeruginosa have been studied by multiple groups using diverse methodologies and culturing conditions. Depending upon the study, the QS regulon usually comprises over 300 genes, of which about a hundred are common between the different studies. This suggests that the QS regulon is fairly plastic, changing with environmental conditions. Nevertheless, considering that the PAO1 genome has 5688 genes, over 5% of its genes are either directly or indirectly QS controlled, emphasizing the global regulatory nature of QS. Among the functions regulated by the las and rhl systems are several virulence factors that have been associated with biofilm formation, such as lectins, rhamnolipids, and siderophores [17,18,19].
2. QS-Regulated Factors that Influence Biofilm Formation in P. aeruginosa
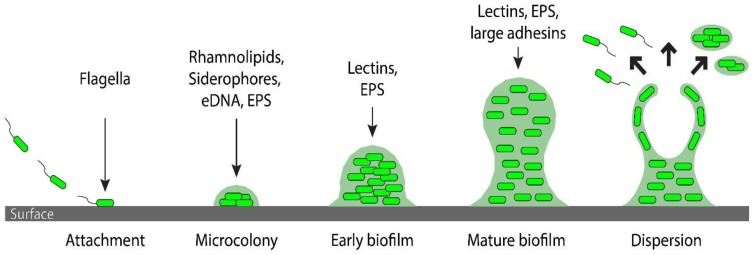
The first report to link P. aeruginosa QS to biofilm formation showed that, contrary to the large aggregates characteristic of wild-type biofilms, the lasI mutant produced a biofilm that was flat, structurally homogenous, and had a densely packed multilayer of cells that was sensitive to detergent treatment [20]. Additionally, a later study showed that furanones, which are AHL analogues that compete for the binding to the LuxR homologs, impacted biofilm formation producing a phenotype similar to that of a lasI mutant [21]. Subsequent studies attempted to identify which QS-controlled factors were responsible for the observed biofilm defect. These studies revealed that a wide range of QS-controlled functions are capable of influencing biofilm formation (Figure 1).
Figure 1.
Schematic of the stages of biofilm development regulated by quorum sensing factors. The first stage of biofilm formation is the attachment of bacteria to a surface, which has been associated with flagella. In the second stage, the ability to produce rhamnolipids (swarming), siderophores (iron availability), eDNA and EPS (matrix formation) are thought to be essential for microcolony formation. In the subsequent stages of biofilm maturation, lectins, adhesins and EPS are important for the proper building of the matrix and localization of its components. The final stage is biofilm dispersion. Little mechanistic data is currently available to establish a role for quorum sensing in this process.
Las- and Rhl-controlled factors. Rhamnolipid production, which is under the control of the rhl QS system, was shown to be required for maintaining the open spaces between biofilm aggregates. Rhamnolipids also affect a type of surface motility called swarming that has been implicated in biofilm development [22,23]. Two QS-dependent carbohydrate-binding lectins, LecA and LecB, were shown to influence biofilm formation, although the mechanism is unclear. Mutant strains were able to initiate biofilm formation by adhering to surfaces, but were not able to fully develop a mature biofilm [24,25]. Another QS-controlled factor is iron siderophores, such as pyoverdine. Mutants unable to produce this iron chelator were not able to generate biofilm aggregates [26].
PQS-controlled factors. Notably, the PQS system controls the expression of biofilm relevant functions that overlap with las and rhl-regulated elements, such as LecA [27] and siderophore production [28]. A unique PQS-controlled factor involved in biofilm formation is a component of the biofilm matrix: extracellular DNA (eDNA). In biofilm formation, the pqs QS system is responsible for an increased production of eDNA, which is capable of interacting with a positively charged exopolysaccharide present in the matrix (Pel). This interaction is thought to be important in generating the initial scaffolding for the biofilm. In strains lacking the ability to make PQS, mature biofilm aggregates never fully develop [27,29,30,31]. Thus, several QS-regulated functions have the potential to influence biofilm production (Figure 1).
3. Environmental Parameters Influencing QS Control of Biofilm Formation
Considering that the QS regulon is fluid and changes in response to environmental conditions, it is not surprising that the importance of QS for biofilm formation can vary tremendously. Therefore, it is not uncommon to find conflicting reports in the literature regarding the importance of a given QS-controlled function for biofilm production.
Nutrition. In P. aeruginosa, the carbon source can have a dramatic effect on the contribution of QS to biofilm formation. When grown on glutamate or glucose as the sole carbon source, QS mutants produce biofilms similar to those of the wild-type strain. However, when succinate is used as the sole carbon source, QS mutants produce biofilms that are structurally distinct from that of wild type. The authors attributed this phenotype to nutritionally-dependent QS control of swarming [32].
pH. QS is impacted by several non-biological elements of the environment. One such element is pH. AHLs are fairly stable under neutral and acidic pH, but at high pH, the half-life of these molecules can be reduced to minutes, due to chemical hydrolysis of the homoserine lactone ring [33,34]. Since the physiological activity of microbes can generate significant pH gradients from the exterior to the interior of biofilm aggregates [35], the microbes in the biofilm may influence and create QS signal concentration gradients.
Mass transfer. Physical parameters may also be important; the impact of flow rates on QS signaling within a biofilm has been demonstrated [36,37]. QS relies on local concentrations of AHLs that freely diffuse across the membrane and into the environment. With high flow rates, the rate of signal accumulation inside the biofilm structure could vary due to signal wash-out [38]. As mass transfer would prevent the build-up of signal to inducing concentrations, this could potentially lead to wild-type and QS mutant strains being nearly indistinguishable. Conversely, at extremely low flow rates, diffusion of AHLs would be very low, creating pockets of high AHL concentrations within the community [39]. Finally, flow rates could influence the onset of QS, with high flow delaying initially attached cells and small cell aggregates from sensing quorum.
Much of the discussion associated with these points emphasize the contextual nature of QS and its importance in biofilm communities. For instance, P. aeruginosa can produce radically different biofilm infections on different surfaces. Biofilms produced in the airways of people suffering from cystic fibrosis are found in thick, static mucus. The bacteria obtain their nutrients from host-derived compounds that are released due to the damage caused by the bacteria. These aggregates are suspended, and are subject to little or almost no flow. On the other hand, P. aeruginosa can also colonize urinary catheters, causing urinary tract infections. These biofilms would experience a completely different environment with regard to the attachment surface and flow. One can imagine that the point at which QS initiates and its contribution to biofilm development could be completely different in these two types of infection.
4. QS-Regulated Factors that Influence Biofilm Formation in Other Species
QS affects biofilm formation in a myriad of species besides P. aeruginosa [40,41,42,43]. In many cases, it is not mechanistically clear how QS impacts biofilm structure. Here, we have focused on those non-Pseudomonad systems for which we have some mechanistic insight.
Attachment and flagellar-based motility. For some species, QS has been shown to regulate flagellar synthesis and activity, which influence the attachment of bacteria to a surface (Figure 1) [44]. In Escherichia coli, mqsR expression is stimulated in the presence of autoinducer-2 (AI-2), a QS signal that is produced by a LuxS-family synthase protein in many bacteria, but not in P. aeruginosa. MqsR positively regulates qseB, fliA, and motA [45], which are involved in flagellar gene expression [46], biosynthesis [47], and motor function [48], respectively. Strains bearing mutations in these flagellum-related genes, as well as motB and fliF, are defective in attachment to many types of surfaces [49]. In addition, the fliA and fliF genes have also been shown to be upregulated 10-fold and 12-fold, respectively, in wild type E. coli strains versus a LuxS mutant. These LuxS mutant strains produce significantly less flagellin and, consequently, fewer flagella [50]. QS has also been shown to regulate flagellar-based motility in Vibrio vulnificus. An smcR (a luxR homolog) mutant strain displayed a reduced swimming diameter compared to that of wild type [51].
In contrast, QS affects biofilm formation by negatively regulating flagellar-based motility in other species. Mutations in the luxR homolog opaR of Vibrio parahaemolyticus result in the production of significantly more lateral flagella, and are hypermotile compared to wild type strains [52]. Mutations within the Burkholderia thailandensis luxR homolog btaR1 exhibit a similar hypermotility phenotype as V. parahaemolyticus opaR mutant strains [53,54]. However, B. thailandensis btaR1 mutant cells show no difference in attachment relative to wild type [55], while a V. parahaemolyticus opaR mutants are attachment deficient [56]. Therefore, the role of QS in regulating motility and surface attachment is highly variable across species.
Formation of mature biofilms. In addition to attachment, QS regulates other aspects of biofilm formation, including accumulation of biofilm biomass, biofilm structure, and the dispersal of biofilm cells. Burkholderia pseudomallei requires a functional bpsR and bpsI (luxR and luxI homologues) for biofilm production; strains harboring mutations in these genes produced biofilms with approximately a 50% and 75% reduction in biomass, respectively, relative to wild type biofilms [57]. Similar to B. pseudomallei, mutations in genes required for AI-2 uptake, lsrR and lsrK, in E. coli result in a reduction of biofilm thickness and total biomass compared to wild type [58]. In addition, these mutant strains show a deficiency in autoaggregation [58]. QS has been shown to affect autoaggregation in B. thailandensis as well. QS system-1 (QS1) of B. thailandensis promotes autoaggregation [53], and mutant strains defective for QS1 formed biofilms with aberrant biofilm structure compared to wild type [55].
Similar to B. pseudomallei, V. vulnificus containing mutations in the luxR homolog smcR are deficient for biofilm formation on glass and polystyrene surfaces [51]. However, as previously discussed for P. aeruginosa, the contribution of QS to biofilm formation is environmentally dependent. Under minimal nutrient conditions, smcR mutant strains can form biofilms on polystyrene surfaces. In such conditions, the smcR mutant cells reached a maximum biomass sooner, and formed thicker biofilms than those of wild-type cells. At later stages, smcR mutant biofilms retained relatively high levels of biofilm biomass, suggesting that these strains exhibit a defect in biofilm dispersal compared to wild-type [59].
Similar to P. aeruginosa, QS can also regulate the production of biofilm matrix components, such as exopolysaccharides and cell surface proteins, in other organisms (Figure 1). In many Vibrio species, colony morphology correlates to exopolysaccharide production. Specifically, opaque colonies indicate an increased quantity of the exopolysaccharide Vps [42]. In V. parahaemolyticus, induction of the luxR homolog opaR produced opaque colonies from previously translucent strains [60]. Similarly, in V. vulnificus, mutant strains of the luxR homolog smcR displayed more translucent colonies than that of wild type, indicating a decrease in exopolysaccharide production [61]. This role of QS in biofilm matrix production is also seen in E. coli and B. cenocepacia. In E. coli, ydgG mutants (renamed tqsA), an exporter of AI-2 molecules, have increased levels of intracellular AI-2, and had approximately a four-fold increase in the production of curli fibers, which are important in adhesion and biofilm maturation [62]. In B. cenocepacia, QS positively regulates the cell surface protein BapA, which is required of normal biofilm formation [63]. Strains with a mutation in bapA formed biofilms characterized by separated discontinuous aggregates, with a biofilm structure that was more porous compared to the wild type biofilm [63].
The curious case of Vibrio cholerae. In contrast to the species mentioned above, V. cholerae presents a very different scenario in terms of the effect of QS regulation on biofilm formation. In the organisms mentioned above, high cell density conditions initiate QS-regulated functions that promote biofilm formation. However, this is not the case in V. cholerae. LuxO is a two-component response regulator in V. cholerae involved in the response to QS signals. Mutations in luxO are locked in a state mimicking high cell density. These mutants show an inability to form biofilms [2]. Additionally, mutations in the luxR homolog hapR that result in a state of mimicking low cell density conditions form larger pellicles than wild-type, and have been suggested to be deficient in biofilm dispersal [64]. Mutant strains of hapR also overproduce the exopolysaccharide Vps, and are defective in attachment and colonization in mouse models [64]. An organism that behaves in a similar fashion to V. cholerae is the Gram-positive pathogen Staphylococcus aureus. While QS is very different in S. aureus in comparison to the Gram-negative organisms described in this review, mutants for agr, a QS system in S. aureus, form biofilms with increased biomass in comparison to those of wild-type [65]. Additionally, similar to that suggested for V. cholerae, the agr system controls the expression of the psm genes, which encode surfactants that promote biofilm dispersal [66].
5. Future Questions/Directions
To date, much research has focused on the impact of QS on biofilm structure through the study of QS mutant strains. Since biofilm structure has been intimately linked to antimicrobial susceptibility, this has given rise to an interest in therapies directed towards QS as a way to treat biofilm infections. However, a number of interesting questions remain regarding the basic biology of QS and biofilm communities.
Moving beyond the impact of QS on biofilm structure. For many species, QS controls the expression of secreted and/or extracellular functions. In many cases, the extracellular activity of these enzymes in the context of a single cell would be irrelevant. In the presence of a quorum of cells however, the enzyme levels and activity are sufficient to provide a benefit to the cells producing them. If a quorum is achieved in a planktonic community, the assumption is that this must represent, in some fashion, a closed system. In other words, these cells are present in a fixed volume that is not subject to the effects of mass transfer. Thus, it is assumed that secreted QS-controlled enzymes, and the QS signals that precede them, are present in a fixed concentration in the surrounding bulk fluid.
In the context of a biofilm, this point is superimposed on an additional layer of complexity. The stationary/sessile nature of the community might be influenced by external forces, such as fluid flow, that might remove QS-controlled enzymes from the biofilm. One simple inference may be that if QS signals can achieve a high enough concentration in the biofilm system, that QS-controlled extracellular functions may provide a transient benefit. However, much of the energy invested in producing these enzymes would be wasted as these enzymes would eventually be removed from the community by mass transfer. This begs the question as to whether there are mechanisms by which a biofilm community can selectively retain extracellular enzymes. There are many potential mechanisms by which this might occur, including specific interactions of these enzymes with biofilm exopolysaccharides.
Another simple question that remains largely unanswered is how QS can influence a biofilm community apart from structure? Depending upon the species, there are many different classes of QS-regulated functions. These might include functions involved in nutrient acquisition, protecting the community from environmental stressors, and antimicrobial activity. One can imagine that mutant strains defective for these functions may have a tremendous impact on the community that is independent of biofilm structure. How these functions would work in a structured system like a biofilm is an interesting question.
Ecology of cheating in structured systems. An expanding area of research in QS draws upon classic ecological theory. The appearance and significance of cheater subpopulations within a QS community is one such question that has been investigated and has relevance to disease in some species. The concept of public and private goods is also central to many of the research directions in this area. However, most of this research has been conducted on planktonic populations of cells.
Considering how these concepts would play out in a biofilm is intriguing. What would be the distribution and frequency of cheaters in a biofilm system? What would be the carrying capacity of cheaters in such a system, and what parameters would influence this? As cheaters appear in the system, would they give rise to clonal pockets of cheaters whose size would be limited by the mass transfer efficiency of public goods? These points are important since in many cases the “real-world” significance of cheating would pertain to biofilm communities. For example, the ability to form biofilms is a feature of many chronic diseases that P. aeruginosa can cause. Additionally, since QS mutant strains are frequently isolated from these infections, the importance of QS cheaters has been hypothesized to be important as well. Thus, if QS cheating occurs in a biofilm, how it occurs and what influences it may be very important questions for the scientific community to address.
Acknowledgments
D.P.d.S. and M.R.P. were funded by the NIH (R01 AI077628, R01 AI097511, P30 DK089507) and the Cystic Fibrosis Foundation (CFR565-CR11). M.C.S. and B.S.T. were funded by the NIH (K22 AI121097).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- 1.Fuqua C., Greenberg E.P. Self perception in bacteria: Quorum sensing with acylated homoserine lactones. Curr. Opin. Microbiol. 1998;1:183–189. doi: 10.1016/S1369-5274(98)80009-X. [DOI] [PubMed] [Google Scholar]
- 2.Hammer B.K., Bassler B.L. Quorum sensing controls biofilm formation in Vibrio cholerae. Mol. Microbiol. 2003;50:101–104. doi: 10.1046/j.1365-2958.2003.03688.x. [DOI] [PubMed] [Google Scholar]
- 3.McKnight S.L., Iglewski B.H., Pesci E.C. The Pseudomonas quinolone signal regulates rhl quorum sensing in Pseudomonas aeruginosa. J. Bacteriol. 2000;182:2702–2708. doi: 10.1128/JB.182.10.2702-2708.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Papenfort K., Bassler B.L. Quorum sensing signal-response systems in gram-negative bacteria. Nat. Rev. Microbiol. 2016;14:576–588. doi: 10.1038/nrmicro.2016.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kaplan H.B., Greenberg E.P. Overproduction and purification of the luxR gene product: Transcriptional activator of the Vibrio fischeri luminescence system. Proc. Natl. Acad. Sci. USA. 1987;84:6639–6643. doi: 10.1073/pnas.84.19.6639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schaefer A.L., Val D.L., Hanzelka B.L., Cronan J.E., Jr., Greenberg E.P. Generation of cell-to-cell signals in quorum sensing: Acyl homoserine lactone synthase activity of a purified Vibrio fischeri luxi protein. Proc. Natl. Acad. Sci. USA. 1996;93:9505–9509. doi: 10.1073/pnas.93.18.9505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gelencser Z., Choudhary K.S., Coutinho B.G., Hudaiberdiev S., Galbats B., Venturi V., Pongor S. Classifying the topology of ahl-driven quorum sensing circuits in proteobacterial genomes. Sensors. 2012;12:5432–5444. doi: 10.3390/s120505432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schuster M., Greenberg E.P. A network of networks: Quorum-sensing gene regulation in Pseudomonas aeruginosa. Int. J. Med. Microbiol. 2006;296:73–81. doi: 10.1016/j.ijmm.2006.01.036. [DOI] [PubMed] [Google Scholar]
- 9.Venturi V. Regulation of quorum sensing in Pseudomonas. FEMS Microbiol. Rev. 2006;30:274–291. doi: 10.1111/j.1574-6976.2005.00012.x. [DOI] [PubMed] [Google Scholar]
- 10.Pearson J.P., Pesci E.C., Iglewski B.H. Roles of Pseudomonas aeruginosa las and rhl quorum-sensing systems in control of elastase and rhamnolipid biosynthesis genes. J. Bacteriol. 1997;179:5756–5767. doi: 10.1128/jb.179.18.5756-5767.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pesci E.C., Pearson J.P., Seed P.C., Iglewski B.H. Regulation of las and rhl quorum sensing in Pseudomonas aeruginosa. J. Bacteriol. 1997;179:3127–3132. doi: 10.1128/jb.179.10.3127-3132.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chugani S.A., Whiteley M., Lee K.M., D’Argenio D., Manoil C., Greenberg E.P. Qscr, a modulator of quorum-sensing signal synthesis and virulence in Pseudomonas aeruginosa. Proc. Natl. Acad. Sci. USA. 2001;98:2752–2757. doi: 10.1073/pnas.051624298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Diggle S.P., Winzer K., Lazdunski A., Williams P., Camara M. Advancing the quorum in Pseudomonas aeruginosa: Mvat and the regulation of N-acylhomoserine lactone production and virulence gene expression. J. Bacteriol. 2002;184:2576–2586. doi: 10.1128/JB.184.10.2576-2586.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rampioni G., Bertani I., Zennaro E., Polticelli F., Venturi V., Leoni L. The quorum-sensing negative regulator rsaL of Pseudomonas aeruginosa binds to the lasI promoter. J. Bacteriol. 2006;188:815–819. doi: 10.1128/JB.188.2.815-819.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rampioni G., Falcone M., Heeb S., Frangipani E., Fletcher M.P., Dubern J.F., Visca P., Leoni L., Camara M., Williams P. Unravelling the genome-wide contributions of specific 2-alkyl-4-quinolones and pqsE to quorum sensing in Pseudomonas aeruginosa. PLoS Pathog. 2016;12:e1006029. doi: 10.1371/journal.ppat.1006029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Venturi V. Control of rpoS transcription in Escherichia coli and pseudomonas: Why so different? Mol. Microbiol. 2003;49:1–9. doi: 10.1046/j.1365-2958.2003.03547.x. [DOI] [PubMed] [Google Scholar]
- 17.Schuster M., Lostroh C.P., Ogi T., Greenberg E.P. Identification, timing, and signal specificity of Pseudomonas aeruginosa quorum-controlled genes: A transcriptome analysis. J. Bacteriol. 2003;185:2066–2079. doi: 10.1128/JB.185.7.2066-2079.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wagner V.E., Bushnell D., Passador L., Brooks A.I., Iglewski B.H. Microarray analysis of Pseudomonas aeruginosa quorum-sensing regulons: Effects of growth phase and environment. J. Bacteriol. 2003;185:2080–2095. doi: 10.1128/JB.185.7.2080-2095.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Whiteley M., Lee K.M., Greenberg E.P. Identification of genes controlled by quorum sensing in Pseudomonas aeruginosa. Proc. Natl. Acad. Sci. USA. 1999;96:13904–13909. doi: 10.1073/pnas.96.24.13904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Davies D.G., Parsek M.R., Pearson J.P., Iglewski B.H., Costerton J.W., Greenberg E.P. The involvement of cell-to-cell signals in the development of a bacterial biofilm. Science. 1998;280:295–298. doi: 10.1126/science.280.5361.295. [DOI] [PubMed] [Google Scholar]
- 21.Hentzer M., Riedel K., Rasmussen T.B., Heydorn A., Andersen J.B., Parsek M.R., Rice S.A., Eberl L., Molin S., Hoiby N., et al. Inhibition of quorum sensing in Pseudomonas aeruginosa biofilm bacteria by a halogenated furanone compound. Microbiology. 2002;148:87–102. doi: 10.1099/00221287-148-1-87. [DOI] [PubMed] [Google Scholar]
- 22.Boles B.R., Horswill A.R. Agr-mediated dispersal of Staphylococcus aureus biofilms. PLoS Pathog. 2008;4:e1000052. doi: 10.1371/journal.ppat.1000052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Davey M.E., Caiazza N.C., O’Toole G.A. Rhamnolipid surfactant production affects biofilm architecture in Pseudomonas aeruginosa PAO1. J. Bacteriol. 2003;185:1027–1036. doi: 10.1128/JB.185.3.1027-1036.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tielker D., Hacker S., Loris R., Strathmann M., Wingender J., Wilhelm S., Rosenau F., Jaeger K.E. Pseudomonas aeruginosa lectin LecB is located in the outer membrane and is involved in biofilm formation. Microbiology. 2005;151:1313–1323. doi: 10.1099/mic.0.27701-0. [DOI] [PubMed] [Google Scholar]
- 25.Winzer K., Falconer C., Garber N.C., Diggle S.P., Camara M., Williams P. The Pseudomonas aeruginosa lectins PA-IL and PA-IIL are controlled by quorum sensing and by rpoS. J. Bacteriol. 2000;182:6401–6411. doi: 10.1128/JB.182.22.6401-6411.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Banin E., Vasil M.L., Greenberg E.P. Iron and Pseudomonas aeruginosa biofilm formation. Proc. Natl. Acad. Sci. USA. 2005;102:11076–11081. doi: 10.1073/pnas.0504266102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Diggle S.P., Winzer K., Chhabra S.R., Worrall K.E., Camara M., Williams P. The Pseudomonas aeruginosa quinolone signal molecule overcomes the cell density-dependency of the quorum sensing hierarchy, regulates rhl-dependent genes at the onset of stationary phase and can be produced in the absence of LasR. Mol. Microbiol. 2003;50:29–43. doi: 10.1046/j.1365-2958.2003.03672.x. [DOI] [PubMed] [Google Scholar]
- 28.Heeb S., Fletcher M.P., Chhabra S.R., Diggle S.P., Williams P., Camara M. Quinolones: From antibiotics to autoinducers. FEMS Microbiol. Rev. 2011;35:247–274. doi: 10.1111/j.1574-6976.2010.00247.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Allesen-Holm M., Barken K.B., Yang L., Klausen M., Webb J.S., Kjelleberg S., Molin S., Givskov M., Tolker-Nielsen T. A characterization of DNA release in Pseudomonas aeruginosa cultures and biofilms. Mol. Microbiol. 2006;59:1114–1128. doi: 10.1111/j.1365-2958.2005.05008.x. [DOI] [PubMed] [Google Scholar]
- 30.Jennings L.K., Storek K.M., Ledvina H.E., Coulon C., Marmont L.S., Sadovskaya I., Secor P.R., Tseng B.S., Scian M., Filloux A., et al. Pel is a cationic exopolysaccharide that cross-links extracellular DNA in the Pseudomonas aeruginosa biofilm matrix. Proc. Natl. Acad. Sci. USA. 2015;112:11353–11358. doi: 10.1073/pnas.1503058112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yang L., Barken K.B., Skindersoe M.E., Christensen A.B., Givskov M., Tolker-Nielsen T. Effects of iron on DNA release and biofilm development by Pseudomonas aeruginosa. Microbiology. 2007;153:1318–1328. doi: 10.1099/mic.0.2006/004911-0. [DOI] [PubMed] [Google Scholar]
- 32.Shrout J.D., Chopp D.L., Just C.L., Hentzer M., Givskov M., Parsek M.R. The impact of quorum sensing and swarming motility on Pseudomonas aeruginosa biofilm formation is nutritionally conditional. Mol. Microbiol. 2006;62:1264–1277. doi: 10.1111/j.1365-2958.2006.05421.x. [DOI] [PubMed] [Google Scholar]
- 33.Chopp D.L., Kirisits M.J., Moran B., Parsek M.R. The dependence of quorum sensing on the depth of a growing biofilm. Bull. Math. Biol. 2003;65:1053–1079. doi: 10.1016/S0092-8240(03)00057-0. [DOI] [PubMed] [Google Scholar]
- 34.Schaefer A.L., Hanzelka B.L., Parsek M.R., Greenberg E.P. Detection, purification, and structural elucidation of the acylhomoserine lactone inducer of Vibrio fischeri luminescence and other related molecules. Methods Enzymol. 2000;305:288–301. doi: 10.1016/s0076-6879(00)05495-1. [DOI] [PubMed] [Google Scholar]
- 35.Wilton M., Charron-Mazenod L., Moore R., Lewenza S. Extracellular DNA acidifies biofilms and induces aminoglycoside resistance in Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 2015;60:544–553. doi: 10.1128/AAC.01650-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pereira M.O., Kuehn M., Wuertz S., Neu T., Melo L.F. Effect of flow regime on the architecture of a Pseudomonas fluorescens biofilm. Biotechnol. Bioeng. 2002;78:164–171. doi: 10.1002/bit.10189. [DOI] [PubMed] [Google Scholar]
- 37.Purevdorj B., Costerton J.W., Stoodley P. Influence of hydrodynamics and cell signaling on the structure and behavior of Pseudomonas aeruginosa biofilms. Appl. Environ. Microbiol. 2002;68:4457–4464. doi: 10.1128/AEM.68.9.4457-4464.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dingemans J., Monsieurs P., Yu S.H., Crabbe A., Forstner K.U., Malfroot A., Cornelis P., Van Houdt R. Effect of shear stress on Pseudomonas aeruginosa isolated from the cystic fibrosis lung. mBio. 2016;7 doi: 10.1128/mBio.00813-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.West S.A., Winzer K., Gardner A., Diggle S.P. Quorum sensing and the confusion about diffusion. Trends Microbiol. 2012;20:586–594. doi: 10.1016/j.tim.2012.09.004. [DOI] [PubMed] [Google Scholar]
- 40.Beloin C., Roux A., Ghigo J.M. Escherichia coli biofilms. Curr. Top. Microbiol. Immunol. 2008;322:249–289. doi: 10.1007/978-3-540-75418-3_12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fazli M., Almblad H., Rybtke M.L., Givskov M., Eberl L., Tolker-Nielsen T. Regulation of biofilm formation in Pseudomonas and Burkholderia species. Environ. Microbiol. 2014;16:1961–1981. doi: 10.1111/1462-2920.12448. [DOI] [PubMed] [Google Scholar]
- 42.Yildiz F.H., Visick K.L. Vibrio biofilms: So much the same yet so different. Trends Microbiol. 2009;17:109–118. doi: 10.1016/j.tim.2008.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hmelo L.R. Quorum sensing in marine microbial environments. Ann. Rev. Mar. Sci. 2017;9:257–281. doi: 10.1146/annurev-marine-010816-060656. [DOI] [PubMed] [Google Scholar]
- 44.Petrova O.E., Sauer K. Sticky situations: Key components that control bacterial surface attachment. J. Bacteriol. 2012;194:2413–2425. doi: 10.1128/JB.00003-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.González Barrios A.F., Zuo R., Hashimoto Y., Yang L., Bentley W.E., Wood T.K. Autoinducer 2 controls biofilm formation in Escherichia coli through a novel motility quorum-sensing regulator (mqsr, b3022) J. Bacteriol. 2006;188:305–316. doi: 10.1128/JB.188.1.305-316.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sperandio V., Torres A.G., Kaper J.B. Quorum sensing Escherichia coli regulators b and c (qseBC): A novel two-component regulatory system involved in the regulation of flagella and motility by quorum sensing in E. Coli. Mol. Microbiol. 2002;43:809–821. doi: 10.1046/j.1365-2958.2002.02803.x. [DOI] [PubMed] [Google Scholar]
- 47.Chilcott G.S., Hughes K.T. Coupling of flagellar gene expression to flagellar assembly in Salmonella enterica serovar typhimurium and Escherichia coli. Microbiol. Mol. Biol. Rev. 2000;64:694–708. doi: 10.1128/MMBR.64.4.694-708.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.DeRosier D.J. The turn of the screw: The bacterial flagellar motor. Cell. 1998;93:17–20. doi: 10.1016/S0092-8674(00)81141-1. [DOI] [PubMed] [Google Scholar]
- 49.Pratt L.A., Kolter R. Genetic analysis of Escherichia coli biofilm formation: Roles of flagella, motility, chemotaxis and type I pili. Mol. Microbiol. 1998;30:285–293. doi: 10.1046/j.1365-2958.1998.01061.x. [DOI] [PubMed] [Google Scholar]
- 50.Sperandio V., Torres A.G., Girón J.A., Kaper J.B. Quorum sensing is a global regulatory mechanism in enterohemorrhagic Escherichia coli o157:H7. J. Bacteriol. 2001;183:5187–5197. doi: 10.1128/JB.183.17.5187-5197.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lee J.H., Rhee J.E., Park U., Ju H.M., Lee B.C., Kim T.S., Jeong H.S., Choi S.H. Identification and functional analysis of Vibrio vulnificus SmcR, a novel global regulator. J. Microbiol. Biotechnol. 2007;17:325–334. [PubMed] [Google Scholar]
- 52.Jaques S., McCarter L.L. Three new regulators of swarming in Vibrio parahaemolyticus. J. Bacteriol. 2006;188:2625–2635. doi: 10.1128/JB.188.7.2625-2635.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chandler J.R., Duerkop B.A., Hinz A., West T.E., Herman J.P., Churchill M.E., Skerrett S.J., Greenberg E.P. Mutational analysis of Burkholderia thailandensis quorum sensing and self-aggregation. J. Bacteriol. 2009;191:5901–5909. doi: 10.1128/JB.00591-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ulrich R.L., Hines H.B., Parthasarathy N., Jeddeloh J.A. Mutational analysis and biochemical characterization of the Burkholderia thailandensis dw503 quorum-sensing network. J. Bacteriol. 2004;186:4350–4360. doi: 10.1128/JB.186.13.4350-4360.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tseng B.S., Majerczyk C.D., Passos da Silva D., Chandler J.R., Greenberg E.P., Parsek M.R. Quorum sensing influences Burkholderia thailandensis biofilm development and matrix production. J. Bacteriol. 2016;198:2643–2650. doi: 10.1128/JB.00047-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Güvener Z.T., McCarter L.L. Multiple regulators control capsular polysaccharide production in Vibrio parahaemolyticus. J. Bacteriol. 2003;185:5431–5441. doi: 10.1128/JB.185.18.5431-5441.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gamage A.M., Shui G., Wenk M.R., Chua K.L. N-octanoylhomoserine lactone signalling mediated by the bpsI-bpsR quorum sensing system plays a major role in biofilm formation of Burkholderia pseudomallei. Microbiology. 2011;157:1176–1186. doi: 10.1099/mic.0.046540-0. [DOI] [PubMed] [Google Scholar]
- 58.Li J., Attila C., Wang L., Wood T.K., Valdes J.J., Bentley W.E. Quorum sensing in Escherichia coli is signaled by ai-2/LsrR: Effects on small RNA and biofilm architecture. J. Bacteriol. 2007;189:6011–6020. doi: 10.1128/JB.00014-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kim S.M., Park J.H., Lee H.S., Kim W.B., Ryu J.M., Han H.J., Choi S.H. LuxR homologue SmcR is essential for Vibrio vulnificus pathogenesis and biofilm detachment, and its expression is induced by host cells. Infect. Immun. 2013;81:3721–3730. doi: 10.1128/IAI.00561-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.McCarter L.L. OpaR, a homolog of Vibrio harveyi LuxR, controls opacity of Vibrio parahaemolyticus. J. Bacteriol. 1998;180:3166–3173. doi: 10.1128/jb.180.12.3166-3173.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lee K.J., Kim J.A., Hwang W., Park S.J., Lee K.H. Role of capsular polysaccharide (CPS) in biofilm formation and regulation of CPS production by quorum-sensing in Vibrio vulnificus. Mol. Microbiol. 2013;90:841–857. doi: 10.1111/mmi.12401. [DOI] [PubMed] [Google Scholar]
- 62.Herzberg M., Kaye I.K., Peti W., Wood T.K. YdgG (TqsA) controls biofilm formation in Escherichia coli k-12 through autoinducer 2 transport. J. Bacteriol. 2006;188:587–598. doi: 10.1128/JB.188.2.587-598.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Inhülsen S., Aguilar C., Schmid N., Suppiger A., Riedel K., Eberl L. Identification of functions linking quorum sensing with biofilm formation in Burkholderia cenocepacia H111. Microbiologyopen. 2012;1:225–242. doi: 10.1002/mbo3.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhu J., Mekalanos J.J. Quorum sensing-dependent biofilms enhance colonization in Vibrio cholerae. Dev. Cell. 2003;5:647–656. doi: 10.1016/S1534-5807(03)00295-8. [DOI] [PubMed] [Google Scholar]
- 65.Vuong C., Saenz H.L., Götz F., Otto M. Impact of the agr quorum-sensing system on adherence to polystyrene in Staphylococcus aureus. J. Infect. Dis. 2000;182:1688–1693. doi: 10.1086/317606. [DOI] [PubMed] [Google Scholar]
- 66.Guilhen C., Forestier C., Balestrino D. Biofilm dispersal: Multiple elaborate strategies for dissemination of bacteria with unique properties. Mol. Microbiol. 2017;105:188–210. doi: 10.1111/mmi.13698. [DOI] [PubMed] [Google Scholar]